Delta Opioid Peptide Targets Brain Microvascular Endothelial Cells Reducing Apoptosis to Relieve Hypoxia-Ischemic/Reperfusion Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatment
2.2. Intracerebroventricular Administration
2.3. MCAO/R Model
2.4. Evaluation of Neurologic Deficit Scores
2.5. Infarct Volume Assessment
2.6. Cell Cultures
2.7. TdT-Mediated dUTP Nick-End Labeling (TUNEL) Assay
2.8. Oxygen Glucose Deprivation/Reperfusion (OGD/R) and Drug Treatment
2.9. Cell Viability Assay
2.10. SiRNA Transfection
2.11. Immunofluorescence
2.12. Western Blotting
2.13. Statistical Analysis
3. Results
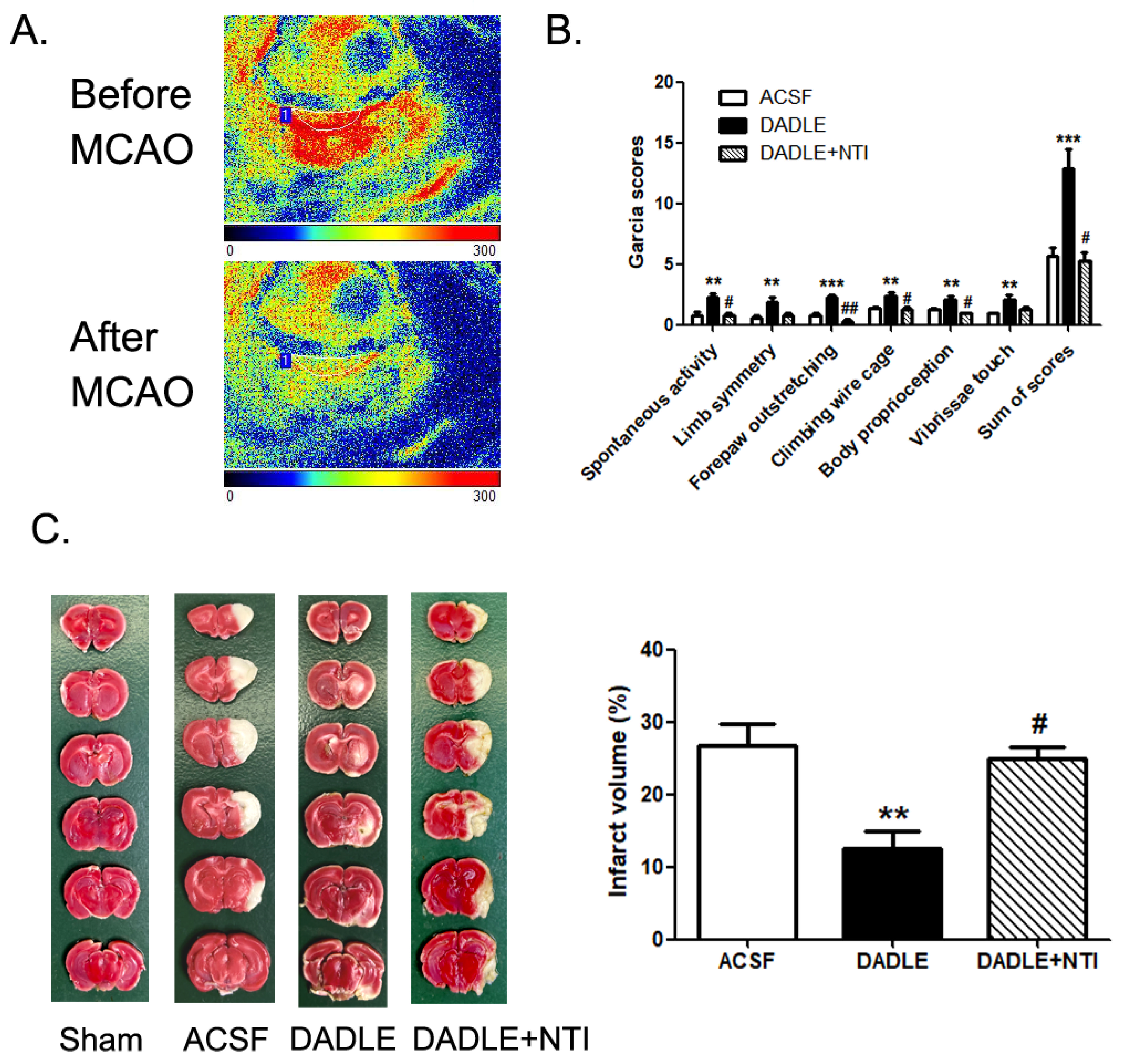
3.1. The Administration of Delta Opioid Peptide DADLE Reduces MCAO/R-Induced Brain Infarct Volume and Improves Neurologic Function
3.2. MCAO/R-Induced Apoptosis Mostly Appears in Cerebral Microvascular Endothelial Cells but Not in Neurons and Glia
3.3. Primarily Cultured Brain Microvascular Endothelial Cells (BMECs) Express Endogenous Delta Opioid Receptors
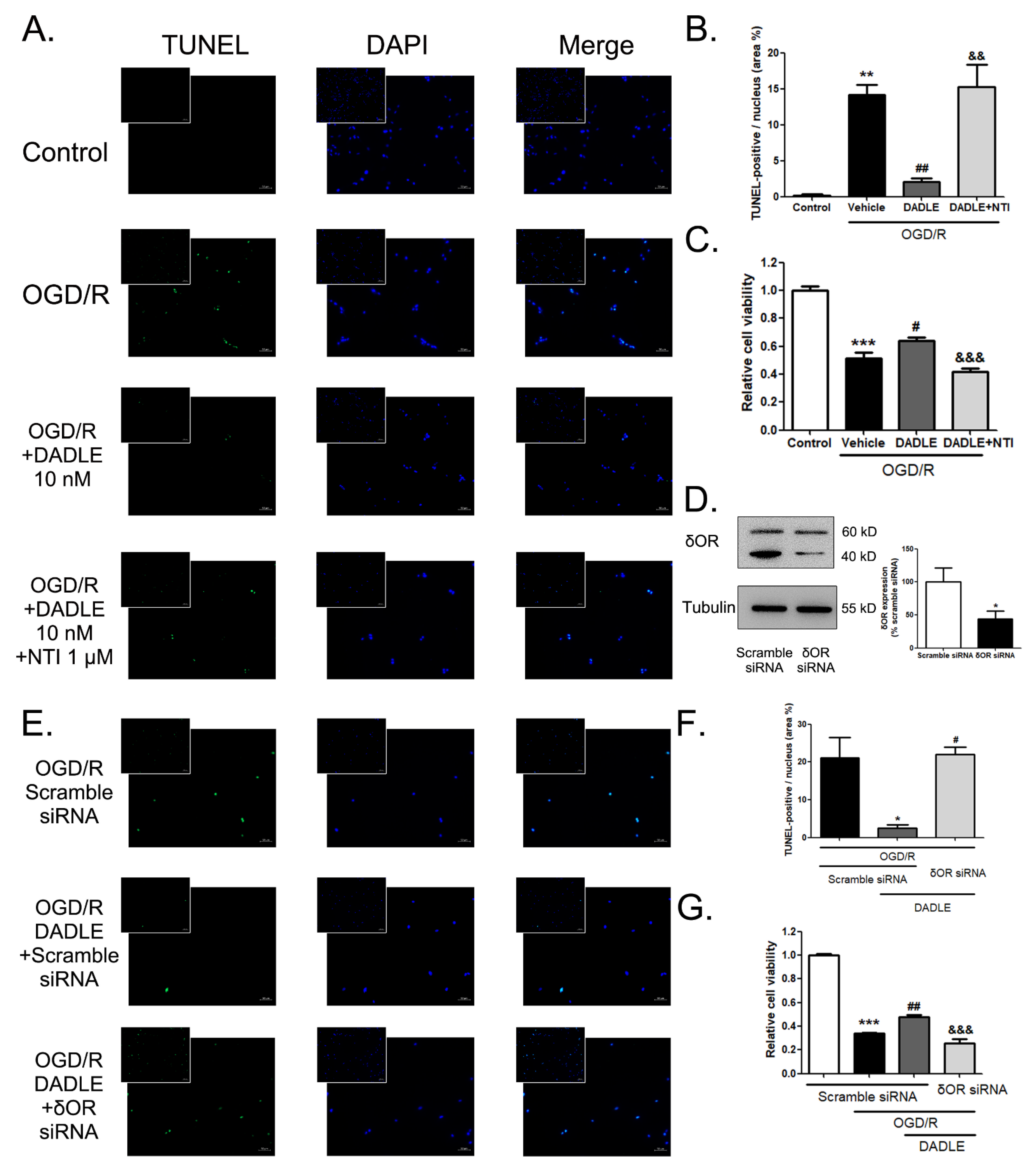
3.4. DALDE Could Decrease OGD/R-Induced Apoptosis and Strengthen the Cell Viability of BMECs by Reducing the Cleaved Caspase-9 and the Ratio of Bax and Bcl-2
3.5. Both δOR Antagonist NTI and the Interference of δOR Could Block the Anti-Apoptosis and Pro-Survival Effects of DADLE on the OGD/R-Damaged BMECs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hankey, G.J. Stroke. Lancet 2016, 389, 641–654. [Google Scholar] [CrossRef]
- Boldsen, J.K.; Engedal, T.S.; Pedraza, S.; Cho, T.-H.; Thomalla, G.; Nighoghossian, N.; Baron, J.-C.; Fiehler, J.; Østergaard, L.; Mouridsen, K.; et al. Better Diffusion Segmentation in Acute Ischemic Stroke Through Automatic Tree Learning Anomaly Segmentation. Front. Neuroinform. 2018, 12, 21. [Google Scholar] [CrossRef]
- Lapchak, P.A. Critical early thrombolytic and endovascular reperfusion therapy for acute ischemic stroke victims: A call for adjunct neuroprotection. Transl. Stroke Res. 2015, 6, 345–354. [Google Scholar] [CrossRef]
- Wesley, U.V.; Sutton, I.C.; Cunningham, K.; Jaeger, J.W.; Phan, A.Q.; Hatcher, J.F.; Dempsey, R.J. Galectin-3 protects against ischemic stroke by promoting neuro-angiogenesis via apoptosis inhibition and Akt/Caspase regulation. J. Cereb. Blood Flow Metab. 2021, 41, 857–873. [Google Scholar] [CrossRef]
- Xue, X.; Wang, H.; Su, J. Inhibition of MiR-122 Decreases Cerebral Ischemia-reperfusion Injury by Upregulating DJ-1-Phosphatase and Tensin Homologue Deleted on Chromosome 10 (PTEN)/Phosphonosinol-3 Kinase (PI3K)/AKT. Med. Sci. Monit. 2020, 26, e915825. [Google Scholar] [CrossRef]
- Ueda, H. Prothymosin alpha plays a key role in cell death mode-switch, a new concept for neuroprotective mechanisms in stroke. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 377, 315–323. [Google Scholar] [CrossRef]
- Chung, P.C.S.; Kieffer, B.L. Delta opioid receptors in brain function and diseases. Pharmacol. Ther. 2013, 140, 112–120. [Google Scholar] [CrossRef]
- Beal, E.W.; Kim, J.-L.; Reader, B.F.; Akateh, C.; Maynard, K.; Washburn, W.K.; Zweier, J.L.; Whitson, B.A.; Black, S.M. [D-Ala(2), D-Leu(5)] Enkephalin Improves Liver Preservation During Normothermic Ex Vivo Perfusion. J. Surg. Res. 2019, 241, 323–335. [Google Scholar] [CrossRef]
- Qiu, J.; Chao, D.; Sheng, S.; Khiati, D.; Zhou, X.; Xia, Y. Δ-Opioid Receptor-Nrf-2-Mediated Inhibition of Inflammatory Cytokines in Neonatal Hypoxic-Ischemic Encephalopathy. Mol. Neurobiol. 2019, 56, 5229–5240. [Google Scholar] [CrossRef]
- Wang, J.-W.; Xue, Z.-Y.; Wu, A.-S. Mechanistic insights into delta-opioid-induced cardioprotection: Involvement of caveolin translocation to the mitochondria. Life Sci. 2019, 247, 116942. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, M.; Guo, Q.-L.; Zhu, C.-Q.; Guo, J.-C. Prolonged DADLE exposure epigenetically promotes Bcl-2 expression and elicits neuroprotection in primary rat cortical neurons via the PI3K/Akt/NF-κB pathway. Acta Pharmacol. Sin. 2018, 39, 1582–1589. [Google Scholar] [CrossRef]
- Wang, S.; Cao, X.; Duan, Y.; Zhang, G. Delta Opioid Peptide [d-Ala2, d-Leu5] Enkephalin (DADLE) Exerts a Cytoprotective Effect in Astrocytes Exposed to Oxygen-Glucose Deprivation by Inducing Autophagy. Cell Transplant. 2019, 28, 775–782. [Google Scholar] [CrossRef]
- Lai, Z.; Gu, L.; Yu, L.; Chen, H.; Yu, Z.; Zhang, C.; Xu, X.; Zhang, M.; Zhang, M.; Ma, M.; et al. Delta opioid peptide [d-Ala2, d-Leu5] enkephalin confers neuroprotection by activating delta opioid receptor-AMPK-autophagy axis against global ischemia. Cell Biosci. 2020, 10, 79. [Google Scholar] [CrossRef]
- Schoos, A.; Gabriel, C.; Knab, V.M.; Fux, D.A. Activation of HIF-1α by δ-Opioid Receptors Induces COX-2 Expression in Breast Cancer Cells and Leads to Paracrine Activation of Vascular Endothelial Cells. J. Pharmacol. Exp. Ther. 2019, 370, 480–489. [Google Scholar] [CrossRef]
- Arendt, R.M.; Schmoeckel, M.; Wilbert-Lampen, U.; Plasse, A.; Heucke, L.; Werdan, K. Bidirectional effects of endogenous opioid peptides on endothelin release rates in porcine aortic endothelial cell culture: Mediation by delta opioid receptor and opioid receptor antagonist-insensitive mechanisms. J. Pharmacol. Exp. Ther. 1995, 272, 1–7. [Google Scholar]
- Saeed, R.W.; Stefano, G.; Murga, J.D.; Short, T.W.; Qi, F.; Bilfinger, T.V.; Magazine, H.I. Expression of functional delta opioid receptors in vascular smooth muscle. Int. J. Mol. Med. 2000, 6, 673–680. [Google Scholar] [CrossRef]
- Parra, L.; Perez-Vizcaino, F.; Alsasua, A.; Martín, M.I.; Tamargo, J. Mu- and delta-opioid receptor-mediated contractile effects on rat aortic vascular smooth muscle. Eur. J. Pharmacol. 1995, 277, 99–105. [Google Scholar] [CrossRef]
- Han, D.; Zhang, S.; Fan, B.; Wen, L.-L.; Sun, M.; Zhang, H.; Feng, J. Ischemic postconditioning protects the neurovascular unit after focal cerebral ischemia/reperfusion injury. J. Mol. Neurosci. 2014, 53, 50–58. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, X.; Zhang, L.; Pu, H.; Hu, X.; Zhang, W.; Cai, W.; Gao, Y.; Leak, R.K.; Keep, R.F.; et al. Endothelium-targeted overexpression of heat shock protein 27 ameliorates blood-brain barrier disruption after ischemic brain injury. Proc. Natl. Acad. Sci. USA 2017, 114, E1243–E1252. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLOS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Guarda, I.F.M.S.; Saad, W.A.; de Arruda Camargo, L.A. Nitric oxide and angiotensin II receptors mediate the pressor effect of angiotensin II: A study in conscious and zoletil-anesthetized rats. Anesth. Analg. 2007, 105, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Limprasutr, V.; Sharp, P.; Jampachaisri, K.; Pacharinsak, C.; Durongphongtorn, S. Tiletamine/zolazepam and dexmedetomidine with tramadol provide effective general anesthesia in rats. Anim. Model. Exp. Med. 2021, 4, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, S.; Shen, B.; Fan, Q.; Zhang, R.; Zhou, Y.; Zhang, P.; Wang, L.; Zhang, L. Activation of the δ opioid receptor relieves cerebral ischemic injury in rats via EGFR transactivation. Life Sci. 2021, 273, 119292. [Google Scholar] [CrossRef]
- Su, D.-S.; Wang, Z.-H.; Zheng, Y.-J.; Zhao, Y.-H.; Wang, X.-R. Dose-dependent neuroprotection of delta opioid peptide [D-Ala2, D-Leu5] enkephalin in neuronal death and retarded behavior induced by forebrain ischemia in rats. Neurosci. Lett. 2007, 423, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Liu, H.; Zhu, H.; Li, S.; Yao, J. Protective effect of delta opioid receptor agonist (D-Ala2, D-Leu5) enkephalin on permanent focal cerebral ischemia in rats. NeuroReport 2016, 27, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.H.; Wagner, S.; Liu, K.-F.; Hu, X.-J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995, 26, 627–635. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Y.; Gao, H.; Zhang, X.; Hu, Y.; Han, T.; Shen, B.; Zhang, L.; Wu, Q. X-ray Causes mRNA Transcripts Change to Enhance Orai2-Mediated Ca2+ Influx in Rat Brain Microvascular Endothelial Cells. Front. Mol. Biosci. 2021, 8, 646730. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.-C.; Zhang, D.-Q.; Ma, S.-W.; Huang, Y.-Y.; Shuster, S.J.; Porreca, F.; Lai, J. An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Mol. Pain 2005, 1, 29. [Google Scholar] [CrossRef]
- Lana, D.; Iovino, L.; Nosi, D.; Wenk, G.L.; Giovannini, M. G. The neuron-astrocyte-microglia triad involvement in neuroinflammaging mechanisms in the CA3 hippocampus of memory-impaired aged rats. Exp Geronto. 2016, 83, 71–88. [Google Scholar] [CrossRef]
- Wang, J.; Sareddy, G.R.; Lu, Y.; Pratap, U.P.; Tang, F.; Greene, K.M.; Meyre, P.L.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and is Neuroprotective following Ischemic Brain Injury. J. Neurosci. 2020, 40, 9751–9771. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Z.; Cheng, M.; Zhao, Y.; Wang, M.; Sai, N.; Wang, X.; Liu, H.; Huang, G.; Zhang, X. Homocysteine exaggerates microglia activation and neuroinflammation through microglia localized STAT3 overactivation following ischemic stroke. J. Neuroinflammation 2017, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Potter, D.E.; Crosson, C.E. Opioid receptor-activation: Retina protected from ischemic injury. Investig. Opthalmology Vis. Sci. 2009, 50, 3853–3859. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Li, H.-W.; Song, S.-J.; Teng, X.; Ma, H.-J.; Guo, Z.; Zhang, Y.; Zhou, Z.-N. Opioid receptors mediate enhancement of Ach-induced aorta relaxation by chronic intermittent hypobaric hypoxia. Acta Physiol. Sin. 2013, 65, 269–275. [Google Scholar]
- McNutt, M.C.; Lagace, T.A.; Horton, J.D. Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J. Biol. Chem. 2007, 282, 20799–20803. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Cui, R.; Chen, C.-H.; Du, J. Oxidized low-density lipoprotein stimulates p53-dependent activation of proapoptotic Bax leading to apoptosis of differentiated endothelial progenitor cells. Endocrinology 2007, 148, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.O.; Fu, N.Y.; Sukumaran, S.K.; Chan, S.-L.; Kang, J.H.; Poon, K.L.; Chen, B.S.; Yu, V.C. MAP-1 is a mitochondrial effector of Bax. Proc. Natl. Acad. Sci. USA 2005, 102, 14623–14628. [Google Scholar] [CrossRef]
- Förster, K.; Kuno, A.; Solenkova, N.; Felix, S.B.; Krieg, T. The delta-opioid receptor agonist DADLE at reperfusion protects the heart through activation of pro-survival kinases via EGF receptor transactivation. Am. J. Physiol. Circ. Physiol. 2007, 293, H1604–H1608. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Yanaga, K.; Okudaira, S.; Eguchi, S.; Furui, J.; Kanematsu, T. [D-Ala2, D-Leu5] enkephalin (DADLE) protects liver against ischemia-reperfusion injury in the rat. J. Surg. Res. 2003, 114, 72–77. [Google Scholar] [CrossRef]
- Cesar, V.B.; Borlongan, C.V.; Wang, Y.; Su, T.-P. Delta opioid peptide (D-Ala 2, D-Leu 5) enkephalin: Linking hibernation and neuroprotection. Front. Biosci. 2004, 9, 2298–3392. [Google Scholar] [CrossRef]
- Nikonenko, A.G.; Radenovic, L.; Andjus, P.R.; Skibo, G.G. Structural features of ischemic damage in the hippocampus. Anat. Rec. 2009, 292, 1914–1921. [Google Scholar] [CrossRef]
- Naseh, M.; Bayat, M.; Akbari, S.; Vatanparast, J.; Shabani, M.; Haghighi, A.B.; Haghani, M. Neuroprotective effects of sodium valproate on hippocampal cell and volume, and cognitive function in a rat model of focal cerebral ischemia. Physiol. Behav. 2022, 251, 113806. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Song, H.-J.; Cui, S.-G.; Wang, T.; Liu, Q.; Wang, R. The stimulative effects of endogenous opioids on endothelial cell proliferation, migration and angiogenesis in vitro. Eur. J. Pharmacol. 2010, 628, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Poonawala, T.; Levay-Young, B.K.; Hebbel, R.P.; Gupta, K. Opioids heal ischemic wounds in the rat. Wound Repair Regen. 2005, 13, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gui, Z.; Liu, H.; Qian, S.; Jia, Y.; Luo, X. Remifentanil pretreatment ameliorates hypoxia/reoxygenation-induced cardiac microvascular endothelial cell dysfunction by regulating the PI3K/Akt/HIF-1α signaling pathway. Bioengineered 2021, 12, 7872–7881. [Google Scholar] [CrossRef]
- Kao, T.-K.; Ou, Y.-C.; Liao, S.-L.; Chen, W.-Y.; Wang, C.-C.; Chen, S.-Y.; Chiang, A.-N.; Chen, C.-J. Opioids modulate post-ischemic progression in a rat model of stroke. Neurochem. Int. 2008, 52, 1256–1265. [Google Scholar] [CrossRef]
- Duan, Y.-L.; Wang, S.-Y.; Zeng, Q.-W.; Su, D.-S.; Li, W.; Wang, X.-R.; Zhao, Z. Astroglial reaction to delta opioid peptide [D-Ala2, D-Leu5] enkephalin confers neuroprotection against global ischemia in the adult rat hippocampus. Neuroscience 2011, 192, 81–90. [Google Scholar] [CrossRef]
- Livingston, K.E.; Traynor, J.R. Allostery at opioid receptors: Modulation with small molecule ligands. Br. J. Pharmacol. 2018, 175, 2846–2856. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Chen, M.; Deng, Z.; Kong, L.; Shen, B.; Zhang, L. Delta Opioid Peptide Targets Brain Microvascular Endothelial Cells Reducing Apoptosis to Relieve Hypoxia-Ischemic/Reperfusion Injury. Pharmaceutics 2023, 15, 46. https://doi.org/10.3390/pharmaceutics15010046
Zhang R, Chen M, Deng Z, Kong L, Shen B, Zhang L. Delta Opioid Peptide Targets Brain Microvascular Endothelial Cells Reducing Apoptosis to Relieve Hypoxia-Ischemic/Reperfusion Injury. Pharmaceutics. 2023; 15(1):46. https://doi.org/10.3390/pharmaceutics15010046
Chicago/Turabian StyleZhang, Ran, Meixuan Chen, Zhongfang Deng, Lingchao Kong, Bing Shen, and Lesha Zhang. 2023. "Delta Opioid Peptide Targets Brain Microvascular Endothelial Cells Reducing Apoptosis to Relieve Hypoxia-Ischemic/Reperfusion Injury" Pharmaceutics 15, no. 1: 46. https://doi.org/10.3390/pharmaceutics15010046
APA StyleZhang, R., Chen, M., Deng, Z., Kong, L., Shen, B., & Zhang, L. (2023). Delta Opioid Peptide Targets Brain Microvascular Endothelial Cells Reducing Apoptosis to Relieve Hypoxia-Ischemic/Reperfusion Injury. Pharmaceutics, 15(1), 46. https://doi.org/10.3390/pharmaceutics15010046





