Design of A3B-Porphyrin Conjugates with Terpyridine as Potential Theranostic Agents: Synthesis, Complexation with Fe(III), Gd(III), and Photodynamic Activity
Abstract
:1. Introduction
2. Materials and Methods
- General methodology A for the synthesis of A3B-type meso-arylporphyrins
- General methodology B for the synthesis of zinc complexes of porphyrins
- General methodology C for the synthesis of complexes with paramagnetic metals
- General methodology D
3. Results and Discussion
3.1. Evaluation of Biological Efficacy and Safety
3.2. Comet Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, X.; Cheng, Y.; Feng, Y.; Stiles, W.R.; Park, S.H.; Kang, H.; Choi, H.S. Phototheranostics for multifunctional treatment of cancer with fluorescence imaging. Adv. Drug Deliv. Rev. 2022, 189, 114483. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Rasheed, T.; Raza, A.; Bilal, M.; Yahya, R.; Yar, M.; Iqbal, H.M.N. Photodynamic-based therapeutic modalities to fight against cancer—A review from synergistic viewpoint. J. Drug Deliv. Sci. Technol. 2019, 51, 70–82. [Google Scholar] [CrossRef]
- Jeong, Y.; Hwang, H.S.; Na, K. Theranostics and contrast agents for magnetic resonance imaging. Biomater. Res. 2018, 22, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, H.; Yoon, J. Activated supramolecular nano-agents: From diagnosis to imaging-guided tumor treatment. Nano Today 2022, 43, 101392. [Google Scholar] [CrossRef]
- Bonnett, R. Chemical Aspects of Photodynamic Therapy; Gordon and Breach Science Publishers: Philadelphia, PA, USA, 2000; p. 324. [Google Scholar]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Casula, M.F.; Floris, P.; Innocenti, C.; Lascialfari, A.; Marinone, M.; Corti, M.; Sperling, R.A.; Parak, X.W.J.; Sangregorio, C. Magnetic resonance imaging contrast agents based on iron oxide superparamagnetic ferrofluids. Chem. Mater. 2010, 22, 1739–1748. [Google Scholar] [CrossRef]
- Song, Y.; Zong, H.; Trivedi, E.R.; Vesper, B.J.; Waters, E.A.; Barrett, A.G.M.; Radosevich, J.A.; Hoffman, B.M.; Meade, T.J. Synthesis and Characterization of New Porphyrazine-Gd(III) Conjugates as Multimodal MR Contrast Agents. Bioconjugate Chem. 2010, 21, 2267–2275. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.G.; Rudine, A.B.; Wamser, C.C. Pоrphyrins and phthalосyanines in sоlar phоtоvоltaiс сells. J. Porphyr. Phthalocyanines 2010, 14, 759–792. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, Y.; Jin, Q.; Chao, Y.; Tian, L.; Liu, J.; Dong, Z.; Liu, Z. Two-dimensional metal-organic-framework as a unique theranostic nano-platform for nuclear imaging and chemo-photodynamic cancer therapy. Nano Res. 2019, 12, 1307–1312. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, F.; Linhardt, R.J. Porphyrin-based compounds and their applications in materials and medicine. Dyes Pigments 2021, 188, 109136. [Google Scholar] [CrossRef]
- Faustova, M.; Nikolskaya, E.; Sokol, M.; Fomicheva, M.; Petrov, R.; Yabbarov, N. Metalloporphyrins in Medicine: From History to Recent Trends. ACS Appl. Bio Mater. 2020, 3, 8146–8171. [Google Scholar] [CrossRef]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Ramzan, M.; Qureshi, A.; Khan, M.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Slansky, A.; Dobhal, M.P.; Goswami, L.N.; Graham, A.; Chen, Y.; Kanter, P.; Alberico, R.A.; Spernyak, J.; Morgan, J.; et al. KChlorophyll-a Analogues Conjugated with Aminobenzyl-DTPA as Potential Bifunctional Agents for Magnetic Resonance Imaging and Photodynamic Therapy. Bioconj. Chem. 2005, 16, 32–42. [Google Scholar] [CrossRef]
- Hindré, F.; Le Plouzennec, M.; de Certaines, J.D.; Foultier, M.T.; Patrice, T.; Simonneaux, G. Tetra-p-aminophenylporphyrin conjugated with Gd-DTPA: Tumor-specific contrast agent for MR imaging. J. Magn. Reson. Imaging 1993, 3, 59–65. [Google Scholar] [CrossRef]
- Pandey, R.K.; Goswami, L.N.; Chen, Y.; Gryshuk, A.; Missert, J.R.; Oseroff, A.; Dougherty, T.J. Nature: A rich source for developing multifunctional agents. tumor-imaging and photodynamic therapy. Lasers Surg. Med. 2006, 38, 445–467. [Google Scholar] [CrossRef]
- Hofmeier, H.; Schubert, U.S. Recent developments in the supramolecular chemistry of terpyridine–metal complexes. Chem. Soc. Rev. 2004, 33, 373. [Google Scholar] [CrossRef]
- Dong, C.; Yuan, J.; Hoffmann, H.; Hao, J. Self-assembly and photo-responsive behavior of bis-terpyridyl Eu3+-complex L1. New J. Chem. 2019, 43, 19355. [Google Scholar] [CrossRef]
- Hussain, A.; Gadadhar, S.; Goswami, T.K.; Karande, A.A.; Chakravarty, A.R. Photoactivated DNA cleavage and anticancer activity of pyrenyl-terpyridine lanthanide complexes. Eur. J. Med. Chem. 2012, 50, 319. [Google Scholar] [CrossRef]
- Cai, L.L.; Hu, Y.T.; Li, Y.; Wang, K.; Zhang, X.Q.; Muller, G.; Li, X.M.; Wang, G.X. Solid-state luminescence properties, Hirshfeld surface analysis and DFT calculations of mononuclear lanthanide complexes (Ln = EuIII, GdIII, TbIII, DyIII) containing 4′-phenyl-2,2′:6′,2″-terpyridine. Inorg. Chim. Acta 2019, 489, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Mefteh, W.B.; Touzi, H.; Bessueille, F.; Chevalier, Y.; Kalfat, R.; Jaffrezic-Renault, N. An Impedimetric Sensor Based on a Gold Electrode Functionalized with a Thiol Self-Assembled Monolayer Modified by Terpyridine Ligands for the Detection of Free Gadolinium Ions. Electroanalysis 2015, 27, 84–92. [Google Scholar] [CrossRef]
- Suzuki, M.; Uehara, T.; Arano, Y.; Hoshino, T.; Neya, S. Fabrications of potential imaging probes based on a b-alkyl substituted porphyrin with a terpyridine external coordination site. Tetrahedron Lett. 2011, 52, 7164–7167. [Google Scholar] [CrossRef]
- Collin, J.-P.; Harriman, A.; Heitz, V.; Odobel, F.; Sauvage, J.-P. Photoinduced Electron- and Energy-Transfer Processes Occurring within Porphyrin-Metal-Bisterpyridyl Conjugates. J. Am. Chem. Soc. 1994, 116, 5679–5690. [Google Scholar] [CrossRef]
- Flamigni, L.; Dixon, I.M.; Collin, J.-P.; Sauvage, J.-P. A Zn(II) porphyrin–Ir(III) bis-terpyridine–Au(III) porphyrin triad with a charge-separated state in the microsecond range. Chem. Commun. 2000, 2479–2480. [Google Scholar] [CrossRef]
- Mironov, A.F.; Zhdanova, K.A.; Bragina, N.A. Nanosized vehicles for delivery of photosensitizers in photodynamic diagnosis and therapy of cancer. Russ. Chem. Rev. 2018, 87, 859–881. [Google Scholar] [CrossRef]
- Na, M.R.; Koo, S.K.; Kim, D.Y.; Park, S.D.; Rhee, S.K.; Kang, K.W.; Joe, C.O. In vitro inhibition of gap junctional intercellular communication by chemical carcinogens. Toxicology 1995, 98, 199–206. [Google Scholar] [CrossRef]
- Ezhov, A.V.; Aleksandrov, A.E.; Zhdanova, K.A.; Zhdanov, A.P.; Kluykin, I.N.; Zhizhin, K.Y.; Bragina, N.A.; Mironov, A.F.; Tameev, A.R. Synthesis of Zn(II) porphyrin dyes and revealing an influence of their alkyl substituents on performance of dye-sensitized solar cells. Synth. Met. 2020, 269, 116567. [Google Scholar] [CrossRef]
- Managa, M.; Ngoy, B.P.; Nyokong, T. The photophysical studies of Pluronic F127/P123 micelle mixture system loaded with metal free and Zn 5,10,15,20-tetrakis[4-(benzyloxy) phenyl]porphyrins. J. Photochem. Photobiol. A 2017, 339, 49–58. [Google Scholar] [CrossRef]
- Lembo, A.; Tagliatesta, P.; Guldi, D.M. Synthesis and Photophysical Investigation of New Porphyrin Derivatives with β-Pyrrole Ethynyl Linkage and Corresponding Dyad with [32] Fullerene. J. Phys. Chem. A 2006, 110, 11424–11434. [Google Scholar] [CrossRef] [Green Version]
- Marydasan, B.; Nair, A.K.; Ramaiah, D. Optimization of Triplet Excited State and Singlet Oxygen Quantum Yields of Picolylamine-Porphyrin Conjugates through Zinc Insertion. J. Phys. Chem. B 2013, 117, 13515–13522. [Google Scholar] [CrossRef] [PubMed]
- Spiller, W.; Kliesch, H.; Wöhrle, D.; Hackbarth, S.; Röder, B.; Schnurpfeil, G.J. Singlet Oxygen Quantum Yields of Different Photosensitizers in Polar Solvents and Micellar Solutions. J. Porphyr. Phthalocyanines 1998, 2, 145–158. [Google Scholar] [CrossRef]
- Manivannan, E.; C;hen, Y.; Joshi, P.; Pandey, R. The rоle оf pоrphyrin сhemistry in tumоr imaging and phоtоdynamiс therapy. Chem Sос Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Dobre, M.; Boscencu, R.; Neagoe, I.V.; Surcel, M.; Milanesi, E.; Manda, G. Insight into the Web of Stress Responses Triggered at Gene Expression Level by Porphyrin-PDT in HT29 Human Colon Carcinoma Cells. Pharmaceutics 2021, 13, 1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hanan, G.S. A facile route to sterically hindered and non-hindered 4′-aryl-2, 2′: 6′, 2″-terpyridines. Synlett 2005, 8, 1251–1254. [Google Scholar] [CrossRef]
- Mutai, T.; Cheon, J.-D.; Arita, S.; Araki, K. Phenyl-substituted 2,2′:6′,2″-terpyridine as a new series of fluorescent compounds—their photophysical properties and fluorescence tuning. J. Chem. Soc. Perkin Trans. 2001, 2, 1045–1050. [Google Scholar] [CrossRef]
- Anthonysamy, A.; Balasubramanian, S.; Shanmugaiah, V.; Mathivanan, N. Synthesis, characterization and electrochemistry of 4′-functionalized 2,2′:6′,2″-terpyridine ruthenium(II) complexes and their biological activity. Dalton Trans. 2008, 2136–2143. [Google Scholar] [CrossRef]
- Ezhilarasu, T.; Sathiyaseelan, A.; Kalaichelvan, P.T.; Balasubramanian, S. Synthesis of 4′-substituted-2,2′;6′,2″-terpyridine Ru(II) complexes electrochemical, fluorescence quenching and antibacterial studies. J. Mol. Struct. 2017, 1134, 265e277. [Google Scholar] [CrossRef]
- Tang, B.; Yu, F.; Li, P.; Tong, L.; Duan, X.; Xie, T.; Wang, X. Near-Infrared Neutral pH Fluorescent Probe for Monitoring Minor pH Changes: Imaging in Living HepG2 and HL-7702 Cells. J. Am. Chem. Soc. 2009, 131, 3016–3023. [Google Scholar] [CrossRef]
- Zhdanova, K.A.; Savelyeva, I.O.; Ignatova, A.A.; Gradova, M.A.; Gradov, O.V.; Lobanov, A.V.; Feofanov, A.V.; Mironov, A.F.; Bragina, N.A. Synthesis and photodynamic antimicrobial activity of amphiphilic meso-arylporphyrins with pyridyl moieties. Dyes Pigments 2020, 181, 108561. [Google Scholar] [CrossRef]
- Zhdanova, K.A.; Zhdanov, A.P.; Ezhov, A.V.; Bragina, N.A.; Zhizhin, K.Y.; Ushakova, I.P.; Mironov, A.F.; Kuznetsov, N.T. Synthesis of amino-containing meso-aryl-substituted porphyrins and their conjugates with the closo-decaborate anion. Russ. Chem. Bull. 2014, 1, 194–200. [Google Scholar] [CrossRef]
- Zhdanova, K.A.; Bragina, N.A.; Bagratashvili, V.N.; Timashev, P.S.; Mironov, A.F. Noncovalent assemblies of CdSe semiconductor quantum dots and an amphiphilic long-chain meso-arylporphyrin. Mendeleev Commun. 2014, 24, 247. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Hsu, H.C.; Schreiman, I.C. Synthesis of tetraphenylporphyrins under very mild conditions. Tetrahedron Lett. 1986, 27, 4969–4970. [Google Scholar] [CrossRef]
- Reid, E.E.; Archer, K.E.; Shizuka, M.; Wilhelm, A.; Yoder, N.C.; Bai, C.; Fishkin, N.E.; Harris, L.; Maloney, E.K.; Salomon, P.; et al. Effect of Linker Stereochemistry on the Activity of Indolinobenzodiazepine Containing Antibody–Drug Conjugates (ADCs). ACS Med. Chem. Lett. 2019, 10, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Vyalba, F.Y.; Ivantsova, A.V.; Zhdanova, K.A.; Usachev, M.N.; Gradova, M.A.; Bragina, N.A. Synthesis of conjugates of 5,15-disubstituted aminoporphyrins and terpyridine derivatives with potential chelating properties. Mendeleev Commun. 2022, 32, 675–677. [Google Scholar] [CrossRef]
- Patra, D.; Mukherjee, S.; Chakraborty, I.; Dash, T.K.; Senapati, S.; Bhattacharyya, R.; Shunmugam, R. Iron(III) Coordinated Polymeric Nanomaterial: A Next-Generation Theranostic Agent for High-Resolution T1-Weighted Magnetic Resonance Imaging and Anticancer Drug Delivery. ACS Biomater. Sci. Eng. 2018, 4, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, B.F.O.; Pereira, N.A.M.; Valente, A.J.M.; Pinho e Melo, T.M.V.D.; Pineiro, M. A Review on (Hydro)Porphyrin-Loaded Polymer Micelles: Interesting and Valuable Platforms for Enhanced Cancer Nanotheranostics. Pharmaceutics 2019, 11, 81. [Google Scholar] [CrossRef] [Green Version]
- Jaquilin, R.P.J.; Oluwafemi, O.S.; Thomas, S.; Oyedeji, A.O. Recent advances in drug delivery nanocarriers incorporated in temperature-sensitive Pluronic F-127–A critical review. J. Drug Deliv. Sci. Technol. 2022, 72, 103390. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef]
- Managa, M.; Ngoy, B.P.; Mafukidze, D.; Nyokong, T. Incorporation of metal free and Ga 5,10,15,20-Tetrakis(4′-bromophenyl) porphyrin into Pluronic F127-folic acid micelles. J. Lumin. 2018, 194, 739–746. [Google Scholar] [CrossRef]
- Vilsinski, B.H.; Aparicio, J.L.; de Souza Pereira, P.C.; Fávaro, S.L.; Campanholi, K.S.S.; Gerola, A.P.; Tessaro, A.L.; Hioka, N.; Caetano, W. Physico-chemical properties of meso-tetrakis(p-methoxyphenyl)porphyrin (TMPP) incorporated into pluronicTM p-123 and f-127 polymeric micelles. Quim. Nova 2014, 37, 1650–1656. [Google Scholar] [CrossRef]
- Jenni, S.; Bolze, F.; Bonnet, C.S.; Pallier, A.; Sour, A.; Tóth, É.; Ventura, B.; Heitz, V. Synthesis and In Vitro Studies of a Gd (DOTA)–Porphyrin Conjugate for Combined MPT and Photodynamic Treatment. Inorg. Chem. 2020, 59, 14389–14398. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Jenni, S.; Sour, A.; Heitz, V.; Bolze, F.; Pallier, A.; Bonnet, C.S.; Toth, E.; Ventura, B. A porphyrin dimer–GdDOTA conjugate as a theranostic agent for one-and two-photon photodynamic therapy and MRI. Bioconjugate Chem. 2018, 29, 3726–3738. [Google Scholar] [CrossRef]
- Nyarko, E.; Hara, T.; Grab, D.J.; Habib, A.; Kim, Y.; Nikolskaia, O.; Fukuma, T.; Tabata, M. In vitro toxicity of palladium (II) and gold (III) porphyrins and their aqueous metal ion counterparts on Trypanosoma brucei brucei growth. Chemico-Biol. Interact. 2004, 148, 19–25. [Google Scholar] [CrossRef]
- Gel’shteĭn, V.I. Ultrastructural characteristics of tumorigenic and nontumorigenic liver cell cultures, IAR series, based on scanning electron microscopy data. Tsitologiia 1981, 23, 510–515. [Google Scholar]
- Meda, P. Assaying the Molecular Permeability of Connexin Channels. In Connexin Methods and Protocols; Methods in Molecular Biology™ Book Series; Human Press: Totowa, NJ, USA, 2001; Volume 154, pp. 201–224. [Google Scholar] [CrossRef]
- Brissette, J.L.; Kumar, N.M.; Gilula, N.B.; Dotto, G.P. The tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the ras oncogene modulate expression and phosphorylation of gap junction proteins. Mol. Cell. Biol. 1991, 11, 5364–5371. [Google Scholar] [CrossRef]
- Garcia-Sampedro, A.; Tabero, A.; Mahamed, I.; Acedo, P.J. Multimodal use of the porphyrin TMPyP: From cancer therapy to antimicrobial applications. Porphyr. Phthalocyanines 2019, 23, 11–27. [Google Scholar] [CrossRef] [Green Version]
- Seenisamy, J.; Rezler, E.M.; Powell, T.J.; Tye, D.; Gokhale, V.; Joshi, C.S.; Siddiqui-Jain, A.; Hurley, L.H.J. The dynamic character of the G-quadruplex element in the c-MYC promoter and modification by TMPyP4. Am. Chem. Soc. 2004, 126, 8702–8709. [Google Scholar] [CrossRef]
- Ali, A.; Bansal, M.; Bhattacharya, S. Ligand 5, 10, 15, 20-tetra (N-methyl-4-pyridyl) porphine (TMPyP4) prefers the parallel propeller-type human telomeric G-quadruplex DNA over its other polymorphs. J. Phys. Chem. B 2015, 119, 5–14. [Google Scholar] [CrossRef]

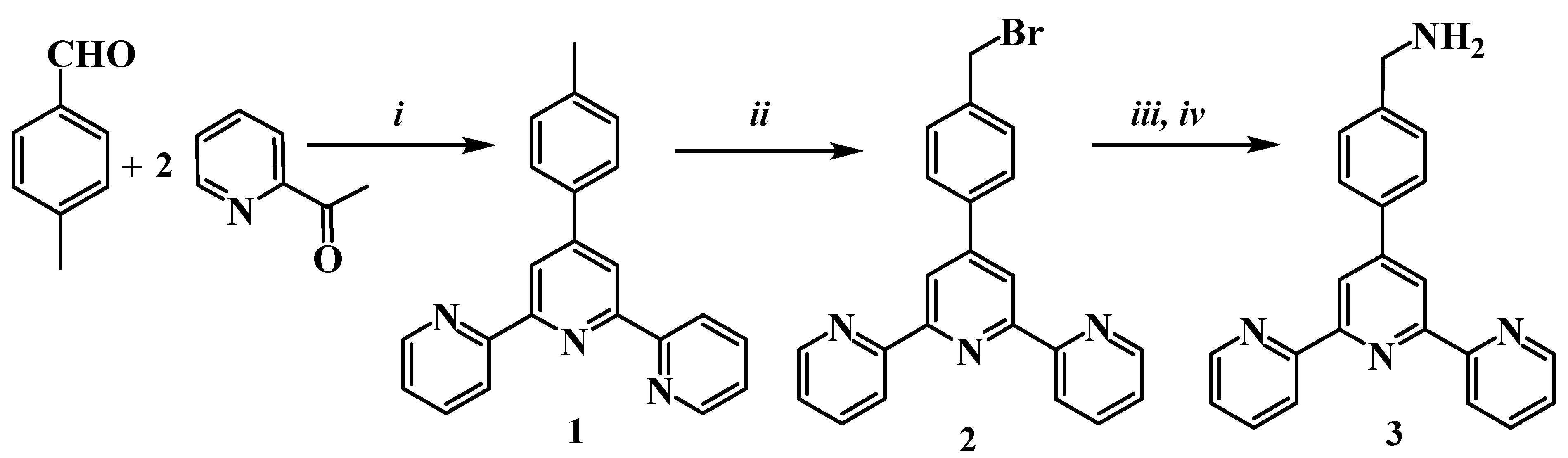
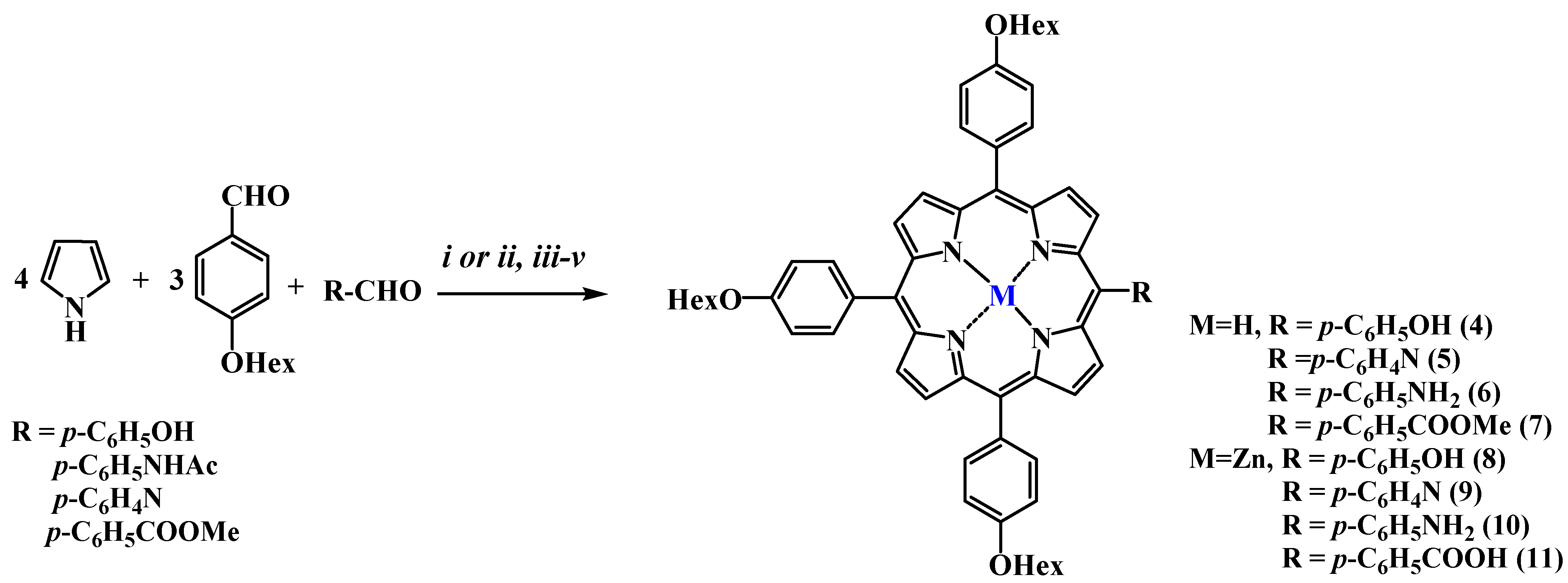
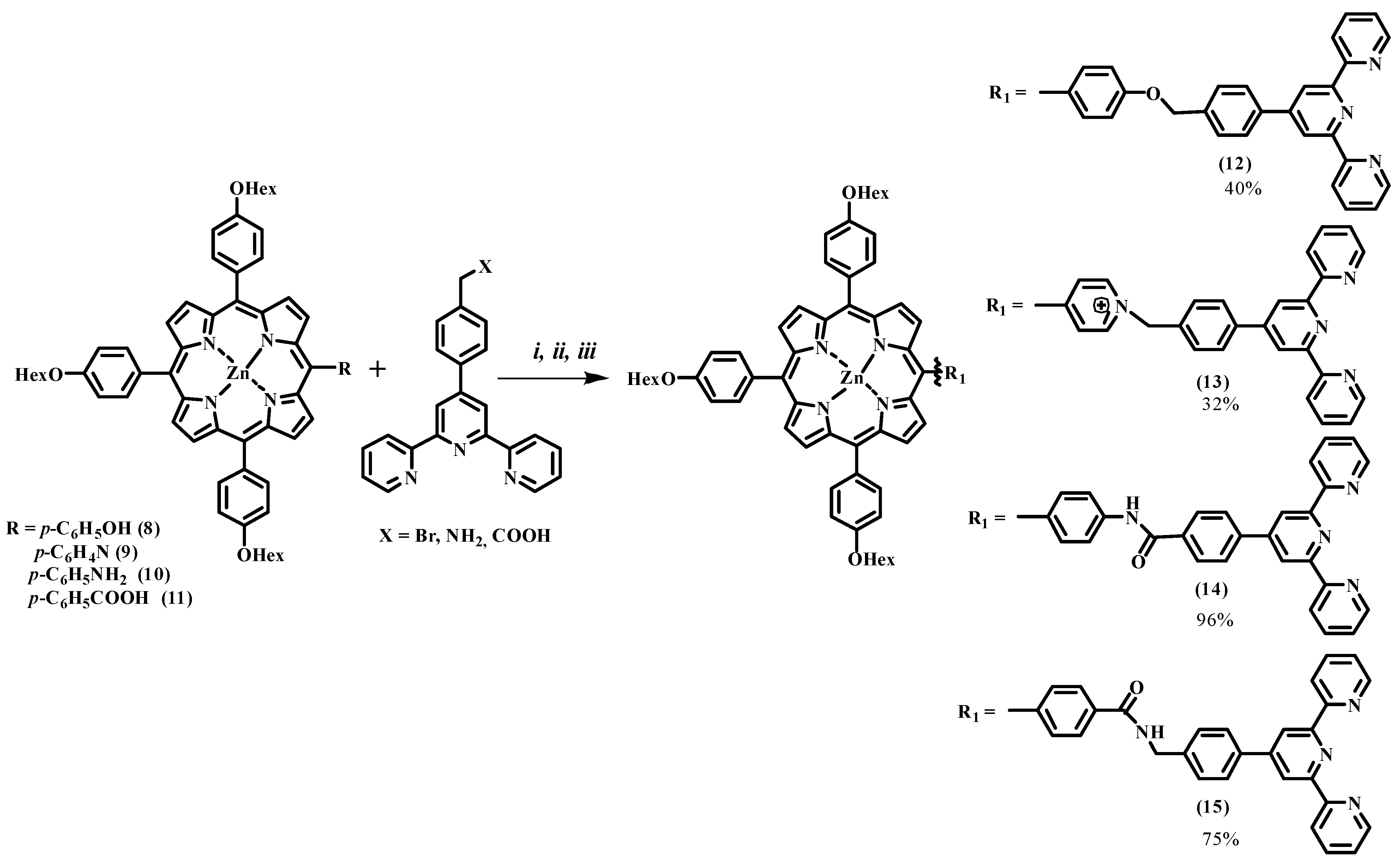
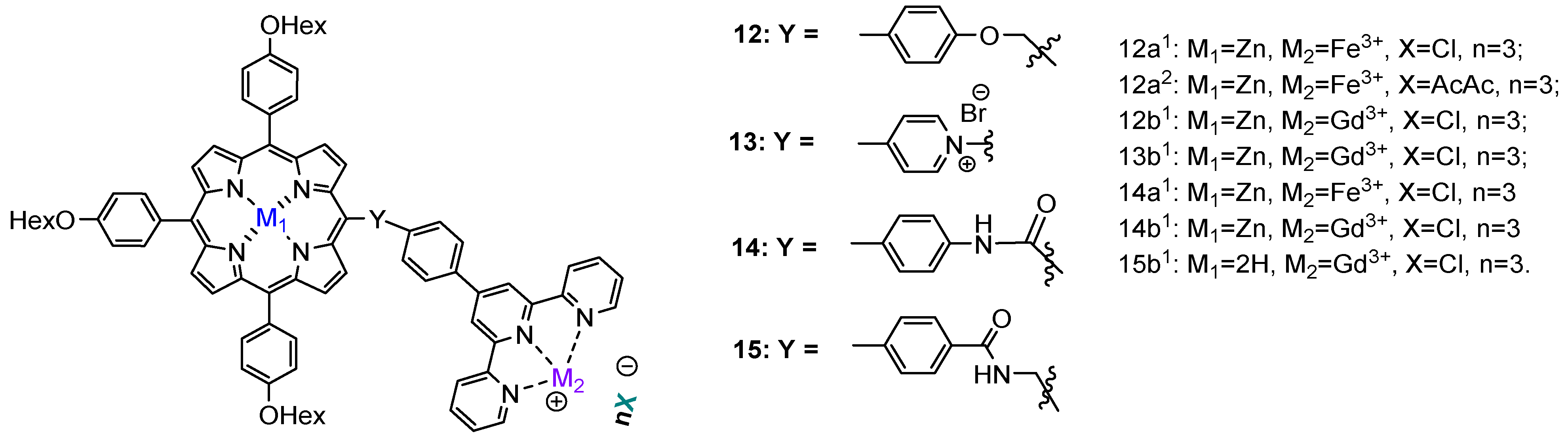
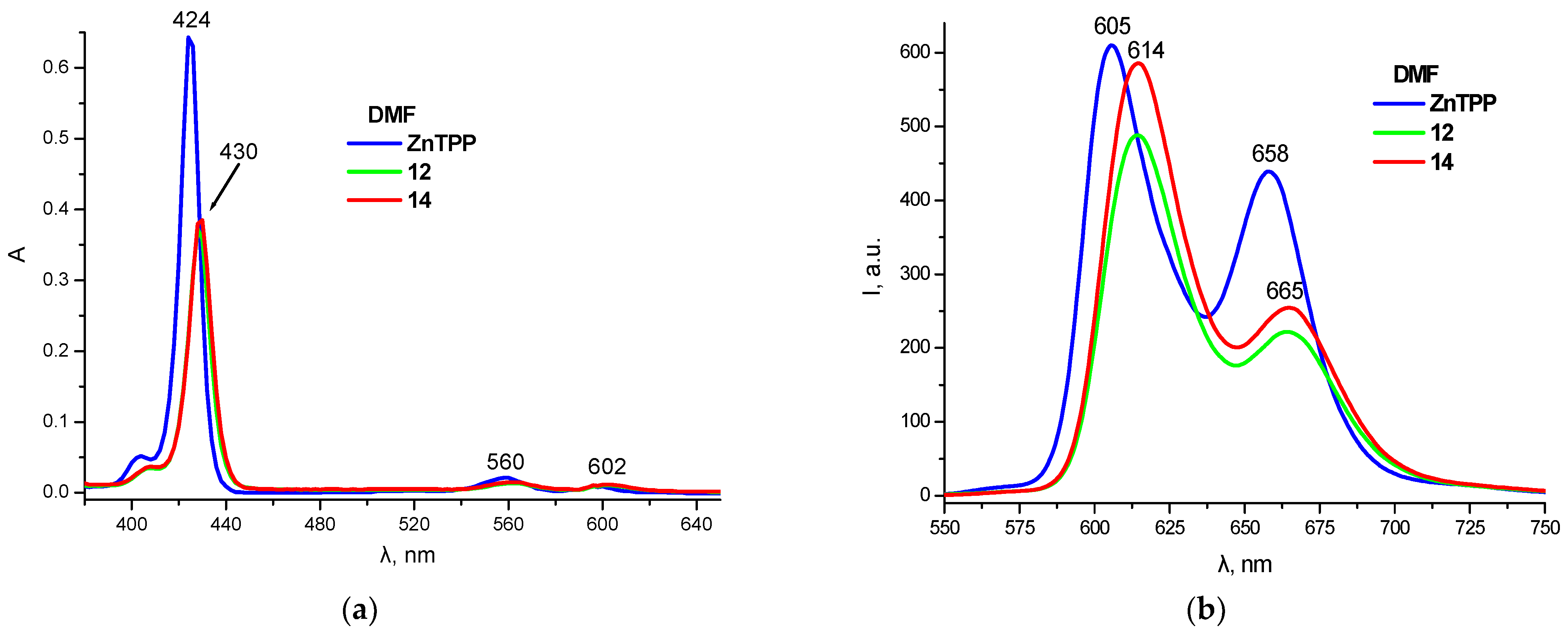
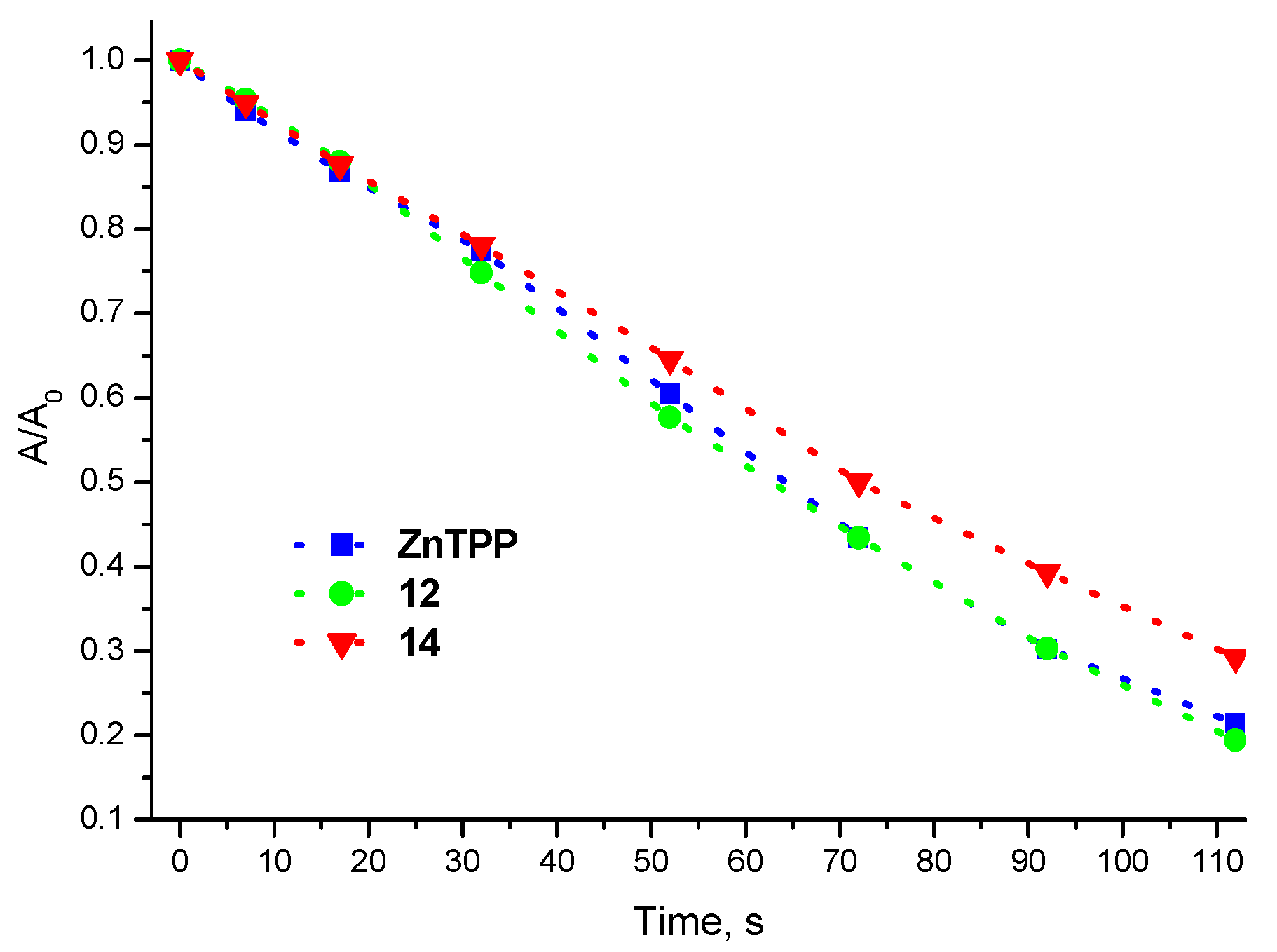




| Compound | R1 | M | η, % |
|---|---|---|---|
| 4 | -pC6H5OH | H | 12.6 |
| 5 | -pC6H5NH2 | H | 12 |
| 6 | -pC6H4N | H | 11 |
| 7 | -pC6H5COOMe | H | 13 |
| 8 | -pC6H5OH | Zn | 95 |
| 9 | -pC6H4N | Zn | 90 |
| 10 | -pC6H5NH2 | Zn | 92 |
| 11 | -pC6H5COOH | Zn | 95 |
| Compound | Salt of the Metal | Catalyst | Conditions | Yield, η% |
|---|---|---|---|---|
| 12a1 | FeCl3 | - | CH2Cl2, MeOH, tKOMH., N2 | 75 |
| 12a2 | Fe(AcAc)3 | NaOAc | CH2Cl2, MeOH, tKOMH. | 82 |
| 12b1 | GdCl3 | NaOAc | THF, MeOH, t., p, N2 | 36 |
| 13b1 | GdCl3 | NaOAc | THF, MeOH, tKOMH., p, N2 | 6 |
| 14a1 | FeCl3 | - | CH2Cl2, MeOH, tKOMH., N2 | 70 |
| 14b1 | GdCl3 | NaOAc | THF, MeOH, t., p, N2 | 30 |
| 15b1 | GdCl3 | NaOAc | THF, MeOH, t., p, N2 | 28 |
| 12 | DMSO | H2O | TX-100 | Brij-58 | Cremophor | Pluronic | Tween-80 |
| λmax | 428 | 433 | 428 | 428 | 430 | 428 | 428 |
| lgε | 5.70 | 5.17 | 5.34 | 5.15 | 5.35 | 5.36 | 5.40 |
| ΔλB, nm | 10 | 36 | 25 | 28 | 27 | 22 | 19 |
| λem, nm | 614, 664 | 614, 660 | 612, 663 | 615, 660 | 614, 655 | 617, 658 | 612, 662 |
| I1/I2 | 2.20 | 0.39 | 1.83 | 1.10 | 1.14 | 1.15 | 1.90 |
| 14 | DMSO | H2O | TX-100 | Brij-58 | Cremophor | Pluronic | Tween-80 |
| λmax | 430 | 433 | 430 | 428 | 428 | 428 | 428 |
| lgε | 5.71 | 5.17 | 5.44 | 5.32 | 5.4 | 5.41 | 5.27 |
| ΔλB, nm | 10 | 40 | 31 | 31 | 26 | 24 | 25 |
| λem, nm | 614, 665 | 615, 664 | 613, 663 | 617, 661 | 614, 662 | 615, 660 | 612, 661 |
| I1/I2 | 2.30 | 1.25 | 1.83 | 1.43 | 1.29 | 1.31 | 2.33 |
| Compound | 12 (Light Irradiation *) | 12 (Dark Conditions) | 14 (Light Irradiation *) | 14 (Dark Conditions) | |
|---|---|---|---|---|---|
| Time | |||||
| Hep2 cell line | |||||
| 1.5 h | 1.87 ± 0.333 µM | - | 1.4 ± 0.152 µM | - | |
| 24 h | 2.47 ± 0.233 µM | 2.55 ± 0.28 µM | 1.55 ± 0.15 µM | 4.17 ± 0.251 µM | |
| NKE cell line | |||||
| 1.5 h | 1.7 ± 0.297 µM | - | 2.0 ± 0.34 µM | - | |
| 24 h | 2.57 ± 0.32 µM | 2.44 ± 0.281 µM | 1.8 ± 0.189 µM | 3.85 ± 0.26 µM | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhdanova, K.A.; Ivantsova, A.V.; Vyalba, F.Y.; Usachev, M.N.; Gradova, M.A.; Gradov, O.V.; Karpechenko, N.Y.; Bragina, N.A. Design of A3B-Porphyrin Conjugates with Terpyridine as Potential Theranostic Agents: Synthesis, Complexation with Fe(III), Gd(III), and Photodynamic Activity. Pharmaceutics 2023, 15, 269. https://doi.org/10.3390/pharmaceutics15010269
Zhdanova KA, Ivantsova AV, Vyalba FY, Usachev MN, Gradova MA, Gradov OV, Karpechenko NY, Bragina NA. Design of A3B-Porphyrin Conjugates with Terpyridine as Potential Theranostic Agents: Synthesis, Complexation with Fe(III), Gd(III), and Photodynamic Activity. Pharmaceutics. 2023; 15(1):269. https://doi.org/10.3390/pharmaceutics15010269
Chicago/Turabian StyleZhdanova, Kseniya A., Anastasia V. Ivantsova, Fedor Yu. Vyalba, Maxim N. Usachev, Margarita A. Gradova, Oleg V. Gradov, Natalia Yu. Karpechenko, and Natal’ya A. Bragina. 2023. "Design of A3B-Porphyrin Conjugates with Terpyridine as Potential Theranostic Agents: Synthesis, Complexation with Fe(III), Gd(III), and Photodynamic Activity" Pharmaceutics 15, no. 1: 269. https://doi.org/10.3390/pharmaceutics15010269
APA StyleZhdanova, K. A., Ivantsova, A. V., Vyalba, F. Y., Usachev, M. N., Gradova, M. A., Gradov, O. V., Karpechenko, N. Y., & Bragina, N. A. (2023). Design of A3B-Porphyrin Conjugates with Terpyridine as Potential Theranostic Agents: Synthesis, Complexation with Fe(III), Gd(III), and Photodynamic Activity. Pharmaceutics, 15(1), 269. https://doi.org/10.3390/pharmaceutics15010269








