Applications of Degradable Hydrogels in Novel Approaches to Disease Treatment and New Modes of Drug Delivery
Abstract
:1. Introduction
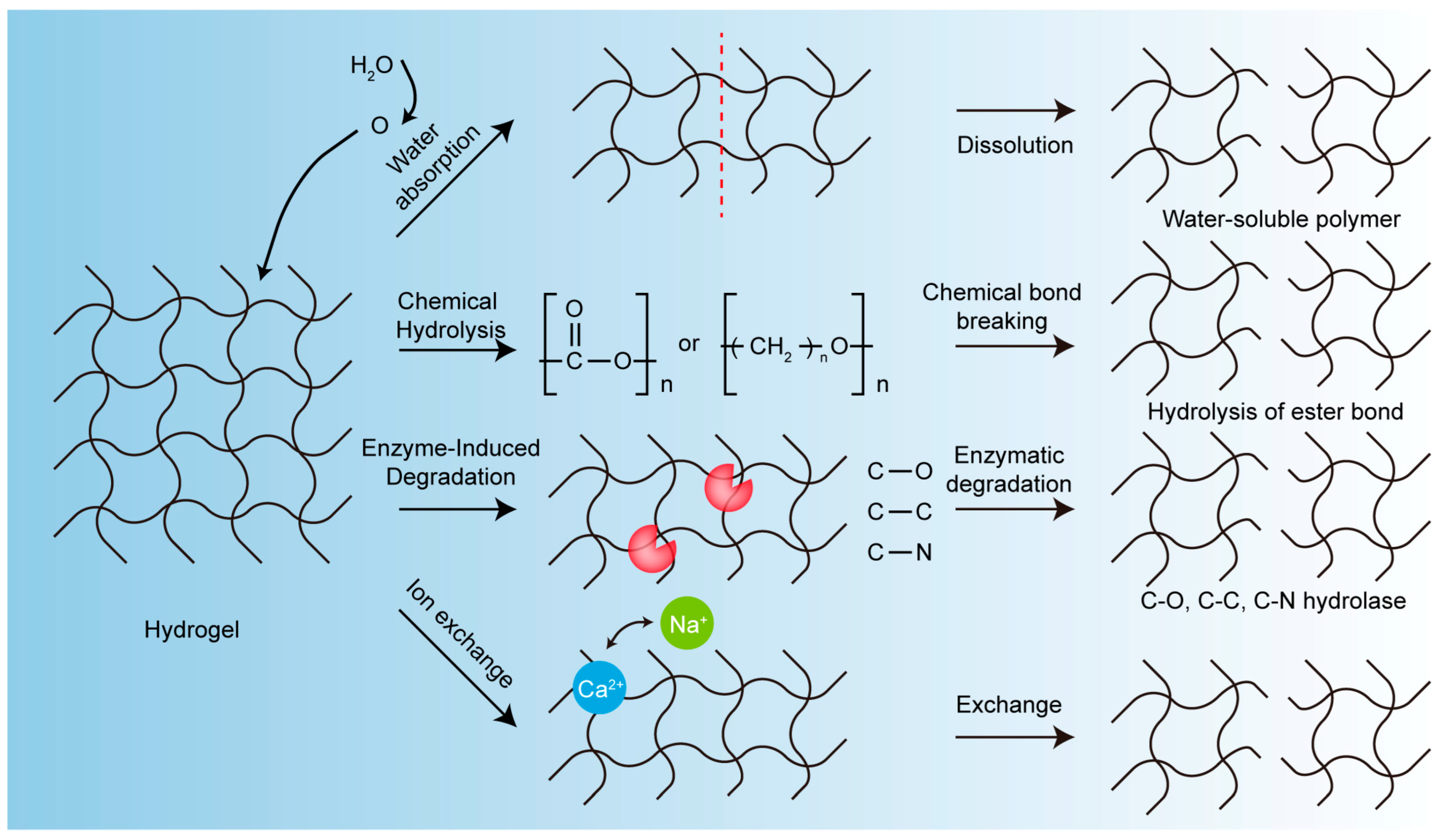
2. The Degradation Mechanism of Biodegradable Hydrogels
- (1)
- Solubilization
- (2)
- Chemical Hydrolysis
- (3)
- Enzyme-Induced Degradation
- (4)
- Other Mechanisms
3. Applications of Biodegradable Hydrogels in Novel Approaches to Disease Treatment
3.1. Wound Treatment
3.1.1. Wound Dressing
3.1.2. Antibacterial Effects
3.1.3. Wound Healing
3.2. Tissue Engineering
3.2.1. Bone Tissue Engineering
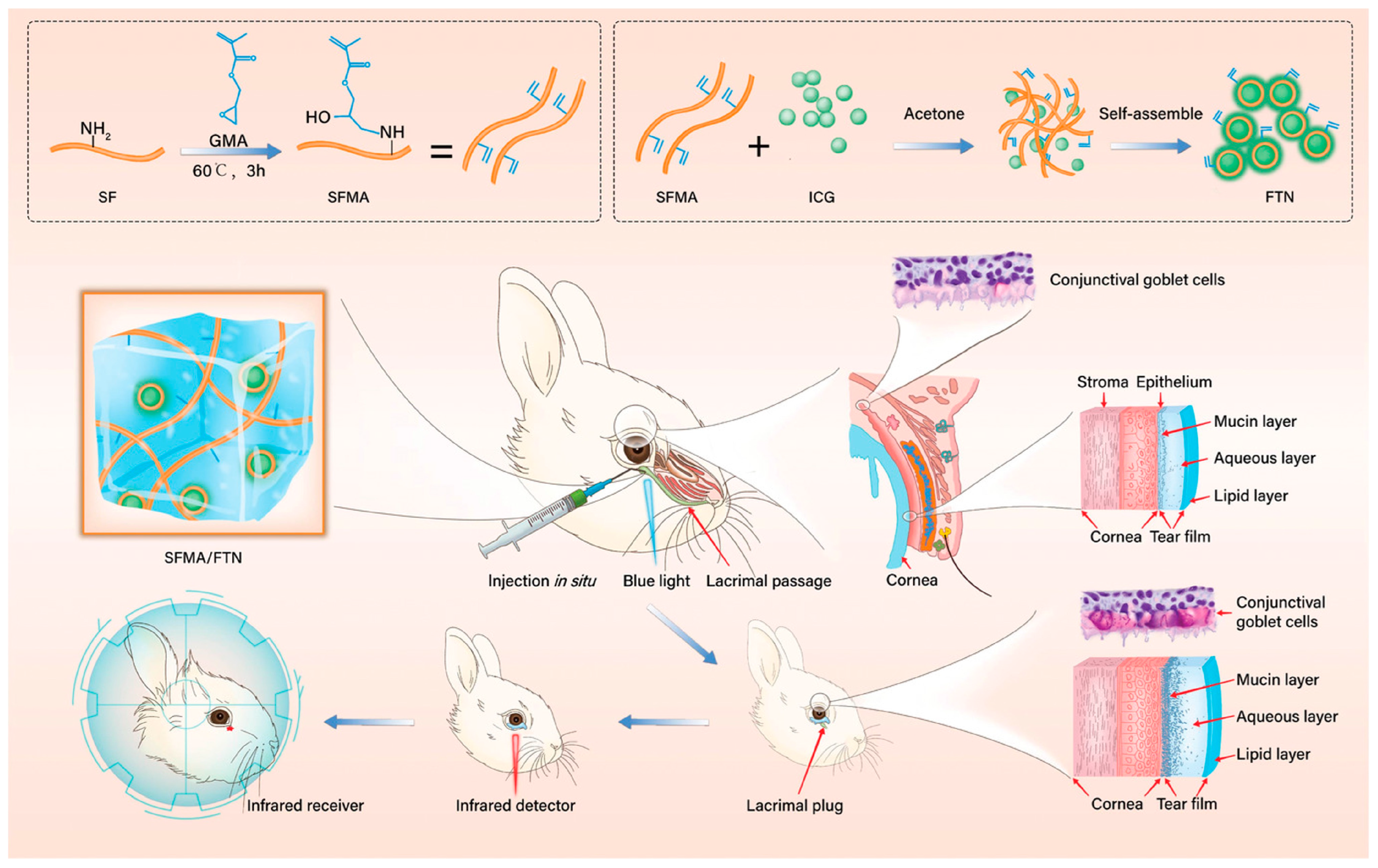
3.2.2. Eye Tissue Engineering
3.2.3. Dental Tissue Engineering
3.3. Cancer Treatment
3.3.1. Biodegradable Hydrogels for Cancer Immunotherapy
3.3.2. Biodegradable Hydrogels for Photothermal Cancer Therapy
3.3.3. Biodegradable Hydrogels for Photodynamic Cancer Therapy
3.3.4. Biodegradable Hydrogels for Combination Cancer Therapy
3.4. Brain Diseases
4. Degradable Hydrogels for Novel Modes of Delivery
4.1. Injectable Hydrogels
4.2. Sprayable Hydrogels
4.3. Indwelling In Vivo
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDT | Photodynamic therapy |
| PTT | Photothermal therapy |
| PTA | Photothermal agent |
| NDDS | Nanodrug Delivery Systems |
| SC | Stratum corneum |
| MNs | Microneedles |
| rGO | Reduced graphene oxide |
| H2O2 | Hydrogen peroxide |
| HRP | Horseradish peroxidase |
| NIR | Near-infrared |
| HA | Hyaluronic acid |
| EDC | 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride |
| NHS | N-hydroxysuccinimide |
| DA | Grafting dopamine |
| HA-DA | Hyaluronic acid-graft-dopamine |
| GO | Graphene oxide |
| PDA | Polydopamine |
| CEC | N-carboxyethyl chitosan |
| OHA-AT | Oxidized hyaluronic acid-aniline tetramer polymer |
| L-Arg | L-arginine |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| ROS | Reactive oxygen species |
| VEGF | Vascular endothelial growth factor |
| ZIF-8 | Zeolite-type imidazolic acid skeleton-8 |
| MeHA | Hyaluronic acid methacrylate |
| Zn-MOF | Zinc metal-organic framework |
| ECM | Extracellular matrix |
| BTE | Bone tissue regeneration |
| OCS | Oxidized chondroitin sulfate |
| Gel | Gelatin |
| MBGNs | Mesoporous bioactive glass nanoparticles |
| H & E | Hematoxylin–eosin staining |
| POSS | Polyhedral oligomeric sesquisiloxane group |
| GelMA | Methacryloyl group |
| TCS | Thio-chitosan |
| PVA | Polyvinyl alcohol |
| TEOS | Tetraethyl orthosilicate |
| OPN | Osteopontin |
| BMSCs | Bone marrow mesenchymal stem cells |
| BP | Black phosphorus |
| BPNs | Black phosphorus nanosheets |
| U-Arg-PEA | Cationic arginine-based unsaturated poly(ester amide)s). |
| FTN | Fluorescent tracer nanoparticles |
| SFMA | Methacrylate-modified silk fibroin |
| SF | Silk fibroin |
| GMA | Glycidyl methacrylate |
| ICG | Indocyanine green |
| RPE | Retinal pigment epithelial |
| AMD | Age-related macular degeneration |
| ESCs | Embryonic stem cells |
| iPSCs | Induced pluripotent stem cells |
| PEG | Polyethylene glycol |
| GG | Gelling glue |
| RT-PCR | Reverse transcription PCR |
| CECs | Corneal endothelial cells |
| CPHFs | Chitosan–PEG hydrogel films |
| ADH | Adipic acid dihydrazide |
| ALD | Acid-aldehyde |
| GH | Hydrogel-nano-hydroxyapatite |
| CS | Chitosan |
| PHMB | Polyhexamethylene guanidine hydrochloride |
| PDL | Periodontal ligament |
| PDLC | Periodontal ligament cells |
| MMP | Matrix metalloproteinase |
| ALP | Alkaline phosphatase |
| IARC | International Agency for Research on Cancer |
| ICB | Immune checkpoint blockade therapy |
| CAR-T | Chimeric antigen receptor T-cell therapy |
| Im DCs | Immature dendritic cells |
| mDCs | Mature dendritic cells |
| CLTs | Cytotoxic T lymphocytes |
| GBM | Glioblastoma |
| STING | The interferon stimulating factor |
| TSPBA | N1-(4-boronobenzyl)-N3-(4-boronophenyl)-N1,N1,N3,N3-tetra- methylpropane-1,3-diaminium |
| AAV | Adeno-associated virus |
| rAAV | Recombinant adeno-associated virus |
| AD | Stimulator of interferon gene (STING) agonist |
| TBK1 | TANK Binding Kinase 1 |
| IRF3 | Interferon regulatory Factor 3 |
| IFNs | Type I interferons |
| DAMPs | Damage-associated molecular patterns |
| GEM | Gemcitabine |
| PDAC | Pancreatic ductal adenocarcinoma |
| DMXAA | 5, 6-dimethylflavone-4-acetic acid |
| TME | Tumor-suppressive immune microenvironment |
| ALG | Iodine-starch-alginate |
| Pp IX | Photosensitizer protoporphyrin IX |
| TK-CHO | Thioaldehyde ketone |
| EDCI | 1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide hydrochloride |
| TK | Thioketal linker |
| DCC | Dicyclohexylcarbodiimide |
| DMAP | 4-(dimethylamino)pyridine |
| DOX | Doxorubicin |
| CDs | Carbon dots |
| DH | DNA CpG hydrogel |
| G/DH | Bis-(3′-5′)-cyclic dimeric guanosine monophosphate |
| DC | Dendritic cells |
| TBI | Traumatic brain injury |
| DX | Dexamethasone |
| HA-DXM | Hyaluronic acid-dexamethasone |
| PEG-bis-AA | Poly (ethylene glycol-bis-(acryl-oxy-acetate)) |
| PLGA | Poly(lactic-co-glycolic acid |
| NFs | Neurotrophins |
| BDNF | Brain-derived neurotrophic factor |
| GDNF | Glial-derived neurotrophic factor |
| MMD | Moyamoya disease |
| TIAs | Transient ischemic attacks |
| EPO | Erythropoietin |
| MSN | Mesoporous silica nanoparticle |
| CPT | Camptothecin |
| PAEU | Polyethylene glycol-poly (β-amino-ester carbamate) |
References
- Perez-Luna, V.H.; Gonzalez-Reynoso, O. Encapsulation of Biological Agents in Hydrogels for Therapeutic Applications. Gels 2018, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef] [PubMed]
- Wichterce, O.; Lim, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Hennink, W.E.; Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2022, 54, 13–36. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Guo, B.; Zhong, Y.; Chen, X.; Yu, S.; Bai, J. 3D printing of electrically conductive and degradable hydrogel for epidermal strain sensor. Compos. Commun. 2023, 37, 101454. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Li, Y.; Chao, M.; Li, M.; Wan, P.; Zhang, L. Healable, Degradable, and Conductive MXene Nanocomposite Hydrogel for Multifunctional Epidermal Sensors. ACS Nano 2021, 15, 7765–7773. [Google Scholar] [CrossRef]
- Krasnopeeva, E.L.; Panova, G.G.; Yakimansky, A.V. Agricultural Applications of Superabsorbent Polymer Hydrogels. Int. J. Mol. Sci. 2022, 23, 15134. [Google Scholar] [CrossRef]
- Grosjean, M.; Gangolphe, L.; Nottelet, B. Degradable Self-healable Networks for Use in Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2205315. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H. Progresses in immunotherapy. Int. Rev. Immunol. 2020, 39, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Xu, Z. Gene therapy: A double-edged sword with great powers. Mol. Cell Biochem. 2020, 474, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.-Q.; Kang, L.; Yin, X.-Z.; Jin, Z.-H.; Gao, Z.-G. Synthesis of PEGylated hyaluronic acid for loading dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt) in nanoparticles for cancer treatment. Chin. Chem. Lett. 2015, 26, 695–699. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.; Yuan, M. Application of the Nano-Drug Delivery System in Treatment of Cardiovascular Diseases. Front Bioeng. Biotechnol. 2019, 7, 489. [Google Scholar] [CrossRef]
- Vora, L.K.; Moffatt, K.; Tekko, I.A.; Paredes, A.J.; Volpe-Zanutto, F.; Mishra, D.; Peng, K.; Raj Singh Thakur, R.; Donnelly, R.F. Microneedle array systems for long-acting drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 44–76. [Google Scholar] [CrossRef]
- Zhou, P.X.; Deng, S.Q.; Gong, Q.F. Recent development of targeted drug delivery system. Acta Pharm. Sin. 2010, 45, 300–306. [Google Scholar] [CrossRef]
- Kalpana, R.K.; Kinam, P. Biodegradable hydrogels in drug delivery. Adv. Drug Deliv. Rev. 1993, 11, 59–84. [Google Scholar]
- Zhang, W. Analysis on the Development and Application of Biodegradable Polymers. Earth Environ. Sci. 2021, 647, 012156. [Google Scholar] [CrossRef]
- Hussein, Y.H.A.; Youssry, M. Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs. Materials 2018, 11, 688. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Mohammadi Nasr, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Bioresorbable composite polymeric materials for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 926–940. [Google Scholar] [CrossRef]
- Banerjee, A.; Chatterjee, K.; Madras, G. Enzymatic degradation of polymers: A brief review. Mater. Sci. Technol. 2014, 30, 567–573. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Bertagnolli, C.; Grishin, A.; Vincent, T.; Guibal, E. Boron removal by a composite sorbent: Polyethylenimine/tannic acid derivative immobilized in alginate hydrogel beads. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2017, 52, 359–367. [Google Scholar] [CrossRef]
- Healy, K.E.; Rezania, A.; Stile, R.A. Designing biomaterials to direct biological responses. Ann. N. Y. Acad. Sci. 1999, 875, 24–35. [Google Scholar] [CrossRef]
- Seliktar, D.; Zisch, A.H.; Lutolf, M.P.; Wrana, J.L.; Hubbell, J.A. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J. Biomed. Mater. Res. Part A 2004, 68, 704–716. [Google Scholar] [CrossRef]
- Pratt, A.B.; Weber, F.E.; Schmoekel, H.G.; Muller, R.; Hubbell, J.A. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol. Bioeng. 2004, 86, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J.; Yang, J. Hydrogels as smart biomaterials. Polym. Int. 2007, 56, 1078–1098. [Google Scholar] [CrossRef]
- Yolanda, M.-M. Adult Stem Cell Therapy in Chronic Wound Healing. J. Stem Cell Res. Ther. 2014, 4, 162. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Health Mater. 2019, 8, e1801210. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiang, Y.W.; Jia, H.R.; Wu, F.G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, R.; Chen, B.; Liu, X.; Jia, Q.; Wang, X.; Yang, Z.; Ning, P.; Wang, Z.; Yang, Y. Injectable Reactive Oxygen Species-Responsive Hydrogel Dressing with Sustained Nitric Oxide Release for Bacterial Ablation and Wound Healing. Adv. Funct. Mater. 2022, 32, 2202857. [Google Scholar] [CrossRef]
- Chen, A.; He, H.; Ma, G.; Li, Y.; Jiang, S.; Xuan, X.; Song, Y.; Zhang, C.; Xiao, J.; Xu, Y.; et al. Biodegradable copolypeptide hydrogel prodrug accelerates dermal wound regeneration by enhanced angiogenesis and epithelialization. RSC Adv. 2018, 8, 10620–10626. [Google Scholar] [CrossRef]
- Donald, O. Wound Healing in Rabbits with a Lysine Deficiency. Nutr. Rev. 1967, 25, 125–127. [Google Scholar]
- Thangavel, P.; Ramachandran, B.; Chakraborty, S.; Kannan, R.; Lonchin, S.; Muthuvijayan, V. Accelerated Healing of Diabetic Wounds Treated with L-Glutamic acid Loaded Hydrogels Through Enhanced Collagen Deposition and Angiogenesis: An In Vivo Study. Sci. Rep. 2017, 7, 10701. [Google Scholar] [CrossRef]
- Liu, G.S.; Kong, Y.; Wang, Y.; Luo, Y.; Fan, X.; Xie, X.; Yang, B.R.; Wu, M.X. Microneedles for transdermal diagnostics: Recent advances and new horizons. Biomaterials 2020, 232, 119740. [Google Scholar] [CrossRef]
- Su, Y.; Mainardi, V.L.; Wang, H.; McCarthy, A.; Zhang, Y.S.; Chen, S.; John, J.V.; Wong, S.L.; Hollins, R.R.; Wang, G.; et al. Dissolvable Microneedles Coupled with Nanofiber Dressings Eradicate Biofilms via Effectively Delivering a Database-Designed Antimicrobial Peptide. ACS Nano 2020, 14, 11775–11786. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.Y.; Lee, J.; Kim, B.J.; Joo, K.I.; Kim, K.H.; Lim, G.; Cha, H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials 2019, 222, 119439. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Z.; Hou, C.; Huang, X.; Yi, B.; Yang, Y.; Zheng, W.; Zhao, X.; Yao, X. Mucus-Inspired Supramolecular Adhesives with Oil-Regulated Molecular Configurations and Long-Lasting Antibacterial Properties. ACS Appl. Mater. Interfaces 2020, 12, 16877–16886. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Chi, J.; Wang, Y.; Zhao, Y.; Luo, Y.; Wang, Y. Zn-MOF Encapsulated Antibacterial and Degradable Microneedles Array for Promoting Wound Healing. Adv. Health Mater. 2021, 10, e2100056. [Google Scholar] [CrossRef]
- Zhu, Y.; Cankova, Z.; Iwanaszko, M.; Lichtor, S.; Mrksich, M.; Ameer, G.A. Potent laminin-inspired antioxidant regenerative dressing accelerates wound healing in diabetes. Proc. Natl. Acad. Sci. USA 2018, 115, 6816–6821. [Google Scholar] [CrossRef]
- Xiang, J.; Zhu, R.; Lang, S.; Yan, H.; Liu, G.; Peng, B. Mussel-inspired immobilization of zwitterionic silver nanoparticles toward antibacterial cotton gauze for promoting wound healing. Chem. Eng. J. 2021, 409, 128291. [Google Scholar] [CrossRef]
- Montaser, A.S.; Rehan, M.; El-Senousy, W.M.; Zaghloul, S. Designing strategy for coating cotton gauze fabrics and its application in wound healing. Carbohydr. Polym. 2020, 244, 116479. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, Z.; Shi, R.; Cui, H.; Liu, Y.; Mao, H.; Wang, B.; Zhu, D.; Yan, F. Integrated Endotoxin Adsorption and Antibacterial Properties of Cationic Polyurethane Foams for Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 2860–2869. [Google Scholar] [CrossRef]
- Liu, X.L.; Cao, J.G.; Chai, X.T.; Liu, J.; Zhao, R.G.; Kong, N. Investigation of forming parameters on springback for ultra high strength steel considering Young’s modulus variation in cold roll forming. J. Manuf. Process. 2017, 29, 289–297. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, Q.; You, J.; Cheng, Y.; Hong, C.; Chen, Z.; Jiang, T.; Hao, T. Adhesion loss mechanism based on carboxymethyl cellulose-filled hydrocolloid dressings in physiological wounds environment. Carbohydr. Polym. 2020, 235, 115953. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, S.Y. Application of a paste-type acellular dermal matrix for coverage of chronic ulcerative wounds. Arch. Plast. Surg. 2018, 45, 564–571. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Pek, Y.S.; Gao, S.J.; Arshad, M.S.M.; Leck, K.J.; Ying, J.Y. Porous collagen-apatite nanocomposite foams as bone regeneration scaffolds. Biomaterials 2008, 29, 4300–4305. [Google Scholar] [CrossRef] [PubMed]
- Munch, E.; Launey, M.E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Tough, Bio-Inspired Hybrid Materials. Science 2008, 322, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Liu, M.; Zhang, Y.J.; Yin, J.B.; Pei, R.J. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.; Tomas, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Chen, C.; Sun, J.; Luo, J.; Cui, W.; Zhu, C.; Zhou, X.; Liu, X.; Yang, H.; et al. NSC-derived extracellular matrix-modified GelMA hydrogel fibrous scaffolds for spinal cord injury repair. NPG Asia Mater. 2022, 14, 20. [Google Scholar] [CrossRef]
- Thoma, D.S.; Payer, M.; Jakse, N.; Bienz, S.P.; Husler, J.; Schmidlin, P.R.; Jung, U.W.; Hammerle, C.H.F.; Jung, R.E. Randomized, controlled clinical two-centre study using xenogeneic block grafts loaded with recombinant human bone morphogenetic protein-2 or autogenous bone blocks for lateral ridge augmentation. J. Clin. Periodontol. 2018, 45, 265–276. [Google Scholar] [CrossRef]
- Sharifi, M.; Kheradmandi, R.; Salehi, M.; Alizadeh, M.; Ten Hagen, T.L.M.; Falahati, M. Criteria, Challenges, and Opportunities for Acellularized Allogeneic/Xenogeneic Bone Grafts in Bone Repairing. ACS Biomater. Sci. Eng. 2022, 8, 3199–3219. [Google Scholar] [CrossRef]
- Li, Y.Q.; Liu, Y.Q.; Xun, X.W.; Zhang, W.; Xu, Y.; Gu, D.Y. Three-Dimensional Porous Scaffolds with Biomimetic Microarchitecture and Bioactivity for Cartilage Tissue Engineering. Acs Appl. Mater. Inter 2019, 11, 36359–36370. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Seo, S.-J.; Won, J.-E.; Lee, E.-J.; Jang, J.-H.; Knowles, J.C.; Kim, H.-W. Therapeutically relevant aspects in bone repair and regeneration. Mater. Today 2015, 18, 573–589. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Zhang, T.X.; Chen, M.; Yao, K.; Huang, X.Q.; Zhang, B.; Li, Y.Z.; Liu, J.; Wang, Y.B.; Zhao, Z.H. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.W.; Chen, Y.H.; Wu, D.Y.; Wang, J.B.; Lv, M.M.; Wang, X.S.; Sun, J.; Zhang, Z.Y. Development of an Accurate and Proactive Immunomodulatory Strategy to Improve Bone Substitute Material-Mediated Osteogenesis and Angiogenesis. Theranostics 2018, 8, 5482–5500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fan, L.; Zhang, F.M.; Jiang, Y.; Cai, M.; Dai, C.; Luo, Y.A.; Tu, L.J.; Zhou, Z.N.; Li, X.J.; et al. Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioact. Mater. 2021, 6, 890–904. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, P.; Jiang, G.; Zhang, M.; Yu, F.; Dong, X.; Wang, L.; Chen, Y.; Zhang, W.; Qi, Y.; et al. A novel magnesium ion-incorporating dual-crosslinked hydrogel to improve bone scaffold-mediated osteogenesis and angiogenesis. Mater. Sci. Eng. C Mater. Biol Appl. 2021, 121, 111868. [Google Scholar] [CrossRef]
- Boriani, F.; Fazio, N.; Fotia, C.; Savarino, L.; Nicoli Aldini, N.; Martini, L.; Zini, N.; Bernardini, M.; Baldini, N. A novel technique for decellularization of allogenic nerves and in vivo study of their use for peripheral nerve reconstruction. J. Biomed. Mater. Res. A 2017, 105, 2228–2240. [Google Scholar] [CrossRef]
- Zheng, S.; Zhong, H.; Cheng, H.; Li, X.; Zeng, G.; Chen, T.; Zou, Y.; Liu, W.; Sun, C. Engineering Multifunctional Hydrogel With Osteogenic Capacity for Critical-Size Segmental Bone Defect Repair. Front Bioeng. Biotechnol. 2022, 10, 899457. [Google Scholar] [CrossRef]
- Goretti Penido, M.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wu, J.; Gu, Z. Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Appl. Mater. Interfaces 2019, 11, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Cholkar, K.; Dasari, S.R.; Pal, D.; Mitra, A.K. Eye: Anatomy, physiology and barriers to drug delivery. Ocul. Transp. Recept. 2013, 39, 1–36. [Google Scholar]
- Ow, V.; Loh, X.J. Recent developments of temperature-responsive polymers for ophthalmic applications. J. Polym. Sci. 2022, 60, 1429–1447. [Google Scholar] [CrossRef]
- Chauhan, A.; Fitzhenry, L.; Serro, A.P. Recent Advances in Ophthalmic Drug Delivery. Pharmaceutics 2022, 14, 2075. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.Z.; Zhu, Y.; Han, Y.; Shen, J.J.; Bao, B.B.; Gao, T.; Lin, J.Q.; Huang, T.L.; Xu, J.; et al. Tunable and Controlled Release of Cobalt Ions from Metal-Organic Framework Hydrogel Nanocomposites Enhances Bone Regeneration. Acs Appl. Mater. Inter 2021, 13, 59051–59066. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Dai, M.; Xu, K.; Xiao, D.; Zheng, Y.; Zheng, Q.; Shen, J.; Qian, Y.; Chen, W. In Situ Forming Hydrogel as a Tracer and Degradable Lacrimal Plug for Dry Eye Treatment. Adv. Health Mater. 2022, 11, e2200678. [Google Scholar] [CrossRef]
- Hageman, G.S.; Luthert, P.J.; Chong, N.H.V.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Gandhi, J.K.; Manzar, Z.; Bachman, L.A.; Andrews-Pfannkoch, C.; Knudsen, T.; Hill, M.; Schmidt, H.; Iezzi, R.; Pulido, J.S.; Marmorstein, A.D. Fibrin hydrogels as a xenofree and rapidly degradable support for transplantation of retinal pigment epithelium monolayers. Acta Biomater. 2018, 67, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, D.; Jeong, Y.W.; Choi, M.J.; Lee, G.W.; Thangavelu, M.; Song, J.E.; Khang, G. Engineering retinal pigment epithelial cells regeneration for transplantation in regenerative medicine using PEG/Gellan gum hydrogels. Int. J. Biol. Macromol. 2019, 130, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, K.; Bednarz, J.; Valtink, M. Prospects for endothelial transplantation. Exp. Eye Res. 2004, 78, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, B.; Brown, K.D.; Blencowe, A.; Daniell, M.; Stevens, G.W.; Qiao, G.G. Ultrathin chitosan-poly(ethylene glycol) hydrogel films for corneal tissue engineering. Acta Biomater. 2013, 9, 6594–6605. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ba, Q.; Niu, S.; Guo, Y.; Duan, Y.; Zhao, P.; Lin, C.; Sun, J. In-situ forming biodegradable glycol chitosan-based hydrogels: Synthesis, characterization, and chondrocyte culture. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 2017–2025. [Google Scholar] [CrossRef]
- Hong, H.; Liu, C.; Wu, W. Preparation and characterization of chitosan/PEG/gelatin composites for tissue engineering. J. Appl. Polym. Sci. 2009, 114, 1220–1225. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Braschler, T.M.; Renaud, P. Advances in the design of macroporous polymer scaffolds for potential applications in dentistry. J. Periodontal. Implant Sci. 2013, 43, 251–261. [Google Scholar] [CrossRef]
- Sanz, M.; Vignoletti, F. Key aspects on the use of bone substitutes for bone regeneration of edentulous ridges. Dent. Mater. 2015, 31, 640–647. [Google Scholar] [CrossRef]
- Jamjoom, A.; Cohen, R.E. Grafts for Ridge Preservation. J. Funct. Biomater. 2015, 6, 833–848. [Google Scholar] [CrossRef]
- Agarwal, R.; Garcia, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- Pan, Y.S.; Zhao, Y.; Kuang, R.; Liu, H.; Sun, D.; Mao, T.J.; Jiang, K.X.; Yang, X.T.; Watanabe, N.; Mayo, K.H.; et al. Injectable hydrogel-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion. Mat. Sci. Eng. C-Mater. 2020, 116, 111158. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Liu, C.R.; Han, Y.J.; Li, C.; Liu, S.D.; Li, X.Y.; Zhao, G.Q.; Jiang, Y.Y. An antibacterial chitosan-based hydrogel as a potential degradable bio-scaffold for alveolar ridge preservation. Rsc Adv. 2022, 12, 32219–32229. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human Periodontal. ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Nguyen, T.; Benoit, D.S.W. Matrix Control. of Periodontal. Ligament Cell Activity Via Synthetic Hydrogel Scaffolds. Tissue Eng. Part A 2021, 27, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018, 9, 2041731418768285. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Fang, F.; Xu, T.; Lan, M.; Zhang, J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 2021, 268, 120557. [Google Scholar] [CrossRef]
- Nomoto, T.; Nishiyama, N. Design of drug delivery systems for physical energy-induced chemical surgery. Biomaterials 2018, 178, 583–596. [Google Scholar] [CrossRef]
- Alamzadeh, Z.; Beik, J.; Pirhajati Mahabadi, V.; Abbasian Ardekani, A.; Ghader, A.; Kamrava, S.K.; Shiralizadeh Dezfuli, A.; Ghaznavi, H.; Shakeri-Zadeh, A. Ultrastructural and optical characteristics of cancer cells treated by a nanotechnology based chemo-photothermal therapy method. J. Photochem. Photobiol. B 2019, 192, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine--challenge and perspectives. Angew. Chem. Int. Ed Engl. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Blattman, J.N.; Greenberg, P.D. Cancer immunotherapy: A treatment for the masses. Science 2004, 305, 200–205. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, Q.; Liu, Z. Smart Injectable Hydrogels for Cancer Immunotherapy. Adv. Funct. Mater. 2019, 30, 1902785. [Google Scholar] [CrossRef]
- Tang, T.; Huang, X.; Zhang, G.; Hong, Z.; Bai, X.; Liang, T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoInt. in cancer immunotherapy. Signal. Transduct. Target. 2021, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.X.; Song, H.J.; Qin, Y.B.; Huang, P.S.; Zhang, C.N.A.; Kong, D.L.; Wang, W.W. Engineering Dendritic-Cell-Based Vaccines and PD-1 Blockade in Self-Assembled Peptide Nanofibrous Hydrogel to Amplify Antitumor T-Cell Immunity. Nano Lett. 2018, 18, 4377–4385. [Google Scholar] [CrossRef]
- Salah, M.; Tayebi, L.; Moharamzadeh, K.; Naini, F.B. Three-dimensional bio-printing and bone tissue engineering: Technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 18. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiang, Y.; Sheng, R.; Tomas, H.; Rodrigues, J.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Polysaccharide-based nanomedicines for cancer immunotherapy: A review. Bioact. Mater. 2021, 6, 3358–3382. [Google Scholar] [CrossRef]
- Cui, R.W.; Wu, Q.; Wang, J.; Zheng, X.M.; Ou, R.Y.; Xu, Y.S.; Qu, S.X.; Li, D.Y. Hydrogel-By-Design: Smart Delivery System for Cancer Immunotherapy. Front Bioeng. Biotechnol. 2021, 9, 723490. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Guo, H.; Li, D.; Hou, Y.; Kuang, T.; Ding, J. Intravesical Hydrogels as Drug Reservoirs. Trends Biotechnol. 2020, 38, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol 2018, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Binder, Z.A.; O’Rourke, D.M. Glioblastoma: The Current State of Biology and Therapeutic Strategies. Cancer Res. 2022, 82, 769–772. [Google Scholar] [CrossRef]
- Sun, S.; Gu, W.; Wu, H.S.; Zhao, Q.Q.; Qian, S.Y.; Xiao, H.; Yang, K.; Liu, J.; Jin, Y.; Hu, C.P.; et al. Immunostimulant In Situ Hydrogel Improves Synergetic Radioimmunotherapy of Malignant Glioblastoma Relapse Post-Resection. Adv. Funct. Mater. 2022, 32, 2205038. [Google Scholar] [CrossRef]
- Jiang, X.; Fitch, S.; Wang, C.; Wilson, C.; Li, J.; Grant, G.A.; Yang, F. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells Target. and eradicate glioblastoma via intracranial delivery. Proc. Natl. Acad. Sci. USA 2016, 113, 13857–13862. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Li, D.; Wang, Q.; Zhou, S.; Zhang, H.; Wang, Y.; He, Z.; Liu, H.; Sun, J. Promising alternatives of CD47 monoclonal antibody: An injectable degradable hydrogel loaded with PQ912 for postoperative immunotherapy effectively blocks CD47-SIRPalpha signal. Theranostics 2022, 12, 4581–4598. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Q.; Huang, J.; Zhang, F.; Yao, Z.; Shao, S.; Zhao, X.; Liang, T. In Situ Formed ROS-Responsive Hydrogel with STING Agonist and Gemcitabine to Intensify Immunotherapy against Pancreatic Ductal Adenocarcinoma. Adv. Health Mater. 2023, 12, e2203264. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef]
- Chen, S.X.; Ma, M.; Xue, F.F.; Shen, S.Z.; Chen, Q.; Kuang, Y.C.; Liang, K.C.; Wang, X.L.; Chen, H.R. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J. Control. Release 2020, 324, 218–227. [Google Scholar] [CrossRef]
- Moon, H.K.; Lee, S.H.; Choi, H.C. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano 2009, 3, 3707–3713. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Jiang, L.M.; Wu, H.H.; Zheng, W.Y.; Kan, D.; Cheng, R.; Yan, J.J.; Yu, C.; Sun, S.K. Biocompatible Iodine-Starch-Alginate Hydrogel for Tumor Photothermal Therapy. Acs Biomater. Sci. Eng. 2019, 5, 3654–3662. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef]
- Bai, J.; Wang, J.T.; Mei, K.C.; Al-Jamal, W.T.; Al-Jamal, K.T. Real-time monitoring of magnetic drug targeting using fibered confocal fluorescence microscopy. J. Control. Release 2016, 244, 240–246. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Huang, Q.; Xiao, J.; Zhang, Q.; Cheng, Y. A degradable hydrogel formed by dendrimer-encapsulated platinum nanoparticles and oxidized dextran for repeated photothermal cancer therapy. J. Mater. Chem. B 2018, 6, 2474–2480. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem. Int. Ed Engl. 2018, 57, 11522–11531. [Google Scholar] [CrossRef]
- Park, K.E.; Noh, Y.W.; Kim, A.; Lim, Y.T. Hyaluronic acid-coated nanoparticles for targeted photodynamic therapy of cancer guided by near-infrared and MR imaging. Carbohyd. Polym. 2017, 157, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with CheckpoInt. Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Zhang, C.; Alfranca, G.; Yang, Y.; Jiang, X.; Yang, Y.; Pan, F.; de la Fuente, J.M.; Cui, D. Near-Infrared Light Triggered ROS-activated Theranostic Platform based on Ce6-CPT-UCNPs for Simultaneous Fluorescence Imaging and Chemo-Photodynamic Combined Therapy. Theranostics 2016, 6, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Zeng, Z.S.; Huang, Z.Q.; Sun, Y.W.; Huang, Y.J.; Chen, J.; Ye, J.X.; Yang, H.L.; Yang, C.Z.; Zhao, C.S. Near-infrared light-triggered degradable hyaluronic acid hydrogel for on-demand drug release and combined chemo-photodynamic therapy. Carbohyd. Polym. 2020, 229, 115394. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Z.; Huang, Z.; Zhang, X.; He, S.; Sun, X.; Shen, Y.; Yan, M.; Zhao, C. Injectable, NIR/pH-Responsive Nanocomposite Hydrogel as Long-Acting Implant for Chemophotothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 20361–20375. [Google Scholar] [CrossRef]
- Hong, J.; Yun, C.O. Emergence of Ad-Mediated Combination Therapy Against Cancer: What to Expect? Curr. Cancer Drug Targets 2018, 18, 139–152. [Google Scholar] [CrossRef]
- Lu, Y.; Hua, J.; Yan, F.; Jiang, C.; Piao, Y.; Ye, Z.; Fu, Z.; Jiang, H.; Wang, F.; Jiang, Y. Combined radiotherapy and chemotherapy versus radiotherapy alone in elderly patients with nasopharyngeal carcinoma: A SEER population-based study. Medicine 2021, 100, e26629. [Google Scholar] [CrossRef]
- Jiang, R.; Dai, J.; Dong, X.; Wang, Q.; Meng, Z.; Guo, J.; Yu, Y.; Wang, S.; Xia, F.; Zhao, Z.; et al. Improving Image-Guided Surgical and Immunological Tumor Treatment Efficacy by Photothermal and Photodynamic Therapies Based on a Multifunctional NIR AIEgen. Adv. Mater. 2021, 33, e2101158. [Google Scholar] [CrossRef]
- Yue, J.; Miao, P.; Li, L.; Yan, R.H.; Dong, W.F.; Mei, Q. Injectable Carbon Dots-Based Hydrogel for Combined Photothermal Therapy and Photodynamic Therapy of Cancer. Acs Appl. Mater. Inter 2022, 14, 49582–49591. [Google Scholar] [CrossRef]
- Wang, D.; Niu, L.; Qiao, Z.Y.; Cheng, D.B.; Wang, J.; Zhong, Y.; Bai, F.; Wang, H.; Fan, H. Synthesis of Self-Assembled Porphyrin Nanoparticle Photosensitizers. ACS Nano 2018, 12, 3796–3803. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Q.Y.; Shim, G.; Oh, Y.K. Melanin-loaded CpG DNA hydrogel for modulation of tumor immune microenvironment. J. Control. Release 2021, 330, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, J.; Chen, Y.; Li, W.; Han, X.; Wang, L. Engineering Microcapsules for Simultaneous Delivery of Combinational Therapeutics. Adv. Mater. Technol. 2020, 5, 2000623. [Google Scholar] [CrossRef]
- Ujcic-Voortman, J.K.; Baan, C.A.; Seidell, J.C.; Verhoeff, A.P. Obesity and cardiovascular disease risk among Turkish and Moroccan migrant groups in Europe: A systematic review. Obes Rev. 2012, 13, 2–16. [Google Scholar] [CrossRef]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014, 35, 2950–2959. [Google Scholar] [CrossRef]
- Cainzos-Achirica, M.; Fedeli, U.; Sattar, N.; Agyemang, C.; Jenum, A.K.; McEvoy, J.W.; Murphy, J.D.; Brotons, C.; Elosua, R.; Bilal, U.; et al. Epidemiology, risk factors, and opportunities for prevention of cardiovascular disease in individuals of South Asian ethnicity living in Europe. Atherosclerosis 2019, 286, 105–113. [Google Scholar] [CrossRef]
- Gersh, B.J.; Sliwa, K.; Mayosi, B.M.; Yusuf, S. Novel therapeutic concepts: The epidemic of cardiovascular disease in the developing world: Global implications. Eur. Heart J. 2010, 31, 642–648. [Google Scholar] [CrossRef]
- Madonna, R.; De Caterina, R. Stem cells and growth factor delivery systems for cardiovascular disease. J. Biotechnol. 2011, 154, 291–297. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Matoba, T.; Koga, J.I.; Nakano, K.; Egashira, K.; Tsutsui, H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J. Cardiol. 2017, 70, 206–211. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Hydera, A.A.; Wunderlichb, C.A.; Gururajc, P.P.G.; Kobusingyed, O.C. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [CrossRef]
- Ahmad, K.; Alrais, Z.F.; Elkholy, H.M.; Elkhouly, A.E.; Beniamein, M.M.; Abdel Hadi, A.; Majeed, S.; Shoaib, A. Effect of Early Correction of Hyponatremia on Neurological Outcome in Traumatic Brain Injury Patients. J. Intensive Crit. Care 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Faden, A.I.; Wu, J.; Stoica, B.A.; Loane, D.J. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br. J. Pharm. 2016, 173, 681–691. [Google Scholar] [CrossRef]
- Macks, C.; Jeong, D.; Bae, S.; Webb, K.; Lee, J.S. Dexamethasone-Loaded Hydrogels Improve Motor and Cognitive Functions in a Rat Mild Traumatic Brain Injury Model. Int. J. Mol. Sci. 2022, 23, 11153. [Google Scholar] [CrossRef]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Approaches to neural tissue engineering using scaffolds for drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 325–338. [Google Scholar] [CrossRef]
- Lampe, K.J.; Kern, D.S.; Mahoney, M.J.; Bjugstad, K.B. The administration of BDNF and GDNF to the brain via PLGA microparticles patterned within a degradable PEG-based hydrogel: Protein distribution and the glial response. J. Biomed. Mater. Res. A 2011, 96, 595–607. [Google Scholar] [CrossRef]
- Pandey, P.; Steinberg, G.K. Neurosurgical advances in the treatment of moyamoya disease. Stroke 2011, 42, 3304–3310. [Google Scholar] [CrossRef]
- Hyun, S.J.; Kim, J.S.; Hong, S.C. Prognostic factors associated with perioperative ischemic complications in adult-onset moyamoya disease. Acta Neurochir. 2010, 152, 1181–1188. [Google Scholar] [CrossRef]
- Deng, X.; Ge, P.; Wang, R.; Zhang, D.; Zhao, J.; Zhang, Y. Risk factors for postoperative ischemic complications in pediatric moyamoya disease. BMC Neurol. 2021, 21, 229. [Google Scholar] [CrossRef]
- Carenza, E.; Jordan, O.; Martinez-San Segundo, P.; Jirik, R.; Starcuk Jr, Z.; Borchard, G.; Rosell, A.; Roig, A. Encapsulation of VEGF(165) into magnetic PLGA nanocapsules for potential local delivery and bioactivity in human brain endothelial cells. J. Mater. Chem. B 2015, 3, 2538–2544. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- des Rieux, A.; Ucakar, B.; Mupendwa, B.P.; Colau, D.; Feron, O.; Carmeliet, P.; Preat, V. 3D systems delivering VEGF to promote angiogenesis for tissue engineering. J. Control. Release 2011, 150, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cao, L.; Shvartsman, D.; Silva, E.A.; Mooney, D.J. Targeted delivery of nanoparticles to ischemic muscle for imaging and therapeutic angiogenesis. Nano Lett. 2011, 11, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Park, G.H.; Shin, H.S.; Choi, E.S.; Yoon, B.S.; Choi, M.H.; Lee, S.J.; Lee, K.E.; Lee, J.S.; Hong, J.M. Cranial burr hole with erythropoietin administration induces reverse arteriogenesis from the enriched extracranium. Neurobiol. Dis. 2019, 132, 104538. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Wang, Y.; Zhang, R.; Chopp, M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 2004, 35, 1732–1737. [Google Scholar] [CrossRef]
- Chen, J.; Cui, X.; Zacharek, A.; Ding, G.L.; Shehadah, A.; Jiang, Q.; Lu, M.; Chopp, M. Niaspan treatment increases tumor necrosis factor-alpha-converting enzyme and promotes arteriogenesis after stroke. J. Cereb. Blood Flow Metab. 2009, 29, 911–920. [Google Scholar] [CrossRef]
- Scholz, D.; Schaper, W. Enhanced arteriogenesis in mice overexpressing erythropoietin. Cell Tissue Res. 2006, 324, 395–401. [Google Scholar] [CrossRef]
- Imazuru, T.; Matsushita, S.; Hyodo, K.; Tokunaga, C.; Kanemoto, S.; Enomoto, Y.; Watanabe, Y.; Hiramatsu, Y.; Sakakibara, Y. Erythropoietin Enhances Arterioles More Significantly Than it Does Capillaries in an Infarcted Rat Heart Model. Int. Heart J. 2009, 50, 801–810. [Google Scholar] [CrossRef]
- Buschmann, I.R.; Busch, H.J.; Mies, G.; Hossmann, K.A. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation 2003, 108, 610–615. [Google Scholar] [CrossRef]
- Duelsner, A.; Gatzke, N.; Glaser, J.; Hillmeister, P.; Li, M.; Lee, E.J.; Lehmann, K.; Urban, D.; Meyborg, H.; Stawowy, P.; et al. Granulocyte colony-stimulating factor improves cerebrovascular reserve capacity by enhancing collateral growth in the circle of Willis. Cereb. Dis. 2012, 33, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Schneeloch, E.; Mies, G.; Busch, H.J.; Buschmann, L.R.; Hossmann, K.A. Granulocyte-macrophage colony-stimulating factor-induced arteriogenesis reduces energy failure in hemodynamic stroke. Proc. Natl. Acad. Sci. USA 2004, 101, 12730–12735. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Wood, K.M.; Blanchette, J.O. Hydrogels for oral delivery of therapeutic proteins. Expert Opin. Biol. Ther. 2004, 4, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Labie, H.; Blanzat, M. Hydrogels for dermal and transdermal drug delivery. Biomater. Sci. 2023, 11, 4073–4093. [Google Scholar] [CrossRef] [PubMed]
- Kauer, T.M.; Figueiredo, J.L.; Hingtgen, S.; Shah, K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat. Neurosci. 2011, 15, 197–204. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, J.; Xu, W.; Zhang, M.; Cui, P.; Guo, Z.; Deng, Y.; Chen, H.; Sun, W. Highly Efficient Far-Red/NIR-Absorbing Neutral Ir(III) Complex Micelles for Potent Photodynamic/Photothermal Therapy. Adv. Mater. 2021, 33, e2100795. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Min Jung, J.; Lip Jung, Y.; Han Kim, S.; Sung Lee, D.; Thambi, T. Injectable hydrogel imbibed with camptothecin-loaded mesoporous silica nanoparticles assoftware an implantable sustained delivery depot for cancer therapy. J. Colloid. Interface Sci. 2023, 636, 328–340. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, Y.; Ryu, H.A.; Jang, Y.; Lee, K.M.; Choi, Y.; Choi, W.J.; Lee, M.; Park, K.M.; Park, K.D.; et al. Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing. Acta Biomater. 2016, 38, 59–68. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Kermanpur, A.; Mokhtari, H. Sprayable and injectable visible-light Kappa-carrageenan hydrogel for in-situ soft tissue engineering. Int. J. Biol. Macromol. 2019, 138, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Ruan, C.; Xie, H.; Li, Z.; Wang, H.; Chu, P.K.; Yu, X.F. Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv. Sci. 2018, 5, 1700848. [Google Scholar] [CrossRef] [PubMed]















| Application | Useful Characteristics |
|---|---|
| Wound Treatment | supports cell adhesion and proliferation, biocompatibility, biodegradability |
| Tissue engineering | biocompatibility, excellent mechanical properties, biodegradability |
| Oncology treatment | high water content, drug delivery, biocompatibility, biodegradability |
| Brain diseases | biodegradability, drug delivery, biocompatibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, B.; Gao, J.; Lu, Y.; Wang, Y. Applications of Degradable Hydrogels in Novel Approaches to Disease Treatment and New Modes of Drug Delivery. Pharmaceutics 2023, 15, 2370. https://doi.org/10.3390/pharmaceutics15102370
Hu B, Gao J, Lu Y, Wang Y. Applications of Degradable Hydrogels in Novel Approaches to Disease Treatment and New Modes of Drug Delivery. Pharmaceutics. 2023; 15(10):2370. https://doi.org/10.3390/pharmaceutics15102370
Chicago/Turabian StyleHu, Bo, Jinyuan Gao, Yu Lu, and Yuji Wang. 2023. "Applications of Degradable Hydrogels in Novel Approaches to Disease Treatment and New Modes of Drug Delivery" Pharmaceutics 15, no. 10: 2370. https://doi.org/10.3390/pharmaceutics15102370





