Nanotechnology-Based Approaches for Voriconazole Delivery Applied to Invasive Fungal Infections
Abstract
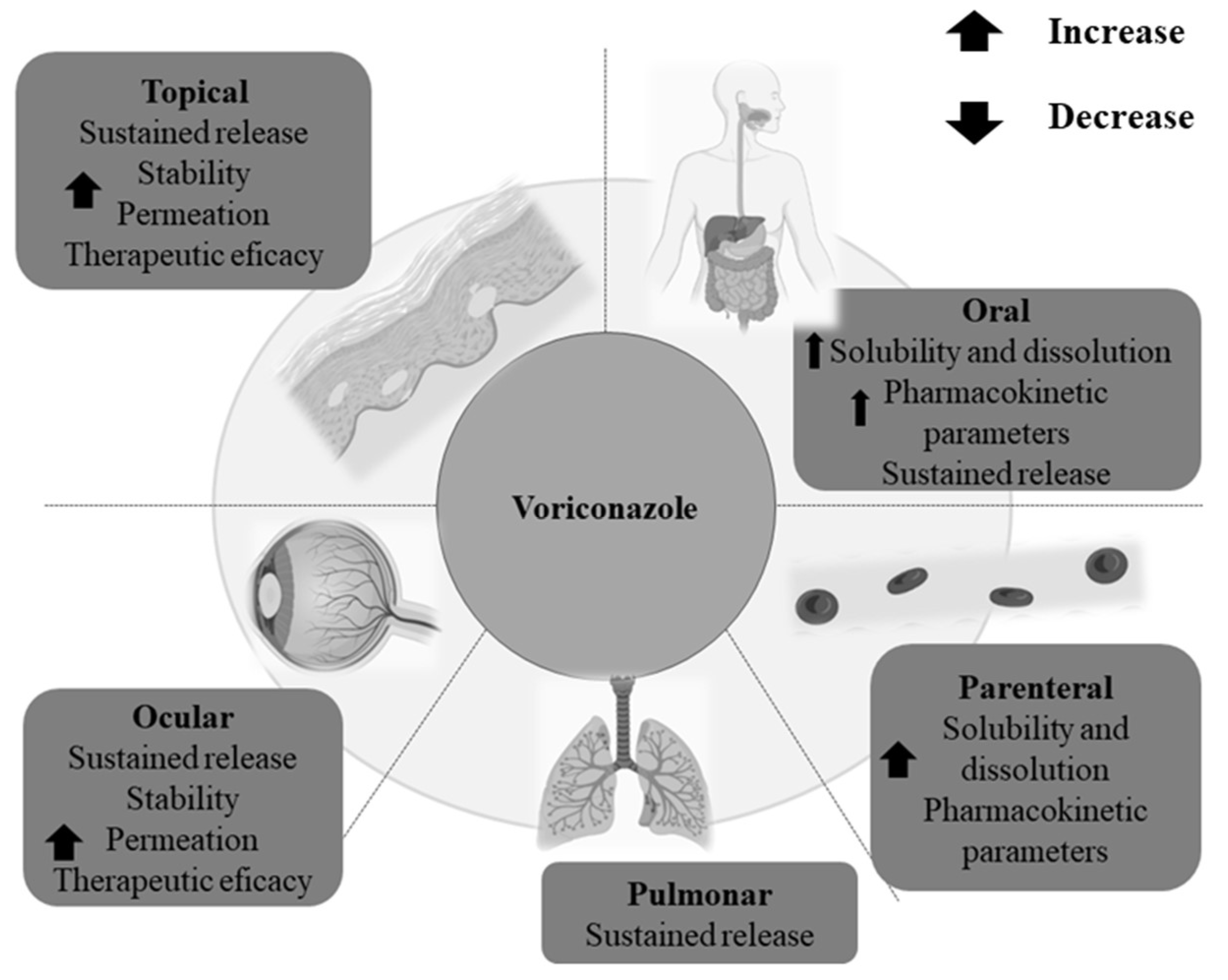
:1. Introduction
2. Fungal Infections
2.1. Pulmonary Aspergillosis
2.1.1. Allergic Bronchopulmonary Aspergillosis (ABPA)
2.1.2. Chronic Pulmonary Aspergillosis (CPA)
2.1.3. Invasive Aspergillosis (IA)
2.2. Candida Infections
2.3. Cryptococcosis
3. Voriconazole
3.1. General Aspects
3.2. Pharmacokinetics
3.2.1. Absorption
3.2.2. Distribution
3.2.3. Metabolism
3.2.4. Excretion
3.3. Toxicity and Drug Monitoring Therapeutics
4. Nanotechnology-Based Voriconazole Delivery Systems
4.1. Lipid Nanoparticles
4.2. Polymeric Nanoparticles
4.3. Protein Nanocarriers
5. Other Systems for Voriconazole Delivery
5.1. Cubosomes
5.2. Cyclodextrins
6. Clinical Trials
7. Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gushiken, A.C.; Saharia, K.K.; Baddley, J.W. Cryptococcosis. Infect. Dis. Clin. N. Am. 2021, 35, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Lakoh, S.; Rickman, H.; Sesay, M.; Kenneh, S.; Burke, R.; Baldeh, M.; Jiba, D.F.; Tejan, Y.S.; Boyle, S.; Koroma, C.; et al. Prevalence and Mortality of Cryptococcal Disease in Adults with Advanced HIV in an Urban Tertiary Hospital in Sierra Leone: A Prospective Study. BMC Infect. Dis. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockhart, S.R.; Guarner, J. Emerging and Reemerging Fungal Infections. Semin. Diagn. Pathol. 2019, 36, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Otto, W.R.; Green, A.M. Fungal Infections in Children with Haematologic Malignancies and Stem Cell Transplant Recipients. Br. J. Haematol. 2020, 189, 607–624. [Google Scholar] [CrossRef]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- David, F.; Rodrigues Morais, J.; Beires, F.; Greefield, H.; Fernandes, G.L. Invasive Pulmonary Aspergillosis after COVID-19 Pneumonia. Eur. J. Case Rep. Intern. Med. 2022, 9, 003209. [Google Scholar] [CrossRef]
- Swain, S.; Ray, A.; Sarda, R.; Vyas, S.; Singh, G.; Jorwal, P.; Kodan, P.; Khanna, P.; Xess, I.; Sinha, S.; et al. COVID-19-Associated Subacute Invasive Pulmonary Aspergillosis. Mycoses 2022, 65, 57–64. [Google Scholar] [CrossRef]
- Blot, S.; Rello, J.; Koulenti, D. Diagnosing Invasive Pulmonary Aspergillosis in ICU Patients: Putting the Puzzle Together. Curr. Opin. Crit. Care 2019, 25, 430–437. [Google Scholar] [CrossRef]
- Hadrich, I.; Ayadi, A. Epidemiology of Antifungal Susceptibility: Review of Literature. J. Mycol. Med. 2018, 28, 574–584. [Google Scholar] [CrossRef]
- Jamzivar, F.; Shams-Ghahfarokhi, M.; Khoramizadeh, M.; Yousefi, N.; Gholami-Shabani, M.; Razzaghi-Abyaneh, M. Unraveling the Importance of Molecules of Natural Origin in Antifungal Drug Development through Targeting Ergosterol Biosynthesis Pathway. Iran. J. Microbiol. 2019, 11, 448–459. [Google Scholar] [CrossRef]
- Poissy, J.; Damonti, L.; Bignon, A.; Khanna, N.; von Kietzell, M.; Boggian, K.; Neofytos, D.; Vuotto, F.; Coiteux, V.; Artru, F.; et al. Risk Factors for Candidemia: A Prospective Matched Case-Control Study. Crit. Care 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Bonifaz, A.; Rojas, R.; Tirado-Sánchez, A.; Chávez-López, D.; Mena, C.; Calderón, L.; María, P.-O.R. Superficial Mycoses Associated with Diaper Dermatitis. Mycopathologia 2016, 181, 671–679. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Santos, C.T.; Fusco-Almeida, A.M.; Mendes, M. In Vitro Characterization of Trichophyton rubrum and T. mentagrophytes Biofilms. Biofouling 2014, 30, 719–727. [Google Scholar] [CrossRef]
- Heidrich, D.; Garcia, M.R.; Stopiglia, C.D.O.; Magagnin, C.M.; Daboit, T.C.; Vetoratto, G.; Schwartz, J.; Amaro, T.G.; Scroferneker, M.L. Dermatophytosis: A 16-Year Retrospective Study in a Metropolitan Area in Southern Brazil. J. Infect. Dev. Ctries. 2015, 9, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Maraki, S.; Mavromanolaki, V.E. Epidemiology of Dermatophytoses in Crete, Greece: A 5-Year Survey. Med. Mycol. J. 2016, 57, e69–e75. [Google Scholar] [CrossRef] [Green Version]
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected Endemic Mycoses. Lancet Infect. Dis. 2017, 17, 367–377. [Google Scholar] [CrossRef]
- Carrasco-Zuber, J.E.; Navarrete-Dechent, C.; Bonifaz, A.; Fich, F.; Vial-Letelier, V.; Berroeta-Mauriziano, D. Afectación Cutánea En Las Micosis Profundas: Una Revisión de La Literatura. Parte II. Micosis Sistémicas. Actas Dermosifiliogr. 2016, 107, 816–822. [Google Scholar] [CrossRef]
- Bollam, R.; Yassin, M.; Phan, T. Disseminated Cryptococcosis in an Immunocompetent Patient. Respir. Med. Case Rep. 2020, 30, 101034. [Google Scholar] [CrossRef]
- Papachristou, S.G.; Iosifidis, E.; Sipsas, N.V.; Gamaletsou, M.N.; Walsh, T.J.; Roilides, E. Management of Osteoarticular Fungal Infections in the Setting of Immunodeficiency. Expert Rev. Anti Infect. Ther. 2020, 18, 461–474. [Google Scholar] [CrossRef]
- El-Baba, F.; Gao, Y.; Soubani, A.O. Pulmonary Aspergillosis: What the Generalist Needs to Know. Am. J. Med. 2020, 133, 668–674. [Google Scholar] [CrossRef]
- WHO. Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Evolution, Mechanisms and Impact HHS Public Access. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The Global Problem of Antifungal Resistance: Prevalence, Mechanisms, and Management. Lancet Infect. Dis. 2017, 17, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Vartak, R.; Menon, S.; Patki, M.; Billack, B.; Patel, K. Ebselen Nanoemulgel for the Treatment of Topical Fungal Infection. Eur. J. Pharm. Sci. 2020, 148, 105323. [Google Scholar] [CrossRef] [PubMed]
- Abbes, S.; Sellami, H.; Neji, S.; Trabelsi, H.; Makni, F.; Ayadi, A. Implication of Efflux Pumps and ERG11 Genes in Resistance of Clinical Trichosporon Asahii Isolates to Fluconazole. J. Med. Microbiol. 2021, 70, 001236. [Google Scholar] [CrossRef] [PubMed]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Andrew, E.C.; Curtis, N.; Coghlan, B.; Cranswick, N.; Gwee, A. Adverse Effects of Amphotericin B in Children; a Retrospective Comparison of Conventional and Liposomal Formulations. Br. J. Clin. Pharmacol. 2018, 84, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Smith, A.R.; Jacobson, P.A.; Alharbi, A.F.; Fisher, J.; Rubin, N.T.; Kirstein, M.N. CYP51A1 Polymorphism and Voriconazoleassociated Hepatotoxicity in Children Undergoing Hematopoietic Cell Transplant. Int. J. Clin. Pharmacol. Ther. 2021, 59, 442–446. [Google Scholar] [CrossRef]
- Donnelly, J.P.; de Pauw, B.E. Voriconazole—A New Therapeutic Agent with an Extended Spectrum of Antifungal Activity. Clin. Microbiol. Infect. 2004, 10, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.B.; Kauffman, C.A.; John, S. Voriconazole: A New Triazole Antifungal Agent. Clin. Infect. Dis. 2003, 36, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Jeu, L.; Piacenti, F.J.; Lyakhovetskiy, A.G.; Fung, H.B. Voriconazole. Clin. Ther. 2003, 25, 1321–1381. [Google Scholar] [CrossRef]
- Limper, A.H.; Knox, K.S.; Sarosi, G.A.; Ampel, N.M.; Bennett, J.E.; Catanzaro, A.; Davies, S.F.; Dismukes, W.E.; Hage, C.A.; Marr, K.A.; et al. An Official American Thoracic Society Statement: Treatment of Fungal Infections in Adult Pulmonary and Critical Care Patients. Am. J. Respir. Crit. Care Med. 2011, 183, 96–128. [Google Scholar] [CrossRef] [Green Version]
- Job, K.M.; Olson, J.; Stockmann, C.; Constance, J.E.; Enioutina, E.Y.; Rower, J.E.; Linakis, M.W.; Balch, A.H.; Yu, T.; Liu, X.; et al. Pharmacodynamic Studies of Voriconazole: Informing the Clinical Management of Invasive Fungal Infections. Expert Rev. Anti Infect. Ther. 2016, 14, 731–746. [Google Scholar] [CrossRef]
- Collins, L.M.; Moore, R.; Sobel, J.D. Prognosis and Long-Term Outcome of Women with Idiopathic Recurrent Vulvovaginal Candidiasis Caused by Candida Albicans. J. Low Genit. Tract. Dis. 2020, 24, 48–52. [Google Scholar] [CrossRef]
- Hassanmoghadam, F.; Shokohi, T.; Hedayati, M.T.; Aslani, N.; Haghani, I.; Nabili, M.; Lotfali, E.; Moazeni, M. High Prevalence of Itraconazole Resistance among Candida Parapsilosis Isolated from Iran. Curr. Med. Mycol. 2019, 5, 43–46. [Google Scholar] [CrossRef]
- Perez-Pitarch, A.; Guglieri-Lopez, B.; Ferriols-Lisart, R.; Pérez, A.; Ezquer-Garín, C.; Hernández-Boluda, J.C.; Piñana, J.L.; Navarro, D.; Solano, C.; Alós-Almiñana, M. Pharmacokinetic/Pharmacodynamic Analysis of Voriconazole against Candida spp. and Aspergillus spp. in Allogeneic Stem Cell Transplant Recipients. Ther. Drug Monit. 2019, 41, 740–747. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, R.; Liu, F.; Li, Y.; Chen, H.; Yao, P.; Sun, F.; Xia, P. Therapeutic Drug Monitoring and Safety of Voriconazole in Elderly Patients. Int. Immunopharmacol. 2020, 78, 106078. [Google Scholar] [CrossRef]
- Zhong, X.; Tong, X.; Ju, Y.; Du, X.; Li, Y. Interpersonal Factors in the Pharmacokinetics and Pharmacodynamics of Voriconazole: Are CYP2C19 Genotypes Enough for Us to Make a Clinical Decision? Curr. Drug Metab. 2018, 19, 1152–1158. [Google Scholar] [CrossRef]
- Resztak, M.; Kosicka, K.; Zalewska, P.; Krawiec, J.; Główka, F.K. Determination of Total and Free Voriconazole in Human Plasma: Application to Pharmacokinetic Study and Therapeutic Monitoring. J. Pharm. Biomed. Anal. 2020, 178, 112952. [Google Scholar] [CrossRef]
- Shi, C.; Xiao, Y.; Mao, Y.; Wu, J.; Lin, N. Voriconazole: A Review of Population Pharmacokinetic Analyses. Clin. Pharmacokinet. 2019, 58, 687–703. [Google Scholar] [CrossRef] [Green Version]
- Theuretzbacher, U.; Ihle, F.; Derendorf, H. Pharmacokinetic/Pharmacodynamic Profile of Voriconazole. Clin. Pharmacokinet. 2006, 45, 649–663. [Google Scholar] [CrossRef]
- Levine, M.T.; Chandrasekar, P.H. Adverse Effects of Voriconazole: Over a Decade of Use. Clin. Transplant. 2016, 30, 1377–1386. [Google Scholar] [CrossRef]
- Musayeva, A.; Riedl, J.C.; Schuster, A.K.; Wasielica-Poslednik, J.; Pfeiffer, N.; Gericke, A. Topical Voriconazole as Supplemental Treatment for Acanthamoeba Keratitis. Cornea 2020, 39, 986–990. [Google Scholar] [CrossRef]
- Voltan, A.R.; Quindós, G.; Alarcón, K.P.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S.; Chorilli, M. Fungal Diseases: Could Nanostructured Drug Delivery Systems Be a Novel Paradigm for Therapy? Int. J. Nanomed. 2016, 11, 3715–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renzi, D.F.; de Almeida Campos, L.; Miranda, E.H.; Mainardes, R.M.; Abraham, W.-R.; Grigoletto, D.F.; Khalil, N.M. Nanoparticles as a Tool for Broadening Antifungal Activities. Curr. Med. Chem. 2021, 28, 1841–1873. [Google Scholar] [CrossRef] [PubMed]
- Khare, A.; Singh, I.; Pawar, P.; Grover, K. Design and Evaluation of Voriconazole Loaded Solid Lipid Nanoparticles for Ophthalmic Application. J. Drug Deliv. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radwan, M.A.; AlQuadeib, B.T.; Šiller, L.; Wright, M.C.; Horrocks, B. Oral Administration of Amphotericin B Nanoparticles: Antifungal Activity, Bioavailability and Toxicity in Rats. Drug Deliv. 2017, 24, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roque, L.; Molpeceres, J.; Reis, C.; Rijo, P.; Pinto Reis, C. Past, Recent Progresses and Future Perspectives of Nanotechnology Applied to Antifungal Agents. Curr. Drug Metab. 2017, 18, 280–290. [Google Scholar] [CrossRef]
- Szalewski, D.A.; Hinrichs, V.S.; Zinniel, D.K.; Barletta, R.G. The Pathogenicity of Aspergillus Fumigatus, Drug Resistance, and Nanoparticle Delivery. Can. J. Microbiol. 2018, 64, 439–453. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, L.T.; Lopes, L.G.; Ramos, S.B.; Martins, C.H.G.; Jamur, M.C.; Pires, R.H. Fungal Biofilms in the Hemodialysis Environment. Microb. Pathog. 2018, 123, 206–212. [Google Scholar] [CrossRef]
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Invasive Aspergillosis: Current Strategies for Diagnosis and Management. Infect. Dis. Clin. N. Am. 2016, 30, 125–142. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and Related Genera (Eurotiales): An Overview of Families, Genera, Subgenera, Sections, Series and Species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoon, J.-A.; Kim, S.-H. Voriconazole-Refractory Invasive Aspergillosis. Korean J. Intern. Med. 2017, 32, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Warris, A. The Biology of Pulmonary Aspergillus Infections. J. Infect. 2014, 44, 36–41. [Google Scholar] [CrossRef]
- Ohara, S.; Tazawa, Y.; Tanai, C.; Tanaka, Y.; Noda, H.; Horiuchi, H.; Usui, K. Clinical Characteristics of Patients with Aspergillus Species Isolation from Respiratory Samples: Comparison of Chronic Pulmonary Aspergillosis and Colonization. Respir. Investig. 2016, 54, 92–97. [Google Scholar] [CrossRef]
- Iqbal, N.; Sheikh, M.D.A.; Jabeen, K.; Awan, S.; Irfan, M. Allergic Bronchopulmonary Aspergillosis Misdiagnosed as Smear Negative Pulmonary Tuberculosis; a Retrospective Study from Pakistan. Ann. Med. Surg. 2021, 72, 103045. [Google Scholar] [CrossRef]
- Tiew, P.Y.; Lim, A.Y.H.; Keir, H.R.; Dicker, A.J.; Aogáin, M.M.; Pang, S.L.; Boon, L.T.; Hassan, T.M.; Poh, M.E.; Xu, H.; et al. High Frequency of Allergic Bronchopulmonary Aspergillosis in Bronchiectasis-COPD Overlap. Chest 2022, 161, 40–53. [Google Scholar] [CrossRef]
- Agarwal, R. Allergic Bronchopulmonary Aspergillosis. Chest 2009, 135, 805–826. [Google Scholar] [CrossRef]
- Asano, K.; Hebisawa, A.; Ishiguro, T.; Takayanagi, N.; Nakamura, Y.; Suzuki, J.; Okada, N.; Tanaka, J.; Fukutomi, Y.; Ueki, S.; et al. New Clinical Diagnostic Criteria for Allergic Bronchopulmonary Aspergillosis/Mycosis and Its Validation. J. Allergy Clin. Immunol. 2021, 147, 1261–1268. [Google Scholar] [CrossRef]
- Tompkins, M.G.; Pettit, R. Beyond the Guidelines: Treatment of Allergic Bronchopulmonary Aspergillosis in Cystic Fibrosis. Ann. Pharmacother. 2022, 56, 181–192. [Google Scholar] [CrossRef]
- Boyle, M.; Mulrennan, S.; Morey, S.; Vekaria, S.; Popowicz, N.; Tai, A. Mepolizumab Use in Cystic Fibrosis-Associated Allergic Bronchopulmonary Aspergillosis. Respirol. Case Rep. 2021, 9, e00696. [Google Scholar] [CrossRef]
- O’Reilly, A.; Dunican, E. The Use of Targeted Monoclonal Antibodies in the Treatment of ABPA—A Case Series. Medicina 2022, 58, 53. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C. Chronic Pulmonary Aspergillosis: Rationale and Clinical Guidelines for Diagnosis and Management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, K.; Suzuki, J.; Watanabe, A.; Arai, T.; Koiwa, T.; Shinfuku, K.; Narumoto, O.; Kawashima, M.; Fukami, T.; Tamura, A.; et al. High Detection Rate of Azole-Resistant Aspergillus Fumigatus after Treatment with Azole Antifungal Drugs among Patients with Chronic Pulmonary Aspergillosis in a Single Hospital Setting with Low Azole Resistance. Med. Mycol. 2021, 59, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, I.S.; Dhooria, S.; Muthu, V.; Prasad, K.T.; Agarwal, R. An Overview of the Available Treatments for Chronic Cavitary Pulmonary Aspergillosis. Expert Rev. Respir. Med. 2020, 14, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Ohba, H.; Miwa, S.; Shirai, M.; Kanai, M.; Eifuku, T.; Suda, T.; Hayakawa, H.; Chida, K. Clinical Characteristics and Prognosis of Chronic Pulmonary Aspergillosis. Respir. Med. 2012, 106, 724–729. [Google Scholar] [CrossRef] [Green Version]
- Im, Y.; Jhun, B.W.; Kang, E.S.; Koh, W.J.; Jeon, K. Impact of Treatment Duration on Recurrence of Chronic Pulmonary Aspergillosis. J. Infect. 2021, 83, 490–495. [Google Scholar] [CrossRef]
- Rodriguez-Goncer, I.; Harris, C.; Kosmidis, C.; Muldoon, E.G.; Newton, P.J.; Denning, D.W. Assessment of Posaconazole Salvage Therapy in Chronic Pulmonary Aspergillosis Using Predefined Response Criteria. Int. J. Antimicrob. Agents 2018, 52, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Jenks, J.D.; Nam, H.H.; Hoenigl, M. Invasive Aspergillosis in Critically Ill Patients: Review of Definitions and Diagnostic Approaches. Mycoses 2021, 64, 1002–1014. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [Green Version]
- Maertens, J.A.; Rahav, G.; Lee, D.G.; Ponce-de-León, A.; Ramírez Sánchez, I.C.; Klimko, N.; Sonet, A.; Haider, S.; Diego Vélez, J.; Raad, I.; et al. Posaconazole versus Voriconazole for Primary Treatment of Invasive Aspergillosis: A Phase 3, Randomised, Controlled, Non-Inferiority Trial. Lancet 2021, 397, 499–509. [Google Scholar] [CrossRef]
- Bongomin, F.; Otu, A.; Harris, C.; Foden, P.; Kosmidis, C.; Denning, D.W. Risk Factors for Relapse of Chronic Pulmonary Aspergillosis after Discontinuation of Antifungal Therapy. Clin. Infect. Pract. 2020, 5, 100015. [Google Scholar] [CrossRef]
- Cadena, J.; Thompson, G.R., III; Patterson, T.F. Aspergillosis Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434. [Google Scholar] [CrossRef]
- Challa, S. Pathogenesis and Pathology of Invasive Aspergillosis. Curr. Fungal Infect. Rep. 2018, 12, 23–32. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Raveendran, S.; Lu, Z. CT Findings and Differential Diagnosis in Adults with Invasive Pulmonary Aspergillosis. Radiol. Infect. Dis. 2018, 5, 14–25. [Google Scholar] [CrossRef]
- Desoubeaux, G.; Bailly, E.; Chandenier, J. Diagnosis of Invasive Pulmonary Aspergillosis: Updates and Recommendations. Med. Mal. Infect. 2014, 44, 89–101. [Google Scholar] [CrossRef]
- Romero-Palacios, A.; Mera Gallardo, O.; Jiménez Aguilar, P.; Borrallo Torrejón, J.J.; Maza Ortega, C.; Rodriguez-Iglesias, M.A. Traqueobronquitis Obstructiva Por Aspergillus Fumigatus En Un Paciente Inmunocompetente. Rev. Iberoam. Micol. 2019, 36, 34–36. [Google Scholar] [CrossRef]
- Tudesq, J.J.; Peyrony, O.; Lemiale, V.; Azoulay, E. Invasive Pulmonary Aspergillosis in Nonimmunocompromised Hosts. Semin. Respir. Crit. Care Med. 2019, 40, 540–547. [Google Scholar] [CrossRef]
- Kanj, A.; Abdallah, N.; Soubani, A.O. The Spectrum of Pulmonary Aspergillosis. Respir. Med. 2018, 141, 121–131. [Google Scholar] [CrossRef]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Flörl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 Guidelines for the Treatment of Invasive Candidiasis, Aspergillosis and Mucormycosis in Leukemia and Hematopoietic Stem Cell Transplant Patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef]
- Astvad, K.M.T.; Hare, R.K.; Arendrup, M.C. Evaluation of the In Vitro Activity of Isavuconazole and Comparator Voriconazole against 2635 Contemporary Clinical Candida and Aspergillus Isolates. Clin. Microbiol. Infect. 2017, 23, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Buil, J.B.; Brüggemann, R.J.M.; Wasmann, R.E.; Zoll, J.; Meis, J.F.; Melchers, W.J.G.; Mouton, J.W.; Verweij, P.E. Isavuconazole Susceptibility of Clinical Aspergillus Fumigatus Isolates and Feasibility of Isavuconazole Dose Escalation to Treat Isolates with Elevated MICs. J. Antimicrob. Chemother. 2018, 73, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolcato, L.; Thiebaut-Bertrand, A.; Stanke-Labesque, F.; Gautier-Veyret, E. Variability of Isavuconazole Trough Concentrations during Longitudinal Therapeutic Drug Monitoring. J. Clin. Med. 2022, 11, 5756. [Google Scholar] [CrossRef] [PubMed]
- Intra, J.; Sala, M.R.; Brambilla, P.; Carcione, D.; Leoni, V. Prevalence and Species Distribution of Microorganisms Isolated among Non-Pregnant Women Affected by Vulvovaginal Candidiasis: A Retrospective Study over a 20 Year-Period. J. Med. Mycol. 2022, 32, 101278. [Google Scholar] [CrossRef] [PubMed]
- Dadar, M.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Shahali, Y.; Dhama, K. Candida Albicans—Biology, Molecular Characterization, Pathogenicity, and Advances in Diagnosis and Control—An Update. Microb. Pathog. 2018, 117, 128–138. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Xie, T.; Wu, L.; Liu, X.Y.; Zhu, L.; Chen, Y.; Mao, E.Q.; Han, L.Z.; Chen, E.Z.; Yang, Z.T. Epidemiology, Species Distribution, and Outcome of Nosocomial Candida spp. Bloodstream Infection in Shanghai: An 11-Year Retrospective Analysis in a Tertiary Care Hospital. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Colombo, A.L.; Agnelli, C.; Kontoyiannis, D.P. Knowledge Gaps in Candidaemia/Invasive Candidiasis in Haematological Cancer Patients. J. Antimicrob. Chemother. 2021, 76, 543–546. [Google Scholar] [CrossRef]
- Eggimann, P.; Garbino, J.; Pittet, D. Management of Candida Species Infections in Critically Ill Patients. Lancet Infect. Dis. 2003, 3, 772–785. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Doi, A.M.; Pignatari, A.C.C.; Edmond, M.B.; Rodrigues Marra, A.; Camargo, L.F.A.; Siqueira, R.A.; Pereira Da Mota, V.; Lopes Colombo, A. Epidemiology and Microbiologic Characterization of Nosocomial Candidemia from a Brazilian National Surveillance Program. PLoS ONE 2016, 11, e0146909. [Google Scholar] [CrossRef]
- Epelbaum, O.; Chasan, R. Candidemia in the Intensive Care Unit. Clin. Chest Med. 2017, 38, 493–509. [Google Scholar] [CrossRef]
- Falces-Romero, I.; Romero-Gómez, M.P.; Moreno-Ramos, F.; Mingorance, J.; García-Rodríguez, J.; Cendejas-Bueno, E. Epidemiology of Bloodstream Candida Species in a Spanish Tertiary Care Hospital as a Guide for Implementation of T2MR (T2CANDIDA®) for Rapid Diagnosis of Candidemia. Med. Mycol. 2021, 59, 350–354. [Google Scholar] [CrossRef]
- Colombo, A.L.; Nucci, M.; Park, B.J.; Nouér, S.A.; Arthington-Skaggs, B.; da Matta, D.A.; Warnock, D.; Morgan, J. Epidemiology of Candidemia in Brazil: A Nationwide Sentinel Surveillance of Candidemia in Eleven Medical Centers. J. Clin. Microbiol. 2006, 44, 2816–2823. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.L.; Júnior, J.N.D.A.; Guinea, J. Emerging Multidrug-Resistant Candida Species. Curr. Opin. Infect. Dis. 2017, 30, 528–538. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida Auris: Epidemiology, Biology, Antifungal Resistance, and Virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Sardi, J.D.C.O.; Silva, D.R.; Mendes-Giannini, M.J.S.; Rosalen, P.L. Candida Auris: Epidemiology, Risk Factors, Virulence, Resistance, and Therapeutic Options. Microb. Pathog. 2018, 125, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Najeeb, H.; Siddiqui, S.A.; Anas, Z.; Ali, S.H.; Usmani, S.U.R.; Jawed, F.; Jatoi, H.N. The Menace of Candida Auris Epidemic Amidst the COVID-19 Pandemic: A Systematic Review. Diseases 2022, 10, 58. [Google Scholar] [CrossRef]
- De Almeida, J.N., Jr.; Francisco, E.C.; Hagen, F.; Brandão, I.B.; Pereira, F.M.; Dias, P.H.P.; de Miranda Costa, M.M.; de Souza Jordão, R.T.; de Groot, T.; Colombo, A.L.; et al. Emergence of Candida Auris in Brazil in a COVID-19 Intensive Care Unit. J. Fungi 2021, 7, 220. [Google Scholar] [CrossRef]
- Alothman, A.F.; Al-Musawi, T.; Al-Abdely, H.M.; Salman, J.A.; Almaslamani, M.; Yared, N.; Butt, A.A.; Raghubir, N.; Morsi, W.; Thaqafi, A.O.; et al. Clinical Practice Guidelines for the Management of Invasive Candida Infections in Adults in the Middle East Region: Expert Panel Recommendations. J. Infect. Public Health 2014, 7, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Arastehfar, A.; Daneshnia, F.; Kord, M.; Roudbary, M.; Zarrinfar, H.; Fang, W.; Hashemi, S.J.; Najafzadeh, M.J.; Khodavaisy, S.; Pan, W.; et al. Comparison of 21-Plex PCR and API 20C AUX, MALDI-TOF MS, and RDNA Sequencing for a Wide Range of Clinically Isolated Yeast Species: Improved Identification by Combining 21-Plex PCR and API 20C AUX as an Alternative Strategy for Developing Countries. Front. Cell. Infect. Microbiol. 2019, 9, 21. [Google Scholar] [CrossRef]
- Contreras, D.A.; Morgan, M.A.; Wang, Y.F.; Desnos-Ollivier, M. Surveillance Diagnostic Algorithm Using Real-Time PCR Assay and Strain Typing Method Development to Assist with the Control of C. auris amid COVID-19 Pandemic. Front. Cell. Infect. Microbiol. 2022, 12, 887754. [Google Scholar] [CrossRef] [PubMed]
- Morace, G.; Perdoni, F.; Borghi, E. Antifungal Drug Resistance in Candida Species. J. Glob. Antimicrob. Resist. 2014, 2, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Tragiannidis, A.; Gkampeta, A.; Vousvouki, M.; Vasileiou, E.; Groll, A.H. Antifungal Agents and the Kidney: Pharmacokinetics, Clinical Nephrotoxicity, and Interactions. Expert Opin. Drug Saf. 2021, 20, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Garzon, A.; Monteoliva, L.; Gil, C.; Alvarez-Moreno, C.; Vega-Vela, N.E.; Engelthaler, D.M.; Bowers, J.; le Pape, P.; Parra-Giraldo, C.M. Genotypic, Proteomic, and Phenotypic Approaches to Decipher the Response to Caspofungin and Calcineurin Inhibitors in Clinical Isolates of Echinocandin-Resistant Candida Glabrata. J. Antimicrob. Chemother. 2022, 77, 585–597. [Google Scholar] [CrossRef]
- Ruhnke, M. Antifungal Stewardship in Invasive Candida Infections. Clin. Microbiol. Infect. 2014, 20, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Yang, X.; Wang, D.; Yu, C.; Sun, S. Antifungal Activity of Immunosuppressants Used Alone or in Combination with Fluconazole. J. Appl. Microbiol. 2019, 126, 1304–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, H.; Matsumoto, T.; Kimura, U.; Hiruma, M.; Kusuhara, M.; Ihn, H. Cutaneous Cryptococcosis. Med. Mycol. J. 2019, 60, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Henao-Martínez, A.F.; Chastain, D.B.; Franco-Paredes, C. Treatment of Cryptococcosis in Non-HIV Immunocompromised Patients. Curr. Opin. Infect. Dis. 2018, 31, 278–285. [Google Scholar] [CrossRef]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef] [Green Version]
- Franco-Paredes, C.; Womack, T.; Bohlmeyer, T.; Sellers, B.; Hays, A.; Patel, K.; Lizarazo, J.; Lockhart, S.R.; Siddiqui, W.; Marr, K.A. Management of Cryptococcus Gattii Meningoencephalitis. Lancet Infect. Dis. 2015, 15, 348–355. [Google Scholar] [CrossRef]
- Jackson, A.; Powderly, W.G. Cryptococcal Infection. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 85, pp. 159–167. ISBN 9780444520104. [Google Scholar]
- Skolnik, K.; Huston, S.; Mody, C.H. Cryptococcal Lung Infections. Clin. Chest Med. 2017, 38, 451–464. [Google Scholar] [CrossRef]
- Leipheimer, J.; Bloom, A.L.M.; Campomizzi, C.S.; Salei, Y.; Panepinto, J.C. Translational Regulation Promotes Oxidative Stress Resistance in the Human Fungal Pathogen Cryptococcus Neoformans. MBio 2019, 10, e02143-19. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Chen, S.; Zhang, J.; Li, X.; Wu, J.; Lin, N. Cryptococcus Neoformans CAP10 Gene Regulates the Immune Response in Mice. J. Med. Mycol. 2021, 31, 101160. [Google Scholar] [CrossRef]
- Wear, M.P.; Jacobs, E.; Wang, S.; McConnell, S.A.; Bowen, A.; Strother, C.; Cordero, R.J.B.; Crawford, C.J.; Casadavall, A. Cryptococcus Neoformans Capsule Regrowth Experiments Reveal Dynamics of Enlargement and Architecture. J. Biol. Chem. 2022, 298, 101769. [Google Scholar] [CrossRef]
- Ball, B.; Woroszchuk, E.; Sukumaran, A.; West, H.; Afaq, A.; Carruthers-Lay, D.; Muselius, B.; Gee, L.; Langille, M.; Pladwig, S.; et al. Proteome and Secretome Profiling of Zinc Availability in Cryptococcus Neoformans Identifies Wos2 as a Subtle Influencer of Fungal Virulence Determinants. BMC Microbiol. 2021, 21, 1–16. [Google Scholar] [CrossRef]
- Brandão, F.; Esher, S.K.; Ost, K.S.; Pianalto, K.; Nichols, C.B.; Fernandes, L.; Bocca, A.L.; Poças-Fonseca, M.J.; Alspaugh, J.A. HDAC Genes Play Distinct and Redundant Roles in Cryptococcus Neoformans Virulence. Sci. Rep. 2018, 8, 5209. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.S.; Coelho, C.; de Leon-Rodriguez, C.M.; Rossi, D.C.P.; Camacho, E.; Jung, E.H.; Kulkarni, M.; Casadevall, A. Cryptococcus neoformans Urease Affects the Outcome of Intracellular Pathogenesis by Modulating Phagolysosomal pH. PLoS Pathog. 2018, 14, e1007144. [Google Scholar] [CrossRef] [Green Version]
- Toplis, B.; Bosch, C.; Schwartz, I.S.; Kenyon, C.; Boekhout, T.; Perfect, J.R.; Botha, A. The Virulence Factor Urease and Its Unexplored Role in the Metabolism of Cryptococcus Neoformans. FEMS Yeast Res. 2020, 20, foaa031. [Google Scholar] [CrossRef]
- Springer, D.J.; Mohan, R.; Heitman, J. Plants Promote Mating and Dispersal of the Human Pathogenic Fungus Cryptococcus. PLoS ONE 2017, 12, e0171695. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.H.; Hu, C.Q.; Shi, Y.; Wu, F.T.; Yang, Q.; Guan, J.; Li, A.C.; Chen, Z. Cryptococcosis in Patients with Liver Cirrhosis: Death Risk Factors and Predictive Value of Prognostic Models. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 460–468. [Google Scholar] [CrossRef]
- Huston, S.M.; Mody, C.H. Cryptococcosis: An Emerging Respiratory Mycosis. Clin. Chest Med. 2009, 30, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Magalhães, T.F.; Costa, M.C.; Holanda, R.A.; Ferreira, G.F.; Dutra Carvalho, V.S.; Cota Freitas, G.J.; Ribeiro, N.Q.; Peres Emídio, E.C.; Fonseca Carmo, P.H.; de Brito, C.B.; et al. N-Acetylcysteine Reduces Amphotericin B Deoxycholate Nephrotoxicity and Improves the Outcome of Murine Cryptococcosis. Med. Mycol. 2020, 58, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A Systematic Review of Fluconazole Resistance in Clinical Isolates of Cryptococcus Species. Mycoses 2018, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Papon, N.; Goldman, G.H. Unraveling Caspofungin Resistance in Cryptococcus Neoformans. MBio 2021, 12, e03225-20. [Google Scholar] [CrossRef] [PubMed]
- Spadari, C.D.C.; Wirth, F.; Lopes, L.B.; Ishida, K. New Approaches for Cryptococcosis Treatment. Microorganisms 2020, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.Q.; Santos, A.P.N.; Emídio, E.C.P.; Costa, M.C.; Freitas, G.J.C.; Carmo, P.H.F.; Silva, M.F.; de Brito, C.B.; de Souza, D.G.; Paixão, T.A.; et al. Pioglitazone as an Adjuvant of Amphotericin B for the Treatment of Cryptococcosis. Int. J. Antimicrob. Agents 2019, 54, 301–308. [Google Scholar] [CrossRef]
- Iyer, K.R.; Revie, N.M.; Fu, C.; Robbins, N.; Cowen, L.E. Treatment Strategies for Cryptococcal Infection: Challenges, Advances and Future Outlook. Nat. Rev. Microbiol. 2021, 19, 454–466. [Google Scholar] [CrossRef]
- Jarvis, J.N.; Lawrence, D.S.; Meya, D.B.; Kagimu, E.; Kasibante, J.; Mpoza, E.; Rutakingirwa, M.K.; Ssebambulidde, K.; Tugume, L.; Rhein, J.; et al. Single-Dose Liposomal Amphotericin B Treatment for Cryptococcal Meningitis. N. Engl. J. Med. 2022, 386, 1109–1120. [Google Scholar] [CrossRef]
- Zaragoza, O. Basic Principles of the Virulence of Cryptococcus. Virulence 2019, 10, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Pearson, M.M.; Rogers, P.D.; Cleary, J.D.; Chapman, S.W. Voriconazole: A New Triazole Antifungal Agent. Ann. Pharmacother. 2003, 37, 420–432. [Google Scholar] [CrossRef]
- Shirasawa, H.; Nagino, K. Voriconazole-Medicalneeds, Evidence, Potential for the Future. Nippon. Ishinkin Gakkai Zasshi 2005, 46, 223–228. [Google Scholar] [CrossRef]
- DrugBank. Voriconazole. Available online: https://go.drugbank.com/drugs/DB00582 (accessed on 6 December 2022).
- Malani, A.; Kerr, L.; Kauffman, C. Voriconazole: How to Use This Antifungal Agent and What to Expect. Semin. Respir. Crit. Care Med. 2015, 36, 786–795. [Google Scholar] [CrossRef]
- Niwa, T.; Imagawa, Y.; Yamazaki, H. Drug Interactions between Nine Antifungal Agents and Drugs Metabolized by Human Cytochromes P450. Curr. Drug Metab. 2014, 15, 651–679. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Guarro, J. In Vitro Activities of Four Novel Triazoles against Scedosporium spp. Antimicrob. Agents Chemother. 2001, 45, 2151–2153. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A. In Vitro Fungicidal Activities of Voriconazole, Itraconazole, and Amphotericin B against Opportunistic Moniliaceous and Dematiaceous Fungi. J. Clin. Microbiol. 2001, 39, 954–958. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, H.L.; Rathbun, R.C. Review of the Safety and Efficacy of Voriconazole. Expert Opin. Investig. Drugs 2002, 11, 410–429. [Google Scholar]
- Fothergill, A.W.; Sutton, D.A.; Mccarthy, D.I.; Wiederhold, N.P. Impact of New Antifungal Breakpoints on Antifungal Resistance in Candida Species. J. Clin. Microbiol. 2014, 52, 994–997. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Pfaller, M.A.; Bustamante, B.; Canton, E.; Fothergill, A.; Fuller, J.; Gonzalez, G.M.; Lass-Flörl, C.; Lockhart, S.R.; Martin-Mazuelos, E.; et al. Multilaboratory Study of Epidemiological Cutoff Values for Detection of Resistance in Eight Candida Species to Fluconazole, Posaconazole, and Voriconazole. Antimicrob. Agents Chemother. 2014, 58, 2006–2012. [Google Scholar] [CrossRef] [Green Version]
- Chaabane, F.; Graf, A.; Jequier, L.; Coste, A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida Auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef]
- Kane, A.; Carter, D.A. Augmenting Azoles with Drug Synergy to Expand the Antifungal Toolbox. Pharmaceuticals 2022, 15, 482. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Aller, A.I.; Canton, E.; Castañón-Olivares, L.R.; Chowdhary, A.; Cordoba, S.; Cuenca-Estrella, M.; Fothergill, A.; Fuller, J.; Govender, N.; et al. Cryptococcus Neoformans-Cryptococcus Gattii Species Complex: An International Study of Wild-Type Susceptibility Endpoint Distributions and Epidemiological Cutoff Values for Fluconazole, Itraconazole, Posaconazole, and Voriconazole. Antimicrob. Agents Chemother. 2012, 56, 5898–5906. [Google Scholar] [CrossRef] [PubMed]
- Hazirolan, G.; Canton, E.; Sahin, S.; Arikan-Akdagli, S. Head-to-Head Comparison of Inhibitory and Fungicidal Activities of Fluconazole, Itraconazole, Voriconazole, Posaconazole, and Isavuconazole against Clinical Isolates of Trichosporon asahii. Antimicrob. Agents Chemother. 2013, 57, 4841–4847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.-K.; Ciblak, M.A.; Nordoff, N.; Pasarell, L.; Warnock, D.W.; Mcginnis, M.R. In Vitro Activities of Voriconazole, Itraconazole, and Amphotericin B against Blastomyces Dermatitidis, Coccidioides Immitis, and Histoplasma Capsulatum. Antimicrob. Agents Chemother. 2000, 44, 1734–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroz-Telles, F.; Goldani, L.Z.; Schlamm, H.T.; Goodrich, J.M.; Espinel-Ingroff, A.; Shikanai-Yasuda, M.A. An Open-Label Comparative Pilot Study of Oral Voriconazole and Itraconazole for Long-Term Treatment of Paracoccidioidomycosis. Clin. Infect. Dis. 2007, 45, 1462–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freifeld, A.; Proia, L.; Andes, D.; Baddour, L.M.; Blair, J.; Spellberg, B.; Arnold, S.; Lentnek, A.; Wheat, L.J. Voriconazole Use for Endemic Fungal Infections. Antimicrob. Agents Chemother. 2009, 53, 1648–1651. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.N.; Salem, F.; Jamei, M.; Rostami-Hodjegan, A. Prediction of Voriconazole Non-Linear Pharmacokinetics Using a Paediatric Physiologically Based Pharmacokinetic Modelling Approach. Clin. Pharmacokinet. 2015, 54, 567–568. [Google Scholar] [CrossRef]
- Luong, M.-L.; Al-Dabbagh, M.; Groll, A.H.; Racil, Z.; Nannya, Y.; Mitsani, D.; Husain, S. Utility of Voriconazole Therapeutic Drug Monitoring: A Meta-Analysis. J. Antimicrob. Chemother. 2016, 71, 1786–1799. [Google Scholar] [CrossRef]
- Yi, W.M.; Schoeppler, K.E.; Jaeger, J.; Mueller, S.W.; MacLaren, R.; Fish, D.N.; Kiser, T.H. Voriconazole and Posaconazole Therapeutic Drug Monitoring: A Retrospective Study. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1–14. [Google Scholar] [CrossRef]
- Benitez, L.L.; Carver, P.L. Adverse Effects Associated with Long-Term Administration of Azole Antifungal Agents. Drugs 2019, 79, 833–853. [Google Scholar] [CrossRef]
- Samanta, P.; Clancy, C.J.; Marini, R.V.; Rivosecchi, R.M.; McCreary, E.K.; Shields, R.K.; Falcione, B.A.; Viehman, A.; Sacha, L.; Kwak, E.J.; et al. Isavuconazole Is as Effective as and Better Tolerated than Voriconazole for Antifungal Prophylaxis in Lung Transplant Recipients. Clin. Infect. Dis. 2021, 73, 416–426. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, F.; Zaib, S.; Shah, H.S.; Jamil, Q.A.; Akbar Sheikh, F.; Khan, A.; Rabea, S.; Hagras, S.A.A.; El-Saber Batiha, G.; et al. Fabrication and Evaluation of Voriconazole Loaded Transethosomal Gel for Enhanced Antifungal and Antileishmanial Activity. Molecules 2022, 27, 3347. [Google Scholar] [CrossRef]
- Mathieu, A.; Thiboutot, Z.; Ferreira, V.; Benoit, P.; Grandjean Lapierre, S.; Hétu, P.-O.; Halwagi, A. Voriconazole Sequestration during Extracorporeal Membrane Oxygenation for Invasive Lung Aspergillosis: A Case Report. ASAIO J. 2022, 68, e56–e58. [Google Scholar] [CrossRef]
- Rosanova, M.T.; Bes, D.; Serrano Aguilar, P.; Sberna, N.; Lede, R. Efficacy and Safety of Voriconazole in Immunocompromised Patients: Systematic Review and Meta-Analysis. Infect. Dis. 2018, 50, 489–494. [Google Scholar] [CrossRef]
- Füredi, P.; Pápay, Z.E.; Kovács, K.; Kiss, B.D.; Ludányi, K.; Antal, I.; Klebovich, I. Development and Characterization of the Voriconazole Loaded Lipid-Based Nanoparticles. J. Pharm. Biomed. Anal. 2017, 132, 184–189. [Google Scholar] [CrossRef]
- Chung, H.; Lee, H.; Han, H.; An, H.; Lim, K.S.; Lee, Y.; Cho, J.-Y.; Yoon, S.H.; Jang, I.-J.; Yu, K.-S. A Pharmacokinetic Comparison of Two Voriconazole Formulations and the Effect of CYP2C19 Polymorphism on Their Pharmacokinetic Profiles. Drug Des. Devel. Ther. 2015, 13, 2609–2616. [Google Scholar] [CrossRef] [Green Version]
- Salem, H.F.; Kharshoum, R.M.; Abdel Hakim, L.F.; Abdelrahim, M.E. Edge Activators and a Polycationic Polymer Enhance the Formulation of Porous Voriconazole Nanoagglomerate for the Use as a Dry Powder Inhaler. J. Liposome Res. 2016, 26, 324–335. [Google Scholar] [CrossRef]
- Song, S.H.; Lee, K.M.; Kang, J.B.; Lee, S.G.; Kang, M.J.; Choi, Y.W. Improved Skin Delivery of Voriconazole with a Nanostructured Lipid Carrier-Based Hydrogel Formulation. Chem. Pharm. Bull. 2014, 62, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, N.; Kocheise, F.; Carls, A.; Burhenne, J.; Weiss, J.; Haefeli, W.E.; Mikus, G. Dose-Dependent Bioavailability and CYP3A Inhibition Contribute to Non-Linear Pharmacokinetics of Voriconazole. Clin. Pharmacokinet. 2016, 55, 1535–1545. [Google Scholar] [CrossRef]
- Allegra, S.; Fatiguso, G.; de Francia, S.; Favata, F.; Pirro, E.; Carcieri, C.; de Nicolò, A.; Cusato, J.; di Perri, G.; D’Avolio, A. Therapeutic Drug Monitoring of Voriconazole for Treatment and Prophylaxis of Invasive Fungal Infection in Children. Br. J. Clin. Pharmacol. 2018, 84, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Kadam, R.S.; van den Anker, J.N. Pediatric Clinical Pharmacology of Voriconazole: Role of Pharmacokinetic/Pharmacodynamic Modeling in Pharmacotherapy. Clin. Pharmacokinet. 2016, 55, 1031–1043. [Google Scholar] [CrossRef]
- Tang, D.; Song, B.; Yan, M.; Zou, J.; Zhang, M.; Zhou, H.; Wang, F.; Xiao, Y.; Xu, P.; Zhang, B.; et al. Identifying Factors Affecting the Pharmacokinetics of Voriconazole in Patients with Liver Dysfunction: A Population Pharmacokinetic Approach. Basic Clin. Pharmacol. Toxicol. 2019, 125, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zubiaur, P.; Kneller, L.A.; Ochoa, D.; Mejía, G.; Saiz-Rodríguez, M.; Borobia, A.M.; Koller, D.; García, I.G.; Navares-Gómez, M.; Hempel, G.; et al. Evaluation of Voriconazole CYP2C19 Phenotype-Guided Dose Adjustments by Physiologically Based Pharmacokinetic Modeling. Clin. Pharmacokinet. 2021, 60, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Wyeth Vfend®: Voriconazol. 2021, pp. 1–30. Available online: https://www.pfizer.com.br/files/Vfend_Comprimidos_Profissional_de_Saude_33.pdf (accessed on 29 March 2022).
- Dolton, M.J.; McLachlan, A.J. Voriconazole Pharmacokinetics and Exposure-Response Relationships: Assessing the Links between Exposure, Efficacy and Toxicity. Int. J. Antimicrob. Agents 2014, 44, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Frechen, S.; Moj, D.; Lehr, T.; Taubert, M.; Hsin, C.; Mikus, G.; Neuvonen, P.J.; Olkkola, K.T.; Saari, T.I.; et al. A Physiologically Based Pharmacokinetic Model of Voriconazole Integrating Time-Dependent Inhibition of CYP3A4, Genetic Polymorphisms of CYP2C19 and Predictions of Drug–Drug Interactions. Clin. Pharmacokinet. 2020, 59, 781–808. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Eto, D.; Goto, K.; Ohchi, Y.; Yasuda, N.; Suzuki, Y.; Tatsuta, R.; Kitano, T.; Itoh, H. Pharmacokinetic and Adsorptive Analyses of Administration of Oral Voriconazole Suspension via Enteral Feeding Tube in Intensive Care Unit Patients. Biol. Pharm. Bull. 2021, 44, 737–741. [Google Scholar] [CrossRef]
- Vanstraelen, K.; Wauters, J.; de Loor, H.; Vercammen, I.; Annaert, P.; Lagrou, K.; Spriet, I. Protein-Binding Characteristics of Voriconazole Determined by High-Throughput Equilibrium Dialysis. J. Pharm. Sci. 2014, 103, 2565–2570. [Google Scholar] [CrossRef]
- Yuan, Z.-Q.-Y.; Qiao, C.; Yang, Z.-C.; Yu, L.; Sun, L.-N.; Qian, Y.; Zhang, X.-H.; Meng, L.; Zhang, X.-Y.; Wang, Y.-Q. The Impact of Plasma Protein Binding Characteristics and Unbound Concentration of Voriconazole on Its Adverse Drug Reactions. Front. Pharmacol. 2020, 11, 505. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wu, S.; Gong, W.; Cao, P.; Chen, X.; Liu, W.; Xiang, L.; Wang, Y.; Huang, J. Application of Population Pharmacokinetic Analysis to Characterize CYP2C19 Mediated Metabolic Mechanism of Voriconazole and Support Dose Optimization. Front. Pharmacol. 2022, 12, 3828. [Google Scholar] [CrossRef]
- Geist, M.J.P.; Egerer, G.; Burhenne, J.; Riedel, K.-D.; Weiss, J.; Mikus, G. Steady-State Pharmacokinetics and Metabolism of Voriconazole in Patients. J. Antimicrob. Chemother. 2013, 68, 2592–2599. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, N.; Galdames, J.; Navea, D.; Farfán, M.J.; Salas, C. Frequency of the CYP2C19*17 Polymorphism in a Chilean Population and Its Effect on Voriconazole Plasma Concentration in Immunocompromised Children. Sci. Rep. 2019, 9, 8863. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Oh, J.; Kim, A.H.; Ji, S.C.; Park, S.-I.; Yoon, S.H.; Chung, J.-Y.; Yu, K.-S.; Jang, I.-J.; Lee, S. Oral Absorption of Voriconazole Is Affected by SLCO2B1 c.*396T>C Genetic Polymorphism in CYP2C19 Poor Metabolizers. Pharm. J. 2020, 20, 792–800. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, H.; Sun, J.; Cheng, X.; Xie, J.; Dong, H.; Chen, L.; Wang, X.; Xing, J.; Dong, Y. Efficacy and Safety of Voriconazole and CYP2C19 Polymorphism for Optimised Dosage Regimens in Patients with Invasive Fungal Infections. Int. J. Antimicrob. Agents 2014, 44, 436–442. [Google Scholar] [CrossRef]
- Mikus, G.; Scholz, I.M.; Weiss, J. Pharmacogenomics of the Triazole Antifungal Agent Voriconazole. Pharmacogenomics 2011, 12, 861–872. [Google Scholar] [CrossRef]
- Hafner, V.; Czock, D.; Burhenne, J.; Riedel, K.-D.; Bommer, J.; Mikus, G.; Machleidt, C.; Weinreich, T.; Haefeli, W.E. Pharmacokinetics of Sulfobutylether-Beta-Cyclodextrin and Voriconazole in Patients with End-Stage Renal Failure during Treatment with Two Hemodialysis Systems and Hemodiafiltration. Antimicrob. Agents Chemother. 2010, 54, 2596–2602. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Yang, Y.-Q.; Zeng, N.-J.; Zhang, H.-W.; Sun, L.-N.; Wang, Y.-Q. Simultaneous Determination of Voriconazole and Its Voriconazole N-Oxide Metabolite in Human Urine by Liquid Chromatography/Tandem Mass Spectrometry. J. Chromatogr. Sci. 2022, 60, 800–806. [Google Scholar] [CrossRef]
- Farrokh, S.; Avdic, E. Voriconazole Autoinduction and Saturable Metabolism after Cessation of Rifampin in a Patient with Invasive Central Nervous System Aspergillus: Importance of Therapeutic Drug Monitoring. J. Pharm. Pract. 2019, 32, 589–594. [Google Scholar] [CrossRef]
- Jović, Z.; Janković, S.M.; Ružić Zečević, D.; Milovanović, D.; Stefanović, S.; Folić, M.; Milovanović, J.; Kostić, M. Clinical Pharmacokinetics of Second-Generation Triazoles for the Treatment of Invasive Aspergillosis and Candidiasis. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 139–157. [Google Scholar] [CrossRef]
- Tucker, L.; Higgins, T.; Egelund, E.F.; Zou, B.; Vijayan, V.; Peloquin, C.A. Voriconazole Monitoring in Children with Invasive Fungal Infections. J. Pediatr. Pharmacol. Ther. 2015, 20, 17–23. [Google Scholar] [CrossRef]
- Zonios, D.; Yamazaki, H.; Murayama, N.; Natarajan, V.; Palmore, T.; Childs, R.; Skinner, J.; Bennett, J.E. Voriconazole Metabolism, Toxicity, and the Effect of Cytochrome P450 2C19 Genotype. J. Infect. Dis. 2014, 209, 1941–1948. [Google Scholar] [CrossRef] [Green Version]
- Elewa, H.; El-Mekaty, E.; El-Bardissy, A.; Ensom, M.H.H.; Wilby, K.J. Therapeutic Drug Monitoring of Voriconazole in the Management of Invasive Fungal Infections: A Critical Review. Clin. Pharmacokinet. 2015, 54, 1223–1235. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, L.; Feng, Y.; Zhou, Y.; Zhai, Y.; Lu, J. Meta-Analysis of the Safety of Voriconazole in Definitive, Empirical, and Prophylactic Therapies for Invasive Fungal Infections. BMC Infect. Dis. 2017, 17, 798. [Google Scholar] [CrossRef] [PubMed]
- Epaulard, O.; Leccia, M.-T.; Blanche, S.; Chosidow, O.; Mamzer-Bruneel, M.-F.; Ravaud, P.; Thiebaut, A.; Villier, C.; Lortholary, O. Phototoxicity and Photocarcinogenesis Associated with Voriconazole. Med. Mal. Infect. 2011, 41, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Lempers, V.J.; Meuwese, E.; Mavinkurve-Groothuis, A.M.; Henriet, S.; van der Sluis, I.M.; Hanff, L.M.; Warris, A.; Koch, B.C.P.; Brüggemann, R.J. Impact of Dose Adaptations Following Voriconazole Therapeutic Drug Monitoring in Pediatric Patients. Med. Mycol. 2019, 57, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Valle-T-Figueras, J.M.; Renedo Miró, B.; Benítez Carabante, M.I.; Díaz-de-Heredia, C.; Vima Bofarull, J.; Mendoza-Palomar, N.; Martín-Gómez, M.T.; Soler-Palacín, P. Voriconazole Use in Children: Therapeutic Drug Monitoring and Control of Inflammation as Key Points for Optimal Treatment. J. Fungi 2021, 7, 456. [Google Scholar] [CrossRef] [PubMed]
- Van Wanrooy, M.J.P.; Rodgers, M.G.G.; Span, L.F.R.; Zijlstra, J.G.; Uges, D.R.A.; Kosterink, J.G.W.; van der Werf, T.S.; Alffenaar, J.-W.C. Voriconazole Therapeutic Drug Monitoring Practices in Intensive Care. Ther. Drug Monit. 2016, 38, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Ng, P.; Hamandi, B.; Husain, S.; Lefebvre, M.J.; Battistella, M. Effect of Therapeutic Drug Monitoring and Cytochrome P450 2C19 Genotyping on Clinical Outcomes of Voriconazole: A Systematic Review. Ann. Pharmacother. 2021, 55, 509–529. [Google Scholar] [CrossRef]
- Pasqualotto, A.C.; Xavier, M.O.; Andreolla, H.F.; Linden, R. Voriconazole Therapeutic Drug Monitoring: Focus on Safety. Expert Opin. Drug Saf. 2010, 9, 125–137. [Google Scholar] [CrossRef]
- Amigues, I.; Cohen, N.; Chung, D.; Seo, S.K.; Plescia, C.; Jakubowski, A.; Barker, J.; Papanicolaou, G.A. Hepatic Safety of Voriconazole after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2010, 16, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Li, Z.; Yan, M.; Zhang, B.; Liang, W.; Wang, F.; Xu, P.; Xiang, D.; Xie, X.; Yu, S.; et al. Population Pharmacokinetics of Voriconazole and CYP2C19 Polymorphisms for Optimizing Dosing Regimens in Renal Transplant Recipients. Br. J. Clin. Pharmacol. 2018, 84, 1587–1597. [Google Scholar] [CrossRef] [Green Version]
- Chawla, P.K.; Dherai, A.J.; Ashavaid, T.F. Plasma Voriconazole Estimation by HPLC. Indian J. Clin. Biochem. 2016, 31, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Yousefian, S.; Dastan, F.; Marjani, M.; Tabarsi, P.; Barati, S.; Shahsavari, N.; Kobarfard, F. Determination of Voriconazole Plasma Concentration by HPLC Technique and Evaluating Its Association with Clinical Outcome and Adverse Effects in Patients with Invasive Aspergillosis. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 5497427. [Google Scholar] [CrossRef]
- Chu, H.Y.; Jain, R.; Xie, H.; Pottinger, P.; Fredricks, D.N. Voriconazole Therapeutic Drug Monitoring: Retrospective Cohort Study of the Relationship to Clinical Outcomes and Adverse Events. BMC Infect. Dis. 2013, 13, 105. [Google Scholar] [CrossRef] [Green Version]
- Hanai, Y.; Hamada, Y.; Kimura, T.; Matsumoto, K.; Takahashi, Y.; Fujii, S.; Nishizawa, K.; Takesue, Y. Optimal trough Concentration of Voriconazole with Therapeutic Drug Monitoring in Children: A Systematic Review and Meta-Analysis. J. Infect. Chemother. 2021, 27, 151–160. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Yan, J.; Gao, L.; Zhou, L.; Wang, Y.; Li, Q.; Wang, J.; Chen, T.; Wang, T.; et al. Voriconazole Therapeutic Drug Monitoring in Critically Ill Patients Improves Efficacy and Safety of Antifungal Therapy. Basic Clin. Pharmacol. Toxicol. 2020, 127, 495–504. [Google Scholar] [CrossRef]
- Risum, M.; Vestergaard, M.-B.; Weinreich, U.M.; Helleberg, M.; Vissing, N.H.; Jørgensen, R. Therapeutic Drug Monitoring of Isavuconazole: Serum Concentration Variability and Success Rates for Reaching Target in Comparison with Voriconazole. Antibiotics 2021, 10, 487. [Google Scholar] [CrossRef]
- Duehlmeyer, S.; Klockau, C.; Yu, D.; Rouch, J. Characterization of Therapeutic Drug Monitoring Practices of Voriconazole and Posaconazole at a Pediatric Hospital. J. Pediatr. Pharmacol. Ther. 2021, 26, 26–32. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on In Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.J.; Jung, J.H. Sustainable Drug Release Using Nanoparticle Encapsulated Microneedles. Chem. Asian J. 2022, 17, e202200333. [Google Scholar] [CrossRef]
- Jeong Yoo, Y.; Hoon Lee, C.; Hyun Park, S.; Taik Lim, Y. Nanoparticle-Based Delivery Strategies of Multifaceted Immunomodulatory RNA for Cancer Immunotherapy. J. Control. Release 2022, 343, 564–583. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; He, F.; Chen, G. Fabrication of Pea Protein-Curcumin Nanocomplexes via Microfluidization for Improved Solubility, Nano-Dispersibility and Heat Stability of Curcumin: Insight on Interaction Mechanisms. Int. J. Biol. Macromol. 2021, 168, 686–694. [Google Scholar] [CrossRef]
- Antonio, E.; dos Reis Antunes, O., Jr.; Marcano, R.G.D.J.V.; Diedrich, C.; da Silva Santos, J.; Machado, C.S.; Khalil, N.M.; Mainardes, R.M. Chitosan Modified Poly (Lactic Acid) Nanoparticles Increased the Ursolic Acid Oral Bioavailability. Int. J. Biol. Macromol. 2021, 172, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kanwar, R.; Mehta, S.K. Development of Phosphatidylcholine/Tween 80 Based Biocompatible Clove Oil-in-Water Nanoemulsion as a Green Nanocarrier for Controlled Herbicide Delivery. Environ. Pollut. 2022, 293, 118558. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.J.; Wei, G.; Xiong, C.; Yu, Y.; Li, S.; Hu, L.; Ma, S.; Tian, J. Efficient Oral Insulin Delivery Enabled by Transferrin-Coated Acid-Resistant Metal-Organic Framework Nanoparticles. Sci. Adv. 2022, 8, eabm4677. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, Y.; Zhang, Y.; Faheem, S.; Rizvi, A. A PH-Targeted and NIR-Responsive NaCl-Nanocarrier for Photothermal Therapy and Ion-Interference Therapy. Nanomedicine 2022, 39, 102460. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Lu, N.; Han, S.; Lu, Z.; Xiao, Y.; Zhao, Z.; Zhang, M. Simultaneous Detection of Dual Biomarkers Using Hierarchical MoS2 Nanostructuring and Nano-Signal Amplification-Based Electrochemical Aptasensor toward Accurate Diagnosis of Prostate Cancer. Biosens. Bioelectron. 2022, 197, 113797. [Google Scholar] [CrossRef]
- Ghader, A.; Behruzi, M.; Hossein, M.; Ara, M.; Ghaznavi, H.; Abbasian, A. Photodiagnosis and Photodynamic Therapy Nonlinear Optical Response of Cancer Cells Following Conventional and Nano-Technology Based Treatment Strategies: Results of Chemo-, Thermo- and Radiation Therapies. Photodiagnosis Photodyn. Ther. 2022, 37, 102686. [Google Scholar] [CrossRef]
- Mishra, D.; Hubenak, J.R.; Mathur, A.B. Nanoparticle Systems as Tools to Improve Drug Delivery and Therapeutic Efficacy. J. Biomed. Mater. Res. A 2013, 101, 3646–3660. [Google Scholar] [CrossRef]
- Qamar, Z.; Qizilbash, F.F.; Iqubal, M.K.; Ali, A.; Narang, J.K.; Ali, J.; Baboota, S. Nano-Based Drug Delivery System: Recent Strategies for the Treatment of Ocular Disease and Future Perspective. Recent Pat. Drug Deliv. Formul. 2019, 13, 246–254. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnology 2022, 20, 1–29. [Google Scholar] [CrossRef]
- Muluh, T.A.; Chen, Z.; Li, Y.; Xiong, K.; Jin, J.; Fu, S.; Wu, J. Enhancing Cancer Immunotherapy Treatment Goals by Using Nanoparticle Delivery System. Int. J. Nanomed. 2021, 16, 2389–2404. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Pardeshi, S.R.; More, M.P.; Patil, P.B.; Mujumdar, A.; Naik, J.B. Statistical Optimization of Voriconazole Nanoparticles Loaded Carboxymethyl Chitosan-Poloxamer Based in Situ Gel for Ocular Delivery: In Vitro, Ex Vivo, and Toxicity Assessment. Drug Deliv. Transl. Res. 2022, 12, 3063–3082. [Google Scholar] [CrossRef]
- Shah, M.K.A.; Azad, A.K.; Nawaz, A.; Ullah, S.; Latif, M.S.; Rahman, H.; Alsharif, K.F.; Alzahrani, K.J.; El-Kott, A.F.; Albrakati, A.; et al. Formulation Development, Characterization and Antifungal Evaluation of Chitosan NPs for Topical Delivery of Voriconazole In Vitro and Ex Vivo. Polymers 2022, 14, 135. [Google Scholar] [CrossRef]
- Kaur, R.; Dennison, S.R.; Burrow, A.J.; Rudramurthy, S.M.; Swami, R.; Gorki, V.; Katare, O.P.; Kaushik, A.; Singh, B.; Singh, K.K. Nebulised Surface-Active Hybrid Nanoparticles of Voriconazole for Pulmonary Aspergillosis Demonstrate Clathrin-Mediated Cellular Uptake, Improved Antifungal Efficacy and Lung Retention. J. Nanobiotechnol. 2021, 19, 1–19. [Google Scholar] [CrossRef]
- Qin, T.; Dai, Z.; Xu, X.; Zhang, Z.; You, X.; Sun, H.; Liu, M.; Zhu, H. Nanosuspension as an Efficient Carrier for Improved Ocular Permeation of Voriconazole. Curr. Pharm. Biotechnol. 2020, 22, 245–253. [Google Scholar] [CrossRef]
- Abd-Elsalam, W.H.; Nagy, Y.I.; Abouelatta, S.M. Tailoring Thixotropic Mixed-Lipid Nanoconstructs of Voriconazole for the Management of Vulvovaginal Candidiasis: Formulation, Statistical Optimization, In Vitro Characterization and In Vivo Assessment. Drug Deliv. 2021, 28, 1877–1889. [Google Scholar] [CrossRef]
- Mumtaz, T.; Ahmed, N.; Hassan, N.U.; Badshah, M.; Khan, S.; Rehman, A. Voriconazole Nanoparticles-Based Film Forming Spray: An Efficient Approach for Potential Treatment of Topical Fungal Infections. J. Drug Deliv. Sci. Technol. 2021, 70, 102973. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Liu, B.; Pan, H.; Liu, H. Establishment and Optimization of Voriconazole/HS15/SBE-β-CD Complex System: Based on Micellization Thermodynamics. J. Mol. Liq. 2021, 321, 114453. [Google Scholar] [CrossRef]
- Krawczyk-Santos, A.P.; da Rocha, P.B.R.; Kloppel, L.L.; Souza, B.D.S.; Anjos, J.L.V.; Alonso, A.; de Faria, D.L.A.; Gil, O.M.; Gratieri, T.; Marreto, R.N.; et al. Enhanced Nail Delivery of Voriconazole-Loaded Nanomicelles by Thioglycolic Acid Pretreatment: A Study of Protein Dynamics and Disulfide Bond Rupture. Int. J. Pharm. 2021, 602, 120597. [Google Scholar] [CrossRef]
- Said, M.; Aboelwafa, A.A.; Elshafeey, A.H.; Elsayed, I. Central Composite Optimization of Ocular Mucoadhesive Cubosomes for Enhanced Bioavailability and Controlled Delivery of Voriconazole. J. Drug Deliv. Sci. Technol. 2021, 61, 102075. [Google Scholar] [CrossRef]
- Rasoanirina, B.N.V.; Lassoued, M.A.; Kamoun, A.; Bahloul, B.; Miladi, K.; Sfar, S. Voriconazole-Loaded Self-Nanoemulsifying Drug Delivery System (SNEDDS) to Improve Transcorneal Permeability. Pharm. Dev. Technol. 2020, 25, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Rasoanirina, B.N.V.; Lassoued, M.A.; Miladi, K.; Razafindrakoto, Z.; Chaâbane-Banaoues, R.; Ramanitrahasimbola, D.; Cornet, M.; Sfar, S. Self-Nanoemulsifying Drug Delivery System to Improve Transcorneal Permeability of Voriconazole: In-Vivo Studies. J. Pharm. Pharmacol. 2020, 72, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, T.; Bao, S.; Liu, Y.; Xu, X. Formulation and Characterization of Voriconazole Nanospray Dried Powders. Pharm. Dev. Technol. 2020, 25, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, C.; Hu, L.; Liu, H.; Pan, H.C. Effect of Freeze-Drying Process on the Physical Stability and Properties of Voriconazole Complex System. Dry. Technol. 2018, 36, 871–878. [Google Scholar] [CrossRef]
- Rençber, S.; Karavana, S.Y. Development and In Vitro Evaluation of Voriconazole Nanoparticle Formulation for Mucosal Application. Turk. J. Pharm. Sci. 2018, 15, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Sengupta, S.; Mukherjee, B.; Shaw, T.K.; Gaonkar, R.H.; Debnath, M.C. Chitosan-Coated Nanoparticles Enhanced Lung Pharmacokinetic Profile of Voriconazole upon Pulmonary Delivery in Mice. Nanomedicine 2018, 13, 501–520. [Google Scholar] [CrossRef]
- Füredi, P.; Kovács, K.; Ludányi, K.; Antal, I.; Klebovich, I. Development and Characterization of Voriconazole Loaded Nanoparticles for Parenteral Delivery. Int. J. Pharm. 2016, 510, 159–163. [Google Scholar] [CrossRef]
- Andrade, L.M.; Rocha, K.A.D.; de Sá, F.A.P.; Marreto, R.N.; Lima, E.M.; Gratieri, T.; Taveira, S.F. Voriconazole-Loaded Nanostructured Lipid Carriers for Ocular Drug Delivery. Cornea 2016, 35, 866–871. [Google Scholar] [CrossRef]
- Vass, P.; Démuth, B.; Farkas, A.; Hirsch, E.; Szabó, E.; Nagy, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; Csontos, I.; et al. Continuous Alternative to Freeze Drying: Manufacturing of Cyclodextrin-Based Reconstitution Powder from Aqueous Solution Using Scaled-up Electrospinning. J. Control. Release 2019, 298, 120–127. [Google Scholar] [CrossRef]
- Moon, C.; Watts, A.B.; Lu, X.; Su, Y.; Williams, R.O. Enhanced Aerosolization of High Potency Nanoaggregates of Voriconazole by Dry Powder Inhalation. Mol. Pharm. 2019, 16, 1799–1812. [Google Scholar] [CrossRef]
- Tian, B.; Yan, Q.; Wang, J.; Ding, C.; Sai, S. Enhanced Antifungal Activity of Voriconazole-Loaded Nanostructured Lipid Carriers against Candida Albicans with a Dimorphic Switching Model. Int. J. Nanomed. 2017, 12, 7131–7141. [Google Scholar] [CrossRef]
- Deshkar, S.S.; Palve, V.K. Formulation and Development of Thermosensitive Cyclodextrin-Based In Situ Gel of Voriconazole for Vaginal Delivery. J. Drug Deliv. Sci. Technol. 2019, 49, 277–285. [Google Scholar] [CrossRef]
- Liao, Q.; Yip, L.; Chow, M.Y.T.; Chow, S.F.; Chan, H.K.; Kwok, P.C.L.; Lam, J.K.W. Porous and Highly Dispersible Voriconazole Dry Powders Produced by Spray Freeze Drying for Pulmonary Delivery with Efficient Lung Deposition. Int. J. Pharm. 2019, 560, 144–154. [Google Scholar] [CrossRef]
- Kelidari, H.R.; Babaei, R.; Nabili, M.; Shokohi, T.; Saeedi, M.; Gholami, S.; Moazeni, M.; Nokhodchi, A. Improved Delivery of Voriconazole to Aspergillus Fumigatus through Solid Lipid Nanoparticles as an Effective Carrier. Colloids Surf. A 2018, 558, 338–342. [Google Scholar] [CrossRef]
- Veloso, D.F.M.C.; Benedetti, N.I.G.M.; Avila, R.I.; Bastos, T.S.A.; Silva, T.C.; Silva, M.R.R.; Batista, A.C.; Valadares, M.C.; Lima, E.M. Intravenous Delivery of a Liposomal Formulation of Voriconazole Improves Drug Pharmacokinetics, Tissue Distribution, and Enhances Antifungal Activity. Drug Deliv. 2018, 25, 1585–1594. [Google Scholar] [CrossRef] [Green Version]
- Santos, G.A.; Angelo, T.; Andrade, L.M.; Silva, S.M.M.; Magalhães, P.O.; Cunha-Filho, M.; Gelfuso, G.M.; Taveira, S.F.; Gratieri, T. The Role of Formulation and Follicular Pathway in Voriconazole Cutaneous Delivery from Liposomes and Nanostructured Lipid Carriers. Colloids Surf. B Biointerfaces 2018, 170, 341–346. [Google Scholar] [CrossRef]
- Qurt, M.S.; Esentürk, İ.; Tan, S.B.; Erdal, M.S.; Araman, A.; Güngör, S. Voriconazole and Sertaconazole Loaded Colloidal Nano-Carriers for Enhanced Skin Deposition and Improved Topical Fungal Treatment. J. Drug Deliv. Sci. Technol. 2018, 48, 215–222. [Google Scholar] [CrossRef]
- Esentürk, İ.; Balkan, T.; Özhan, G.; Döşler, S.; Güngör, S.; Erdal, M.S.; Sarac, A.S. Voriconazole Incorporated Nanofiber Formulations for Topical Application: Preparation, Characterization and Antifungal Activity Studies against Candida Species. Pharm. Dev. Technol. 2020, 25, 440–453. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, V.R. Solid Lipid Nanoparticle: An Efficient Carrier for Improved Ocular Permeation of Voriconazole. Drug Dev. Ind. Pharm. 2016, 42, 1956–1967. [Google Scholar] [CrossRef]
- Pandurangan, D.; Bodagala, P.; Palanirajan, V.; Govindaraj, S. Formulation and Evaluation of Voriconazole Ophthalmic Solid Lipid Nanoparticles in Situ Gel. Int. J. Pharm. Investig. 2016, 6, 56–62. [Google Scholar] [CrossRef] [Green Version]
- De Sá, F.A.P.; Taveira, S.F.; Gelfuso, G.M.; Lima, E.M.; Gratieri, T. Liposomal Voriconazole (VOR) Formulation for Improved Ocular Delivery. Colloids Surf. B Biointerfaces 2015, 133, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Pawar, P.; Kashyap, H.; Malhotra, S.; Sindhu, R. Hp-β-CD-Voriconazole In Situ Gelling System for Ocular Drug Delivery: In Vitro, Stability, and Antifungal Activities Assessment. Biomed. Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-F.; Zhong, J.; Chen, G.-P.; Lin, Z.-T.; Deng, Y.; Liu, Y.-L.; Cao, P.-Y.; Wang, B.; Wei, Y.; Wu, T.; et al. A Hydrogel-Based Hybrid Theranostic Contact Lens for Fungal Keratitis. ACS Nano 2016, 10, 6464–6473. [Google Scholar] [CrossRef] [PubMed]
- Das, P.J.; Paul, P.; Mukherjee, B.; Mazumder, B.; Mondal, L.; Baishya, R.; Debnath, M.C.; Dey, K.S. Pulmonary Delivery of Voriconazole Loaded Nanoparticles Providing a Prolonged Drug Level in Lungs: A Promise for Treating Fungal Infection. Mol. Pharm. 2015, 12, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- El-Hadidy, G.N.; Ibrahim, H.K.; Mohamed, M.I.; El-Milligi, M.F. Microemulsions as Vehicles for Topical Administration of Voriconazole: Formulation and In Vitro Evaluation. Drug Dev. Ind. Pharm. 2012, 38, 64–72. [Google Scholar] [CrossRef]
- Carbone, C.; Fuochi, V.; Zielińska, A.; Musumeci, T.; Souto, E.B.; Bonaccorso, A.; Puglia, C.; Petronio Petronio, G.; Furneri, P.M. Dual-Drugs Delivery in Solid Lipid Nanoparticles for the Treatment of Candida Albicans Mycosis. Colloids Surf. B Biointerfaces 2020, 186, 110705. [Google Scholar] [CrossRef]
- Mu, H.; Holm, R. Solid Lipid Nanocarriers in Drug Delivery: Characterization and Design. Expert Opin. Drug Deliv. 2018, 15, 771–785. [Google Scholar] [CrossRef]
- Lankalapalli, S.; Vemuri, V.D.; Tenneti, V.S.V.K.; Guntaka, P.R. Bioavailability Enhancement of Voriconazole Using Liposomal Pastilles: Formulation and Experimental Design Investigation. J. Liposome Res. 2021, 20, 1–15. [Google Scholar] [CrossRef]
- Lima, T.W.K.D.; Renzi, D.F.; Campos, L.D.A. Solid Lipidic Nanoparticles and Their Application in the Delivery of Natural Active Compounds. Braz. J. Dev. 2021, 7, 110306–110330. [Google Scholar] [CrossRef]
- Sheoran, R.; Khokra, S.L.; Chawla, V.; Dureja, H. Recent Patents, Formulation Techniques, Classification and Characterization of Liposomes. Recent Pat. Nanotechnol. 2019, 13, 17–27. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the Design of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Targeting Brain Diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- González-Fernández, F.M.; Bianchera, A.; Gasco, P.; Nicoli, S.; Pescina, S. Lipid-Based Nanocarriers for Ophthalmic Administration: Towards Experimental Design Implementation. Pharmaceutics 2021, 13, 447. [Google Scholar] [CrossRef]
- Makoni, P.A.; Ranchhod, J.; WaKasongo, K.; Khamanga, S.M.; Walker, R.B. The Use of Quantitative Analysis and Hansen Solubility Parameter Predictions for the Selection of Excipients for Lipid Nanocarriers to Be Loaded with Water Soluble and Insoluble Compounds. Saudi Pharm. J. 2020, 28, 308–315. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Z.; Yang, J.; Lu, P.; Zhou, T.; Li, J.; Zhang, J. Assessment to the Antifungal Effects In Vitro and the Ocular Pharmacokinetics of Solid-Lipid Nanoparticle in Rabbits. Int. J. Nanomed. 2021, 16, 7847–7857. [Google Scholar] [CrossRef]
- Faustino, C.; Pinheiro, L. Lipid Systems for the Delivery of Amphotericin B in Antifungal Therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, M.; Tian, B.; Yang, X.; Du, W.; Wang, X.; Zhou, H.; Ding, C.; Sai, S. Calcofluor White-Cholesteryl Hydrogen Succinate Conjugate Mediated Liposomes for Enhanced Targeted Delivery of Voriconazole into Candida Albicans. Biomater. Sci. 2023. [Google Scholar] [CrossRef]
- Ahmed, A.; Sarwar, S.; Hu, Y.; Munir, M.U.; Nisar, M.F.; Ikram, F.; Asif, A.; Rahman, S.U.; Chaudhry, A.A.; Rehman, I.U. Surface-Modified Polymeric Nanoparticles for Drug Delivery to Cancer Cells. Expert Opin. Drug Deliv. 2021, 18, 1–24. [Google Scholar] [CrossRef]
- Li, L.; He, H.; Jiang, S.; Qi, J.; Lu, Y.; Ding, N.; Lin, H.-S.; Wu, W.; Xiang, X. Simulation of the In Vivo Fate of Polymeric Nanoparticles Traced by Environment-Responsive Near-Infrared Dye: A Physiologically Based Pharmacokinetic Modelling Approach. Molecules 2021, 26, 1271. [Google Scholar] [CrossRef]
- Afsharzadeh, M.; Hashemi, M.; Babaei, M.; Abnous, K.; Ramezani, M. PEG-PLA Nanoparticles Decorated with Small-molecule PSMA Ligand for Targeted Delivery of Galbanic Acid and Docetaxel to Prostate Cancer Cells. J. Cell. Physiol. 2020, 235, 4618–4630. [Google Scholar] [CrossRef]
- Ding, D.; Zhu, Q. Recent Advances of PLGA Micro/Nanoparticles for the Delivery of Biomacromolecular Therapeutics. Mater. Sci. Eng. C 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef] [PubMed]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Sagharjoghi Farahani, M.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of Curcumin Loaded Chitosan Nanoparticle within Poly (ε-Caprolactone) and Gelatin Fiber Mat for Wound Healing and Layered Dermal Reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Hernández, J.A.; Rodríguez-Felix, F.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Robles-García, M.A.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-Polysaccharide Nanoparticles as Matrices for Antioxidant Compounds: A Strategy for Prevention of Chronic Degenerative Diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiao, L.; Yu, C.; Jin, P.; Qin, D.; Xu, Y.; Yin, J.; Liu, Z.; Du, Q. Enhanced Antiarthritic Efficacy by Nanoparticles of (−)-Epigallocatechin Gallate–Glucosamine–Casein. J. Agric. Food Chem. 2019, 67, 6476–6486. [Google Scholar] [CrossRef]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Y.; Chen, X.; Zeng, C.; Hu, Q.; Yin, W.; Li, W.; Xie, H.; Zhang, B.; Huang, X.; et al. Nanoparticle-Modified Chitosan-Agarose-Gelatin Scaffold for Sustained Release of SDF-1 and BMP-2. Int. J. Nanomed. 2018, 13, 7395–7408. [Google Scholar] [CrossRef] [Green Version]
- Roviello, G.; Conter, F.U.; Mini, E.; Generali, D.; Traversini, M.; Lavacchi, D.; Nobili, S.; Sobhani, N. Nanoparticle Albumin-Bound Paclitaxel: A Big Nano for the Treatment of Gastric Cancer. Cancer Chemother. Pharmacol. 2019, 84, 669–677. [Google Scholar] [CrossRef]
- Fan, X.; Yang, J.; Loh, X.J.; Li, Z. Polymeric Janus Nanoparticles: Recent Advances in Synthetic Strategies, Materials Properties, and Applications. Macromol. Rapid Commun. 2019, 40, 1800203. [Google Scholar] [CrossRef]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric Nanoparticle Technologies for Oral Drug Delivery. Clin. Gastroenterol. Hepatol. 2014, 12, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What Has Been Done and the Challenges Remain Ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Joshi, M.D.; Patravale, V.; Prabhu, R. Polymeric Nanoparticles for Targeted Treatment in Oncology: Current Insights. Int. J. Nanomed. 2015, 10, 1001–1018. [Google Scholar] [CrossRef] [Green Version]
- Muniswamy, V.J.; Raval, N.; Gondaliya, P.; Tambe, V.; Kalia, K.; Tekade, R.K. ‘Dendrimer-Cationized-Albumin’ Encrusted Polymeric Nanoparticle Improves BBB Penetration and Anticancer Activity of Doxorubicin. Int. J. Pharm. 2019, 555, 77–99. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-Mediated Brain Drug Delivery: Overcoming Blood–Brain Barrier to Treat Neurodegenerative Diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Matoba, T.; Koga, J.; Nakano, K.; Egashira, K.; Tsutsui, H. Nanoparticle-Mediated Drug Delivery System for Atherosclerotic Cardiovascular Disease. J. Cardiol. 2017, 70, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Pechanova, O.; Barta, A.; Koneracka, M.; Zavisova, V.; Kubovcikova, M.; Klimentova, J.; Török, J.; Zemancikova, A.; Cebova, M. Protective Effects of Nanoparticle-Loaded Aliskiren on Cardiovascular System in Spontaneously Hypertensive Rats. Molecules 2019, 24, 2710. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, H.; Gorain, B.; Pandey, M.; Khurana, R.K.; Kesharwani, P. Strategizing Biodegradable Polymeric Nanoparticles to Cross the Biological Barriers for Cancer Targeting. Int. J. Pharm. 2019, 565, 509–522. [Google Scholar] [CrossRef]
- Nemati Shizari, L.; Mohammadpour Dounighi, N.; Bayat, M.; Mosavari, N. A New Amphotericin B-Loaded Trimethyl Chitosan Nanoparticles as a Drug Delivery System and Antifungal Activity on Candida Albicans Biofilm. Arch. Razi Inst. 2021, 76, 571–586. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Abouelmagd, S.A. Surface Functionalization of Polymeric Nanoparticles for Tumor Drug Delivery: Approaches and Challenges. Expert Opin. Drug Deliv. 2017, 14, 201–214. [Google Scholar] [CrossRef]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric Nanoparticles: The Future of Nanomedicine. WIREs Nanomed. Nanobiotechnology 2016, 8, 271–299. [Google Scholar] [CrossRef]
- Sinha, B.; Mukherjee, B.; Pattnaik, G. Poly-Lactide-Co-Glycolide Nanoparticles Containing Voriconazole for Pulmonary Delivery: In Vitro and In Vivo Study. Nanomedicine 2013, 9, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-Based Nanocarriers as Promising Drug and Gene Delivery Systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Kariduraganavar, M.Y.; Heggannavar, G.B.; Amado, S.; Mitchell, G.R. Protein Nanocarriers for Targeted Drug Delivery for Cancer Therapy. In Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 173–204. ISBN 9780128140338. [Google Scholar]
- Samrot, A.V.; Sean, T.C.; Kudaiyappan, T.; Bisyarah, U.; Mirarmandi, A.; Faradjeva, E.; Abubakar, A.; Ali, H.H.; Angalene, J.L.A.; Suresh Kumar, S. Production, Characterization and Application of Nanocarriers Made of Polysaccharides, Proteins, Bio-Polyesters and Other Biopolymers: A Review. Int. J. Biol. Macromol. 2020, 165, 3088–3105. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Chen, J.; Wang, Z.; Wang, X.; Zhong, J. Protein Nanoparticles for Pickering Emulsions: A Comprehensive Review on Their Shapes, Preparation Methods, and Modification Methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- Ghosh, G.; Panicker, L. Protein–Nanoparticle Interactions and a New Insight. Soft Matter 2021, 17, 3855–3875. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Banerjee, S.; Khan, S.; Jha, N.; Gupta, G.; Dua, K.; Hasnain, S.; Kumar Nayak, A.; Kumar Dubey, S.; Singhvi, G. QbD-Driven Formulation Development and Evaluation of Topical Hydrogel Containing Ketoconazole Loaded Cubosomes. Mater. Sci. Eng. C 2020, 119, 111548. [Google Scholar] [CrossRef]
- Victorelli, F.D.; Salvati Manni, L.; Biffi, S.; Bortot, B.; Buzzá, H.H.; Lutz-Bueno, V.; Handschin, S.; Calixto, G.; Murgia, S.; Chorilli, M.; et al. Potential of Curcumin-Loaded Cubosomes for Topical Treatment of Cervical Cancer. J. Colloid Interface Sci. 2022, 620, 419–430. [Google Scholar] [CrossRef]
- Villalva, D.G.; França, C.G.; Loh, W. Characterization of Cubosomes Immobilized in Hydrogels of Hyaluronic Acid and Their Use for Diclofenac Controlled Delivery. Colloids Surf. B Biointerfaces 2022, 212, 112352. [Google Scholar] [CrossRef]
- Miletic, T.; Kyriakos, K.; Graovac, A.; Ibric, S. Spray-Dried Voriconazole-Cyclodextrin Complexes: Solubility, Dissolution Rate and Chemical Stability. Carbohydr. Polym. 2013, 98, 122–131. [Google Scholar] [CrossRef]
- Venturini, C.D.G.; Nicolini, J.; Machado, C.; Machado, V.G. Propriedades e Aplicações Recentes Das Ciclodextrinas. Quim. Nova 2008, 31, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Tomé, V.; García-Otero, X.; Varela-Fernández, R.; Martín-Pastor, M.; Conde-Penedo, A.; Aguiar, P.; González-Barcia, M.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. In Situ Forming and Mucoadhesive Ophthalmic Voriconazole/HPβCD Hydrogels for the Treatment of Fungal Keratitis. Int. J. Pharm. 2021, 597, 120318. [Google Scholar] [CrossRef]
- Botros, S. Clinical Assessment of Voriconazole Self Nano Emulsifying Drug Delivery System Intermediate Gel. Available online: https://clinicaltrials.gov/ct2/show/NCT04110860?term=voriconazole&draw=5&rank=115 (accessed on 28 March 2022).
- Samyang Biopharmaceuticals Corporation. Study to Evaluate the Pharmacokinetic Properties of SYP-1018 (Voriconazole-Loaded PNP) and Voriconazole (Vfend). Available online: https://clinicaltrials.gov/ct2/show/NCT01657201?term=voriconazole&draw=4&rank=70 (accessed on 28 March 2022).
| Species and Varieties | Serotype | Molecular Types |
|---|---|---|
| C. neoformans var. grubii 1 | A | VN I, VN II |
| C. neoformans var. neoformans | D | VN IV |
| C. neoformans | AD | VN III |
| C. gattii | B | VG I, VG II, VG III, VG IV |
| C. gattii | C | VG I, VG II, VG III, VG IV |
| Composition | Method of Preparation | Route of Administration | Size | Toxicity | In Vitro | In Vivo | Reference |
|---|---|---|---|---|---|---|---|
| Chitosan, Sodium Lauryl Sulfate, Poloxamer, Benzalkonium Chloride | O/W Solvent Emulsification Technique | Ocular | 219.3 nm | A study on egg chorioallantoic membrane indicated nanoparticle is not irritating | In C. albicans there was a reduction in MIC compared to free VCZ; NPs loaded in situ gel had MIC at 0.06 µg/mL over 1 µg/mL of free VCZ. Mucoadhesion was increased with nanoparticles and prolonged release for up to 8 h. An Ex vivo study revealed increased permeation of the VRC from the nanoparticles in the cornea. | [216] | |
| Chitosan, sodium lauryl sulfate, propylene glycol, Polyethylene glycol-4000 | Spray Dryer | Topical | 160–500 nm | There was greater retention in the skin and low retention in the stratum corneum. The nanoparticles showed even greater inhibitory activity on C. albicans than on free VCZ, with an inhibition halo of 17.55 mm for the nanoparticle and 9.25 mm for free VCZ. | [217] | ||
| Chitosan, Sodium Tripolyphosphate (TPP), dipalmitoylphosphatidylcholine (DPPC) | Ionic gelation | Pulmonary | 228–255 nm | Cell viability and uptake studies showed cytocompatibility in A549 and Calu-3 lung epithelial cells. | Higher efficacy in Candida sp. and Aspergillus sp. about free VCZ in laboratory strains and equal inhibition in clinical isolates. | Pharmacokinetic study showed an increase of almost 5, 4, and 3 times in the area under the curve, Tmax, and residence time in the lungs. | [218] |
| Eudragit RS 100 PVP (Polyvinylpyrrolidone) PVA (Polyvynil Alcohol) | Quasi-emulsion solvent evaporation | Ophthalmic | 138 nm | The system showed a higher inhibitory effect on C. albicans at lower concentrations than VCZ injection. | Increased corneal permeability in rats | [219] | |
| Glyceryl monooleate Glyceryl monostearate Maisine | High Shear Homogenization and Ultrasonication | Vaginal | 322.72 nm | Normal morphological features on histopathology, with results similar to the negative control | The in vitro release profile compared the aqueous suspension of VCZ and the optimized formulation and verified a sustained release profile, where 70% was released after 8 h. | The reduction of fungal load in Wistar rats was higher when using the nanoparticle about the VCZ suspension. | [220] |
| Chitosan, Tween 80, Sodium tripolyphosphate | Ionic gelation | Topic | 199–232 nm | A skin irritation study was performed in albino rabbits, and no signs of skin irritation and inflammatory cell infiltration were observed. | The release profile was slower in PBS medium pH 7.4 compared to pH 5.5, releasing 82% VCZ in 24 h at the most acidic pH. The ex vivo test using mouse skin (mice) demonstrated that a limited amount of VCZ permeated in the receptor medium, higher for the film with suspended VCZ than the film with VCZ-polymeric nanoparticles. The latter presented higher deposition (15.05%) in the stratum corneum concerning the other formulations and the deposition of 54.76% in the epidermis and dermis. In the antifungal test, the film based on VCZ-PNPs showed the highest retention zone against Candida sp. (20 mm) and Aspergillus sp. (17 mm). | The histological study confirmed its safety, which makes it suitable for topical application. | [221] |
| Surfactant, cyclodextrin | Micellization thermodynamic | Topic | 13–16 nm | A 24-h dialysis membrane release test was performed with different formulations and the release varied from 72 to 75%. The model that best explained the release was the first-order model (r2 = 0.99). | [222] | ||
| Sodium taurodeoxycholate | Emulsification | Topic | 21–24 nm | The Franz cell release test had performed for 10 h, and the amount released corresponded to 30% of the formulation’s VCZ. The kinetic model that best explains it was Higuchi’s (r2 = 0.9842). The permeation test was performed on hulls for 24 h, and no amount of VCZ was detected in the receiving medium. The concentration found in the hull increase 10×. | [223] | ||
| Monoolein, Pluronic F127, chitosan | Cubosomes by Melt dispersion emulsification | Ocular | 109–243 nm | The release test had performed in a dialysis bag for 24 h. The cubosomes showed a biphasic release profile, with a burst effect at 30 min followed by a sustained release for 24 h. | Pharmacokinetic was performed in albino rabbits by ocular instillation. The results showed that the chitosan-coated cubosomes showed higher Cmax than the VCZ-suspension (4.44 and 3.52 ng/mL, respectively; p < 0.0001). A similar performance had obtained for parameters AUC0–8 and AUC0-∞. | [224] | |
| Isopropyl myristate, PEG 400, Tween 80®, Span 80®. | Self-nano emulsifying | Ocular | 21 nm | Ex vivo: nanostructured VCZ formulation demonstrated an increase in VCZ transcorneal permeation compared to the commercial formulation. | [225] | ||
| IPM, PEG 400, Tween 80®, Span 80®. | Self-nano emulsifying (SNEDDS) | Ocular | 21 nm | Eye irritation and damage tests were performed on rabbits. The findings demonstrate that the VCZ nanoformulation was well tolerated and capable of ocular delivery. | When comparing VCZ marketed formulation and VCZ SNEDDS the antifungal activity (MIC) showed similar results for Candida sp. and significantly lower MIC (p < 0.001) for A. fumigatus. | The pharmacokinetic evaluation was superior to the commercial one, presenting the following results: AUC0–8 h: 16,200 µg/mL; Tmax: 2 h; Cmax: 5577 µg/mL. | [226] |
| Polaxâmero 188, Dodecilsulfato de sódio, cloreto de cetiltrimetilamônio | Nanospray dryer | Oral | 421 nm | Subacute treatment with API-NP up to a concentration 80 mg/kg of body weight did not cause adverse toxicological effects in the organs evaluated. | Improved solubility, dissolution, and release of VCZ in the aqueous medium. | API-NP showed improved in pharmacokinetic parameters (AUC, Cmax) compared to API tablets and VFEND®. Increased bioavailability, sustained release, and less inter-individual variability. | [227] |
| Kolliphor® HS 15, Sulfobutyl ether-β-Cyclodextrin | Self-assembly method | 13–15 nm | [228] | ||||
| L-α-Fosfatidilcolina, Polissorbato 80, Witepsol W35, Ácido esteárico e Compritol 888 ATO | High-pressure homogenization | Ophthalmic | 182 nm | The dialysis release test demonstrated that the SLNs were able to release VCZ. The formulation demonstrated antifungal efficacy against Aspergillus flavus and Candida glabrata. | [157] | ||
| Chitosan, EUD RS 100 | Spontaneous Emulsification | Topic | 217 nm | Desired physicochemical characteristics of the formulation for administration in mucosa showing mucoadhesion and release mechanism by dialysis and constant diffusion in vitro. | [229] | ||
| Chitosan, PLGA, PVA | Multiple emulsion by solvent evaporation | Pulmonary | 154–277 nm | After six hours, a greater accumulation of VCZ was detected in the liver than in the urine, suggesting that urinary clearance decreases. | Both formulations, PLGA-NP, coated or not with chitosan, showed sustained VCZ release after 24 h and followed the Korsmeyer–Peppas kinetic model. | Both formulations showed uniform distribution in the alveoli and sustained release up to 72 h, about free VCZ. VCZ levels were detected in the lung and plasma after administration. The Cmax was achieved earlier by the chitosan-coated formulation, but the time required was the same when compared. | [230] |
| Albumin | nabTM-technology | Parenteral | 81 nm | Up to 2× increase in VCZ solubility. | [231] | ||
| NLC (Tween 80, capric caprylic triglycerides, Span 85, cetylpyridinium chloride (CPC), Compritol 888 ATO) | Microemulsion | Ocular | 250 nm | The weak irritant in HET-CAM irritation test. | Therapeutic delivery after 30 min in ex vivo permeability assessment. | [232] | |
| SBE-β-CD | Electrospinning | Parenteral | * | It demonstrated ease in promoting rapid dissolution for reconstitution of the pharmaceutical form. | [233] | ||
| Mannitol (MAN) | Thin Film Freezing (TFF) | Pulmonary | 3 µm | The VCZ nanoaggregates 95:5 formulation showed better in vitro performance in the aerosol performance test, FPF 73.6%, and in dissolution test. | [234] | ||
| Compritol 888 ATO, Miglyol 812N, Gelucire 44/14, Solutol HS 15 e Tween 80. | Melt High-Pressure Homogenization | 45 nm | There was no difference in MIC for VCZ–NLC and free VCZ. However, at low concentrations, the inhibition rate of planktonic cells of C. albicans was higher for VCZ–NLC; there was also a reduction in the biofilm cell density. There is an increase in the efficiency of the VCZ. | [235] | |||
| HP-β-CD + P407, P188 | Spray drying | Vaginal | * | The addition of mucoadhesive polymers increases mucoadhesion and sustained drug release of VCZ. | VCZ uptake was higher when administered in the VCZ HP-β-CD formulation, whose Cmax was 7.13 µg/g at two h post-dose, an increase 3.4× greater than HP-β-CD or VCZ in dispersion. | [236] | |
| Mannitol, TBA (tert-Butyl alcohol) | Spray freezes drying | Pulmonary | 2–4 µm | All test formulations showed complete dissolution within the first 5 min. | V8 (intratracheal) had a higher concentration of VCZ in the lungs when compared to VFEND®(IV). After 30 min, the concentration of VCZ was lower in the liver and spleen, and there was no significant difference in the kidneys. | [237] | |
| Solid Lipid (Compritol 888 ATO or Stearic Acid), Span 80/60, Tween 80 | High-shear homogenization followed by probe ultrasonication | 286 nm | VCZ-SLN reduced the MIC50 value for all the tested Aspergillus fumigates (susceptible and resistant) about free VCZ. | [238] | |||
| Phosphatidylcholine, cholesterol, α-tocopherol | Lipid-film hydration followed by extrusion | Intravenous | 95 nm | Accumulation of VCZ in the liver and kidneys was lower in the liposomal form. | Candida sp. was more susceptible than strains of Aspergillus sp., but there was no difference between VCZ liposome and VFEND®. | There was a difference in the pharmacokinetic parameters for liposome and VFEND®. Liposome reduced the deputation by half; the AUC0–24 increased 2.5× and reduced the volume of distribution of the VCZ. | [239] |
| Phosphatidylcholine (Liposomes) Compritol, Miglyol, Tween 80 e Span 85 (NLC) | Film hydration followed by extrusion (Liposomes) and microemulsion (NLC) | Topic | 114 nm | The percentage of VCZ release was higher for NLC (40%) than for liposome (15%) after six hours. The gel formulation showed significant accumulation only with liposomes. Liposome deposition in the follicle produces the greatest amount in the stratum corneum. While NLC has a faster and deeper release. The MIC50 to Trichophyton rubrum result was similar for both formulations. | [240] | ||
| Tween 80, ethanol and oleic acid. | Microemulsion | Topic | 10 nm | In the pork skin permeation test, the accumulation of VCZ in the stratum corneum and the rest of the skin was higher for the microemulsion concerning the commercial formulation. The antifungal activity was better for the microemulsion containing VCZ compared to the one without VCZ in Candida sp. | [241] | ||
| PVA, SA (Sodium Alginate) | Electrospinning | Topic | 242–542 nm | Cell viability of rat fibroblast cells was higher after crosslinking VCZ nanofibers. | There was a release of 38% of VCZ in 30 min and 89% in 8 h for the nanofibers. The kinetic model that explains the release was from Higuchi. Crosslinked or not with the nanofibers was more effective in promoting penetration into the skin layers than the control. After cross-linking, there was a reduction in the MIC value for Candida sp. | [242] | |
| Compritol 888 ATO, Palmitic Acid, Stearic Acid, Glycerol, Soy Lecithin, Pluronic F-68, Sodium Tauracholate. | Emulsification Solvent Evaporation | Ocular | 139–344 nm | In vitro studies of corneal hydration, histopathology and HET-CAM suggested a non-irritating property of the formulation. | Sustained release > 60% in 12 h of study. | Sustained release compared to VCZ suspension. A significantly lower amount of the drug was also observed in the plasma, suggesting nasolacrimal drainage. | [243] |
| Lecithin, Cholesterol | Film hydration | Ocular | * | Eye irritation studies in rabbits showed no irritation. | In vitro sustained release. | [244] | |
| Soy Phosphatidylcholine, Cholesterol | Thin film hydration | Ocular | 116 nm | HET-CAM irritability study indicated a non-irritating formulation, therefore, safe. | Mucin permeation study showed a good affinity with the mucous layer of the eye, showing ophthalmic viability. | [245] | |
| Pluronic F-127 and F-68, Sodium Alginate | In situ gelation | Ocular | * | Antifungal activity in C. albicans and A. fumigatus depends on increased VCZ release from the gel. The formulation showed prolonged stability. | [246] | ||
| Chitosan, Silver, and Graphene Oxide | Electrostatic Interaction | Ocular | * | A study performed with corneal cells did not show cytotoxicity to the cells, demonstrating biocompatibility. | Sustained release of VCZ was observed through the hydrogel. The hydrogel showed inhibitory activity against Fusarium solani and A. fumigatus with MIC of 2.5 µg/mL and 2.5–5.0 µg/mL. The matrix activity of the contact lenses produced was also evaluated and presented a MIC of 1.25 µg/mL, suggesting an increase in therapeutic efficacy and a synergistic effect of the matrix with the hydrogel. | Clinical evaluation of rats treated with the lenses containing the VCZ hydrogel exhibited a reduction in fungal keratitis during the treatment period. | [247] |
| PLGA | Multiple Emulsion and Solvent Evaporation | Pulmonary | 300 nm | In PBS medium, the drug release was limited by the dissolution rate of the drug particles, while in SLF medium, the release occurred by the diffusion/erosion mechanism. | There was variation in the concentration of VCZ in the pulmonary lobes of the animals during treatment with intravenous injection, a situation that did not occur with pulmonary administration. In addition, there was greater retention of VCZ in lung tissue from the nanoparticles. | [248] | |
| Polietilenoimina, Stearic acid, sodium deoxycholate | Emulsification | Pulmonary | 353 nm | Cytotoxicity in human lung carcinoma cells (A549) was dependent on polyethyleneimine concentration. | From the results of the mass mean aerodynamic diameter, the use of VCZ in the form of aerosols from agglomerates or nanoparticles can result in better pulmonary deposition than with the use of pure powder. | [159] | |
| Carbopol 934, stearic acid, Tween 80 | Ultrasonication and Microemulsion | Ocular | 234–288 nm | The corneal hydration level remained between 76% and 79%, causing no damage to the corneal tissue. | Formulation using ultrasonication allowed controlled release for 12 h and prolonged stability. An Ex vivo transcorneal permeation showed controlled release in the cornea. | [46] | |
| Jojoba Oil, Brij 97 and Sorbitol | Microemulsion | Topic | * | Microemulsion showed higher therapeutic efficacy than supersaturated VCZ solution against C. albicans ATCC 90028. | [249] | ||
| Precirol ATO 5, Labrafil 1944 CS, Tween 80 | High Pressure Homogenization | Topic | 210 nm | Sustained and controlled Release of VCZ for 24 h. The hydrogel formulation showed a higher amount of VCZ permeated in 12 h. | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida Campos, L.; Fin, M.T.; Santos, K.S.; de Lima Gualque, M.W.; Freire Cabral, A.K.L.; Khalil, N.M.; Fusco-Almeida, A.M.; Mainardes, R.M.; Mendes-Giannini, M.J.S. Nanotechnology-Based Approaches for Voriconazole Delivery Applied to Invasive Fungal Infections. Pharmaceutics 2023, 15, 266. https://doi.org/10.3390/pharmaceutics15010266
de Almeida Campos L, Fin MT, Santos KS, de Lima Gualque MW, Freire Cabral AKL, Khalil NM, Fusco-Almeida AM, Mainardes RM, Mendes-Giannini MJS. Nanotechnology-Based Approaches for Voriconazole Delivery Applied to Invasive Fungal Infections. Pharmaceutics. 2023; 15(1):266. https://doi.org/10.3390/pharmaceutics15010266
Chicago/Turabian Stylede Almeida Campos, Laís, Margani Taise Fin, Kelvin Sousa Santos, Marcos William de Lima Gualque, Ana Karla Lima Freire Cabral, Najeh Maissar Khalil, Ana Marisa Fusco-Almeida, Rubiana Mara Mainardes, and Maria José Soares Mendes-Giannini. 2023. "Nanotechnology-Based Approaches for Voriconazole Delivery Applied to Invasive Fungal Infections" Pharmaceutics 15, no. 1: 266. https://doi.org/10.3390/pharmaceutics15010266
APA Stylede Almeida Campos, L., Fin, M. T., Santos, K. S., de Lima Gualque, M. W., Freire Cabral, A. K. L., Khalil, N. M., Fusco-Almeida, A. M., Mainardes, R. M., & Mendes-Giannini, M. J. S. (2023). Nanotechnology-Based Approaches for Voriconazole Delivery Applied to Invasive Fungal Infections. Pharmaceutics, 15(1), 266. https://doi.org/10.3390/pharmaceutics15010266








