Pharmacokinetic/Pharmacodynamic Evaluation of Aztreonam/Amoxicillin/Clavulanate Combination against New Delhi Metallo-β-Lactamase and Serine-β-Lactamase Co-Producing Escherichia coli and Klebsiella pneumoniae
Abstract
:1. Introduction
2. Methods
2.1. Antimicrobial Agents
2.2. Bacterial Isolates and Susceptibility Testing
2.3. Time–Kill Kinetic Assay
2.4. Population Pharmacokinetic Simulations
2.5. Pharmacodynamic Metrics to Quantify Suppression of Emergent Mutant
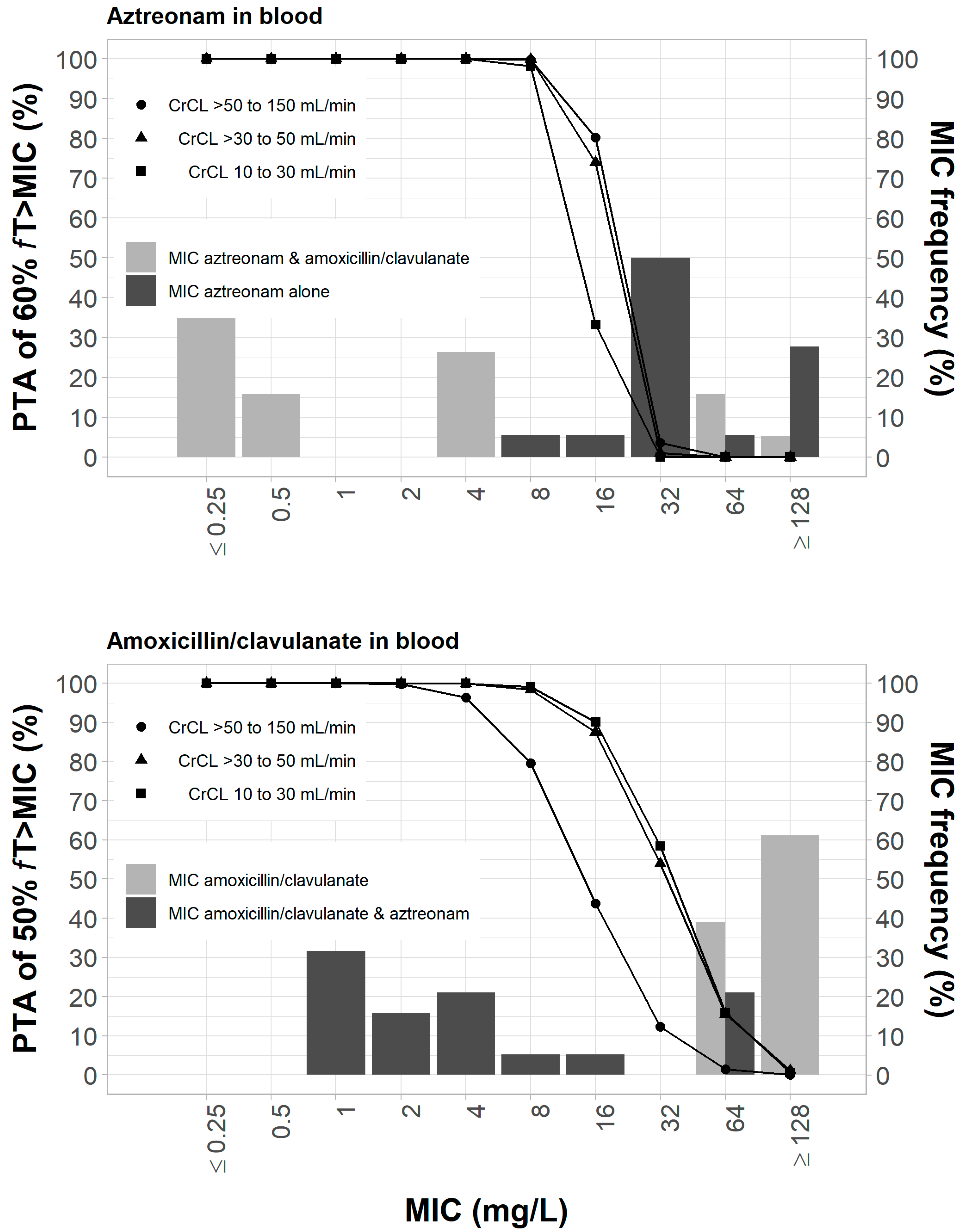
2.6. Target Pharmacodynamic Indices and Probability of Target Attainment
2.7. Software
3. Results
3.1. In Vitro Antimicrobial Susceptibility
3.2. Time–Kill Kinetics
3.3. Pharmacodynamic Analysis of Resistant Mutant Selection in Blood
3.4. Pharmacodynamic Analysis of Resistant Mutant Selection in ELF
4. Discussion
| ESBLs | IC50 (μM) | Reference | |||
|---|---|---|---|---|---|
| Clavulanic Acid | Tazobactam | Sulbactam | Avibactam | ||
| P99 | >100 | 1.3 | 21.1 | 0.1 | Stachyra et al., 2010 [20] |
| AmpC | >100 | 4.6 | 27 | 0.128 | |
| CTM-M-15 | 0.012 | 0.006 | 0.23 | 0.005 | |
| TEM-1 | 0.058 | 0.032 | 1.56 | 0.008 | |
| KPC-2 | >100 | 50 | 57 | 0.17 | |
| SHV-4 | 0.004 | 0.055 | 0.26 | 0.003 | |
| SHV-1 | 0.06 | 0.14 | 3.1 | Giakkoupi et al., 1999 [45] | |
| SHV-5 | 0.02 | 0.08 | 1.8 | ||
| OXA-24 | 50 | 0.5 | 40 | Bou et al., 2000 [22] | |
| TEM-1 | 0.09 | 0.04 | 6.1 | Payne et al., 1994 [21] | |
| TEM-2 | 0.18 | 0.05 | 8.7 | ||
| TEM-3 | 0.03 | 0.01 | 0.03 | ||
| TEM-5 | 0.03 | 0.28 | 1.2 | ||
| TEM-6 | 0.12 | 0.17 | 0.45 | ||
| TEM-7 | 0.10 | 0.18 | 0.62 | ||
| TEM-9 | 0.29 | 0.34 | 0.9 | ||
| TEM-10 | 0.03 | 0.08 | 0.34 | ||
| SHV-1 | 0.03 | 0.14 | 17 | ||
| SHV-2 | 0.05 | 0.13 | 2.8 | ||
| SHV-3 | 0.04 | 0.1 | 2.7 | ||
| SHV-5 | 0.01 | 0.08 | 0.63 | ||
| Enzyme A | 0.09 | 0.07 | 0.48 | ||
| Enzyme B | 0.01 | 0.01 | 0.12 | ||
| Enzyme C | 0.33 | 0.09 | 10 | ||
| Enzyme D | 0.04 | 0.01 | 0.57 | ||
| Enzyme E | 0.09 | 0.11 | 3.6 | ||
| TEM-E1 | 0.05 | 0.02 | 0.64 | ||
| TEM-E2 | 0.09 | 0.05 | 1.6 | ||
| TEM-E3 | 0.02 | 0.06 | 0.2 | ||
| TEM-E4 | 0.06 | 0.04 | 0.79 | ||
| CAZ-3 | 0.13 | 0.06 | 2.5 | ||
| DJP-1 | 0.01 | 0.02 | 0.21 | ||
| TLE-1 | 0.11 | 0.05 | 5.5 | ||
| MJ-1 | 0.09 | 0.43 | 40 | ||
| PSE-4 | 0.15 | 0.1 | 3.7 | ||
| BRO-1 | 0.02 | 0.02 | 0.02 | ||
| OXA-1 | 1.8 | 1.4 | 4.7 | ||
| OXA-2 | 1.4 | 0.01 | 0.14 | ||
| OXA-4 | 8.4 | 5.6 | 16 | ||
| OXA-5 | 3.1 | 0.25 | 18 | ||
| OXA-6 | 1.6 | 1.7 | 51 | ||
| OXA-7 | 0.36 | 0.61 | 40 | ||
| PSE-2 | 0.81 | 0.94 | 37 | ||
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Chan, E.W.-C.; Zhou, H.; Chen, S. Prevalence and genetic characteristics of carbapenem-resistant Enterobacteriaceae strains in China. Lancet Infect. Dis. 2017, 17, 256–257. [Google Scholar] [CrossRef] [Green Version]
- Effah, C.Y.; Sun, T.W.; Liu, S.H.; Wu, Y.J. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microb. Anti 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Giacobbe, D.R.; Castaldo, N. Management of Infections caused by Multidrug-resistant Gram-negative Pathogens: Recent Advances and Future Directions. Arch. Med. Res. 2021, 52, 817–827. [Google Scholar] [CrossRef]
- El-Ahmed, M.A.E.G.; Zhong, L.L.; Shen, C.; Yang, Y.Q.; Doi, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Song, C.; Zhang, J.; Diao, S.; Heinrichs, T.M.; Martins, F.S.; Lv, Z.; Zhu, Y.; Yu, M.; Sy, S.K.B. Effects of amikacin, polymyxin-B, and sulbactam combination on the pharmacodynamic indices of mutant selection against multi-drug resistant Acinetobacter baumannii. Front. Microbiol. 2022, 13, 1013939. [Google Scholar] [CrossRef]
- Zhang, J.; Diao, S.; Liu, Y.; Wang, H.; Liu, Y.; Zhu, S.; Feng, K.; Tang, X.; Oo, C.; Zhu, P.; et al. The combination effect of meropenem/sulbactam/polymyxin-B on the pharmacodynamic parameters for mutant selection windows against carbapenem-resistant Acinetobacter baumannii. Front. Microbiol. 2022, 13, 1024702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Song, C.; Liu, Y.; Oo, C.; Heinrichs, M.T.; Lv, Z.; Zhu, Y.; Sy, S.K.B.; Deng, P.; et al. Metabolomic profiling of polymyxin-B in combination with meropenem and sulbactam against multi-drug resistant Acinetobacter baumannii. Front. Microbiol. 2022, 13, 1013934. [Google Scholar] [CrossRef] [PubMed]
- Menegucci, T.C.; Fedrigo, N.H.; Lodi, F.G.; Albiero, J.; Nishiyama, S.A.B.; Mazucheli, J.; Carrara-Marroni, F.E.; Voelkner, N.M.F.; Gong, H.; Sy, S.K.B.; et al. Pharmacodynamic effects of sulbactam/meropenem/polymyxin-B combination against extremely drug resistant Acinetobacter baumannii using checkerboard information. Microbial Drug Resist. 2019, 25, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Bergen, P.J.; Smith, N.M.; Bedard, T.B.; Bulman, Z.P.; Cha, R.; Tsuji, B.T. Rational Combinations of Polymyxins with Other Antibiotics. Adv. Exp. Med. Biol. 2019, 1145, 251–288. [Google Scholar] [CrossRef]
- Yahav, D.; Giske, C.G.; Gramatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New beta-Lactam-beta-Lactamase Inhibitor Combinations. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef]
- Feng, K.; Jia, N.; Zhu, P.; Sy, S.; Liu, Y.; Dong, D.; Zhu, S.; Zhang, J.; Liu, Y.; Martins, F.S.; et al. Aztreonam/avibactam effect on pharmacodynamic indices for mutant selection of Escherichia coli and Klebsiella pneumoniae harbouringserine- and New Delhi metallo-beta-lactamases. J. Antimicrob. Chemother. 2021, 76, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Maraolo, A.E.; Di Bella, S.; Luzzaro, F.; Principe, L. The Revival of Aztreonam in Combination with Avibactam against Metallo-beta-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases. Antibiotics 2021, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Cervino, I.; Gonzalez, D.; Nastro, M.; Vega, J.; Reyes, A.P.; Buriano, G.; Vay, C.; Famiglietti, A.; Rodriguez, C.H. In vitro synergistic activity of aztreonam (AZT) plus novel and old β-lactamase inhibitor combinations against metallo-β-lactamase-producing AZT-resistant Enterobacterales. J. Med. Microbiol. 2021, 70, 001427. [Google Scholar] [CrossRef] [PubMed]
- Morroni, G.; Bressan, R.; Fioriti, S.; D’Achille, G.; Mingoia, M.; Cirioni, O.; Di Bella, S.; Piazza, A.; Comandatore, F.; Mauri, C.; et al. Antimicrobial Activity of Aztreonam in Combination with Old and New beta-Lactamase Inhibitors against MBL and ESBL Co-Producing Gram-Negative Clinical Isolates: Possible Options for the Treatment of Complicated Infections. Antibiotics 2021, 10, 1341. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Kotetishvili, M.; Shurland, S.; Johnson, J.A.; Morris, J.G.; Nemoy, L.L.; Johnson, J.K. How important is patient-to-patient transmission in extended-spectrum beta-lactamase Escherichia coli acquisition. Am. J. Infect. Control. 2007, 35, 97–101. [Google Scholar] [CrossRef]
- Harris, A.D.; Perencevich, E.N.; Johnson, J.K.; Paterson, D.L.; Morris, J.G.; Strauss, S.M.; Johnson, J.A. Patient-to-patient transmission is important in extended-spectrum beta-lactamase-producing Klebsiella pneumoniae acquisition. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 45, 1347–1350. [Google Scholar] [CrossRef]
- Fedrigo, N.H.; Shinohara, D.R.; Mazucheli, J.; Nishiyama, S.A.B.; Carrara-Marroni, F.E.; Martins, F.S.; Zhu, P.; Yu, M.; Sy, S.K.B.; Tognim, M.C.B. Pharmacodynamic evaluation of suppression of in vitro resistance in Acinetobacter baumannii strains using polymyxin B-based combination therapy. Sci. Rep. 2021, 11, 11339. [Google Scholar] [CrossRef]
- Filozov, A.; Visintainer, P.; Carbonaro, C.; Aguero-Rosenfeld, M.; Wormser, G.P.; Montecalvo, M.A. Epidemiology of an outbreak of antibiotic-resistant Klebsiella pneumoniae at a tertiary care medical center. Am. J. Infect. Control. 2009, 37, 723–728. [Google Scholar] [CrossRef]
- Doi, Y.; Adams-Haduch, J.M.; Peleg, A.Y.; D’Agata, E.M. The role of horizontal gene transfer in the dissemination of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates in an endemic setting. Diagn. Microbiol. Infect. Dis. 2012, 74, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Stachyra, T.; Pechereau, M.C.; Bruneau, J.M.; Claudon, M.; Frere, J.M.; Miossec, C.; Coleman, K.; Black, M.T. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam beta-lactamase inhibitor. Antimicrob. Agents Chemother. 2010, 54, 5132–5138. [Google Scholar] [CrossRef]
- Payne, D.J.; Cramp, R.; Winstanley, D.J.; Knowles, D.J. Comparative activities of clavulanic acid, sulbactam, and tazobactam against clinically important beta-lactamases. Antimicrob. Agents Chemother. 1994, 38, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Bou, G.; Oliver, A.; Martinez-Beltran, J. OXA-24, a novel class D beta-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob. Agents Chemother. 2000, 44, 1556–1561. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Fu, Y.; Ji, J.; Du, X.; Yu, Y. In vitro Effect of the Combination of Aztreonam and Amoxicillin/Clavulanic Acid Against Carbapenem-Resistant Gram-Negative Organisms Producing Metallo-β-Lactamase. Infect. Drug Resist. 2021, 14, 833–839. [Google Scholar] [CrossRef]
- M100; Performance Standards for Antimicrobial Susceptibility Testing-Thirty Edition. CLSI: Wayne, PA, USA, 2020.
- Xu, H.; Zhou, W.; Zhou, D.; Li, J.; Al-Huniti, N. Evaluation of Aztreonam Dosing Regimens in Patients with Normal and Impaired Renal Function: A Population Pharmacokinetic Modeling and Monte Carlo Simulation Analysis. J. Clin. Pharmacol. 2017, 57, 336–344. [Google Scholar] [CrossRef]
- Cornely, O.A.; Cisneros, J.M.; Torre-Cisneros, J.; Rodriguez-Hernandez, M.J.; Tallon-Aguilar, L.; Calbo, E.; Horcajada, J.P.; Queckenberg, C.; Zettelmeyer, U.; Arenz, D.; et al. Pharmacokinetics and safety of aztreonam/avibactam for the treatment of complicated intra-abdominal infections in hospitalized adults: Results from the REJUVENATE study. J. Antimicrob. Chemother. 2020, 75, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; Noe, M.; De Waele, J.J.; Stove, V.; Verstraete, A.G.; Lipman, J.; Roberts, J.A. Population pharmacokinetics and dosing simulations of amoxicillin/clavulanic acid in critically ill patients. J. Antimicrob. Chemother. 2013, 68, 2600–2608. [Google Scholar] [CrossRef] [Green Version]
- Cies, J.J.; LaCoursiere, R.J.; Moore, W.S., 2nd; Chopra, A. Therapeutic Drug Monitoring of Prolonged Infusion Aztreonam for Multi-Drug Resistant Pseudomonas aeruginosa: A Case Report. J. Pediatr. Pharmacol. Ther. JPPT Off. J. PPAG 2017, 22, 467–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, P.J.; Andrews, J.M.; Woodcock, J.; Wise, R.; Honeybourne, D. Concentration of amoxycillin and clavulanate in lung compartments in adults without pulmonary infection. Thorax 1994, 49, 1134–1138. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Drlica, K. A unified anti-mutant dosing strategy. J. Antimicrob. Chemother. 2008, 62, 434–436. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, R.; Sy, S.K.B.; Li, Z.; Zhu, S.; Zhou, M.; Song, C.; Zhang, J.; Lv, Z.; Liu, J.; et al. Florfenicol/Chlortetracycline Effect on Pharmacodynamic Indices for Mutant Selection of Riemerella anatipestifer in Ducks. Microb. Drug Resist. 2022, 28, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, C.; MacGowan, A.P. A review of the pharmacokinetics and pharmacodynamics of aztreonam. J. Antimicrob. Chemother. 2016, 71, 2704–2712. [Google Scholar] [CrossRef] [Green Version]
- Sy, S.K.; Beaudoin, M.E.; Zhuang, L.; Loblein, K.I.; Lux, C.; Kissel, M.; Tremmel, R.; Frank, C.; Strasser, S.; Heuberger, J.A.; et al. In vitro pharmacokinetics/pharmacodynamics of the combination of avibactam and aztreonam against MDR organisms. J. Antimicrob. Chemother. 2016, 71, 1866–1880. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Zhang, Y.; Yang, J.; Wang, Y.; Zhang, J.; Zhao, Y.; Dong, W. Prediction of the pharmacokinetics and tissue distribution of levofloxacin in humans based on an extrapolated PBPK model. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 395–402. [Google Scholar] [CrossRef]
- Gould, I.M.; Harvey, G.; Golder, D.; Reid, T.M.; Watt, S.J.; Friend, J.A.; Legge, J.S.; Douglas, J.G. Penetration of amoxycillin/clavulanic acid into bronchial mucosa with different dosing regimens. Thorax 1994, 49, 999–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stachyra, T.; Levasseur, P.; Pechereau, M.C.; Girard, A.M.; Claudon, M.; Miossec, C.; Black, M.T. In vitro activity of the {beta}-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J. Antimicrob. Chemother. 2009, 64, 326–329. [Google Scholar] [CrossRef] [Green Version]
- Hentschke, M.; Kotsakis, S.D.; Wolters, M.; Heisig, P.; Miriagou, V.; Aepfelbacher, M. CMY-42, a novel plasmid-mediated CMY-2 variant AmpC beta-lactamase. Microb. Drug Resist. 2011, 17, 165–169. [Google Scholar] [CrossRef]
- Wu, P.J.; Shannon, K.; Phillips, I. Effect of hyperproduction of TEM-1 beta-lactamase on in vitro susceptibility of Escherichia coli to beta-lactam antibiotics. Antimicrob. Agents Chemother. 1994, 38, 494–498. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, P.; Wu, P.J.; King, A.; Shannon, K.; French, G.; Phillips, I. Incidence and mechanisms of resistance to the combination of amoxicillin and clavulanic acid in Escherichia coli. Antimicrob. Agents Chemother. 1995, 39, 2478–2483. [Google Scholar] [CrossRef] [Green Version]
- Emeraud, C.; Escaut, L.; Boucly, A.; Fortineau, N.; Bonnin, R.A.; Naas, T.; Dortet, L. Aztreonam plus clavulanate, tazobactam, or avibactam for treatment of infections caused by metallo-beta-lactamase-producing Gram-negative bacteria. Antimicrob. Agents Chemother. 2019, 63, e00010-19. [Google Scholar] [CrossRef] [Green Version]
- Lister, P.D.; Gardner, V.M.; Sanders, C.C. Clavulanate induces expression of the Pseudomonas aeruginosa AmpC cephalosporinase at physiologically relevant concentrations and antagonizes the antibacterial activity of ticarcillin. Antimicrob. Agents Chemother. 1999, 43, 882–889. [Google Scholar] [CrossRef]
- Sy, S.K.; Beaudoin, M.E.; Schuck, V.J.; Derendorf, H. Modeling the potentiation of in vitro aztreonam activities by avibactam against four beta-lactam-resistant bacterial strains. In Proceedings of the 53rd Interscience Conference of Antimicrobial Agents and Chemotherapy, Denver, CO, USA, 10–13 September 2013; p. abstr A-1014. [Google Scholar]
- Ract, P.; Compain, F.; Robin, F.; Decre, D.; Gallah, S.; Podglajen, I. Synergistic in vitro activity between aztreonam and amoxicillin-clavulanate against Enterobacteriaceae-producing class B and/or class D carbapenemases with or without extended-spectrum beta-lactamases. J. Med. Microbiol. 2019, 68, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- De Cock, P.A.; Standing, J.F.; Barker, C.I.; de Jaeger, A.; Dhont, E.; Carlier, M.; Verstraete, A.G.; Delanghe, J.R.; Robays, H.; De Paepe, P. Augmented renal clearance implies a need for increased amoxicillin-clavulanic acid dosing in critically ill children. Antimicrob. Agents Chemother. 2015, 59, 7027–7035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giakkoupi, P.; Tzelepi, E.; Legakis, N.J.; Tzouvelekis, L.S. Aspartic acid for asparagine substitution at position 276 reduces susceptibility to mechanism-based inhibitors in SHV-1 and SHV-5 beta-lactamases. J. Antimicrob. Chemother. 1999, 43, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Diverse Populations Collaborative, G. Weight-height relationships and body mass index: Some observations from the Diverse Populations Collaboration. Am. J. Phys. Anthropol. 2005, 128, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Sy, S.K.; Asin-Prieto, E.; Derendorf, H.; Samara, E. Predicting pediatric age-matched weight and body mass index. AAPS J. 2014, 16, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.A.; Fryar, C.D.; Ogden, C.L.; Flegal, K.M. Anthropometric Reference Data for Children and Adults: United States, 2003–2006; National Health Statistics Reports; National Center for Health Statistics: Hyattsville, MD, USA, 2008; pp. 1–48. [Google Scholar]
- Sy, S.K.; Derendorf, H. Pharmacokinetics I: PK-PD Approach, the Case of Antibiotic Drug Development. In Clinical Pharmacology: Current Topics and Case Studies; Müller, M., Ed.; Springer: New York, NY, USA, 2016; pp. 185–217. [Google Scholar]


| Creatinine Clearance | Aztreonam (High Dose) | Amoxicillin/Clavulanate |
|---|---|---|
| >50 to 150 mL/min | 2g LD 1.5g MD q6h | 1/0.2 g q6h |
| >30 to 50 mL/min | 2g LD 750g MD q6h | 1/0.2 g q6h |
| 10 to 30 mL/min | 2g LD 500 mg MD q8h | 1/0.2 g followed by 500/100 mg q12h |
| Strains | ATM Alone MIC (mg/L) | AMC/CA MIC (mg/L) | ATM/AMC/CA MIC (mg/L) | FICI | β-Lactamase Encoded |
|---|---|---|---|---|---|
| Control | |||||
| E. coli ATCC 25922 | 0.5 | 2/1 | - | - | |
| E. coli ATCC 35218 | 0.5 | 16/2 | - | - | |
| K. pneumoniae ATCC 700603 | 16 | 32/16 | 1/1/0.5 | 0.0937 | |
| C. braakii HDC438 | >128 | >128/64 | 64/64/32 | 1 | CMY-79, NDM-1, KPC-2 |
| K. pneumonia | |||||
| LW1 | >128 | 64/32 | 128/64/32 | 2.00 | TEM-1B, SHV-1, CTX-M-15, NDM-1 |
| LW4 | 32 | 64/32 | 0.25/2/1 | 0.0391 | CTX-M-15, NDM-1, TEM-1B, SHV-1 |
| LW5 | 32 | 64/32 | 0.25/1/0.5 | 0.0234 | NDM-1, TEM-1B, SHV-1 |
| LW8 | 32 | >128/64 | 0.5/1/0.5 | 0.0234 | CTX-M-15, TEM-1B, SHV-1, NDM-1 |
| LW9 | 32 | >128/64 | 0.5/2/1 | 0.0313 | SHV-1, CTX-M-15, NDM-1, TEM-1B |
| LW10 | 32 | >128/64 | 0.25/4/2 | 0.0391 | CTX-M-15, TEM-1B, NDM-1, SHV-25 |
| LW13 | 32 | 64/32 | 0.25/2/1 | 0.0391 | SHV-1, TEM-1B, NDM-1, CTX-M-15 |
| LW15 | >128 | >128/64 | 64/64/32 | 1.00 | SHV-26, TEM-1B, CTX-M-15, NDM-1 |
| LW16 | 32 | >128/64 | 0.5/1/0.5 | 0.0234 | CTX-M-15, TEM-1B, SHV-1, NDM-1 |
| MIC50 | 32 | >128/64 | |||
| MIC90 | >128 | >128/64 | |||
| E. coli | |||||
| EC9 | >128 | >128/64 | 64/64/32 | 1.00 | NDM-1, CMY-42 |
| EC13 | >128 | >128/64 | 4/4/2 | 0.0625 | NDM-5, CTX-M-55 |
| EC36 | 32 | >128/64 | 4/8/4 | 0.1875 | NDM-1, TEM-20, CMY-6, TEM-1C |
| EC37 | 32 | >128/64 | 0.25/1/0.5 | 0.0156 | NDM-1, CTX-M-55, TEM-1B |
| EC38 | >128 | >128/64 | 4/16/8 | 0.1563 | NDM-7, OXA-1, CTX-M-55 |
| EC42 | 16 | >128/64 | 4/4/2 | 0.2813 | TEM-20, NDM-1, CMY-6, TEM-32 |
| EC45 | 64 | 64/32 | 0.25/1/0.5 | 0.0195 | TEM-1B, NDM-13, TEM-141, CTX-M-55, OXA-1 |
| EC48 | >128 | 64/32 | 4/4/2 | 0.0938 | CTX-M-65, TEM-34, NDM-9, TEM-1B, OXA-1 |
| EC52 | 8 | 64/32 | 0.25/1/0.5 | 0.0469 | TEM-1B, NDM-1 |
| MIC50 | 32 | >128 | |||
| MIC90 | >128 | >128 |
| Mutant Prevention Concentration (mg/L) | |||
|---|---|---|---|
| ATM Alone | AMC/CA | ATM/AMC/CA | |
| K. pneumonia | |||
| LW5 | >256 | >256/128 | 1/1/0.5 |
| LW8 | >256 | >256/128 | 1/1/0.5 |
| LW13 | >256 | 128/64 | 4/4/2 |
| E. coli | |||
| EC13 | >256 | >256/128 | 8/8/4 |
| EC37 | >256 | >256/128 | 2/2/1 |
| EC45 | >256 | >256/128 | 1/1/0.5 |
| EC48 | >256 | >256/128 | 8/8/4 |
| Dosing Regimen ‡ in CrCL > 50 to 150 mL/min | Dosing Regimen ‡ in CrCL > 30 to 50 mL/min | Dosing Regimen ‡ in CrCL 10 to 30 mL/min | ||||
|---|---|---|---|---|---|---|
| Bacteria Isolate | fTMSW (Mean ± SE) | fT>MPC (Mean ± SE) | fTMSW (Mean ± SE) | fT>MPC (Mean ± SE) | fTMSW (Mean ± SE) | fT>MPC (Mean ± SE) |
| Aztreonam | ||||||
| EC13, EC48 | 3.2 ± 0.07% | 96.3 ± 0.00082% | 4.0 ± 0.09% | 95.8 ± 0.09% | 7.2 ± 0.12% | 92.2 ± 0.13% |
| LW13 | 0.4 ± 0.023% | 99.6 ± 0.023% | 0.2 ± 0.015% | 99.8 ± 0.02% | 0.6 ± 0.03% | 99.3 ± 0.03% |
| EC37 | 0.12 ± 0.0053% | 99.9 ± 0.005% | 0.1 ± 0.003% | 99.9 ± 0.003% | 0.1 ± 0.007% | 99.8 ± 0.007% |
| LW8 | 0.03 ± 0.0007% | 99.9 ± 0.001% | 0.04 ± 0.004% | 99.9 ± 0.005% | 0.03 ± 0.0014% | 99.9 ± 0.002% |
| EC45 | 0.04 ± 0.0007% | 99.9 ± 0.001% | 0.02 ± 0.0002% | 100 ± 0.0003% | 0.05 ± 0.0015% | 99.9 ± 0.002% |
| Amoxicillin/clavulanate | ||||||
| EC13, EC48 | 14.4 ± 0.16% | 75.8 ± 0.29% | 2.4 ± 7.7% | 96.9 ± 0.11% | 1.0 ± 0.06% | 98.8 ± 0.07% |
| LW13 | 5.8 ± 0.09% | 90.0 ± 0.17% | 0.5 ± 3.0% | 99.4 ± 0.04% | 0.1 ± 0.02% | 99.8 ± 0.02% |
| EC37 | 2.6 ± 0.06% | 95.6 ± 0.11% | 0.2 ± 1.3% | 99.7 ± 0.02% | 0.1 ± 0.005% | 99.8 ± 0.005% |
| LW8 | 0% | 98.2 ± 0.07% | 0% | 99.9 ± 0.007% | 0% | 99.9 ± 0.002% |
| EC45 | 0% | 98.2 ± 0.07% | 0% | 99.9 ± 0.007% | 0% | 99.9 ± 0.002% |
| Dosing Regimen ‡ in CrCL > 50 to 150 mL/min | Dosing Regimen ‡ in CrCL > 30 to 50 mL/min | Dosing Regimen ‡ in CrCL 10 to 30 mL/min | ||||
|---|---|---|---|---|---|---|
| Bacteria Isolate | fTMSW (Mean ± SE) | fT>MPC (Mean ± SE) | fTMSW (Mean ± SE) | fT>MPC (Mean ± SE) | fTMSW (Mean ± SE) | fT>MPC (Mean ± SE) |
| Aztreonam | ||||||
| EC13, EC48 | 4.3 ± 0.0008% | 94.2 ± 0.1% | 5.8 ± 0.11% | 93.9 ± 0.12% | 9.5 ± 0.13% | 89.7 ± 0.15% |
| LW13 | 0.6 ± 0.0003% | 99.4 ± 0.03% | 0.3 ± 0.02% | 99.7 ± 0.02% | 0.8 ± 0.04% | 99.1 ± 0.04% |
| EC37 | 0.14 ± 0.0064% | 99.8 ± 0.006% | 0.1 ± 0.003% | 99.8 ± 0.003% | 0.2 ± 0.009% | 99.8 ± 0.009% |
| LW8 | 0.03 ± 0.001% | 99.9 ± 0.001% | 0.03 ± 0.0002% | 99.9 ± 0.0004% | 0.04 ± 0.002% | 99.9 ± 0.002% |
| EC45 | 0.04 ± 0.001% | 99.9 ± 0.001% | 0.04 ± 0.0003% | 99.9 ± 0.0004% | 0.05 ± 0.002% | 99.9 ± 0.002% |
| Amoxicillin/clavulanate | ||||||
| EC13, EC48 | 35.0 ± 0.27% | 28.2 ± 0.38% | 19.3 ± 0.27% | 74.3 ± 0.36% | 14.3 ± 0.28% | 82.5 ± 0.34% |
| LW13 | 22.0 ± 0.2% | 63.2 ± 0.36% | 4.9 ± 0.12% | 93.6 ± 0.17% | 2.7 ± 0.11% | 96.8 ± 0.13% |
| EC37 | 8.9 ± 0.02% | 84.5 ± 0.22% | 1.4 ± 0.05% | 98.0 ± 0.07% | 0.6 ± 0.03% | 99.1 ± 0.04% |
| LW8 | 0% | 93.5 ± 0.13% | 0% | 99.4 ± 0.03% | 0% | 99.8 ± 0.01% |
| EC45 | 0% | 93.5 ± 0.13% | 0% | 99.4 ± 0.03% | 0% | 99.8 ± 0.01% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wu, M.; Diao, S.; Zhu, S.; Song, C.; Yue, J.; Martins, F.S.; Zhu, P.; Lv, Z.; Zhu, Y.; et al. Pharmacokinetic/Pharmacodynamic Evaluation of Aztreonam/Amoxicillin/Clavulanate Combination against New Delhi Metallo-β-Lactamase and Serine-β-Lactamase Co-Producing Escherichia coli and Klebsiella pneumoniae. Pharmaceutics 2023, 15, 251. https://doi.org/10.3390/pharmaceutics15010251
Zhang J, Wu M, Diao S, Zhu S, Song C, Yue J, Martins FS, Zhu P, Lv Z, Zhu Y, et al. Pharmacokinetic/Pharmacodynamic Evaluation of Aztreonam/Amoxicillin/Clavulanate Combination against New Delhi Metallo-β-Lactamase and Serine-β-Lactamase Co-Producing Escherichia coli and Klebsiella pneumoniae. Pharmaceutics. 2023; 15(1):251. https://doi.org/10.3390/pharmaceutics15010251
Chicago/Turabian StyleZhang, Jiayuan, Mengyuan Wu, Shuo Diao, Shixing Zhu, Chu Song, Jiali Yue, Frederico S. Martins, Peijuan Zhu, Zhihua Lv, Yuanqi Zhu, and et al. 2023. "Pharmacokinetic/Pharmacodynamic Evaluation of Aztreonam/Amoxicillin/Clavulanate Combination against New Delhi Metallo-β-Lactamase and Serine-β-Lactamase Co-Producing Escherichia coli and Klebsiella pneumoniae" Pharmaceutics 15, no. 1: 251. https://doi.org/10.3390/pharmaceutics15010251





