Natural Compounds and Biopolymers-Based Hydrogels Join Forces to Promote Wound Healing
Abstract
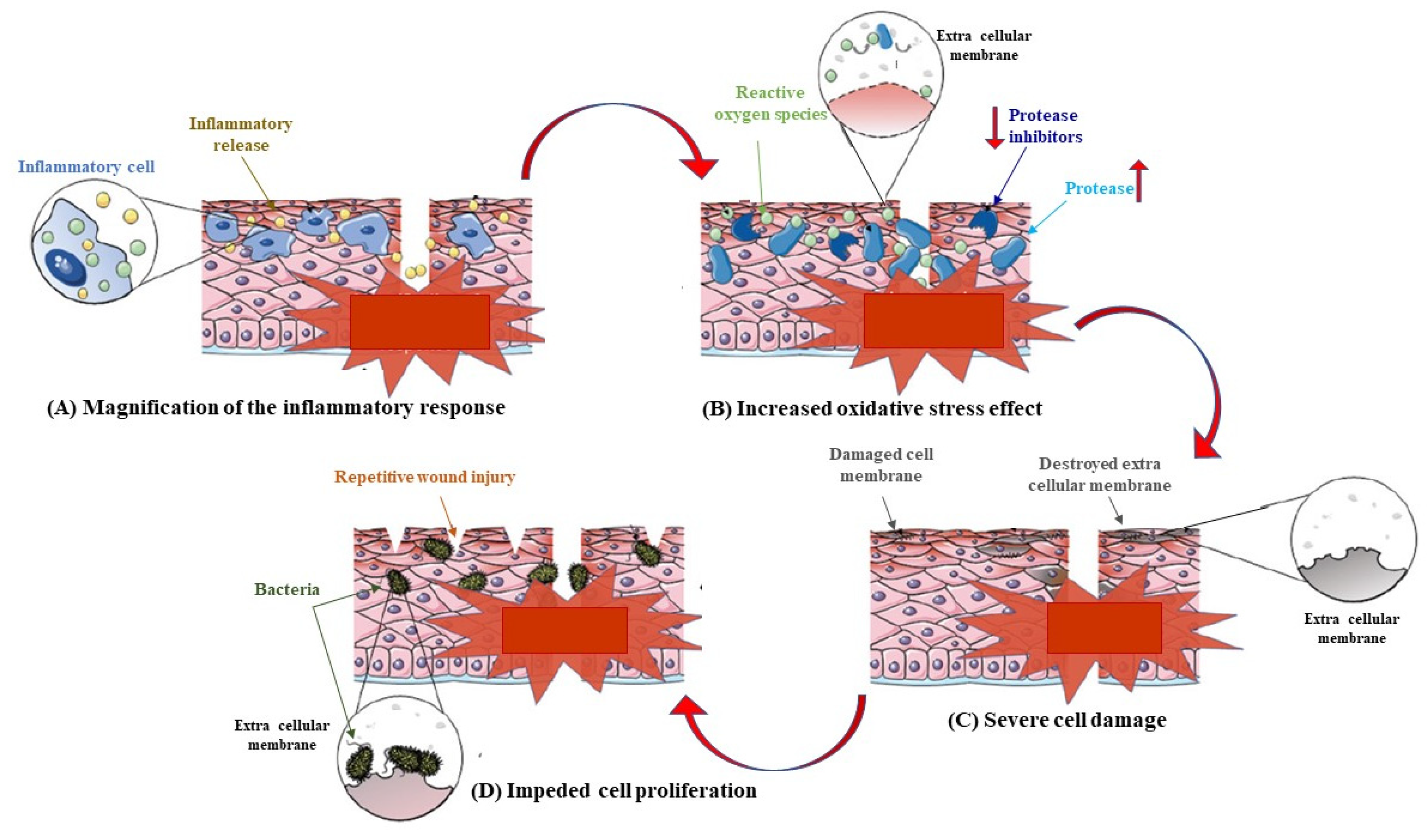
1. Introduction
2. Phenolic Compounds
2.1. Flavonoids
2.1.1. Quercetin
2.1.2. Curcumin
2.1.3. Pinocembrin
2.1.4. Chrysin
2.1.5. Luteolin
2.1.6. Catechin and Epigallocatechin-3-Gallate (EGCG)
2.2. Tannins
Tannic Acid
2.3. Terpenoids
2.3.1. Terpinolene and α-Phellandrene
2.3.2. Thymol
2.4. Alkaloids
Taspine
3. Polymeric Compounds
3.1. Chitosan-Based Hydrogels
| Hydrogel Composition | Delivery Properties | Wound Healing | Ref. | |||
|---|---|---|---|---|---|---|
| Polysaccharide | Component | Bioactive Agent | Concentration | Mechanism | Time (Day) | |
| CH | PEG | Silver nanoparticle | 0.1% (w/w) | Antioxidant Antibacterial | 14 | [66] |
| CH | Collagen | Silver nanoparticles | - | Antibacterial | 6 | [68] |
| CH | - | Silver nanoparticles Calendula extract | 10.9–14.5% (v/v) 3.6–27.3% (v/v) | Anti-inflammatory Antibacterial | 15 | [74] |
| CH | Carbopol | Pterocarpus marsupium heartwood extract | 10% (w/w) | Antibacterial Antioxidant | 18 | [75] |
| CH | - | Ag+ Cu2+ | 0.37 mol L−1 0.15 mol L−1 | Antibacterial Pro-angiogenesis | 14 | [70] |
| CH | PVAc | Ag+ Epidermal growth factor | 0–60 μg mL−1 0–96 mM | Antibacterial Pro-angiogenesis | 14 | [73] |
| CH | - | Curcumin | 0.5–1.5% (w/w) | Antibacterial Antioxidant | - | [76] |
| CH | PVA | Tibetan dangshen pills | 5–20% (w/w) | Antibacterial Antioxidant | 21 | [3] |
| CH | PVA | Polyhexamethylene biguanide Epidermal growth factor Perfluorocarbon | 60 μg mL−1 60 μg mL−1 50 mg mL−1 | Anti-inflammatory Antimicrobial Pro-angiogenesis | 15 | [73] |
| CHMA-g-GA | - | F127/chlorhexidine nanoparticles | 0–0.1 mg mL−1 | Antibacterial Antioxidant | 20 | [77] |
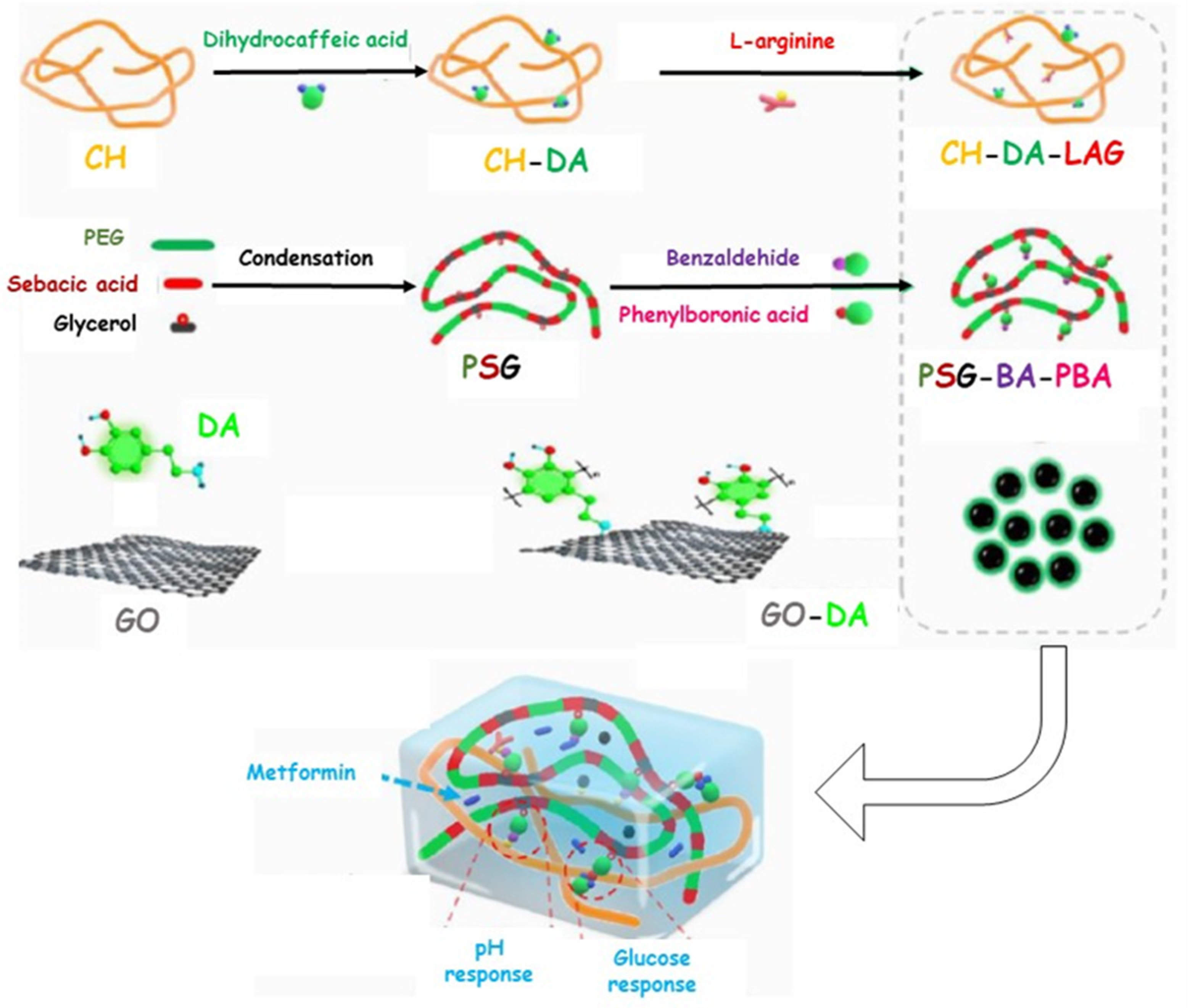
| CH-DA-LAG | PEG-co-poly(glycerol sebacic acid) GO-Polydopamine | Metformin | 1 mg mL−1 | Anti-inflammatory Pro-angiogenesis Antioxidant Antibacterial | 21 | [78] |
| CMCH | - | Fibroblast growth factor | 4200 IU mL−1 | Antibacterial Pro-angiogenesis | 14 | [83] |
| CMCH | PVPI | - | - | Antibacterial | 14 | [90] |
| QCH | - | Tannic acid | 0.05% (w/w) | Antibacterial Antioxidant | 15 | [86] |
| QCH | ε-poly-L-lysine grafted graphene quantum dots | - | - | Antibacterial | 14 | [91] |
| QCH | N-acryloyl glycinamide Polyaniline | - | - | Antibacterial Antioxidant | 14 | [88] |
| QCH | F108-CHO | CORM-401 Insulin | 1–3% (w/w) 0.5–1.5% (w/w) | Anti-inflammatory Antibacterial Antioxidant Anti-glycaemic | 15 | [87] |
| QCH | POSS-PEG-CHO | - | - | Antibacterial | 21 | [89] |
3.2. Others Polysaccharides-Based Hydrogels
3.2.1. Sodium Alginate (SA)
3.2.2. Hyaluronic Acid (HA)
3.2.3. Cellulose
| Hydrogel Composition | Delivery Properties | Wound Healing | Ref. | |||
|---|---|---|---|---|---|---|
| Polysaccharides | Component | Bioactive Agent | Concentration | Mechanism | Time (Day) | |
| SA | PF127 CS | Curcumin | 5 mg mL−1 | Antibacterial Antioxidant | 20 | [95] |
| HA | HP407; FND; EGFN; PHNB | - | - | Anti-inflammatory Pro-angiogenesis Antioxidant Antibacterial | 15 | [100] |
| BCL | - | - | - | Antibacterial | 21 | [105] |
| CA | Dimethyloxallyl Glycine Silver nanoparticles | 2.5% (w/w) 3.2% (w/w) | Pro-angiogenesis Antibacterial | - | [107] | |
3.3. Mixing of Polysaccharides-Based Hydrogel in the Treatment of Diabetic Wounds
| Hydrogel Composition | Delivery Properties | Wound Healing | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Polysaccharides | Others | Bioactive Agent | Concentration | Mechanism | Time (Day) | ||
| Component1 | Component2 | ||||||
| CH | AL | - | - | - | Antimicrobial | 14 | [108] |
| CH | CMC | - | Mequinol | 0.3% (w/w) | Antioxidant Antibacterial | - | [109] |
| CH | KCA | PVA | Cefotaxime sodium | 1% (w/w) | Antibacterial | 21 | [112] |
| CMCH | OHA | - | Modified curcumin Epidermal growth factor | - | Anti-inflammatory Pro-angiogenesis Antioxidant Antibacterial | 15 | [113] |
| CECH | OHA | GO | Polymyxin B | 1% (w/w) | Antioxidant Antibacterial | 18 | [114] |
| QCH | OHA | - | α-lipoic acid | 1–5% (w/w) | Antioxidant Antibacterial | 11 | [116] |
| QCH | OGL | - | Polydomaine nanoparticles | 0.5–2 mg mL−1 | Pro-angiogenesis Antioxidant Antibacterial | 15 | [117] |
| QCH | OGL | - | Poly(tannic acid) nanorods | 0.5–2 mg mL−1 | Pro-angiogenesis Antioxidant Antibacterial | 35 | [84] |
| CaAL | HAO | - | Protamine | 2 mg mL−1 | Pro-angiogenesis Antibacterial | 14 | [118] |
| HA-peptide modified | ODX | - | Platelet-rich plasma | 14% (v/v) | Pro-angiogenesis Antibacterial | 14 | [119] |
| CMC | KCA KG | GO | Ag-ZnO nanoparticles | 2.0% (w/w) | Antibacterial | - | [120] |
3.4. Gelatin-Based Hydrogels in the Treatment of Diabetic Wounds
| Hydrogel Composition | Delivery Properties | Wound Healing | Ref. | |||
|---|---|---|---|---|---|---|
| Protein | Component | Bioactive Agent | Concentration | Mechanism | Time (Day) | |
| GL | PAC | PLy | 10% (w/v) | Antibacterial | 18 | [122] |
| GL-MA | AA | Cu2+ | 0.5–1.5 mg mL−1 | Antibacterial | 21 | [123] |
| GL-MA | - | Ce-BG | 1% (w/v) | Antibacterial Pro-angiogenesis | 21 | [124] |
| GL-MA | PEGDA; VI; DMAPS; AP | Ag nanoparticles | 10% (w/w) | Antibacterial Anti-inflammatory | 14 | [127] |
| GL-CPBA | PVA | VAN-AgNCL NIM | 0.04 mg mL−1 0.03 mg mL−1 | Pro-angiogenesis Antibacterial Anti-inflammatory | 14 | [128] |
| GL-THB | Fe3+ | - | - | Antibacterial Antioxidant Pro-angiogenesis | 21 | [129] |
| GL-GA | ODX | Fe3+ | 0–14 mM | Pro-angiogenesis Antibacterial Antioxidant | 18 | [130] |
4. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Eming, S.A.; Tomic-Canic, M. Updates in wound healing: Mechanisms and translation. Exp. Dermatol. 2017, 26, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.F.; Bernstein, L.; Anton-Culver, H.; Deapen, D.; Horn-Ross, P.L.; Mohrenweiser, H.; Peel, D.; Pinder, R.; Purdie, D.M.; Reynolds, P.; et al. Nonsteroidal Anti-Inflammatory Drug Use and Breast Cancer Risk by Stage and Hormone Receptor Status. J. Natl. Cancer Inst. 2005, 97, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Napavichayanun, S.; Amornsudthiwat, P.; Pienpinijtham, P.; Aramwit, P. Interaction and effectiveness of antimicrobials along with healing-promoting agents in a novel biocellulose wound dressing. Mater. Sci. Eng. C 2015, 55, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Sharma, A.K.; Kalonia, A.; Shukla, S.K. Vascular perfusion: A predictive tool for thermal burn injury. J. Tissue Viability 2020, 29, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Liu, D.; He, X.; Shen, Y.; Zhao, Y.; Hou, X.; Chen, B.; OuYang, W.; Dai, J.; Li, X. A dual functional collagen scaffold coordinates angiogenesis and inflammation for diabetic wound healing. Biomater. Sci. 2020, 8, 6337–6349. [Google Scholar] [CrossRef]
- Vatankhah, N.; Jahangiri, Y.; Landry, G.J.; Moneta, G.L.; Azarbal, A.F. Effect of systemic insulin treatment on diabetic wound healing. Wound Repair Regen. 2017, 25, 288–291. [Google Scholar] [CrossRef]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Invest. 2007, 117, 1219–1222. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Yuan, Q.; Gu, Z.; Wu, J. Tofu-Based Hybrid Hydrogels with Antioxidant and Low Immunogenicity Activity for Enhanced Wound Healing. J. Biomed. Nanotechnol. 2019, 15, 1371–1383. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Cheon, K.-H.; Song, E.-H.; Seong, Y.-J.; Park, J.-U.; Kim, H.-E.; Jeong, S.-H. Fluorine-ion-releasing injectable alginate nanocomposite hydrogel for enhanced bioactivity and antibacterial property. Int. J. Biol. Macromol. 2018, 123, 866–877. [Google Scholar] [CrossRef]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef]
- Deng, H.; Yu, Z.; Chen, S.; Fei, L.; Sha, Q.; Zhou, N.; Chen, Z.; Xu, C. Facile and eco-friendly fabrication of polysaccharides-based nanocomposite hydrogel for photothermal treatment of wound infection. Carbohydr. Polym. 2020, 230, 115565. [Google Scholar] [CrossRef]
- Dalisson, B.; Barralet, J. Bioinorganics and Wound Healing. Adv. Healthc. Mater. 2019, 8, e1900764. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Alves, A.; Miguel, S.P.; Araujo, A.R.; de Jesús Valle, M.J.; Sánchez Navarro, A.; Correia, I.J.; Ribeiro, M.P.; Coutinho, P. Xanthan Gum-Konjac Glucomannan Blend Hydrogel for Wound Healing. Polymers 2020, 12, 99. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Curcio, M.; Altimari, I.; Spizzirri, U.G.; Cirillo, G.; Vittorio, O.; Puoci, F.; Picci, N.; Iemma, F. Biodegradable gelatin-based nanospheres as pH-responsive drug delivery systems. J. Nanopart. Res. 2013, 15, 1581. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Valli, E.; Farfalla, A.; Leggio, A.; Nicoletta, F.P.; Iemma, F. Chitosan–Quercetin Bioconjugate as Multi-Functional Component of Antioxidants and Dual-Responsive Hydrogel Networks. Macromol. Mater. Eng. 2019, 304, 728. [Google Scholar] [CrossRef]
- Thambi, T.; Phan, V.H.G.; Lee, D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, M.; Kubes, P. The Healing Power of Neutrophils. Trends Immunol. 2019, 40, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.; Gu, Z.; Liu, G.; Wu, J. Whole wheat flour coating with antioxidant property accelerates tissue remodeling for enhanced wound healing. Chin. Chem. Lett. 2020, 31, 1612–1615. [Google Scholar] [CrossRef]
- Moore, A.N.; Silva, T.L.L.; Carrejo, N.C.; Marmolejo, C.A.O.; Li, I.C.; Hartgerink, J.D. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials 2018, 161, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Noguera, A.E.; Ciriza, J.; Cañibano-Hernández, A.; Fernandez, L.; Ochoa, I.; del Burgo, L.S.; Pedraz, J.L. Tunable injectable alginate-based hydrogel for cell therapy in Type 1 Diabetes Mellitus. Int. J. Biol. Macromol. 2018, 107, 1261–1269. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Benito-Garzón, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; San Román, J. Bioadhesive functional hydrogels: Controlled release of catechol species with antioxidant and antiinflammatory behavior. Mater. Sci. Eng. C 2019, 105, 110040. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, A.P.; Kumar, S.; Negi, A.; Asha Maurya, V.K. Herbal wound healing agents. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 169–184. [Google Scholar]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Amrati, F.E.-Z.; Lyoussi, B.; Derwich, E. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: A review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abdollahi, M.; Rahimi, R. Role of dietary polyphenols in the management of peptic ulcer. World J. Gastroenterol. 2015, 21, 6499–6517. [Google Scholar] [CrossRef]
- Chabane, S.; Boudjelal, A.; Keller, M.; Doubakh, S.; Potterat, O. Teucrium polium—Wound healing potential, toxicity and polyphenolic profile. S. Afr. J. Bot. 2021, 137, 228–235. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, D.-H.; Liu, P.; Cao, J.-J.; Wang, Y.-P.; Yin, J.; Zhu, Y.; Rahman, K. Bioactive components from the tea polyphenols influence on endogenous antioxidant defense system and modulate inflammatory cytokines after total-body irradiation in mice. Phytomedicine 2011, 18, 970–975. [Google Scholar] [CrossRef]
- Vicentini, F.; He, T.; Shao, Y.; Fonseca, M.J.V.; Verri, W.A., Jr.; Fisher, G.J.; Xu, Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-κB pathway. J Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef]
- Phan, T.; Sun, L.; Bay, B.; Chan, S.; Lee, S. Dietary Compounds Inhibit Proliferation and Contraction of Keloid and Hypertrophic Scar-Derived Fibroblasts In Vitro: Therapeutic Implication for Excessive Scarring. J. Trauma 2003, 54, 1212–1224. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Yang, P.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Polyphenol scaffolds in tissue engineering. Mater. Horizons 2020, 8, 145–167. [Google Scholar] [CrossRef]
- Manivannan, R.; Kalaivanan, P.; Sivagnanam, I. Antibacterial and wound healing activities of quercetin-3-O-A-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside isolated from Salvia leucantha. Int. J. Pharm. Sci. Rev. Res. 2013, 48, 264–268. [Google Scholar]
- Gopalakrishnan, A.; Ram, M.; Kumawat, S.; Tandan, S.; Kumar, D. Quercetin accelerated cutaneous wound healing in rats by increasing levels of VEGF and TGF-β1. Indian J Exp Biol. 2016, 54, 187–195. [Google Scholar]
- El Goweini, M.F.; El Din, N.M. Effect of quercetin on excessive dermal scarring. Egypt. J. Dermatol. 2005, 1, 1. [Google Scholar]
- Chittasupho, C.; Manthaisong, A.; Okonogi, S.; Tadtong, S.; Samee, W. Effects of Quercetin and Curcumin Combination on Antibacterial, Antioxidant, In Vitro Wound Healing and Migration of Human Dermal Fibroblast Cells. Int. J. Mol. Sci. 2022, 23, 142. [Google Scholar] [CrossRef]
- Carullo, G.; Governa, P.; Leo, A.; Gallelli, L.; Citraro, R.; Cione, E.; Caroleo, M.C.; Biagi, M.; Aiello, F.; Manetti, F. Quercetin-3-Oleate Contributes to Skin Wound Healing Targeting FFA1/GPR40. Chem. Sel. 2019, 4, 8429–8433. [Google Scholar] [CrossRef]
- Carullo, G.; Aiello, F. Quercetin-3-oleate. Molbank 2018, 2018, M1006. [Google Scholar] [CrossRef]
- Carullo, G.; Perri, M.; Manetti, F.; Aiello, F.; Caroleo, M.C.; Cione, E. Quercetin-3-Oleoyl Derivatives as New GPR40 Agonists: Molecular Docking Studies and Functional Evaluation. Bioorg. Med. Chem. Lett. 2019, 29, 1761–1764. [Google Scholar] [CrossRef]
- Weiß, K.T.; Fante, M.; Köhl, G.; Schreml, J.; Haubner, F.; Kreutz, M.; Haverkampf, S.; Berneburg, M.; Schreml, S. Proton-sensing G protein-coupled receptors as regulators of cell proliferation and migration during tumor growth and wound healing. Exp. Dermatol. 2017, 26, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Kumar, N.; Chauhan, N.S. Curcumin Encapsulated PEGylated Nanoliposomes: A Potential Anti-Infective Therapeutic Agent. Indian J. Microbiol. 2019, 59, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, V.M.; Peram, M.R.; Kugaji, M.S.; Shah, T.; Patil, S.P.; Muddapur, U.M.; Bhat, K.G. Effect of curcumin on growth, bioflm formation and virulence factor gene expression of Porphyromonas gingivalis. Odontology 2021, 109, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Hussain, T.; Arshad, M.; Ansari, A.R.; Irshad, A.; Nisar, J.; Hussain, F.; Masood, N.; Nazir, A.; Iqbal, M. Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int. J. Biol. Macromol. 2019, 140, 871–876. [Google Scholar] [CrossRef]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
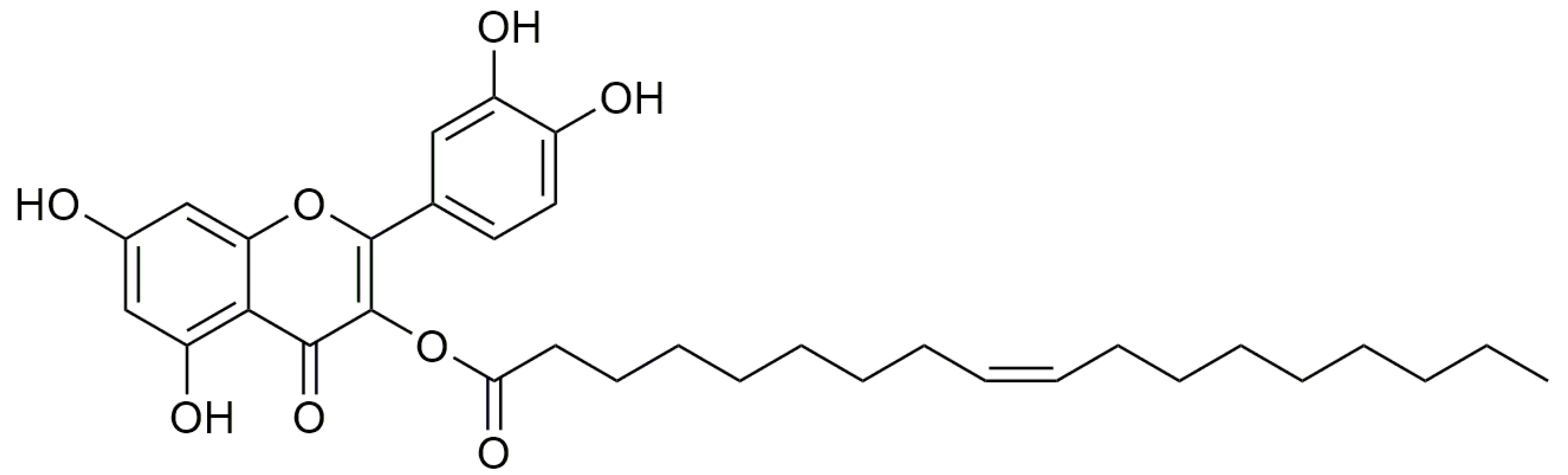
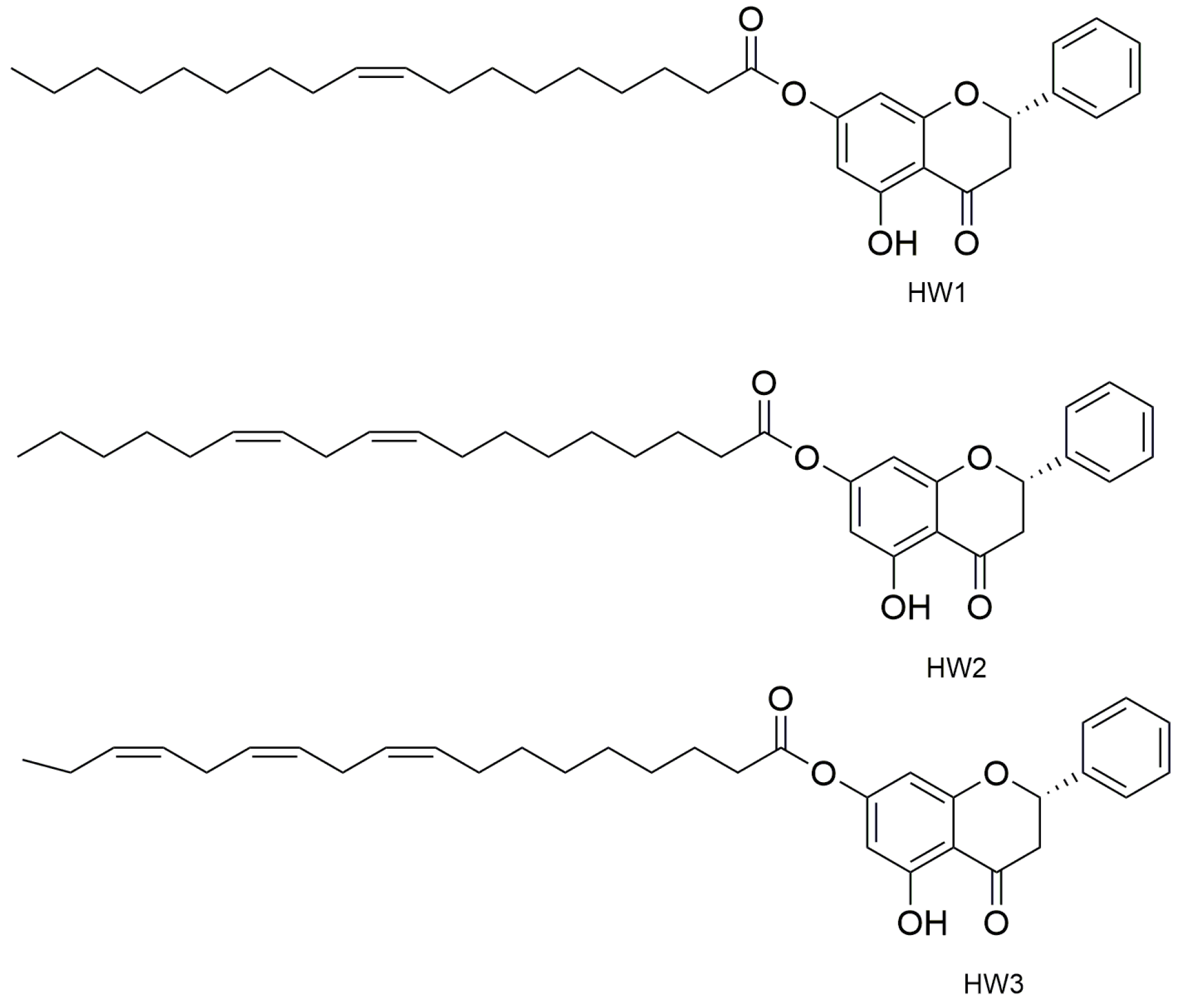
- Mazzotta, S.; Governa, P.; Borgonetti, V.; Marcolongo, P.; Nanni, C.; Gamberucci, A.; Manetti, F.; Pessina, F.; Carullo, G.; Brizzi, A.; et al. Pinocembrin and its linolenoyl ester derivative induce wound healing activity in HaCaT cell line potentially involving a GPR120/FFA4 mediated pathway. Bioorg. Chem. 2021, 108, 104657. [Google Scholar] [CrossRef]
- Governa, P.; Carullo, G.; Biagi, M.; Rago, V.; Aiello, F. Evaluation of the In Vitro Wound-Healing Activity of Calabrian Honeys. Antioxidants 2019, 8, 36. [Google Scholar] [CrossRef]
- Kaparekar, P.S.; Poddar, N.; Anandasadagopan, S.K. Fabrication and characterization of Chrysin—A plant polyphenol loaded alginate -chitosan composite for wound healing application. Colloids Surf. B Biointerfaces 2021, 206, 111922. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Sharif Zak, M.; Majdi, H.; Mostafavi, E.; Barati, M.; Lotfimehr, H.; Ghaseminasab, K.; Pazoki Toroudi, H.; Webster, T.J.; Akbarzade, A. The effect of chrysin–curcumin-loaded nanofibers on the wound-healing process in male rats. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1642–1652. [Google Scholar] [CrossRef]
- Xu, P.; Kumar Kankala, R.; Li, Y.; Wang, S.; Chen, A. Synergistic chemo-/photothermal therapy based on supercritical technology-assisted chitosan–indocyanine green/luteolin nanocomposites for wound healing. Regen. Biomater. 2022, 9, rbac072. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, A.; Alarcon-Aguilar, F.; Almanza-Perez, J.; Nieto-Yañez, O.; Olivares-Sanchez, J.; Duran-Diaz, A.; Rodriguez-Monroy, M.; Canales-Martinez, M. Antimicrobial and anti-inflammatory activities, wound-healing effectiveness and chemical characterization of the latex of Jatropha neopauciflora Pax. J. Ethnopharmacol. 2017, 204, 1–7. [Google Scholar] [CrossRef]
- De Sousa Leal, A.; De Carvalho, L.; da Silva, D.; Cunha, L.; Arimateia, N.; Arimateia, J.; Lopes, D. Incorporation of tannic acid in formulations for topical use in wound healing: A technological prospecting. Afr. J. Pharm. Pharmacol. 2015, 9, 662–674. [Google Scholar]
- Li, K.; Diao, Y.; Zhang, H.; Wang, S.; Zhang, Z.; Yu, B.; Huang, S.; Yang, H. Tannin extracts from immature fruits of Terminalia chebula Fructus Retz. promote cutaneous wound healing in rats. BMC Complement. Altern. Med. 2011, 11, 86. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound. ACS Appl. Mater. Interfaces 2016, 8, 42. [Google Scholar] [CrossRef]
- Scherera, M.; Marquesa, F.; Figueira, M.; Peisinoa, M.; Schmitta, E.; Kondratyukb, T.P.; Endringera, D.; Scherera, R.; Fronza, M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.C.; Thomazzi, S.M. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol. 2012, 143, 656–663. [Google Scholar] [CrossRef]
- Porras-Reyes, B.H.; Lewis, W.H.; Roman, J.; Simchowitz, L.; Mustoe, T.A. Enhancement of Wound Healing by the Alkaloid Taspine Defining Mechanism of Action. Exp. Biol. Med. 1993, 203, 18–25. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Tripathy, S.; Adhikary, J.; Chattopadhyay, S.; Mandal, D.; Dash, S.K.; Das, S.; Dey, A.; Dey, S.K.; Das, D.; et al. Surface modification minimizes the toxicity of silver nanoparticles: An in vitro and in vivo study. JBIC J. Biol. Inorg. Chem. 2017, 22, 893–918. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef]
- Shagdarova, B.; Konovalova, M.; Zhuikova, Y.; Lunkov, A.; Zhuikov, V.; Khaydapova, D.; Il’ina, A.; Svirshchevskaya, E.; Varlamov, V. Collagen/Chitosan Gels Cross-Linked with Genipin for Wound Healing in Mice with Induced Diabetes. Materials 2022, 15, 15. [Google Scholar] [CrossRef]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, S.; Cai, B.; Wang, Y.; Deng, D.; Wang, X. An injectable and self-healing hydrogel with antibacterial and angiogenic properties for diabetic wound healing. Biomater. Sci. 2022, 10, 3480–3492. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Bhadauriya, P.; Mamtani, H.; Ashfaq, M.; Raghav, A.; Teotia, A.K.; Kumar, A.; Verma, N. Synthesis of Yeast-Immobilized and Copper Nanoparticle-Dispersed Carbon Nanofiber-Based Diabetic Wound Dressing Material: Simultaneous Control of Glucose and Bacterial Infections. ACS Appl. Biomater. 2018, 1, 246–258. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lin, S.J. Chitosan/PVA Hetero-Composite Hydrogel Containing Antimicrobials, Perfluorocarbon Nanoemulsions, and Growth Factor-Loaded Nanoparticles as a Multifunctional Dressing for Diabetic Wound Healing: Synthesis, Characterization, and In Vitro/In Vivo Evaluation. Pharmaceutics 2022, 14, 537. [Google Scholar] [CrossRef]
- Rodríguez-Acosta, H.; Rivera, J.M.T.; Guerrero-Guzmán, A.; Hernández-Elizarraráz, E.; Díaz, J.A.H.; García, J.J.G.; Ramírez, P.E.P.; Ramírez, S.F.V.; Anguiano, A.C.R.; Juárez, G.V.; et al. Chronic wound healing by controlled release of chitosan hydrogels loaded with silver nanoparticles and calendula extract. J. Tissue Viability 2022, 31, 173–179. [Google Scholar] [CrossRef]
- Manne, A.A.; Arigela, B.; Giduturi, A.K.; Komaravolu, R.K.; Mangamuri, U.; Poda, S. Pterocarpus marsupium Roxburgh heartwood extract/chitosan nanoparticles loaded hydrogel as an innovative wound healing agent in the diabetic rat model. Mater. Today Commun. 2021, 26, 101916. [Google Scholar] [CrossRef]
- Megha, G.; Arpit, S.; Chandra Shekhar, B.; Priyanka, T. FTIR and GC-MS Analysis of Curcumin Loaded Chitosan Hydrogel for Diabetic Wound. Res. J. Biotechnol. 2022, 17, 111–117. [Google Scholar]
- Xu, Z.; Liu, G.; Zheng, L.; Wu, J. A polyphenol-modified chitosan hybrid hydrogel with enhanced antimicrobial and antioxidant activities for rapid healing of diabetic wounds. Nano Res. 2022; in press. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Z.; Yu, Q.; Ma, T. Preparation of reactive oxygen species-responsive antibacterial hydrogels for efficient anti-infection therapy. Mater. Lett. 2020, 263, 127254. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, Y.; Lu, W.; Zhu, W.; Li, Y.; Chen, K.; Zhang, G.; Xu, J.; Deng, Z.; Wang, D. Characterization of a novel polyvinyl alcohol/chitosan porous hydrogel combined with bone marrow mesenchymal stem cells and its application in articular cartilage repair. BMC Musculoskelet. Disord. 2019, 20, 257. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl chitosan-based hydrogels containing fibroblast growth factors for triggering diabetic wound healing. Carbohydr. Polym. 2022, 287, 119336. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Huang, Y.; Mao, R.; Xiang, Y.; Cai, E.; Chen, Y.; Shen, J.; Dong, W.; Qi, X. Together is better: Poly(tannic acid) nanorods functionalized polysaccharide hydrogels for diabetic wound healing. Ind. Crops Prod. 2022, 186, 115273. [Google Scholar] [CrossRef]
- Abueva, C.; Ryu, H.S.; Min, J.W.; Chung, P.S.; You, H.S.; Yang, M.S.; Woo, S.H. Quaternary ammonium N, N, N-trimethyl chitosan derivative and povidoneiodin ecomplex as a potent antiseptic with enhanced wound healing property. Int. J. Biol. Macromol. 2021, 182, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Qi, X.; Xiang, Y.; You, S.; Cai, E.; Gao, T.; Tong, X.; Hu, R.; Shen, J.; Deng, H. Facile formation of injectable quaternized chitosan/tannic acid hydrogels with antibacterial and ROS scavenging capabilities for diabetic wound healing. Int. J. Biol. Macromol. 2022, 195, 190–197. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Chen, J.; Shen, T.; Jin, T.; Zeng, B.; Li, L.; Yang, C.; Mu, Z.; Deng, H.; et al. An all-in-one CO gas therapy-based hydrogel dressing with sustained insulin release, anti-oxidative stress, antibacterial, and anti-inflammatory capabilities for infected diabetic wounds. Acta Biomater. 2022, 146, 49–65. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Y.; Wu, C.; Chen, J.; Ning, N.; Yang, Z.; Guo, Y.; Zhang, J.; Hu, X.; Wang, Y. Conductive dual hydrogen bonding hydrogels for the electrical stimulation of infected chronic wounds. J. Mater. Chem. B 2021, 9, 8138–8146. [Google Scholar] [CrossRef]
- Li, C.; Jiang, T.; Zhou, C.; Jiang, A.; Lu, C.; Yang, G.; Nie, J.; Wang, F.; Yang, X.; Chen, Z. Injectable self-healing chitosan-based POSS-PEG hybrid hydrogel as wound dressing to promote diabetic wound healing. Carbohydr. Polym. 2023, 299, 120198. [Google Scholar] [CrossRef]
- Yu, L.D.; Hu, P.; Chen, Y. Gas-Generating Nanoplatforms: Material Chemistry, Multifunctionality, and Gas Therapy. Adv. Mater. 2018, 30, 1801964. [Google Scholar] [CrossRef]
- Sattary, M.; Rafienia, M.; Khorasani, M.T.; Salehi, H. The effect of collector type on the physical, chemical, and biological properties of polycaprolactone/gelatin/nano-hydroxyapatite electrospun scaffold. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 933–950. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a polyvinyl alcohol sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Naghizadeh, Z.; Karkhaneh, A.; Khojasteh, A. Self-crosslinking effect of chitosan and gelatin on alginate based hydrogels: Injectable in situ forming scaffolds. Mater. Sci. Eng. C 2018, 89, 256–264. [Google Scholar] [CrossRef]
- Qin, Y.; Hu, H.; Luo, A. The conversion of calcium alginate fibers into alginic acid fibers and sodium alginate fibers. J. Appl. Polym. Sci. 2006, 101, 4216–4221. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.A.; Kousar, M. Improved drug delivery and accelerated diabetic wound healing by chondroitin sulfate grafted alginate-based thermoreversible hydrogels. Mater. Sci. Eng. C 2021, 126, 112169. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Larson, B.J.; Longaker, M.T.; Lorenz, H.P. Scarless Fetal Wound Healing: A Basic Science Review. Plast. Reconstr. Surg. 2010, 126, 1172–1180. [Google Scholar] [CrossRef]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with miRNA-Laden Nanoparticles Promotes Wound Healing. Small 2019, 15, 1902232. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chien, B.-Y.C.; Lee, Y.-H. Injectable and thermoresponsive hybrid hydrogel with Antibacterial, Anti-inflammatory, oxygen Transport, and enhanced cell growth activities for improved diabetic wound healing. Eur. Polym. J. 2022, 175, 111364. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Żywicka, A.; Peitler, D.; Rakoczy, R.; Junka, A.F.; Fijałkowski, K. Wet and Dry Forms of Bacterial Cellulose Synthetized by Different Strains of Gluconacetobacter xylinus as Carriers for Yeast Immobilization. Appl. Biochem. Biotechnol. 2016, 180, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Netravali, A.N. A Review of Fabrication and Applications of Bacterial Cellulose Based Nanocomposites. Polym. Rev. 2014, 54, 598–626. [Google Scholar] [CrossRef]
- Mohamad, N.; Amin, M.C.I.M.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Lin, S.-C.; Wu, Y.-H.; Hu, C.-Y.; Chen, Y.-T.; Chen, Y.-C. The Antimicrobial Effects of Bacterial Cellulose Produced by Komagataeibacter intermedius in Promoting Wound Healing in Diabetic Mice. Int. J. Mol. Sci. 2022, 23, 5456. [Google Scholar] [CrossRef]
- Lei, L.; Huang, W.; Liu, K.E.; Liu, X.; Dai, M.; Liu, Z.; Zhiao, Y. Trilazad mesylate-loaded electrospun cellulose acetate nanofibrous wound dressings promote diabetic wound healing by modulation of immune response and protection against oxidative damage. J. Drug Deliv. Sci. Technol. 2022, 69, 102863. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Liu, S.; Tiwari, S.K.; Thummavichai, K.; Ola, O.; Ma, Z.; Zhang, S.; Wang, N.; Zhu, Y. Antibacterial properties and drug release study of cellulose acetate nanofibers containing ear-like Ag-NPs and Dimethyloxallyl Glycine/beta-cyclodextrin. Appl. Surf. Sci. 2022, 590, 153132. [Google Scholar] [CrossRef]
- Sheir, M.M.; Nasra, M.M.; Abdallah, O.Y. Chitosan alginate nanoparticles as a platform for the treatment of diabetic and non-diabetic pressure ulcers: Formulation and in vitro/in vivo evaluation. Int. J. Pharm. 2021, 607, 120963. [Google Scholar] [CrossRef]
- Abdelbasset, W.K.; Elkholi, S.M.; Ismail, K.A.; Al-Ghamdi, H.S.; Mironov, S.; Ridha, H.S.H.; Maashi, M.S.; Thangavelu, L.; Mahmudiono, T.; Mustafa, Y.F. Mequinol-loaded carboxymethyl cellulose/chitosan electrospun wound dressing as a potential candidate to treat diabetic wounds. Cellulose 2022, 29, 7863–7881. [Google Scholar] [CrossRef]
- Draelos, Z.D. The combination of 2% 4-hydroxyanisole (mequinol) and 0.01% tretinoin effectively improves the appearance of solar lentigines in ethnic groups. J. Cosmet. Dermatol. 2006, 5, 239–244. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favorable physical features versus adverse biological effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Minhas, M.U.; Shah, S.A.; Jabeen, N.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Rashid, H. Self-crosslinked chitosan/κ-carrageenan-based biomimetic membranes to combat diabetic burn wound infections. Int. J. Biol. Macromol. 2022, 197, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gao, M.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.; Zhang, X.-Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Guan, L.; Guo, W.; Zhang, X.; Wu, S.; Guo, D.; Li, R.; Zvyagin, A.V.; Lin, Q.; Qu, W. Graphene oxide-based injectable conductive hydrogel dressing with immunomodulatory for chronic infected diabetic wounds. Mater. Des. 2022, 224, 111284. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef]
- Li, Q.; Liu, K.; Jiang, T.; Ren, S.; Kang, Y.; Li, W.; Yao, H.; Yang, X.; Dai, H.; Chen, Z. Injectable and self-healing chitosan-based hydrogel with MOF-loaded α-lipoic acid promotes diabetic wound healing. Mater. Sci. Eng. C 2021, 131, 112519. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, Y.; Cai, E.; You, S.; Gao, T.; Lan, Y.; Deng, H.; Li, Z.; Hu, R.; Shen, J. All-in-one: Harnessing multifunctional injectable natural hydrogels for ordered therapy of bacteria-infected diabetic wounds. Chem. Eng. J. 2022, 439, 135691. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, Y.; Shi, Y.; Zhao, L. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Deliv. Transl. Res. 2019, 9, 227–239. [Google Scholar] [CrossRef]
- Wei, S.; Xu, P.; Yao, Z.; Cui, X.; Lei, X.; Li, L.; Dong, Y.; Zhu, W.; Guo, R.; Cheng, B. A composite hydrogel with co-delivery of antimicrobial peptides and platelet-rich plasma to enhance healing of infected wounds in diabetes. Acta Biomater. 2021, 124, 205–218. [Google Scholar] [CrossRef]
- Li, X.-X.; Dong, J.-Y.; Li, Y.-H.; Zhong, J.; Yu, H.; Yu, Q.-Q.; Lei, M. Fabrication of Ag–ZnO@ carboxymethyl cellulose/K-carrageenan/graphene oxide/konjac glucomannan hydrogel for effective wound dressing in nursing care for diabetic foot ulcers. Appl. Nanosci. 2020, 10, 729–738. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Tucci, P.; Picci, N.; Iemma, F.; Hampel, S.; Nicoletta, F.P. Carbon nanotubes hybrid hydrogels for electrically tunable release of Curcumin. Eur. Polym. J. 2017, 90, 1–12. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Zhao, Y.; Feng, Y.; Zvyagin, A.V.; Wang, J.; Yang, X.; Yang, B.; Lin, Q. Novel Diabetic Foot Wound Dressing Based on Multifunctional Hydrogels with Extensive Temperature-Tolerant, Durable, Adhesive, and Intrinsic Antibacterial Properties. ACS Appl. Mater. Interfaces 2021, 13, 26770–26781. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, J.; Yang, Y.; Qiao, L.; Hu, J.; Zhang, J.; Guo, B. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater. 2022, 146, 119–130. [Google Scholar] [CrossRef]
- Chen, Y.; Rao, Z.; Liu, Y.; Liu, X.; Liu, Y.; Xu, L.; Wang, Z.; Guo, J.; Zhang, L.; Dong, Y.; et al. Multifunctional Injectable Hydrogel Loaded withCerium-Containing Bioactive Glass Nanoparticles for Diabetic Wound Healing. Biomolecules 2021, 11, 702. [Google Scholar] [CrossRef]
- Li, J.; Zhai, D.; Lv, F.; Yu, Q.; Ma, H.; Yin, J.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016, 36, 254–266. [Google Scholar] [CrossRef]
- Goh, Y.-F.; Alshemary, A.Z.; Akram, M.; Kadir, M.R.A.; Hussain, R. In-vitro characterization of antibacterial bioactive glass containing ceria. Ceram. Int. 2014, 40, 729–737. [Google Scholar] [CrossRef]
- Xie, X.; Jin, X.; He, B.; Zou, Y.; Yang, J.; Liu, C.; Kong, X.; Liu, W.; Wang, W. A change-prone zwitterionic hyperbranched terpolymer-base d diab etic wound dressing. Appl. Mater. Today 2022, 27, 101477. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef]
- Han, N.; Xu, Z.; Cui, C.; Li, Y.; Zhang, D.; Xiao, M.; Fan, C.; Wu, T.; Yang, J.; Liu, W. A Fe3+-crosslinked pyrogallol-tethered gelatin adhesive hydrogel with antibacterial activity for wound healing. Biomater. Sci. 2020, 8, 3164–3172. [Google Scholar] [CrossRef]
- He, Y.; Liu, K.; Guo, S.; Chang, R.; Zhang, C.; Guan, F.; Yao, M. Multifunctional hydrogel with reactive oxygen species scavenging and photothermal antibacterial activity accelerates infected diabetic wound healing. Acta Biomater. 2023, 155, 199–217. [Google Scholar] [CrossRef]

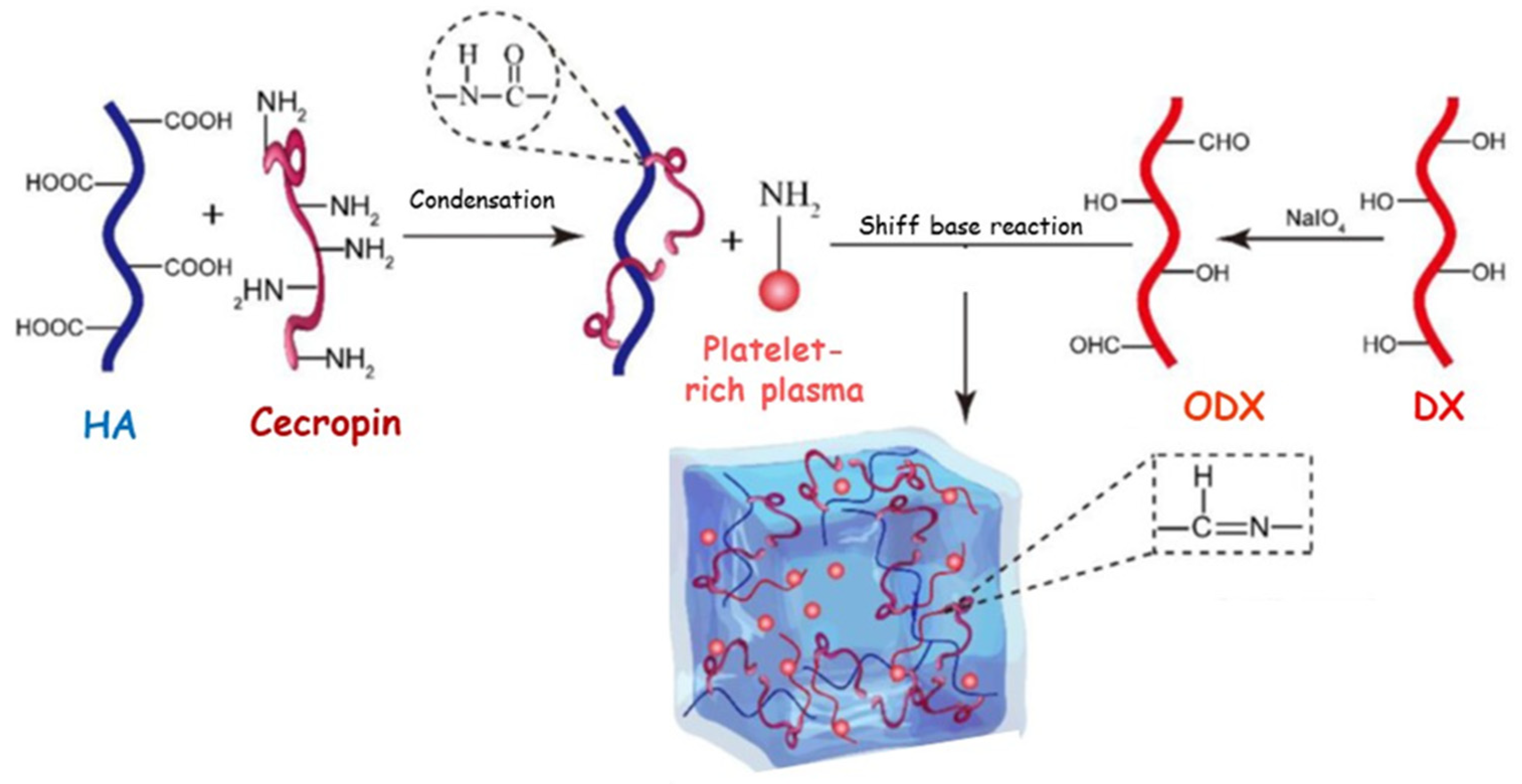
| Poliphenol | Structure | Characteristics |
|---|---|---|
| Quercetin | 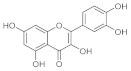 | It modulates the activity of fibroblasts. It up-regulates vascular the endothelial growth factor and transforms growth factor-β1. It is a wound healing agent for diabetic scars. Limitations: low bioavaibility and low systemic and topic absorption |
| Curcumin |  | It is involved in tissue remodeling, the formation of granulation tissue, and collagen deposition. It can induce the regeneration of epithelial tissue and increases fibroblasts proliferation and vascular density. It is poorly absorbed following oral administration. It is involved in extensive first-pass metabolism. It is a light-sensitive molecule. Topical formulations are preferred. |
| Pinocembrin | 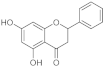 | It is able to modulate the production of inflammatory cytokines. It can accelerate in vitro skin wound healing, improving the migration of keratinocytes. Limitations are associated to its low bioavavibility. |
| Chrysin |  | It can take a reduction of p53 and iNOS expression. Limitations: low stability in vivo due to its poor acqueos solubility and low bioavaibility Assocations with polymers or other compounds can ameliorate the situation. |
| Luteolin |  | It can inhibit TNF-α and IL-6 and iNOS secretion in LPS-activated macrophages. It can modulate IGF, PDGF, and FGF. It can also suppress NETs in activated human neutrophils, and improve immune system by inhibiting the production of ROS. Limitations: it has low bioavability so local use is preferred but also other ways can be undertaken, such as intraperitoneal injection for systemic effects. |
| Catechin and Epigallocatechin-3-gallate |  | They are able to reduce TNF-α secretiona and NFκB activity. They can inhibit the production of NO regulating inflammatory processes. Limitations: they have poor systemic absorption, bad biodistribution, suffer of first-pass metabolism, and have low stability, which take to the formation of degradation products. Nanoformulation can get around these problems. |
| Tannic acid |  | It can inhibit lipid oxidation by removing free radicals. Its application can be topical, local, or systemic. It can be combined with polymers to become more resistant to proteolytic enzymes. There are controversial hypotesis about its cytotoxic effects but at the current state it is considered a safe food additive. |
| Terpinolene and α-phellandrene | 
 | They can improve the migration and proliferation of fibroblasts, suppress IL-6 and TNF-α, inhibit NO production, and suppress NF-κB activity. Limitations: They are lipophilic so they present low bioavaibility but nanoformulation are used to improve their use. |
| Thymol |  | It improves the edema formation and the influx of leukocytes to the wound area. It improves granulation reaction. Limitations: it is rapidly absorbed in vivo, but nanoformulations can be used to increase solubility and stability. |
| Taspine | 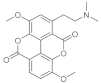 | It shows chemotactic properties on fibroblasts. Limitations: Low bioavaibility, but the association with other compounds or polymers can be used in its advantage in the use of the molecule. |
| Day | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| 0 | 0% | 0% | 0% |
| 4 | 24.30% | 37.50% | 33.33% |
| 8 | 29.00% | 58.33% | 65.07% |
| 12 | 66.66% | 79.85% | 87.50% |
| 16 | 83.33% | 91.66% | 95.83% |
| Curcumin + Chitosan-PVA Mixture | E. coli a | P. itocida a | B. subtilis a | S. aureus a |
|---|---|---|---|---|
| Curcumin (10 mg/mL) | 12 ± 2.45 | 14 ± 3.5 | 11 ± 1.23 | 13 ± 0.95 |
| Curcumin (20 mg/mL) | 15 ± 4.23 | 16 ± 2.52 | 14 ± 2.18 | 14 ± 0.90 |
| Curcumin (30 mg/mL) | 17 ± 5.50 | 20 ± 2.24 | 13 ± 3.27 | 15 ± 2.50 |
| Curcumin (10 mg + Chitosan-PVP 80) | 22 ± 3.56 | 24 ± 0.90 | 15 ± 3.52 | 17 ± 0.96 |
| Curcumin (20 mg + Chitosan-PVP 80) | 25 ± 1.90 | 23 ± 0.8 | 17 ± 0.54 | 20 ± 2.50 |
| Curcumin (30 mg + Chitosan-PVP 80) | 28 ± 2.7 | 26 ± 3.8 | 25 ± 3.1 | 23 ± 1.50 |
| Chitosan-PVA 80 | 18 ± 0.5 | 20 ± 0.7 | 13 ± 0.4 | 18 ± 3.59 |
| Rifampicin | 36 ± 0.9 | 32 ± 1.4 | 30 ± 2.8 | 36 ± 4.3 |
| Polymer | Structure | Characteristics |
|---|---|---|
| Chitosan | 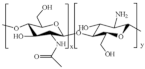 | Antimicrobial, wound healing, antidiabetic, biodegradability, nontoxicity, biocompatibility, anti-inflammatory, hemostasis |
| Alginate | 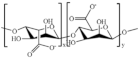 | Antimicrobial, moisture absorbing, hydrophilicity, biocompatibility, gelation |
| Hyaluronic acid |  | Antimicrobial, biodegradability, anti-adhesive, viscoelasticity lubricity, biocompatibility, immunostimulatory |
| β-Glucan | 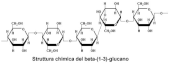 | Antiproliferative, blood glucose regulation, immunomodulatory |
| Cellulose | 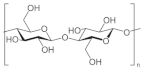 | Biodegradable, biocompatible, non-carcinogenic, non-toxic, retain moisture, absorb exudates, gelation |
| Konjac glucomannan | 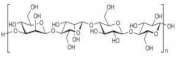 | Biocompatibility, gelling agent, biodegradability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falbo, F.; Spizzirri, U.G.; Restuccia, D.; Aiello, F. Natural Compounds and Biopolymers-Based Hydrogels Join Forces to Promote Wound Healing. Pharmaceutics 2023, 15, 271. https://doi.org/10.3390/pharmaceutics15010271
Falbo F, Spizzirri UG, Restuccia D, Aiello F. Natural Compounds and Biopolymers-Based Hydrogels Join Forces to Promote Wound Healing. Pharmaceutics. 2023; 15(1):271. https://doi.org/10.3390/pharmaceutics15010271
Chicago/Turabian StyleFalbo, Federica, Umile Gianfranco Spizzirri, Donatella Restuccia, and Francesca Aiello. 2023. "Natural Compounds and Biopolymers-Based Hydrogels Join Forces to Promote Wound Healing" Pharmaceutics 15, no. 1: 271. https://doi.org/10.3390/pharmaceutics15010271
APA StyleFalbo, F., Spizzirri, U. G., Restuccia, D., & Aiello, F. (2023). Natural Compounds and Biopolymers-Based Hydrogels Join Forces to Promote Wound Healing. Pharmaceutics, 15(1), 271. https://doi.org/10.3390/pharmaceutics15010271









