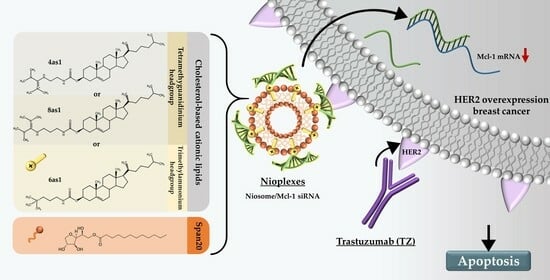
Dual-Targeted Therapy in HER2-Overexpressing Breast Cancer with Trastuzumab and Novel Cholesterol-Based Nioplexes Silencing Mcl-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cationic Niosome Preparation
2.3. Cationic Niosome Characterization
2.4. Cytotoxicity of Niosomes
2.5. Nioplexes Preparation and Characterization
2.6. Cellular Uptake Ability of Nioplexes
2.6.1. Flow Cytometry
2.6.2. Fluorescence Imaging
2.7. Cell-Growth Inhibition by the Single Treatment and Dual-Targeted Approach
2.7.1. Cell-Growth Inhibition by Mcl-1 Nioplexes
2.7.2. Cell-Growth Inhibition by Dual-Targeted Approach
2.8. Analysis of Dual Therapy by CompuSyn Software
2.9. mRNA Expression Level Determination by RT-qPCR
2.10. Cell Death and Apoptosis Determination
2.11. Statistical Analysis
3. Results
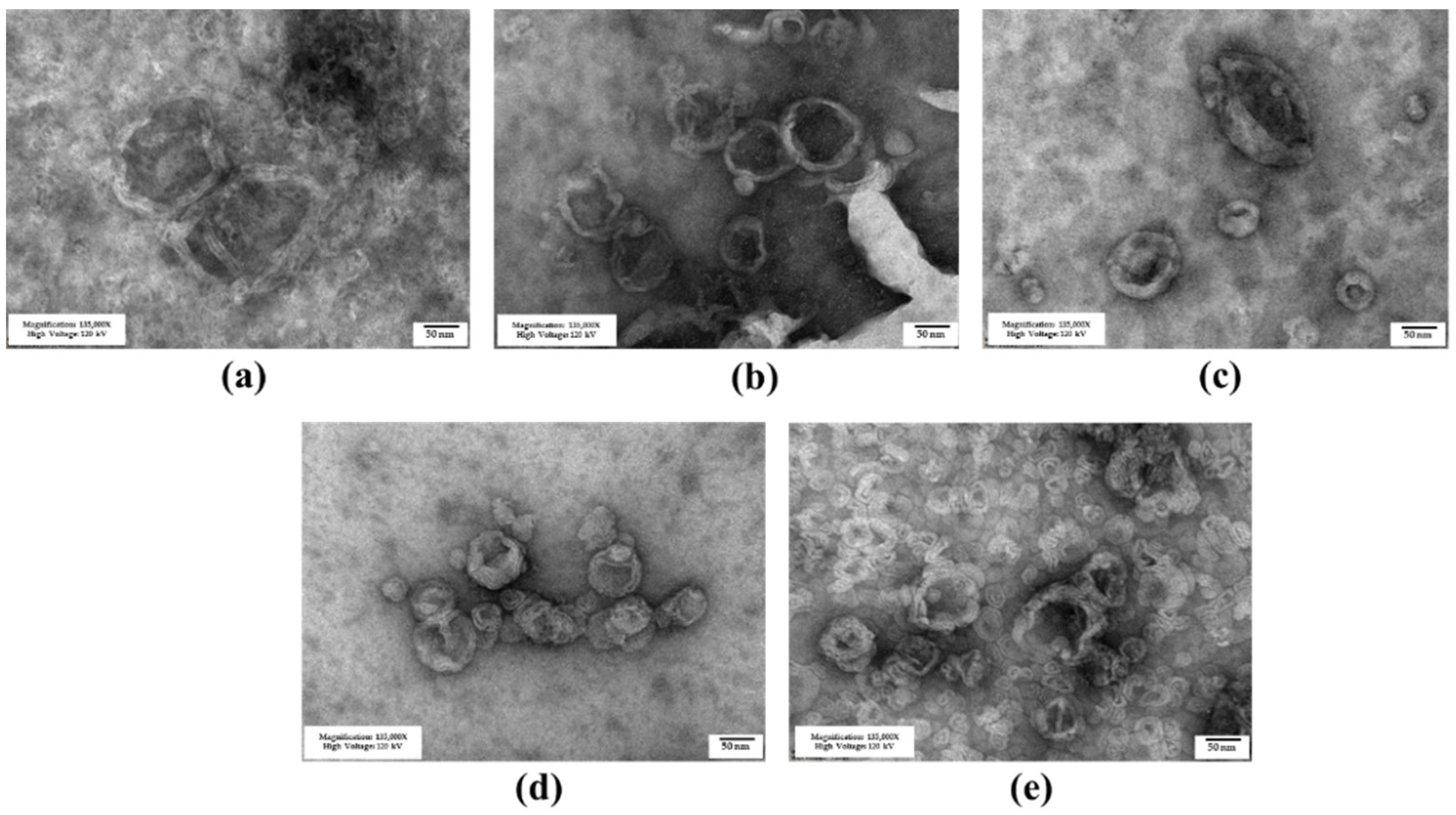
3.1. Niosome Preparation and Characterization
3.1.1. Niosome Characteristics
3.1.2. Niosome Stability
3.2. Cellular Uptake in Breast Cancer Cells by Flow Cytometry and Fluorescence Microscope
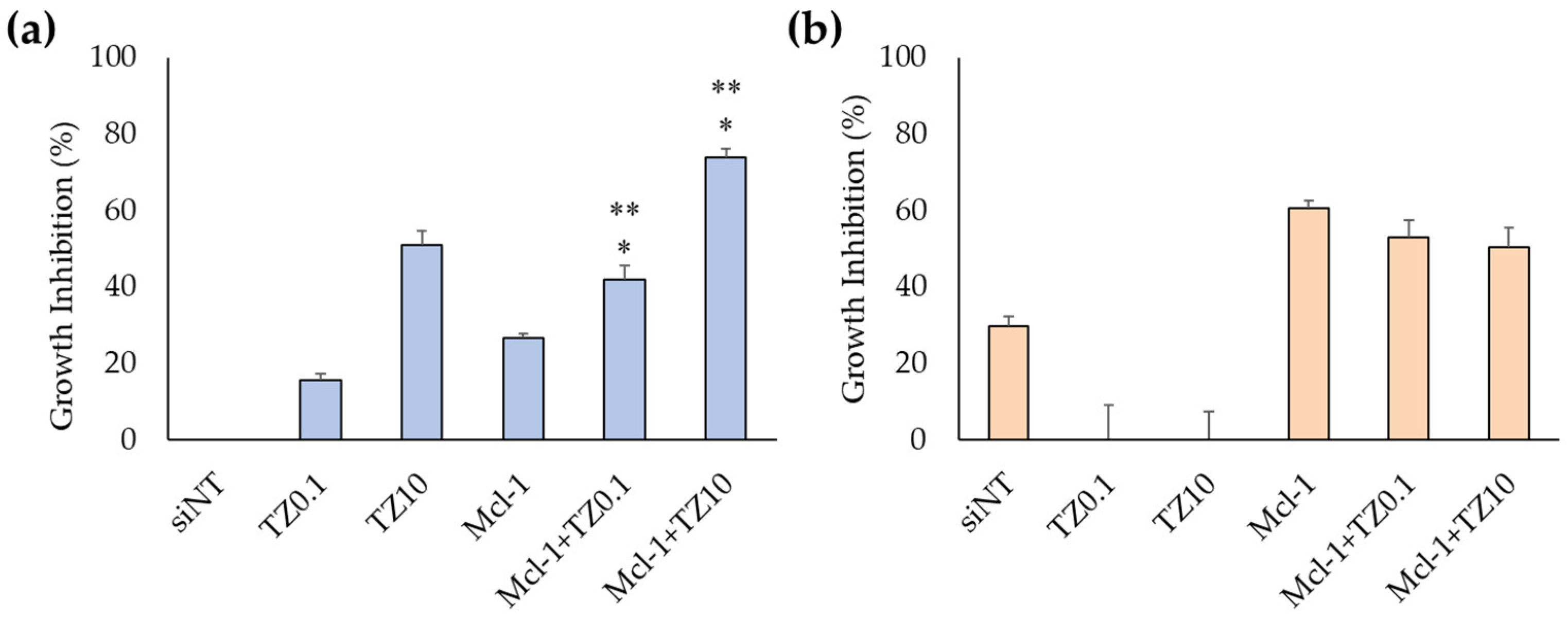
3.3. Cell-Growth Inhibition of Mcl-1 siRNA Nioplexes and Mcl-1 siRNA Nioplex/TZ Dual-Targeted Therapy
3.3.1. Cell-Growth Inhibition of Mcl-1 siRNA Nioplexes
3.3.2. Cell-Growth Inhibition of Mcl-1 siRNA Nioplex/TZ Dual-Targeted Therapy and Synergistic Analysis
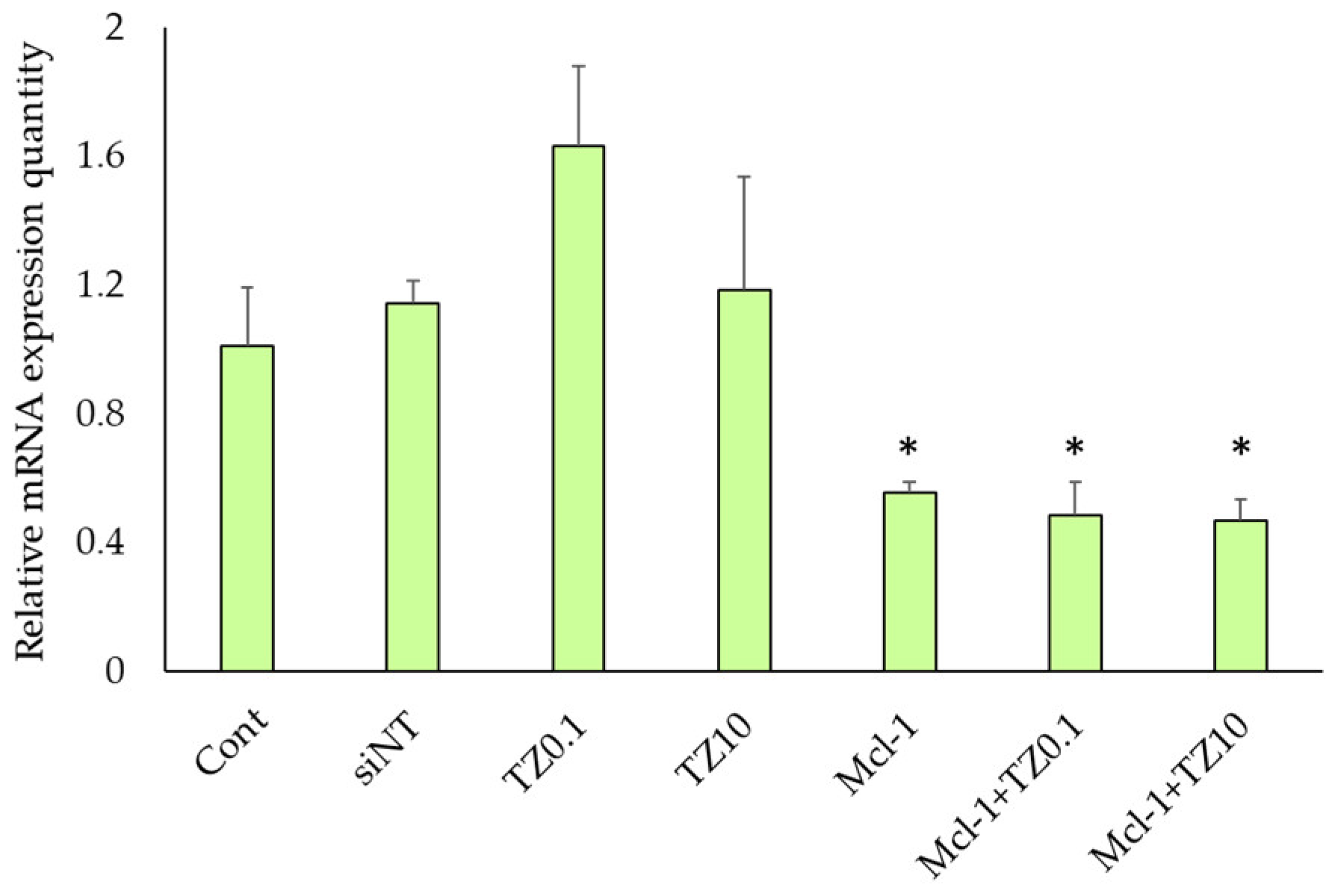
3.4. mRNA Expression Level Determination by RT-qPCR
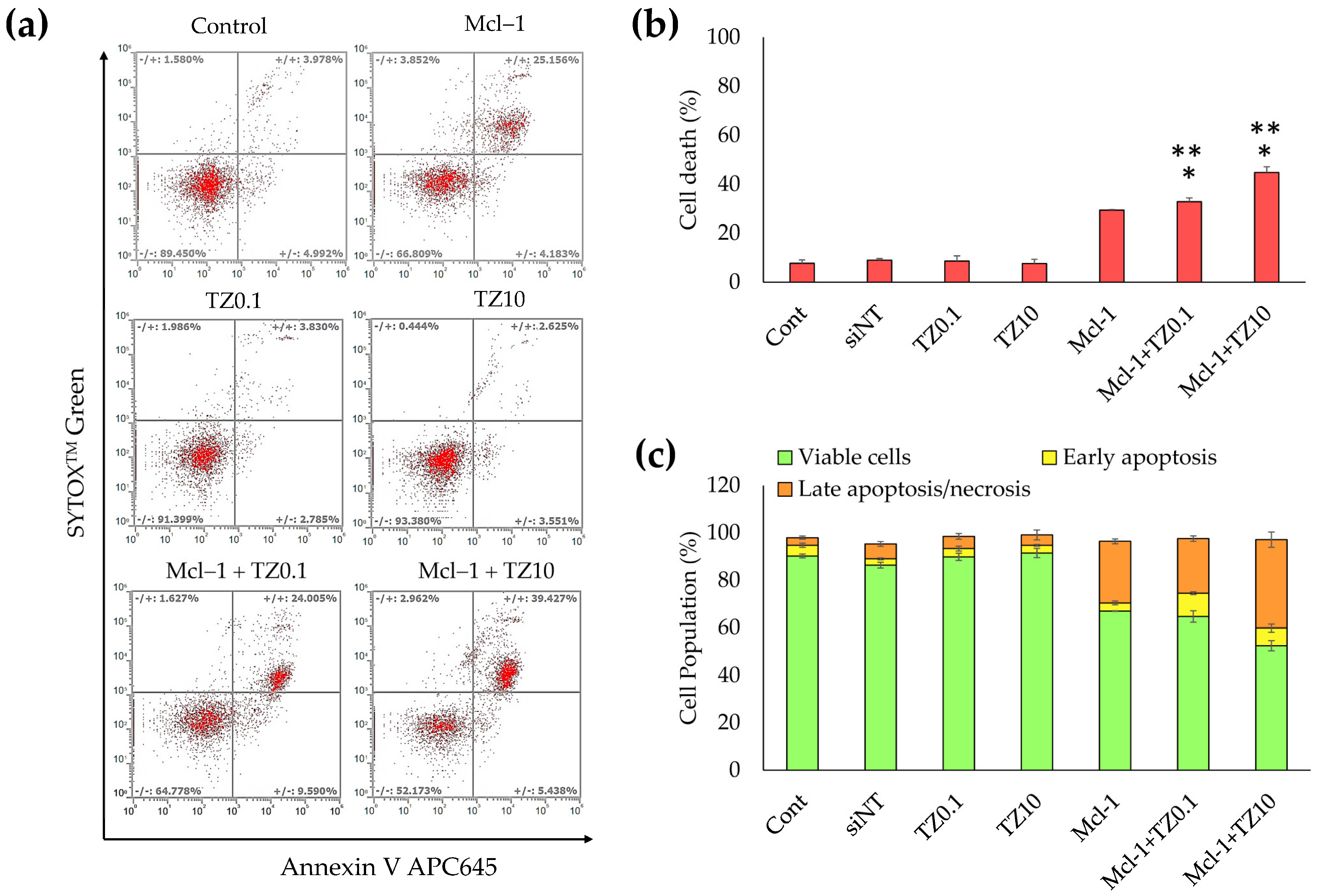
3.5. Cell Death and Apoptosis Determination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Mastoraki, A.; Kazani, A.; Mastoraki, S.; Konstantiadou, I.; Kokoropoulos, P.; Smyrniotis, V.; Arkadopoulos, N. Breast-Cancer Subtyping in Clinical Practice: Clinicopathologic Features and Outcomes. J. Gynecol. Surg. 2014, 30, 260–264. [Google Scholar] [CrossRef]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Onitilo, A.A.; Engel, J.M.; Greenlee, R.T.; Mukesh, B.N. Breast cancer subtypes based on ER/PR and Her2 expression: Comparison of clinicopathologic features and survival. Clin. Med. Res. 2009, 7, 4–13. [Google Scholar] [CrossRef]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef]
- Sledge, G.W.; Mamounas, E.P.; Hortobagyi, G.N.; Burstein, H.J.; Goodwin, P.J.; Wolff, A.C. Past, present, and future challenges in breast cancer treatment. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 1979–1986. [Google Scholar] [CrossRef]
- Tai, W.; Mahato, R.; Cheng, K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control. Release 2010, 146, 264–275. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Sliwkowski, M.X.; Osborne, C.K.; Perez, E.A.; Puglisi, F.; Gianni, L. Treatment of HER2-positive breast cancer: Current status and future perspectives. Nat. Rev. Clin. Oncol. 2012, 9, 16–32. [Google Scholar] [CrossRef]
- Leyland-Jones, B. Trastuzumab: Hopes and realities. Lancet Oncol. 2002, 3, 137–144. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007, 18, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Bang, Y.-J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Kreutzfeldt, J.; Rozeboom, B.; Dey, N.; De, P. The trastuzumab era: Current and upcoming targeted HER2+ breast cancer therapies. Am. J. Cancer Res. 2020, 10, 1045–1067. [Google Scholar] [PubMed]
- de Melo Gagliato, D.; Jardim, D.L.; Marchesi, M.S.; Hortobagyi, G.N. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget 2016, 7, 64431–64446. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Unni, N.; Peng, Y. The Changing Paradigm for the Treatment of HER2-Positive Breast Cancer. Cancers 2020, 12, 2081. [Google Scholar] [CrossRef]
- Nowsheen, S.; Viscuse, P.V.; O’Sullivan, C.C.; Sandhu, N.P.; Haddad, T.C.; Blaes, A.; Klemp, J.; Nhola, L.; Herrmann, J.; Ruddy, K.J. Incidence, Diagnosis, and Treatment of Cardiac Toxicity From Trastuzumab in Patients With Breast Cancer. Curr. Breast Cancer Rep. 2017, 9, 173–182. [Google Scholar] [CrossRef]
- Campbell, K.J.; Dhayade, S.; Ferrari, N.; Sims, A.H.; Johnson, E.; Mason, S.M.; Dickson, A.; Ryan, K.M.; Kalna, G.; Edwards, J.; et al. MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis. 2018, 9, 19. [Google Scholar] [CrossRef]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M.; et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef]
- Winder, M.L.; Campbell, K.J. MCL-1 is a clinically targetable vulnerability in breast cancer. Cell Cycle 2022, 21, 1439–1455. [Google Scholar] [CrossRef]
- Lee, K.-m.; Giltnane, J.M.; Balko, J.M.; Schwarz, L.J.; Guerrero-Zotano, A.L.; Hutchinson, K.E.; Nixon, M.J.; Estrada, M.V.; Sánchez, V.; Sanders, M.E. MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metab. 2017, 26, 633–647.e637. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.L.; Lo, H.W. Regulation of Apoptosis by HER2 in Breast Cancer. J. Carcinog. Mutagen. 2013, 2013, 003. [Google Scholar] [CrossRef]
- Campone, M.; Noël, B.; Couriaud, C.; Grau, M.; Guillemin, Y.; Gautier, F.; Gouraud, W.; Charbonnel, C.; Campion, L.; Jézéquel, P.; et al. c-Myc dependent expression of pro-apoptotic Bim renders HER2-overexpressing breast cancer cells dependent on anti-apoptotic Mcl-1. Mol. Cancer 2011, 10, 110. [Google Scholar] [CrossRef]
- Modugno, M.; Banfi, P.; Gasparri, F.; Borzilleri, R.; Carter, P.; Cornelius, L.; Gottardis, M.; Lee, V.; Mapelli, C.; Naglich, J.G.; et al. Mcl-1 antagonism is a potential therapeutic strategy in a subset of solid cancers. Exp. Cell Res. 2015, 332, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Diepstraten, S.T.; Anderson, M.A.; Czabotar, P.E.; Lessene, G.; Strasser, A.; Kelly, G.L. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat. Rev. Cancer 2022, 22, 45–64. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Devi, G.R. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006, 13, 819–829. [Google Scholar] [CrossRef]
- Singh, A.; Trivedi, P.; Jain, N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 274–283. [Google Scholar] [CrossRef]
- Pengnam, S.; Plianwong, S.; Yingyongnarongkul, B.-e.; Patrojanasophon, P.; Opanasopit, P. Delivery of small interfering RNAs by nanovesicles for cancer therapy. Drug Metab. Pharmacokinet. 2022, 42, 100425. [Google Scholar] [CrossRef]
- Dumkliang, E.; Pamornpathomkul, B.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Yoksan, S.; Opanasopit, P. Feasibility of chitosan-based nanoparticles approach for intranasal immunisation of live attenuated Japanese encephalitis vaccine. Int. J. Biol. Macromol. 2021, 183, 1096–1105. [Google Scholar] [CrossRef]
- Paecharoenchai, O.; Teng, L.; Yung, B.C.; Teng, L.; Opanasopit, P.; Lee, R.J. Nonionic surfactant vesicles for delivery of RNAi therapeutics. Nanomedicine 2013, 8, 1865–1873. [Google Scholar] [CrossRef]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an alternative for liposomal delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef]
- Masjedi, M.; Montahaei, T. An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: Fabrication, characterization, pharmaceutical, and cosmetic applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102234. [Google Scholar] [CrossRef]
- Grijalvo, S.; Puras, G.; Zárate, J.; Sainz-Ramos, M.; Qtaish, N.A.L.; López, T.; Mashal, M.; Attia, N.; Díaz Díaz, D.; Pons, R.; et al. Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery. Pharmaceutics 2019, 11, 50. [Google Scholar] [CrossRef]
- Nowroozi, F.; Almasi, A.; Javidi, J.; Haeri, A.; Dadashzadeh, S. Effect of Surfactant Type, Cholesterol Content and Various Downsizing Methods on the Particle Size of Niosomes. Iran. J. Pharm. Res. IJPR 2018, 17 (Suppl. S2), 1. [Google Scholar]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Di Marzio, L.; Esposito, S.; Rinaldi, F.; Marianecci, C.; Carafa, M. Polysorbate 20 vesicles as oral delivery system: In vitro characterization. Colloids Surf. B Biointerfaces 2013, 104, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef] [PubMed]
- Mashal, M.; Attia, N.; Puras, G.; Martínez-Navarrete, G.; Fernández, E.; Pedraz, J.L. Retinal gene delivery enhancement by lycopene incorporation into cationic niosomes based on DOTMA and polysorbate 60. J. Control. Release 2017, 254, 55–64. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Yu, B.; Phelps, M.A.; Lee, L.J.; Lee, R.J. Comparative cellular pharmacokinetics and pharmacodynamics of siRNA delivery by SPANosomes and by cationic liposomes. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 504–513. [Google Scholar] [CrossRef]
- Villate-Beitia, I.; Gallego, I.; Martínez-Navarrete, G.; Zárate, J.; López-Méndez, T.; Soto-Sánchez, C.; Santos-Vizcaíno, E.; Puras, G.; Fernández, E.; Pedraz, J.L. Polysorbate 20 non-ionic surfactant enhances retinal gene delivery efficiency of cationic niosomes after intravitreal and subretinal administration. Int. J. Pharm. 2018, 550, 388–397. [Google Scholar] [CrossRef]
- Martin, B.; Sainlos, M.; Aissaoui, A.; Oudrhiri, N.; Hauchecorne, M.; Vigneron, J.-P.; Lehn, J.-M.; Lehn, P. The design of cationic lipids for gene delivery. Curr. Pharm. Des. 2005, 11, 375–394. [Google Scholar] [CrossRef]
- Gao, X.; Huang, L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem. Biophys. Res. Commun. 1991, 179, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Ponti, F.; Campolungo, M.; Melchiori, C.; Bono, N.; Candiani, G. Cationic lipids for gene delivery: Many players, one goal. Chem. Phys. Lipids 2021, 235, 105032. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, D.A.; Maslov, M.A.; Serikov, R.N.; Morozova, N.G.; Serebrenikova, G.A.; Sheglov, D.V.; Latyshev, A.V.; Vlassov, V.V.; Zenkova, M.A. Novel Cholesterol-Based Cationic Lipids for Gene Delivery. J. Med. Chem. 2009, 52, 6558–6568. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Eritja, R.; Martinez-Navarrete, G.; Soto-Sánchez, C.; Diaz-Tahoces, A.; Aviles-Trigueros, M.; et al. The influence of the polar head-group of synthetic cationic lipids on the transfection efficiency mediated by niosomes in rat retina and brain. Biomaterials 2016, 77, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; Li, H.; Mao, K.; Wang, H.; Meng, X.; Wang, J.; Wu, C.; Chen, H.; Wang, X.; et al. An optimized ionizable cationic lipid for brain tumor-targeted siRNA delivery and glioblastoma immunotherapy. Biomaterials 2022, 287, 121645. [Google Scholar] [CrossRef] [PubMed]
- Radchatawedchakoon, W.; Thongbamrer, C.; Konbamrung, W.; Khattawee, P.; Sakee, U.; Roobsoong, W.; Sattabongkot, J.; Opanasopit, P.; Yingyongnarongkul, B.-e. The effect of polar headgroups and spacer length on the DNA transfection of cholesterol-based cationic lipids. RSC Med. Chem. 2020, 11, 212–224. [Google Scholar] [CrossRef]
- Thongbamrer, C.; Niyomtham, N.; Chaiwut, C.; Posa, C.; Apiratikul, N.; Sangvichien, E.; Opanasopit, P.; Sakee, U.; Yingyongnarongkul, B.-e.; Radchatawedchakoon, W. Headgroup modification of cholesterol-based cationic lipids: Synthesis, transfection efficiency evaluation, and serum compatibility. Songklanakarin J. Sci. Technol. 2020, 42, 213–221. [Google Scholar]
- Aung, N.N.; Pengnam, S.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. Fabrication of polyvinyl pyrrolidone-K90/Eudragit RL100-based dissolving microneedle patches loaded with alpha-arbutin and resveratrol for skin depigmentation. Biomater. Sci. 2023, 11, 4583–4601. [Google Scholar] [CrossRef]
- Paecharoenchai, O.; Niyomtham, N.; Leksantikul, L.; Ngawhirunpat, T.; Rojanarata, T.; Yingyongnarongkul, B.-e.; Opanasopit, P. Nonionic Surfactant Vesicles Composed of Novel Spermine-Derivative Cationic Lipids as an Effective Gene Carrier In Vitro. AAPS PharmSciTech 2014, 15, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Pengnam, S.; Patrojanasophon, P.; Rojanarata, T.; Ngawhirunpat, T.; Yingyongnarongkul, B.-e.; Radchatawedchakoon, W.; Opanasopit, P. A novel plier-like gemini cationic niosome for nucleic acid delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 325–333. [Google Scholar] [CrossRef]
- Varshosaz, J.; Pardakhty, A.; Hajhashemi, V.-i.; Najafabadi, A.R. Development and Physical Characterization of Sorbitan Monoester Niosomes for Insulin Oral Delivery. Drug Deliv. 2003, 10, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Atale, N.; Gupta, S.; Yadav, U.C.S.; Rani, V. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014, 255, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Aparajay, P.; Dev, A. Functionalized niosomes as a smart delivery device in cancer and fungal infection. Eur. J. Pharm. Sci. 2022, 168, 106052. [Google Scholar] [CrossRef] [PubMed]
- Somjid, S.; Krongsuk, S.; Johns, J.R. Cholesterol concentration effect on the bilayer properties and phase formation of niosome bilayers: A molecular dynamics simulation study. J. Mol. Liq. 2018, 256, 591–598. [Google Scholar] [CrossRef]
- Nematollahi, M.H.; Pardakhty, A.; Torkzadeh-Mahanai, M.; Mehrabani, M.; Asadikaram, G. Changes in physical and chemical properties of niosome membrane induced by cholesterol: A promising approach for niosome bilayer intervention. RSC Adv. 2017, 7, 49463–49472. [Google Scholar] [CrossRef]
- Xu, L.; Anchordoquy, T.J. Cholesterol domains in cationic lipid/DNA complexes improve transfection. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2008, 1778, 2177–2181. [Google Scholar] [CrossRef]
- Kolašinac, R.; Kleusch, C.; Braun, T.; Merkel, R.; Csiszár, A. Deciphering the Functional Composition of Fusogenic Liposomes. Int. J. Mol. Sci. 2018, 19, 346. [Google Scholar] [CrossRef]
- Mashal, M.; Attia, N.; Grijalvo, S.; Eritja, R.; Puras, G.; Pedraz, J.L. Stability of polymeric cationic niosomes and their plasmid DNA-based complexes as gene delivery carriers. Drug Deliv. 2023, 30, 2219420. [Google Scholar] [CrossRef]
- Grosfils, P.; Losada-Pérez, P. Kinetic control of liposome size by direct lipid transfer. J. Colloid Interface Sci. 2023, 652, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Mishra, S.K.; Kondaiah, P.; Bhattacharya, S. Effect of the headgroup variation on the gene transfer properties of cholesterol based cationic lipids possessing ether linkage. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Niculescu-Duvaz, D.; Heyes, J.; Springer, C.J. Structure-Activity Relationship in Cationic Lipid Mediated Gene Transfection. Curr. Med. Chem. 2003, 10, 1233–1261. [Google Scholar] [CrossRef]
- Hirko, A.; Tang, F.; Hughes, J.A. Cationic Lipid Vectors for Plasmid DNA Delivery. Curr. Med. Chem. 2003, 10, 1185–1193. [Google Scholar] [CrossRef]
- Zhi, D.; Zhang, S.; Cui, S.; Zhao, Y.; Wang, Y.; Zhao, D. The Headgroup Evolution of Cationic Lipids for Gene Delivery. Bioconjugate Chem. 2013, 24, 487–519. [Google Scholar] [CrossRef] [PubMed]
- Man-Zhou, Z.; Qi-Hua, W.; Guisheng, Z.; Tan, R.; Dexi, L.; Qing-Xiang, G. Synthesis and Evaluation of Cationic Lipids Bearing Cholesteryl Groups for Gene Delivery In Vitro. Bull. Chem. Soc. Jpn. 2002, 75, 2207–2213. [Google Scholar] [CrossRef]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, J.-P.; Oudrhiri, N.; Fauquet, M.; Vergely, L.; Bradley, J.-C.; Basseville, M.; Lehn, P.; Lehn, J.-M. Guanidinium-cholesterol cationic lipids: Efficient vectors for the transfection of eukaryotic cells. Proc. Natl. Acad. Sci. USA 1996, 93, 9682–9686. [Google Scholar] [CrossRef]
- Sen, J.; Chaudhuri, A. Design, Syntheses, and Transfection Biology of Novel Non-Cholesterol-Based Guanidinylated Cationic Lipids. J. Med. Chem. 2005, 48, 812–820. [Google Scholar] [CrossRef]
- Aissaoui, A.; Oudrhiri, N.; Petit, L.; Hauchecorne, M.; Kan, E.; Sainlos, M.; Julia, S.; Navarro, J.; Vigneron, J.P.; Lehn, J.M.; et al. Progress in Gene Delivery by Cationic Lipids: Guanidinium-Cholesterol-Based Systems as an Example. Curr. Drug Targets 2002, 3, 1–16. [Google Scholar] [CrossRef]
- Zhi, D.; Zhang, S.; Qureshi, F.; Zhao, Y.; Cui, S.; Wang, B.; Chen, H.; Wang, Y.; Zhao, D. Synthesis and biological activity of carbamate-linked cationic lipids for gene delivery in vitro. Bioorganic Med. Chem. Lett. 2012, 22, 3837–3841. [Google Scholar] [CrossRef]
- Sarker, S.R.; Takeoka, S. Amino acid-based liposomal assemblies: Intracellular plasmid DNA delivery nanoparticles. J. Nanomed 2018, 2, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, S.; Jiang, H.; Zhao, B.; Lv, H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J. Control. Release 2007, 123, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Turek, J.; Dubertret, C.; Jaslin, G.; Antonakis, K.; Scherman, D.; Pitard, B. Formulations which increase the size of lipoplexes prevent serum-associated inhibition of transfection. J. Gene Med. 2000, 2, 32–40. [Google Scholar] [CrossRef]
- Lian, T.; Ho, R.J. Design and characterization of a novel lipid-DNA complex that resists serum-induced destabilization. J. Pharm. Sci. 2003, 92, 2373–2385. [Google Scholar] [CrossRef]
- Yinan, Z.; Defu, Z.; Shubiao, Z. Cationic Liposomes in Different Structural Levels for Gene Delivery. In Non-Viral Gene Therapy; Xu-bo, Y., Ed.; IntechOpen: Rijeka, Yugoslavia, 2011; p. 13. [Google Scholar]
- Bolomsky, A.; Vogler, M.; Köse, M.C.; Heckman, C.A.; Ehx, G.; Ludwig, H.; Caers, J. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J. Hematol. Oncol. 2020, 13, 173. [Google Scholar] [CrossRef]
- Booy, E.P.; Henson, E.S.; Gibson, S.B. Epidermal growth factor regulates Mcl-1 expression through the MAPK-Elk-1 signalling pathway contributing to cell survival in breast cancer. Oncogene 2011, 30, 2367–2378. [Google Scholar] [CrossRef]
- Floros, K.V.; Jacob, S.; Kurupi, R.; Fairchild, C.K.; Hu, B.; Puchalapalli, M.; Koblinski, J.E.; Dozmorov, M.G.; Boikos, S.A.; Scaltriti, M.; et al. Targeting transcription of MCL-1 sensitizes HER2-amplified breast cancers to HER2 inhibitors. Cell Death Dis. 2021, 12, 179. [Google Scholar] [CrossRef]
- Pandya Martin, A.; Mitchell, C.; Rahmani, M.; Nephew, K.P.; Grant, S.; Dent, P. Inhibition of MCL-1 enhances Lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol. Ther. 2009, 8, 2084–2096. [Google Scholar] [CrossRef]
- Arnould, L.; Gelly, M.; Penault-Llorca, F.; Benoit, L.; Bonnetain, F.; Migeon, C.; Cabaret, V.; Fermeaux, V.; Bertheau, P.; Garnier, J.; et al. Trastuzumab-based treatment of HER2-positive breast cancer: An antibody-dependent cellular cytotoxicity mechanism? Br. J. Cancer 2006, 94, 259–267. [Google Scholar] [CrossRef]
- Merino, D.; Whittle, J.R.; Vaillant, F.; Serrano, A.; Gong, J.-N.; Giner, G.; Maragno, A.L.; Chanrion, M.; Schneider, E.; Pal, B.; et al. Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci. Transl. Med. 2017, 9, eaam7049. [Google Scholar] [CrossRef]
- Piro, G.; Carbone, C.; Cataldo, I.; Di Nicolantonio, F.; Giacopuzzi, S.; Aprile, G.; Simionato, F.; Boschi, F.; Zanotto, M.; Mina, M.M.; et al. An FGFR3 Autocrine Loop Sustains Acquired Resistance to Trastuzumab in Gastric Cancer Patients. Clin. Cancer Res. 2016, 22, 6164–6175. [Google Scholar] [CrossRef]
- Gong, C.; Yao, Y.; Wang, Y.; Liu, B.; Wu, W.; Chen, J.; Su, F.; Yao, H.; Song, E. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J. Biol. Chem. 2011, 286, 19127–19137. [Google Scholar] [CrossRef]
- Kamashev, D.; Shaban, N.; Suntsova, M.; Raevskiy, M.; Efimov, V.; Moisseev, A.; Sorokin, M.; Buzdin, A. Human Blood Serum Inhibits Ductal Carcinoma Cells BT474 Growth and Modulates Effect of HER2 Inhibition. Biomedicines 2022, 10, 1914. [Google Scholar] [CrossRef]
- Zhang, N.; Fu, J.N.; Chou, T.C. Synergistic combination of microtubule targeting anticancer fludelone with cytoprotective panaxytriol derived from panax ginseng against MX-1 cells in vitro: Experimental design and data analysis using the combination index method. Am. J. Cancer Res. 2016, 6, 97–104. [Google Scholar]
- Ting-Chao, C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621. [Google Scholar] [CrossRef]
- Bashari, M.H.; Fan, F.; Vallet, S.; Sattler, M.; Arn, M.; Luckner-Minden, C.; Schulze-Bergkamen, H.; Zörnig, I.; Marme, F.; Schneeweiss, A.; et al. Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: Therapeutic implications. Breast Cancer Res. 2016, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Subik, K.; Lee, J.-F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.-C.; Bonfiglio, T.; Hicks, D.G.; et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer: Basic Clin. Res. 2010, 4, 117822341000400004. [Google Scholar] [CrossRef]
- Brockhoff, G.; Heckel, B.; Schmidt-Bruecken, E.; Plander, M.; Hofstaedter, F.; Vollmann, A.; Diermeier, S. Differential impact of Cetuximab, Pertuzumab and Trastuzumab on BT474 and SK-BR-3 breast cancer cell proliferation. Cell Prolif. 2007, 40, 488–507. [Google Scholar] [CrossRef]
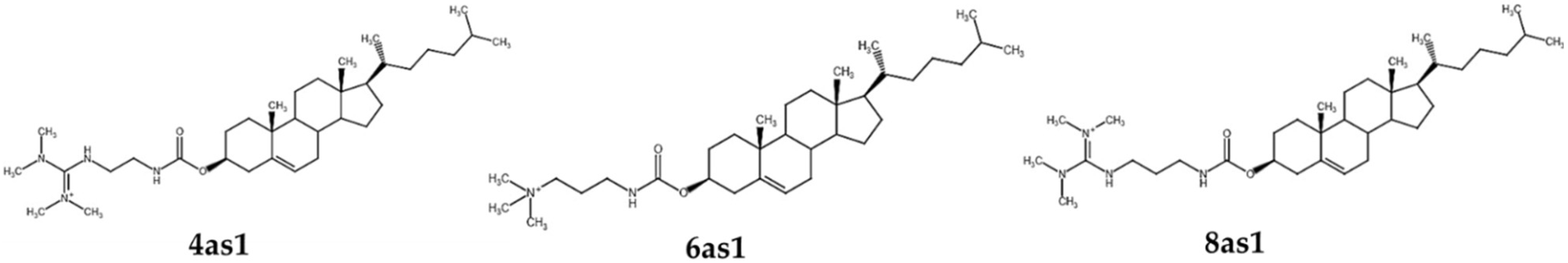




| Formulations (Molar Ratios) | Hydrodynamic Size | Zeta Potential (mV) ± SD | |
|---|---|---|---|
| Diameter (nm) ± SD | PDI ± SD | ||
| 6as1 (1.5:2.5) | 161.6 ± 4.7 | 0.3 ± 0.02 | 25.8 ± 4.4 |
| 6as1 (2.5:2.5) | 105.8 ± 9.6 | 0.3 ± 0.06 | 24.9 ± 4.5 |
| 6as1 (3.5:2.5) | 90.8 ± 3.3 | 0.4 ± 0.03 | 21.6 ± 1.5 |
| 4as1 (2.5:2.5) | 110.9 ± 2.8 | 0.5 ± 0.01 | 16.0 ± 3.1 |
| 8as1 (2.5:2.5) | 242.3 ± 62.0 | 0.6 ± 0.13 | 19.1 ± 2.6 |
| Drug Combo (μg/mL) | Dose Reduction Index (DRI) | Doses of Each Drug Required to Achieve Specific Fa Level | |||||
|---|---|---|---|---|---|---|---|
| TZ | Mcl-1 siRNA | Fa | CI | TZ | Mcl-1 siRNA | TZ | Mcl-1 siRNA |
| 0.1 | 1.4 | 0.42 | 1.04 | 13.92 | 1.04 | 1.39 | 1.45 |
| 10 | 1.4 | 0.74 | 0.57 | 28.35 | 1.89 | 283.48 | 2.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pengnam, S.; Opanasopit, P.; Rojanarata, T.; Yingyongnarongkul, B.-e.; Thongbamrer, C.; Plianwong, S. Dual-Targeted Therapy in HER2-Overexpressing Breast Cancer with Trastuzumab and Novel Cholesterol-Based Nioplexes Silencing Mcl-1. Pharmaceutics 2023, 15, 2424. https://doi.org/10.3390/pharmaceutics15102424
Pengnam S, Opanasopit P, Rojanarata T, Yingyongnarongkul B-e, Thongbamrer C, Plianwong S. Dual-Targeted Therapy in HER2-Overexpressing Breast Cancer with Trastuzumab and Novel Cholesterol-Based Nioplexes Silencing Mcl-1. Pharmaceutics. 2023; 15(10):2424. https://doi.org/10.3390/pharmaceutics15102424
Chicago/Turabian StylePengnam, Supusson, Praneet Opanasopit, Theerasak Rojanarata, Boon-ek Yingyongnarongkul, Chopaka Thongbamrer, and Samarwadee Plianwong. 2023. "Dual-Targeted Therapy in HER2-Overexpressing Breast Cancer with Trastuzumab and Novel Cholesterol-Based Nioplexes Silencing Mcl-1" Pharmaceutics 15, no. 10: 2424. https://doi.org/10.3390/pharmaceutics15102424
APA StylePengnam, S., Opanasopit, P., Rojanarata, T., Yingyongnarongkul, B.-e., Thongbamrer, C., & Plianwong, S. (2023). Dual-Targeted Therapy in HER2-Overexpressing Breast Cancer with Trastuzumab and Novel Cholesterol-Based Nioplexes Silencing Mcl-1. Pharmaceutics, 15(10), 2424. https://doi.org/10.3390/pharmaceutics15102424