Development of a Nanoemulgel for the Topical Application of Mupirocin
Abstract
:1. Introduction
2. Materials and Methods
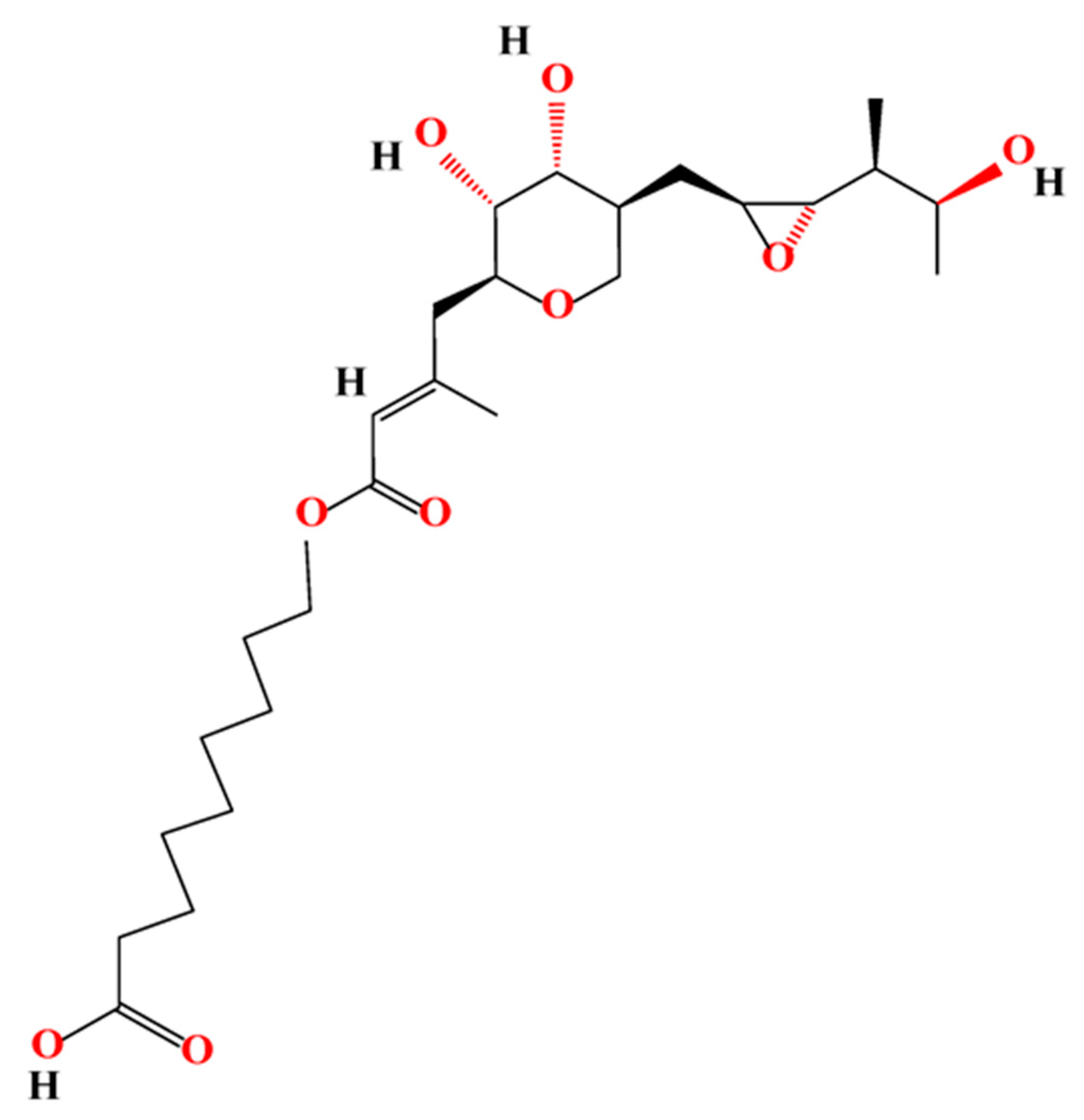
2.1. Materials
2.2. Solubility Determination
2.3. HPLC Method
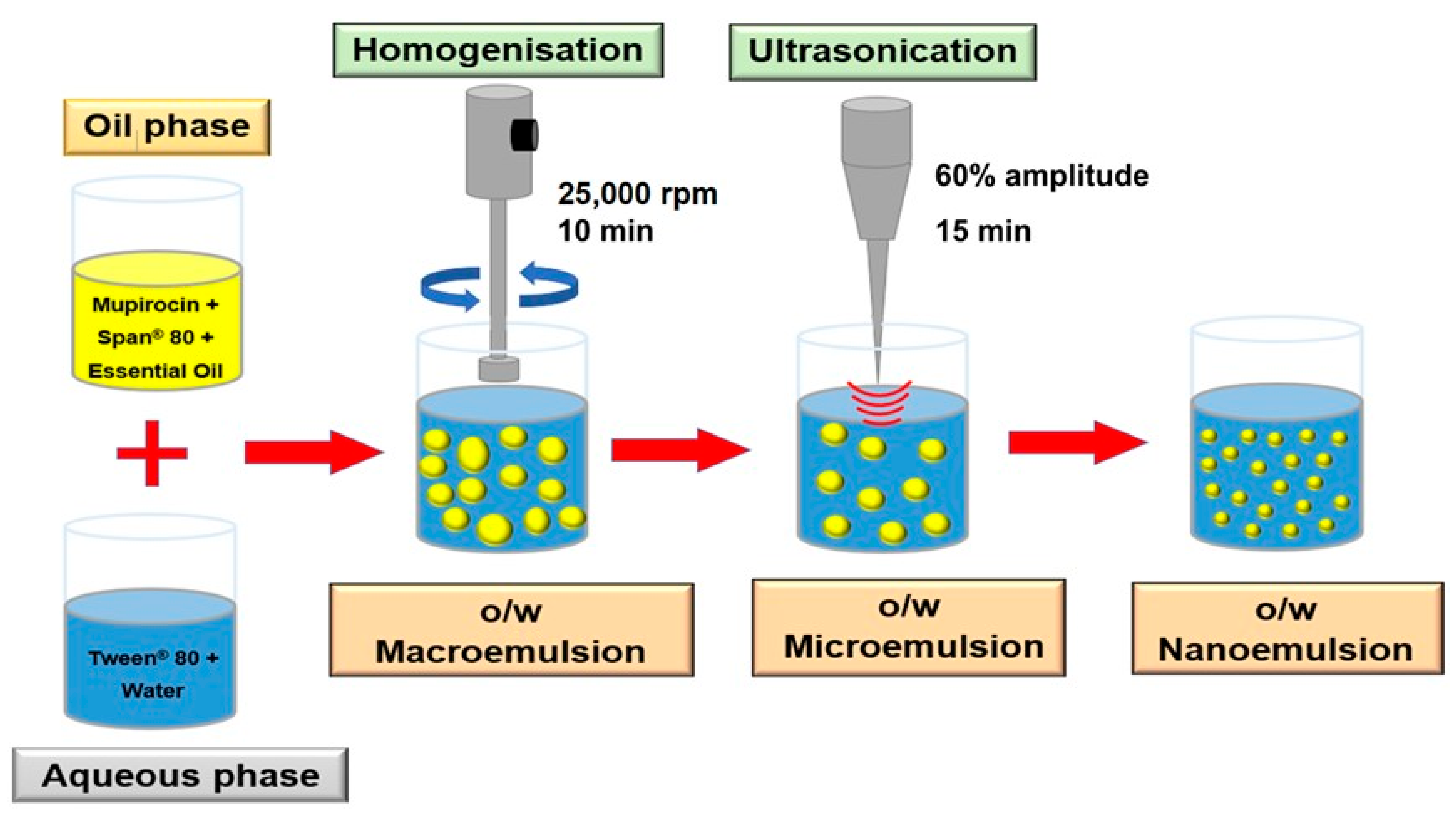
2.4. Preparation of Nanoemulsions
2.5. Preparation of MUP-Loaded Nanoemulsions
2.6. Measurement of Size, Polydispersity Index, and Zeta Potential of Nanoemulsions
2.7. Determination of Entrapment Efficiency (EE%)
2.8. Preparation of Hydrogel
2.9. Selection of the Gel for Nanoemulgel Formulation
2.9.1. Measurement of Viscosity of Hydrogel and Nanoemulgel
2.9.2. Texture Analysis Profile
2.10. Preparation of Nanoemulgel with/without MUP
2.11. Characterisation of Nanoemulgel
2.11.1. Visual Examination
2.11.2. Determination of Particle Size, Polydispersity, and Zeta Potential
2.11.3. Determination of the pH of Gel and Nanoemulgel
2.11.4. Assessment of Spreadability of Nanoemulgel
2.12. Thermodynamic Stability Study
2.12.1. Long-Term Stability Studies
2.12.2. Accelerated Stability Studies
2.13. In Vitro Permeation Studies of MUP through Strat-M® Membrane and Porcine Skin
2.13.1. In Vitro Permeation Studies of Nanoemulgel Using Strat-M® Membrane
2.13.2. Preparation of the Skin and In Vitro Skin Permeation Studies of Nanoemulgel
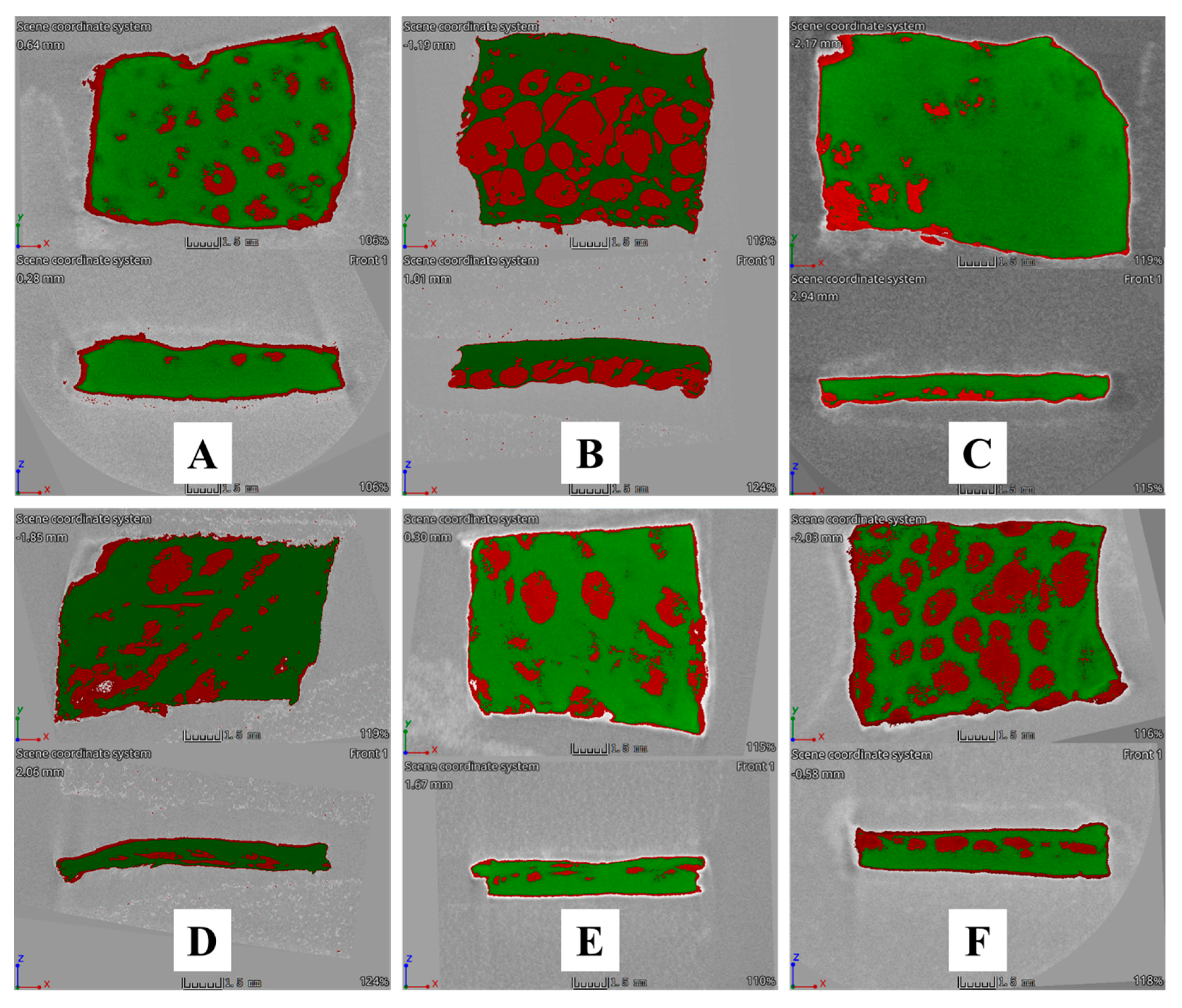
2.14. Qualitative Determination of MUP Deposited in Skin Using a Micro-CT Scan
2.15. Antibacterial Testing
2.16. Statistical Data Analysis
3. Results and Discussion
3.1. Preparation and Optimisation of Nanoemulgel
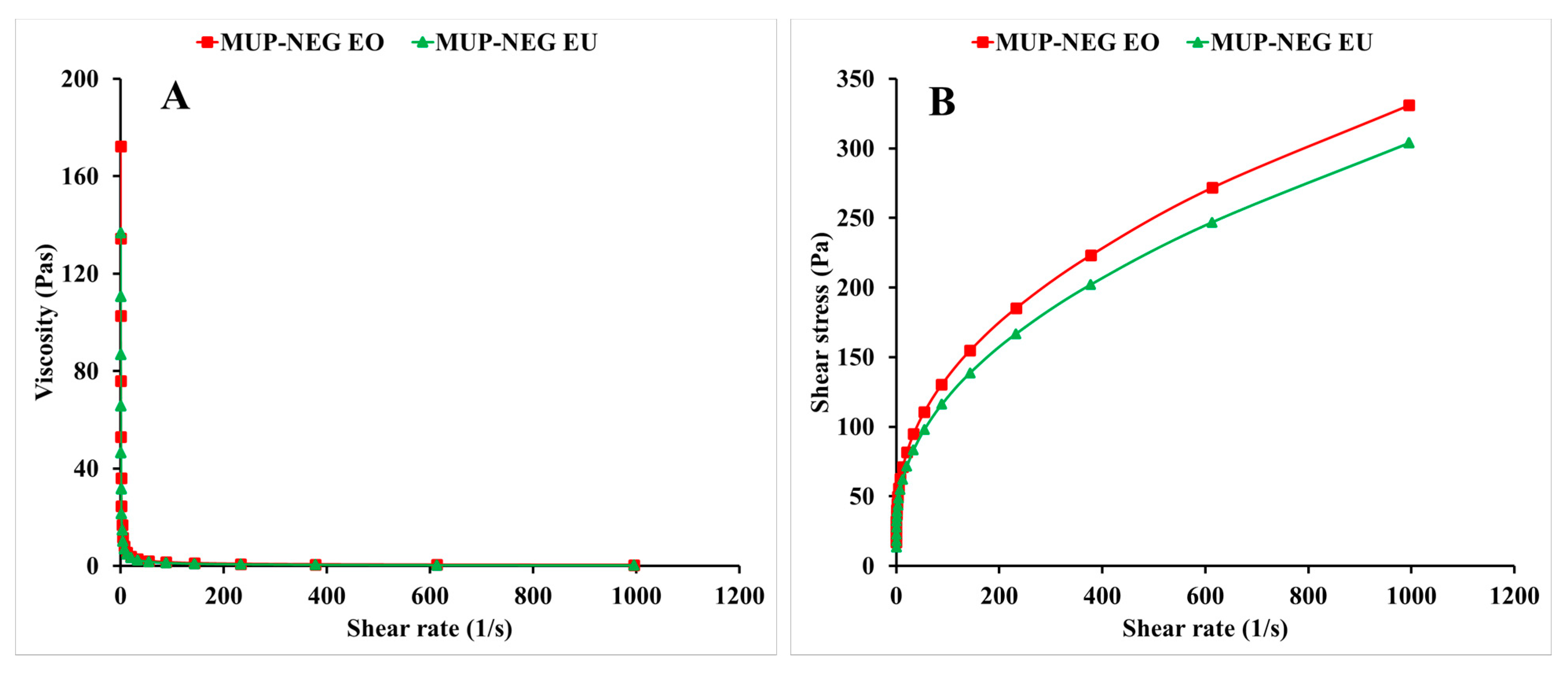
3.1.1. Viscosity Measurement
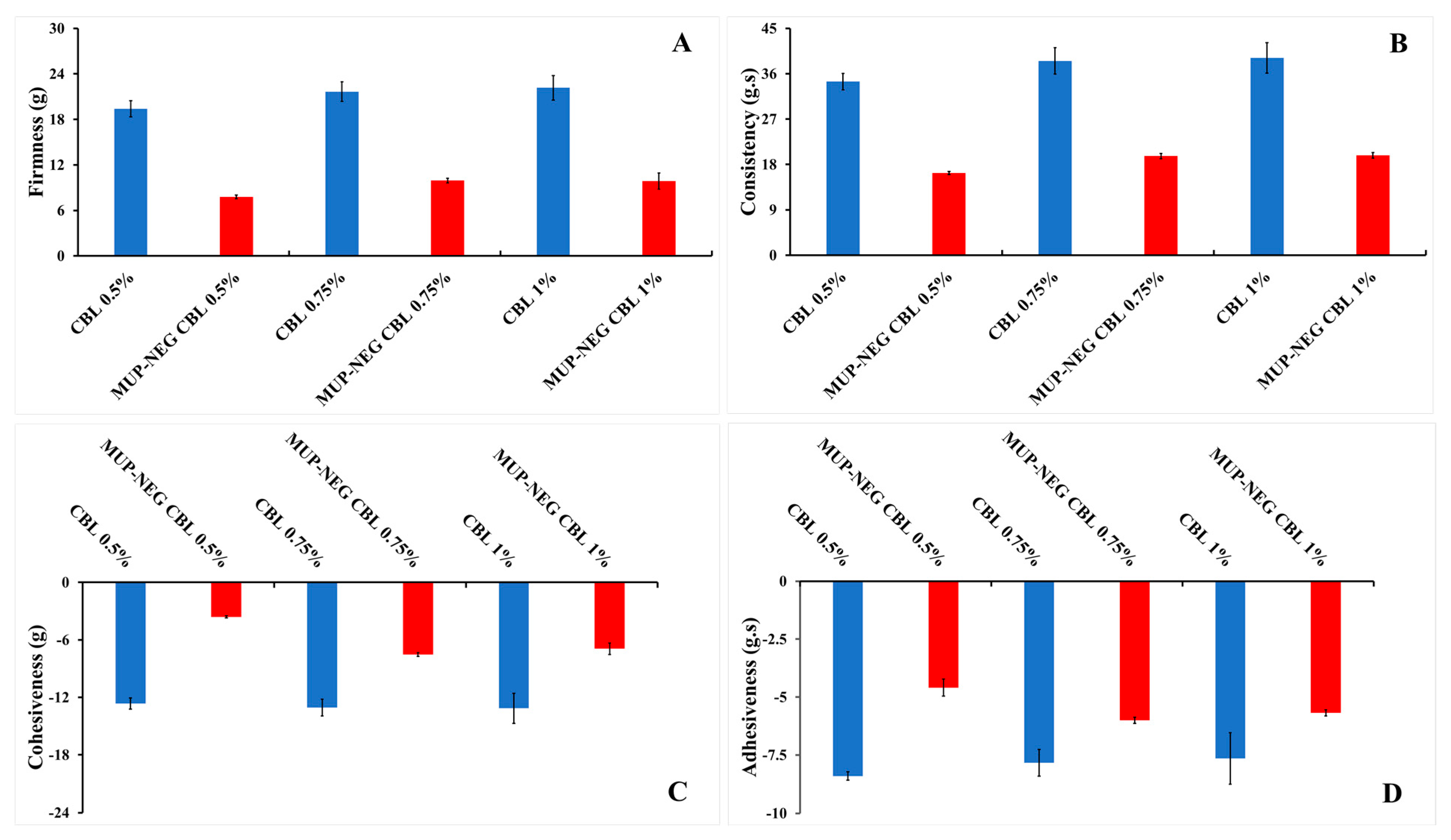
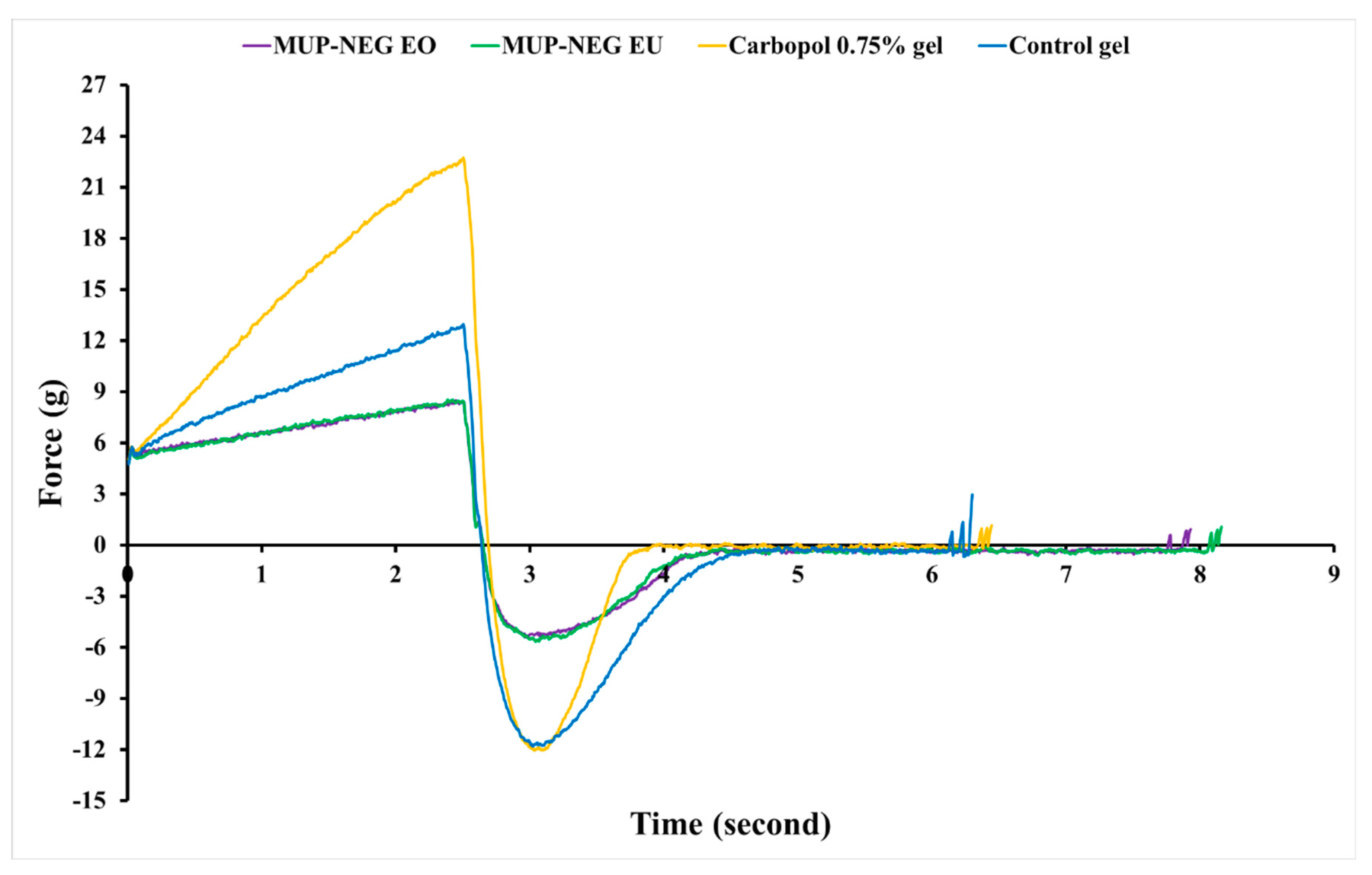
3.1.2. Texture Analysis
3.2. Physicochemical Characterisation of Nanoemulgel Formulations
3.2.1. Organoleptic Properties
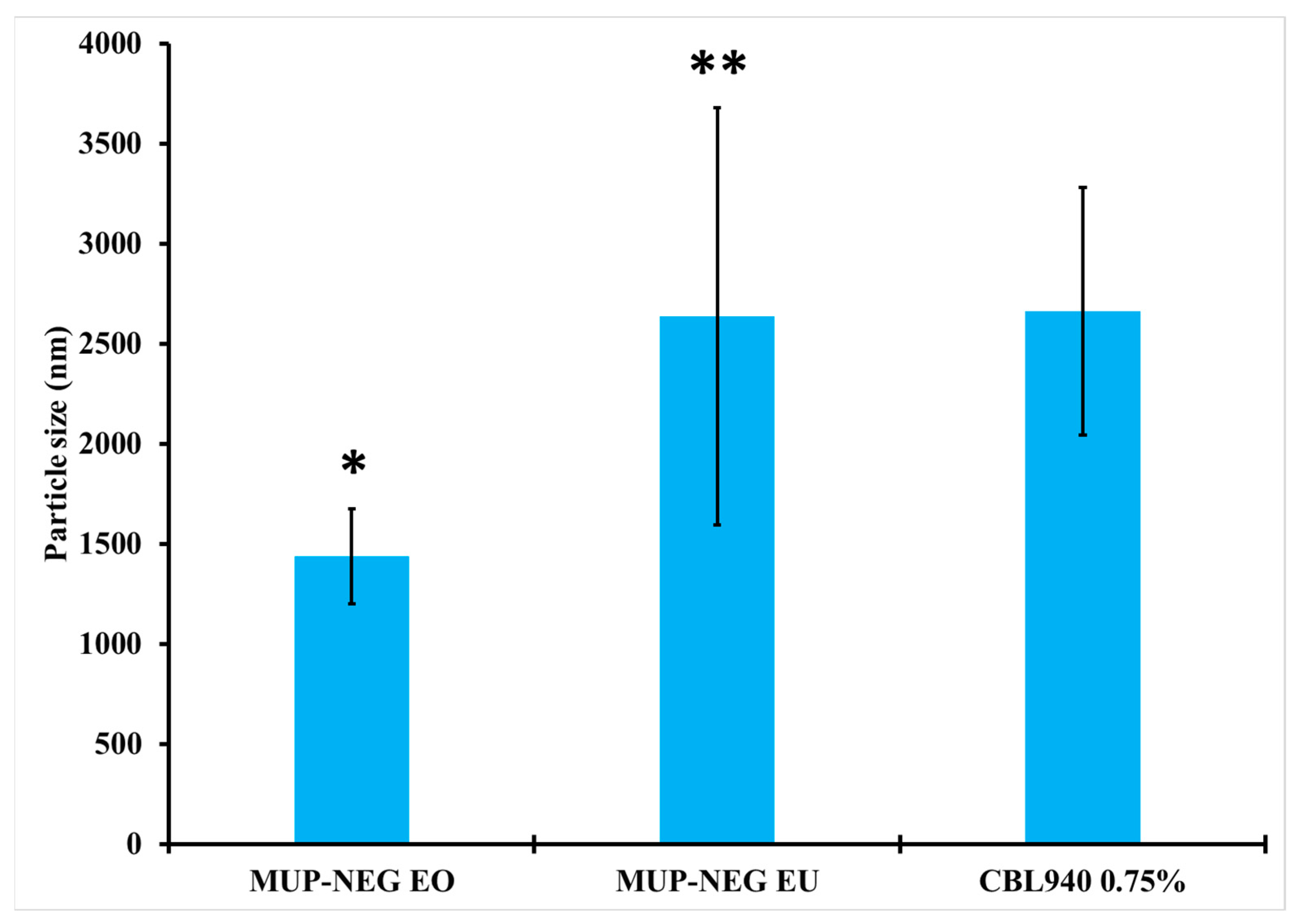
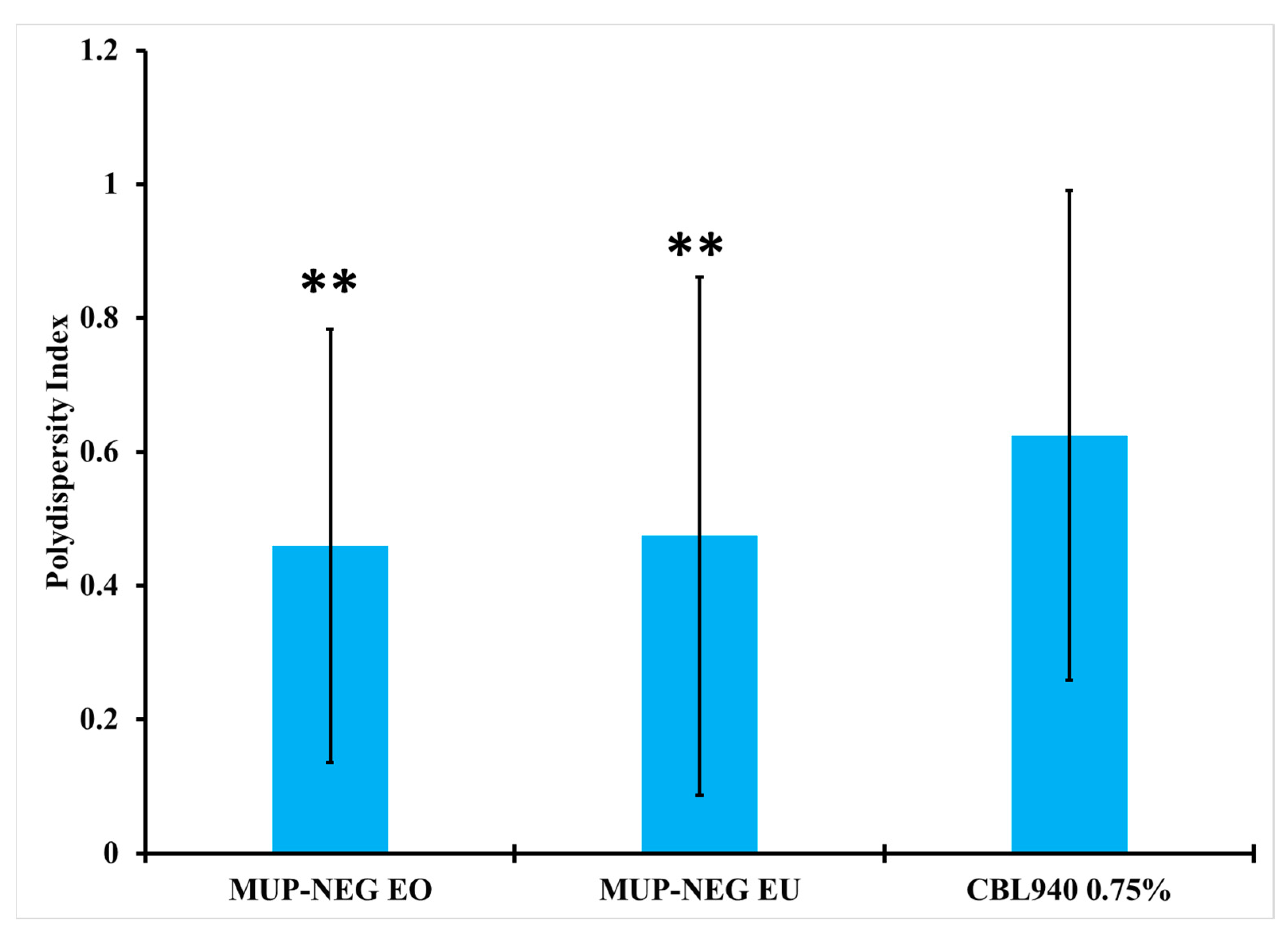
3.2.2. Measurement of Particle Size and Polydispersity Index (PDI)
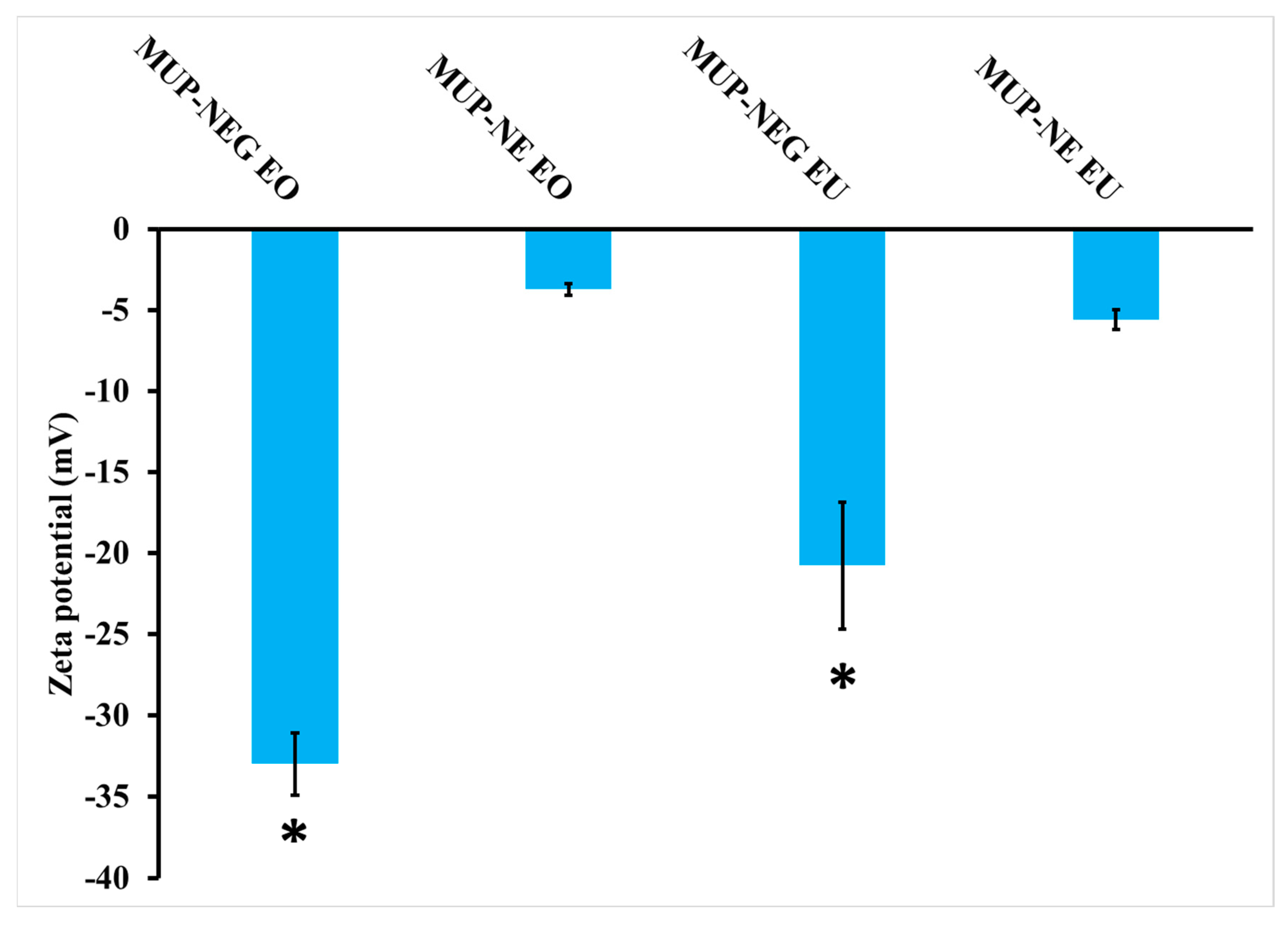
3.2.3. Determination of Zeta Potential
3.2.4. Measurement of pH
3.2.5. Determination of Viscosity
3.2.6. Determination of Spreadability
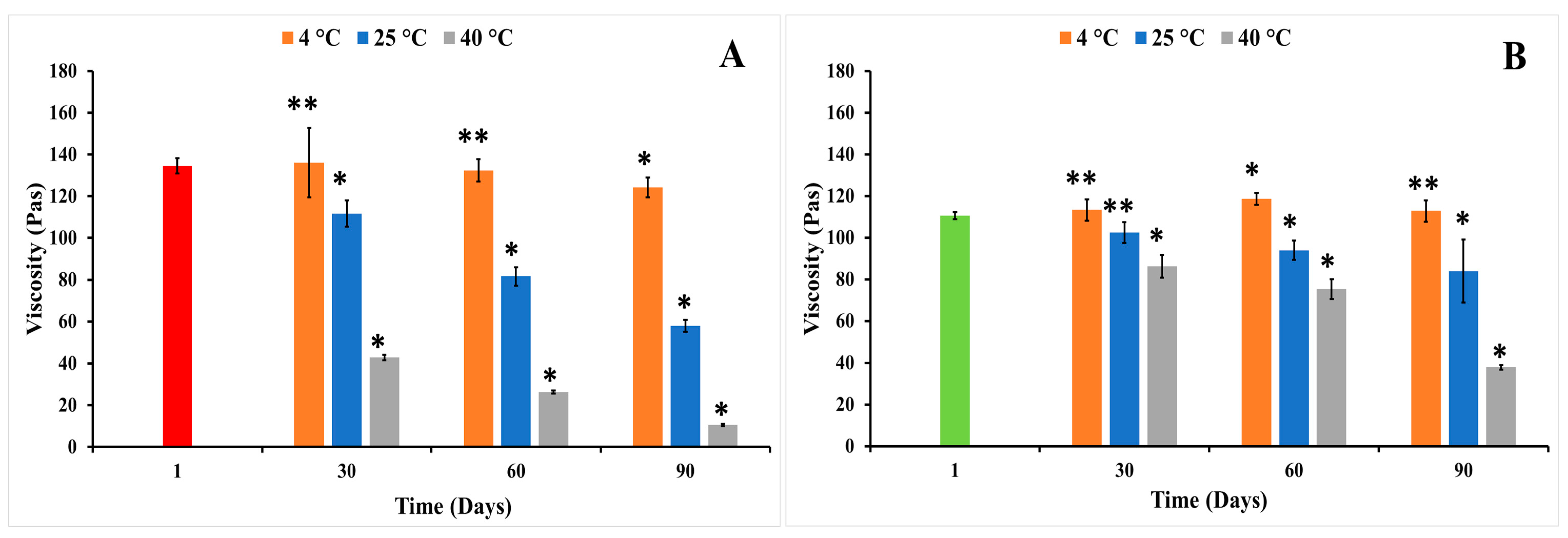
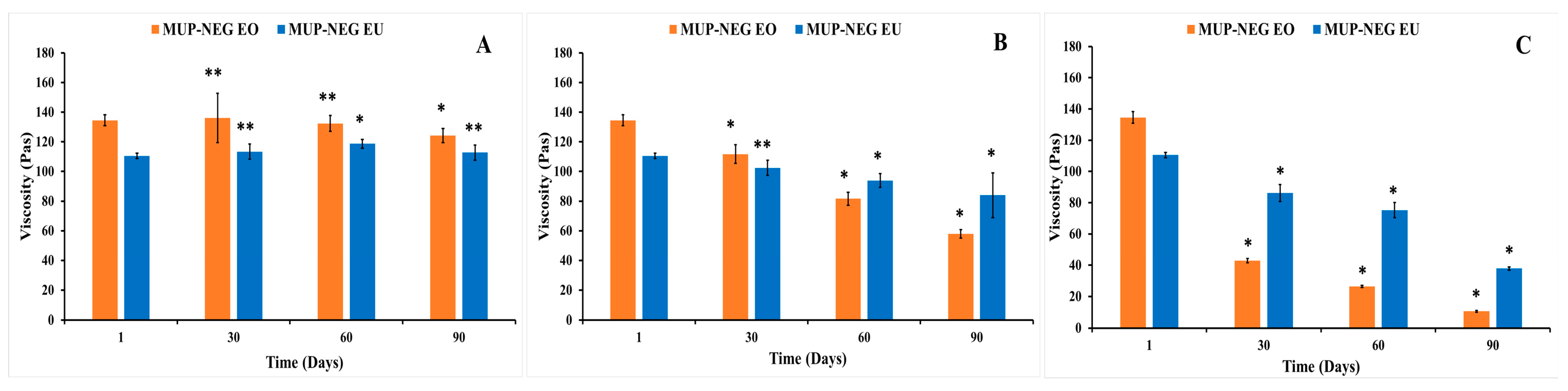
3.3. Thermodynamic Stability Studies
Long-Term Stability Studies
3.4. In Vitro Permeation Studies of MUP through Strat-M® Membrane and Porcine Skin
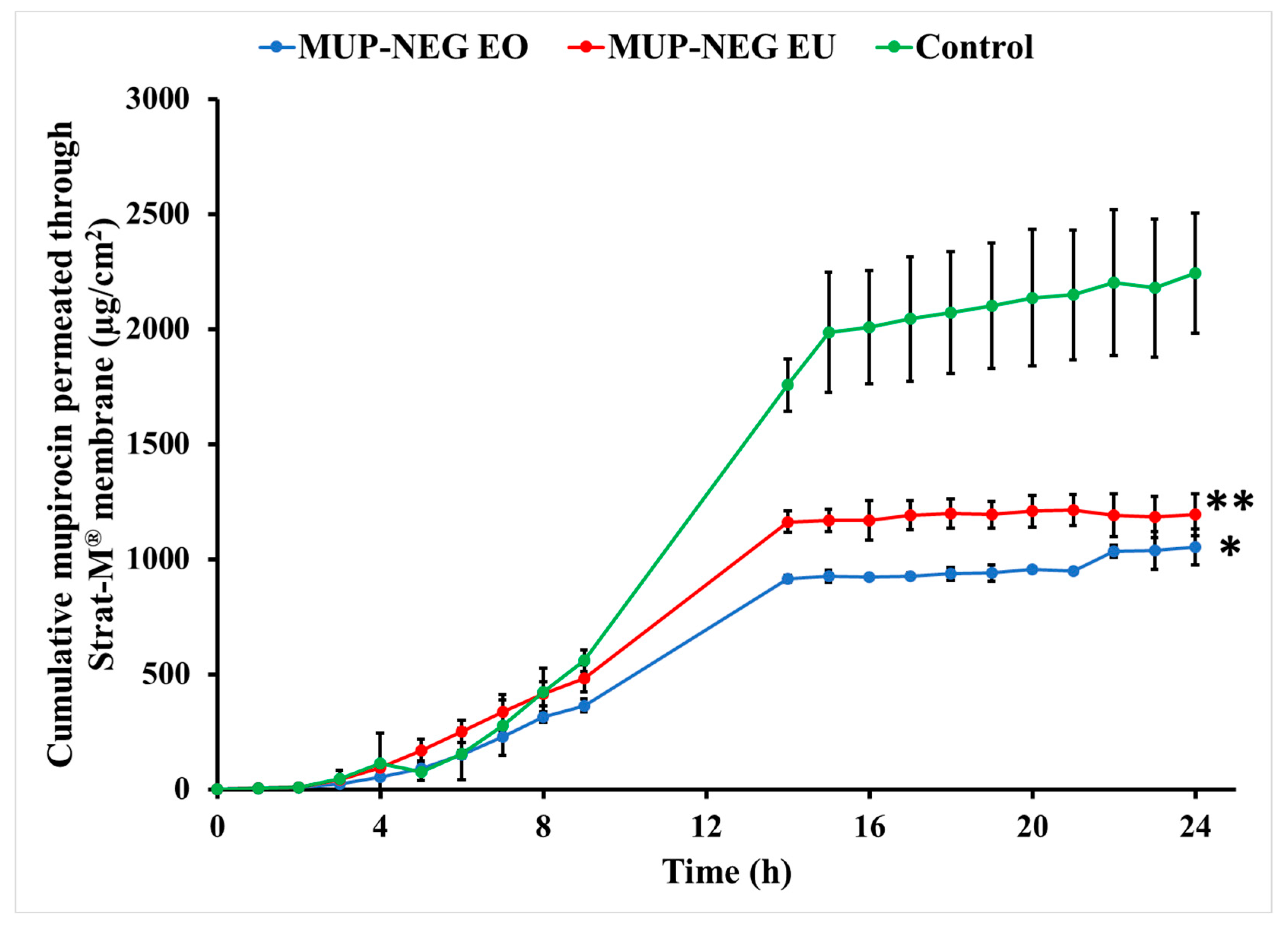
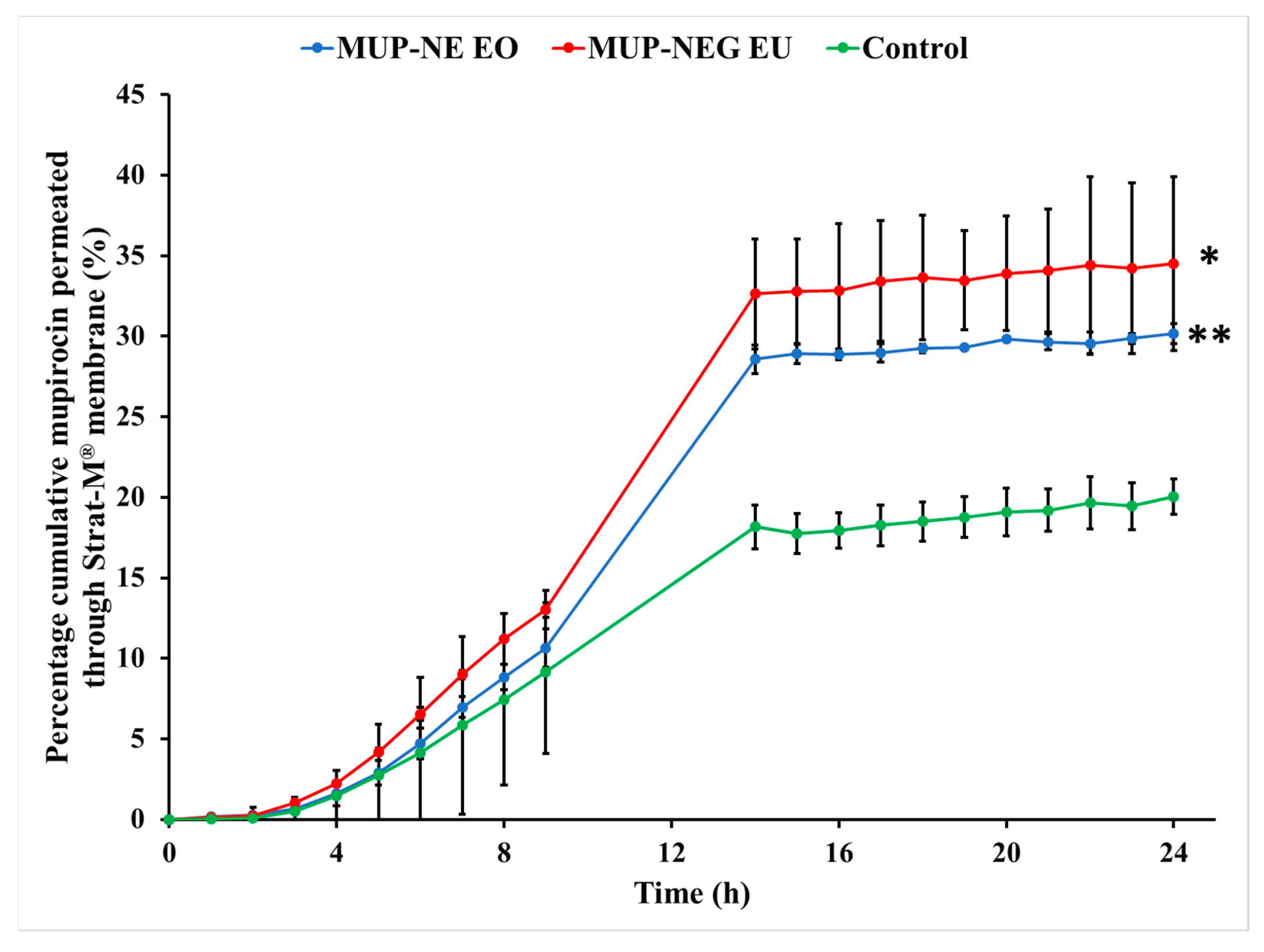
3.4.1. In Vitro Permeation Study of MUP from Nanoemulgel Formulations Using Strat-M® Membrane
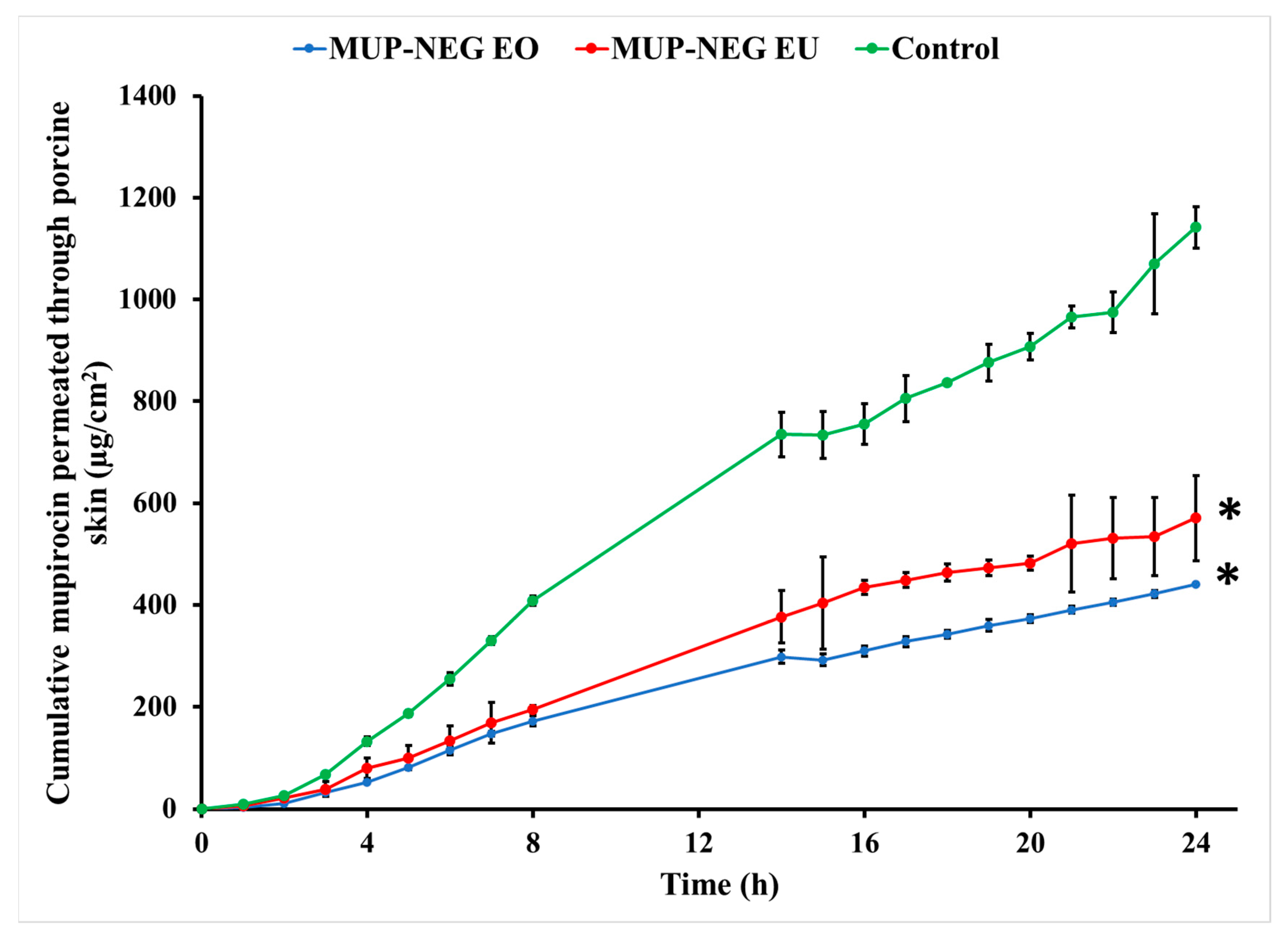
3.4.2. In Vitro Skin Permeation Study of MUP from Nanoemulgel Formulation
3.5. Quantification of MUP in Skin
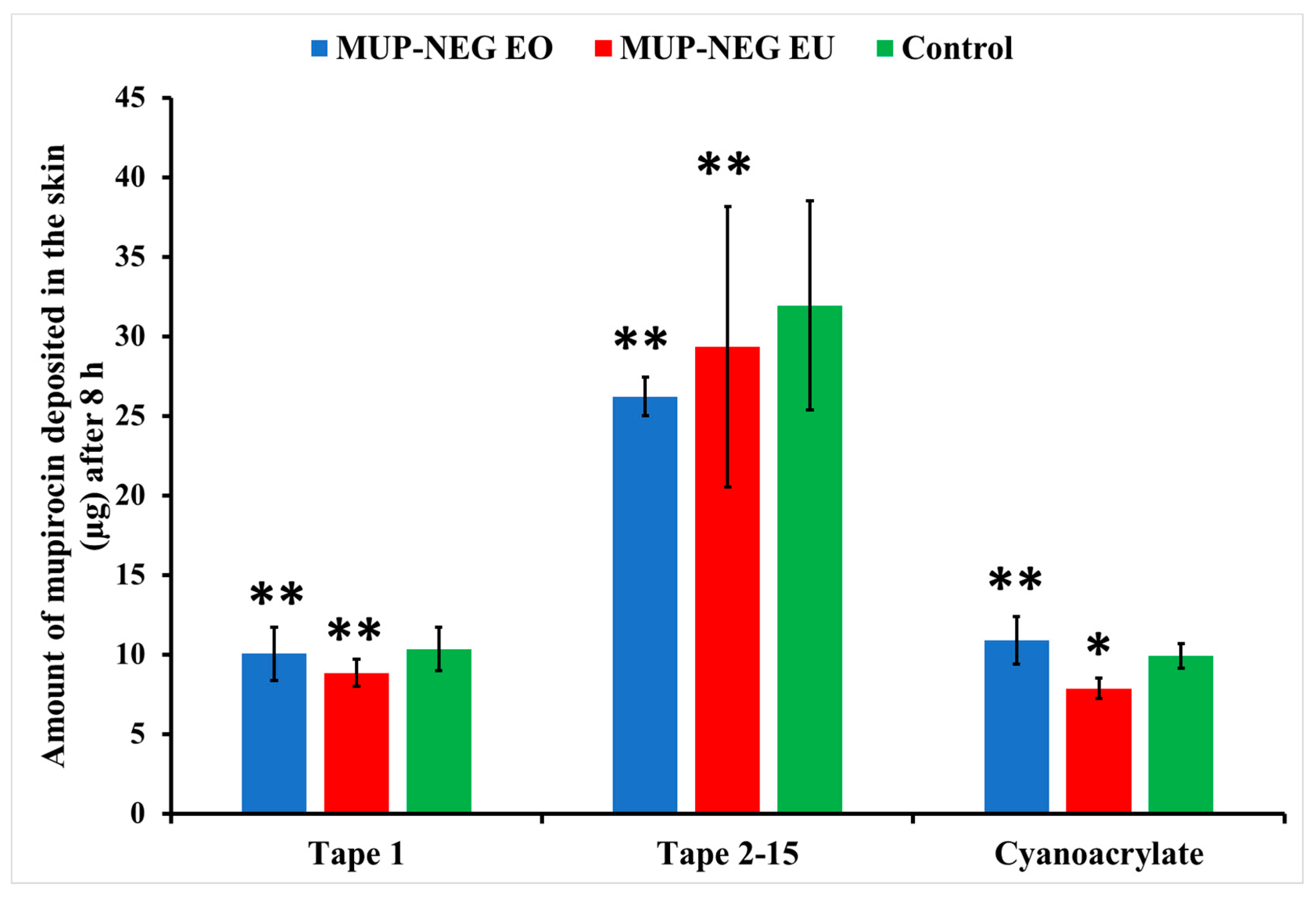
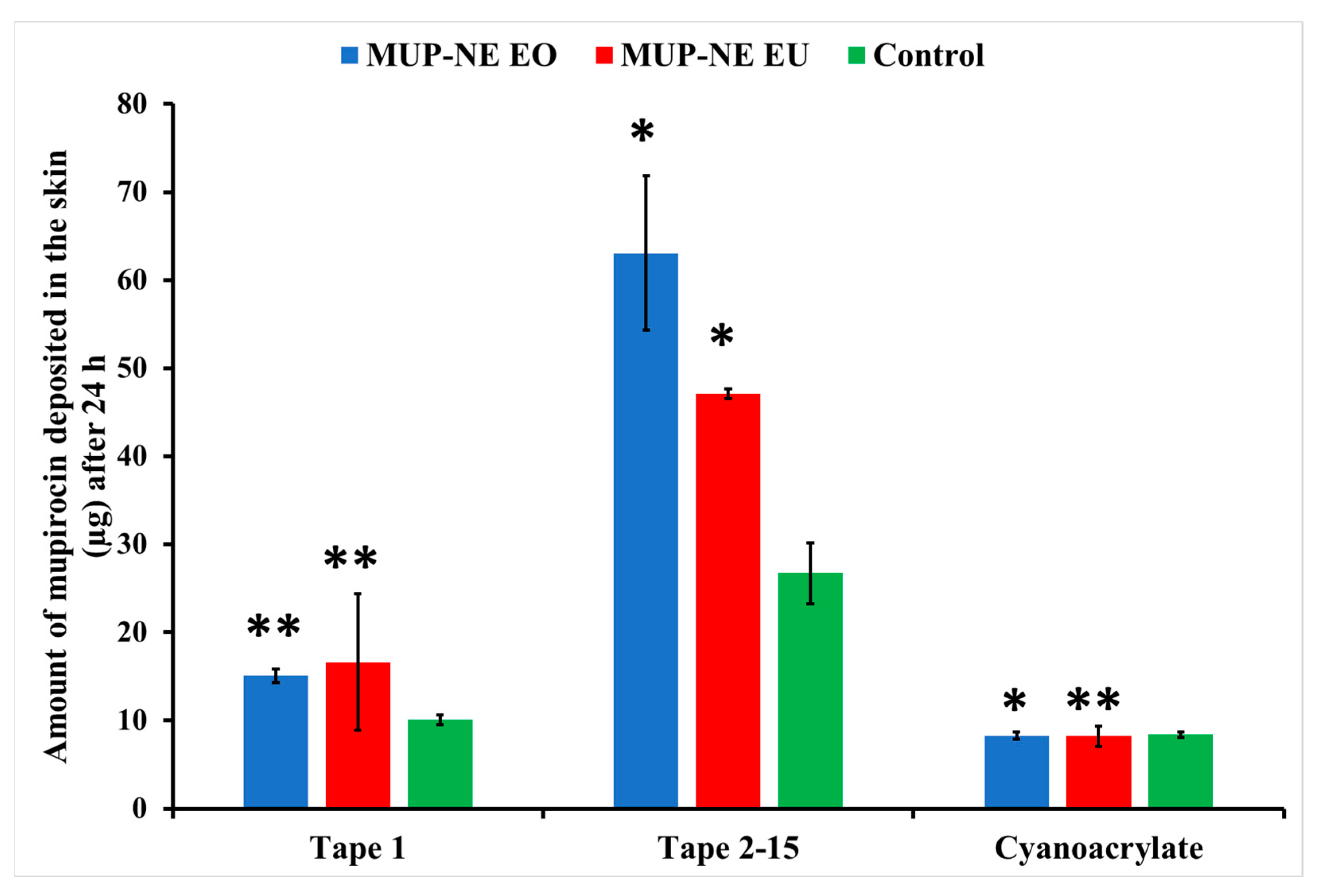
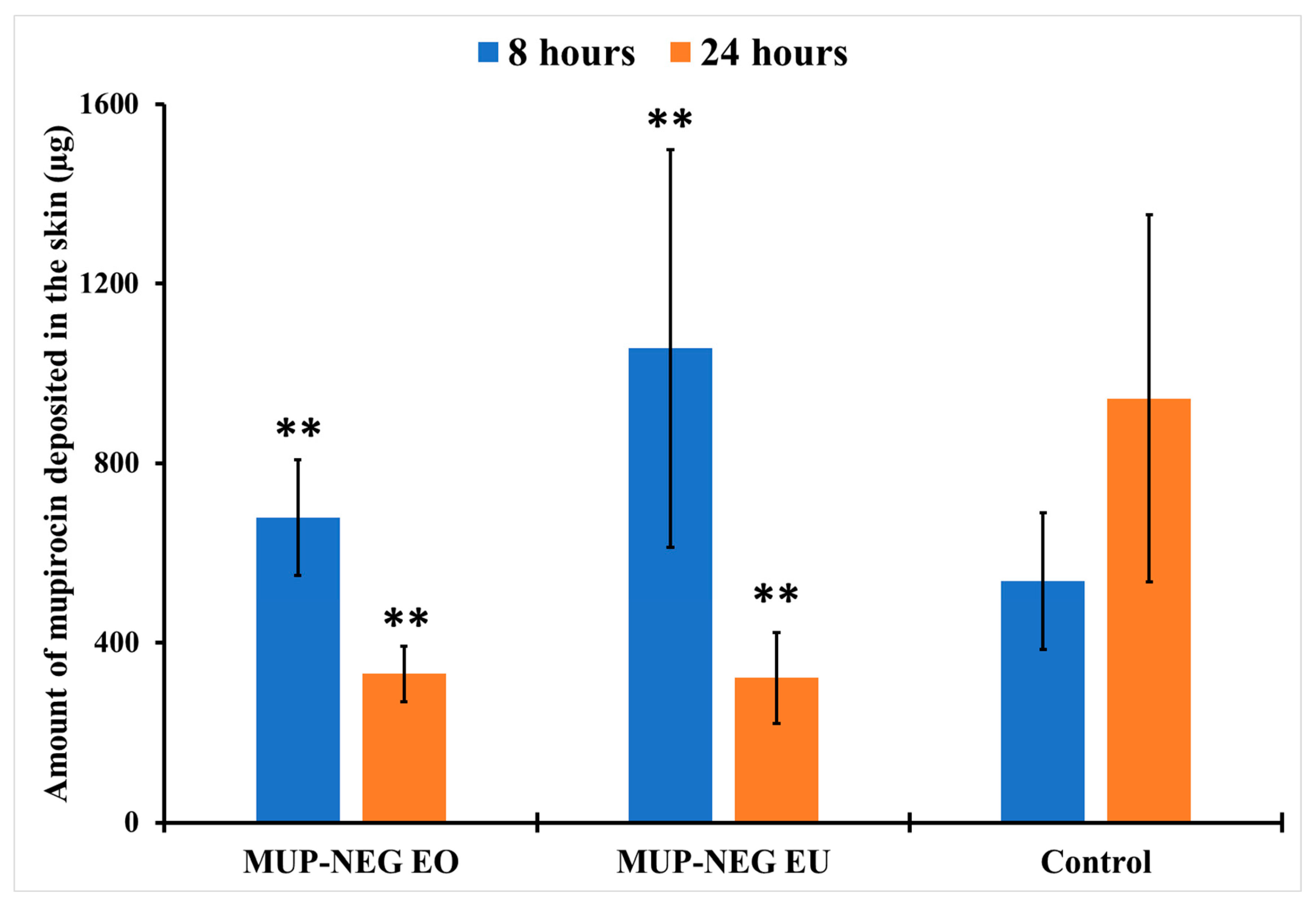
3.5.1. Quantitative Method: Differential Stripping Techniques
3.5.2. Qualitative Method: Micro-CT
3.6. Evaluation of the Antibacterial Activity of MUP Nanoemulgels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- DeLouise, L.A. Applications of nanotechnology in dermatology. J. Investig. Dermatol. 2012, 132, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Hirose, A.; Nishimura, T.; Kanno, J. Research strategy for evaluation methods of the manufactured nanomaterials in NIHS and importance of the chronic health effects studies. Bull. Natl. Inst. Health Sci. 2009, 127, 15–25. [Google Scholar]
- Saini, R.; Saini, S.; Sharma, S. Nanotechnology: The future medicine. J. Cutan. Aesthetic Surg. 2010, 3, 32–33. [Google Scholar] [CrossRef]
- Pawar, K.R.; Babu, R.J. Lipid materials for topical and transdermal delivery of nanoemulsions. Crit. Rev.TM Ther. Drug Carr. Syst. 2014, 31, 429–458. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Abd, E.; Namjoshi, S.; Mohammed, Y.H.; Roberts, M.S.; Grice, J.E. Synergistic skin penetration enhancer and nanoemulsion formulations promote the human epidermal permeation of caffeine and naproxen. J. Pharm. Sci. 2016, 105, 212–220. [Google Scholar] [CrossRef]
- Md Saari, N.H.; Chua, L.S.; Hasham, R.; Yuliati, L. Curcumin-loaded nanoemulsion for better cellular permeation. Sci. Pharm. 2020, 88, 44. [Google Scholar] [CrossRef]
- Alhasso, B.; Ghori, M.U.; Conway, B.R. Development of Nanoemulsions for Topical Application of Mupirocin. Pharmaceutics 2023, 15, 378. [Google Scholar] [CrossRef]
- Tagne, J.-B.; Kakumanu, S.; Ortiz, D.; Shea, T.; Nicolosi, R.J. A nanoemulsion formulation of tamoxifen increases its efficacy in a breast cancer cell line. Mol. Pharm. 2008, 5, 280–286. [Google Scholar] [CrossRef]
- Khurana, S.; Jain, N.; Bedi, P. Nanoemulsion based gel for transdermal delivery of meloxicam: Physico-chemical, mechanistic investigation. Life Sci. 2013, 92, 383–392. [Google Scholar] [CrossRef]
- Dhawan, B.; Aggarwal, G.; Harikumar, S. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int. J. Pharm. Investig. 2014, 4, 65–76. [Google Scholar] [PubMed]
- Aparna, C.; Srinivas, P.; Patnaik, K. Enhanced transdermal permeability of telmisartan by a novel nanoemulsion gel. Int. J. Pharm. Pharm. Sci. 2015, 7, 335–342. [Google Scholar]
- Radhika, P.R.; Guruprasad, S. Nanoemulsion based emulgel formulation of lipophilic drug for topical delivery. Int. J. PharmTech Res. 2016, 9, 210–223. [Google Scholar]
- Mou, D.; Chen, H.; Du, D.; Mao, C.; Wan, J.; Xu, H.; Yang, X. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int. J. Pharm. 2008, 353, 270–276. [Google Scholar] [CrossRef]
- Sengupta, P.; Chatterjee, B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int. J. Pharm. 2017, 526, 353–365. [Google Scholar] [CrossRef]
- Chellapa, P.; Mohamed, A.T.; Keleb, E.I.; Elmahgoubi, A.; Eid, A.M.; Issa, Y.S.; Elmarzugi, N.A. Nanoemulsion and Nanoemulgel as a Topical Formulation. IOSR J. Pharm. 2015, 5, 43–47. [Google Scholar]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and emerging topical antibacterials and antiseptics: Agents, action, and resistance patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef]
- Bakkiyaraj, D.; Sritharadol, R.; Padmavathi, A.R.; Nakpheng, T.; Srichana, T. Anti-biofilm properties of a mupirocin spray formulation against Escherichia coli wound infections. Biofouling 2017, 33, 591–600. [Google Scholar] [CrossRef]
- Sutherland, R.; Boon, R.; Griffin, K.; Masters, P.; Slocombe, B.; White, A. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob. Agents Chemother. 1985, 27, 495–498. [Google Scholar] [CrossRef]
- Nakama, T.; Nureki, O.; Yokoyama, S. Structural basis for the recognition of isoleucyl-adenylate and an antibiotic, mupirocin, by isoleucyl-tRNA synthetase. J. Biol. Chem. 2001, 276, 47387–47393. [Google Scholar] [CrossRef]
- Pappa, K.A. The clinical development of mupirocin. J. Am. Acad. Dermatol. 1990, 22, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Mupirocin [Internet]. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Mupirocin (accessed on 22 March 2021).
- Conly, J.M.; Johnston, B.L. Mupirocin—Are we in danger of losing it? Can. J. Infect. Dis. 2002, 13, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, R. Martindale: The Complete Drug Reference; Pharmaceutical Press: London, UK, 2020. [Google Scholar]
- Werner, A.; Russell, A. Mupirocin, fusidic acid and bacitracin: Activity, action and clinical uses of three topical antibiotics. Vet. Dermatol. 1999, 10, 225–240. [Google Scholar] [CrossRef]
- Tucaliuc, A.; Blaga, A.C.; Galaction, A.I.; Cascaval, D. Mupirocin: Applications and production. Biotechnol. Lett. 2019, 41, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, O.; Cern, A.; Müsken, M.; Rohde, M.; Weiss, W.; Barenholz, Y.; Medina, E. Liposomal mupirocin holds promise for systemic treatment of invasive Staphylococcus aureus infections. J. Control. Release 2019, 316, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-K.; Kim, Y.-N.; Park, K.-S.; Yang, J.-W.; Kim, K.-J.; Ha, N.-J. Antimicrobial activity of mupirocin, daptomycin, linezolid, quinupristin/dalfopristin and tigecycline against vancomycin-resistant enterococci (VRE) from clinical isolates in Korea (1998 and 2005). BMB Rep. 2007, 40, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Alhasso, B.; Ghori, M.U.; Conway, B.R. Systematic review on the effectiveness of essential and carrier oils as skin penetration enhancers in pharmaceutical formulations. Sci. Pharm. 2022, 90, 14. [Google Scholar] [CrossRef]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. MicrobiologyOpen 2017, 6, e00459–e00465. [Google Scholar] [CrossRef]
- Pitarokili, D.; Tzakou, O.; Loukis, A.; Harvala, C. Volatile metabolites from Salvia fruticosa as antifungal agents in soilborne pathogens. J. Agric. Food Chem. 2003, 51, 3294–3301. [Google Scholar] [CrossRef]
- Tolba, H.; Moghrani, H.; Benelmouffok, A.; Kellou, D.; Maachi, R. Essential oil of Algerian Eucalyptus citriodora: Chemical composition, antifungal activity. J. Mycol. Medicale 2015, 25, e128–e133. [Google Scholar] [CrossRef]
- Gaikwad, S.G.; Pandit, A.B. Ultrasound emulsification: Effect of ultrasonic and physicochemical properties on dispersed phase volume and droplet size. Ultrason. Sonochem. 2008, 15, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of High-Drug-Loading Nanoparticles. ChemPlusChem 2020, 85, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, R.; Li, L.; Li, B.; Zhang, X.; Su, J. Mechanical, rheological and release behaviors of a poloxamer 407/poloxamer 188/carbopol 940 thermosensitive composite hydrogel. Molecules 2013, 18, 12415–12425. [Google Scholar] [CrossRef]
- Sabale, V.; Kunjwani, H.; Sabale, P. Formulation and in vitro evaluation of the topical antiageing preparation of the fruit of Benincasa hispida. J. Ayurveda Integr. Med. 2011, 2, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Khullar, R.; Kumar, D.; Seth, N.; Saini, S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm. J. 2012, 20, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Iqbal, M.; Khan, B.A.; Khan, M.K.; Huwaimel, B.; Alshehri, S.; Alamri, A.H.; Alzhrani, R.M.; Bukhary, D.M.; Safhi, A.Y. Fabrication, in vitro, and in vivo assessment of eucalyptol-loaded nanoemulgel as a novel paradigm for wound healing. Pharmaceutics 2022, 14, 1971. [Google Scholar] [CrossRef]
- Shinde, U.; Pokharkar, S.; Modani, S. Design and evaluation of microemulsion gel system of nadifloxacin. Indian J. Pharm. Sci. 2012, 74, 237–247. [Google Scholar] [CrossRef]
- Arianto, A.; Cella, G.; Bangun, H. Preparation and evaluation of sunscreen nanoemulsions with synergistic efficacy on SPF by combination of soybean oil, avobenzone, and octyl methoxycinnamate. Open Access Maced. J. Med. Sci. 2019, 7, 2751–2756. [Google Scholar] [CrossRef]
- Teichmann, A.; Heuschkel, S.; Jacobi, U.; Presse, G.; Neubert, R.H.; Sterry, W.; Lademann, J. Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur. J. Pharm. Biopharm. 2007, 67, 699–706. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Madan, P.; Lin, S. Formulation and rheological evaluation of ethosome-loaded carbopol hydrogel for transdermal application. Drug Dev. Ind. Pharm. 2016, 42, 1315–1324. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; Nicoli, S.; Padula, C.; Santi, P.; Rossi, F.; e Silva, K.G.d.H.; Mansur, C.R.E. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: Permeation and stability studies. Eur. J. Pharm. Biopharm. 2017, 116, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Abu Alata, W.a.; Abu-Sini, M.; Abulebdah, D.H.; Hammad, A.M.; Aburayya, R. Development and Comparative Evaluation of Ciprofloxacin Nanoemulsion-Loaded Bigels Prepared Using Different Ratios of Oleogel to Hydrogels. Gels 2023, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, S.; Basavarajappa, G.M.; Attimarad, M.; Pund, S. Topical nanoemulgel for the treatment of skin cancer: Proof-of-technology. Pharmaceutics 2021, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- Al-Awady, M.J.; Fauchet, A.; Greenway, G.M.; Paunov, V.N. Enhanced antimicrobial effect of berberine in nanogel carriers with cationic surface functionality. J. Mater. Chem. B 2017, 5, 7885–7897. [Google Scholar] [CrossRef]
- Srivastava, R.; Srivastava, S.; Singh, S.P. Thermoreversible in-situ nasal gel formulations and their pharmaceutical evaluation for the treatment of allergic rhinitis containing extracts of moringa olifera and embelia ribes. Int. J. Appl. Pharm. 2017, 9, 16–20. [Google Scholar] [CrossRef]
- Almostafa, M.M.; Elsewedy, H.S.; Shehata, T.M.; Soliman, W.E. Novel formulation of fusidic acid incorporated into a myrrh-oil-based nanoemulgel for the enhancement of skin bacterial infection treatment. Gels 2022, 8, 245. [Google Scholar] [CrossRef]
- Pressi, G.; Barbieri, E.; Rizzi, R.; Tafuro, G.; Costantini, A.; Di Domenico, E.; Semenzato, A. Formulation and Physical Characterization of a Polysaccharidic Gel for the Vehiculation of an Insoluble Phytoextract for Mucosal Application. Polysaccharides 2022, 3, 728–744. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Miastkowska, M. Polymeric gels and their application in the treatment of psoriasis vulgaris: A review. Int. J. Mol. Sci. 2021, 22, 5124. [Google Scholar] [CrossRef]
- Bhatia, A.; Singh, B.; Raza, K.; Wadhwa, S.; Katare, O.P. Tamoxifen-loaded lecithin organogel (LO) for topical application: Development, optimization and characterization. Int. J. Pharm. 2013, 444, 47–59. [Google Scholar] [CrossRef]
- Huang, M.; Kennedy, J.; Li, B.; Xu, X.; Xie, B. Characters of rice starch gel modified by gellan, carrageenan, and glucomannan: A texture profile analysis study. Carbohydr. Polym. 2007, 69, 411–418. [Google Scholar] [CrossRef]
- Garala, K.; Joshi, P.; Shah, M.; Ramkishan, A.; Patel, J. Formulation and evaluation of periodontal in situ gel. Int. J. Pharm. Investig. 2013, 3, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Patel, B.; Banwait, H.; Parmar, K.; Patel, M. Formulation and evaluation of topical aceclofenac gel using different gelling agent. Int. J. Drug Dev. Res. 2011, 3, 156–164. [Google Scholar]
- Kaur, L.P.; Guleri, T.K. Topical gel: A recent approach for novel drug delivery. Asian J. Biomed. Pharm. Sci. 2013, 3, 1–5. [Google Scholar]
- Bhattacharya, S.; Prajapati, B.G. Formulation and optimization of celecoxib nanoemulgel. Asian J. Pharm. Clin. Res. 2017, 10, 353–365. [Google Scholar] [CrossRef]
- Contreras, M.F.; Diéguez, A.R.R.; Soriano, M.J. Viscosity and temperature relationship in ethanol/water mixtures gelified with Carbopol® Ultrez™ 10. Il Farm. 2001, 56, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Bonacucina, G.; Cespi, M.; Misici-Falzi, M.; Palmieri, G.F. Rheological evaluation of silicon/carbopol hydrophilic gel systems as a vehicle for delivery of water insoluble drugs. Am. Assoc. Pharm. Sci. J. 2008, 10, 84–91. [Google Scholar] [CrossRef]
- de Almeida Borges, V.R.; Simon, A.; Sena, A.R.C.; Cabral, L.M.; de Sousa, V.P. Nanoemulsion containing dapsone for topical administration: A study of in vitro release and epidermal permeation. Int. J. Nanomed. 2013, 8, 535–544. [Google Scholar]
- Nair, A.; Jacob, S.; Al-Dhubiab, B.; Attimarad, M.; Harsha, S. Basic considerations in the dermatokinetics of topical formulations. Braz. J. Pharm. Sci. 2013, 49, 423–434. [Google Scholar] [CrossRef]
- Otto, A.; Du Plessis, J.; Wiechers, J. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using Strat-M® membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Patra, K.C.; Pareta, S.K.; Singh, J.; Rahman, M.A. Nanoemulsions as vehicles for transdermal delivery of glycyrrhizin. Braz. J. Pharm. Sci. 2011, 47, 769–778. [Google Scholar] [CrossRef]
- Wavikar, P.; Vavia, P. Nanolipidgel for enhanced skin deposition and improved antifungal activity. AAPS PharmSciTech 2013, 14, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ouyang, W.-Q.; Wei, Y.-P.; Syed, S.F.; Hao, C.-S.; Wang, B.-Z.; Shang, Y.-H. Effects of Carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: A skin permeation study. Int. J. Nanomed. 2016, 11, 5971–5987. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chang, F.-Y.; Hung, D.-K. Terpene microemulsions for transdermal curcumin delivery: Effects of terpenes and cosurfactants. Colloids Surf. B Biointerfaces 2011, 82, 63–70. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef]
- Marslin, G.; Selvakesavan, R.K.; Franklin, G.; Sarmento, B.; Dias, A.C. Antimicrobial activity of cream incorporated with silver nanoparticles biosynthesized from Withania somnifera. Int. J. Nanomed. 2015, 10, 5955–5963. [Google Scholar]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; De Bruyne, T.; Hermans, N.; Totté, J.; Pieters, L.; Vlietinck, A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J. Infect. Chemother. 2001, 7, 251–254. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Repellent, larvicidal and pupicidal properties of essential oils and their formulations against the housefly, Musca domestica. Med. Vet. Entomol. 2011, 25, 302–310. [Google Scholar] [CrossRef]
- Sugumar, S.; Clarke, S.; Nirmala, M.; Tyagi, B.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull. Entomol. Res. 2014, 104, 393–402. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control 2008, 19, 346–352. [Google Scholar] [CrossRef]
- Assali, M.; Zaid, A.N.; Abdallah, F.; Almasri, M.; Khayyat, R. Single-walled carbon nanotubes-ciprofloxacin nanoantibiotic: Strategy to improve ciprofloxacin antibacterial activity. Int. J. Nanomed. 2017, 12, 6647–6659. [Google Scholar] [CrossRef] [PubMed]
- Mayaud, L.; Carricajo, A.; Zhiri, A.; Aubert, G. Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Lett. Appl. Microbiol. 2008, 47, 167–173. [Google Scholar] [CrossRef] [PubMed]

| Polymer | Concentration (% w/w) | Mixing Rate (rpm) | Processing Temperature (°C) | Triethanolamine (mL) |
|---|---|---|---|---|
| Carbopol 940 | 0.5 0.75 1 | 400 | 25 | 0.15 |
| Xanthan gum | 1 1.5 2 2.5 | 400 | 40 | 0.25 |
| HPMC | 1 2 | 400 | 40 | 0.25 |
| Formulations | Mechanical Properties | |||
|---|---|---|---|---|
| Firmness (g) | Cohesiveness (g) | Consistency (g·s) | Adhesiveness (g·s) | |
| MUP-NEG EO | 8.55 ± 0.32 | −5.53 ± 0.04 | 17.80 ± 0.52 | −5.72 ± 0.05 |
| MUP-NEG EU | 8.67 ± 0.21 | −5.72 ± 0.26 | 17.98 ± 0.29 | −5.95 ± 0.70 |
| Carbopol 0.75% gel | 21.66 ± 1.30 | −13.07 ± 0.87 | 38.54 ± 2.62 | −7.84 ± 0.57 |
| Control gel | 12.99 ± 0.39 | −11.91 ± 0.39 | 24.22 ± 0.67 | −12.03 ± 0.32 |
| Formulation | Spreadability (g·mm/s) | Viscosity (Pas) |
|---|---|---|
| NEG EO | 20.14 ± 0.99 | 252.97 ± 11.67 |
| NEG EU | 28.17 ± 0.88 | 68.47 ± 2.19 |
| MUP-NEG EO | 17.12 ± 0.64 | 134.53 ± 3.69 |
| MUP-NEG EU | 17.26 ± 0.46 | 110.53 ± 1.69 |
| Control | 25.97 ± 0.82 | 79.13 ± 1.61 |
| Formulation | Centrifugation | Heating and Cooling | |
|---|---|---|---|
| 4 °C | 40 °C | ||
| MUP-NEG EO1 | Stable (Pass) | Stable (Pass) | Stable (Pass) |
| MUP-NEG EU | Stable (Pass) | Stable (Pass) | Stable (Pass) |
| Parameters | MUP-NEG EO | MUP-NEG EU | Control |
|---|---|---|---|
| tlag (h) | 2.06 | 1.89 | 2.64 |
| Jmax (µg/cm2) | 1053.41 ± 78.82 | 1194.07 ± 91.96 | 2242.79 ± 262.17 |
| Jss (µg/cm2/h) | 53.53 ± 2.89 | 64.92 ± 7.52 | 112.42 ± 3.75 |
| Kp (×10−4 cm/h) | 55.69 ± 0.69 | 60.67 ± 0.22 | 107.14 ± 2.73 |
| Enhancement ratio (ER) | 0.47 | 0.53 | 1 |
| Parameters | MUP-NEG EO | MUP-NEG EU | Control |
|---|---|---|---|
| tlag (h) | 2.08 | 1.47 | 1.86 |
| Jmax (µg/cm2) | 440.43 ± 24.33 | 570.97 ± 83.67 | 1141.61 ± 40.45 |
| Jss (µg/cm2/h) | 16.39 ± 1.09 | 26.46 ± 2.44 | 39.32 ± 4.27 |
| Kp (×10−4 cm/h) | 15.02 ± 0.07 | 21.27 ± 0.03 | 40.5 ± 4.4 |
| ER | 0.39 | 0.50 | 1 |
| Parameters | MUP-NEG EO | MUP-NEG EU | Control |
|---|---|---|---|
| LAC (8 h) | 3.74 ± 0.25 | 4.33 ± 0.42 | 1.45 ± 0.36 |
| LAC (24 h) | 0.91 ± 0.19 | 0.75 ± 0.39 | 0.87 ± 0.38 |
| Formulations | Inhibition Zone Radius (mm) | |
|---|---|---|
| S. aureus (NCIMB9518) | MRSA (NCTC13142) | |
| Control | 0 | 0 |
| Bactroban cream | 20.67 ± 1.03 | 21.67 ± 1.89 |
| NEG EU | 6.67 ± 0.52 | 6.17 ± 0.75 |
| NEG EO | 7.67 ± 0.52 | 8.33 ± 1.03 |
| MUP-NEG EU | 20.00 ± 2.37 | 22.17 ± 1.60 |
| MUP-NEG EO | 20.67 ± 0.52 | 20.83 ± 1.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhasso, B.; Ghori, M.U.; Rout, S.P.; Conway, B.R. Development of a Nanoemulgel for the Topical Application of Mupirocin. Pharmaceutics 2023, 15, 2387. https://doi.org/10.3390/pharmaceutics15102387
Alhasso B, Ghori MU, Rout SP, Conway BR. Development of a Nanoemulgel for the Topical Application of Mupirocin. Pharmaceutics. 2023; 15(10):2387. https://doi.org/10.3390/pharmaceutics15102387
Chicago/Turabian StyleAlhasso, Bahjat, Muhammad Usman Ghori, Simon P. Rout, and Barbara R. Conway. 2023. "Development of a Nanoemulgel for the Topical Application of Mupirocin" Pharmaceutics 15, no. 10: 2387. https://doi.org/10.3390/pharmaceutics15102387







