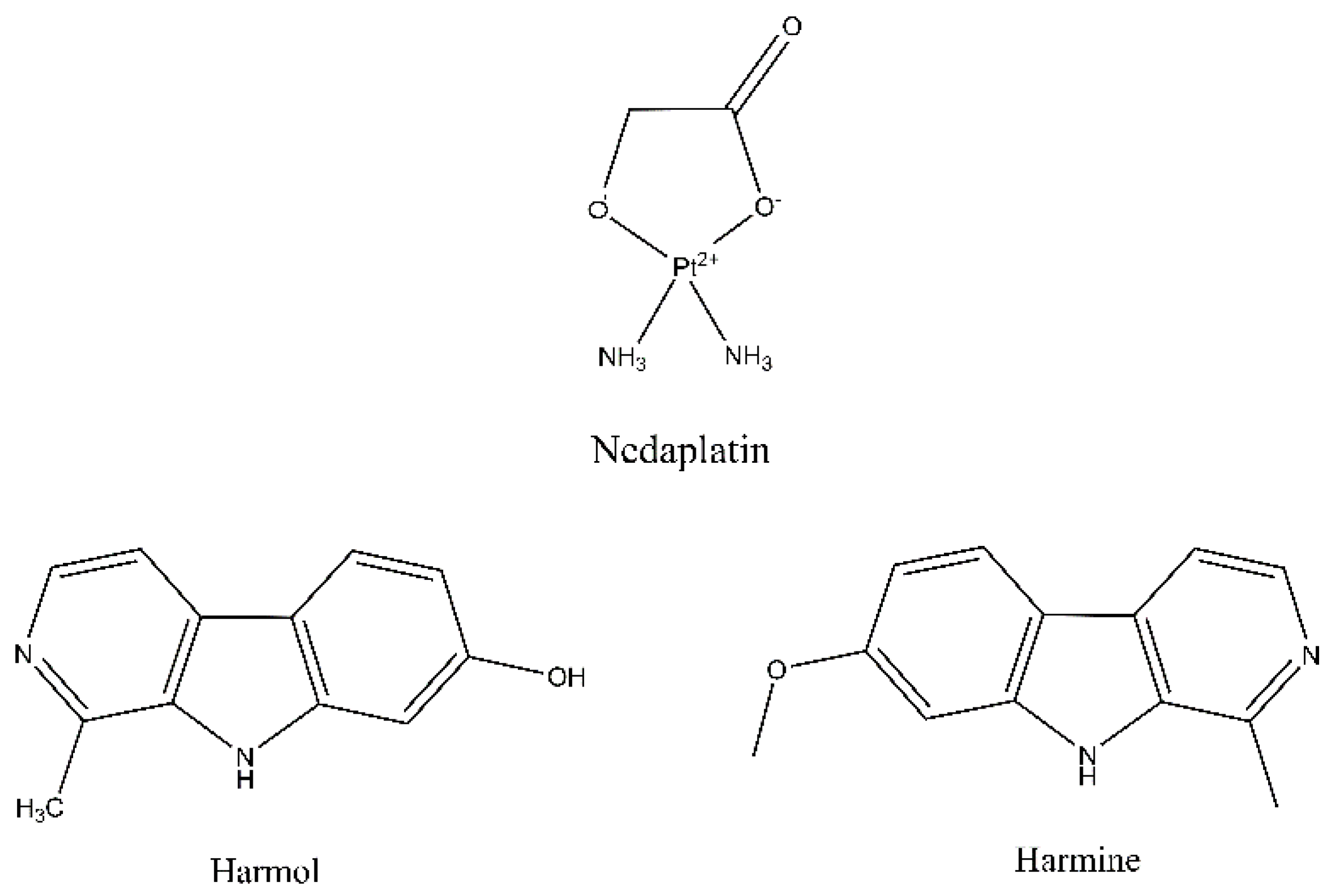
Nedaplatin/Peganum harmala Alkaloids Co-Loaded Electrospun, Implantable Nanofibers: A Chemopreventive Nano-Delivery System for Treating and Preventing Breast Cancer Recurrence after Tumorectomy
Abstract
:1. Introduction
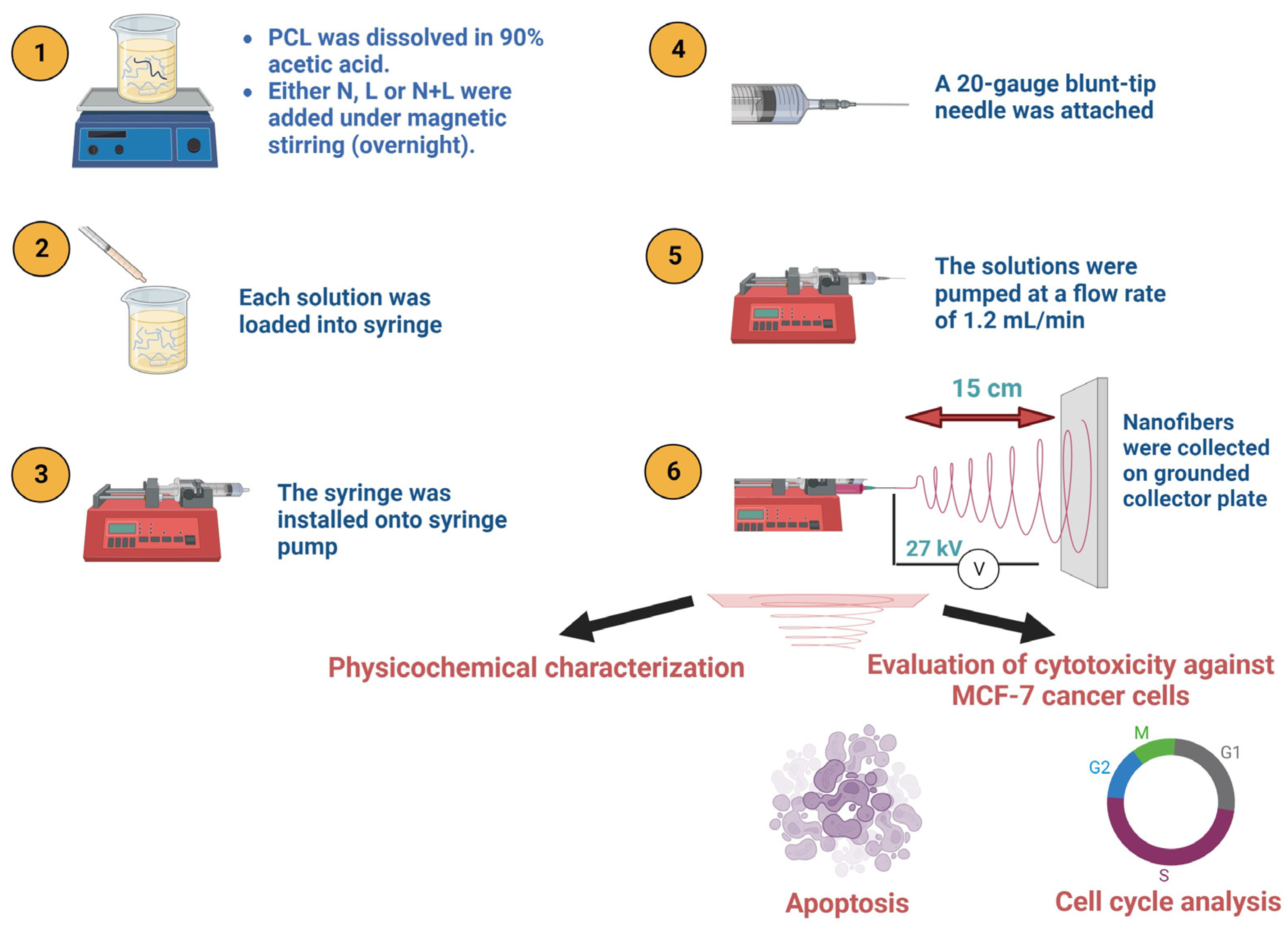
2. Materials and Methods
2.1. Materials
2.2. Preparation of Peganum harmala Alkaloid-Rich Fraction (L)
2.3. Preparation of Nanofiber Mats
2.3.1. Preparation of Electrospinning Solutions
2.3.2. Electrospinning Process
2.4. Characterization and Loading Efficiency
2.5. In Vitro Degradation Study
2.6. In Vitro Release Study
2.7. Release Kinetics
2.8. Cell Culture
2.9. Cytotoxicity Assay
2.10. Apoptosis Assay
2.11. Cell Cycle Analysis
2.12. Statistical Analysis
3. Results
3.1. Size and Morphology Characterization of Nanofibers
3.2. Characterization and Loading Efficiency
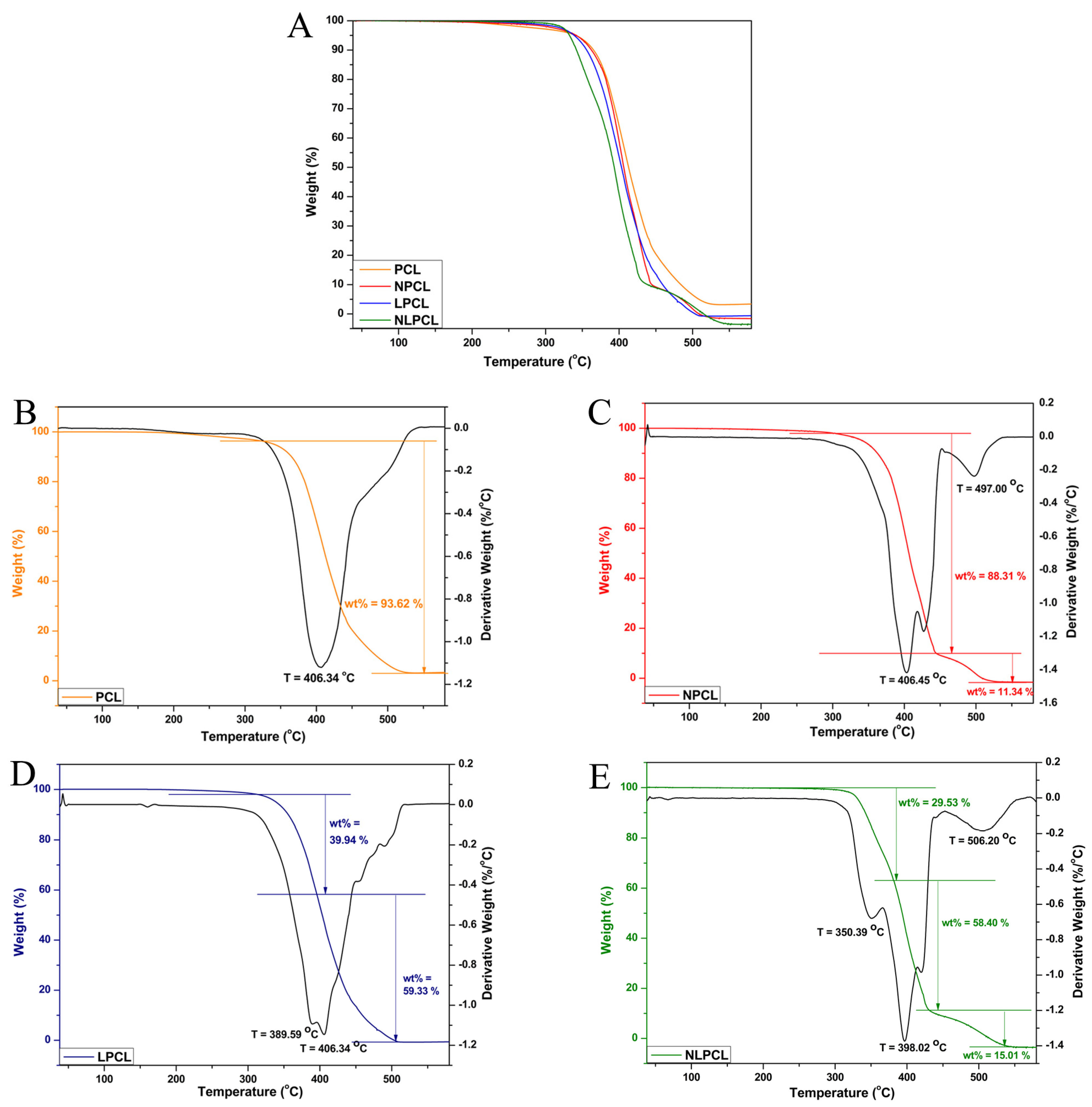
3.3. In Vitro Degradation Study
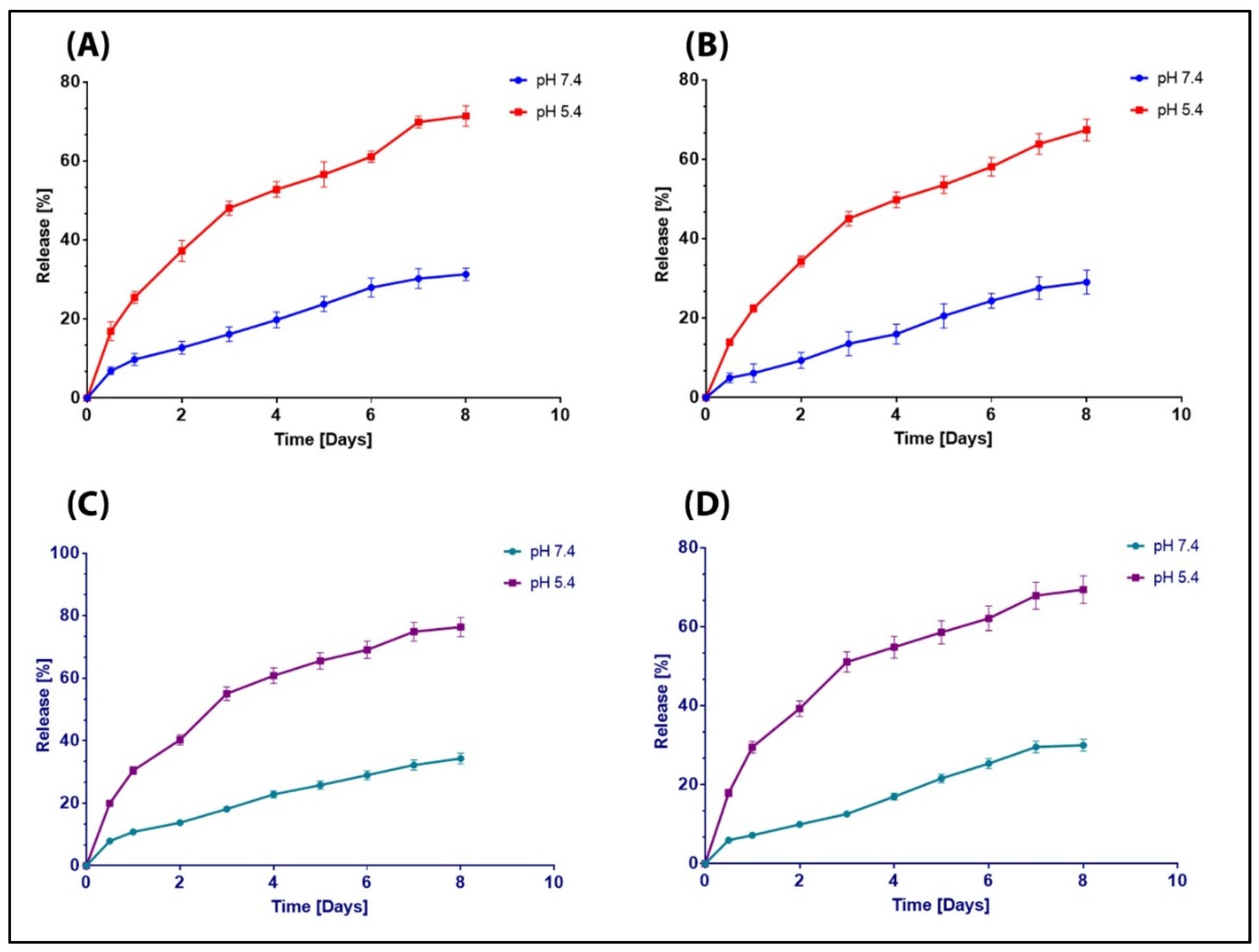
3.4. Release Study
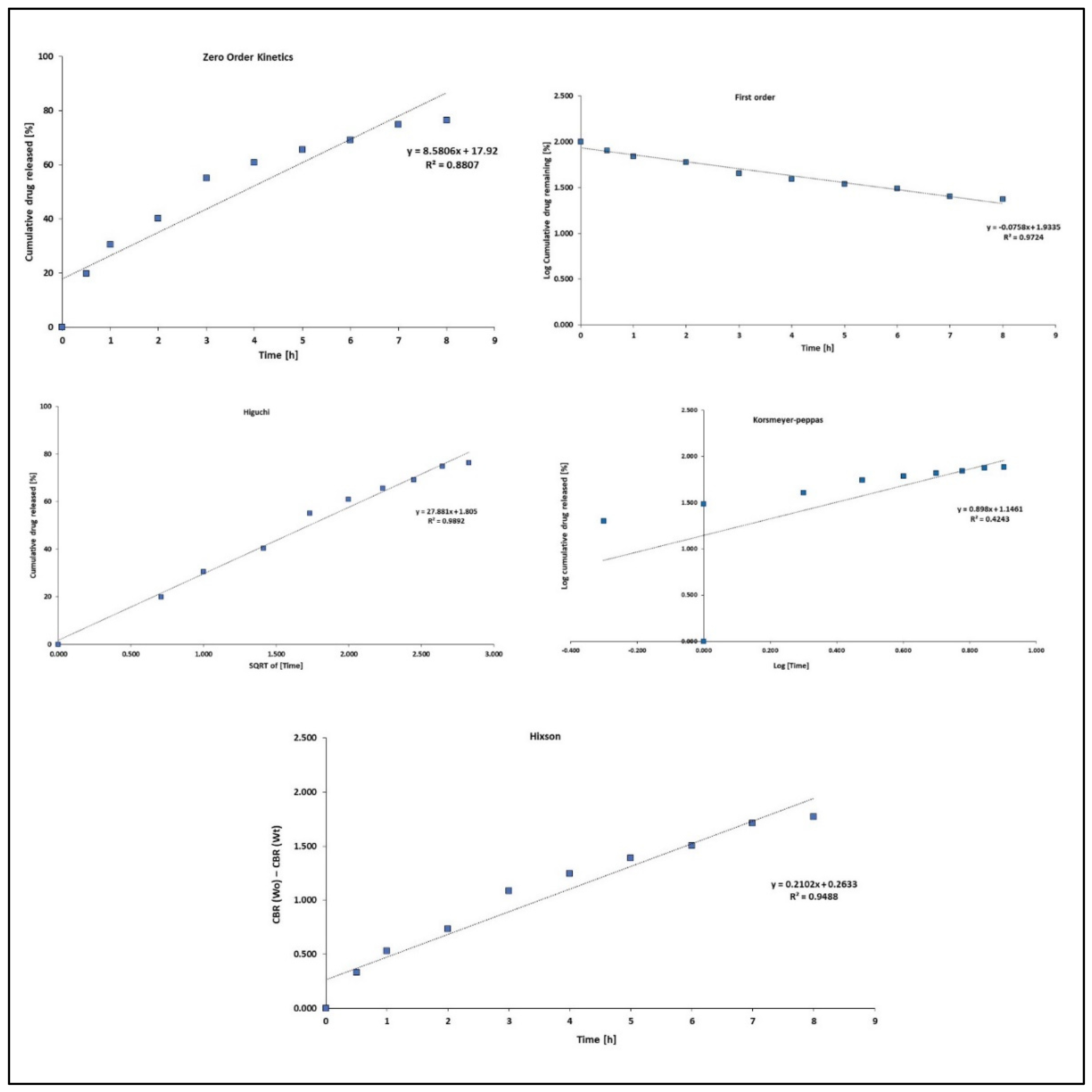
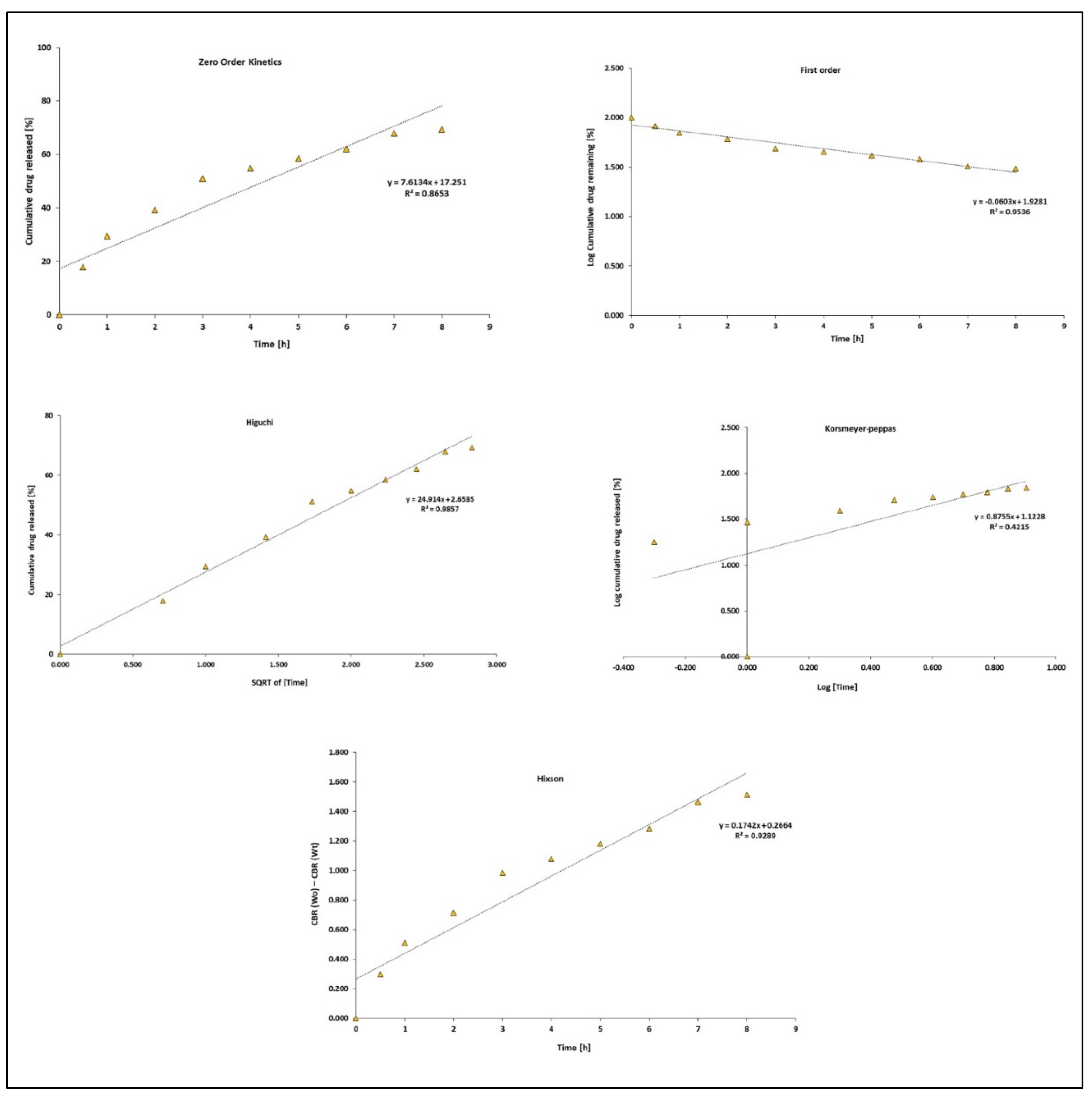
3.5. Release Kinetics
3.6. Cytotoxicity Assay
3.7. Apoptosis Assay
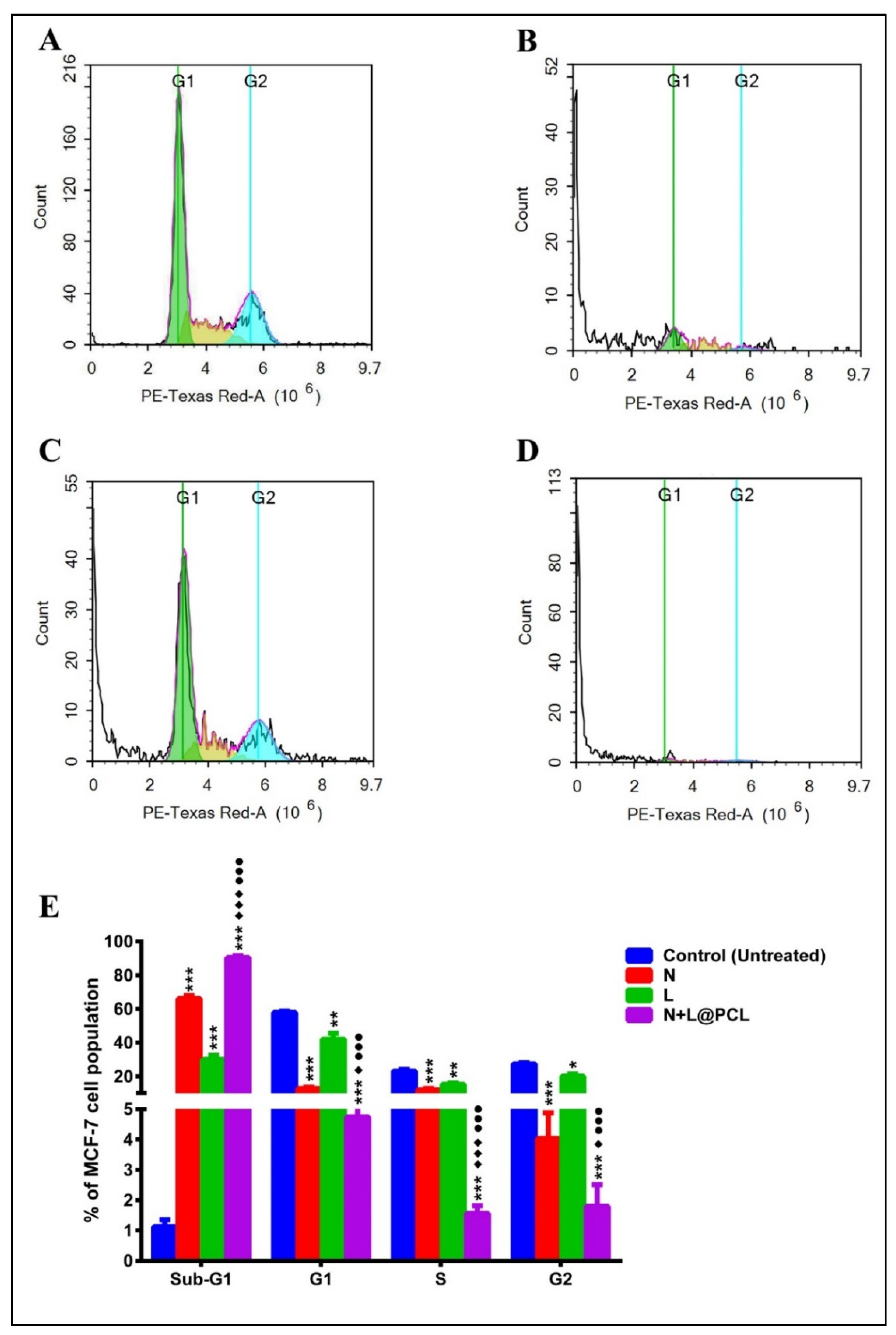
3.8. Cell Cycle Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calabrese, C.; Casella, D.; Di Taranto, G.; Marcasciano, M.; Kothari, A.; Sordi, S.; Barellini, L.; Lo Torto, F.; Tarallo, M.; Perra, A. Oncoplastic conservative surgery for breast cancer: Long-term outcomes of our first ten years experience. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7333–7342. [Google Scholar] [PubMed]
- Hussain, T.; Ramakrishna, S.; Abid, S. Nanofibrous drug delivery systems for breast cancer: A review. Nanotechnology 2021, 33, 102001. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, Q.; Zhang, J.; Huang, Y.; Deng, L.; Li, C.; Tai, G.; Ruan, B. Local penetration of doxorubicin via intrahepatic implantation of PLGA based doxorubicin-loaded implants. Drug Deliv. 2019, 26, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Caceres, R.; Cabeza, L.; Perazzoli, G.; Diaz, A.; Lopez-Romero, J.M.; Melguizo, C.; Prados, J. Electrospun Nanofibers: Recent Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2019, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Dominguez-Robles, J.; Donnelly, R.F.; Larraneta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef]
- Kumar, A.; Pillai, J. Implantable drug delivery systems: An overview. Nanostructures Eng. Cells Tissues Organs 2018, 2018, 473–511. [Google Scholar]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Kamath, S.M.; Sridhar, K.; Jaison, D.; Gopinath, V.; Ibrahim, B.M.; Gupta, N.; Sundaram, A.; Sivaperumal, P.; Padmapriya, S.; Patil, S.S. Fabrication of tri-layered electrospun polycaprolactone mats with improved sustained drug release profile. Sci. Rep. 2020, 10, 18179. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Jwa, S.J.; Won, J.M.; Kim, D.H.; Kim, K.B.; Lee, J.B.; Heo, M.; Shim, K.S.; Jo, H.S.; Lee, W.J.; Roh, T.S.; et al. Breast Tissue Restoration after the Partial Mastectomy Using Polycaprolactone Scaffold. Polymer 2022, 14, 3817. [Google Scholar] [CrossRef] [PubMed]
- Pinzón-García, A.D.; Sinisterra, R.; Cortes, M.; Mesa, F.; Ramírez-Clavijo, S. Polycaprolactone nanofibers as an adjuvant strategy for Tamoxifen release and their cytotoxicity on breast cancer cells. PeerJ 2021, 9, e12124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Liu, Y.; Huang, Y.; Wang, F.; Qian, Y.; Wang, Y. Preparation and Properties of (Sc2O3-MgO)/Pcl/Pvp Electrospun Nanofiber Membranes for the Inhibition of Escherichia coli Infections. Int. J. Mol. Sci. 2023, 24, 7649. [Google Scholar] [CrossRef]
- Aboeita, N.M.; Fahmy, S.A.; El-Sayed, M.M.; Azzazy, H.M.E.-S.; Shoeib, T. Enhanced anticancer activity of nedaplatin loaded onto copper nanoparticles synthesized using red algae. Pharmaceutics 2022, 14, 418. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Preis, E.; Dayyih, A.A.; Alawak, M.; El-Said Azzazy, H.M.; Bakowsky, U.; Shoeib, T. Thermosensitive Liposomes Encapsulating Nedaplatin and Picoplatin Demonstrate Enhanced Cytotoxicity against Breast Cancer Cells. ACS Omega 2022, 7, 42115–42125. [Google Scholar] [CrossRef]
- Pang, H.; Feng, T.; Lu, H.; Meng, Q.; Chen, X.; Shen, Q.; Dong, X.; Cai, L. Efficacy and Safety of Nedaplatin in Advanced Breast Cancer Therapy. Cancer Investig. 2016, 34, 167–172. [Google Scholar] [CrossRef]
- Tsvetkova, D.; Ivanova, S. Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases. Molecules 2022, 27, 2466. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Q.; Zhang, P.; Yuan, P.; Ma, F.; Wang, J.; Luo, Y.; Cai, R.; Chen, S.; Li, Q. Vinorelbine plus platinum in patients with metastatic triple negative breast cancer and prior anthracycline and taxane treatment. Medicine 2015, 94, e1928. [Google Scholar]
- Sobhani, A.M.; Ebrahimi, S.A.; Mahmoudian, M. An in vitro evaluation of human DNA topoisomerase I inhibition by Peganum harmala L. seeds extract and its beta-carboline alkaloids. J. Pharm. Pharm. Sci. 2002, 5, 19–23. [Google Scholar]
- Hamsa, T.P.; Kuttan, G. Harmine activates intrinsic and extrinsic pathways of apoptosis in B16F-10 melanoma. Chin. Med. 2011, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.R.; Li, Q.; Liu, Z.L.; Liu, H.H.; Wang, W.; Liao, X.L.; Pan, Y.L.; Jiang, J.W. Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Wang, C.; Yi, X.; Li, M.; He, X. Anticancer activities of harmine by inducing a pro-death autophagy and apoptosis in human gastric cancer cells. Phytomedicine 2017, 28, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chourasiya, R.K.; Agrawal, R.K.; Vaidya, A. Promising Anticancer Activity of β-Carboline Derivatives: Design, Synthesis, and Pharmacological Evaluation. Chemistry 2022, 4, 1395–1406. [Google Scholar] [CrossRef]
- Shabani, S.H.S.; Tehrani, S.S.H.; Rabiei, Z.; Enferadi, S.T.; Vannozzi, G.P. Peganum harmala L.’s anti-growth effect on a breast cancer cell line. Biotechnol. Rep. 2015, 8, 138–143. [Google Scholar] [CrossRef]
- Roshankhah, S.; Arji Rodsari, B.; Jalili, C.; Salahshoor, M.R. The role of harmine in up-regulating p53 gene expression and inducing apoptosis in MCF-7 cell line. Middle East. J. Cancer 2020, 11, 34–41. [Google Scholar]
- Fahmy, S.A.; Issa, M.Y.; Saleh, B.M.; Meselhy, M.R.; Azzazy, H.M.E.-S. Peganum harmala alkaloids self-assembled supramolecular nanocapsules with enhanced antioxidant and cytotoxic activities. ACS Omega 2021, 6, 11954–11963. [Google Scholar] [CrossRef]
- Agnes Mary, S.; Giri Dev, V. Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J. Text. Inst. 2015, 106, 886–895. [Google Scholar] [CrossRef]
- Khalil, N.K.; Abo Dena, A.S.; El-Sherbiny, I.M. Boosting the mechanical strength and solubility-enhancement properties of hydroxypropyl-β-cyclodextrin nanofibrous films. Drug Dev. Ind. Pharm. 2021, 47, 1413–1423. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Mahdy, N.K.; Al Mulla, H.; ElMeshad, A.N.; Issa, M.Y.; Azzazy, H.M.E.-S. PLGA/PEG nanoparticles loaded with cyclodextrin-Peganum harmala alkaloid complex and ascorbic acid with promising antimicrobial activities. Pharmaceutics 2022, 14, 142. [Google Scholar] [CrossRef]
- Youness, R.A.; Al-Mahallawi, A.M.; Mahmoud, F.H.; Atta, H.; Braoudaki, M.; Fahmy, S.A. Oral Delivery of Psoralidin by Mucoadhesive Surface-Modified Bilosomes Showed Boosted Apoptotic and Necrotic Effects against Breast and Lung Cancer Cells. Polymers 2023, 15, 1464. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.T.; Vu, M.T.; Nguyen, N.H.; Nguyen-Huu, A.-M.; Nguyen, D.H. Preparation and in vitro evaluation of PEGylated liposomes as effective nanocarrier for delivery of oxaliplatin. J. Mater. Res. 2021, 36, 475–486. [Google Scholar] [CrossRef]
- Ioniţă, S.; Lincu, D.; Mitran, R.-A.; Ziko, L.; Sedky, N.K.; Deaconu, M.; Brezoiu, A.-M.; Matei, C.; Berger, D. Resveratrol encapsulation and release from pristine and functionalized mesoporous silica carriers. Pharmaceutics 2022, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Karuppuswamy, P.; Venugopal, J.R.; Navaneethan, B.; Laiva, A.L.; Ramakrishna, S. Polycaprolactone nanofibers for the controlled release of tetracycline hydrochloride. Mater. Lett. 2015, 141, 180–186. [Google Scholar] [CrossRef]
- Mozaffari, S.; Seyedabadi, S.; Alemzadeh, E. Anticancer efficiency of doxorubicin and berberine-loaded PCL nanofibers in preventing local breast cancer recurrence. J. Drug Deliv. Sci. Technol. 2022, 67, 102984. [Google Scholar] [CrossRef]
- Azzazy, H.M.E.-S.; Sawy, A.M.; Abdelnaser, A.; Meselhy, M.R.; Shoeib, T.; Fahmy, S.A. Peganum harmala Alkaloids and Tannic Acid Encapsulated in PAMAM Dendrimers: Improved Anticancer Activities as Compared to Doxorubicin. ACS Appl. Polym. Mater. 2022, 4, 7228–7239. [Google Scholar] [CrossRef]
- Torres, M.; Khan, S.; Duplanty, M.; Lozano, H.C.; Morris, T.J.; Nguyen, T.; Rostovtsev, Y.V.; DeYonker, N.J.; Mirsaleh-Kohan, N. Raman and infrared studies of platinum-based drugs: Cisplatin, carboplatin, oxaliplatin, nedaplatin, and heptaplatin. J. Phys. Chem. A 2018, 122, 6934–6952. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Ramzy, A.; Mandour, A.A.; Nasr, S.; Abdelnaser, A.; Bakowsky, U.; Azzazy, H.M.E.-S. PEGylated chitosan nanoparticles encapsulating ascorbic acid and oxaliplatin exhibit dramatic apoptotic effects against breast cancer cells. Pharmaceutics 2022, 14, 407. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Ramzy, A.; Sawy, A.M.; Nabil, M.; Gad, M.Z.; El-Shazly, M.; Aboul-Soud, M.A.; Azzazy, H.M.E.-S. Ozonated Olive Oil: Enhanced Cutaneous Delivery via Niosomal Nanovesicles for Melanoma Treatment. Antioxidants 2022, 11, 1318. [Google Scholar] [CrossRef]
- Pompa-Monroy, D.A.; Figueroa-Marchant, P.G.; Dastager, S.G.; Thorat, M.N.; Iglesias, A.L.; Miranda-Soto, V.; Pérez-González, G.L.; Villarreal-Gómez, L.J. Bacterial biofilm formation using pcl/curcumin electrospun fibers and its potential use for biotechnological applications. Materials 2020, 13, 5556. [Google Scholar] [CrossRef]
- Alahmmar, M.; Prabhakaran, P.; Jaganathan, S.; Nik, N.A.N. Fabrication and Characterization of Polycaprolactone with Retinoic Acid and Cerium Oxide for Anticancer Applications. Biointerface Res. Appl. Chem. 2023, 13, 1–15. [Google Scholar]
- Babadi, D.; Dadashzadeh, S.; Shahsavari, Z.; Shahhosseini, S.; Ten Hagen, T.L.; Haeri, A. Piperine-loaded electrospun nanofibers, an implantable anticancer controlled delivery system for postsurgical breast cancer treatment. Int. J. Pharm. 2022, 624, 121990. [Google Scholar] [CrossRef] [PubMed]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomater. Silver Jubil. Compend. 1996, 17, 117–128. [Google Scholar]
- Unalan, I.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Frank, G.; Boccaccini, A.R. Physical and antibacterial properties of peppermint essential oil loaded poly (ε-caprolactone)(PCL) electrospun fiber mats for wound healing. Front. Bioeng. Biotechnol. 2019, 7, 346. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.E.-S.; Abdelnaser, A.; Al Mulla, H.; Sawy, A.M.; Shamma, S.N.; Elhusseiny, M.; Alwahibi, S.; Mahdy, N.K.; Fahmy, S.A. Essential Oils Extracted from Boswellia sacra Oleo Gum Resin Loaded into PLGA–PCL Nanoparticles: Enhanced Cytotoxic and Apoptotic Effects against Breast Cancer Cells. ACS Omega 2022, 8, 1017–1025. [Google Scholar] [CrossRef]
- Knutson, C.M.; Hilker, A.P.; Tolstyka, Z.P.; Anderson, C.B.; Wilbon, P.A.; Mathers, R.T.; Wentzel, M.T.; Perkins, A.L.; Wissinger, J.E. Dyeing to Degrade: A Bioplastics Experiment for College and High School Classrooms. J. Chem. Educ. 2019, 96, 2565–2573. [Google Scholar] [CrossRef]
- Sedky, N.K.; Abdel-Kader, N.M.; Issa, M.Y.; Abdelhady, M.M.; Shamma, S.N.; Bakowsky, U.; Fahmy, S.A. Co-Delivery of Ylang Ylang Oil of Cananga odorata and Oxaliplatin Using Intelligent pH-Sensitive Lipid-Based Nanovesicles for the Effective Treatment of Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 8392. [Google Scholar] [CrossRef]
- Narum, S.M.; Le, T.; Le, D.P.; Lee, J.C.; Donahue, N.D.; Yang, W.; Wilhelm, S. Passive targeting in nanomedicine: Fundamental concepts, body interactions, and clinical potential. In Nanoparticles For Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 37–53. [Google Scholar]
- Gouda, R.; Baishya, H.; Qing, Z. Application of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. J. Dev. Drugs 2017, 6, 1–8. [Google Scholar]
- Rehman, F.; Volpe, P.L.; Airoldi, C. The applicability of ordered mesoporous SBA-15 and its hydrophobic glutaraldehyde-bridge derivative to improve ibuprofen-loading in releasing system. Colloids Surf. B Biointerfaces 2014, 119, 82–89. [Google Scholar] [CrossRef]
- Moydeen, A.M.; Padusha, M.S.A.; Aboelfetoh, E.F.; Al-Deyab, S.S.; El-Newehy, M.H. Fabrication of electrospun poly (vinyl alcohol)/dextran nanofibers via emulsion process as drug delivery system: Kinetics and in vitro release study. Int. J. Biol. Macromol. 2018, 116, 1250–1259. [Google Scholar] [CrossRef]
- Mirzaeei, S.; Mansurian, M.; Asare-Addo, K.; Nokhodchi, A. Metronidazole-and amoxicillin-loaded PLGA and PCL nanofibers as potential drug delivery systems for the treatment of periodontitis: In vitro and in vivo evaluations. Biomedicines 2021, 9, 975. [Google Scholar] [CrossRef]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef]
- Seyed Hassan Tehrani, S.; Hashemi Sheikh Shabani, S.; Tahmasebi Enferadi, S.; Rabiei, Z. Growth inhibitory impact of Peganum harmala L. on two breast cancer cell lines. Iran. J. Biotechnol. 2014, 12, 8–14. [Google Scholar] [CrossRef]
- Su, X.-Y.; Yin, H.-T.; Li, S.-Y.; Huang, X.-E.; Tan, H.-Y.; Dai, H.-Y.; Shi, F.-F. Intervention effects of nedaplatin and cisplatin on proliferation and apoptosis of human tumour cells in vitro. Asian Pac. J. Cancer Prev. 2012, 13, 4531–4536. [Google Scholar] [CrossRef] [PubMed]
- Arafa, K.K.; Fytory, M.; Mousa, S.A.; El-Sherbiny, I.M. Nanosized biligated metal-organic framework systems for enhanced cellular and mitochondrial sequential targeting of hepatic carcinoma. Biomater. Sci. 2021, 9, 6609–6622. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, M.M.; Shahini, A.; Mottahedi, M.; Garousi, S.; Shariat Razavi, S.A.; Pouyamanesh, G.; Afshari, A.R.; Ferns, G.A.; Bahrami, A. Harmaline exerts potentially anticancer effects on U-87 human malignant glioblastoma cells in vitro. Mol. Biol. Rep. 2023, 50, 4357–4366. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Wang, Z.; Lou, R.; Wu, J.; Shi, C.; Chen, D.; Ma, R.; Liu, S.; Cao, H.; Feng, J. Nedaplatin reduces multidrug resistance of non-small cell lung cancer by downregulating the expression of long non-coding RNA MVIH. J. Cancer 2020, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Takii, Y.; Shibata, Y.; Ariyama, H.; Qin, B.; Baba, E.; Kusaba, H.; Mitsugi, K.; Harada, M.; Nakano, S. In vitro sequence-dependent interaction between nedaplatin and paclitaxel in human cancer cell lines. Cancer Chemother. Pharmacol. 2005, 56, 279–285. [Google Scholar] [CrossRef]
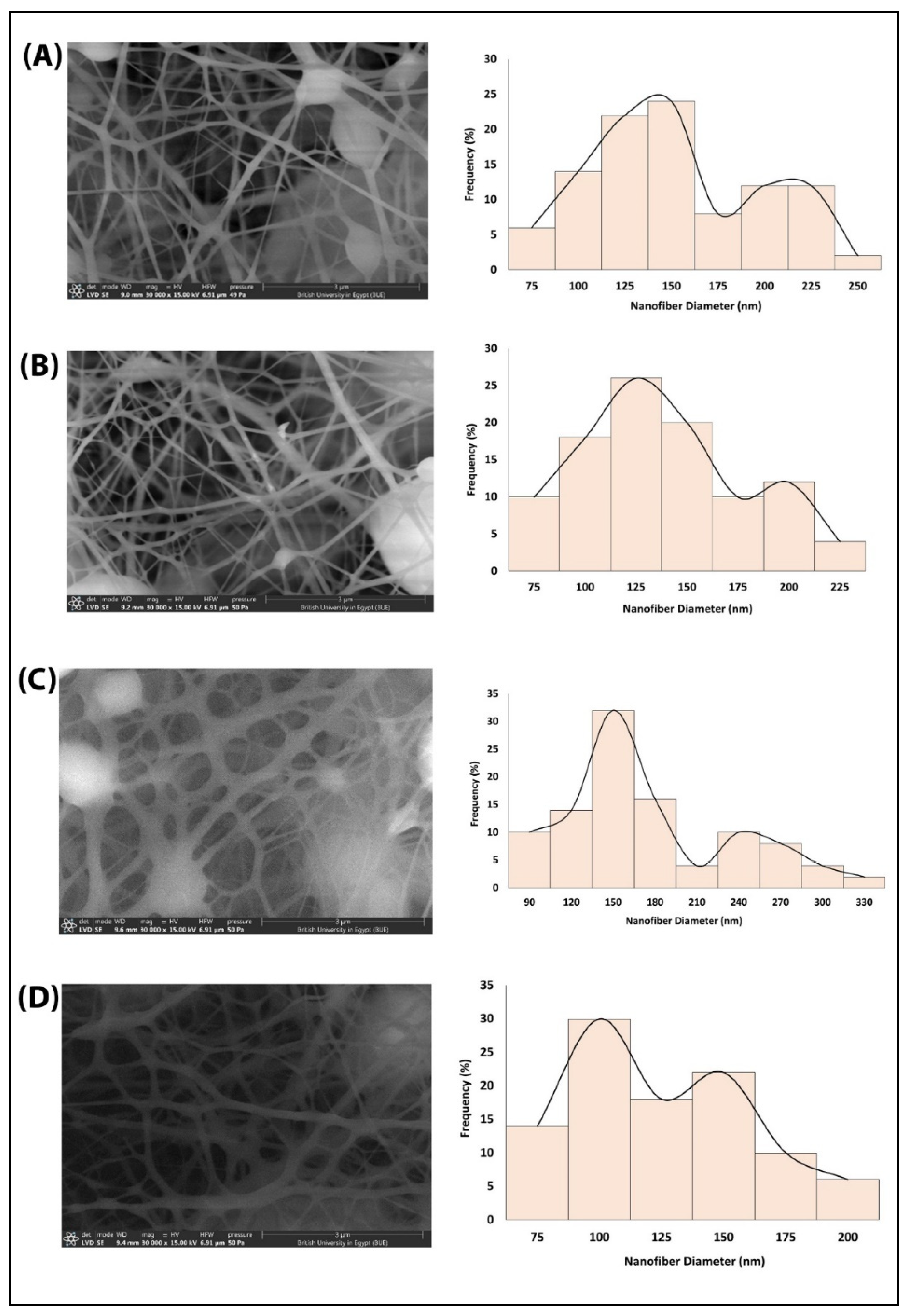


| Nanofiber Film | Mean Nanofiber Diameter (nm) ± SD | Nanofiber Diameter Range (nm) |
|---|---|---|
| PCL | 140.4 ± 44.9 | 64.7–231.6 |
| N@PCL | 128.1 ± 40.8 | 63.4–204.2 |
| L@PCL | 157.9 ± 60.7 | 67.3–300.9 |
| N + L@PCL | 112.0 ± 34.5 | 64.3–184.3 |
| Models | N from N + L@PCL | L from N + L@PCL |
|---|---|---|
| Zero-order | ||
| k0 | 8.58 | 7.61 |
| R2 | 0.88 | 0.86 |
| First-order | ||
| kf | 0.08 | 0.06 |
| R2 | 0.97 | 0.95 |
| Higuchi | ||
| kH | 27.88 | 24.91 |
| R2 | 0.99 | 0.98 |
| Korsmeyer–Peppas | ||
| n | 0.89 | 0.87 |
| R2 | 0.42 | 0.42 |
| Hixson | ||
| κ t | 0.21 | 0.17 |
| R2 | 0.95 | 0.93 |
| Test Sample | IC50 on MCF-7 Cells (µg/mL) | CC50 on MCF10A Cells (µg/mL) | SI (CC50/IC50) |
|---|---|---|---|
| L | 4.93 ± 0.13 | 62.53 ± 1.38 | 12.68 |
| N | 4.59 ± 0.37 | 24.24 ± 1.15 | 5.28 |
| L@PCL | 4.40 ± 0.56 | 67.07 ± 1.64 | 15.24 |
| N@PCL | 3.42 ± 0.17 | 27.92 ± 0.92 | 8.16 |
| N + L@PCL | 3.21 ± 0.86 | 65.63 ± 1.29 | 20.45 |
| Apoptotic Stage | Control (Untreated) | N | L | N + L@PCL |
|---|---|---|---|---|
| Necrosis (Q2-1) | 1.903 ± 0.3035 | * 2.830 ± 0.04359 | 2.223 ±0.08327 | ***♦♦♦●●● 6.773 ± 0.1986 |
| Late apoptosis (Q2-2) | 0.6367 ± 0.05508 | 0.9600 ± 0.1253 | 0.5833 ±0.1286 | ***♦♦♦●●● 3.300 ± 0.2524 |
| Viable cells (Q2-3) | 97.37 ± 0.2950 | * 96.12 ± 0.1168 | 97.18 ±0.1909 | ***♦♦♦●●● 89.88 ± 0.4148 |
| Early apoptosis (Q2-4) | 0.0900 ± 0.01732 | 0.0900 ± 0.0200 | 0.01333 ±0.005774 | 0.0400 ± 0.0200 |
| Phases | Control (Untreated) | N | L | N + L@PCL |
|---|---|---|---|---|
| Freq Sub-G1 | 1.127 ± 0.2309 | *** 66.09 ± 1.752 | *** 29.99 ± 2.435 | ***♦♦♦●●● 90.44 ± 1.172 |
| Freq G1 | 57.89 ± 0.7967 | *** 12.81 ± 0.659 | ** 42.03 ± 3.516 | ***♦●●● 4.740 ± 2.941 |
| Freq S | 23.16 ± 0.9295 | *** 11.92 ± 0.8504 | ** 15.28 ± 0.8675 | ***♦♦♦●●● 1.557 ± 0.260 |
| Freq G2 | 27.45 ± 0.6293 | *** 4.033 ± 0.842 | * 20.11 ± 1.364 | ***♦●●● 1.800 ± 0.7118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedky, N.K.; Arafa, K.K.; Abdelhady, M.M.M.; Issa, M.Y.; Abdel-Kader, N.M.; Mahdy, N.K.; Mokhtar, F.A.; Alfaifi, M.Y.; Fahmy, S.A. Nedaplatin/Peganum harmala Alkaloids Co-Loaded Electrospun, Implantable Nanofibers: A Chemopreventive Nano-Delivery System for Treating and Preventing Breast Cancer Recurrence after Tumorectomy. Pharmaceutics 2023, 15, 2367. https://doi.org/10.3390/pharmaceutics15102367
Sedky NK, Arafa KK, Abdelhady MMM, Issa MY, Abdel-Kader NM, Mahdy NK, Mokhtar FA, Alfaifi MY, Fahmy SA. Nedaplatin/Peganum harmala Alkaloids Co-Loaded Electrospun, Implantable Nanofibers: A Chemopreventive Nano-Delivery System for Treating and Preventing Breast Cancer Recurrence after Tumorectomy. Pharmaceutics. 2023; 15(10):2367. https://doi.org/10.3390/pharmaceutics15102367
Chicago/Turabian StyleSedky, Nada K., Kholoud K. Arafa, Manal M. M. Abdelhady, Marwa Y. Issa, Nour M. Abdel-Kader, Noha Khalil Mahdy, Fatma A. Mokhtar, Mohammad Y. Alfaifi, and Sherif Ashraf Fahmy. 2023. "Nedaplatin/Peganum harmala Alkaloids Co-Loaded Electrospun, Implantable Nanofibers: A Chemopreventive Nano-Delivery System for Treating and Preventing Breast Cancer Recurrence after Tumorectomy" Pharmaceutics 15, no. 10: 2367. https://doi.org/10.3390/pharmaceutics15102367
APA StyleSedky, N. K., Arafa, K. K., Abdelhady, M. M. M., Issa, M. Y., Abdel-Kader, N. M., Mahdy, N. K., Mokhtar, F. A., Alfaifi, M. Y., & Fahmy, S. A. (2023). Nedaplatin/Peganum harmala Alkaloids Co-Loaded Electrospun, Implantable Nanofibers: A Chemopreventive Nano-Delivery System for Treating and Preventing Breast Cancer Recurrence after Tumorectomy. Pharmaceutics, 15(10), 2367. https://doi.org/10.3390/pharmaceutics15102367








