Preparation and Characterization of Transethosome Formulation for the Enhanced Delivery of Sinapic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SA-Transethosomes
2.2. Analysis of the Vesicles Sizes, and Polydispersity Index (PDI) of SA-Transethosomes
2.3. Assessment of Zeta Potential of SA-Transethosomes
2.4. Assessment of Entrapment Efficiency (EE) of SA-Transethosomes
2.5. Surface Morphology of SA-Transethosomes
2.6. Assessment of Antioxidant Activity of SA-Transethosomes
2.7. In-Vitro Penetration Study
2.8. Statistical Analysis
3. Result and Discussion
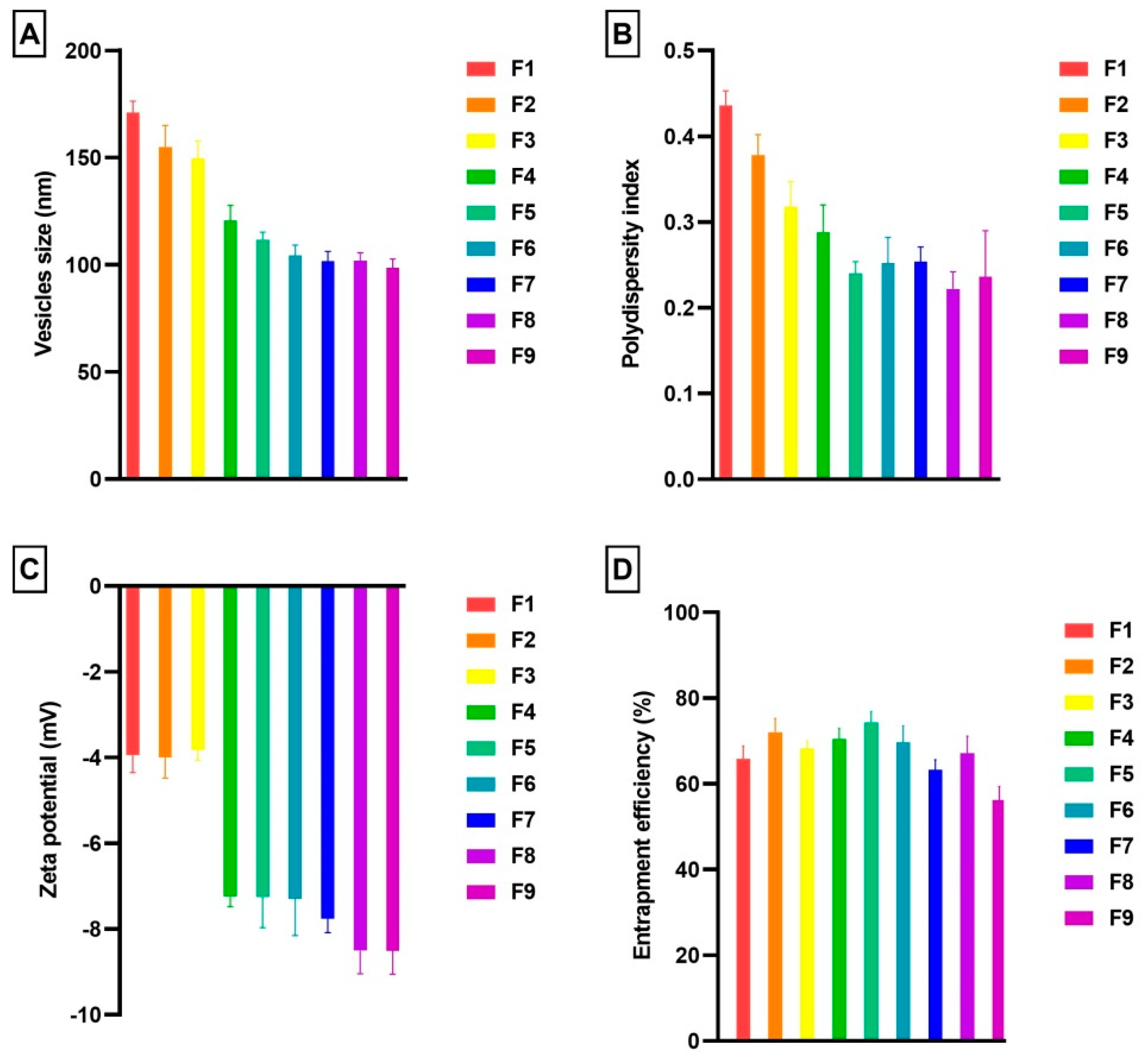
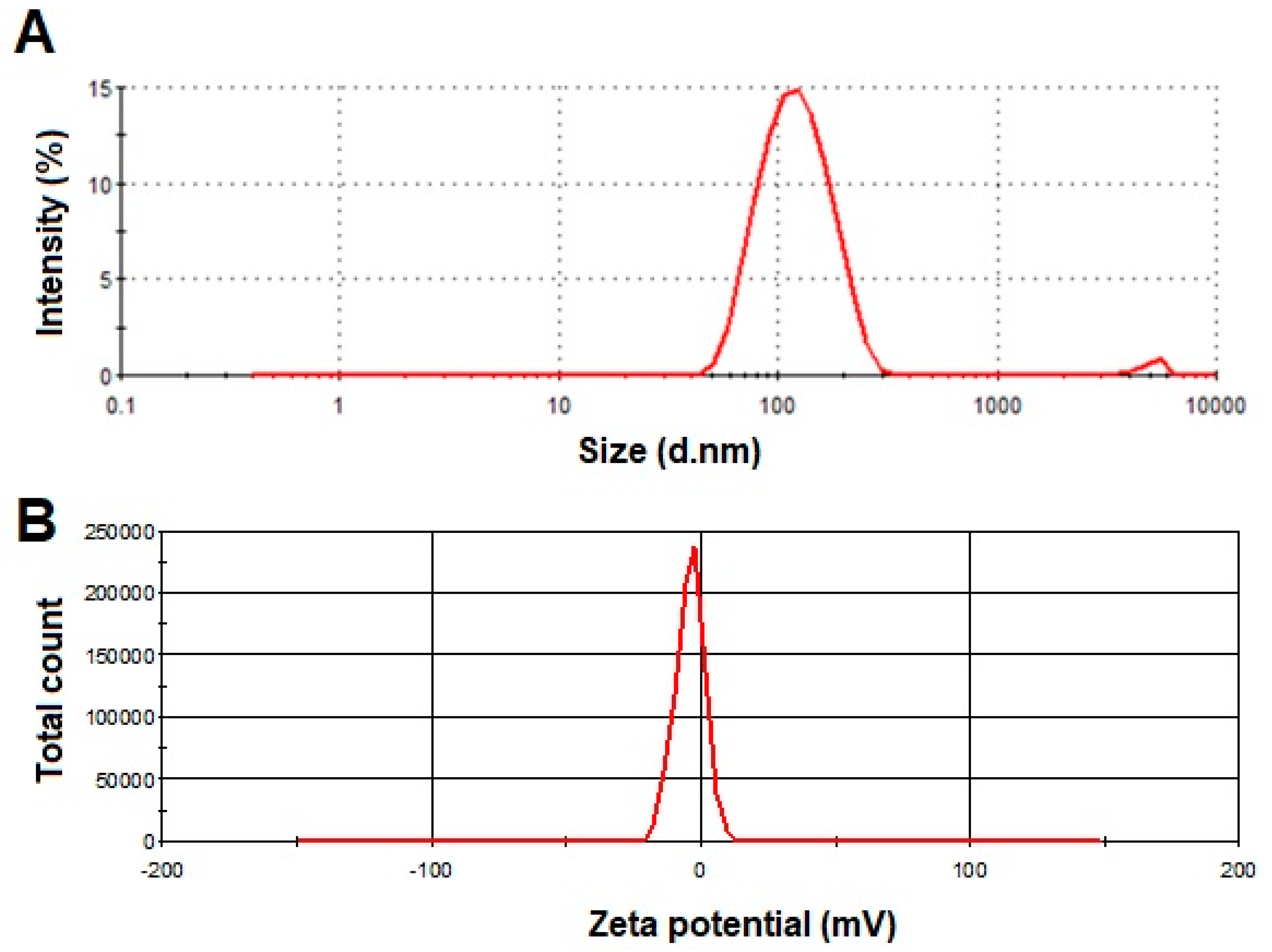
3.1. Vesicles Size and PDI
3.2. Zeta Potential
3.3. Entrapment Efficiency
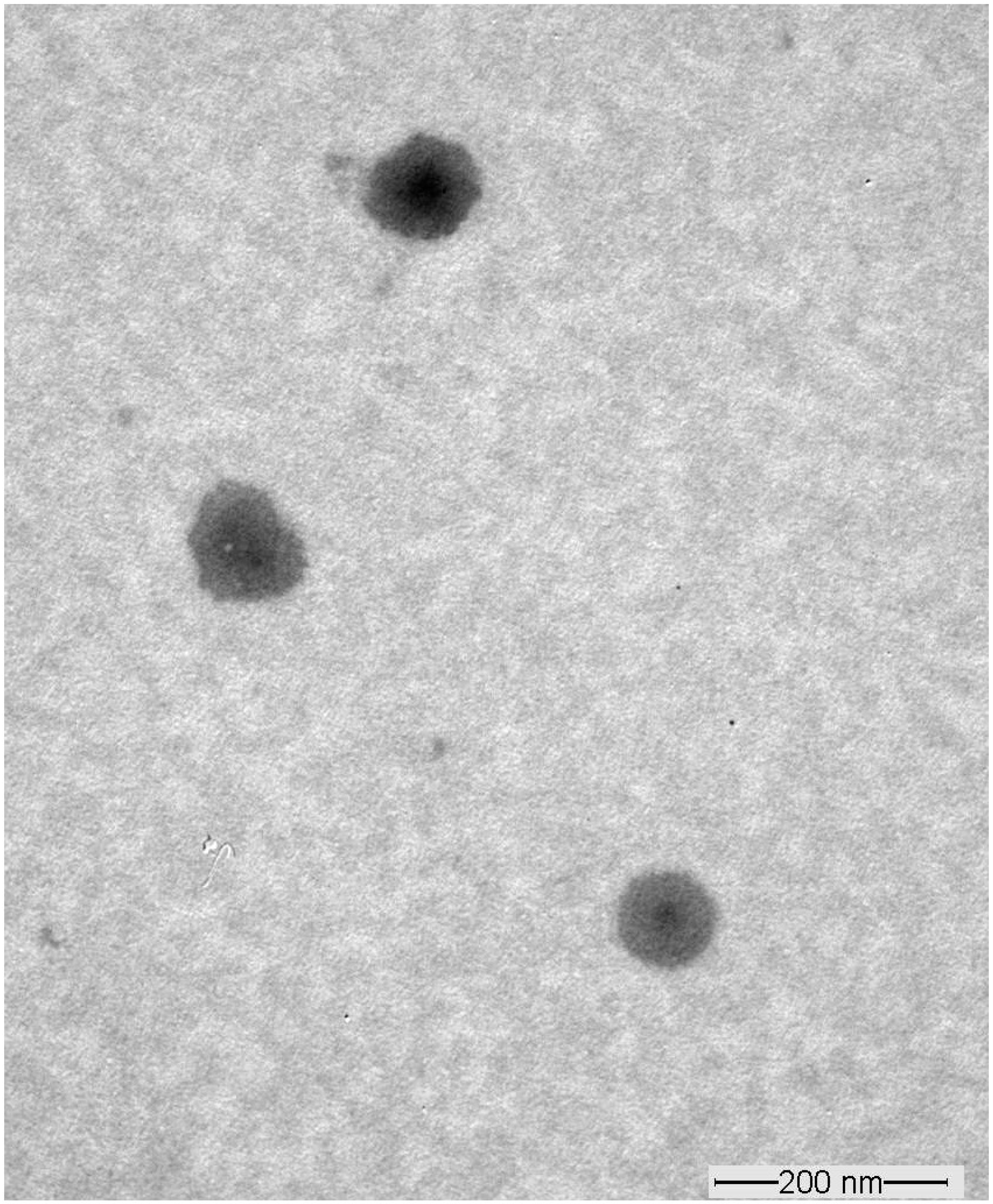
3.4. Transmission Electron Microscopy
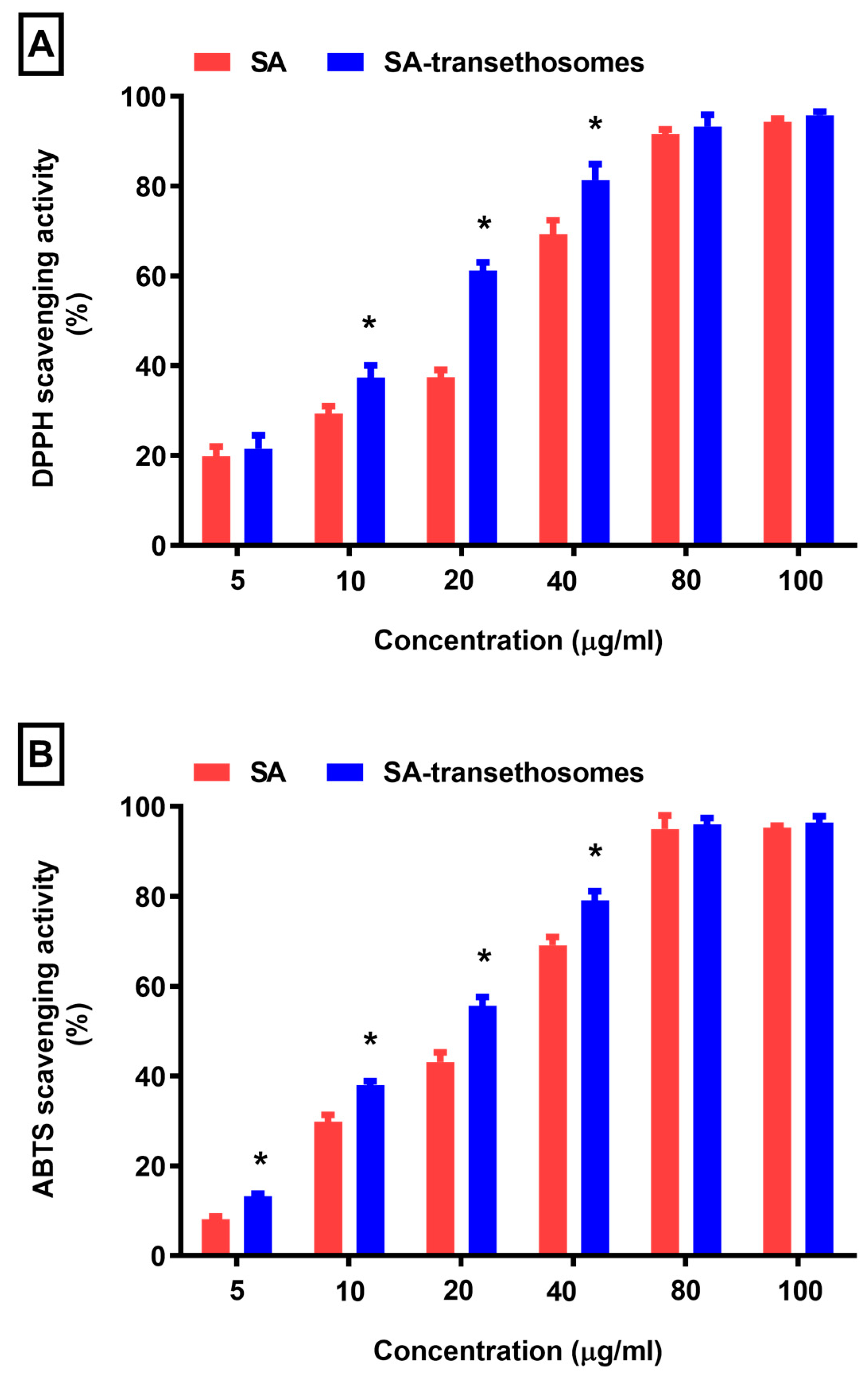
3.5. Antioxidant Activity
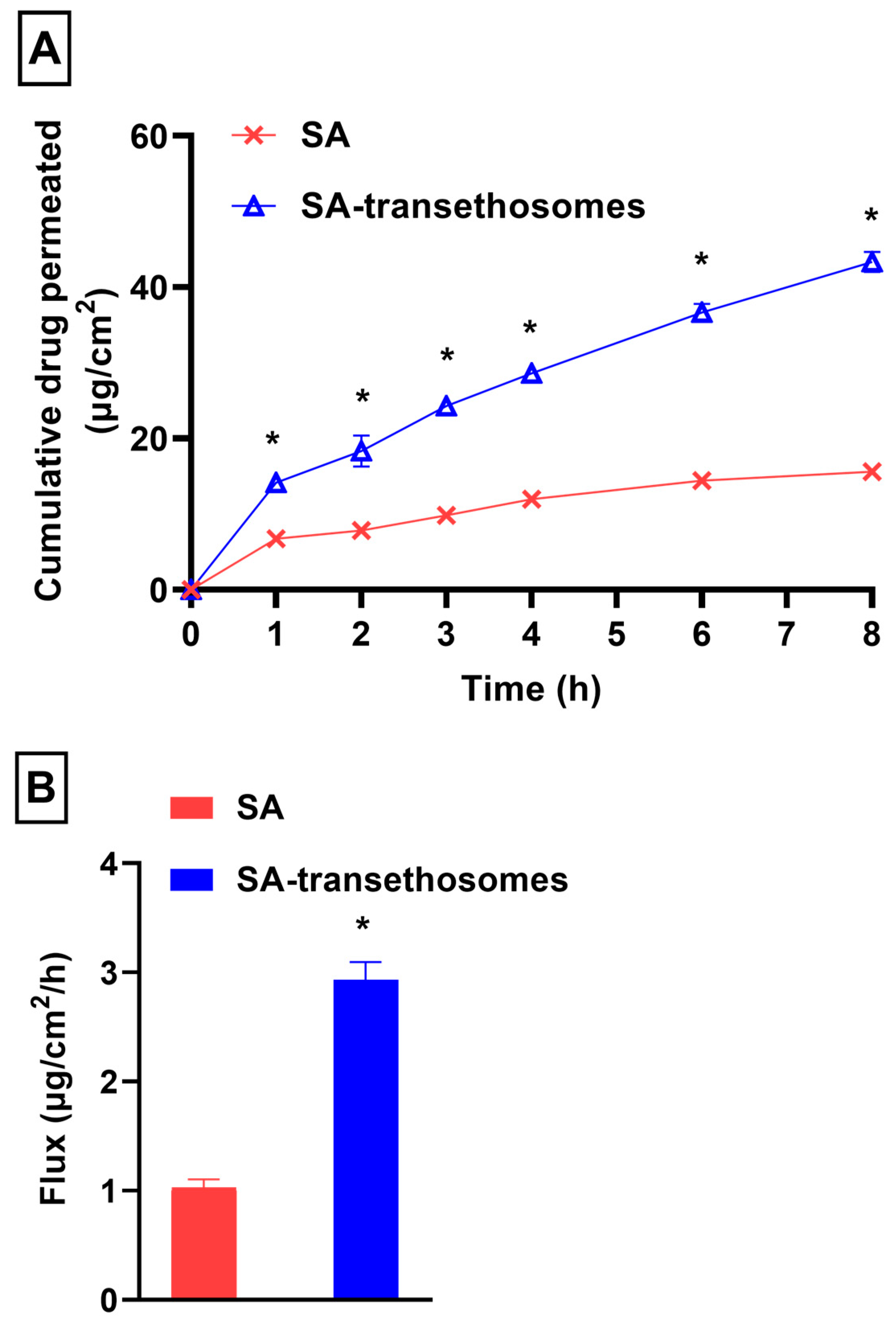
3.6. In-Vitro Penetration Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Mezei, M.; Gulasekharam, V. Liposomes—A selective drug delivery system for the topical route of administration. Lotion dosage form. Life Sci. 1980, 26, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Formulation and characterization of novel soft nanovesicles for enhanced transdermal delivery of eprosartan mesylate. Saudi Pharm. J. 2017, 25, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Blume, G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim. Biophys. Acta 1992, 1104, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M. Enhanced transdermal delivery of an anti-hypertensive agent via nanoethosomes: Statistical optimization, characterization and pharmacokinetic assessment. Int. J. Pharm. 2013, 443, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M.; Ali, A. Formulation and optimization of nanotransfersomes using experimental design technique for accentuated transdermal delivery of valsartan. Nanomedicine 2012, 8, 237–249. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Ahmad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Development and biological evaluation of vesicles containing bile salt of telmisartan for the treatment of diabetic nephropathy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 532–539. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Alam, M.A. Enhanced anti-inflammatory activity of carbopol loaded meloxicam nanoethosomes gel. Int. J. Biol. Macromol. 2014, 67, 99–104. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Bendas, E.R.; Tadros, M.I. Enhanced transdermal delivery of salbutamol sulfate via ethosomes. AAPS PharmSciTech 2007, 8, E107. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Kang, L.; Chan, S.Y. Nanosized ethosomes bearing ketoprofen for improved transdermal delivery. Results Pharma Sci. 2011, 1, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Balakrishnan, P.; Shim, C.K.; Chung, S.J.; Chong, S.; Kim, D.D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box-Behnken design, optimization, in vitro skin penetration, vesicles-skin interaction and dermatokinetic studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and Transethosomes for Mangiferin Transdermal Delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef]
- Ferrara, F.; Benedusi, M.; Sguizzato, M.; Cortesi, R.; Baldisserotto, A.; Buzzi, R.; Valacchi, G.; Esposito, E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics 2022, 14, 1038. [Google Scholar] [CrossRef]
- Verma, S.; Utreja, P. Transethosomes of Econazole Nitrate for Transdermal Delivery: Development, In-vitro Characterization, and Ex-vivo Assessment. Pharm. Nanotechnol. 2018, 6, 171–179. [Google Scholar] [CrossRef]
- Chen, Z.X.; Li, B.; Liu, T.; Wang, X.; Zhu, Y.; Wang, L.; Wang, X.H.; Niu, X.; Xiao, Y.; Sun, Q. Evaluation of paeonol-loaded transethosomes as transdermal delivery carriers. Eur. J. Pharm. Sci. 2017, 99, 240–245. [Google Scholar] [CrossRef]
- Chen, M.; Liu, X.; Fahr, A. Skin delivery of ferulic acid from different vesicular systems. J. Biomed. Nanotechnol. 2010, 6, 577–585. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Mariani, P.; Cortesi, R.; Huang, N.; Simeliere, F.; Marchetti, N.; Drechsler, M.; Ruzgas, T.; Esposito, E. Design and Characterization of Ethosomes for Transdermal Delivery of Caffeic Acid. Pharmaceutics 2020, 12, 740. [Google Scholar] [CrossRef]
- Kuwahara, H.; Kanazawa, A.; Wakamatu, D.; Morimura, S.; Kida, K.; Akaike, T.; Maeda, H. Antioxidative and antimutagenic activities of 4-vinyl-2,6-dimethoxyphenol (canolol) isolated from canola oil. J. Agric. Food Chem. 2004, 52, 4380–4387. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, H.; Lv, X.; Wang, D.; Chen, H.; Wei, F. Comprehensive review of composition distribution and advances in profiling of phenolic compounds in oilseeds. Front. Nutr. 2022, 9, 1044871. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 1163–1170. [Google Scholar] [PubMed]
- Liu, X.; Liu, M.; Cheng, C.; Deng, B.; Xie, J. Sinapic acid attenuates muscle atrophy in streptozotocin-induced diabetic mice. Iran. J. Basic. Med. Sci. 2021, 24, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Alaofi, A.L. Sinapic Acid Ameliorates the Progression of Streptozotocin (STZ)-Induced Diabetic Nephropathy in Rats via NRF2/HO-1 Mediated Pathways. Front. Pharmacol. 2020, 11, 1119. [Google Scholar] [CrossRef]
- Niciforovic, N.; Abramovic, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Engels, C.; Schieber, A.; Gänzle, M.G. Sinapic acid derivatives in defatted Oriental mustard (Brassica juncea L.) seed meal extracts using UHPLC-DAD-ESI-MSn and identification of compounds with antibacterial activity. Eur. Food Res. Technol. 2012, 234, 535–542. [Google Scholar] [CrossRef]
- Nithya, R.; Subramanian, S. Sinapic acid, a naturally occurring carboxylic acid derivative ameliorates hyperglycemia in high fat diet-low dose stz induced experimental diabetic rats. Int. J. Sci. Eng. Tech. Res. 2015, 4, 5746–5750. [Google Scholar]
- Yoon, B.H.; Jung, J.W.; Lee, J.J.; Cho, Y.W.; Jang, C.G.; Jin, C.; Oh, T.H.; Ryu, J.H. Anxiolytic-like effects of sinapic acid in mice. Life Sci. 2007, 81, 234–240. [Google Scholar] [CrossRef]
- Lan, H.; Zhang, L.Y.; He, W.; Li, W.Y.; Zeng, Z.; Qian, B.; Wang, C.; Song, J.L. Sinapic Acid Alleviated Inflammation-Induced Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide- (LPS-) Treated Caco-2 Cells. Mediators Inflamm. 2021, 2021, 5514075. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella Fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Ito, H.; Masuoka, T.; Kamei, C.; Hatano, T. Effect of Polygala tenuifolia root extract on scopolamine-induced impairment of rat spatial cognition in an eight-arm radial maze task. Biol. Pharm. Bull. 2007, 30, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Raish, M.; Anwer, M.K.; Al-Shdefat, R. Self-nanoemulsifying drug delivery system of sinapic acid: In vitro and in vivo evaluation. J. Mol. Liq. 2016, 224, 351–358. [Google Scholar] [CrossRef]
- Ahad, A.; Jardan, Y.A.B.; Raish, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Ternary inclusion complex of sinapic acid with hydroxypropyl-β-cyclodextrin and hydrophilic polymer prepared by microwave technology. Processes 2022, 10, 2637. [Google Scholar] [CrossRef]
- Ahad, A.; Bin Jardan, Y.A.; Raish, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Hydroxypropyl-β-cyclodextrin for delivery of sinapic acid via inclusion complex prepared by solvent evaporation method. Processes 2022, 10, 2046. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Ahmad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Eprosartan mesylate loaded bilosomes as potential nano-carriers against diabetic nephropathy in streptozotocin-induced diabetic rats. Eur. J. Pharm. Sci. 2018, 111, 409–417. [Google Scholar] [CrossRef]
- Dragicevic-Curic, N.; Scheglmann, D.; Albrecht, V.; Fahr, A. Development of different temoporfin-loaded invasomes-novel nanocarriers of temoporfin: Characterization, stability and in vitro skin penetration studies. Colloids Surf. B Biointerfaces 2009, 70, 198–206. [Google Scholar] [CrossRef]
- Jangdey, M.S.; Gupta, A.; Saraf, S. Development and optimization of apigenin-loaded transfersomal system for skin cancer delivery: In vitro evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1452–1462. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Altamimi, M.A.; Almalki, R.K.H.; Hussain, A.; Bukhari, S.I.; Mahdi, W.A.; Qamar, W. Formulation of Chitosan-Coated Apigenin Bilosomes: In Vitro Characterization, Antimicrobial and Cytotoxicity Assessment. Polymers 2022, 14, 921. [Google Scholar] [CrossRef]
- Khattab, R.; Eskin, M.; Aliani, M.; Thiyam, U. Determination of Sinapic Acid Derivatives in Canola Extracts Using High-Performance Liquid Chromatography. J. Am. Oil Chem. Soc. 2010, 87, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Formulation and characterization of Phospholipon 90 G and tween 80 based transfersomes for transdermal delivery of eprosartan mesylate. Pharm. Dev. Technol. 2017, 23, 787–793. [Google Scholar] [CrossRef]
- da Silva Mourao, L.C.; Ribeiro Batista, D.R.M.; Honorato, S.B.; Ayala, A.P.; de Alencar Morais, W.; Barbosa, E.G.; Raffin, F.N.; de Lima e Moura, T.F.A. Effect of hydroxypropyl methylcellulose on beta cyclodextrin complexation of praziquantel in solution and in solid state. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 151–160. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kalave, S.; Chatterjee, B.; Shah, P.; Misra, A. Transdermal Delivery of Macromolecules Using Nano Lipid Carriers. Curr. Pharm. Des. 2021, 27, 4330–4340. [Google Scholar] [CrossRef] [PubMed]
- Harshita; Barkat, M.A.; Das, S.S.; Pottoo, F.H.; Beg, S.; Rahman, Z. Lipid-Based Nanosystem as Intelligent Carriers for Versatile Drug Delivery Applications. Curr. Pharm. Des. 2020, 26, 1167–1180. [Google Scholar] [CrossRef]
- Imam, S.S.; Ahad, A.; Aqil, M.; Akhtar, M.; Sultana, Y.; Ali, A. Formulation by design based risperidone nano soft lipid vesicle as a new strategy for enhanced transdermal drug delivery: In-vitro characterization, and in-vivo appraisal. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1198–1205. [Google Scholar] [CrossRef]
- Pierre, M.B.; Dos Santos Miranda Costa, I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch. Dermatol. Res. 2011, 303, 607–621. [Google Scholar] [CrossRef]
- Natsheh, H.; Touitou, E. Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules 2020, 25, 2959. [Google Scholar] [CrossRef]
- Rakesh, R.; Anoop, K.R. Formulation and optimization of nano-sized ethosomes for enhanced transdermal delivery of cromolyn sodium. J. Pharm. Bioallied Sci. 2012, 4, 333–340. [Google Scholar]
- Farrah, A.Y.; Al-Mahallawi, A.M.; Basalious, E.B.; Nesseem, D.I. Investigating the Potential of Phosphatidylcholine-Based Nano-Sized Carriers in Boosting the Oto-Topical Delivery of Caroverine: In vitro Characterization, Stability Assessment and ex vivo Transport Studies. Int. J. Nanomed. 2020, 15, 8921–8931. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and Transfersomes: Principles, Perspectives and Practices. Curr. Drug Deliv. 2017, 14, 613–633. [Google Scholar] [CrossRef]
- Zhang, J.P.; Wei, Y.H.; Zhou, Y.; Li, Y.Q.; Wu, X.A. Ethosomes, binary ethosomes and transfersomes of terbinafine hydrochloride: A comparative study. Arch. Pharm. Res. 2012, 35, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Santos, A.C.; Silva, A.L.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Castro, R.; Veiga, F. Ethosomes as Nanocarriers for the Development of Skin Delivery Formulations. Pharm. Res. 2021, 38, 947–970. [Google Scholar] [CrossRef]
- Bodade, S.S.; Shaikh, K.S.; Kamble, M.S.; Chaudhari, P.D. A study on ethosomes as mode for transdermal delivery of an antidiabetic drug. Drug Deliv. 2013, 20, 40–46. [Google Scholar] [CrossRef]
- Paolino, D.; Lucania, G.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control Release 2005, 106, 99–110. [Google Scholar] [CrossRef]
- Niu, X.Q.; Zhang, D.P.; Bian, Q.; Feng, X.F.; Li, H.; Rao, Y.F.; Shen, Y.M.; Geng, F.N.; Yuan, A.R.; Ying, X.Y.; et al. Mechanism investigation of ethosomes transdermal permeation. Int. J. Pharm. X 2019, 1, 100027. [Google Scholar] [CrossRef]
- Sudhakar, K.; Mishra, V.; Jain, S.; Rompicherla, N.C.; Malviya, N.; Tambuwala, M.M. Development and evaluation of the effect of ethanol and surfactant in vesicular carriers on Lamivudine permeation through the skin. Int. J. Pharm. 2021, 610, 121226. [Google Scholar] [CrossRef]
- Albash, R.; Abdelbary, A.A.; Refai, H.; El-Nabarawi, M.A. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: In vitro, ex vivo, and in vivo evaluation. Int. J. Nanomed. 2019, 14, 1953–1968. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Chen, J.; Lai, J.; Sun, J.; Hu, F.; Wu, W. Enhanced bioavailability of the poorly water-soluble drug fenofibrate by using liposomes containing a bile salt. Int. J. Pharm. 2009, 376, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Amselem, S.; Gabizon, A.; Barenholz, Y. Optimization and upscaling of doxorubicin-containing liposomes for clinical use. J. Pharm. Sci. 1990, 79, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Lu, Y.; Qi, J.; Niu, M.; Lian, R.; Hu, F.; Wu, W. Enhanced oral bioavailability of cyclosporine A by liposomes containing a bile salt. Int. J. Nanomed. 2011, 6, 965–974. [Google Scholar]
- Yang, L.; Yang, W.; Bi, D.; Zeng, Q. A novel method to prepare highly encapsulated interferon-alpha-2b containing liposomes for intramuscular sustained release. Eur. J. Pharm. Biopharm. 2006, 64, 9–15. [Google Scholar] [CrossRef]
- Sun, W.; Zou, W.; Huang, G.; Li, A.; Zhang, N. Pharmacokinetics and targeting property of TFu-loaded liposomes with different sizes after intravenous and oral administration. J. Drug Target. 2008, 16, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.J.; Martinez-Navarrete, M.; Garrigues, T.M.; Melero, A. Skin drug delivery using lipid vesicles: A starting guideline for their development. J. Control Release 2023, 355, 624–654. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Mohanty, B.; Mishra, B.; Roy, H.; Nandi, S. Transethosomes: Cutting edge approach for drug permeation enhancement in transdermal drug delivery system. Chem. Biol. Drug Des. 2023, 102, 653–667. [Google Scholar] [CrossRef]
- Romero, E.L.; Morilla, M.J. Highly deformable and highly fluid vesicles as potential drug delivery systems: Theoretical and practical considerations. Int. J. Nanomed. 2013, 8, 3171–3186. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Assi, R.A.; Khan, N.A.K. Transethosomal gels as carriers for the transdermal delivery of colchicine: Statistical optimization, characterization, and ex vivo evaluation. Drug Des. Devel Ther. 2018, 12, 795–813. [Google Scholar] [CrossRef]
- Vasanth, S.; Dubey, A.; Ravi, G.S.; Lewis, S.A.; Ghate, V.M.; El-Zahaby, S.A.; Hebbar, S. Development and Investigation of Vitamin C-Enriched Adapalene-Loaded Transfersome Gel: A Collegial Approach for the Treatment of Acne Vulgaris. AAPS PharmSciTech 2020, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Kharshoum, R.M.; Awad, S.M.; Ahmed Mostafa, M.; Abou-Taleb, H.A. Tailoring of Retinyl Palmitate-Based Ethosomal Hydrogel as a Novel Nanoplatform for Acne Vulgaris Management: Fabrication, Optimization, and Clinical Evaluation Employing a Split-Face Comparative Study. Int. J. Nanomed. 2021, 16, 4251–4276. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Alzahrani, M.M.; Sirwi, A.; Alhakamy, N.A. The Antifungal and Ocular Permeation of Ketoconazole from Ophthalmic Formulations Containing Trans-Ethosomes Nanoparticles. Pharmaceutics 2021, 13, 151. [Google Scholar] [CrossRef]
- Nasr, A.M.; Moftah, F.; Abourehab, M.A.S.; Gad, S. Design, Formulation, and Characterization of Valsartan Nanoethosomes for Improving Their Bioavailability. Pharmaceutics 2022, 14, 2268. [Google Scholar] [CrossRef] [PubMed]
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; Sahafi, S.M. Formulation and characterization of novel nanostructured lipid carriers made from beeswax, propolis wax and pomegranate seed oil. Food Chem. 2018, 244, 83–92. [Google Scholar] [CrossRef]
- Ogiso, T.; Yamaguchi, T.; Iwaki, M.; Tanino, T.; Miyake, Y. Effect of positively and negatively charged liposomes on skin permeation of drugs. J. Drug Target. 2001, 9, 49–59. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; AbouTaleb, H.A.; AbouElhassan, K.M. Progesterone-loaded nanosized transethosomes for vaginal permeation enhancement: Formulation, statistical optimization, and clinical evaluation in anovulatory polycystic ovary syndrome. J. Liposome Res. 2019, 29, 183–194. [Google Scholar] [CrossRef]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M. Design, formulation and optimization of valsartan transdermal gel containing iso-eucalyptol as novel permeation enhancer: Preclinical assessment of pharmacokinetic in wistar albino rats. Expert. Opin. Drug Deliv. 2014, 11, 1149–1162. [Google Scholar] [CrossRef]
- Mishra, D.; Garg, M.; Dubey, V.; Jain, S.; Jain, N.K. Elastic liposomes mediated transdermal delivery of an anti-hypertensive agent: Propranolol hydrochloride. J. Pharm. Sci. 2007, 96, 145–155. [Google Scholar] [CrossRef]
- Lopez, O.; de la Maza, A.; Coderch, L.; Lopez-Iglesias, C.; Wehrli, E.; Parra, J.L. Direct formation of mixed micelles in the solubilization of phospholipid liposomes by Triton X-100. FEBS Lett. 1998, 426, 314–318. [Google Scholar] [CrossRef]
- Nayak, D.; Tippavajhala, V.K. A Comprehensive Review on Preparation, Evaluation and Applications of Deformable Liposomes. Iran. J. Pharm. Res. 2021, 20, 186–205. [Google Scholar] [CrossRef] [PubMed]
- Ita, K.B.; Du Preez, J.; Lane, M.E.; Hadgraft, J.; du Plessis, J. Dermal delivery of selected hydrophilic drugs from elastic liposomes: Effect of phospholipid formulation and surfactants. J. Pharm. Pharmacol. 2007, 59, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Kovacik, A.; Kopecna, M.; Vavrova, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert. Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Aqil, M.; Kohli, K.; Chaudhary, H.; Sultana, Y.; Mujeeb, M.; Talegaonkar, S. Chemical penetration enhancers: A patent review. Expert. Opin. Ther. Pat. 2009, 19, 969–988. [Google Scholar] [CrossRef] [PubMed]
| Formulations | PL90 (%, w/w) | SDC (%, w/w) | Ethanol (%, v/v) |
|---|---|---|---|
| F1 | 90 | 10 | 20 |
| F2 | 90 | 10 | 30 |
| F3 | 90 | 10 | 40 |
| F4 | 85 | 15 | 20 |
| F5 | 85 | 15 | 30 |
| F6 | 85 | 15 | 40 |
| F7 | 80 | 20 | 20 |
| F8 | 80 | 20 | 30 |
| F9 | 80 | 20 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Jardan, Y.A.; Ahad, A.; Raish, M.; Al-Jenoobi, F.I. Preparation and Characterization of Transethosome Formulation for the Enhanced Delivery of Sinapic Acid. Pharmaceutics 2023, 15, 2391. https://doi.org/10.3390/pharmaceutics15102391
Bin Jardan YA, Ahad A, Raish M, Al-Jenoobi FI. Preparation and Characterization of Transethosome Formulation for the Enhanced Delivery of Sinapic Acid. Pharmaceutics. 2023; 15(10):2391. https://doi.org/10.3390/pharmaceutics15102391
Chicago/Turabian StyleBin Jardan, Yousef A., Abdul Ahad, Mohammad Raish, and Fahad I. Al-Jenoobi. 2023. "Preparation and Characterization of Transethosome Formulation for the Enhanced Delivery of Sinapic Acid" Pharmaceutics 15, no. 10: 2391. https://doi.org/10.3390/pharmaceutics15102391







