Preparation and Evaluation of Vitamin D3 Supplementation as Transdermal Film-Forming Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. HPLC Analysis Method
Chromatographic Conditions
2.4. Formulation
2.5. General Method of Preparation
2.6. Preparation of the Primary Trial Formulations (PTFs)
2.7. Characterization of PTFs
2.7.1. Drying Time Stickiness and Flexibility
2.7.2. Cosmetic Appearance
2.7.3. Adhesiveness
- Low: Not attached/removed without effort.
- Good: Moderate attachment/removed with some effort.
- High: Strong attachment/removed with effort.
2.7.4. Flexibility
- Low: Bent, rolled, and twisted with effort and could be broken or cracked easily.
- Good: Bent, rolled, and twisted with little effort and without breaking.
- High: Bent, rolled, and twisted easily without effort and without breaking.
2.7.5. pH, Viscosity, and Elasticity
- η2: Viscosity of water;
- : Density of tested liquid;
- : Density of water.
2.8. Vitamin D3 Loading
2.9. Release and Permeation Studies
2.10. In Vitro Permeation Study Using Strat M® Membrane
2.11. Stability Studies
3. Results and Discussion
3.1. HPLC Analysis
Linearity, Range, and Sensitivity
3.2. Formulation Development
3.2.1. The Primary Trial Formulations (PTFs)
3.2.2. Successful Trial Formulation STFs
3.3. Vitamin D Loading
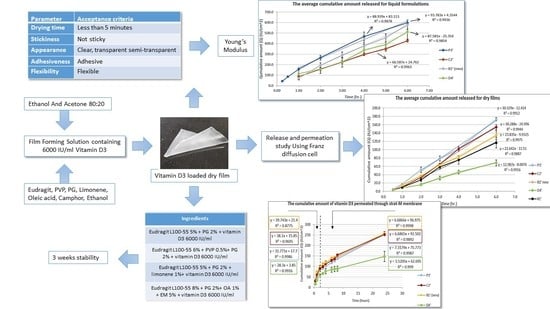
3.4. In Vitro Release Study Using Franz Diffusion Cell
3.5. In Vitro Permeation Study Using Strat-M® Membrane
3.6. Stability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and transdermal drug delivery: From simple potions to smart technologies. Bentham Sci. 2019, 16, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tanner, T.; Marks, R. Delivering drugs by the transdermal route: Review and comment. Ski. Res. Technol. 2008, 14, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.S. Handbook of Non-Invasive Drug Delivery Systems, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 4–36. [Google Scholar]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 427–432. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; De Sousa, V.P. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo-in vitro correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, K.; Paul, S.; Singh, S.; Singh, S.; Jain, S.K. A Mechanistic study to determine the structural similarities between artificial membrane Strat-MTM and biological membranes and its application to carry out skin permeation study of amphotericin B nanoformulations. AAPS PharmSciTech 2018, 19, 1606–1624. [Google Scholar] [CrossRef]
- Haq, A.; Dorrani, M.; Goodyear, B.; Joshi, V.; Michniak-Kohn, B. Membrane properties for permeability testing: Skin versus synthetic membranes. Int. J. Pharm. 2018, 539, 58–64. [Google Scholar] [CrossRef]
- Osmałek, T.; Milanowski, B.; Froelich, A.; Górska, S.; Białas, W.; Szybowicz, M.; Kapela, M. Novel organogels for topical delivery of naproxen: Design, physicochemical characteristics and in vitro drug permeation. Pharm. Dev. 2017, 22, 521–536. [Google Scholar] [CrossRef]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-M™. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef]
- Zsikó, S.; Cutcher, K.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Baki, G.; Csányi, E.; Berkó, S. Nanostructured lipid carrier gel for the dermal application of lidocaine: Comparison of skin penetration testing methods. Pharmaceutics 2019, 11, 310. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, L.; Seabra, A.; Pelegrino, M.; De Araujo, D.; Bernardes, J.; Haddad, P. Superparamagnetic iron oxide nanoparticles dispersed in pluronic F127 hydrogel: Potential uses in topical applications. RSC Adv. 2017, 7, 14496–14503. [Google Scholar] [CrossRef] [Green Version]
- Uchida, T.; Nishioka, K.; Motoki, A.; Yakumaru, M.; Sano, T.; Todo, H.; Sugibayashi, K. Effect of Esters on the Permeation of Chemicals with Different Polarities through Synthetic Artificial Membranes Using a High-Throughput Diffusion Cell Array. Chem. Pharm. Bull. 2016, 64, 1597–1606. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health. Am. J. Clin. Nutr. 2018, 87, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Alsaqr, A.; Rasoully, M.; Musteata, F.M. Investigating transdermal delivery of vitamin D3. AAPS PharmSciTech 2015, 16, 963–972. [Google Scholar] [CrossRef]
- Holick, M.F. Cancer, sunlight and vitamin D. J. Clin. Transl. Endocrinol. 2014, 1, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Zittermann, A.; Koerfer, R. Vitamin D in the prevention and treatment of coronary heart disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 752–757. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M. Vitamin D effects on lung immunity and respiratory diseases. In Vitamins and Hormones; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 217–232. [Google Scholar]
- Murakami, M.; Morita, Y.; Koide, T.; Saito, H.; Tanimoto, T. Cholecalciferol reference standard (control 031) of national institute of health sciences. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku 2003, 121, 65–67. [Google Scholar]
- Maekawa, K.; Iwata, M.; Koide, T.; Saito, H.; Tanimoto, T.; Okada, S. Cholecalciferol reference standard (control 001) of national institute of health sciences. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku 2001, 119, 107–109. [Google Scholar]
- National Institution of Health. Vitamin D Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 17 December 2022).
- Von Brenken, S.; Jensen, J.M.; Fartasch, M.; Proksch, E. Topical vitamin D3 derivatives impair the epidermal permeability barrier in normal mouse skin. Dermatology 1997, 194, 151–156. [Google Scholar] [CrossRef]
- D’Angelo Costa, G.M.; Sales de Oliveira Pinto, C.A.; Rodrigues Leite-Silva, V.; Rolim Baby, A.; Robles Velasco, M.V. Is vitamin D3 transdermal formulation feasible? an ex vivo skin retention and permeation. AAPS PharmSciTech 2018, 19, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Schwarzrock, T.; Cleary, G. Transdermal Delivery Formulation; World Intellectual Property Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Sawarkar, S.; Ashtekar, A. Transdermal vitamin D supplementation—A potential vitamin D deficiency treatment. J. Cosmet. Dermatol. 2020, 19, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.D.; Tran, P.H.L. Controlled release film forming systems in drug delivery. Pharmaceutics 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Baber, N. Validation of analytical procedures: Text and methodology Q2(R1). Br. J. Clin. Pharmacol. 1994, 37, 447–460. [Google Scholar]
- United States Pharmacopeia and National Formulary (USP 30-NF 25). United States Pharmacopeial Convention. <467> Residual solvents. 2007.
- Paradkar, M.; Thakkar, V.; Soni, T.; Gandhi, T.; Gohel, M. Formulation and evaluation of clotrimazole transdermal spray. Drug Dev. Ind. Pharm. 2015, 41, 1718–1725. [Google Scholar] [CrossRef]
- Lu, W.; Luo, H.; Wu, Y.; Zhu, Z.; Wang, H. Preparation and characterization of a metered dose transdermal spray for testosterone. Acta Pharm. Sin. B 2013, 3, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.; Kumar, P.; Thakkar, H.P. Lopinavir metered-dose transdermal spray through microporated skin: Permeation enhancement to achieve therapeutic needs. J. Drug Deliv. Sci. Technol. 2015, 29, 173–180. [Google Scholar] [CrossRef]
- Gohel, M.C.; Nagori, S.A. Fabrication of modified transport fluconazole transdermal spray containing ethyl cellulose and eudragit® RS100 as film formers. AAPS PharmSciTech 2009, 10, 684–691. [Google Scholar] [CrossRef]
- Mori, N.M.; Patel, P.; Sheth, N.R.; Rathod, L.V.; Ashara, K.C. Fabrication and characterization of film-forming voriconazole transdermal spray for the treatment of fungal infection. Sciencedirect 2017, 55, 41–51. [Google Scholar] [CrossRef]
- Hearn, E.J. Mechanics of materials. In Structural and Stress Analysis, 3rd ed.; Butterworrth Heinemann: Oxford, UK, 1997; p. 481. [Google Scholar]
- Fauth, C.; Wiedersberg, S.; Neubert, R.H.H.; Dittgen, M. Adhesive backing foil interactions affecting the elasticity, adhesion strength of laminates, and how to interpret these properties of branded transdermal patches. Drug Dev. Ind. Pharm. 2002, 28, 1251–1259. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 7th ed.; Buffer solutions; Council of Europe: Strasbourg, France, 2011; p. 492.
- Zhan, X.; Mao, Z.; Chen, S.; Chen, S.; Wang, L. Formulation and evaluation of transdermal drug-delivery system of isosorbide dinitrate. Braz. J. Pharm. Sci. 2015, 51, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipient, 6th ed.; Pharmacutical Press: London, UK, 2009; pp. 7–18. [Google Scholar]
- Poplavko, Y. Electronic Materials, Principle ans Applied Science; Dos Santos, K., Ed.; Elsevier: Gurgaon, India, 2019; pp. 71–93. [Google Scholar]
- Edwards, A.; Qi, S.; Liu, F.; Brown, M.; McAuley, W. Rationalising polymer selection for supersaturated film forming systems produced by an aerosol spray for the transdermal delivery of methylphenidate. Eur. J. Pharm. Biopharm. 2017, 114, 164–174. [Google Scholar] [CrossRef]
- Ibrahim, S.A. Spray-on transdermal drug delivery systems. Expert Opin. Drug Deliv. 2015, 12, 195–205. [Google Scholar] [CrossRef]
- Pünnel, L.C.; Lunter, D.J. Film-forming systems for dermal drug delivery. Pharmaceutics 2021, 13, 932. [Google Scholar] [CrossRef]
- De Jaeghere, F.; Allémann, E.; Doelker, E.; Gurny, R.; Cerny, R.; Galli, B.; Steulet, A.F.; Müller, I.; Schütz, H. pH-dependent dissolving nano- and microparticles for improved peroral delivery of a highly lipophilic compound in dogs. AAPS PharmSciTech 2001, 3, 92–99. [Google Scholar] [CrossRef]








| Parameter | Acceptance Criteria |
|---|---|
| Drying time | Less than 5 min [31] |
| Stickiness | Not sticky [31,32,33] |
| Cosmetic appearance | Clear, transparent or semi-transparent [31,32,33,34,35] |
| Adhesiveness | Adhesive [34,35] |
| Flexibility | Flexible [31,33,34,35] |
| No. | Eudragit | PVP | PEG | PG | OA | IPM | SLS | limonene | Eucalyptol | Transcutol | EM | Ethanol | Ethanol:Acetone (80:20) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L100-55 | L100 | S100 | |||||||||||||
| X1 | 5% | - | - | - | - | - | - | - | - | - | - | - | - | Up to volume | - |
| X2 | - | 5% | - | - | - | - | - | - | - | - | - | - | - | - | |
| X3 | - | - | 5% | - | - | - | - | - | - | - | - | - | - | - | |
| X4 | - | - | - | 5% | - | - | - | - | - | - | - | - | - | - | |
| XP1 | 5% | - | - | 5% | 2% | - | - | - | - | - | - | - | - | - | |
| XP2 | 5% | - | - | 5% | - | - | 2% | - | - | - | - | - | - | - | |
| XP3 | 5% | - | - | 5% | - | 2% | - | - | - | - | - | - | - | - | |
| XP4 | - | 5% | - | - | 2% | - | - | - | - | - | - | - | - | - | |
| XP5 | - | 5% | - | - | - | 2% | - | - | - | - | - | - | - | - | |
| XP6 | - | 5% | - | - | - | - | 2% | - | - | - | - | - | - | - | |
| XP7 | - | - | 5% | - | 2% | - | - | - | - | - | - | - | - | - | |
| XP8 | - | - | 5% | - | - | 2% | - | - | - | - | - | - | - | - | |
| XP9 | - | - | 5% | - | - | - | 2% | - | - | - | - | - | - | - | |
| 1 | 5% | - | - | - | - | - | - | - | - | - | - | - | - | - | Up to volume |
| 2 | - | 5% | - | - | - | - | - | - | - | - | - | - | - | - | |
| 3 | - | - | 5% | - | - | - | - | - | - | - | - | - | - | - | |
| 4 | - | - | - | 5% | - | - | - | - | - | - | - | - | - | - | |
| P1 | 5% | - | - | - | 2% | - | - | - | - | - | - | - | - | - | |
| P2 | 5% | - | - | - | - | - | 2% | - | - | - | - | - | - | - | |
| P3 | 5% | - | - | - | - | 2% | - | - | - | - | - | - | - | - | |
| P4 | - | 5% | - | - | 2% | - | - | - | - | - | - | - | - | - | |
| P5 | - | 5% | - | - | - | 2% | - | - | - | - | - | - | - | - | |
| P6 | - | 5% | - | - | - | - | 2% | - | - | - | - | - | - | - | |
| P7 | - | - | 5% | - | 2% | - | - | - | - | - | - | - | - | - | |
| P8 | - | - | 5% | - | - | 2% | - | - | - | - | - | - | - | - | |
| P9 | - | - | 5% | - | - | - | 2% | - | - | - | - | - | - | - | |
| A1 | 8% | - | - | - | - | 2% | 1% | - | - | - | - | - | - | - | |
| A2 | 8% | - | - | - | 2% | - | 1% | - | - | - | - | - | - | - | |
| A3 | 8% | - | - | - | 2% | 2% | - | - | - | - | - | - | - | - | |
| A4 | 8% | - | - | - | - | 2% | - | 2% | - | - | - | - | - | - | |
| A5 | 8% | - | - | - | 2% | - | - | 2% | - | - | - | - | - | - | |
| A6 | - | 8% | - | - | 2% | - | - | 2% | - | - | - | - | - | - | |
| A7 | - | 5% | - | - | 2% | - | 1% | - | - | - | - | - | - | - | |
| B1 | 6% | - | - | 1% | - | 2% | - | - | - | - | - | - | - | - | |
| B1 new | 6% | - | - | 0.5% | - | 2% | - | - | - | - | - | - | - | - | |
| B2 | 4% | 4% | - | - | 2% | 2% | - | - | - | - | - | - | - | - | |
| B3 | 6% | 2% | - | - | 2% | 2% | - | - | - | - | - | - | - | - | |
| B4 | 7% | 1% | - | - | 2% | 2% | - | - | - | - | - | - | - | - | |
| C1 | 5% | - | - | - | - | 2% | - | - | 1% | - | - | - | - | - | |
| C2 | 5% | - | - | - | - | 2% | - | - | - | 1% | - | - | - | - | |
| C3 | 5% | - | - | - | - | 2% | - | - | - | - | 1% | - | - | - | |
| C4 | 5% | - | - | - | - | 2% | - | - | - | - | - | 1% | - | - | |
| D1 | 8% | - | - | - | 2% | - | 1% | - | - | - | - | - | 2% | - | |
| D2 | 8% | - | - | - | 2% | - | 1% | - | - | - | - | - | 5% | - | |
| D3 | 5% | - | - | - | - | 2% | - | - | - | 1% | - | - | 5% | - | |
| D4 | 8% | - | - | - | - | 2% | 1% | - | - | - | - | - | 5% | - | |
| D5 | 6% | - | - | 1% | - | 2% | - | - | - | - | - | - | 5% | - | |
| No. | Drying Time | Stickiness | Cosmetic Appearance (Thin Film) | Adhesiveness | Flexibility | Pass/Fail |
|---|---|---|---|---|---|---|
| X1 | 5 min 15 s | Non-sticky | Transparent, clear | Low | Not flexible | Fail |
| X2 | 4 min 30 s | Not sticky | White | Low | Not flexible | Fail |
| X3 | 6 min 45 s | Not sticky | White | Low | Not flexible | Fail |
| X4 | 4 min | Not sticky | Transparent, clear | High | N.A. | Fail |
| XP1 | 5 min 45 s | Non-sticky | Transparent | High | High | Fail |
| XP2 | 5 min 45 s | Non-sticky | Transparent | Low | Not flexible | Fail |
| XP3 | 6 min | Non-sticky | Transparent | Good | Good | Fail |
| XP4 | 5 min 45 s | Non-sticky | Transparent | Low | Not flexible | Fail |
| XP5 | 5 min 45 s | Non-sticky | Transparent | Low | Not flexible | Fail |
| XP6 | 5 min 30 s | Non-sticky | Transparent | Low | Not flexible | Fail |
| XP7 | 5 min | Non-sticky | Transparent | Low | Low | Fail |
| XP8 | 5 min 15 s | Non-sticky | Transparent | Low | Not flexible | Fail |
| XP9 | 5 min | Non-sticky | Transparent | Low | Low | Fail |
| P1 | 4 min and 30 s | Non-sticky | Transparent | High | High | Pass |
| P2 | 4 min and 30 s | Non-sticky | Transparent | Low | Not flexible | Fail |
| P3 | 4 min and 30 s | Non-sticky | Transparent | Good | Good | Pass |
| P4 | 3 min and 45 s | Non-sticky | Transparent | Low | Not flexible | Fail |
| P5 | 3 min and 45 s | Non-sticky | Transparent | Low | Not flexible | Fail |
| P6 | 3 min and 30 s | Non-sticky | Transparent | Low | Not flexible | Fail |
| P7 | 3 min | Non-sticky | Transparent | Low | Low | Fail |
| P8 | 3 min | Non-sticky | Transparent | Low | Not flexible | Fail |
| P9 | 3 min | Non-sticky | Transparent | Low | Low | Fail |
| A1 | 5 min 30 s | Non-sticky | Transparent | Good | Not flexible | Fail |
| A2 | 3 min 30 s | Non-sticky | Transparent | Good | High | Pass |
| A3 | 5 min | Sticky | Transparent | High | High | Fail |
| A4 | 4 min 14 s | Non-sticky | Transparent | Good | Low | Fail |
| A5 | 4 min | Non-sticky | Transparent | Good | Good | Fail |
| A6 | 2 min 45 s | Not sticky | Transparent | Low | Good | Fail |
| A7 | 3 min 30 s | Not sticky | Transparent | Low | Low | Fail |
| B1 | 3 min 15 s | Not sticky | Transparent, | Good | Good | Pass |
| B1 new | 3 min 15 s | Not sticky | Transparent, | Good | Good | Pass |
| B2 | 4 min 15 s | Not sticky | Transparent, not smooth | Good | High | Fail |
| B3 | 3 min 15 s | Not sticky | Transparent | Good | Good | Pass |
| B4 | 3 min | Not sticky | Transparent | High | High | Pass |
| C1 | 3 min 15 s | Sticky | Semi-Transparent | Good | Good | Fail |
| C2 | 3 min 30 s | Not sticky | Transparent | Good | Good | Pass |
| C3 | 3 min 30 s | Not sticky | Transparent | Good | Good | Pass |
| C4 | 4 min | Not sticky | Transparent | Good | Good | Pass |
| D1 | 3 min 30 s | Non-sticky | Transparent | Good | Good | Pass |
| D2 | 4 min 30 s | Slightly sticky | Transparent | Good | Good | Fail |
| D3 | 3 min 45 s | Very sticky | Transparent | Good | Good | Fail |
| D4 | 3 min 45 s | Non-sticky | Transparent | Good | Good | Pass |
| D5 | 4 min 15 s | Slightly sticky | Transparent | Good | Good | Fail |
| No. | Ingredients Dissolved in Ethanol and Acetone (80:20) | pH | Viscosity (CP) | Young’s Modulus kPa |
|---|---|---|---|---|
| P1 | Eudragit L100-55 5% + PEG 2% | 3.5 ± 0.1 | 5.1 ± 0.0 | N.A. |
| P3 | Eudragit L100-55 5% + PG 2% | 3.9 ± 0.1 | 5.5 ± 0.0 | 1114.6 |
| A2 | Eudragit L100-55 8% + PEG 2% + OA 1% | 3.6 ± 0.1 | 8.2 ± 0.1 | 87.4 |
| B1 | Eudragit L100-55 6% + PVP 1% + PG 2% | 3.5 ± 0.1 | 8.7 ± 0.0 | 1459.0 |
| B3 | Eudragit L100 2% + Eudragit L100-55 6% + PG 2% + PEG 2% | 3.4 ± 0.1 | 12.1 ± 0.1 | N.A. |
| B4 | Eudragit L100 1% + Eudragit L100-55 7% + PG 2% + PEG 2% | 3.4 ± 0.1 | 18.9 ± 0.0 | 82.9 |
| C2 | Eudragit L100-55 5% + PG 2% + limonene 1% | 4.1 ± 0.1 | 5.5 ± 0.0 | 12,598.0 |
| C3 | Eudragit L100-55 5% + PG 2% + Eucalyptol 1% | 5.0 ± 0.1 | 5.6 ± 0.0 | 3785.7 |
| C4 | Eudragit L100-55 5% + PG 2% + transcutol 1% | 4.2 ± 0.1 | 5.8 ± 0.0 | 705.3 |
| D1 | Eudragit L100-55 8% + PEG 2% + OA 1% + EM 2% | 4.7 ± 0.1 | 14.3 ± 0.1 | 264.0 |
| D4 | Eudragit L100-55 8% + PG 2% + OA 1% + EM 5% | 5.1 ± 0.1 | 11.1 ± 0.2 | 55.5 |
| Ingredients | Drying Time | Stickiness | Cosmetic Appearance | Adhesiveness | Flexibility | |
|---|---|---|---|---|---|---|
| P3′ | Eudragit L100-55 5% + PG 2% + vitamin D3 6000 IU/mL | 3 min 30 s | Non-sticky | Transparent | Good | Good |
| B1′ (new) | Eudragit L100-55 6% + PVP 0.5% + PG 2% + vitamin D3 6000 IU/mL | 3 min 45 s | Non-sticky | Transparent | Good | Good |
| C2′ | Eudragit L100-55 5% + PG 2% + limonene 1% + vitamin D3 6000 IU/mL | 3 min 30 s | Non-sticky | Transparent | Good | Good |
| D4′ | Eudragit L100-55 8% + PG 2% + OA 1% + EM 5% + vitamin D3 6000 IU/mL | 3 min 45 s | Non-sticky | Transparent | Good | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittaneh, M.; Qurt, M.; Malkieh, N.; Naseef, H.; Muqedi, R. Preparation and Evaluation of Vitamin D3 Supplementation as Transdermal Film-Forming Solution. Pharmaceutics 2023, 15, 39. https://doi.org/10.3390/pharmaceutics15010039
Kittaneh M, Qurt M, Malkieh N, Naseef H, Muqedi R. Preparation and Evaluation of Vitamin D3 Supplementation as Transdermal Film-Forming Solution. Pharmaceutics. 2023; 15(1):39. https://doi.org/10.3390/pharmaceutics15010039
Chicago/Turabian StyleKittaneh, Majd, Moammal Qurt, Numan Malkieh, Hani Naseef, and Ramzi Muqedi. 2023. "Preparation and Evaluation of Vitamin D3 Supplementation as Transdermal Film-Forming Solution" Pharmaceutics 15, no. 1: 39. https://doi.org/10.3390/pharmaceutics15010039
APA StyleKittaneh, M., Qurt, M., Malkieh, N., Naseef, H., & Muqedi, R. (2023). Preparation and Evaluation of Vitamin D3 Supplementation as Transdermal Film-Forming Solution. Pharmaceutics, 15(1), 39. https://doi.org/10.3390/pharmaceutics15010039