Hydrogel-Based Therapeutics for Pancreatic Ductal Adenocarcinoma Treatment
Abstract
:1. Introduction
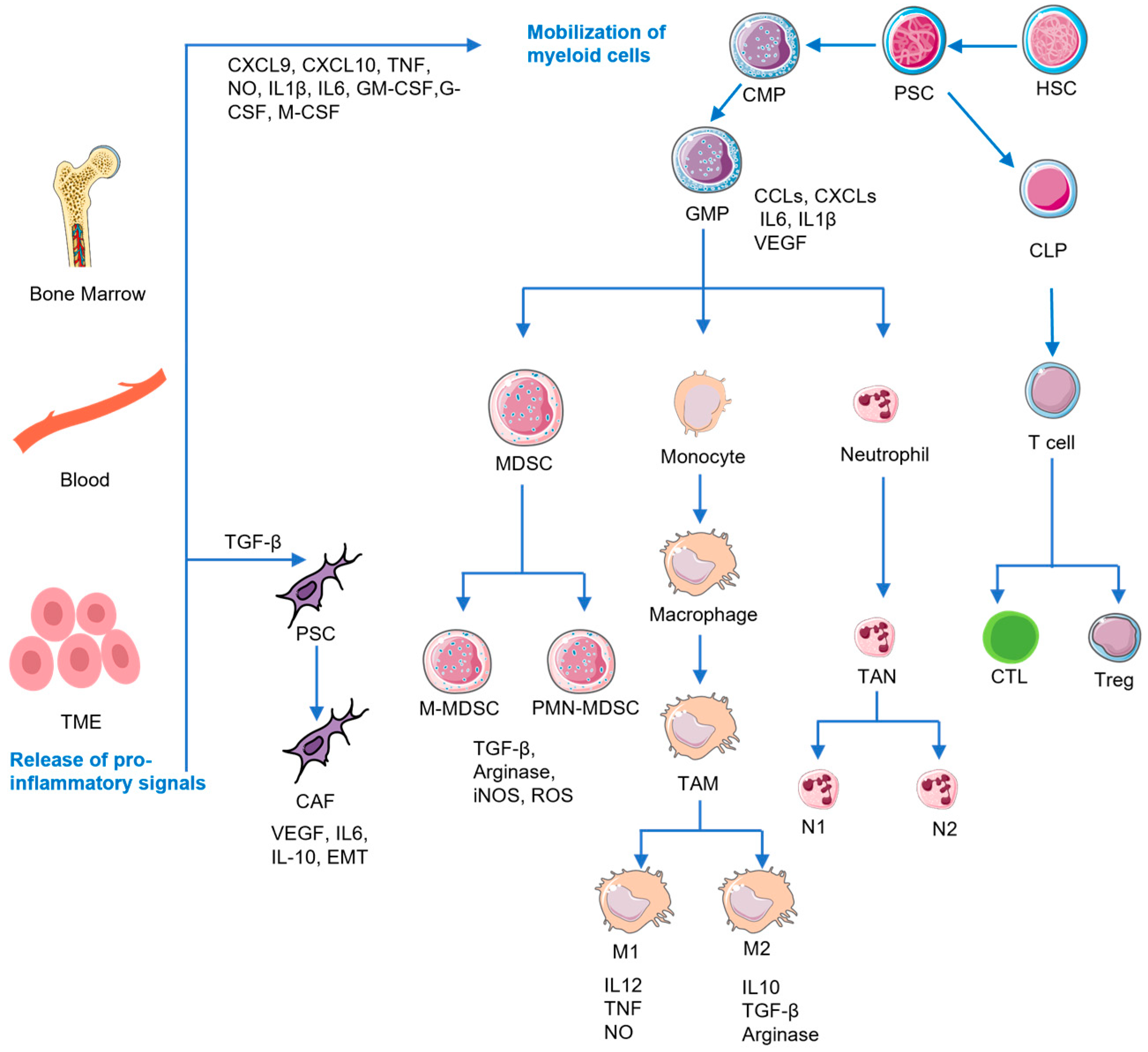
2. The Immunosuppressive TME of PDAC
2.1. MDSCs
2.2. TAMs
2.3. Tregs
2.4. CAFs
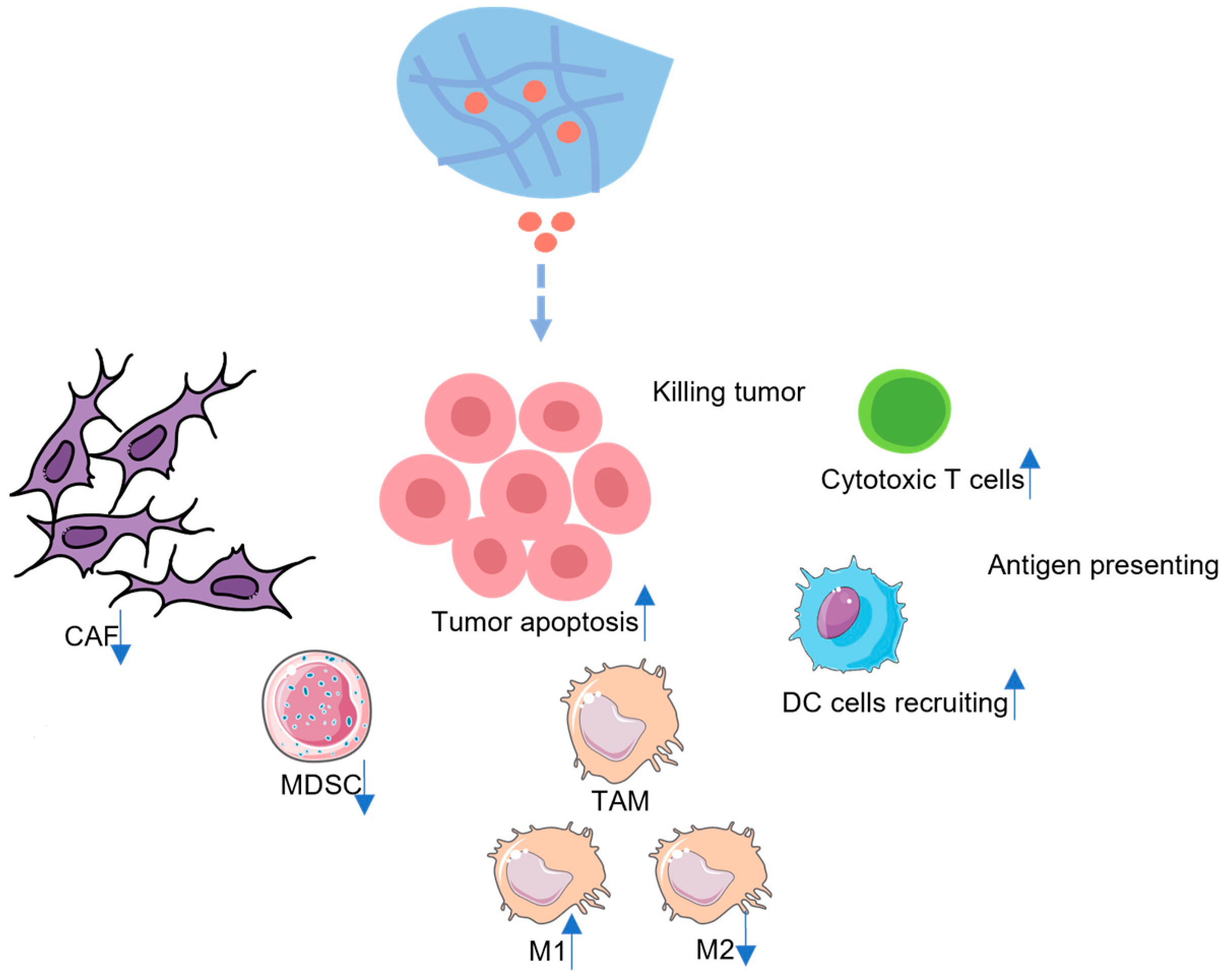
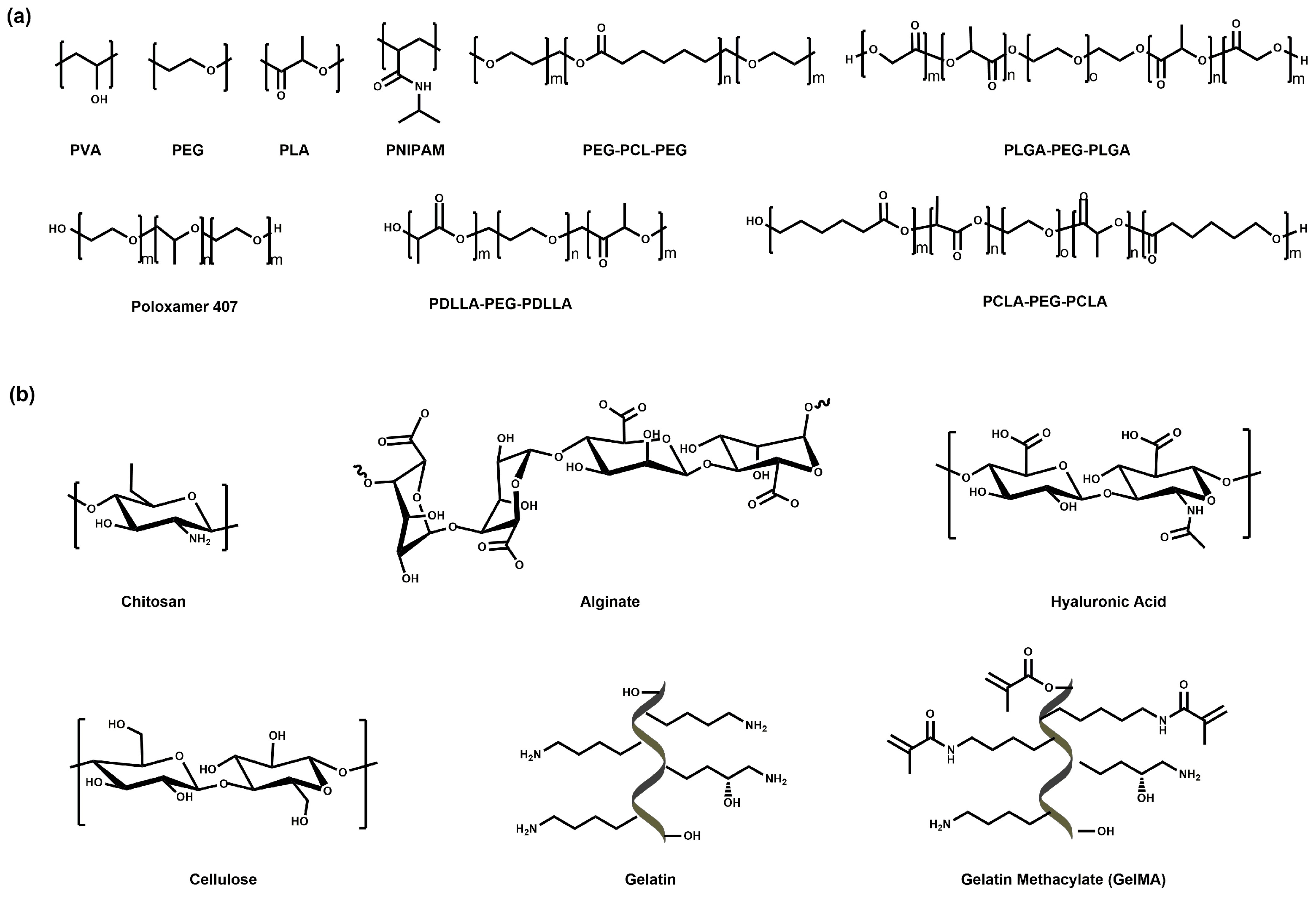
3. Hydrogel-Based Therapeutics for PDAC
3.1. Hydrogel-Based Small-Molecule Drug Therapy for PDAC
3.1.1. Hydrogel as the Platform for Synergistic Therapy
| Hydrogel | Drug | Hydrogel Size | Delivery Route | Characteristics | Antitumor Effect | Ref. |
|---|---|---|---|---|---|---|
| DNA | Anti-miRNA21 antisense nucleic acid, GEM | Nanogel | Unreported | A miRNA 21-responsive hydrogel which could simultaneously release drug and anti-miRNA. | Inducing the apoptosis of tumor cells by targeting miRNA21. | [117] |
| OCMS, CMCS | GEM | Macroscopic hydrogel | Intratumoral injection | An injectable and thermosensitive hydrogel to sustainably release GEM. | Inducing the apoptosis of tumor cells. | [125] |
| Alginate | Tumor cell lysate, GM-CSF | Macroscopic hydrogel | Surgical implantation | A personalized hydrogel vaccine which sustainably released drug through the porous stereo structure. | Recruiting DCs and enhancing the targeted antitumor immune response of CD8+ T cells. | [126] |
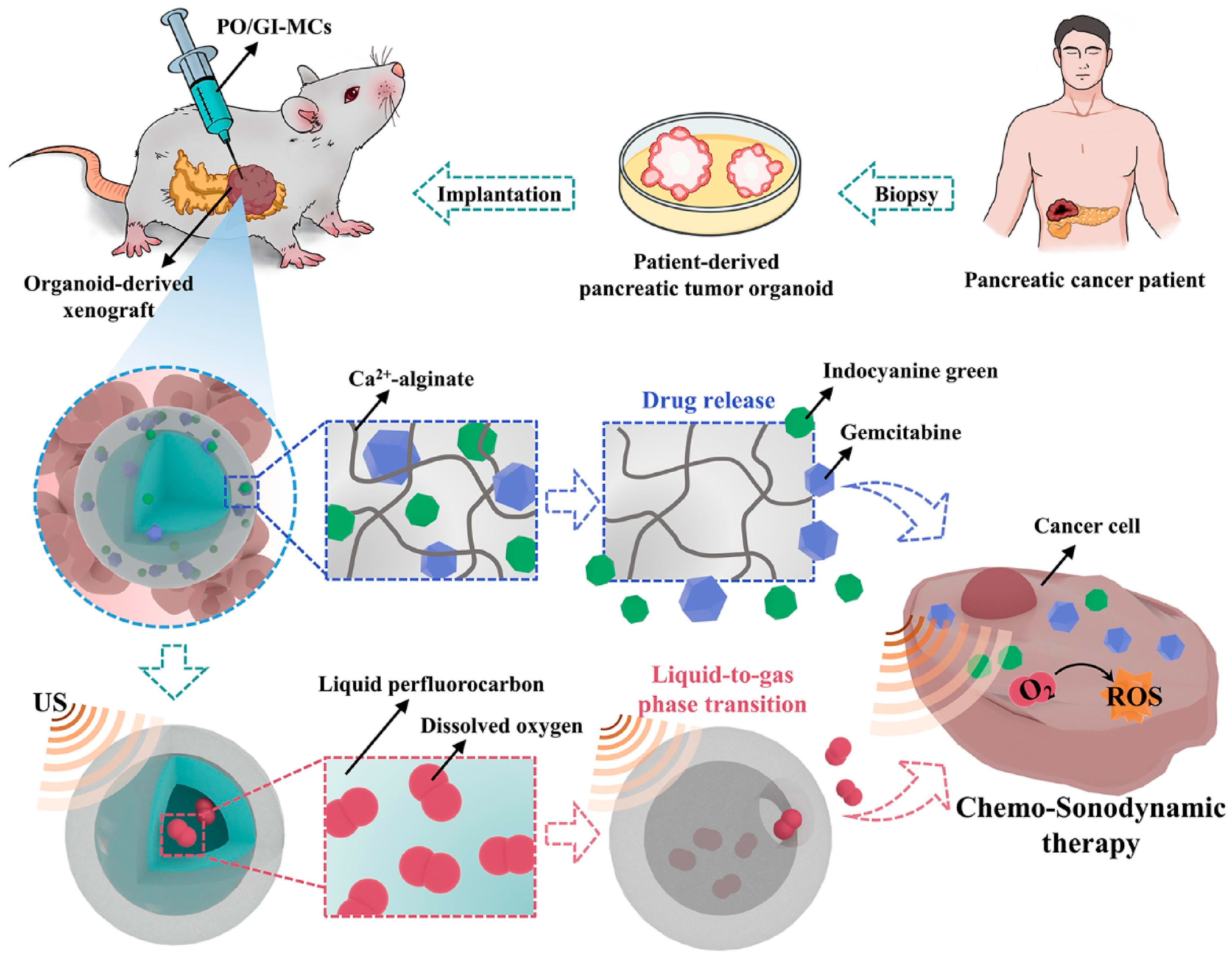
| Alginate | GEM, ICG | Microgel | Intratumoral injection | A core–shell microcapsule which can release oxygen and drug in presence of low intensity ultrasound. | Hypoxic microenvironment reverse and apoptosis of PDAC cells activated by ROS. | [124] |
| Chitosan | IRF5 mRNA, CCL5 siRNA | Macroscopic hydrogel | Intratumoral injection | An in situ-injectable thermosensitive hydrogel with sustained RNA release. | Inducing macrophage polarization and increasing the infiltration of CD8+ T cells into the TME, thus reshaping the immunosuppressive TME. | [127] |
| GelMA | GEM | Macroscopic hydrogel | Surgical implantation | An adhesive microneedle patch that could efficiently penetrate the tumor tissue to release GEM. | PDAC cell apoptosis. | [128] |
| Alginate, PLA | GEM | Macroscopic hydrogel | Surgical implantation | A hydrogel patch with reduced swelling ratio exhibiting prolonged drug release. | PDAC cell apoptosis | [75] |
| PDLLA-PEG-PDLLA | GEM, DPP-BTz | Macroscopic hydrogel | Intratumoral injection | Thermosensitive liposomal hydrogels with NIR-II light-triggered drug release. | PDAC cell apoptosis | [129] |
| PNIPAM, Alginate, PVA | GEM, H2S | Microgel | Intratumoral injection | Ultrasound responsive microbubble hydrogel, which contracted under the increasing temperature resulted from ultrasound, thus releasing GEM and H2S. | Contributing to PDAC cell apoptosis and inhibiting PDAC cell proliferation. | [114] |
| HA | Neoantigen peptide | Macroscopic hydrogel | Surgical implantation | A hydrogel vaccine with sustained adjuvant release. | Enhanced T-cell activation in the draining lymph node and expansion of neoantigen-specific T cells in the spleen. | [130] |
| Alginate | GEM or DOX | Macroscopic hydrogel | Surgical implantation | Coaxial hydrogel fibers exhibiting a slower release profile due to the core–shell structure for controlled release and diffusion barrier. | Inhibiting the growth of PDAC cells. | [131] |
| PNIPAM, CS, PEG, GNR | Unreported | Macroscopic hydrogel | Intratumoral injection | A thermal-sensitive hydrogel which shrunk with the increased temperature induced by an 808 nm laser. | Inducing tumor internal stresses, hypoxia, and apoptosis. | [19] |
| PDLLAPEG-PDLLA | GEM, cisplatin | Macroscopic hydrogel | Intratumoral injection | A thermal-sensitive hydrogel gelated in situ at physiological temperature, exhibiting delayed drug release from the micelle networks. | Inducing PDAC cell apoptosis and inhibiting proliferation. | [132] |
| Terpolypeptide | GEM | Macroscopic hydrogel | Peritumoral injection | A self-healing hydrogel that can deliver drugs sustainably due to its pH- and enzyme-responsive nature. | PDAC cell apoptosis. | [18] |
| PCLA-PEG-PCLA) | GEM | Macroscopic hydrogel | Subcutaneous injection | A thermal-sensitive nano-biohybrid hydrogel with sustained drug release. | PDAC cell apoptosis. | [133] |
| Poloxamer | PTX | Macroscopic hydrogel | Intratumoral injection | A thermosensitive hydrogel with paclitaxel liposome showed a slower release than liposome. | Unreported | [134] |
| PLGA-bPEG-b-PLGA | DOX | Macroscopic hydrogel | Intratumoral injection | A thermosensitive hydrogel with micelle networks. | Unreported | [135] |
| PEG, HSA | TRIAL | Macroscopic hydrogel | Intratumoral injection | A PEG-modified albumin hydrogel, gelated in situ. | PDAC cell apoptosis. | [136] |
| Gelatin | EGFR-lyric | Nanogel | Intravenous injection | Hydrogel nanoparticles formed by electrostatic interaction exhibiting a longer circulation time in vivo. | Unreported | [101] |
| HA | TRIAL | Macroscopic hydrogel | Intratumoral injection | PEG-TRAIL HA hydrogels with stability and controlled drug release. | PDAC cell apoptosis. | [137] |
| PVA | DOX, mitoxantrone, irinotecan | Microgel | Intraperitoneal injection | Drug eluting hydrogel beads. | PDAC cell apoptosis. | [138] |
| PEG-PCL-PEG | LPS, FGF | Macroscopic hydrogel | Subcutaneous injections | A hydrogel vaccine with adjuvant release. | Enhancing both cellular and humoral immune response against PDAC. | [139] |
3.1.2. Encapsulating Drugs in Microneedles (MNs) to Achieve Sustained Release
3.1.3. Design of TME-Responsive Hydrogel Degradation to Achieve Sustained Release
3.1.4. Incorporating the Drugs into the Polymer Network of Hydrogel to Release Sustainably
3.1.5. Encapsulating the Drugs into Hydrophobic Nanoparticles to Achieve Sustained Release
3.2. Hydrogel-Based Nucleic Acid Therapy for PDAC
3.3. Hydrogel-Based Protein Therapy for PDAC
4. The Potential Drug Candidates for Hydrogel-Based Immunotherapeutic Options for PDAC Treatment
4.1. MDSCs Directed Therapy
4.2. TAMs-Directed Therapy
4.3. CAFs-Directed Therapy
5. Discussion and Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMP | adenosine |
| APC | antigen-presenting cell |
| CaCO3 | calcium carbonate |
| CAF | cancer-related fibroblast |
| CCL2 | chemokine ligand 2 |
| CMCS | carboxymethyl chitosan |
| CMP | common myeloid progenitor |
| CSF1 | colony-stimulating factor 1 |
| CTL | cytotoxic T lymphocyte |
| CXCL | chemokine |
| CXCR | chemokine receptor |
| DC | dendritic cell |
| DDS | drug-delivery system |
| DOTAP | 1,2-Dioleoyl-3-trimethylammonium-propane |
| DOX | doxorubicin |
| DPPC | dipalmitoyl phosphatidylcholine |
| ECM | extracellular matrix |
| EDC | 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride |
| EGFR | epidermal growth factor receptors |
| EPR | permeability and resident effect |
| FAP | fibroblast-activating protein |
| FGF | fibroblast growth factor |
| FSP1 | fibroblast-specific protein 1 |
| GelMA | gelatin methacryloyl |
| GEM | gemcitabine |
| GEMM | genetically engineered and mutant mice |
| GRZB | granzyme B |
| H2S | hydrogen sulfide |
| HA | hyaluronic acid |
| HLA-DR | human leukocyte antigen DR isotype |
| HSC | hematopoietic stem cell |
| ICG | indocyanine green |
| IDO | indolamine 2,3-dioxygenase |
| IFNG | Interferon-γ |
| IL-10 | Interleukin 10 |
| IL12 | Interleukin 12 |
| IL-2 | Interleukin 2 |
| IL-6 | Interleukin 6 |
| IMC | immature myeloid cell |
| iNOS | inducible nitric oxide synthase |
| LPS | lipopolysaccharides |
| MDSCs | myeloid-derived suppressor cell |
| MHC-I | major histocompatibility complex class I |
| MHC-II | major histocompatibility complex class II |
| M-MDSC | monocytes-like MDSC |
| MMT | montmorillonite |
| MNs | microneedles |
| MSN | mesoporous silica nanoparticle |
| NHC | N-heterocyclic carbene |
| OCMC | oxidized-carboxymethylcellulose |
| PCLA-PEG-PCLA | Poly(ε-caprolactone-co-lactide)-b-poly(ε-caprolactone-co-lactide) |
| PDAC | pancreatic ductal adenocarcinoma |
| PDGFR | platelet-derived growth factor receptor |
| PDGFRβ | platelet-derived growth factor receptor β |
| PD-L1 | programmed cell death-Ligand 1 |
| PECE | Poly(ethylene glycol)poly(N-caprolactone)-poly(ethylene glycol) |
| PEG | polyethylene glycol |
| PFS | perfluorocarbon |
| PLA | polylactic acid |
| PLEL | Poly(D,L-lactide)-polyethylene glycol-poly(D,L-lactide) |
| PMN-MDSC | polymorphonuclear MDSC |
| PNIPAM | Poly(N-isopropyl acrylamide) |
| PSC | pancreatic stellate cell |
| PTT | photodynamic therapy |
| PTX | paclitaxel |
| ROS | reactive oxygen species |
| SDT | acoustic dynamic therapy |
| TAM | tumor-associated macrophage |
| TGFβ1 | transforming growth factor beta 1 |
| TME | tumor microenvironment |
| TMSPMA | 3-(trimethoxysilyl)propyl methacrylate |
| TNF | tumor necrosis factor |
| TRAIL | tumor-necrosis-factor-associated apoptosis-inducing ligand |
| Treg | regulatory T cells |
| VEGF | vascular endothelial growth factor |
| α-SMA | α-smooth actin |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e381. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Kennedy, E.B.; Khorana, A.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Krishnamurthi, S.; Moravek, C.; O’Reilly, E.M.; Philip, P.A.; et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Chen, Y.; Huang, D.; Guo, C.; Zhang, Q.; Li, X.; Zhang, X.; Gao, S.; Que, R.; Shen, Y.; et al. Randomized phase III study of sintilimab in combination with modified folfrinox versus folfrinox alone in patients with metastatic and recurrent pancreatic cancer in China: The CISPD3 trial. J. Clin. Oncol. 2022, 40, 560. [Google Scholar] [CrossRef]
- Gao, Q.; Feng, J.; Liu, W.; Wen, C.; Wu, Y.; Liao, Q.; Zou, L.; Sui, X.; Xie, T.; Zhang, J.; et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv. Drug Deliv. Rev. 2022, 188, 114445. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, C.; Xie, K.-P. Therapeutic resistance of pancreatic cancer: Roadmap to its reversal. Biochim. Biophys. Acta-Rev. Cancer 2021, 75, 2800–2810. [Google Scholar] [CrossRef]
- Lo, A.; Wang, L.-C.S.; Scholler, J.; Monslow, J.; Avery, D.; Newick, K.; O’Brien, S.; Evans, R.A.; Bajor, D.J.; Clendenin, C.; et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015, 75, 2800–2810. [Google Scholar] [CrossRef]
- Sherman, M.H.; Beatty, G.L. Tumor Microenvironment in Pancreatic Cancer Pathogenesis and Therapeutic Resistance. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 123–148. [Google Scholar] [CrossRef]
- Pereira, B.A.; Vennin, C.; Papanicolaou, M.; Chambers, C.R.; Herrmann, D.; Morton, J.P.; Cox, T.R.; Timpson, P. CAF Subpopulations: A New Reservoir of Stromal Targets in Pancreatic Cancer. Trends Cancer 2019, 5, 724–741. [Google Scholar] [CrossRef]
- Helms, E.J.; Berry, M.W.; Chaw, R.C.; DuFort, C.C.; Sun, D.; Onate, M.K.; Oon, C.; Bhattacharyya, S.; Sanford-Crane, H.; Horton, W.; et al. Mesenchymal Lineage Heterogeneity Underlies Nonredundant Functions of Pancreatic Cancer-Associated Fibroblasts. Cancer Discov. 2022, 12, 484–501. [Google Scholar] [CrossRef]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef]
- Boyd, L.N.C.; Andini, K.D.; Peters, G.J.; Kazemier, G.; Giovannetti, E. Heterogeneity and plasticity of cancer-associated fibroblasts in the pancreatic tumor microenvironment. Semin. Cancer Biol. 2022, 82, 184–196. [Google Scholar] [CrossRef]
- Siret, C.; Collignon, A.; Silvy, F.; Robert, S.; Cheyrol, T.; Andre, P.; Rigot, V.; Iovanna, J.; van de Pavert, S.; Lombardo, D.; et al. Deciphering the Crosstalk Between Myeloid-Derived Suppressor Cells and Regulatory T Cells in Pancreatic Ductal Adenocarcinoma. Front. Immunol. 2020, 10, 3070. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, Y.; Tazawa, H.; Yamada, M.; Kanaya, N.; Fushimi, T.; Kikuchi, S.; Kuroda, S.; Ohara, T.; Noma, K.; Yoshida, R.; et al. Oncolytic virus-mediated reducing of myeloid-derived suppressor cells enhances the efficacy of PD-L1 blockade in gemcitabine-resistant pancreatic cancer. Cancer Immunol. Immunother. 2023, 72, 1285–1300. [Google Scholar] [CrossRef]
- Zhang, Y.; Lazarus, J.; Steele, N.G.; Yan, W.; Lee, H.-J.; Nwosu, Z.C.; Halbrook, C.J.; Menjivar, R.E.; Kemp, S.B.; Sirihorachai, V.R.; et al. Regulatory T-cell Depletion Alters the Tumor Microenvironment and Accelerates Pancreatic Carcinogenesis. Cancer Discov. 2020, 10, 422–439. [Google Scholar] [CrossRef]
- Steele, N.G.; Carpenter, E.S.; Kemp, S.B.; Sirihorachai, V.R.; The, S.; Delrosario, L.; Lazarus, J.; Amir, E.-a.D.; Gunchick, V.; Espinoza, C.; et al. Multimodal mapping of the tumor and peripheral blood immune landscape in human pancreatic cancer. Nat. Cancer 2020, 1, 1097–1112. [Google Scholar] [CrossRef]
- Garcia, C.J.G.; Huang, Y.; Fuentes, N.R.; Turner, M.C.; Monberg, M.E.; Lin, D.; Nguyen, N.D.; Fujimoto, T.N.; Zhao, J.; Lee, J.J.; et al. Stromal HIF2 Regulates Immune Suppression in the Pancreatic Cancer Microenvironment. Gastroenterology 2022, 162, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, P.; Skoulas, D.; Karatzas, A.; Marakis, J.; Stamogiannos, A.; Tsimblouli, C.; Sereti, E.; Stratikos, E.; Dimas, K.; Vlassopoulos, D.; et al. Self-Healing pH- and Enzyme Stimuli-Responsive Hydrogels for Targeted Delivery of Gemcitabine To Treat Pancreatic Cancer. Biomacromolecules 2018, 19, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fang, Y.; He, Y.; Yin, H.; Guan, X.; Pu, Y.; Zhou, B.; Yue, W.; Ren, W.; Du, D.; et al. Extravascular gelation shrinkage-derived internal stress enables tumor starvation therapy with suppressed metastasis and recurrence. Nat. Commun. 2019, 10, 5380. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug-delivery systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Li, G.; Cai, Z.; Wu, J.; Wang, L.; Deng, L.; Cai, M.; Cui, W. Injectable Microfluidic Hydrogel Microspheres for Cell and Drug Delivery. Adv. Funct. Mater. 2021, 31, 2103339. [Google Scholar] [CrossRef]
- Yang, Z.; McClements, D.J.; Li, C.; Sang, S.; Chen, L.; Long, J.; Qiu, C.; Jin, Z. Targeted delivery of hydrogels in human gastrointestinal tract: A review. Food Hydrocoll. 2023, 134, 108013. [Google Scholar] [CrossRef]
- Hamarsheh, S.a.; Gross, O.; Brummer, T.; Zeiser, R. Immune modulatory effects of oncogenic KRAS in cancer. Nat. Commun. 2020, 11, 5439. [Google Scholar] [CrossRef]
- Kerk, S.A.; Papagiannakopoulos, T.; Shah, Y.M.; Lyssiotis, C.A. Metabolic networks in mutant KRAS-driven tumours: Tissue specificities and the microenvironment. Nat. Rev. Cancer 2021, 21, 510–525. [Google Scholar] [CrossRef]
- Singh, K.; Pruski, M.; Bland, R.; Younes, M.; Guha, S.; Thosani, N.; Maitra, A.; Cash, B.D.; McAllister, F.; Logsdon, C.D.; et al. Kras mutation rate precisely orchestrates ductal derived pancreatic intraepithelial neoplasia and pancreatic cancer. Lab. Investig. 2021, 101, 177–192. [Google Scholar] [CrossRef]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS mutation: From undruggable to druggable in cancer. Signal Transduct. Target. Ther. 2021, 6, 386. [Google Scholar] [CrossRef]
- Kim, M.P.; Li, X.; Deng, J.; Zhang, Y.; Dai, B.; Allton, K.L.; Hughes, T.G.; Siangco, C.; Augustine, J.J.; Kang, Y.a.; et al. Oncogenic KRAS Recruits an Expansive Transcriptional Network through Mutant p53 to Drive Pancreatic Cancer Metastasis. Cancer Discov. 2021, 11, 2094–2111. [Google Scholar] [CrossRef]
- Yamamoto, K.; Venida, A.; Yano, J.; Biancur, D.E.; Kakiuchi, M.; Gupta, S.; Sohn, A.S.W.; Mukhopadhyay, S.; Lin, E.Y.; Parker, S.J.; et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 2020, 581, 100–105. [Google Scholar] [CrossRef]
- Ye, Z.-H.; Yu, W.-B.; Huang, M.-Y.; Chen, J.; Lu, J.-J. Building on the backbone of CD47-based therapy in cancer: Combination strategies, mechanisms, and future perspectives. Acta Pharm. Sin. B 2023, 13, 1467–1487. [Google Scholar] [CrossRef]
- Wesch, D.; Kabelitz, D.; Oberg, H.-H. Tumor resistance mechanisms and their consequences on gamma delta T cell activation. Immunol. Rev. 2020, 298, 84–98. [Google Scholar] [CrossRef]
- Winograd, R.; Byrne, K.T.; Evans, R.A.; Odorizzi, P.M.; Meyer, A.R.L.; Bajor, D.L.; Clendenin, C.; Stanger, B.Z.; Furth, E.E.; Wherry, E.J.; et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol. Res. 2015, 3, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu. Rev. Med. 2020, 71, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Porembka, M.R.; Mitchem, J.B.; Belt, B.A.; Hsieh, C.-S.; Lee, H.-M.; Herndon, J.; Gillanders, W.E.; Linehan, D.C.; Goedegebuure, P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol. Immunother. 2012, 61, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.E.; Hingorani, S.R.; Mick, R.; Combs, C.; Tuveson, D.A.; Vonderheide, R.H. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007, 67, 9518–9527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Obermann, S.; von Wasielewski, R.; Haile, L.; Manns, M.P.; Korangy, F.; Greten, T.F. Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology 2009, 128, 141–149. [Google Scholar] [CrossRef]
- Sahraei, M.; Bose, M.; Sanders, J.A.; De, C.; DasRoy, L.; Nath, S.; Brouwer, C.R.; Mukherjee, P. Repression of MUC1 Promotes Expansion and Suppressive Function of Myeloid-Derived Suppressor Cells in Pancreatic and Breast Cancer Murine Models. Int. J. Mol. Sci. 2021, 22, 5587. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Galkin, A.; Dupont, J. GB1275, a first-in-class CD11b modulator: Rationale for immunotherapeutic combinations in solid tumors. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.; Weaver, J.D.; Pal, R.; Asara, J.; Patsoukis, N.; Boussiotis, V.A. Metabolic Reprogramming of Myeloid Cells in Response to Factors of "Emergency" Myelopoiesis By Myeloid-Specific PD-1 Ablation, Regulates Myeloid Lineage Fate Commitment and Anti-Tumor Immunity. Blood 2018, 132, 14. [Google Scholar] [CrossRef]
- Strauss, L.; Weaver, J.D.; Patsoukis, N.; Boussiotis, V.A. PD-1 Mediates Lineage Fate Determination and Function of Myeloid Cells in Response to Tumor-Mediated "Emergency" Myelopoiesis. Blood 2017, 130, 445. [Google Scholar]
- Hegde, S.; Leader, A.M.; Merad, M. MDSC: Markers, development, states, and unaddressed complexity. Immunity 2021, 54, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Greene, S.; Robbins, Y.; Mydlarz, W.K.; Huynh, A.P.; Schmitt, N.C.; Friedman, J.; Horn, L.A.; Palena, C.; Schlom, J.; Maeda, D.Y.; et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin. Cancer Res. 2020, 26, 1420–1431. [Google Scholar] [CrossRef]
- Sun, L.; Clavijo, P.E.; Robbins, Y.; Patel, P.; Friedman, J.; Greene, S.; Das, R.; Silvin, C.; Van Waes, C.; Horn, L.A.; et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight 2019, 4, e126853. [Google Scholar] [CrossRef]
- Haist, M.; Stege, H.; Grabbe, S.; Bros, M. The Functional Crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the Immunosuppressive Tumor Microenvironment. Cancers 2021, 13, 210. [Google Scholar] [CrossRef]
- Peng, P.; Lou, Y.; Wang, S.; Wang, J.; Zhang, Z.; Du, P.; Zheng, J.; Liu, P.; Xu, L.X. Activated NK cells reprogram MDSCs via NKG2D-NKG2DL and IFN-gamma to modulate antitumor T-cell response after cryo-thermal therapy. J. Immunother. Cancer 2022, 10, e005769. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Ji, J.; Luo, J.; Zhao, Y.; Zhou, X.; Zheng, J.; Guo, M.; Liu, Y. Orthotopic and Heterotopic Murine Models of Pancreatic Cancer Exhibit Different Immunological Microenvironments and Different Responses to Immunotherapy. Front. Immunol. 2022, 13, 863346. [Google Scholar] [CrossRef] [PubMed]
- Raber, P. Differential Mechanisms of Suppression by Subsets of Tumor Infiltrating Myeloid Derived Suppressor Cells and Their Surprising Potential to Enhance T Cell Immunotherapy. Ph.D. Thesis, Louisiana State University Health Sciences Center, Baton Rouge, LA, USA, 2014. [Google Scholar]
- Bleve, A.; Consonni, F.M.; Porta, C.; Garlatti, V.; Sica, A. Evolution and Targeting of Myeloid Suppressor Cells in Cancer: A Translational Perspective. Cancers 2022, 14, 510. [Google Scholar] [CrossRef]
- Clark, C.E. The dynamics of immunosurveillance in pancreatic cancer from inception to invasion and metastasis. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2007. [Google Scholar]
- Oweida, A.J.; Mueller, A.C.; Piper, M.; Milner, D.; Van Court, B.; Bhatia, S.; Phan, A.; Bickett, T.; Jordan, K.; Proia, T.; et al. Response to radiotherapy in pancreatic ductal adenocarcinoma is enhanced by inhibition of myeloid-derived suppressor cells using STAT3 anti-sense oligonucleotide. Cancer Immunol. Immunother. 2021, 70, 989–1000. [Google Scholar] [CrossRef]
- Pittet, M.J.; Michielin, O.; Migliorini, D. Clinical relevance of tumour-associated macrophages. Nat. Rev. Clin. Oncol. 2022, 19, 402–421. [Google Scholar] [CrossRef]
- Zhu, Y.; Herndon, J.M.; Sojka, D.K.; Kim, K.-W.; Knolhoff, B.L.; Zuo, C.; Cullinan, D.R.; Luo, J.; Bearden, A.R.; Lavine, K.J.; et al. Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity 2017, 47, 323. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Ino, Y.; Yamazaki-Itoh, R.; Shimada, K.; Iwasaki, M.; Kosuge, T.; Kanai, Y.; Hiraoka, N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer 2013, 108, 914–923. [Google Scholar] [CrossRef]
- Koyama, S. Mechanisms of regulatory T cell infiltration in tumors: Implications for innovative immune precision therapies. Cancer Sci. 2022, 113, e002591. [Google Scholar] [CrossRef]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef]
- Jang, J.-E.; Hajdu, C.H.; Liot, C.; Miller, G.; Dustin, M.L.; Bar-Sagi, D. Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-tumor Immunity in Pancreatic Cancer. Cell Rep. 2017, 20, 558–571. [Google Scholar] [CrossRef]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017, 216, 3799–3816. [Google Scholar] [CrossRef]
- Murray, E.R.; Menezes, S.; Henry, J.C.; Williams, J.L.; Alba-Castellon, L.; Baskaran, P.; Quetier, I.; Desai, A.; Marshall, J.J.T.; Rosewell, I.; et al. Disruption of pancreatic stellate cell myofibroblast phenotype promotes pancreatic tumor invasion. Cell Rep. 2022, 38, 110227. [Google Scholar] [CrossRef]
- Chu, X.; Yang, Y.; Tian, X. Crosstalk between Pancreatic Cancer Cells and Cancer-Associated Fibroblasts in the Tumor Microenvironment Mediated by Exosomal MicroRNAs. Int. J. Mol. Sci. 2022, 23, 9512. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Jeong, J.; Lee, D.S.; Hyeon, D.Y.; Park, G.W.; Jeon, S.; Lee, K.B.; Jang, J.-Y.; Hwang, D.; Kim, H.M.; et al. PD-L1-directed PlGF/VEGF blockade synergizes with chemotherapy by targeting CD141+ cancer-associated fibroblasts in pancreatic cancer. Nat. Commun. 2022, 13, 6292. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.E. Crosstalk between pancreatic cancer cells and pancreatic cancer-associated fibroblasts through cytokines and a GPCR. Ph.D. Thesis, University of California, San Diego, CA, USA, 2016. [Google Scholar]
- Guan, J.; Zhang, H.; Wen, Z.; Gu, Y.; Cheng, Y.; Sun, Y.; Zhang, T.; Jia, C.; Lu, Z.; Chen, J. Retinoic acid inhibits pancreatic cancer cell migration and EMT through the downregulation of IL-6 in cancer associated fibroblast cells. Cancer Lett. 2014, 345, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Schuth, S.; Le Blanc, S.; Krieger, T.G.; Jabs, J.; Schenk, M.; Giese, N.A.; Buchler, M.W.; Eils, R.; Conrad, C.; Strobel, O. Patient-specific modeling of stroma-mediated chemoresistance of pancreatic cancer using a three-dimensional organoid-fibroblast co-culture system. J. Exp. Clin. Cancer Res. 2022, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ren, Y.; Yang, P.; Wang, J.; Zhou, H. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Cell Death Dis. 2022, 13, 897. [Google Scholar] [CrossRef]
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-associated fibroblasts in the single-cell era. Nat. Cancer 2022, 3, 793–807. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, Classification, Properties and Potential Applications-A Brief Review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Bruns, J.; Egan, T.; Mercier, P.; Zustiak, S.P. Glioblastoma spheroid growth and chemotherapeutic responses in single and dual-stiffness hydrogels. Acta Biomater. 2023, 163, 400–414. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Darvishan, S.; Abdouss, M.; Yazdian, F.; Rahdar, A.; Diez-Pascual, A.M. pH-responsive polyacrylic acid (PAA)-carboxymethyl cellulose (CMC) hydrogel incorporating halloysite nanotubes (HNT) for controlled curcumin delivery. Ind. Crops Prod. 2023, 197, 116654. [Google Scholar] [CrossRef]
- Eshaghi, M.M.; Pourmadadi, M.; Rahdar, A.; Diez-Pascual, A.M. Improving quercetin anticancer activity through a novel polyvinylpyrrolidone/polyvinyl alcohol/TiO2 nanocomposite. J. Drug Deliv. Sci. Technol. 2023, 8, 104304. [Google Scholar] [CrossRef]
- Talebian, S.; Shim, I.K.; Foroughi, J.; Orive, G.; Vine, K.L.; Kim, S.C.; Wallace, G.G. 3D-Printed Coaxial Hydrogel Patches with Mussel-Inspired Elements for Prolonged Release of Gemcitabine. Polymers 2021, 13, 4367. [Google Scholar] [CrossRef] [PubMed]
- Gardel, M.L. MATERIALS SCIENCE Synthetic polymers with biological rigidity. Nature 2013, 493, 618–619. [Google Scholar] [CrossRef]
- Sun, H.; Xu, J.; Wang, Y.; Shen, S.; Xu, X.; Zhang, L.; Jiang, Q. Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact. Mater. 2023, 24, 477–496. [Google Scholar] [CrossRef]
- Li, Z.; Xu, W.; Yang, J.; Wang, J.; Wang, J.; Zhu, G.; Li, D.; Ding, J.; Sun, T. A Tumor Microenvironments-Adapted Polypeptide Hydrogel/Nanogel Composite Boosts Antitumor Molecularly Targeted Inhibition and Immunoactivation. Adv. Mater. 2022, 34, e2200449. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, D. Rational Design of Peptide-based Smart Hydrogels for Therapeutic Applications. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Rosa, E.; Gallo, E.; Sibillano, T.; Giannini, C.; Rizzuti, S.; Gianolio, E.; Scognamiglio, P.L.; Morelli, G.; Accardo, A.; Diaferia, C. Incorporation of PEG Diacrylates (PEGDA) Generates Hybrid Fmoc-FF Hydrogel Matrices. Gels 2022, 8, 831. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kumar, M.; Miravet, J.F.; Ulijn, R.V.; Escuder, B. Peptide-Based Molecular Hydrogels as Supramolecular Protein Mimics. Chem.-A Eur. J. 2017, 23, 981–993. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, J.; Meng, Q.; Zhong, S.; Gao, Y.; Cui, X. Recent advances in polysaccharide-based self-healing hydrogels for biomedical applications. Carbohydr. Polym. 2022, 283, 119161. [Google Scholar] [CrossRef]
- Kahn, J.S.; Hu, Y.; Willner, I. Stimuli-Responsive DNA-Based Hydrogels: From Basic Principles to Applications. Acc. Chem. Res. 2017, 50, 680–690. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Li, H.; Peng, L.; Wu, M.; Zhao, X.; Cui, X.; Ruan, C.; Liu, W. Osteochondral Regeneration with 3D-Printed Biodegradable High-Strength Supramolecular Polymer Reinforced-Gelatin Hydrogel Scaffolds. Adv. Sci. 2019, 6, 1900867. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; Gonzalez-Garcia, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B-Biointerfaces 2023, 222, 113096. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Huang, X.; Du, X.; Mo, L.; Ma, C.; Wang, H. Facile synthesis of pH-responsive sodium alginate/carboxymethyl chitosan hydrogel beads promoted by hydrogen bond. Carbohydr. Polym. 2022, 278, 118993. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, Q.; Peng, X.; Chen, Y.; Yang, H.; Xu, W.; Zhao, Y.; Xiao, P.; Zhou, Y. Self-Healing Hyaluronic Acid Nanocomposite Hydrogels with Platelet-Rich Plasma Impregnated for Skin Regeneration. Acs Nano 2022, 16, 11346–11359. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. -JmrT 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Xing, X.; Han, Y.; Cheng, H. Biomedical applications of chitosan/silk fibroin composites: A review. Int. J. Biol. Macromol. 2023, 240, 124407. [Google Scholar] [CrossRef]
- Rahbar, M.R.; Galeh, H.E.G.; Khalili, S.; Jahangiri, A. Chitosan: A Promising Protective Component Against SARS-CoV-2 and Influenza Virus. Lett. Drug Des. Discov. 2021, 18, 418–421. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 509–533. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Wu, W.; Dong, Y.; Liu, H.; Jiang, X.; Yang, L.; Luo, J.; Hu, Y.; Gou, M. 3D printed elastic hydrogel conduits with 7,8-dihydroxyflavone release for peripheral nerve repair. Mater. Today Bio 2023, 20, 100652. [Google Scholar] [CrossRef]
- Gaowa, A.; Horibe, T.; Kohno, M.; Sato, K.; Harada, H.; Hiraoka, M.; Tabata, Y.; Kawakami, K. Combination of hybrid peptide with biodegradable gelatin hydrogel for controlled release and enhancement of anti-tumor activity in vivo. J. Control. Release 2014, 176, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Boike, L.; Henning, N.J.; Nomura, D.K. Advances in covalent drug discovery. Nat. Rev. Drug Discov. 2022, 21, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Yang, D. Recent Advances in Hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Culebras, M.; Pishnamazi, M.; Walker, G.M.; Collins, M.N. Facile Tailoring of Structures for Controlled Release of Paracetamol from Sustainable Lignin Derived Platforms. Molecules 2021, 26, 1593. [Google Scholar] [CrossRef] [PubMed]
- Asikainen, S.; Seppala, J. Photo-crosslinked anhydride-modified polyester and -ethers for pH-sensitive drug release. Eur. J. Pharm. Biopharm. 2020, 150, 33–42. [Google Scholar] [CrossRef]
- Lin, F.; Zheng, J.; Guo, W.; Zhu, Z.; Wang, Z.; Dong, B.; Lin, C.; Huang, B.; Lu, B. Smart cellulose-derived magnetic hydrogel with rapid swelling and deswelling properties for remotely controlled drug release. Cellulose 2019, 26, 6861–6877. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Yang, S.; Tan, S.; Li, X.; Dai, H.; Yu, X.; Zhang, X.; Weng, N.; Jian, B.; et al. Double-Network Hydrogel with High Mechanical Strength Prepared from Two Biocompatible Polymers. J. Appl. Polym. Sci. 2009, 112, 3063–3070. [Google Scholar] [CrossRef]
- Young, M.E.; Carroad, P.A.; Bell, R.L. Estimation of Diffusion-Coefficients of Proteins. Biotechnol. Bioeng. 1980, 22, 947–955. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- O’Shea, T.M.; Aimetti, A.A.; Kim, E.; Yesilyurt, V.; Langer, R. Synthesis and Characterization of a Library of In-Situ Curing, Nonswelling Ethoxylated Polyol Thiol-ene Hydrogels for Tailorable Macromolecule Delivery. Adv. Mater. 2015, 27, 65–72. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Xu, F.; Xu, Z.; Wei, D.; Feng, Y.; Wang, Y.; Jia, D.; Zhou, Y. UV-crosslinkable and thermo-responsive chitosan hybrid hydrogel for NIR-triggered localized on-demand drug delivery. Carbohydr. Polym. 2017, 174, 904–914. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Liu, B.; He, S.; Dai, G.; Xu, B.; Zhong, W. NIR light-responsive short peptide/2D NbSe2 nanosheets composite hydrogel with controlled-release capacity. J. Mater. Chem. B 2019, 7, 3134–3142. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, X.; Zhao, C.; Fu, X.; Zhang, W.; Kong, W.; Zhang, B.; Zhao, Y. Ultrasound-Responsive Microfluidic Microbubbles for Combination Tumor Treatment. Adv. Ther. 2021, 4, 2100050. [Google Scholar] [CrossRef]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef]
- Szilagyi, B.A.; Nemethy, A.; Magyar, A.; Szabo, I.; Bosze, S.; Gyarmati, B.; Szilagyi, A. Amino acid based polymer hydrogel with enzymatically degradable cross-links. React. Funct. Polym. 2018, 133, 21–28. [Google Scholar] [CrossRef]
- Yan, J.; Zou, H.; Zhou, W.; Yuan, X.; Li, Z.; Ma, X.; Liu, C.; Wang, Y.; Rosenholm, J.M.; Cui, W.; et al. Self-assembly of DNA nanogels with endogenous microRNA toehold self-regulating switches for targeted gene regulation therapy. Biomater. Sci. 2022, 10, 4119–4125. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, B.; Shao, H.; Zhang, S.; Chen, X.; Li, F.; Liang, W. Hydrogels for localized chemotherapy of liver cancer: A possible strategy for improved and safe liver cancer treatment. Drug Deliv. 2022, 29, 1457–1476. [Google Scholar] [CrossRef]
- Nowak, K.M.M.; Schwartz, M.R.R.; Breza, V.R.R.; Price, R.J.J. Sonodynamic therapy: Rapid progress and new opportunities for non-invasive tumor cell killing with sound. Cancer Lett. 2022, 532, 215592. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Lin, H.; Yu, L.; Chen, Y. Hypoxia-Irrelevant Photonic Thermodynamic Cancer Nanomedicine. Acs Nano 2019, 13, 2223–2235. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, C.; Wen, B.; Fu, X.; Shang, L.; Kong, W.; Zhao, Y. Oxygen-carrying microfluidic microcapsules for enhancing chemo-sonodynamic therapy on patient-derived tumor organoid models. Chem. Eng. J. 2022, 435, 134871. [Google Scholar] [CrossRef]
- Xu, L.; Tang, S.; Yang, H.; Liang, M.; Ren, P.; Wei, D.; He, J.; Kong, W.; Liu, P.; Zhang, T. Sustained delivery of gemcitabine via in situ injectable mussel-inspired hydrogels for the local therapy of pancreatic cancer. J. Mater. Chem. B 2022, 10, 6338–6350. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, C.; Yang, Y.; Chen, X.; Ge, F.; Wang, J.; Deng, J. Inhibition of tumor recurrence and metastasis via a surgical tumor-derived personalized hydrogel vaccine. Biomater. Sci. 2022, 10, 1352–1363. [Google Scholar] [CrossRef]
- Gao, C.; Cheng, K.; Li, Y.; Gong, R.; Zhao, X.; Nie, G.; Ren, H. Injectable Immunotherapeutic Hydrogel Containing RNA-Loaded Lipid Nanoparticles Reshapes Tumor Microenvironment for Pancreatic Cancer Therapy. Nano Lett. 2022, 22, 8801–8809. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, X.; Huang, D.; Mao, L.; Qiu, Y.; Zhao, Y. Bioinspired adhesive microneedle patch with gemcitabine encapsulation for pancreatic cancer treatment. Chem. Eng. J. 2022, 431, 133362. [Google Scholar] [CrossRef]
- Kong, Y.; Dai, Y.; Qi, D.; Du, W.; Ni, H.; Zhang, F.; Zhao, H.; Shen, Q.; Li, M.; Fan, Q. Injectable and Thermosensitive Liposomal Hydrogels for NIR-II Light-Triggered Photothermal-Chemo Therapy of Pancreatic Cancer. Acs Appl. Bio Mater. 2021, 4, 7595–7604. [Google Scholar] [CrossRef]
- Delitto, D.; Zabransky, D.J.; Chen, F.; Thompson, E.D.; Zimmerman, J.W.; Armstrong, T.D.; Leatherman, J.M.; Suri, R.; Lopez-Vidal, T.Y.; Huff, A.L.; et al. Implantation of a neoantigen-targeted hydrogel vaccine prevents recurrence of pancreatic adenocarcinoma after incomplete resection. Oncoimmunology 2021, 10, 2001159. [Google Scholar] [CrossRef]
- Talebian, S.; Shim, I.K.; Kim, S.C.; Spinks, G.M.; Vine, K.L.; Foroughi, J. Coaxial mussel-inspired biofibers: Making of a robust and efficacious depot for cancer drug delivery. J. Mater. Chem. B 2020, 8, 5064–5079. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Xue, B.; Jia, Y.; Yuan, L.; Han, R.; Yang, F.; Peng, J.; Qian, Z. Sustained co-delivery of gemcitabine and cis-platinum via biodegradable thermo-sensitive hydrogel for synergistic combination therapy of pancreatic cancer. Nano Res. 2019, 12, 1389–1399. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Lee, E.; Maeng, J.H.; Thambi, T.; Kim, B.S.; Lee, D.; Lee, D.S. Pancreatic cancer therapy using an injectable nanobiohybrid hydrogel. Rsc. Adv. 2016, 6, 41644–41655. [Google Scholar] [CrossRef]
- Mao, Y.; Li, X.; Chen, G.; Wang, S. Thermosensitive Hydrogel System With Paclitaxel Liposomes Used in Localized Drug Delivery System for In Situ Treatment of Tumor: Better Antitumor Efficacy and Lower Toxicity. J. Pharm. Sci. 2016, 105, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, J.; Sun, D.; Sun, H.; Zhuang, X.; Chang, F.; Wang, J.; Chen, X. Thermogel-mediated sustained drug delivery for in situ malignancy chemotherapy. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 49, 262–268. [Google Scholar] [CrossRef]
- Kim, I.; Choi, J.S.; Lee, S.; Byeon, H.J.; Lee, E.S.; Shin, B.S.; Choi, H.-G.; Lee, K.C.; Youn, Y.S. In situ facile-forming PEG cross-linked albumin hydrogels loaded with an apoptotic TRAIL protein. J. Control. Release 2015, 214, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H.J.; Choi, S.H.; Choi, J.S.; Kim, I.; Shin, B.S.; Lee, E.S.; Park, E.-S.; Lee, K.C.; Youn, Y.S. Four-arm PEG cross-linked hyaluronic acid hydrogels containing PEGylated apoptotic TRAIL protein for treating pancreatic cancer. Acta Biomater. 2014, 10, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Yagublu, V.; Caliskan, N.; Lewis, A.L.; Jesenofsky, R.; Gasimova, L.; Loehr, J.M.; Keese, M. Treatment of experimental pancreatic cancer by doxorubicin-, mitoxantrone-, and irinotecan-drug eluting beads. Pancreatology 2013, 13, 79–87. [Google Scholar] [CrossRef]
- Shi, H.S.; Gong, C.Y.; Zhang, H.L.; Wang, Y.S.; Zhang, J.; Luo, Z.C.; Qian, Z.Y.; Wei, Y.Q.; Yang, L. Novel vaccine adjuvant LPS-Hydrogel for truncated basic fibroblast growth factor to induce antitumor immunity. Carbohydr. Polym. 2012, 89, 1101–1109. [Google Scholar] [CrossRef]
- Shen, Z.; Ma, Q.; Zhou, X.; Zhang, G.; Hao, G.; Sun, Y.; Cao, J. Strategies to improve photodynamic therapy efficacy by relieving the tumor hypoxia environment. Npg Asia Mater. 2021, 13, 39. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef]
- Zhang, Z.; Ai, S.; Yang, Z.; Li, X. Peptide-based supramolecular hydrogels for local drug delivery. Adv. Drug Deliv. Rev. 2021, 174, 482–503. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Z.; Lu, S.; Zhang, T.; Zhou, N.; Ren, P.; Wang, F.; Yang, Y.; Ji, Z. Assembled anti-adhesion polypropylene mesh with self-fixable and degradable in situ mussel-inspired hydrogel coating for abdominal wall defect repair. Biomater. Sci. 2018, 6, 3030–3041. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Z.; Zhu, L.; Wen, Y.; Zhang, T.; Ren, P.; Wang, F.; Ji, Z. Combination of Polypropylene Mesh and in Situ Injectable Mussel-Inspired Hydrogel in Laparoscopic Hernia Repair for Preventing Post-Surgical Adhesions in the Piglet Model. Acs Biomater. Sci. Eng. 2020, 6, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C.; Sleiman, H.F. DNA nanotechnology. Nat. Rev. Mater. 2018, 3, 17068. [Google Scholar] [CrossRef]
- Steele, C.W.; Karim, S.A.; Leach, J.D.G.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z.; et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef]
- Dominguez, G.A.; Condamine, T.; Mony, S.; Hashimoto, A.; Wang, F.; Liu, Q.; Forero, A.; Bendell, J.; Witt, R.; Hockstein, N.; et al. Selective Targeting of Myeloid-Derived Suppressor Cells in Cancer Patients Using DS-8273a, an Agonistic TRAIL-R2 Antibody. Clin. Cancer Res. 2017, 23, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.S.; Hezel, A.F.; Linehan, D.; Wang-Gillam, A.; Eskens, F.; Sleijfer, S.; Desar, I.; Erdkamp, F.; Wilmink, J.; Diehl, J.; et al. Orally administered CCR2 selective inhibitor CCX872-b clinical trial in pancreatic cancer. J. Clin. Oncol. 2017, 35, 276. [Google Scholar] [CrossRef]
- Nywening, T.M.; Belt, B.A.; Cullinan, D.R.; Panni, R.Z.; Han, B.J.; Sanford, D.E.; Jacobs, R.C.; Ye, J.; Patel, A.A.; Gillanders, W.E.; et al. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2018, 67, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Knolhoff, B.L.; Meyer, M.A.; Nywening, T.M.; West, B.L.; Luo, J.; Wang-Gillam, A.; Goedegebuure, S.P.; Linehan, D.C.; DeNardo, D.G. CSF1/CSF1R Blockade Reprograms Tumor-Infiltrating Macrophages and Improves Response to T-cell Checkpoint Immunotherapy in Pancreatic Cancer Models. Cancer Res. 2014, 74, 5057–5069. [Google Scholar] [CrossRef]
- Trabulo, S.; Aires, A.; Aicher, A.; Heeschen, C.; Cortajarena, A.L. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells. Biochim. Et. Biophys. Acta-Gen. Subj. 2017, 1861, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; O’Connor, R.S.; Trefely, S.; Graham, K.; Snyder, N.W.; Beatty, G.L. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat. Immunol. 2019, 20, 265–275. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Elahi-Gedwillo, K.Y.; Carlson, M.; Zettervall, J.; Provenzano, P.P. Antifibrotic Therapy Disrupts Stromal Barriers and Modulates the Immune Landscape in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2019, 79, 372–386. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, W.; Lytle, N.K.; Huang, P.; Yuan, X.; Dann, A.M.; Ridinger-Saison, M.; DelGiorno, K.E.; Antal, C.E.; Liang, G.; et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 2019, 569, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Moatassim-Billah, S.; Duluc, C.; Samain, R.; Jean, C.; Perraud, A.; Decaup, E.; Cassant-Sourdy, S.; Bakri, Y.; Selves, J.; Schmid, H.; et al. Anti-metastatic potential of somatostatin analog SOM230: Indirect pharmacological targeting of pancreatic cancerassociated fibroblasts. Oncotarget 2016, 7, 41584–41598. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Falcomata, C.; Baerthel, S.; Widholz, S.A.; Schneeweis, C.; Montero, J.J.; Toska, A.; Mir, J.; Kaltenbacher, T.; Heetmeyer, J.; Swietlik, J.J.; et al. Selective multi-kinase inhibition sensitizes mesenchymal pancreatic cancer to immune checkpoint blockade by remodeling the tumor microenvironment. Nat. Cancer 2022, 3, 318–336. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tempero, M.A.; Sigal, D.; Oh, D.Y.; Fazio, N.; Macarulla, T.; Hitre, E.; Hammel, P.; Hendifar, A.E.; Bates, S.E.; et al. Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients With Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2020, 38, 3185–3194. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Li, C.; Liang, X.; Li, C.; Liu, Y.; Yi, Z.; Zhang, L.; Fu, S.; Zeng, Y. Role of CD47 in tumor immunity: A potential target for combination therapy. Sci. Rep. 2022, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; Trabulo, S.; Hidalgo, M.; Costello, E.; Greenhalf, W.; Erkan, M.; Kleeff, J.; Sainz, B., Jr.; Heeschen, C. Inhibition of CD47 Effectively Targets Pancreatic Cancer Stem Cells via Dual Mechanisms. Clin. Cancer Res. 2015, 21, 2325–2337. [Google Scholar] [CrossRef]
- Michaels, A.D.; Newhook, T.E.; Adair, S.J.; Morioka, S.; Gaudreau, B.J.; Nagdas, S.; Mullen, M.G.; Persily, J.B.; Bullock, T.N.J.; Slingluff, C.L., Jr.; et al. CD47 Blockade as an Adjuvant Immunotherapy for Resectable Pancreatic Cancer. Clin. Cancer Res. 2018, 24, 1415–1425. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, F.; Fei, Q.; Yu, X.; Xiong, P.; Yu, X.; Dang, Y.; Hou, Z.; Lin, W.; Lin, X.; et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J. Hematol. Oncol. 2019, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.-P.; Rodier, F.; Patil, C.K.; Freund, A.; Desprez, P.-Y.; Campisi, J. Tumor Suppressor and Aging Biomarker p16(INK4a) Induces Cellular Senescence without the Associated Inflammatory Secretory Phenotype. J. Biol. Chem. 2011, 286, 36396–36403. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.W.; Deonarine, A.; Jones, J.O.; Denton, A.E.; Feig, C.; Lyons, S.K.; Espeli, M.; Kraman, M.; McKenna, B.; Wells, R.J.; et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 2013, 210, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.E.; Go, G.-Y.; Koh, E.-Y.; Yoon, H.-N.; Seo, M.; Hong, S.-M.; Jeong, J.H.; Kim, J.-C.; Cho, D.; Kim, T.S.; et al. Synergistic therapeutic combination with a CAF inhibitor enhances CAR-NK-mediated cytotoxicity via reduction of CAF-released IL-6. J. ImmunoTherapy Cancer 2023, 11, e006130. [Google Scholar] [CrossRef]
- Jacobetz, M.A.; Chan, D.S.; Neesse, A.; Bapiro, T.E.; Cook, N.; Frese, K.K.; Feig, C.; Nakagawa, T.; Caldwell, M.E.; Zecchini, H.I.; et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013, 62, 112–120. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Harris, W.P.; Beck, J.T.; Berdov, B.A.; Wagner, S.A.; Pshevlotsky, E.M.; Tjulandin, S.A.; Gladkov, O.A.; Holcombe, R.F.; Korn, R.; et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 2848–2854. [Google Scholar] [CrossRef]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Liao, W.-C.; Sohn, Y.S.; Fadeev, M.; Cecconello, A.; Nechushtai, R.; Willner, I. Stimuli-Responsive Nucleic Acid-Based Polyacrylamide Hydrogel-Coated Metal-Organic Framework Nanoparticles for Controlled Drug Release. Adv. Funct. Mater. 2018, 28, 1705137. [Google Scholar] [CrossRef]
- Fan, Q.; Ma, Q.; Bai, J.; Xu, J.; Fei, Z.; Dong, Z.; Maruyama, A.; Leong, K.W.; Liu, Z.; Wang, C. An implantable blood clot-based immune niche for enhanced cancer vaccination. Sci. Adv. 2020, 6, eabb4639. [Google Scholar] [CrossRef]


| Drug | Therapeutic Target | Preclinical Model/Clinical Trial | Publish Year | Ref. |
|---|---|---|---|---|
| anti-GM-CSF monoclonal antibody | PMN-MDSCs | Subcutaneous transplantation tumor in mice | 2023 | [14] |
| CXCR2 inhibitors | PMN-MDSCs | GEMM | 2016 | [146] |
| STAT3 antisense nucleotides | PMN-MDSCs | Subcutaneous transplantation tumor in mice | 2021 | [51] |
| TRAIL-R agonist DS-8273a | PMN-MDSCs | Phase I trial | 2017 | [147] |
| CCL2 inhibitor CCX872-B | TAMs | Phase Ib trial | 2017 | [148] |
| CCR2 inhibitors | TAMs | Orthotopic transplantation tumor in mice | 2018 | [149] |
| CSF1R blockade | TAMs | GEMM | 2014 | [150] |
| anti-CD47 monoclonal antibody | TAMs | Hepatic micro-metastatic tumor model in mice | 2018 | [151] |
| CpG oligodeoxynucleotides | TAMs | Orthotopic transplantation tumor in mice | 2019 | [152] |
| IPI926 | CAFs | Orthotopic transplantation tumor in mice | 2009 | [153] |
| Halofuginone | CAFs | GEMM | 2019 | [154] |
| LIF mAb | CAFs | GEMM | 2019 | [155] |
| SOM230 | CAFs | Orthotopic transplantation tumor in mice | 2016 | [156] |
| Calcipotriene | CAFs | GEMM | 2014 | [157] |
| Nintedanib | CAFs | GEMM | 2022 | [158] |
| PEGPH20 | CAFs | Phase III trial | 2020 | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wu, W.; Zhu, Q.; Zhu, H. Hydrogel-Based Therapeutics for Pancreatic Ductal Adenocarcinoma Treatment. Pharmaceutics 2023, 15, 2421. https://doi.org/10.3390/pharmaceutics15102421
Liu J, Wu W, Zhu Q, Zhu H. Hydrogel-Based Therapeutics for Pancreatic Ductal Adenocarcinoma Treatment. Pharmaceutics. 2023; 15(10):2421. https://doi.org/10.3390/pharmaceutics15102421
Chicago/Turabian StyleLiu, Jinlu, Wenbi Wu, Qing Zhu, and Hong Zhu. 2023. "Hydrogel-Based Therapeutics for Pancreatic Ductal Adenocarcinoma Treatment" Pharmaceutics 15, no. 10: 2421. https://doi.org/10.3390/pharmaceutics15102421
APA StyleLiu, J., Wu, W., Zhu, Q., & Zhu, H. (2023). Hydrogel-Based Therapeutics for Pancreatic Ductal Adenocarcinoma Treatment. Pharmaceutics, 15(10), 2421. https://doi.org/10.3390/pharmaceutics15102421





