Combined Use of Antimicrobial Peptides with Antiseptics against Multidrug-Resistant Bacteria: Pros and Cons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Antimicrobial Peptides
2.1.2. Antiseptics and Surfactants
2.1.3. Bacterial Strains
2.2. Methods for Examining Antibacterial Effects
2.2.1. Broth Microdilution Assay: Evaluation of Minimal Inhibitory Concentrations against Planktonic Bacteria
2.2.2. Checkerboard Titration for Analyzing Combined Antibacterial Effect against Planktonic Bacteria
2.2.3. Bacterial Membrane Permeability Assays
2.2.4. Fluorometric Resazurin Dye-Based Assay to Monitor Bacterial Metabolic Activity and Viability
2.2.5. Crystal Violet Assay to Evaluate Formation of Biofilms
2.3. Methods for Examining Toxicity toward Eukaryotic Cells
2.3.1. Hemolysis Test
2.3.2. MTT Test
2.3.3. Assessment of Viability of Adherent Cells by Light Microscopy
2.4. General Principles Used for Combined Effect Analysis
3. Results
3.1. Preliminary Overview of the Tested Compounds
3.2. Antimicrobial Action of the Individual Compounds against Planktonic Bacteria
3.3. Effects of Combinations against Planktonic Bacteria
3.4. Effects of the Individual Compounds and Their Combinations on the Permeability of Bacterial Membranes
3.5. Effects of the Individual Compounds and Their Combinations on the Metabolic Activity of Bacteria
3.6. Effects of the Individual Compounds and Their Combinations against Forming Monobacterial Biofilms
3.7. Toxicity of the Individual Compounds and Their Combinations against Host Cells
3.7.1. Hemolytic Activity
3.7.2. Cytotoxic Action toward Human Dermal Fibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Report on Antimicrobial Resistance. 2016. Available online: https://amr-review.org (accessed on 31 October 2022).
- Denamur, E.; Matic, I. Evolution of mutation rates in bacteria. Mol. Microbiol. 2006, 60, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, M. Role of antibiotic stewardship in extending the age of modern medicine. S. Afr. Med. J. 2015, 105, 414–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Barriere, S.L. Clinical, economic and societal impact of antibiotic resistance. Expert. Opin. Pharmacother. 2015, 16, 151–153. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. World Health Organization: Geneva, Switzerland, 2015. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 31 October 2022).
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Bjorn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [Green Version]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef]
- Zhang, D.; He, Y.; Ye, Y.; Ma, Y.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. Little Antimicrobial Peptides with Big Therapeutic Roles. Protein Pept. Lett. 2019, 26, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Wilson, D.N. Intracellular Antimicrobial Peptides Targeting the Protein Synthesis Machinery. Adv. Exp. Med. Biol. 2019, 1117, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski Lda, S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4, 353. [Google Scholar] [CrossRef] [Green Version]
- Wimley, W.C.; Hristova, K. Antimicrobial peptides: Successes, challenges and unanswered questions. J. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Baeder, D.Y.; Regoes, R.R.; Rolff, J. Predicting drug resistance evolution: Insights from antimicrobial peptides and antibiotics. Proc. Biol. Sci. 2018, 285, 20172687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fuente-Núñez, C.; Cardoso, M.H.; de Souza Cândido, E.; Franco, O.L.; Hancock, R.E. Synthetic antibiofilm peptides. Biochim. Biophys. Acta 2016, 1858, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Shahrour, H.; Ferrer-Espada, R.; Dandache, I.; Bárcena-Varela, S.; Sánchez-Gómez, S.; Chokr, A.; Martinez-de-Tejada, G. AMPs as Anti-biofilm Agents for Human Therapy and Prophylaxis. Adv. Exp. Med. Biol. 2019, 1117, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Jaśkiewicz, M.; Neubauer, D.; Kazor, K.; Bartoszewska, S.; Kamysz, W. Antimicrobial Activity of Selected Antimicrobial Peptides Against Planktonic Culture and Biofilm of Acinetobacter baumannii. Probiotics Antimicrob. Proteins. 2019, 11, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Langham, A.A.; Khandelia, H.; Kaznessis, Y.N. Correlation between simulated physicochemical properties and hemolycity of protegrin-like antimicrobial peptides: Predicting experimental toxicity. Peptides 2008, 29, 1085–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef]
- Mabrouk, D.M. Antimicrobial peptides: Features, applications and the potential use against covid-19. Mol. Biol. Rep. 2022, 49, 10039–10050. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Diana, J. Mining the bacterial genome to discover new antimicrobial molecules. EMBO Mol. Med. 2022, 14, e15409. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Borchard, G.; Jordan, O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol Sci. 2020, 21, 7047. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Cheng, H.; Chabok, R.; Alvarez, M.M.; de la Fuente-Nunez, C.; Phillips, K.S.; Khademhosseini, A. Strategies for antimicrobial peptide coatings on medical devices: A review and regulatory science perspective. Crit. Rev. Biotechnol. 2021, 41, 94–120. [Google Scholar] [CrossRef]
- Gomes, A.P.; Mano, J.F.; Queiroz, J.A.; Gouveia, I.C. Incorporation of antimicrobial peptides on functionalized cotton gauzes for medical applications. Carbohydr. Polym. 2015, 127, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Salvagni, E.; García, C.; Manresa, À.; Müller-Sánchez, C.; Reina, M.; Rodríguez-Abreu, C.; García-Celma, M.J.; Esquena, J. Short and ultrashort antimicrobial peptides anchored onto soft commercial contact lenses inhibit bacterial adhesion. Colloids Surf. B. Biointerfaces. 2020, 196, 111283. [Google Scholar] [CrossRef]
- Babalska, Z.Ł.; Korbecka-Paczkowska, M.; Karpiński, T.M. Wound Antiseptics and European Guidelines for Antiseptic Application in Wound Treatment. Pharmaceuticals 2021, 14, 1253. [Google Scholar] [CrossRef]
- Dumville, J.C.; McFarlane, E.; Edwards, P.; Lipp, A.; Holmes, A.; Liu, Z. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst. Rev. 2015, 2015, CD003949. [Google Scholar] [CrossRef]
- Letzelter, J.; Hill, J.B.; Hacquebord, J. An Overview of Skin Antiseptics Used in Orthopaedic Surgery Procedures. J. Am. Acad. Orthop. Surg. 2019, 27, 599–606. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [Green Version]
- Percival, S.L.; Finnegan, S.; Donelli, G.; Vuotto, C.; Rimmer, S.; Lipsky, B.A. Antiseptics for treating infected wounds: Efficacy on biofilms and effect of pH. Crit. Rev. Microbiol. 2016, 42, 293–309. [Google Scholar] [CrossRef]
- Walker, J.T.; Bradshaw, D.J.; Fulford, M.R.; Marsh, P.D. Microbiological evaluation of a range of disinfectant products to control mixed-species biofilm contamination in a laboratory model of a dental unit water system. Appl. Environ. Microbiol. 2003, 69, 3327–3332. [Google Scholar] [CrossRef] [Green Version]
- Echols, K.; Graves, M.; LeBlanc, K.G.; Marzolf, S.; Yount, A. Role of Antiseptics in the Prevention of Surgical Site Infections. Dermatol. Surg. 2015, 41, 667–676. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, T.; Koerber, A.; Jacobsen, F.; Dissemond, J.; Steinau, H.U.; Gatermann, S.; Al-Benna, S.; Kesting, M.; Seipp, H.M.; Steinstraesser, L. Evaluation of toxic side effects of clinically used skin antiseptics in vitro. J. Surg. Res. 2010, 164, 344–350. [Google Scholar] [CrossRef]
- Turnidge, J. Drug–Drug Combinations. In Fundamentals of Antimicrobial Pharmacokinetics and Pharmacodynamics; Vinks, A., Derendorf, H., Mouton, J., Eds.; Springer: New York, NY, USA, 2014; pp. 153–198. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 2: Management strategies and new agents. Pharm. Ther. 2015, 40, 344–352. [Google Scholar]
- Zimmerman, G.R.; Lehar, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug. Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical Basis, Experimental Design and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Kopeikin, P.M.; Zharkova, M.S.; Kolobov, A.A.; Smirnova, M.P.; Sukhareva, M.S.; Umnyakova, E.S.; Kokryakov, V.N.; Orlov, D.S.; Milman, B.L.; Balandin, S.V.; et al. Caprine Bactenecins as Promising Tools for Developing New Antimicrobial and Antitumor Drugs. Front. Cell. Infect. Microbiol. 2020, 10, 552905. [Google Scholar] [CrossRef]
- Tossi, A.; Scocchi, M.; Zanetti, M.; Genaro, R.; Storici, P.; Romeo, D. An approach combining cDNA amplification and chemical synthesis for the identification of novel, cathelicidin-derived, antimicrobial peptides. In Antibacterial Peptide Protocols. Methods in Molecular Biology; Shafer, W., Ed.; Humana Press Inc.: Totowa, NJ, USA, 1997; Volume 78, pp. 133–151. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Greco, W.; Unkelbach, H.-D.; Pöch, G.; Sühnel, J.; Kundi, M.; Bödeker, W. Consensus on concepts and terminology for combined-action assessment: The Saariselkä agreement. Arch. Complex Environ. Stud. 1992, 4, 65–69. [Google Scholar]
- Breitinger, H.-G. Drug Synergy—Mechanisms and Methods of Analysis. In Toxicity and Drug Testing; Acree, B., Ed.; IntechOpen: London, UK, 2012; pp. 143–166. ISBN 978-953-51-0004-1. Available online: https://www.intechopen.com/chapters/28118 (accessed on 31 October 2022). [CrossRef] [Green Version]
- Berenbaum, M.C. What is synergy? Pharmacol. Rev. 1989, 41, 93–141. [Google Scholar]
- Hsieh, M.H.; Yu, C.M.; Yu, V.L.; Chow, J.W. Synergy assessed by checkerboard. A critical analysis. Diagn. Microbiol. Infect. Dis. 1993, 16, 343–349. [Google Scholar] [CrossRef]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy Tests by E Test and Checkerboard Methods of Antimicrobial Combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Fehri, L.F.; Wróblewski, H.; Blanchard, A. Activities of Antimicrobial Peptides and Synergy with Enrofloxacin against Mycoplasma pulmonis. Antimicrob. Agents Chemother. 2007, 51, 468–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehrer, R.I.; Barton, A.; Ganz, T. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J. Immunol. Methods 1988, 108, 153–158. [Google Scholar] [CrossRef]
- Casale, A.; Clark, S.; Grasso, M.; Kryschuk, M.; Ritzer, L.; Trudeau, M.; Williams, L.E. Complete Genome Sequence of Escherichia coli ML35. Genome Announc. 2018, 6, e00034-18. [Google Scholar] [CrossRef] [Green Version]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Zalata, A.A.; Lammertijn, N.; Christophe, A.; Comhaire, F.H. The correlates and alleged biochemical background of the resazurin reduction test in semen. Int. J. Androl. 1998, 21, 289–294. [Google Scholar] [CrossRef]
- Magnani, E.; Bettini, E. Resazurin detection of energy metabolism changes in serum-starved PC12 cells and of neuroprotective agent effect. Brain Res. Protoc. 2000, 5, 266–272. [Google Scholar] [CrossRef]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 2006, 22, 1B.1.1–1B.1.18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zharkova, M.S.; Orlov, D.S.; Golubeva, O.Y.; Chakchir, O.B.; Eliseev, I.E.; Grinchuk, T.M.; Shamova, O.V. Application of Antimicrobial Peptides of the Innate Immune System in Combination With Conventional Antibiotics-A Novel Way to Combat Antibiotic Resistance? Front. Cell. Infect. Microbiol. 2019, 9, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef] [PubMed]
- Kokryakov, V.N.; Harwig, S.S.; Panyutich, E.A.; Shevchenko, A.A.; Aleshina, G.M.; Shamova, O.V.; Korneva, H.A.; Lehrer, R.I. Protegrins: Leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993, 327, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, D.A.; Hurst, M.A.; Fujii, C.A.; Kung, A.H.C.; Ho, J.F.; Cheng, F.C.; Loury, D.J.; Fiddes, J.C. Protegrin-1: A broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 1997, 41, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Bolintineanu, D.S.; Kaznessis, Y.N. Computational studies of protegrin antimicrobial peptides: A review. Peptides 2011, 32, 188–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazaridis, T.; He, Y.; Prieto, L. Membrane interactions and pore formation by the antimicrobial peptide protegrin. Biophys. J. 2013, 104, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.; Hong, M. Structure and mechanism of beta-hairpin antimicrobial peptides in lipid bilayers from solid-state NMR spectroscopy. Mol. Biosyst. 2009, 5, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Edwards, I.A.; Elliott, A.G.; Kavanagh, A.M.; Zuegg, J.; Blaskovich, M.A.; Cooper, M.A. Contribution of Amphipathicity and Hydrophobicity to the Antimicrobial Activity and Cytotoxicity of β-Hairpin Peptides. ACS Infect. Dis. 2016, 2, 442–450. [Google Scholar] [CrossRef]
- Soundrarajan, N.; Park, S.; Le Van Chanh, Q.; Cho, H.S.; Raghunathan, G.; Ahn, B.; Song, H.; Kim, J.H.; Park, C. Protegrin-1 cytotoxicity towards mammalian cells positively correlates with the magnitude of conformational changes of the unfolded form upon cell interaction. Sci. Rep. 2019, 9, 11569. [Google Scholar] [CrossRef] [Green Version]
- Shamova, O.; Orlov, D.; Stegemann, C.; Czihal, P.; Hoffmann, R.; Brogden, K.; Kolodkin, N.; Sakuta, G.; Tossi, A.; Sahl, H.-G.; et al. ChBac3.4: A Novel Proline-Rich Antimicrobial Peptide from Goat Leukocytes. Int. J. Pept. Res. Therap. 2009, 15, 31–42. [Google Scholar] [CrossRef]
- Mattiuzzo, M.; Bandiera, A.; Gennaro, R.; Benincasa, M.; Pacor, S.; Antcheva, N.; Scocchi, M. Role of the Escherichia coli SbmA in the antimicrobial activity of prolinerich peptides. Mol. Microbiol. 2007, 66, 151–163. [Google Scholar] [CrossRef]
- Krizsan, A.; Prahl, C.; Goldbach, T.; Knappe, D.; Hoffmann, R. Short proline-rich antimicrobial peptides inhibit either the bacterial 70S ribosome or the assembly of its large 50S subunit. Chembiochem. 2015, 16, 2304–2308. [Google Scholar] [CrossRef]
- Otvos, L. The short proline-rich family. Cell Mol. Life Sci. 2002, 59, 1138–1150. [Google Scholar] [CrossRef]
- Mohammadi, Z. Sodium hypochlorite in endodontics: An update review. Int. Dent. J. 2008, 58, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Guida, A. Mechanism of action of sodium hypochlorite and its effects on dentin. Minerva Stomatol. 2006, 55, 471–482. [Google Scholar] [PubMed]
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol. Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Estrela, C.; Estrela, C.R.; Barbin, E.L.; Spanó, J.C.; Marchesan, M.A.; Pécora, J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef]
- Fadeeva, N.I.; Gerasina, S.F.; Degtyareva, I.N.; Ryabokon’, N.A.; Kamzolova, S.G.; Pershin, G.N. Change in the activity of enzymes under the action of dioxidine and florenal. Pharm. Chem. J. 1980, 14, 674–677. [Google Scholar] [CrossRef]
- Degtiareva, I.N.; Fadeeva, N.I.; Gerasina, S.F.; Pershin, G.N.; Permogorov, V.I. Effect of dioxidine on DNA and RNA synthesis in Staphylococcus aureus. Farmakol. Toksikol. 1981, 44, 217–220. (In Russian) [Google Scholar]
- Bekbergenov, B.M.; Antipov, A.V.; Danil’iants, E.V.; Korolev, P.N.; Glezer, G.A. Clinical and experimental study of dioxidine. Its antibacterial action. Antibiotiki 1982, 27, 349–352. (In Russian) [Google Scholar] [PubMed]
- Kryukov, A.I.; Kunelskaya, N.L.; Gurov, A.V.; Izotova, G.N.; Romanenko, S.G.; Pavlikhin, O.G.; Muratov, D.L. Prospects for antiseptics in the treatment of laryngeal and tonsillar pathology. Meditsinskiy Sov. = Med. Counc. 2016, 6, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Kryukov, A.I.; Kunelskaya, N.L.; Tsarapkin, G.Y.; Izotova, G.N.; Tovmasyan, A.S.; Sedinkin, A.A.; Fedotkina, K.M. A study of the efficacy and safety of local antibiotic treatment of acute purulent maxillary sinusitis. Meditsinskiy Sov. Med. Counc. 2015, 15, 12–19. (In Russian) [Google Scholar] [CrossRef]
- SKTB Technolog. Poviargolum. Available online: http://sktb-technolog.ru/pharma/poviargolum/ (accessed on 31 October 2022).
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter baumannii and Other Bacteria. Front. Microbiol. 2021, 12, 652863. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- B.Braun Medical Inc. Prontosan® Wound Irrigation Solution. Prontosan Wound Bed Preparation Taken Seriously. Available online: https://www.bbraun.com/en/products/b/prontosan-wound-irrigation-solution-for-wounds-and-burns.html (accessed on 31 October 2022).
- Sowlati-Hashjin, S.; Carbone, P.; Karttunen, M. Insights into the Polyhexamethylene Biguanide (PHMB) Mechanism of Action on Bacterial Membrane and DNA: A Molecular Dynamics Study. J. Phys. Chem. B. 2020, 124, 4487–4497. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Ohmizo, C.; Katsu, T. Potassium and tetraphenylphosphonium ion-selective electrodes for monitoring changes in the permeability of bacterial outer and cytoplasmic membranes. J. Microbiol. Methods 2003, 54, 111–115. [Google Scholar] [CrossRef]
- Kaehn, K. Polihexanide: A safe and highly effective biocide. Skin Pharmacol. Physiol. 2010, 23 (Suppl. 1), 7–16. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; White, G.F.; Morby, A.P. The response of Escherichia coli to exposure to the biocide polyhexamethylene biguanide. Microbiology 2006, 152 Pt 4, 989–1000. [Google Scholar] [CrossRef] [Green Version]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [Green Version]
- Lukoyanova, T.V.; Bulgakov, V.S.; Kravtsov, E.G.; Zaslavskaya, M.I.; Lukova, O.A.; Tsarev, V.N. Efficiency of etidronic acid for the prevention of inflammatory processes in the oral cavity. Russ. Stomatol. 2011, 4, 26–28. (In Russian) [Google Scholar]
- Afinogenova, A.G.; Voroshilova, T.M.; Afinogenov, G.E.; Maday, D.Y. The new metall-beta-lactamase’s inhibitor efficacy in a model system in vitro. Russ. J. Infect. Immun. 2016, 6, 335–344. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Kropinski, A.M.; Parr, T.R., Jr.; Angus, B.L.; Hancock, R.E.; Ghiorse, W.C.; Greenberg, E.P. Isolation of the outer membrane and characterization of the major outer membrane protein from Spirochaeta aurantia. J. Bacteriol. 1987, 169, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Ravaoarinoro, M.; Ciurli, C.; Toma, E.; Morisset, R. Rapid method for isolating detergent-insoluble outer membrane proteins from Pseudomonas aeruginosa. Electrophoresis 1994, 15, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Yapar, S.; Ateş, M.; Özdemir, G. Preparation and characterization of sodium lauroyl sarcosinate adsorbed on cetylpyridinium-montmorillonite as a possible antibacterial agent. Appl. Clay Sci. 2017, 150, 16–22. [Google Scholar] [CrossRef]
- Kabara, J.J.; Orth, D.S. (Eds.) Preservative-Free and Self-Preserving Cosmetics and Drugs: Principles and Practice; Marcel Dekker, Inc.: New York, NY, USA, 1997; ISBN 0-8247-9366-8. [Google Scholar]
- Krasowska, A.; Biegalska, A.; Lukaszewicz, M. Comparison of antimicrobial activity of three commercially used quaternary ammonium surfactants. Sepsis 2012, 5, 170–174. [Google Scholar]
- Publishing Group «GEOTAR-Media». Reference Book of Medicines. Dioxydin. 2015. Available online: https://www.lsgeotar.ru/dioxidin-14759.html (accessed on 31 October 2022).
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Dehnavi, A.S.; Raisi, A.; Aroujalian, A. Control size and stability of colloidal silver nanoparticles with antibacterial activity prepared by a green synthesis method. Synth. React. Inorg. Met-Org. Nano-Met. Chem. 2013, 43, 543–551. [Google Scholar] [CrossRef]
- Ruden, S.; Hilpert, K.; Berditsch, M.; Wadhwani, P.; Ulrich, A.S. Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrob. Agents Chemother. 2009, 53, 3538–3540. [Google Scholar] [CrossRef] [Green Version]
- Salouti, M.; Mirzaei, F.; Shapouri, R.; Ahangari, A. Synergistic Antibacterial Activity of Plant Peptide MBP-1 and Silver Nanoparticles Combination on Healing of Infected Wound Due to Staphylococcus aureus. Jundishapur J. Microbiol. 2016, 9, e27997. [Google Scholar] [CrossRef] [Green Version]
- Zharkova, M.S.; Golubeva, O.Y.; Orlov, D.S.; Vladimirova, E.V.; Dmitriev, A.V.; Tossi, A.; Shamova, O.V. Silver Nanoparticles Functionalized With Antimicrobial Polypeptides: Benefits and Possible Pitfalls of a Novel Anti-infective Tool. Front. Microbiol. 2021, 12, 750556. [Google Scholar] [CrossRef]
- Darwish, R.M.; Salama, A.H. Study the Effect of Conjugate Novel Ultra-Short Antimicrobial Peptide with Silver Nanoparticles against Methicillin Resistant S. aureus and ESBL E. coli. Antibiotics 2022, 11, 1024. [Google Scholar] [CrossRef]
- Rhein, L. Surfactant action on skin and hair: Cleansing and skin reactivity mechanisms. In Handbook for Cleaning/Decontamination of Surfaces; Johansson, I., Somasundaran, P., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 305–369. [Google Scholar] [CrossRef]
- Yan, H.; Hancock, R.E. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 2001, 45, 1558–1560. [Google Scholar] [CrossRef] [Green Version]
- Hanson, M.A.; Dostálová, A.; Ceroni, C.; Poidevin, M.; Kondo, S.; Lemaitre, B. Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach. eLife 2019, 8, e44341. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Baeder, D.Y.; Regoes, R.R.; Rolff, J. Combination Effects of Antimicrobial Peptides. Antimicrob. Agents Chemother. 2016, 60, 1717–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Gomez, S.; Japelj, B.; Jerala, R.; Moriyon, I.; Fernandez, A.M.; Leiva, J.; Blondelle, S.E.; Andrä, J.; Brandenburg, K.; Lohner, K.; et al. Structural features governing the activity of lactoferricin-derived peptides that act in synergy with antibiotics against Pseudomonas aeruginosa in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Huang, Y.; Chen, M.; Li, G.; Chen, Y. Functional synergy of alpha-helical antimicrobial peptides and traditional antibiotics against Gram-negative and Gram-positive bacteria in vitro and in vivo. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 197–204. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Del Prete, M.S.; Paggi, A.M.; D’Errico, M.M.; Scalise, G. Combination studies between polycationic peptides and clinically used antibiotics against Gram-positive and Gram-negative bacteria. Peptides 2000, 21, 1155–1160. [Google Scholar] [CrossRef]
- Chen, H.; Wubbolts, R.W.; Haagsman, H.P.; Veldhuizen, E.J.A. Inhibition and Eradication of Pseudomonas aeruginosa Biofilms by Host Defence Peptides. Sci. Rep. 2018, 8, 10446. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofims: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Dhar, Y.; Han, Y. Current developments in biofilm treatments: Wound and implant infections. Eng. Regen. 2020, 1, 64–75. [Google Scholar] [CrossRef]
- Devanga Ragupathi, N.K.; Veeraraghavan, B.; Karunakaran, E.; Monk, P.N. Editorial: Biofilm-mediated nosocomial infections and its association with antimicrobial resistance: Detection, prevention, and management. Front. Med. 2022, 9, 987011. [Google Scholar] [CrossRef]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob. Resist. Infect. Control. 2020, 9, 162. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, P.; Lourenço, A.; Pereira, M.O. New trends in peptide-based anti-biofilm strategies: A review of recent achievements and bioinformatic approaches. Biofouling 2012, 28, 1033–1061. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Hill, K.E.; Malic, S.; Thomas, D.W.; Williams, D.W. Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair Regen. 2011, 19, 1–9. [Google Scholar] [CrossRef]
- Morroni, G.; Simonetti, O.; Brenciani, A.; Brescini, L.; Kamysz, W.; Kamysz, E.; Neubauer, D.; Caffarini, M.; Orciani, M.; Giovanetti, E.; et al. In vitro activity of Protegrin-1, alone and in combination with clinically useful antibiotics, against Acinetobacter baumannii strains isolated from surgical wounds. Med. Microbiol. Immunol. 2019, 208, 877–883. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, X.; Jiao, Z.; Peng, J.; Zhou, L.; Yang, L.; Huang, M.; Tian, C.; Guo, G. The Antimicrobial Peptide AMP-17 Derived from Musca domestica Inhibits Biofilm Formation and Eradicates Mature Biofilm in Candida albicans. Antibiotics 2022, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Ciandrini, E.; Morroni, G.; Cirioni, O.; Kamysz, W.; Kamysz, E.; Brescini, L.; Baffone, W.; Campana, R. Synergistic combinations of antimicrobial peptides against biofilms of methicillin-resistant Staphylococcus aureus (MRSA) on polystyrene and medical devices. J. Glob. Antimicrob. Resist. 2020, 21, 203–210. [Google Scholar] [CrossRef]
- Nair, B. Clinical Trial Designs. Indian Dermatol. Online J. 2019, 10, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zemlyanoj, A.B.; Afinogenova, A.G.; Matveev, S.A. The use of antiseptics in the treatment of wounds with a high risk of infection. Bull. Pirogov Natl. Med. Surg. Cent. 2020, 15, 129–137. [Google Scholar] [CrossRef]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10 (Suppl. 1), 9–14. [Google Scholar] [CrossRef]
- Umnyakova, E.S.; Zharkova, M.S.; Berlov, M.N.; Shamova, O.V.; Kokryakov, V.N. Human antimicrobial peptides in autoimmunity. Autoimmunity 2020, 53, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Diana, J. The Dual Role of Antimicrobial Peptides in Autoimmunity. Front. Immunol. 2020, 11, 2077. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Paulmann, M.; Senyürek, I.; Steffen, H. The role of antimicrobial peptides in human skin and in skin infectious diseases. Infect. Disord. Drug Targets 2008, 8, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Cokol, M.; Chua, H.N.; Tasan, M.; Mutlu, B.; Weinstein, Z.B.; Suzuki, Y.; Nergiz, M.E.; Costanzo, M.; Baryshnikova, A.; Giaever, G.; et al. Systematic exploration of synergistic drug pairs. Mol. Syst. Biol. 2011, 7, 544. [Google Scholar] [CrossRef]
- Naish, J.; Syndercombe Court, D. (Eds.) Medical Sciences, 3rd ed.; Elsevier: Edinburgh, UK, 2019; ISBN 9780702073373. [Google Scholar]
- Lin, J.H. Bisphosphonates: A review of their pharmacokinetic properties. Bone 1996, 18, 75–85. [Google Scholar] [CrossRef]
- Amaral, L.; Martins, A.; Spengler, G.; Molnar, J. Efflux pumps of Gram-negative bacteria: What they do, how they do it, with what and how to deal with them. Front. Pharmacol. 2014, 4, 168. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-Z.; Nikaido, H.; Williams, K.E. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J. Bacteriol. 1997, 179, 6127–6132. [Google Scholar] [CrossRef] [Green Version]
- Randall, C.P.; Gupta, A.; Jackson, N.; Busse, D.; O’Neill, A.J. Silver resistance in Gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015, 70, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Ding, J.; Li, H.; Li, L.; Zhao, R.; Shen, Z.; Fan, X.; Xi, T. Effects of cations and pH on antimicrobial activity of thanatin and s-thanatin against Escherichia coli ATCC25922 and B. subtilis ATCC 21332. Curr. Microbiol. 2008, 57, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Smart, M.; Rajagopal, A.; Liu, W.K.; Ha, B.Y. Opposing effects of cationic antimicrobial peptides and divalent cations on bacterial lipopolysaccharides. Phys. Rev. E. 2017, 96, 042405. [Google Scholar] [CrossRef]
- Walkenhorst, W.F. Using adjuvants and environmental factors to modulate the activity of antimicrobial peptides. Biochim. Biophys. Acta 2016, 1858, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Walkenhorst, W.F.; Sundrud, J.N.; Laviolette, J.M. Additivity and synergy between an antimicrobial peptide and inhibitory ions. Biochim. Biophys. Acta 2014, 1838, 2234–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, C.; Scott, M.G.; Karunaratne, N.; Yan, H.; Hancock, R.E. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob. Agents Chemother. 1999, 43, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
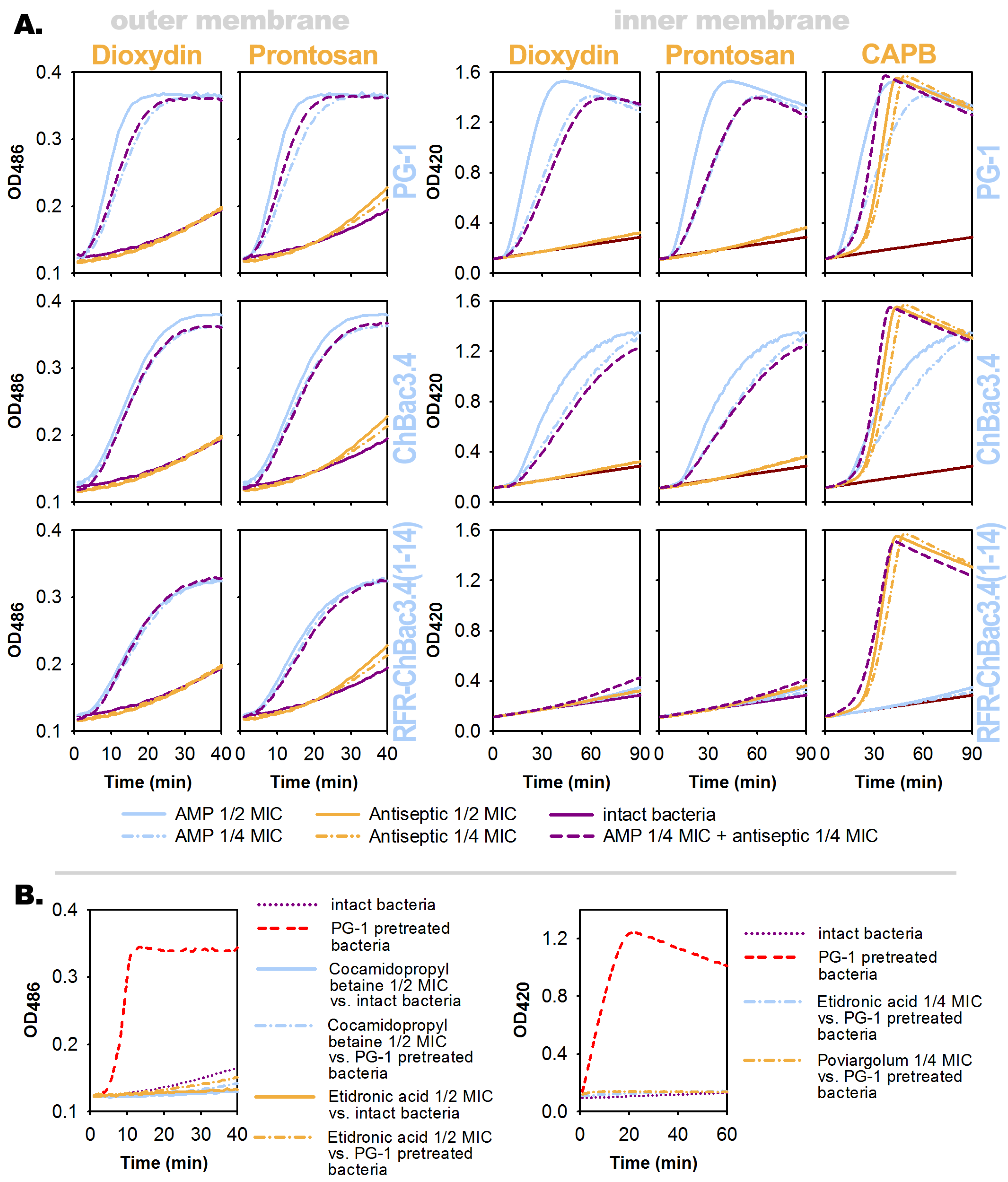
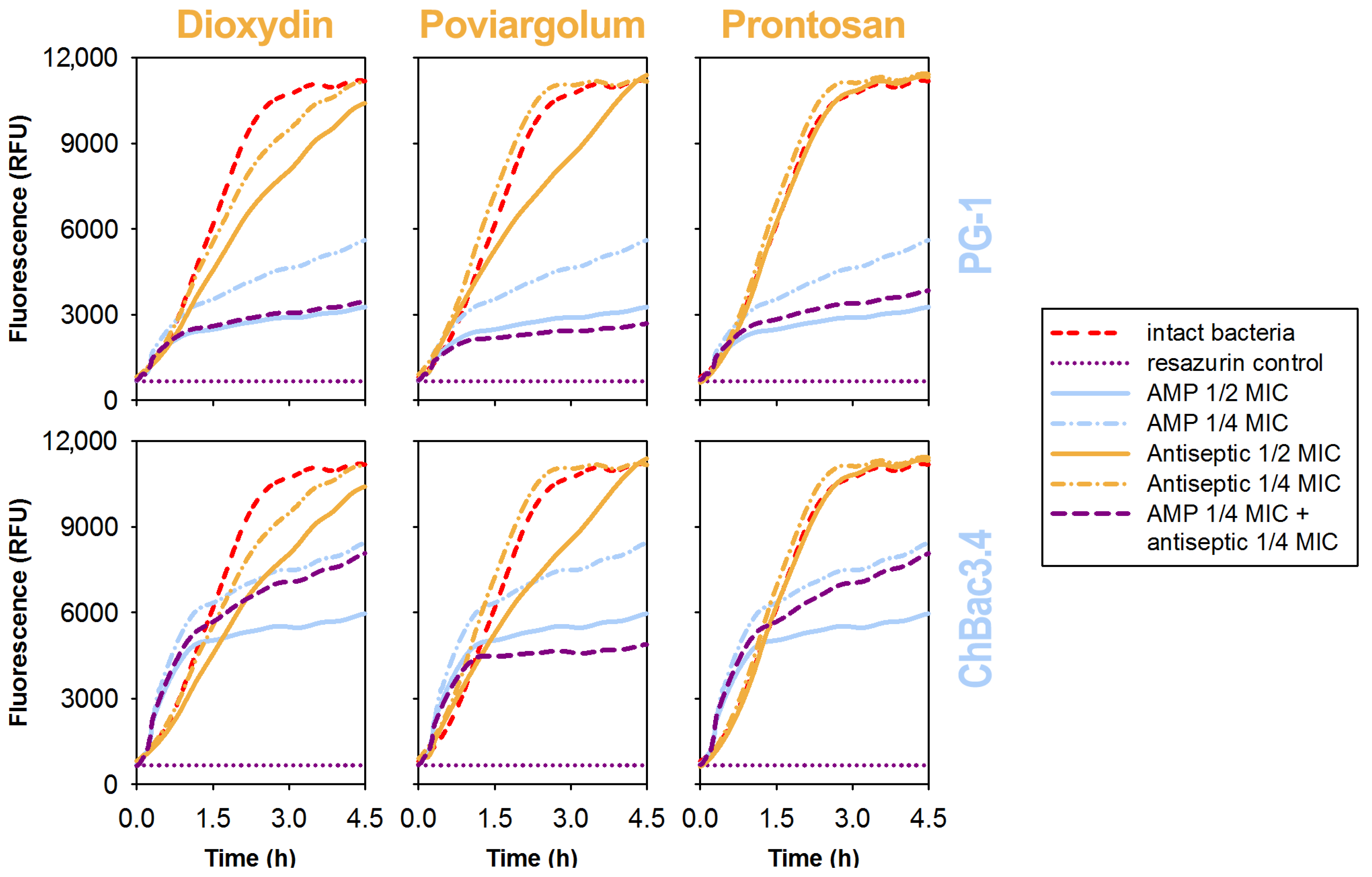
| Substances | MIC a against Drug-Resistant Bacteria | |||||
|---|---|---|---|---|---|---|
| Gram− | Gram+ | |||||
| E. coli ML-35p | E. coli ESBL 521/17 | A. baumannii 7226/16 | P. aeruginosa MDR 522/17 | K. pneumoniae ESBL 344/17 | S. aureus 1399/17 | |
| AMPs (µM) b: | ||||||
| PG-1 | 0.8 | 0.4 | 6.2 | 6.2 | 3.1 | 0.4 |
| ChBac3.4 | 1.6 | 3.1 | 6.2 | 6.2 | 3.1 | 3.1 |
| RFR-ChBac3.4 (1–14) | 3.1 | 6.2 | 12.5 | 16 | 6.2 | 6.2 |
| Antiseptics (µg/mL): | ||||||
| Dioxydin | 15.6 | 15.6 | 31.2 | 125 | 15.6 | 500 |
| Poviargolum | 39.1 | 39.1 | 39.1 | 39.1 | 39.1 | 156.2 |
| Prontosan * | 0.4 | 0.4 | 31.2 | 3.1 | 1.6 | 0.8 |
| Etidronic acid | 50,000 | 50,000 | 12,500 | 25,000 | 50,000 | 50,000 |
| Sodium hypochlorite | 5000 | 5000 | 2500 | 5000 | 5000 | 10,000 |
| Surfactants (µg/mL): | ||||||
| Cocamidopropyl betaine | >50,000 | >50,000 | 78.1 | >50,000 | 156.2 | >50,000 |
| Sodium lauroyl sarcosinate | 6250 | 12,500 | 25,000 | 50,000 | 12,500 | >50,000 |
| Minimal FICIs a of Surfactants or Antiseptics (AS) Combinations with AMPs against Drug-Resistant Bacteria | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli ML-35p (Gram–) | E. coli ESBL 521/17 (Gram–) | ||||||||||
| AMP\AS | DXD | PVG | PTS | CAPB | ETA | AMP\AS | DXD | PVG | PTS | CAPB | ETA |
| PG-1 | 1.12 | 0.5 | 0.5 | 0.31 | 0.5 | PG-1 | 1.12 | 0.56 | 0.75 | 0.25 | 0.56 |
| ChBac3.4 | 0.62 | 0.75 | 0.75 | 0.38 | 0.5 | ChBac3.4 | 0.75 | 0.38 | 1 | 0.38 | 0.25 |
| RFR-ChBac3.4 (1–14) | 0.75 | 0.62 | 0.75 | 0.38 | - | RFR-ChBac3.4 (1–14) | 0.5 | 0.5 | 0.56 | 0.38 | - |
| A. baumannii 7226/16 (Gram–) | P. aeruginosa MDR 522/17 (Gram–) | ||||||||||
| AMP\AS | DXD | PVG | PTS | CAPB | ETA | AMP\AS | DXD | PVG | PTS | CAPB | ETA |
| PG-1 | 0.75 | 0.62 | 0.62 | 0.5 | 1 | PG-1 | 1 | 0.5 | 1 | 0.56 | 1.12 |
| ChBac3.4 | 0.5 | 0.75 | 0.75 | 0.38 | 0.5 | ChBac3.4 | 1 | 0.62 | 0.75 | 0.56 | 0.75 |
| RFR-ChBac3.4 (1–14) | 1.12 | 0.38 | 0.38 | 0.5 | - | RFR-ChBac3.4 (1–14) | 0.31 | 0.25 | 0.31 | 0.25 | - |
| K. pneumoniae ESBL 344/17 (Gram–) | S. aureus 1399/17 (Gram+) | ||||||||||
| AMP\AS | DXD | PVG | PTS | CAPB | ETA | AMP\AS | DXD | PVG | PTS | CAPB | ETA |
| PG-1 | 0.62 | 0.5 | 0.75 | 0.38 | 1.12 | PG-1 | 0.62 | 0.5 | 1 | 0.31 | 0.5 |
| ChBac3.4 | 0.5 | 0.5 | 0.62 | 0.38 | 1 | ChBac3.4 | 0.75 | 0.5 | 0.38 | 0.5 | 0.38 |
| RFR-ChBac3.4 (1–14) | 0.62 | 0.62 | 0.5 | 0.38 | - | RFR-ChBac3.4 (1–14) | 0.5 | 0.25 | 0.75 | 0.12 | - |
| Substances | Effects of Sub-MIC Concentrations on Biofilm Formation | Toxic Effects against Human Cells | ||
|---|---|---|---|---|
| MECBF a Partially Inhibiting Bacterial Biofilms | MECH a of Hemolysis of Human Erythrocytes | MECF a of Cytotoxic Action toward Normal Human Dermal Fibroblasts | ||
| A. baumannii 7226/16 | P. aeruginosa MDR 522/17 | |||
| AMPs: | MIC ratio | µM b | ||
| PG-1 | 1/32 | 1/64 | 0.5 | 5 |
| ChBac3.4 | 1/16 | 1/64 | >40 {80} | 40 |
| RFR-ChBac3.4(1–14) | 1 | 1/128 | >50 {100} | >40 {80} |
| Antiseptics & surfactants: | MIC ratio | µg/mL | ||
| Dioxydin | 1/16 | 1/16 | 500 | 500 |
| Poviargolum | 1 | 1/4 | 500 | 12.5 |
| Prontosan | 1/16 | 1/256 | 1.6 * | 3.1 * |
| Etidronic acid | 1/128 | 1/32 | 5000 | <2500 |
| Cocamidopropyl betaine | 1/32 | 1/512 | 1 | 12.5 |
| Minimal FECIs a of Surfactants or Antiseptics (AS) Combinations with AMPs Partially Inhibiting Bacterial Biofilms’ Formation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. baumannii 7226/16 (Gram–) | P. aeruginosa MDR 522/17 (Gram–) | ||||||||||
| AMP\AS | DXD | PVG | PTS | CAPB | ETA | AMP\AS | DXD | PVG | PTS | CAPB | ETA |
| PG-1 | 1.12 | 1.12 | 0.25 | 0.5 | 1.12 | PG-1 | 1 | 0.25 | 0.12 | 0.12 | 2 |
| ChBac3.4 | 1 | 1 | 0.5 | 0.5 | 1.12 | ChBac3.4 | 1.12 | 1 | 0.12 | 0.5 | 2 |
| RFR-ChBac3.4 (1–14) | 1.12 | 1.12 | 1 | 1 | - | RFR-ChBac3.4 (1–14) | 0.5 | 1 | 0.12 | 0.5 | - |
| AMP (A) | Hemolytic Action of (½ MEC A + ½ MEC B) and (¼ MEC A + ¼ MEC B) Combinations toward Human Erythrocytes and Corresponding FECIs a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiseptic (B) | |||||||||||||||
| Dioxydin | Poviargolum | Prontosan | Cocamidopropyl Betaine | Etidronic Acid | |||||||||||
| ½A & ½B | ¼A & ¼B | FECI | ½A & ½B | ¼A & ¼B | FECI | ½A & ½B | ¼A & ¼B | FECI | ½A & ½B | ¼A & ¼B | FECI | ½A & ½B | ¼A & ¼B | FECI | |
| PG-1 | + + − [+] | − − − [−] | 1.0 | + + − [+] | − − − [−] | 1.0 | + + + [+] | + + + [+] | 0.5 | + + + [+] | + − − [−] | 1.0 | + + + [+] | − − − [−] | 1.0 |
| ChBac3.4 | − − − [−] | − − − [−] | >1.0 | + + + [+] | − − − [−] | 1.0 | + + − [+] | − − − [−] | 1.0 | + + − [+] | − − − [−] | 1.0 | − − − [−] | − − − [−] | >1.0 |
| RFR-ChBac3.4 (1–14) | + − − [−] | + − − [−] | >1.0 | − − − [−] | − − − [−] | >1.0 | + + + [+] | − − − [−] | 1.0 | + + + [+] | + − − [−] | 1.0 | |||
| AMP (A) | Toxicity of (½ MEC A + ½ MEC B) and (¼ MEC A + ¼ MEC B) Combinations toward Human Dermal Fibroblasts and Corresponding FECIs a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiseptic (B) | ||||||||||||
| Dioxydin | Poviargolum | Prontosan | Cocamidopropyl Betaine | |||||||||
| ½ A & ½ B | ¼ A & ¼ B | FECI | ½ A & ½ B | ¼ A & ¼ B | FECI | ½ A & ½ B | ¼ A & ¼ B | FECI | ½ A & ½ B | ¼ A & ¼ B | FECI | |
| PG-1 | − − − [−] | − − − [−] | >1.0 | + + + [+] | + − − [−] | 1.0 | + + − [−] | − − − [−] | 1.0 | + + + [+] | + − − [−] | 1.0 |
| ChBac3.4 | − − − [−] | − − − [−] | >1.0 | + + + [+] | + − − [−] | 1.0 | + + + [+] | + − − [−] | 1.0 | + + + [+] | − − − [−] | 1.0 |
| RFR-ChBac3.4 (1–14) | + − − [−] | + − − [−] | >1.0 | + + + [+] | + − − [−] | 1.0 | + + + [+] | + − − [−] | 1.0 | + − − [−] | + − − [−] | >1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zharkova, M.S.; Komlev, A.S.; Filatenkova, T.A.; Sukhareva, M.S.; Vladimirova, E.V.; Trulioff, A.S.; Orlov, D.S.; Dmitriev, A.V.; Afinogenova, A.G.; Spiridonova, A.A.; et al. Combined Use of Antimicrobial Peptides with Antiseptics against Multidrug-Resistant Bacteria: Pros and Cons. Pharmaceutics 2023, 15, 291. https://doi.org/10.3390/pharmaceutics15010291
Zharkova MS, Komlev AS, Filatenkova TA, Sukhareva MS, Vladimirova EV, Trulioff AS, Orlov DS, Dmitriev AV, Afinogenova AG, Spiridonova AA, et al. Combined Use of Antimicrobial Peptides with Antiseptics against Multidrug-Resistant Bacteria: Pros and Cons. Pharmaceutics. 2023; 15(1):291. https://doi.org/10.3390/pharmaceutics15010291
Chicago/Turabian StyleZharkova, Maria S., Aleksey S. Komlev, Tatiana A. Filatenkova, Maria S. Sukhareva, Elizaveta V. Vladimirova, Andrey S. Trulioff, Dmitriy S. Orlov, Alexander V. Dmitriev, Anna G. Afinogenova, Anna A. Spiridonova, and et al. 2023. "Combined Use of Antimicrobial Peptides with Antiseptics against Multidrug-Resistant Bacteria: Pros and Cons" Pharmaceutics 15, no. 1: 291. https://doi.org/10.3390/pharmaceutics15010291
APA StyleZharkova, M. S., Komlev, A. S., Filatenkova, T. A., Sukhareva, M. S., Vladimirova, E. V., Trulioff, A. S., Orlov, D. S., Dmitriev, A. V., Afinogenova, A. G., Spiridonova, A. A., & Shamova, O. V. (2023). Combined Use of Antimicrobial Peptides with Antiseptics against Multidrug-Resistant Bacteria: Pros and Cons. Pharmaceutics, 15(1), 291. https://doi.org/10.3390/pharmaceutics15010291






