The Path from Nasal Tissue to Nasal Mucosa on Chip: Part 2—Advanced Microfluidic Nasal In Vitro Model for Drug Absorption Testing
Abstract
:1. Introduction
2. Materials and Methods
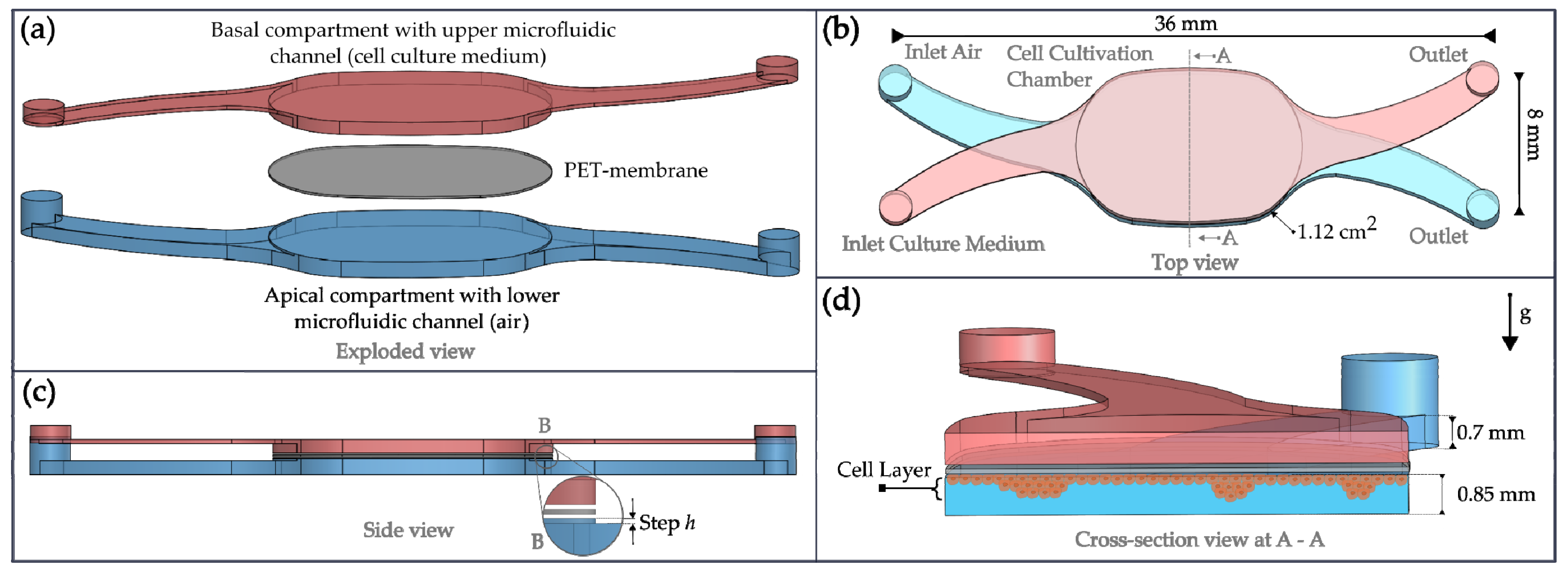
2.1. NM-Chip Design
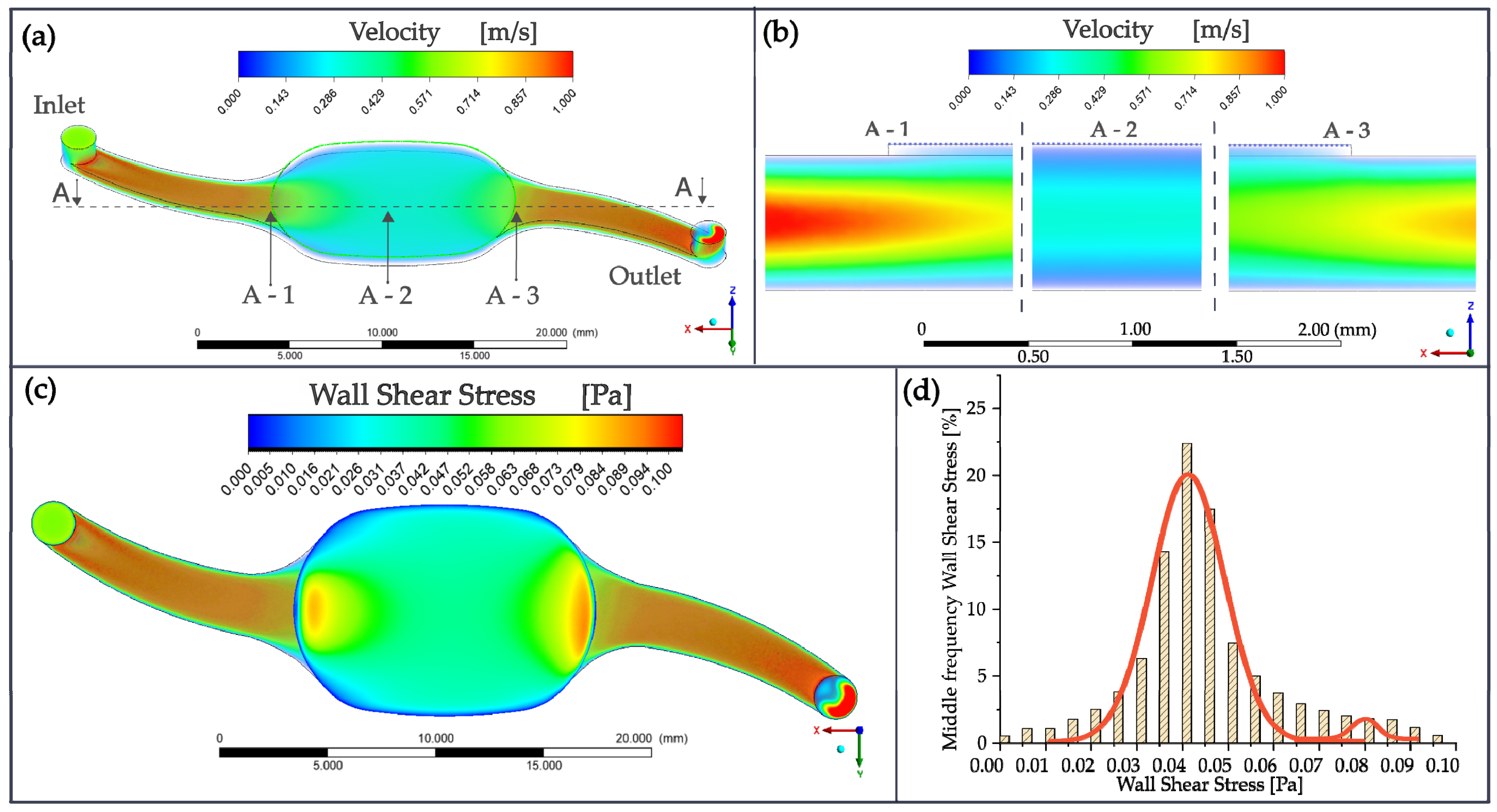
2.2. Computational Fluid Dynamics
2.3. Microfabrication of the Chip
2.4. Membrane Transfer Tooling and Chip Mount
2.5. Cell Cultivation
2.6. Experimental Setup
2.7. Mucin Staining
2.8. Immunofluorescence Staining of Cilia
2.9. PIV Analysis
2.10. Permeation Experiments
2.11. Statistical Analysis
3. Results and Discussion
3.1. Numerical Simulation
3.2. TEER Measurements
3.3. Mucus Production
3.4. Formation of Cilia
3.5. Mucociliary Transport
3.6. Permeation Coefficient for FITC-Dextran 4000
| Nasal Mucosa Model | FITC-Dextran 4000 |
|---|---|
| ANaMuc Transwell insert | 5.13 ± 0.76 A |
| NM-Chip 14 d static | 4.366 ± 0.645 |
| NM-Chip 14 d dynamic | 3.419 ± 0.499 |
| MucilAir™ | 0.28 ± 0.04 A |
| Human nasal primary cells | 1.8 ± 0.4 B |
| RPMI 2650 | 6.9 ± 0.4 C |
| Excised nasal mucosa | 4.6 (sheep) D 5.2–12.75 (rabbit) E |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007, 337, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, A.N.; Jain, S.K. Nasal-nanotechnology: Revolution for efficient therapeutics delivery. Drug Deliv. 2016, 23, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Bitter, C.; Suter-Zimmermann, K.; Surber, C. Nasal drug delivery in humans. Curr. Probl. Dermatol. 2011, 40, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Protopapa, C.; Siamidi, A.; Pavlou, P.; Vlachou, M. Excipients Used for Modified Nasal Drug Delivery: A Mini-Review of the Recent Advances. Materials 2022, 15, 6547. [Google Scholar] [CrossRef] [PubMed]
- Merkle, H.P.; Ditzinger, G.; Lang, S.R.; Peter, H.; Schmidt, M.C. In vitro cell models to study nasal mucosal permeability and metabolism. Adv. Drug Deliv. Rev. 1998, 29, 51–79. [Google Scholar] [CrossRef]
- Gizurarson, S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 2015, 38, 497–506. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- Inoue, D.; Tanaka, A.; Kimura, S.; Kiriyama, A.; Katsumi, H.; Yamamoto, A.; Ogawara, K.-I.; Kimura, T.; Higaki, K.; Yutani, R.; et al. The relationship between in vivo nasal drug clearance and in vitro nasal mucociliary clearance: Application to the prediction of nasal drug absorption. Eur. J. Pharm. Sci. 2018, 117, 21–26. [Google Scholar] [CrossRef]
- Felicetti, S.A.; Wolff, R.K.; Muggenburg, B.A. Comparison of tracheal mucous transport in rats, guinea pigs, rabbits, and dogs. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 1612–1617. [Google Scholar] [CrossRef]
- Gerasimenko, T.; Nikulin, S.; Zakharova, G.; Poloznikov, A.; Petrov, V.; Baranova, A.; Tonevitsky, A. Impedance Spectroscopy as a Tool for Monitoring Performance in 3D Models of Epithelial Tissues. Front. Bioeng. Biotechnol. 2019, 7, 474. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Ong, H.X.; Bradbury, P.; Kourmatzis, A.; Traini, D.; Young, P.; Li, M.; Cheng, S. Real-time quantitative monitoring of in vitro nasal drug delivery by a nasal epithelial mucosa-on-a-chip model. Expert Opin. Drug Deliv. 2021, 18, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Lee, S.-N.; Baek, J.; Yoon, J.-H.; Lee, H. Mechanical compression enhances ciliary beating through cytoskeleton remodeling in human nasal epithelial cells. Acta Biomater. 2021, 128, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Brooks, Z.; Kim, K.; Zhao, K.; Goswami, T.; Hussain, S.; Dixon, A.R. 3D printed transwell-integrated nose-on-chip model to evaluate effects of air flow-induced mechanical stresses on mucous secretion. Biomed. Microdevices 2022, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Elad, D.; Naftali, S.; Rosenfeld, M.; Wolf, M. Physical stresses at the air-wall interface of the human nasal cavity during breathing. J. Appl. Physiol. 2006, 100, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yan, Y.; Li, C.W.; Xia, H.M.; Chao, S.S.; Wang, D.Y.; Wang, Z.P. Live human nasal epithelial cells (hNECs) on chip for in vitro testing of gaseous formaldehyde toxicity via airway delivery. Lab Chip 2014, 14, 677–680. [Google Scholar] [CrossRef]
- Byun, J.; Song, B.; Lee, K.; Kim, B.; Hwang, H.W.; Ok, M.-R.; Jeon, H.; Lee, K.; Baek, S.-K.; Kim, S.-H.; et al. Identification of urban particulate matter-induced disruption of human respiratory mucosa integrity using whole transcriptome analysis and organ-on-a chip. J. Biol. Eng. 2019, 13, 88. [Google Scholar] [CrossRef]
- Becker, K.; Reichl, S. Cultivation of RPMI 2650 cells as an in-vitro model for human transmucosal nasal drug absorption studies: Optimization of selected culture conditions. J. Pharm. Pharmacol. 2012, 64, 1621–1630. [Google Scholar] [CrossRef]
- Bernocchi, B.; Carpentier, R.; Betbeder, D. Nasal nanovaccines. Int. J. Pharm. 2017, 530, 128–138. [Google Scholar] [CrossRef]
- Usman Khan, M.; Cai, X.; Shen, Z.; Mekonnen, T.; Kourmatzis, A.; Cheng, S.; Gholizadeh, H. Challenges in the Development and Application of Organ-on-Chips for Intranasal Drug Delivery Studies. Pharmaceutics 2023, 15, 1557. [Google Scholar] [CrossRef]
- Pozzoli, M.; Ong, H.X.; Morgan, L.; Sukkar, M.; Traini, D.; Young, P.M.; Sonvico, F. Application of RPMI 2650 nasal cell model to a 3D printed apparatus for the testing of drug deposition and permeation of nasal products. Eur. J. Pharm. Biopharm. 2016, 107, 223–233. [Google Scholar] [CrossRef]
- Wengst, A.; Reichl, S. RPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studies. Eur. J. Pharm. Biopharm. 2010, 74, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Bicker, J.; Falcão, A.; Fortuna, A. Air-liquid interface (ALI) impact on different respiratory cell cultures. Eur. J. Pharm. Biopharm. 2023, 184, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yang, T.; Abbruscato, T.J.; Ahsan, F. Evaluation of human nasal RPMI 2650 cells grown at an air–liquid interface as a model for nasal drug transport studies. J. Pharm. Sci. 2008, 97, 1165–1178. [Google Scholar] [CrossRef]
- Ladel, S.; Schlossbauer, P.; Flamm, J.; Luksch, H.; Mizaikoff, B.; Schindowski, K. Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650. Pharmaceutics 2019, 11, 367. [Google Scholar] [CrossRef]
- Roy, Y.; Sivathanu, V.; Das, S.K. Effect of bunching of cilia and their interplay on muco-ciliary transport. Comput. Biol. Med. 2013, 43, 1758–1772. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A. Airway Epithelial Differentiation and Mucociliary Clearance. Ann. Am. Thorac. Soc. 2018, 15, S143–S148. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, L.; Liu, B.; Feng, Y.; Zhou, H.; Zhang, Z.; Wu, Y.; Wang, J.; Gan, Y.; Yuan, T.; et al. Establishment and comparison of air-liquid interface culture systems for primary and immortalized swine tracheal epithelial cells. BMC Cell Biol. 2018, 19, 10. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Forbes, B.; Kim, K.-J. In Vitro Models of the Tracheo-Bronchial Epithelium. In Drug Absorption Studies: In Situ, In Vitro and In Silico Models; Ehrhardt, C., Forbes, B., Kim, K.-J., Eds.; Springer: New York, NY, USA, 2016; pp. 235–257. ISBN 978-1-4899-7754-0. [Google Scholar]
- Wiszniewski, L.; Jornot, L.; Dudez, T.; Pagano, A.; Rochat, T.; Lacroix, J.S.; Suter, S.; Chanson, M. Long-term cultures of polarized airway epithelial cells from patients with cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2006, 34, 39–48. [Google Scholar] [CrossRef]
- Bendas, S.; Koch, E.V.; Nehlsen, K.; May, T.; Dietzel, A.; Reichl, S. The Path from Nasal Tissue to Nasal Mucosa on Chip: Part 1—Establishing a Nasal In Vitro Model for Drug Delivery Testing Based on a Novel Cell Line. Pharmaceutics 2023, 15, 2245. [Google Scholar] [CrossRef]
- Koch, E.V.; Ledwig, V.; Bendas, S.; Reichl, S.; Dietzel, A. Tissue Barrier-on-Chip: A Technology for Reproducible Practice in Drug Testing. Pharmaceutics 2022, 14, 1451. [Google Scholar] [CrossRef]
- Inthavong, K.; Shang, Y.; Wong, E.; Singh, N. Characterization of nasal irrigation flow from a squeeze bottle using computational fluid dynamics. Int. Forum Allergy Rhinol. 2020, 10, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Kratz, S.R.A.; Eilenberger, C.; Schuller, P.; Bachmann, B.; Spitz, S.; Ertl, P.; Rothbauer, M. Characterization of four functional biocompatible pressure-sensitive adhesives for rapid prototyping of cell-based lab-on-a-chip and organ-on-a-chip systems. Sci. Rep. 2019, 9, 9287. [Google Scholar] [CrossRef] [PubMed]
- Mulisch, M.; Welsch, U. Romeis-Mikroskopische Technik; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Thielicke, W.; Sonntag, R. Particle Image Velocimetry for MATLAB: Accuracy and enhanced algorithms in PIVlab. J. Open Res. Softw. 2021, 9, 12. [Google Scholar] [CrossRef]
- Brüning, J.; Hildebrandt, T.; Heppt, W.; Schmidt, N.; Lamecker, H.; Szengel, A.; Amiridze, N.; Ramm, H.; Bindernagel, M.; Zachow, S.; et al. Characterization of the Airflow within an Average Geometry of the Healthy Human Nasal Cavity. Sci. Rep. 2020, 10, 3755. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Sun, X.; Yu, S.; Gao, F. Numerical analysis of respiratory flow patterns within human upper airway. Acta Mech. Sin. 2009, 25, 737–746. [Google Scholar] [CrossRef]
- Shang, Y.; Dong, J.L.; Inthavong, K.; Tu, J.Y. Computational fluid dynamics analysis of wall shear stresses between human and rat nasal cavities. Eur. J. Mech. B/Fluids 2017, 61, 160–169. [Google Scholar] [CrossRef]
- Chatelin, R.; Anne-Archard, D.; Murris-Espin, M.; Thiriet, M.; Poncet, P. Numerical and experimental investigation of mucociliary clearance breakdown in cystic fibrosis. J. Biomech. 2017, 53, 56–63. [Google Scholar] [CrossRef]
- Shang, Y.; Inthavong, K.; Tu, J. Development of a computational fluid dynamics model for mucociliary clearance in the nasal cavity. J. Biomech. 2019, 85, 74–83. [Google Scholar] [CrossRef]
- Brüning, J.J.; Goubergrits, L.; Heppt, W.; Zachow, S.; Hildebrandt, T. Numerical Analysis of Nasal Breathing: A Pilot Study. Facial Plast. Surg. 2017, 33, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.; Goubergrits, L.; Heppt, W.J.; Bessler, S.; Zachow, S. Evaluation of the intranasal flow field through computational fluid dynamics. Facial Plast. Surg. 2013, 29, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, N.E.-T.; Kloog, Y.; Wolf, M.; Elad, D. Mechanophysical stimulations of mucin secretion in cultures of nasal epithelial cells. Biophys. J. 2011, 100, 2855–2864. [Google Scholar] [CrossRef]
- Even-Tzur, N.; Kloog, Y.; Wolf, M.; Elad, D. Mucus secretion and cytoskeletal modifications in cultured nasal epithelial cells exposed to wall shear stresses. Biophys. J. 2008, 95, 2998–3008. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.S.S.; Matias, A.A.; Poejo, J.; Serra, A.T.; Duarte, C.M.M. Application of RPMI 2650 as a cell model to evaluate solid formulations for intranasal delivery of drugs. Int. J. Pharm. 2016, 515, 1–10. [Google Scholar] [CrossRef]
- Trieu, D.; Waddell, T.K.; McGuigan, A.P. A microfluidic device to apply shear stresses to polarizing ciliated airway epithelium using air flow. Biomicrofluidics 2014, 8, 64104. [Google Scholar] [CrossRef]
- Oldenburg, A.L.; Chhetri, R.K.; Hill, D.B.; Button, B. Monitoring airway mucus flow and ciliary activity with optical coherence tomography. Biomed. Opt. Express 2012, 3, 1978–1992. [Google Scholar] [CrossRef]
- Yergin, B.M.; Saketkhoo, K.; Michaelson, E.D.; Serafini, S.M.; Kovitz, K.; Sackner, M.A. A roentgenographic method for measuring nasal mucous velocity. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 44, 964–968. [Google Scholar] [CrossRef]
- Lee, D.F.; Lethem, M.I.; Lansley, A.B. A comparison of three mucus-secreting airway cell lines (Calu-3, SPOC1 and UNCN3T) for use as biopharmaceutical models of the nose and lung. Eur. J. Pharm. Biopharm. 2021, 167, 159–174. [Google Scholar] [CrossRef]
- Werner, U.; Kissel, T. Development of a human nasal epithelial cell culture model and its suitability for transport and metabolism studies under in vitro conditions. Pharm. Res. 1995, 12, 565–571. [Google Scholar] [CrossRef]
- Gerber, W.; Svitina, H.; Steyn, D.; Peterson, B.; Kotzé, A.; Weldon, C.; Hamman, J.H. Comparison of RPMI 2650 cell layers and excised sheep nasal epithelial tissues in terms of nasal drug delivery and immunocytochemistry properties. J. Pharmacol. Toxicol. Methods 2022, 113, 107131. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, K.; Maeno, T.; Ueda, H.; Natsume, H.; Morimoto, Y. Poly-L-arginine predominantly increases the paracellular permeability of hydrophilic macromolecules across rabbit nasal epithelium in vitro. Pharm. Res. 2003, 20, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Hosoya, K.-I.; Natsume, H.; Sugibayashi, K.; Morimoto, Y. In vitro permeation of several model drugs across rabbit nasal mucosa. Int. J. Pharm. 1994, 103, 27–36. [Google Scholar] [CrossRef]






| ALI Cultivation [Days] | NM-Chip TEER [Ω∙cm2] | Transwell® Insert TEER [Ω∙cm2] |
|---|---|---|
| 7 days | 430 b | 293 ± 16 a,d |
| 10 days | 327 b | 189 ± 14 a |
| 14 days | 556 ± 72 c | 412 ± 39 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, E.V.; Bendas, S.; Nehlsen, K.; May, T.; Reichl, S.; Dietzel, A. The Path from Nasal Tissue to Nasal Mucosa on Chip: Part 2—Advanced Microfluidic Nasal In Vitro Model for Drug Absorption Testing. Pharmaceutics 2023, 15, 2439. https://doi.org/10.3390/pharmaceutics15102439
Koch EV, Bendas S, Nehlsen K, May T, Reichl S, Dietzel A. The Path from Nasal Tissue to Nasal Mucosa on Chip: Part 2—Advanced Microfluidic Nasal In Vitro Model for Drug Absorption Testing. Pharmaceutics. 2023; 15(10):2439. https://doi.org/10.3390/pharmaceutics15102439
Chicago/Turabian StyleKoch, Eugen Viktor, Sebastian Bendas, Kristina Nehlsen, Tobias May, Stephan Reichl, and Andreas Dietzel. 2023. "The Path from Nasal Tissue to Nasal Mucosa on Chip: Part 2—Advanced Microfluidic Nasal In Vitro Model for Drug Absorption Testing" Pharmaceutics 15, no. 10: 2439. https://doi.org/10.3390/pharmaceutics15102439
APA StyleKoch, E. V., Bendas, S., Nehlsen, K., May, T., Reichl, S., & Dietzel, A. (2023). The Path from Nasal Tissue to Nasal Mucosa on Chip: Part 2—Advanced Microfluidic Nasal In Vitro Model for Drug Absorption Testing. Pharmaceutics, 15(10), 2439. https://doi.org/10.3390/pharmaceutics15102439







