Efficacy of Apremilast Gels in Mouse Model of Imiquimod-Induced Psoriasis Skin Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. APM Gel Preparations: Carbopol, Pluronic, and Sepigel
2.2.2. Physicochemical Characterization
Swelling and Degradation Studies
Porosity
Rheological Behavior
2.2.3. Stability Studies
2.2.4. Release Profile
2.2.5. Skin Permeation Studies
Determination of the Amount of Drug Retention in the Skin
2.2.6. Cytotoxity Study in HaCaT Cells
2.2.7. Biomechanical Skin Properties
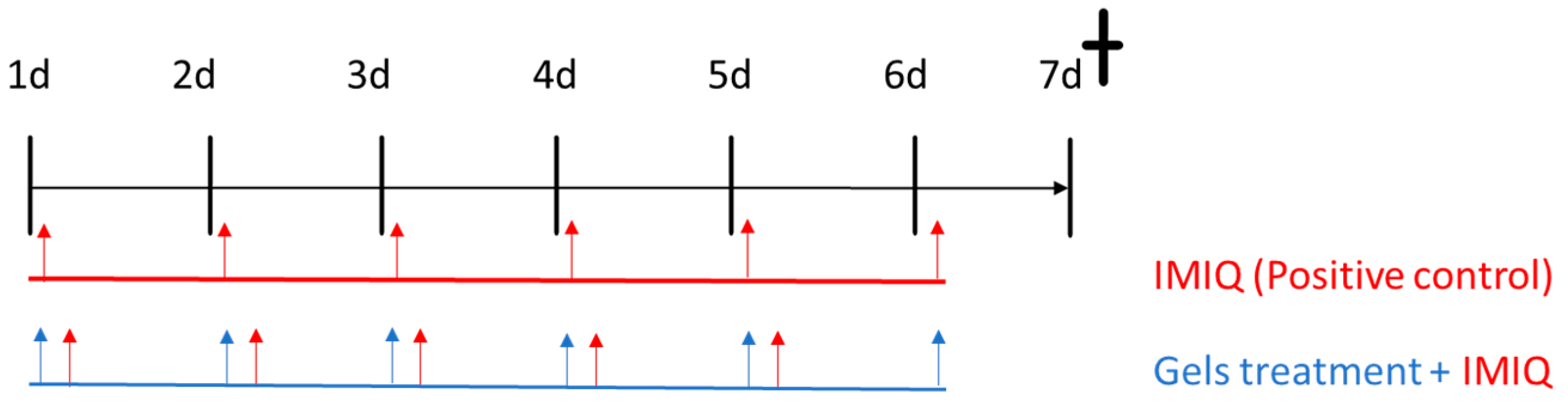
2.2.8. In Vivo Animal study: Imiquimod-Induced Psoriasis Skin Inflammation
Score for Skin Inflammation
Histological Analysis
RNA Extraction and RT-qPCR Assays
2.2.9. Statistical Analysis
3. Results
3.1. Optimization and Preparation of APM Gels
3.2. Swelling, Degradation Tests, and Porosity
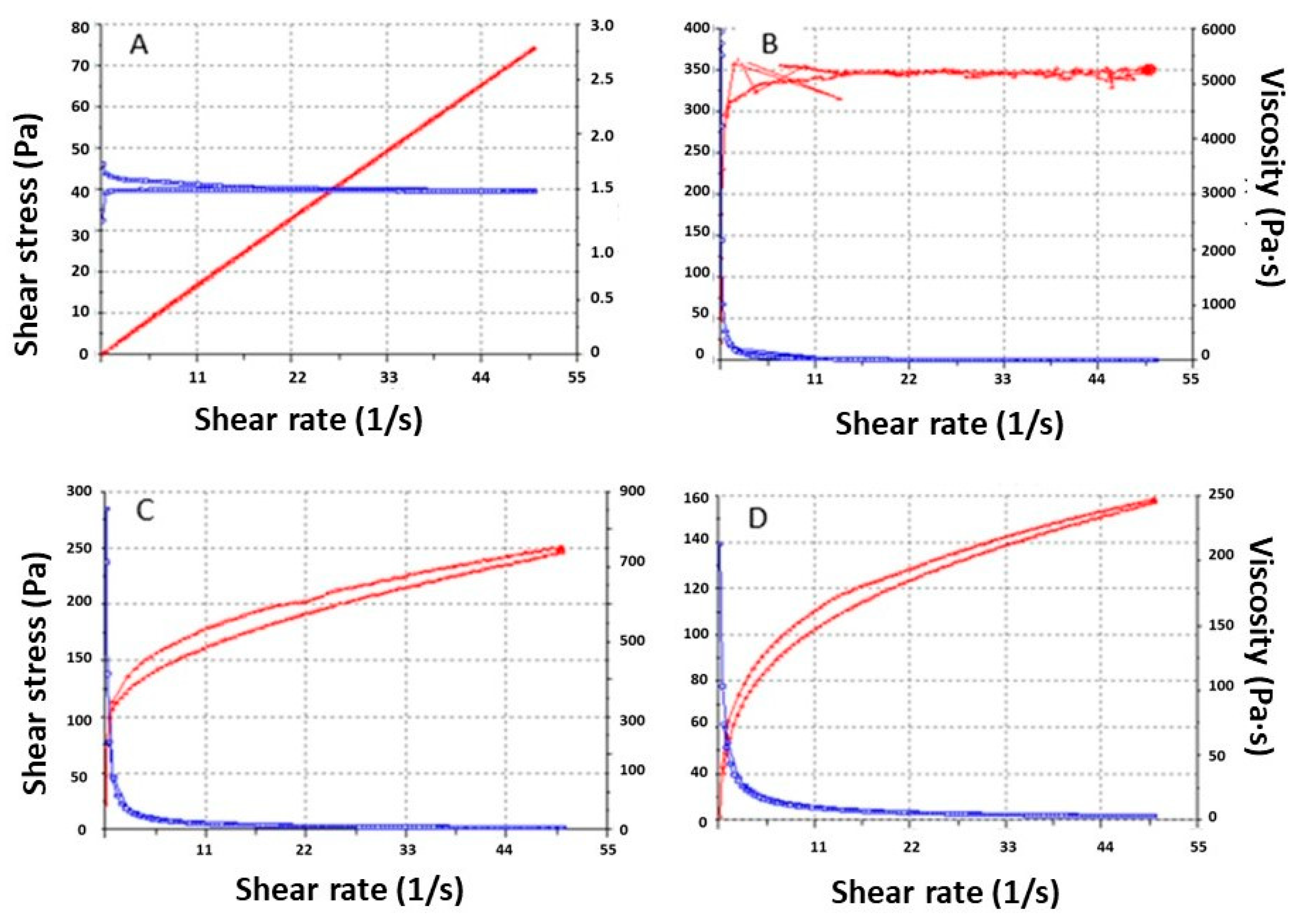
3.3. Rheologic Studies
3.4. Stability Studies
3.5. Release Studies
3.6. Skin Permeation and Retained Amount on the Skin
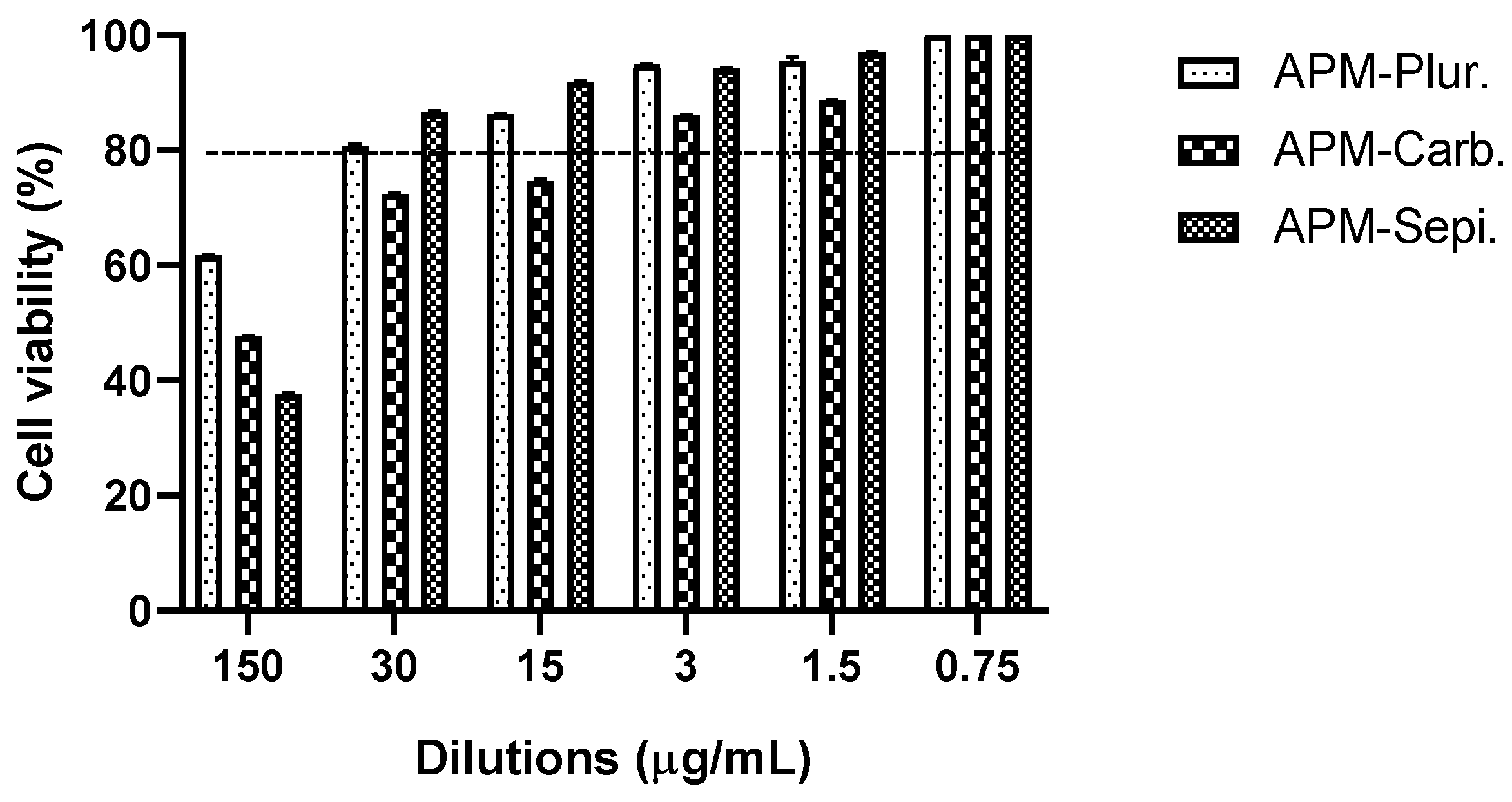
3.7. In Vitro Studies: Cytotoxicity Data
3.8. In Vivo Tolerance Studies: Biomechanical Skin Properties
3.9. In Vivo Animal Study: Imiquimod-Induced Psoriasis Skin Inflammation
3.9.1. Score for Skin Inflammation
3.9.2. Histological Analysis
3.9.3. RNA Extraction and RT-qPCR Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nogueira, S.; Rodrigues, M.A.; Vender, R.; Torres, T. Tapinarof for the treatment of psoriasis. Dermatol. Ther. 2022, 35, e15931. [Google Scholar] [CrossRef] [PubMed]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef] [PubMed]
- Panonnummal, R.; Sabitha, M. Anti-psoriatic and toxicity evaluation of methotrexate loaded chitin nanogel in imiquimod induced mice model. Int. J. Biol. Macromol. 2018, 110, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Rioux, J.D.; Abbas, A.K. Paths to understanding the genetic basis of autoimmune disease. Nature 2005, 435, 584–589. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Miastkowska, M. Polymeric Gels and Their Application in the Treatment of Psoriasis Vulgaris: A Review. Int. J. Mol. Sci. 2021, 22, 5124. [Google Scholar] [CrossRef]
- Korman, N.J. Management of psoriasis as a systemic disease: What is the evidence? Br. J. Dermatol. 2020, 182, 840–848. [Google Scholar] [CrossRef]
- Mahil, S.K.; Capon, F.; Barker, J.N. Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin. Immunopathol. 2016, 38, 11–27. [Google Scholar] [CrossRef]
- Yang, B.-Y.; Cheng, Y.-G.; Liu, Y.; Liu, Y.; Tan, J.-Y.; Guan, W.; Guo, S.; Kuang, H.-X.; Datura Metel, L. Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis and Inhibits Inflammatory Cytokines Production through TLR7/8–MyD88–NF-κB–NLRP3 Inflammasome Pathway. Molecules 2019, 24, 2157. [Google Scholar] [CrossRef]
- Niculet, E.; Radaschin, D.S.; Nastase, F.; Draganescu, M.; Baroiu, L.; Miulescu, M.; Arbune, M.; Tatu, A.L. Influence of phytochemicals in induced psoriasis (Review). Exp. Ther. Med. 2020, 20, 3421–3424. [Google Scholar] [CrossRef]
- Sarango-Granda, P.; Silva-Abreu, M.; Calpena, A.C.; Halbaut, L.; Fábrega, M.J.; Rodríguez-Lagunas, M.J.; Díaz-Garrido, N.; Badia, J.; Espinoza, L.C. Apremilast Microemulsion as Topical Therapy for Local Inflammation: Design, Characterization and Efficacy Evaluation. Pharmaceuticals 2020, 13, 484. [Google Scholar] [CrossRef]
- Sarango-Granda, P.; Espinoza, L.C.; Díaz-Garrido, N.; Alvarado, H.; Rodríguez-Lagunas, M.J.; Baldomá, L.; Calpena, A. Effect of Penetration Enhancers and Safety on the Transdermal Delivery of Apremilast in Skin. Pharmaceutics 2022, 14, 1011. [Google Scholar] [CrossRef]
- Strober, B.; Thaçi, D.; Sofen, H.; Kircik, L.; Gordon, K.B.; Foley, P.; Rich, P.; Paul, C.; Bagel, J.; Colston, E.; et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J. Am. Acad. Dermatol. 2023, 88, 40–51. [Google Scholar] [CrossRef]
- Maloney, N.J.; Zhao, J.; Tegtmeyer, K.; Lee, E.Y.; Cheng, K. Off-label studies on apremilast in dermatology: A review. J. Dermatol. Treat. 2020, 31, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Chaudhary, S.; Chaudhary, A. Formulation Development and Evaluation of Apremilast Nanoemulgel for Enhancing Permeability. Curr. Drug Ther. 2023, 18, 132–150. [Google Scholar] [CrossRef]
- Madan, J.R.; Khobaragade, S.; Dua, K.; Awasthi, R. Formulation, optimization, and in vitro evaluation of nanostructured lipid carriers for topical delivery of Apremilast. Dermatol. Ther. 2020, 33, e13370. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.K.; Bansal, A.K. Novel nanocrystal-based formulations of apremilast for improved topical delivery. Drug Deliv. Transl. Res. 2021, 11, 966–983. [Google Scholar] [CrossRef]
- Thakur, G.; Singh, A.; Singh, I. Formulation and evaluation of transdermal composite films of chitosan-montmorillonite for the delivery of curcumin. Int. J. Pharm. Investig. 2016, 6, 23–31. [Google Scholar] [CrossRef]
- Teixeira, A.; Vasconcelos, V.; Teixeira, M.; Almeida, V.; Azevedo, R.; Torres, T.; Sousa Lobo, J.M.; Costa, P.C.; Almeida, I.F. Mechanical Properties of Topical Anti-Psoriatic Medicines: Implications for Patient Satisfaction with Treatment. AAPS PharmSciTech 2019, 20, 36. [Google Scholar] [CrossRef]
- Rincón, M.; Silva-Abreu, M.; Espinoza, L.C.; Sosa, L.; Calpena, A.C.; Rodríguez-Lagunas, M.J.; Colom, H. Enhanced Transdermal Delivery of Pranoprofen Using a Thermo-Reversible Hydrogel Loaded with Lipid Nanocarriers for the Treatment of Local Inflammation. Pharmaceuticals 2021, 15, 22. [Google Scholar] [CrossRef]
- Berenguer, D.; Alcover, M.M.; Sessa, M.; Halbaut, L.; Guillén, C.; Boix-Montañés, A.; Fisa, R.; Calpena-Campmany, A.C.; Riera, C.; Sosa, L. Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity. Pharmaceutics 2020, 12, 149. [Google Scholar] [CrossRef]
- Hayati, F.; Ghamsari, S.M.; Dehghan, M.M.; Oryan, A. Effects of carbomer 940 hydrogel on burn wounds: An in vitro and in vivo study. J. Dermatol. Treat. 2018, 29, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Madhavikutty, A.S.; Ohta, S.; Chandel, A.K.S.; Qi, P.; Ito, T. Analysis of Endoscopic Injectability and Post-Ejection Dripping of Yield Stress Fluids: Laponite, Carbopol and Xanthan Gum. J. Chem. Eng. Jpn. 2021, 54, 500–511. [Google Scholar] [CrossRef]
- Baptista, S.; Freitas, F. Formulation of the Polysaccharide FucoPol into Novel Emulsified Creams with Improved Physicochemical Properties. Molecules 2022, 27, 7759. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-Assembly of Partially Alkylated Dextran-graft-poly[(2-dimethylamino)ethyl methacrylate] Copolymer Facilitating Hydrophobic/Hydrophilic Drug Delivery and Improving Conetwork Hydrogel Properties. Biomacromolecules 2018, 19, 1142–1153. [Google Scholar] [CrossRef]
- Georgiev, G.Z. Random Number Generator. Available online: https://www.gigacalculator.com/calculators/random-number-generator.php (accessed on 23 September 2022).
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Sullivan, D.W.; Gad, S.C.; Julien, M. A review of the nonclinical safety of Transcutol®, a highly purified form of diethylene glycol monoethyl ether (DEGEE) used as a pharmaceutical excipient. Food Chem. Toxicol. 2014, 72, 40–50. [Google Scholar] [CrossRef]
- Mallandrich, M.; Fernández-Campos, F.; Clares, B.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduño-Ramírez, M.L.; Andrade, B.; Del Pozo, A.; Lane, M.E.; et al. Developing Transdermal Applications of Ketorolac Tromethamine Entrapped in Stimuli Sensitive Block Copolymer Hydrogels. Pharm. Res. 2017, 34, 1728–1740. [Google Scholar] [CrossRef]
- Sosa, L.; Calpena, A.C.; Silva-Abreu, M.; Espinoza, L.C.; Rincón, M.; Bozal, N.; Domenech, O.; Rodríguez-Lagunas, M.J.; Clares, B. Thermoreversible Gel-Loaded Amphotericin B for the Treatment of Dermal and Vaginal Candidiasis. Pharmaceutics 2019, 11, 312. [Google Scholar] [CrossRef]
- Dano, M.E.L.; Said dos Santos, R.; Bassi da Silva, J.; Junqueira, M.V.; de Souza Ferreira, S.B.; Bruschi, M.L. Design of emulgel platforms for local propolis delivery: The influence of type and concentration of carbomer. Journal of Molecular Liquids 2021, 334, 116025. [Google Scholar] [CrossRef]
- Welin-Berger, K.; Neelissen, J.; Bergenståhl, B. In vitro permeation profile of a local anaesthetic compound from topical formulations with different rheological behaviour--verified by in vivo efficacy data. Eur. J. Pharm. Sci. 2001, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yeo, Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Silva-Abreu, M.; Calpena, A.C.; Rodríguez-Lagunas, M.J.; Fábrega, M.J.; Garduño-Ramírez, M.L.; Clares, B. Nanoemulsion strategy of pioglitazone for the treatment of skin inflammatory diseases. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 115–125. [Google Scholar] [CrossRef]
- Shavit, R.; Dierickx, C. A New Method for Percutaneous Drug Delivery by Thermo-Mechanical Fractional Injury. Lasers Surg. Med. 2020, 52, 61–69. [Google Scholar] [CrossRef]
- Choi, E.H.; Man, M.Q.; Wang, F.; Zhang, X.; Brown, B.E.; Feingold, K.R.; Elias, P.M. Is endogenous glycerol a determinant of stratum corneum hydration in humans? J. Investig. Dermatol. 2005, 125, 288–293. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Gudjonsson, J.E.; Ward, N.L. Mouse Models of Psoriasis: A Comprehensive Review. J. Investig. Dermatol. 2022, 142, 884–897. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ricardo-Gonzalez, R.R.; Moro, K. Skin-Resident Innate Lymphoid Cells—Cutaneous Innate Guardians and Regulators. Trends Immunol. 2020, 41, 100–112. [Google Scholar] [CrossRef]
- Matsuzaki, G.; Umemura, M. Interleukin-17 family cytokines in protective immunity against infections: Role of hematopoietic cell-derived and non-hematopoietic cell-derived interleukin-17s. Microbiol. Immunol. 2018, 62, 1–13. [Google Scholar] [CrossRef]
- Chan, T.C.; Hawkes, J.E.; Krueger, J.G. Interleukin 23 in the skin: Role in psoriasis pathogenesis and selective interleukin 23 blockade as treatment. Ther. Adv. Chronic Dis. 2018, 9, 111–119. [Google Scholar] [CrossRef] [PubMed]









| Gene | Primer Sequence (5′ to 3′) |
|---|---|
| GAPDH | FW: AGCTTGTCATCAACGGGAAG |
| RV: TTTGATGTTAGTGGGGTCTCG | |
| IL-8 | FW: GCTGTGACCCTCTCTGTGAAG |
| RV: CAAACTCCATCTTGTTGTGTC | |
| IL-23 | FW: GAGCCTTCTCTGCTCCCTGATA |
| RV: GACTGAGGCTTGGAATCTGCTG | |
| IL-17A | FW: TTTTCAGCAAGGAATGTGGA |
| RV: TTCATTGTGGAGGGCAGAC | |
| IL-17F | FW: TTCCAAAAGC CTGAGAGTTG |
| RV: GCCCAAGTTC CTACACTGG | |
| TNFα | FW: AACTAGTGGTGCCAGCCGAT |
| RV: CTTCACAGAGCAATGACTCC |
| Transcutol-P Concentration (%) | Color | Homogeneity | Consistency | Phase Separation | |
|---|---|---|---|---|---|
| 20 | Turbid | ++ | ++ | None | |
| APM-Plur | 30 | Transp. | +++ | +++ | None |
| 40 | Transp. | +++ | ++ | None | |
| 20 | White | ++ | + | None | |
| APM-Carb | 30 | White | +++ | +++ | None |
| 40 | White | ++ | ++ | None | |
| 20 | White | ++ | + | None | |
| APM-Sepi | 30 | White | +++ | +++ | None |
| 40 | White | ++ | ++ | None |
| Components (%, w/v) | APM-Plur | APM-Carb | APM-Sepi |
|---|---|---|---|
| Apremilast (APM) | 0.15 | 0.15 | 0.15 |
| Transcutol-P | 30 | 30 | 30 |
| Hyaluronic acid (HA) | 0.2 | 0.2 | 0.2 |
| Pluronic F 127 | 20 | - | - |
| Carbomer 940 | - | 2 | - |
| NaOH 1N | 0.4 | ||
| Sepigel 305® | - | - | 2 |
| MQ Water | 49.65 | 67.25 | 67.65 |
| Formulation | Viscosity at 4 °C mPa·S | Viscosity at 25 °C mPa·S | Thixotropy Pa/s | Rheological Behavior (Best Equation) |
|---|---|---|---|---|
| APM-Plur | 1503.00 ± 0.97 | The product does not flow under the shear conditions of the experiment. | 36.62 | Pseudoplastic (Cross model; r2 = 1) |
| APM-Carb | NA | 5097.00 ± 28.83 | 2093.00 | Plastic (Herschel–Bulkley) |
| APM-Sepi | NA | 3086.00 ± 5.50 | 411.50 | Pseudoplastic (Cross model; r2 = 0.9998) |
| Days | pH | Color | Homogeneity | Consistency | Phase Separation | |
|---|---|---|---|---|---|---|
| APM-Plur | 1 | 5.5 ± 0.08 | Transp. | +++ | +++ | None |
| 60 | 5.3 ± 0.06 | Transp. | +++ | +++ | None | |
| APM-Carb | 1 | 6.3 ± 0.07 | White | +++ | +++ | None |
| 60 | 6.4 ± 0.06 | White | +++ | +++ | None | |
| APM-Sepi | 1 | 5.3 ± 0.02 | White | +++ | +++ | None |
| 60 | 5. 5 ± 0.03 | White | +++ | +++ | None |
| APM-Plur | APM-Sepi | APM-Carb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | Mean | SD | n | |
| Plateau (µg) | 92.56 | 5.69 | 5 | 115.00 | 4.03 | 5 | 97.84 | 3.42 | 5 |
| K (1/h) | 0.041 | 0.007 | 5 | 0.094 | 0.018 | 5 | 0.083 | 0.014 | 5 |
| Half-time (h) | 17.04 | 0.04 | 5 | 7.37 | 0.09 | 5 | 8.38 | 0.08 | 5 |
| AUC (µg·h) | 4557 | 471 | 5 | 7232 | 798 | 5 | 6004 | 599 | 5 |
| r2 | 0.9617 | 0.9533 | 0.9606 | ||||||
| Parameters | Plateau | K | Half-Time | AUC |
|---|---|---|---|---|
| Tukey’s Multiple Comparison Test | p-Value | p-Value | p-Value | p-Value |
| APM-Plur vs. APM-Sepi | *** | *** | *** | *** |
| APM-Plur vs. APM-Carb | ns | ** | *** | ** |
| APM-Sepi vs. APM-Carb | *** | ns | *** | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Abreu, M.; Sosa, L.; Espinoza, L.C.; Fábrega, M.-J.; Rodríguez-Lagunas, M.J.; Mallandrich, M.; Calpena, A.C.; Garduño-Ramírez, M.L.; Rincón, M. Efficacy of Apremilast Gels in Mouse Model of Imiquimod-Induced Psoriasis Skin Inflammation. Pharmaceutics 2023, 15, 2403. https://doi.org/10.3390/pharmaceutics15102403
Silva-Abreu M, Sosa L, Espinoza LC, Fábrega M-J, Rodríguez-Lagunas MJ, Mallandrich M, Calpena AC, Garduño-Ramírez ML, Rincón M. Efficacy of Apremilast Gels in Mouse Model of Imiquimod-Induced Psoriasis Skin Inflammation. Pharmaceutics. 2023; 15(10):2403. https://doi.org/10.3390/pharmaceutics15102403
Chicago/Turabian StyleSilva-Abreu, Marcelle, Lilian Sosa, Lupe Carolina Espinoza, María-José Fábrega, María J. Rodríguez-Lagunas, Mireia Mallandrich, Ana Cristina Calpena, María Luisa Garduño-Ramírez, and María Rincón. 2023. "Efficacy of Apremilast Gels in Mouse Model of Imiquimod-Induced Psoriasis Skin Inflammation" Pharmaceutics 15, no. 10: 2403. https://doi.org/10.3390/pharmaceutics15102403
APA StyleSilva-Abreu, M., Sosa, L., Espinoza, L. C., Fábrega, M.-J., Rodríguez-Lagunas, M. J., Mallandrich, M., Calpena, A. C., Garduño-Ramírez, M. L., & Rincón, M. (2023). Efficacy of Apremilast Gels in Mouse Model of Imiquimod-Induced Psoriasis Skin Inflammation. Pharmaceutics, 15(10), 2403. https://doi.org/10.3390/pharmaceutics15102403









