Liposomes for Cancer Theranostics
Abstract
:1. Introduction
2. Common Cancer Therapies
2.1. Chemotherapy
2.2. Gene Therapy
2.3. Immunotherapy
2.4. Photothermal Therapy
2.5. Photodynamic Therapy
2.6. Magneto-Thermal Therapy
2.7. Ultrasound Responsive Therapy
2.8. Radiotherapy
3. Imaging Modalities Available for Cancer Theranostics
3.1. Positron Emission Tomography
3.2. Single-Photon Emission Computed Tomography
3.3. Computed Tomography
3.4. Magnetic Resonance Imaging
3.5. Optical Imaging
3.6. Ultrasound and Photoacoustic Imaging
4. Biomedical Applications of Cancer Theranostic Liposomes
4.1. Breast Cancer
4.2. Cervical Cancer
4.3. Brain Cancer
4.4. Lung Cancer
4.5. Prostate Cancer
4.6. Skin Cancer
| Type | Therapeutic/Imaging Agent(s) | Cancer(s) | Phase(s) | Remarks | ClinicalTrials.gov Identifier | Reference(s) |
|---|---|---|---|---|---|---|
| liposomal doxorubicin | doxorubicin | metastatic breast cancer (MBC) | phase II | Results from clinical trials revealed liposomal doxorubicin is well tolerated and has activity similar to weekly docetaxel. | NCT00193037 | [223] |
| pegylated liposomal doxorubicin (PLD) | bevacizumab doxorubicin | metastatic breast cancer (MBC) | phase II | Combination of bimonthly PLD and antibody bevacizumab led to modest activity for the treatment of MBC. | NCT00445406 | [224] |
| nanoliposomal irinotecan (nal-IRI, MM-398) | irinotecan 5-fluorouracil (5-FU) leucovorin (LV) oxaliplatin | metastatic pancreatic cancer | phase I/II | The nal-IRI with oxaliplatin, 5-FU and LV (NALIRIFOX) was tolerable and generally manageable for patients with locally advanced/metastatic pancreatic ductal adenocarcinoma (mPDAC). | NCT02551991 | [225,226] |
| liposome entrapped paclitaxel easy to use (LEP-ETU) | paclitaxel | many different advanced cancers | phase I | LEP-ETU is well tolerated and safe at doses below 225 mg/m2 and showed bioequivalence with paclitaxel formulated with polyethoxylated castor oil. | NCT00080418 | [227,228] |
| DsiRNA lipid nanoparticle for MYC oncogene silencing (DCR-MYC) | double-stranded RNA | liver cancer | phase Ib/II | The lipid particles can inhibit cancer cell growth by targeting oncogene MYC. | NCT02314052 | [229] |
| cationic liposome-DNA complexes (JVRS-100) | plasmid DNA complex | leukemia | phase I | JVRS-100 can be used for immunotherapy for eliciting cytokines important in mediating host defense against cancer. | NCT00860522 | [230] |
| thermosensitive liposomal doxorubicin (ThermoDox®) | doxorubicin | liver cancer | phase III | There is a therapeutic benefit from combining thermosensitive liposomal doxorubicin with radiofrequency ablation (RFA). | NCT00617981 | [231] |
| 99mTc-labeled, pegylated liposomal doxorubicin (Caelyx®, PLD) | technetium-99m (99mTc) doxorubicin cyclophosphamide trastuzumab | many different advanced cancers | phase II | PLD and cyclophosphamide ± trastuzumab (antibody) followed by docetaxel is highly active in patients with planar gamma scintigraphy images showing accumulation of particles. | NCT01206881 | [232,233] |
| PEGylated liposomal doxorubicin targeted against HER2 (MM-302) | copper-64 (64Cu) doxorubicin | advanced breast cancer | phase I | High 64Cu-MM-302 deposition was associated with more favorable treatment outcomes using radiotherapy and PET imaging. | NCT01304797 | [234] |
5. Future Directions
6. Conclusions
Funding
Conflicts of Interest
References
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Sharmiladevi, P.; Girigoswami, K.; Haribabu, V.; Girigoswami, A. Nano-enabled theranostics for cancer. Mater. Adv. 2021, 2, 2876–2891. [Google Scholar] [CrossRef]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Anderson, R.C.; Lan, X.; Conti, P.S.; Chen, K. Recent advances in the development of nanoparticles for multimodality imaging and therapy of cancer. Med. Res. Rev. 2020, 40, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Fernandes, D.A. Review on the applications of nanoemulsions in cancer theranostics. J. Mater. Res. 2022, 37, 1953–1977. [Google Scholar] [CrossRef]
- Fernandes, D.A. Theranostic Nanoparticles for Therapy and Imaging in Cancer Detection. In Nanomaterials for Cancer Detection Using Imaging Techniques and Their Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 141–177. [Google Scholar]
- Guo, J.; Rahme, K.; He, Y.; Li, L.-L.; Holmes, J.D.; O’Driscoll, C.M. Gold nanoparticles enlighten the future of cancer theranostics. Int. J. Nanomed. 2017, 12, 6131. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, J.; Gao, J.; Zhang, Z.; Zhu, H.; Wang, D. Gold nanoparticles in cancer theranostics. Front. Bioeng. Biotechnol. 2021, 9, 647905. [Google Scholar] [CrossRef]
- Dhas, N.; Pastagia, M.; Sharma, A.; Khera, A.; Kudarha, R.; Kulkarni, S.; Soman, S.; Mutalik, S.; Barnwal, R.P.; Singh, G. Organic quantum dots: An ultrasmall nanoplatform for cancer theranostics. J. Control. Release 2022, 348, 798–824. [Google Scholar] [CrossRef]
- Tripathi, S.; Kaur, G.; Khurana, R.K.; Kapoor, S.; Singh, B. Quantum dots and their potential role in cancer theranostics. Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 461–502. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release 2021, 335, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic nanoparticles in cancer theranostics. Theranostics 2015, 5, 1249. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Li, K.; Hong, E.; Wang, B.; Wang, Z.; Zhang, L.; Hu, R.; Wang, B. Advances in the application of upconversion nanoparticles for detecting and treating cancers. Photodiagn. Photodyn. Ther. 2019, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Gorain, B.; Choudhury, H.; Nair, A.B.; Dubey, S.K.; Kesharwani, P. Theranostic application of nanoemulsions in chemotherapy. Drug Discov. Today 2020, 25, 1174–1188. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Fernandes, D.D.; Malik, A.; Gomes, G.-N.W.; Appak-Baskoy, S.; Berndl, E.; Gradinaru, C.C.; Kolios, M.C. Multifunctional nanoparticles as theranostic agents for therapy and imaging of breast cancer. J. Photochem. Photobiol. B Biol. 2021, 218, 112110. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Appak-Baskoy, S.; Berndl, E.; Kolios, M.C. Laser activatable perfluorocarbon bubbles for imaging and therapy through enhanced absorption from coupled silica coated gold nanoparticles. RSC Adv. 2021, 11, 4906–4920. [Google Scholar] [CrossRef]
- Wang, H.; Picchio, M.L.; Calderon, M. One stone, many birds: Recent advances in functional nanogels for cancer nanotheranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1791. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Jangjou, A.; Meisami, A.H.; Jamali, K.; Niakan, M.H.; Abbasi, M.; Shafiee, M.; Salehi, M.; Hosseinzadeh, A.; Amani, A.M.; Vaez, A. The promising shadow of microbubble over medical sciences: From fighting wide scope of prevalence disease to cancer eradication. J. Biomed. Sci. 2021, 28, 49. [Google Scholar] [CrossRef]
- Zahiri, M.; Taghavi, S.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Theranostic nanobubbles towards smart nanomedicines. J. Control. Release 2021, 339, 164–194. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.H.; Dayton, P.A. Current status and prospects for microbubbles in ultrasound theranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, J.; Pan, T.; Yin, Y.; Mei, Y.; Xiao, Q.; Wang, R.; Yan, Z.; Wang, W. Versatile carbon nanoplatforms for cancer treatment and diagnosis: Strategies, applications and future perspectives. Theranostics 2022, 12, 2290. [Google Scholar] [CrossRef]
- Saleem, J.; Wang, L.; Chen, C. Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Adv. Healthc. Mater. 2018, 7, 1800525. [Google Scholar] [CrossRef] [PubMed]
- Indoria, S.; Singh, V.; Hsieh, M.-F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. Int. J. Pharm. 2020, 582, 119314. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Fang, R.H.; Zhang, L. Lipid-and polymer-based nanostructures for cancer theranostics. Theranostics 2012, 2, 1117. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921. [Google Scholar] [CrossRef]
- Varela-Moreira, A.; Shi, Y.; Fens, M.H.; Lammers, T.; Hennink, W.E.; Schiffelers, R.M. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017, 1, 1485–1501. [Google Scholar] [CrossRef]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef]
- Vahed, S.Z.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C 2017, 71, 1327–1341. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Gerlowski, L.E.; Jain, R.K. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 1986, 31, 288–305. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.A.; Malla, S.; Pasternak, M.A.; Terrero, D.; Brown, N.G.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.-S.; Tiwari, A.K. The role of endolysosomal trafficking in anticancer drug resistance. Drug Resist. Updat. 2021, 57, 100769. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A. Emerging nanotherapeutic approaches to overcome drug resistance in cancers with update on clinical trials. Pharmaceutics 2022, 14, 866. [Google Scholar] [CrossRef]
- Majidinia, M.; Mirza-Aghazadeh-Attari, M.; Rahimi, M.; Mihanfar, A.; Karimian, A.; Safa, A.; Yousefi, B. Overcoming multidrug resistance in cancer: Recent progress in nanotechnology and new horizons. IUBMB Life 2020, 72, 855–871. [Google Scholar] [CrossRef]
- Yadav, P.; Ambudkar, S.V.; Rajendra Prasad, N. Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer. J. Nanobiotechnol. 2022, 20, 423. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles—From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Luiz, M.T.; Dutra, J.A.P.; Tofani, L.B.; de Araújo, J.T.C.; Di Filippo, L.D.; Marchetti, J.M.; Chorilli, M. Targeted liposomes: A nonviral gene delivery system for cancer therapy. Pharmaceutics 2022, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gerbi, S.A. Making ends meet: Targeted integration of DNA fragments by genome editing. Chromosoma 2018, 127, 405–420. [Google Scholar] [CrossRef]
- Xin, Y.; Huang, M.; Guo, W.W.; Huang, Q.; Jiang, G. Nano-based delivery of RNAi in cancer therapy. Mol. Cancer 2017, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Stahel, R.A.; Zangemeister-Wittke, U. Antisense oligonucleotides for cancer therapy—An overview. Lung Cancer 2003, 41, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene therapy in cancer treatment: Why go nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.H. Gene therapy for cancer: Present status and future perspective. Mol. Cell. Ther. 2014, 2, 27. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Darwiche, K.; Sakkas, A.; Yarmus, L.; Huang, H.; Li, Q.; Freitag, L.; Zarogoulidis, K.; Malecki, M. Suicide gene therapy for cancer–current strategies. J. Genet. Syndr. Gene Ther. 2013, 4, 16849. [Google Scholar]
- Maeder, M.L.; Gersbach, C.A. Genome-editing technologies for gene and cell therapy. Mol. Ther. 2016, 24, 430–446. [Google Scholar] [CrossRef]
- Yeh, C.D.; Richardson, C.D.; Corn, J.E. Advances in genome editing through control of DNA repair pathways. Nat. Cell Biol. 2019, 21, 1468–1478. [Google Scholar] [CrossRef]
- Sheng, W.-Y.; Huang, L. Cancer immunotherapy and nanomedicine. Pharm. Res. 2011, 28, 200–214. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, Q.; Zhang, R. PD-1/PD-L1 pathway blockade works as an effective and practical therapy for cancer immunotherapy. Cancer Biol. Med. 2018, 15, 116. [Google Scholar]
- Gao, A.; Hu, X.-l.; Saeed, M.; Chen, B.-f.; Li, Y.-p.; Yu, H.-j. Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol. Sin. 2019, 40, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Alhamhoom, Y.; Kakinani, G.; Rahamathulla, M.; Osmani, R.A.M.; Hani, U.; Thajudeen, K.Y.; Gowda, D.V. Recent Advances in the Liposomal Nanovesicles Based Immunotherapy in the Treatment of Cancer: A Review. Saudi Pharm. J. 2022, 31, 279–294. [Google Scholar] [CrossRef]
- Debele, T.A.; Yeh, C.-F.; Su, W.-P. Cancer immunotherapy and application of nanoparticles in cancers immunotherapy as the delivery of immunotherapeutic agents and as the immunomodulators. Cancers 2020, 12, 3773. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; He, S.; Seare, W.J.; Almutairi, A. Review of the progress toward achieving heat confinement—The holy grail of photothermal therapy. J. Biomed. Opt. 2017, 22, 080901. [Google Scholar] [CrossRef]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, X.; Qi, T.; Wu, Y.; Xie, X.; Chen, F.; Shao, D.; Liao, J. Biomedical applications and prospects of temperature-orchestrated photothermal therapy. MedComm Biomater. Appl. 2022, 1, e25. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics 2007, 2, 107–118. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef]
- Geng, J.; Sun, C.; Liu, J.; Liao, L.D.; Yuan, Y.; Thakor, N.; Wang, J.; Liu, B. Biocompatible conjugated polymer nanoparticles for efficient photothermal tumor therapy. Small 2015, 11, 1603–1610. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, H.; Chen, Y.; Zhu, R.; Yin, L. Photoresponsive drug/gene delivery systems. Biomacromolecules 2018, 19, 1840–1857. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Derycke, A.S.; De Witte, P.A. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 2004, 56, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Moghassemi, S.; Dadashzadeh, A.; Azevedo, R.B.; Feron, O.; Amorim, C.A. Photodynamic cancer therapy using liposomes as an advanced vesicular photosensitizer delivery system. J. Control. Release 2021, 339, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-J.; Chuang, C.-C.; Chen, J.-P. Liposomal IR-780 as a highly stable nanotheranostic agent for improved photothermal/photodynamic therapy of brain tumors by convection-enhanced delivery. Cancers 2021, 13, 3690. [Google Scholar] [CrossRef]
- Brazel, C.S. Magnetothermally-responsive nanomaterials: Combining magnetic nanostructures and thermally-sensitive polymers for triggered drug release. Pharm. Res. 2009, 26, 644–656. [Google Scholar] [CrossRef]
- Shevtsov, M.; Kaesler, S.; Posch, C.; Multhoff, G.; Biedermann, T. Magnetic nanoparticles in theranostics of malignant melanoma. EJNMMI Res. 2021, 11, 127. [Google Scholar] [CrossRef]
- Heidarli, E.; Dadashzadeh, S.; Haeri, A. State of the art of stimuli-responsive liposomes for cancer therapy. Iran. J. Pharm. Res. IJPR 2017, 16, 1273. [Google Scholar]
- TS, A.; Shalumon, K.; Chen, J.-P. Applications of magnetic liposomes in cancer therapies. Curr. Pharm. Des. 2019, 25, 1490–1504. [Google Scholar]
- Shivanna, A.T.; Dash, B.S.; Chen, J.-P. Functionalized magnetic nanoparticles for alternating magnetic field-or near infrared light-induced cancer therapies. Micromachines 2022, 13, 1279. [Google Scholar] [CrossRef] [PubMed]
- TS, A.; Lu, Y.-J.; Chen, J.-P. Optimization of the preparation of magnetic liposomes for the combined use of magnetic hyperthermia and photothermia in dual magneto-photothermal cancer therapy. Int. J. Mol. Sci. 2020, 21, 5187. [Google Scholar]
- Yudina, A.; De Smet, M.; Lepetit-Coiffe, M.; Langereis, S.; Van Ruijssevelt, L.; Smirnov, P.; Bouchaud, V.; Voisin, P.; Grüll, H.; Moonen, C. Ultrasound-mediated intracellular drug delivery using microbubbles and temperature-sensitive liposomes. J. Control. Release 2011, 155, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Staruch, R.; Chopra, R.; Hynynen, K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int. J. Hyperth. 2011, 27, 156–171. [Google Scholar] [CrossRef]
- Ibsen, S.; Benchimol, M.; Simberg, D.; Schutt, C.; Steiner, J.; Esener, S. A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. J. Control. Release 2011, 155, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Rizzitelli, S.; Giustetto, P.; Boffa, C.; Castelli, D.D.; Cutrin, J.C.; Aime, S.; Terreno, E. In vivo MRI visualization of release from liposomes triggered by local application of pulsed low-intensity non-focused ultrasound. Nanomed. Nanotechnol. Biol. Med. 2014, 10, e901–e904. [Google Scholar] [CrossRef]
- Ranjan, A.; Jacobs, G.C.; Woods, D.L.; Negussie, A.H.; Partanen, A.; Yarmolenko, P.S.; Gacchina, C.E.; Sharma, K.V.; Frenkel, V.; Wood, B.J. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J. Control. Release 2012, 158, 487–494. [Google Scholar] [CrossRef]
- Deckers, R.; Moonen, C.T. Ultrasound triggered, image guided, local drug delivery. J. Control. Release 2010, 148, 25–33. [Google Scholar] [CrossRef]
- Zha, Z.; Wang, J.; Qu, E.; Zhang, S.; Jin, Y.; Wang, S.; Dai, Z. Polypyrrole hollow microspheres as echogenic photothermal agent for ultrasound imaging guided tumor ablation. Sci. Rep. 2013, 3, 2360. [Google Scholar] [CrossRef]
- Gao, Y.; Chan, C.U.; Gu, Q.; Lin, X.; Zhang, W.; Yeo, D.C.L.; Alsema, A.M.; Arora, M.; Chong, M.S.K.; Shi, P. Controlled nanoparticle release from stable magnetic microbubble oscillations. NPG Asia Mater. 2016, 8, e260. [Google Scholar] [CrossRef]
- Kang, S.-T.; Lin, J.-L.; Wang, C.-H.; Chang, Y.-C.; Yeh, C.-K. Internal polymer scaffolding in lipid-coated microbubbles for control of inertial cavitation in ultrasound theranostics. J. Mater. Chem. B 2015, 3, 5938–5941. [Google Scholar] [CrossRef] [PubMed]
- Geers, B.; De Wever, O.; Demeester, J.; Bracke, M.; De Smedt, S.C.; Lentacker, I. Targeted liposome-loaded microbubbles for cell-specific ultrasound-triggered drug delivery. Small 2013, 9, 4027–4035. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, F.; Fokong, S.; Koczera, P.; Lederle, W.; Lammers, T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J. Nucl. Med. 2012, 53, 345–348. [Google Scholar] [CrossRef]
- Dwivedi, P.; Kiran, S.; Han, S.; Dwivedi, M.; Khatik, R.; Fan, R.; Mangrio, F.A.; Du, K.; Zhu, Z.; Yang, C. Magnetic targeting and ultrasound activation of liposome–microbubble conjugate for enhanced delivery of anticancer therapies. ACS Appl. Mater. Interfaces 2020, 12, 23737–23751. [Google Scholar] [CrossRef]
- Yang, P.; Li, D.; Jin, S.; Ding, J.; Guo, J.; Shi, W.; Wang, C. Stimuli-responsive biodegradable poly (methacrylic acid) based nanocapsules for ultrasound traced and triggered drug delivery system. Biomaterials 2014, 35, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Kang, S.-T.; Yeh, C.-K. Superparamagnetic iron oxide and drug complex-embedded acoustic droplets for ultrasound targeted theranosis. Biomaterials 2013, 34, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-F.; Zhu, X.-M.; Wang, Y.-X.J.; Xuan, S.-H.; You, Q.; Chan, W.-H.; Wong, C.-H.; Wang, F.; Yu, J.C.; Cheng, C.H. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl. Mater. Interfaces 2013, 5, 1566–1574. [Google Scholar] [CrossRef]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef]
- Min, H.S.; You, D.G.; Son, S.; Jeon, S.; Park, J.H.; Lee, S.; Kwon, I.C.; Kim, K. Echogenic glycol chitosan nanoparticles for ultrasound-triggered cancer theranostics. Theranostics 2015, 5, 1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-E.; Yu, H.-M.; Lu, Y.-C.; Heish, N.-N.; Tseng, Y.-L.; Huang, K.-L.; Chuang, K.-T.; Chen, C.-H.; Hwang, J.-J.; Lin, W.-J. Internal radiotherapy and dosimetric study for 111In/177Lu-pegylated liposomes conjugates in tumor-bearing mice. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 569, 533–537. [Google Scholar] [CrossRef]
- Stolarz, A.J.; Chhetri, B.P.; Borrelli, M.J.; Jenkins, S.V.; Jamshidi-Parsian, A.; Phillips, J.H.; Fologea, D.; Gandy, J.; Griffin, R.J. Liposome formulation for tumor-targeted drug delivery using radiation therapy. Int. J. Mol. Sci. 2022, 23, 11662. [Google Scholar] [CrossRef]
- Bar-Shalom, R.; Valdivia, A.Y.; Blaufox, M.D. PET imaging in oncology. In Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 2000; pp. 150–185. [Google Scholar]
- Adam, M.J.; Wilbur, D.S. Radiohalogens for imaging and therapy. Chem. Soc. Rev. 2005, 34, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A. [74As]-Labeled monoclonal antibody against anionic phospholipids. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information: Bethesda, MD, USA, 2004. [Google Scholar]
- Pressly, E.D.; Rossin, R.; Hagooly, A.; Fukukawa, K.-i.; Messmore, B.W.; Welch, M.J.; Wooley, K.L.; Lamm, M.S.; Hule, R.A.; Pochan, D.J. Structural effects on the biodistribution and positron emission tomography (PET) imaging of well-defined 64Cu-labeled nanoparticles comprised of amphiphilic block graft copolymers. Biomacromolecules 2007, 8, 3126–3134. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Keliher, E.J.; Thurber, G.M.; Nahrendorf, M.; Weissleder, R. 18F labeled nanoparticles for in vivo PET-CT imaging. Bioconjugate Chem. 2009, 20, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Herth, M.M.; Barz, M.; Jahn, M.; Zentel, R.; Rösch, F. 72/74As-labeling of HPMA based polymers for long-term in vivo PET imaging. Bioorganic Med. Chem. Lett. 2010, 20, 5454–5458. [Google Scholar] [CrossRef]
- Roesch, F. Scandium-44: Benefits of a long-lived PET radionuclide available from the 44Ti/44Sc generator system. Curr. Radiopharm. 2012, 5, 187–201. [Google Scholar] [CrossRef]
- Müller, C.; Bunka, M.; Haller, S.; Köster, U.; Groehn, V.; Bernhardt, P.; van der Meulen, N.; Türler, A.; Schibli, R. Promising prospects for 44Sc-/47Sc-based theragnostics: Application of 47Sc for radionuclide tumor therapy in mice. J. Nucl. Med. 2014, 55, 1658–1664. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Hong, H.; Nickles, R.J.; Cai, W. Matching the decay half-life with the biological half-life: ImmunoPET imaging with 44Sc-labeled cetuximab Fab fragment. Bioconjugate Chem. 2014, 25, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Man, F.; Gawne, P.J.; de Rosales, R.T. Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef]
- Chakravarty, R.; Hong, H.; Cai, W. Positron emission tomography image-guided drug delivery: Current status and future perspectives. Mol. Pharm. 2014, 11, 3777–3797. [Google Scholar] [CrossRef] [PubMed]
- de Smet, M.; Langereis, S.; van den Bosch, S.; Bitter, K.; Hijnen, N.M.; Heijman, E.; Grüll, H. SPECT/CT imaging of temperature-sensitive liposomes for MR-image guided drug delivery with high intensity focused ultrasound. J. Control. Release 2013, 169, 82–90. [Google Scholar] [CrossRef]
- Head, H.W.; Dodd, G.D., III; Bao, A.; Soundararajan, A.; Garcia-Rojas, X.; Prihoda, T.J.; McManus, L.M.; Goins, B.A.; Santoyo, C.A.; Phillips, W.T. Combination radiofrequency ablation and intravenous radiolabeled liposomal Doxorubicin: Imaging and quantification of increased drug delivery to tumors. Radiology 2010, 255, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Silindir, M.; Erdoğan, S.; Özer, A.Y.; Doğan, A.L.; Tuncel, M.; Uğur, Ö.; Torchilin, V.P. Nanosized multifunctional liposomes for tumor diagnosis and molecular imaging by SPECT/CT. J. Liposome Res. 2013, 23, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Fouillet, X.; Tournier, H.; Khan, H.; Sabitha, S.; Burkhardt, S.; Terrier, F.; Schneider, M. Enhancement of computed tomography liver contrast using iomeprol-containing liposomes and detection of small liver tumors in rats. Acad. Radiol. 1995, 2, 576–583. [Google Scholar] [CrossRef]
- Xu, H.; Ohulchanskyy, T.Y.; Yakovliev, A.; Zinyuk, R.; Song, J.; Liu, L.; Qu, J.; Yuan, Z. Nanoliposomes co-encapsulating CT imaging contrast agent and photosensitizer for enhanced, imaging guided photodynamic therapy of cancer. Theranostics 2019, 9, 1323. [Google Scholar] [CrossRef]
- Zheng, J.; Jaffray, D.; Allen, C. Quantitative CT imaging of the spatial and temporal distribution of liposomes in a rabbit tumor model. Mol. Pharm. 2009, 6, 571–580. [Google Scholar] [CrossRef]
- Kircher, M.F.; Willmann, J.K. Molecular body imaging: MR imaging, CT, and US. part I. principles. Radiology 2012, 263, 633–643. [Google Scholar] [CrossRef]
- Willmann, J.K.; Van Bruggen, N.; Dinkelborg, L.M.; Gambhir, S.S. Molecular imaging in drug development. Nat. Rev. Drug Discov. 2008, 7, 591–607. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, H.; Lv, R.; Zhao, P.; Sun, X.; Wang, S.; Su, W.; Niu, R.; Chang, J. Polymeric liposomes-coated superparamagnetic iron oxide nanoparticles as contrast agent for targeted magnetic resonance imaging of cancer cells. Langmuir 2011, 27, 3100–3105. [Google Scholar] [CrossRef]
- Di Corato, R.; Béalle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clément, O.; Silva, A.K.; Ménager, C.; Wilhelm, C. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-C.; Ren, W.; Zhang, S.; Zhong, T.; Duan, X.-C.; Yin, Y.-F.; Xu, M.-Q.; Hao, Y.-L.; Li, Z.-T.; Li, H. The theranostic efficiency of tumor-specific, pH-responsive, peptide-modified, liposome-containing paclitaxel and superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2018, 13, 1495. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kattel, K.; Park, J.Y.; Chang, Y.; Kim, T.J.; Lee, G.H. Paramagnetic nanoparticle T 1 and T 2 MRI contrast agents. Phys. Chem. Chem. Phys. 2012, 14, 12687–12700. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.; Kalber, T.L.; Cooper, M.S.; Sunassee, K.; Chalker, S.L.; Shaw, K.P.; Ordidge, K.L.; Badar, A.; Janes, S.M.; Blower, P.J. Incorporation of paramagnetic, fluorescent and PET/SPECT contrast agents into liposomes for multimodal imaging. Biomaterials 2013, 34, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gnanasammandhan, M.K.; Zhang, Y. Optical imaging-guided cancer therapy with fluorescent nanoparticles. J. R. Soc. Interface 2010, 7, 3–18. [Google Scholar] [CrossRef]
- Xing, H.; Hwang, K.; Lu, Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics 2016, 6, 1336. [Google Scholar] [CrossRef]
- Zhou, L.-Q.; Li, P.; Cui, X.-W.; Dietrich, C.F. Ultrasound nanotheranostics in fighting cancer: Advances and prospects. Cancer Lett. 2020, 470, 204–219. [Google Scholar] [CrossRef]
- Lin, X.; Qiu, Y.; Song, L.; Chen, S.; Chen, X.; Huang, G.; Song, J.; Chen, X.; Yang, H. Ultrasound activation of liposomes for enhanced ultrasound imaging and synergistic gas and sonodynamic cancer therapy. Nanoscale Horiz. 2019, 4, 747–756. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yuan, C.; Li, M.; Wang, T.; Chen, B.; Jin, J.; Zhao, P.; Tong, J.; Luo, S. Magnetic nanoliposomes as in situ microbubble bombers for multimodality image-guided cancer theranostics. ACS Nano 2017, 11, 1509–1519. [Google Scholar] [CrossRef]
- Park, B.; Park, S.; Kim, J.; Kim, C. Listening to drug delivery and responses via photoacoustic imaging. Adv. Drug Deliv. Rev. 2022, 184, 114235. [Google Scholar] [CrossRef]
- Mathiyazhakan, M.; Wiraja, C.; Xu, C. A concise review of gold nanoparticles-based photo-responsive liposomes for controlled drug delivery. Nano-Micro Lett. 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Razansky, D.; Ntziachristos, V. Hybrid photoacoustic fluorescence molecular tomography using finite-element-based inversion. Med. Phys. 2007, 34, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 2010, 7, 603–614. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, G.; Lee, W.; Baek, S.; Jung, H.N.; Im, H.-J. Development of theranostic dual-layered Au-liposome for effective tumor targeting and photothermal therapy. J. Nanobiotechnology 2021, 19, 262. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.-J.; Du, X.; Wen, D. Photothermal conversion characteristics of gold nanoparticle dispersions. Sol. Energy 2014, 100, 141–147. [Google Scholar] [CrossRef]
- Goh, D.; Gong, T.; Dinish, U.; Maiti, K.K.; Fu, C.Y.; Yong, K.-T.; Olivo, M. Pluronic triblock copolymer encapsulated gold nanorods as biocompatible localized plasmon resonance-enhanced scattering probes for dark-field imaging of cancer cells. Plasmonics 2012, 7, 595–601. [Google Scholar] [CrossRef]
- Liopo, A.V.; Conjusteau, A.; Chumakova, O.V.; Ermilov, S.A.; Su, R.; Oraevsky, A.A. Highly purified biocompatible gold nanorods for contrasted optoacoustic imaging of small animal models. Nanosci. Nanotechnol. Lett. 2012, 4, 681–686. [Google Scholar] [CrossRef]
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011, 11, 2560–2566. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Huang, J.; Zhu, J. Synthesis and solar photo-thermal conversion of Au, Ag, and Au-Ag blended plasmonic nanoparticles. Energy Convers. Manag. 2016, 127, 293–300. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.F.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall black phosphorus quantum dots: Synthesis and use as photothermal agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; He, Y.; Zhu, J.; Kim, D.R. Enhancement of photo-thermal conversion using gold nanofluids with different particle sizes. Energy Convers. Manag. 2016, 112, 21–30. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. 2013, 125, 14208–14214. [Google Scholar] [CrossRef]
- Santos, G.M.; Zhao, F.; Zeng, J.; Shih, W.-C. Characterization of nanoporous gold disks for photothermal light harvesting and light-gated molecular release. Nanoscale 2014, 6, 5718–5724. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Dai, L.; Li, J.; He, Q. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl. Mater. Interfaces 2016, 8, 9610–9618. [Google Scholar] [CrossRef]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 2015, 5, 946. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yao, D.; Wang, Y.; Yang, W.; Zhang, B.; Wang, D. Enzyme-triggered self-assembly of gold nanoparticles for enhanced retention effects and photothermal therapy of prostate cancer. Chem. Commun. 2018, 54, 9841–9844. [Google Scholar] [CrossRef]
- Xu, C.; Chen, F.; Valdovinos, H.F.; Jiang, D.; Goel, S.; Yu, B.; Sun, H.; Barnhart, T.E.; Moon, J.J.; Cai, W. Bacteria-like mesoporous silica-coated gold nanorods for positron emission tomography and photoacoustic imaging-guided chemo-photothermal combined therapy. Biomaterials 2018, 165, 56–65. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, L.; Wang, G.; Yang, K.; Chen, M.; Tian, R.; Ma, Q.; Zhu, L. Hybrid graphene/Au activatable theranostic agent for multimodalities imaging guided enhanced photothermal therapy. Biomaterials 2016, 79, 36–45. [Google Scholar] [CrossRef]
- You, J.; Zhang, R.; Xiong, C.; Zhong, M.; Melancon, M.; Gupta, S.; Nick, A.M.; Sood, A.K.; Li, C. Effective Photothermal Chemotherapy Using Doxorubicin-Loaded Gold Nanospheres That Target EphB4 Receptors in TumorsConcerted Chemo-Photothermal Therapy Targeting EphB4. Cancer Res. 2012, 72, 4777–4786. [Google Scholar] [CrossRef]
- Sun, M.; Peng, D.; Hao, H.; Hu, J.; Wang, D.; Wang, K.; Liu, J.; Guo, X.; Wei, Y.; Gao, W. Thermally triggered in situ assembly of gold nanoparticles for cancer multimodal imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 10453–10460. [Google Scholar] [CrossRef]
- Askari, A.; Tajvar, S.; Nikkhah, M.; Mohammadi, S.; Hosseinkhani, S. Synthesis, characterization and in vitro toxicity evaluation of doxorubicin-loaded magnetoliposomes on MCF-7 breast cancer cell line. J. Drug Deliv. Sci. Technol. 2020, 55, 101447. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- German, S.; Navolokin, N.; Kuznetsova, N.; Zuev, V.; Inozemtseva, O.; Anis’Kov, A.; Volkova, E.; Bucharskaya, A.; Maslyakova, G.; Fakhrullin, R. Liposomes loaded with hydrophilic magnetite nanoparticles: Preparation and application as contrast agents for magnetic resonance imaging. Colloids Surf. B Biointerfaces 2015, 135, 109–115. [Google Scholar] [CrossRef]
- Mueller, S. Magnetic fluid hyperthermia therapy for malignant brain tumors—An ethical discussion. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Can, H.K.; Kavlak, S.; ParviziKhosroshahi, S.; Güner, A. Preparation, characterization and dynamical mechanical properties of dextran-coated iron oxide nanoparticles (DIONPs). Artif. Cells Nanomed. Biotechnol. 2018, 46, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.; Lung, I.; Soran, M.-L.; Opris, O.; Leostean, C.; Popa, A.; Copaciu, F.; Lazar, M.D.; Kacso, I.; Silipas, T.-D. Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. J. Taiwan Inst. Chem. Eng. 2019, 100, 65–73. [Google Scholar] [CrossRef]
- Herea, D.; Chiriac, H.; Lupu, N.; Grigoras, M.; Stoian, G.; Stoica, B.; Petreus, T. Study on iron oxide nanoparticles coated with glucose-derived polymers for biomedical applications. Appl. Surf. Sci. 2015, 352, 117–125. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Khmara, I.; Strbak, O.; Zavisova, V.; Koneracka, M.; Kubovcikova, M.; Antal, I.; Kavecansky, V.; Lucanska, D.; Dobrota, D.; Kopcansky, P. Chitosan-stabilized iron oxide nanoparticles for magnetic resonance imaging. J. Magn. Magn. Mater. 2019, 474, 319–325. [Google Scholar] [CrossRef]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Ramírez-García, G.; Banu, N.; Vallejo-Cardona, A.A.; Lugo-Fabres, P.; Camacho-Villegas, T.A.; Salas, P.; De la Rosa, E. Ligand-targeted Theranostic Liposomes combining Methylene Blue attached Upconversion nanoparticles for NIR activated Bioimaging and Photodynamic therapy against HER-2 positive breast cancer. J. Lumin. 2021, 237, 118143. [Google Scholar] [CrossRef]
- Hamblin, M.R. Upconversion in photodynamic therapy: Plumbing the depths. Dalton Trans. 2018, 47, 8571–8580. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-L.; Chang, C.A. Optimising FRET-efficiency of Nd 3+-sensitised upconversion nanocomposites by shortening the emitter–photosensitizer distance. Nanoscale 2020, 12, 8742–8749. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Y.; Jiang, Y.; Wu, Y.; Cui, C.; Wang, Y.; Zhang, L.; Teng, I.-T.; Tan, W. Aptamer-based multifunctional ligand-modified UCNPs for targeted PDT and bioimaging. Nanoscale 2018, 10, 10986–10990. [Google Scholar] [CrossRef]
- Feng, L.; He, F.; Liu, B.; Yang, G.; Gai, S.; Yang, P.; Li, C.; Dai, Y.; Lv, R.; Lin, J. g-C3N4 coated upconversion nanoparticles for 808 nm near-infrared light triggered phototherapy and multiple imaging. Chem. Mater. 2016, 28, 7935–7946. [Google Scholar] [CrossRef]
- Liao, G.; He, F.; Li, Q.; Zhong, L.; Zhao, R.; Che, H.; Gao, H.; Fang, B. Emerging graphitic carbon nitride-based materials for biomedical applications. Prog. Mater. Sci. 2020, 112, 100666. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Panikar, S.S.; Ramírez-García, G.; Vallejo-Cardona, A.A.; Banu, N.; Patrón-Soberano, O.A.; Cialla-May, D.; Camacho-Villegas, T.A.; de la Rosa, E. Novel anti-HER2 peptide-conjugated theranostic nanoliposomes combining NaYF 4: Yb, Er nanoparticles for NIR-activated bioimaging and chemo-photodynamic therapy against breast cancer. Nanoscale 2019, 11, 20598–20613. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Yadav, A.S.; Gorain, M.; Chauhan, D.S.; Kundu, G.C.; Srivastava, R.; Selvaraj, K. Graphene oxide supported liposomes as red emissive theranostics for phototriggered tissue visualization and tumor regression. ACS Appl. Bio Mater. 2019, 2, 3312–3320. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Aiyer, S.; Chauhan, D.S.; Srivastava, R.; Selvaraj, K. Bioresponsive carbon nano-gated multifunctional mesoporous silica for cancer theranostics. Nanoscale 2016, 8, 4537–4546. [Google Scholar] [CrossRef] [PubMed]
- Jaimes-Aguirre, L.; Morales-Avila, E.; Ocampo-García, B.E.; Medina, L.A.; López-Téllez, G.; Gibbens-Bandala, B.V.; Izquierdo-Sánchez, V. Biodegradable poly (D, L-lactide-co-glycolide)/poly (L-γ-glutamic acid) nanoparticles conjugated to folic acid for targeted delivery of doxorubicin. Mater. Sci. Eng. C 2017, 76, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2014, 4, 81. [Google Scholar] [CrossRef]
- Lim, C.-K.; Shin, J.; Kwon, I.C.; Jeong, S.Y.; Kim, S. Iodinated photosensitizing chitosan: Self-assembly into tumor-homing nanoparticles with enhanced singlet oxygen generation. Bioconjug. Chem. 2012, 23, 1022–1028. [Google Scholar] [CrossRef]
- Kim, S.; Ohulchanskyy, T.Y.; Bharali, D.; Chen, Y.; Pandey, R.K.; Prasad, P.N. Organically modified silica nanoparticles with intraparticle heavy-atom effect on the encapsulated photosensitizer for enhanced efficacy of photodynamic therapy. J. Phys. Chem. C 2009, 113, 12641–12644. [Google Scholar] [CrossRef]
- Lim, C.K.; Shin, J.; Lee, Y.D.; Kim, J.; Park, H.; Kwon, I.C.; Kim, S. Heavy-Atomic Construction of Photosensitizer Nanoparticles for Enhanced Photodynamic Therapy of Cancer. Small 2011, 7, 112–118. [Google Scholar] [CrossRef]
- Dai, Y.; Su, J.; Wu, K.; Ma, W.; Wang, B.; Li, M.; Sun, P.; Shen, Q.; Wang, Q.; Fan, Q. Multifunctional thermosensitive liposomes based on natural phase-change material: Near-infrared light-triggered drug release and multimodal imaging-guided cancer combination therapy. ACS Appl. Mater. Interfaces 2019, 11, 10540–10553. [Google Scholar] [CrossRef]
- Zheng, X.; Xing, D.; Zhou, F.; Wu, B.; Chen, W.R. Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol. Pharm. 2011, 8, 447–456. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, F.; Wu, B.; Chen, W.R.; Xing, D. Enhanced tumor treatment using biofunctional indocyanine green-containing nanostructure by intratumoral or intravenous injection. Mol. Pharm. 2012, 9, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-J.; Lee, H.-S.; Lim, J.-Y.; Park, J.-H. Liposomal indocyanine green for enhanced photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 5683–5691. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Raczka, E.; Piehler, L.; Lee, I.; Myc, A.; Majoros, I.; Patri, A.K.; Thomas, T.; Mulé, J.; Baker, J.R. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharm. Res. 2002, 19, 1310–1316. [Google Scholar] [CrossRef]
- Reddy, J.; Allagadda, V.; Leamon, C. Targeting therapeutic and imaging agents to folate receptor positive tumors. Curr. Pharm. Biotechnol. 2005, 6, 131–150. [Google Scholar] [CrossRef]
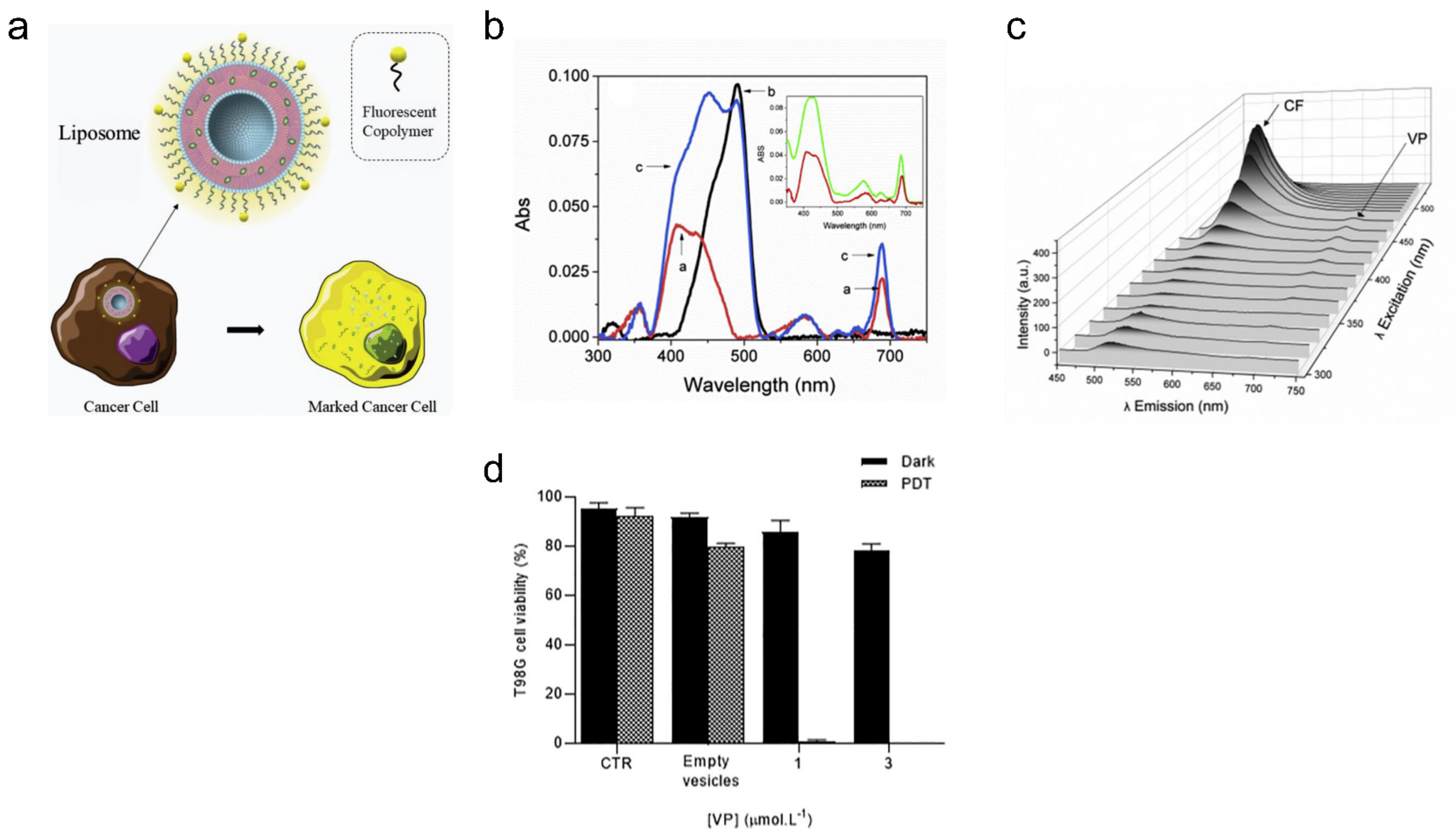
- de Oliveira, D.C.S.; de Freitas, C.F.; Calori, I.R.; Goncalves, R.S.; Cardinali, C.A.E.F.; Malacarne, L.C.; Ravanelli, M.I.; de Oliveira, H.P.M.; Tedesco, A.C.; Caetano, W. Theranostic verteporfin-loaded lipid-polymer liposome for photodynamic applications. J. Photochem. Photobiol. B Biol. 2020, 212, 112039. [Google Scholar] [CrossRef] [PubMed]
- Al-Moujahed, A.; Brodowska, K.; Stryjewski, T.P.; Efstathiou, N.E.; Vasilikos, I.; Cichy, J.; Miller, J.W.; Gragoudas, E.; Vavvas, D.G. Verteporfin inhibits growth of human glioma in vitro without light activation. Sci. Rep. 2017, 7, 7602. [Google Scholar] [CrossRef] [PubMed]
- Mordon, S.; Devoisselle, J.M.; Maunoury, V. In vivo pH measurement and imaging of tumor tissue using a pH-sensitive fluorescent probe (5, 6–Carboxyfluorescein): Instrumental and experimental studies. Photochem. Photobiol. 1994, 60, 274–279. [Google Scholar] [CrossRef]
- Magin, R.L.; Weinstein, J.N. The Design and Characterization of Temperature-Sensitive Liposomes. Liposome Technology, 1st ed.; Gregoriadis, G., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 137–155. [Google Scholar]
- de Freitas, C.F.; Calori, I.R.; da Silva, A.C.P.; de Castro, L.V.; Sato, F.; Pellosi, D.S.; Tessaro, A.L.; Caetano, W.; Hioka, N. PEG-coated vesicles from Pluronic/lipid mixtures for the carrying of photoactive erythrosine derivatives. Colloids Surf. B Biointerfaces 2019, 175, 530–544. [Google Scholar] [CrossRef]
- Sonkar, R.; Jha, A.; Viswanadh, M.K.; Burande, A.S.; Pawde, D.M.; Patel, K.K.; Singh, M.; Koch, B.; Muthu, M.S. Gold liposomes for brain-targeted drug delivery: Formulation and brain distribution kinetics. Mater. Sci. Eng. C 2021, 120, 111652. [Google Scholar] [CrossRef]
- Muthu, M.S.; Kutty, R.V.; Luo, Z.; Xie, J.; Feng, S.-S. Theranostic vitamin E TPGS micelles of transferrin conjugation for targeted co-delivery of docetaxel and ultra bright gold nanoclusters. Biomaterials 2015, 39, 234–248. [Google Scholar] [CrossRef]
- Sonali; Agrawal, P.; Singh, R.P.; Rajesh, C.V.; Singh, S.; Vijayakumar, M.R.; Pandey, B.L.; Muthu, M.S. Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: Preparation, characterization and brain distribution in rats. Drug Deliv. 2016, 23, 1788–1798. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharma, G.; Agrawal, P.; Pandey, B.L.; Koch, B.; Muthu, M.S. Transferrin receptor targeted PLA-TPGS micelles improved efficacy and safety in docetaxel delivery. Int. J. Biol. Macromol. 2016, 83, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Singh, R.P.; Sharma, G.; Mehata, A.K.; Singh, S.; Rajesh, C.V.; Pandey, B.L.; Koch, B.; Muthu, M.S. Bioadhesive micelles of d-α-tocopherol polyethylene glycol succinate 1000: Synergism of chitosan and transferrin in targeted drug delivery. Colloids Surf. B Biointerfaces 2017, 152, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Viswanadh, M.K.; Singh, R.P.; Agrawal, P.; Mehata, A.K.; Pawde, D.M.; Sonkar, R.; Muthu, M.S. Nanotheranostics: Emerging strategies for early diagnosis and therapy of brain cancer. Nanotheranostics 2018, 2, 70. [Google Scholar]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics˗ application and further development of nanomedicine strategies for advanced theranostics. Theranostics 2014, 4, 660. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Feng, S.-S. Theranostic Liposomes for Cancer Diagnosis and Treatment: CURRENT Development and Pre-Clinical Success; Taylor & Francis: Abingdon, UK, 2013; Volume 10, pp. 151–155. [Google Scholar]
- Muthu, M.S.; Sahu, A.K.; Sonali; Abdulla, A.; Kaklotar, D.; Rajesh, C.V.; Singh, S.; Pandey, B.L. Solubilized delivery of paliperidone palmitate by D-alpha-tocopheryl polyethylene glycol 1000 succinate micelles for improved short-term psychotic management. Drug Deliv. 2016, 23, 230–237. [Google Scholar] [CrossRef]
- Jha, A.; Viswanadh, M.K.; Burande, A.S.; Mehata, A.K.; Poddar, S.; Yadav, K.; Mahto, S.K.; Parmar, A.S.; Muthu, M.S. DNA biodots based targeted theranostic nanomedicine for the imaging and treatment of non-small cell lung cancer. Int. J. Biol. Macromol. 2020, 150, 413–425. [Google Scholar] [CrossRef]
- Rani, D.; Somasundaram, V.H.; Nair, S.; Koyakutty, M. Advances in cancer nanomedicine. J. Indian Inst. Sci. 2012, 92, 187–218. [Google Scholar]
- Kuang, L.; Cao, S.-P.; Zhang, L.; Li, Q.-H.; Liu, Z.-C.; Liang, R.-P.; Qiu, J.-D. A novel nanosensor composed of aptamer bio-dots and gold nanoparticles for determination of thrombin with multiple signals. Biosens. Bioelectron. 2016, 85, 798–806. [Google Scholar] [CrossRef]
- Karpuz, M.; Silindir-Gunay, M.; Ozer, A.Y.; Ozturk, S.C.; Yanik, H.; Tuncel, M.; Aydin, C.; Esendagli, G. Diagnostic and therapeutic evaluation of folate-targeted paclitaxel and vinorelbine encapsulating theranostic liposomes for non-small cell lung cancer. Eur. J. Pharm. Sci. 2021, 156, 105576. [Google Scholar] [CrossRef]
- Jung, M.; Grunberg, S.; Timblin, C.; Buder-Hoffman, S.; Vacek, P.; Taatjes, D.J.; Mossman, B.T. Paclitaxel and vinorelbine cause synergistic increases in apoptosis but not in microtubular disruption in human lung adenocarcinoma cells (A-549). Histochem. Cell Biol. 2004, 121, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Huerter, M.M.; Meza, J.L.; Copur, M.S.; Tolentino, A.; Marr, A.S.; Ketcham, M.; DeSpiegelaere, H.; Kruse, S.; Kos, M.E.; Swenson, K. Weekly vinorelbine and paclitaxel in older patients with advanced non-small cell lung cancer: A phase II Fred and Pamela Buffet Cancer Center Clinical Trials Network study. J. Geriatr. Oncol. 2017, 8, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zwicke, G.L.; Ali Mansoori, G.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef]
- Rathmann, S.M.; Ahmad, Z.; Slikboer, S.; Bilton, H.A.; Snider, D.P.; Valliant, J.F. The Radiopharmaceutical Chemistry of Technetium-99m. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 311–333. [Google Scholar] [CrossRef]
- Wang, X.; Tong, J.; He, Z.; Yang, X.; Meng, F.; Liang, H.; Zhang, X.; Luo, L. Paclitaxel-potentiated photodynamic theranostics for synergistic tumor ablation and precise anticancer efficacy monitoring. ACS Appl. Mater. Interfaces 2020, 12, 5476–5487. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, X.; He, X.; He, Z.; Yang, X.; Tian, S.; Meng, F.; Ding, D.; Luo, L.; Tang, B.Z. A dual-functional photosensitizer for ultraefficient photodynamic therapy and synchronous anticancer efficacy monitoring. Adv. Funct. Mater. 2019, 29, 1902673. [Google Scholar] [CrossRef]
- Lan, Y.-Q.; Kong, L.-J.; Lin, X.-Y.; Xu, Q.; Gao, X.-Y.; Wu, R.-P.; Wang, X.-L.; Zhong, D.-T. Combination chemotherapy with paclitaxel and oxaliplatin as first-line treatment in patients with advanced gastric cancer. Cancer Chemother. Pharmacol. 2018, 81, 1007–1015. [Google Scholar] [CrossRef]
- Yoshimura, A.; Chihara, Y.; Date, K.; Tamiya, N.; Takemura, Y.; Imabayashi, T.; Kaneko, Y.; Yamada, T.; Ueda, M.; Arimoto, T. A phase II study of S-1 and paclitaxel combination therapy as a first-line treatment in elderly patients with advanced non-small cell lung cancer. Oncologist 2019, 24, 459-e131. [Google Scholar] [CrossRef]
- Sandercock, J.; Parmar, M.; Torri, V.; Qian, W. First-line treatment for advanced ovarian cancer: Paclitaxel, platinum and the evidence. Br. J. Cancer 2002, 87, 815–824. [Google Scholar] [CrossRef]
- Ye, S.; Rao, J.; Qiu, S.; Zhao, J.; He, H.; Yan, Z.; Yang, T.; Deng, Y.; Ke, H.; Yang, H. Rational design of conjugated photosensitizers with controllable photoconversion for dually cooperative phototherapy. Adv. Mater. 2018, 30, 1801216. [Google Scholar] [CrossRef]
- An, X.; Zhu, A.; Luo, H.; Ke, H.; Chen, H.; Zhao, Y. Rational design of multi-stimuli-responsive nanoparticles for precise cancer therapy. Acs Nano 2016, 10, 5947–5958. [Google Scholar] [CrossRef]
- An, W.; Hwang, S.; Trepel, J.; Blagosklonny, M. Protease inhibitor-induced apoptosis: Accumulation of wt p53, p21WAF1/CIP1, and induction of apoptosis are independent markers of proteasome inhibition. Leukemia 2000, 14, 1276–1283. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, S.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Water-Soluble Tetraphenylethene Derivatives as Fluorescent “Light-Up” Probes for Nucleic Acid Detection and Their Applications in Cell Imaging. Chem. Asian J. 2013, 8, 1806–1812. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Zheng, Z.; Ye, R.; Zhang, Y.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. In situ monitoring apoptosis process by a self-reporting photosensitizer. J. Am. Chem. Soc. 2019, 141, 5612–5616. [Google Scholar] [CrossRef]
- Youden, B.; Wang, F.; Zhang, X.; Curry, D.; Majtenyi, N.; Shaaer, A.; Bingham, K.; Nguyen, Q.; Bragg, L.; Liu, J. Degradable multifunctional gold-liposomes as an all-in-one theranostic platform for image-guided radiotherapy. Int. J. Pharm. 2022, 629, 122413. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Airan, R.; Han, Z.; Xu, J.; Chan, K.W.; Xu, Y.; Bulte, J.W.; Van Zijl, P.C.; McMahon, M.T. CT and CEST MRI bimodal imaging of the intratumoral distribution of iodinated liposomes. Quant. Imaging Med. Surg. 2019, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Delama, A.; Teixeira, M.I.; Dorati, R.; Genta, I.; Conti, B.; Lamprou, D.A. Microfluidic encapsulation method to produce stable liposomes containing iohexol. J. Drug Deliv. Sci. Technol. 2019, 54, 101340. [Google Scholar] [CrossRef]
- Mukundan, S., Jr.; Ghaghada, K.B.; Badea, C.T.; Kao, C.-Y.; Hedlund, L.W.; Provenzale, J.M.; Johnson, G.A.; Chen, E.; Bellamkonda, R.V.; Annapragada, A. A liposomal nanoscale contrast agent for preclinical CT in mice. Am. J. Roentgenol. 2006, 186, 300–307. [Google Scholar] [CrossRef]
- Zheng, J.; Perkins, G.; Kirilova, A.; Allen, C.; Jaffray, D.A. Multimodal contrast agent for combined computed tomography and magnetic resonance imaging applications. Investig. Radiol. 2006, 41, 339–348. [Google Scholar] [CrossRef]
- Chen, C.; Gao, K.; Lian, H.; Chen, C.; Yan, X. Single-particle characterization of theranostic liposomes with stimulus sensing and controlled drug release properties. Biosens. Bioelectron. 2019, 131, 185–192. [Google Scholar] [CrossRef]
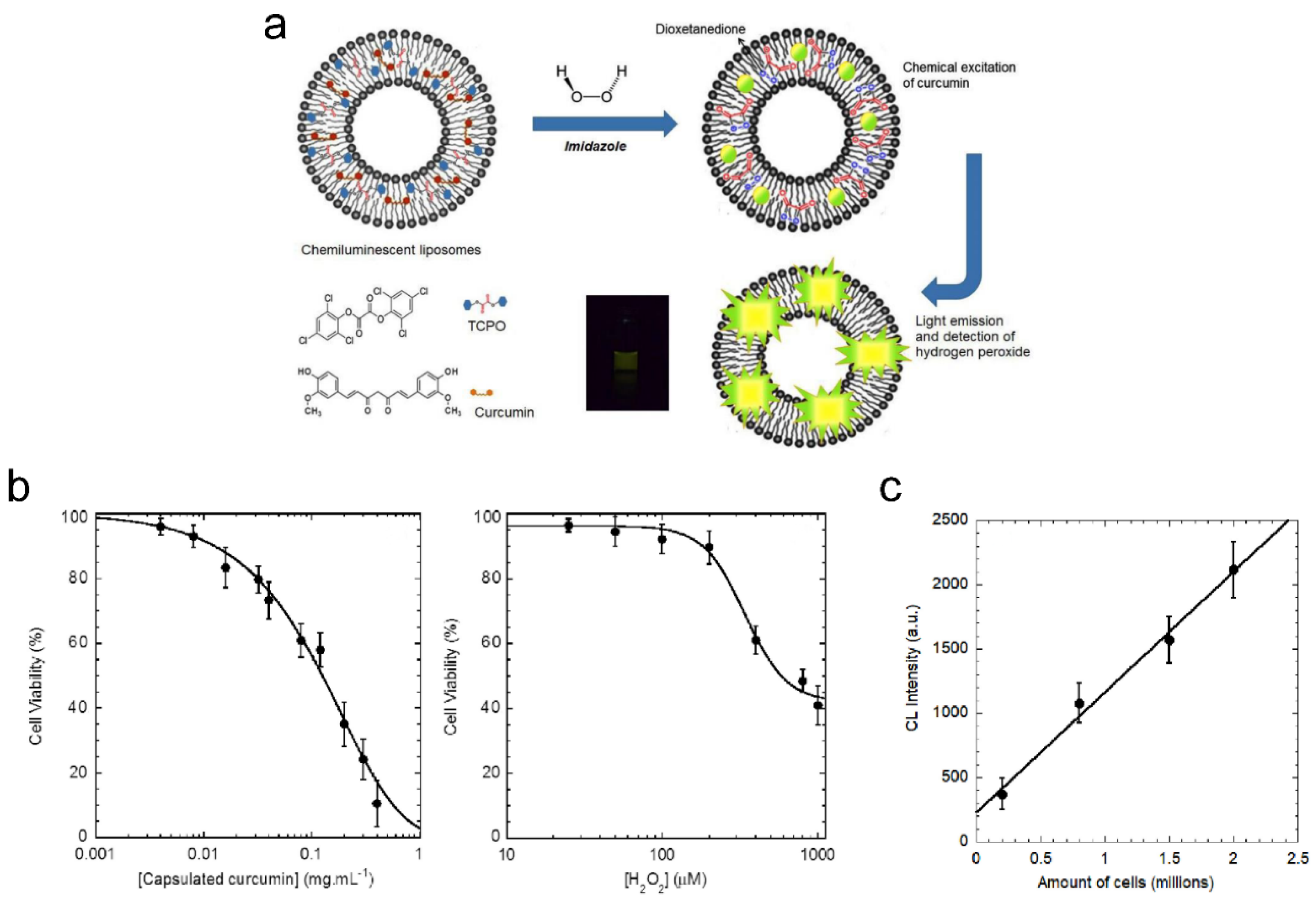
- Mohammadi, S.S.; Vaezi, Z.; Shojaedin-Givi, B.; Naderi-Manesh, H. Chemiluminescent liposomes as a theranostic carrier for detection of tumor cells under oxidative stress. Anal. Chim. Acta 2019, 1059, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.Y.; Abedirad, S.M.; Vaezi, Z.; Ganjali, M.R. A study of chemiluminescence characteristics of a novel peroxyoxalate system using berberine as the fluorophore. Dye. Pigment. 2012, 95, 751–756. [Google Scholar] [CrossRef]
- Chaichi, M.J.; VAAEZI, Z.; Hosseini, M.; Hosseinkhani, S.; Shamsipur, M. The study of chemiluminescence of acridinium ester in presence of rhodamin B as a fluorescer. Iran. J. Chem. Chem. Eng. 2011, 30, 89–96. [Google Scholar]
- Ruby, A.J.; Kuttan, G.; Babu, K.D.; Rajasekharan, K.; Kuttan, R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, P.; Scapin, C.; Vitadello, M.; Florean, C.; Gorza, L. Grp94 acts as a mediator of curcumin-induced antioxidant defence in myogenic cells. J. Cell. Mol. Med. 2010, 14, 970–981. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Yardley, D.A.; Burris, H.A., III; Spigel, D.R.; Clark, B.L.; Vazquez, E.; Shipley, D.; Barton, J.; Thompson, D.; Montes, I.; Greco, F.A. A phase II randomized crossover study of liposomal doxorubicin versus weekly docetaxel in the first-line treatment of women with metastatic breast cancer. Clin. Breast Cancer 2009, 9, 247–252. [Google Scholar] [CrossRef]
- Rochlitz, C.; Ruhstaller, T.; Lerch, S.; Spirig, C.; Huober, J.; Suter, T.; Bühlmann, M.; Fehr, M.; Schönenberger, A.; von Moos, R. Combination of bevacizumab and 2-weekly pegylated liposomal doxorubicin as first-line therapy for locally recurrent or metastatic breast cancer. A multicenter, single-arm phase II trial (SAKK 24/06). Ann. Oncol. 2011, 22, 80–85. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Bekaii-Saab, T.; Boland, P.M.; Dayyani, F.; Macarulla, T.; Mody, K.; Belanger, B.; Maxwell, F.; Moore, Y.; Thiagalingam, A. First-line liposomal irinotecan with oxaliplatin, 5-fluorouracil and leucovorin (NALIRIFOX) in pancreatic ductal adenocarcinoma: A phase I/II study. Eur. J. Cancer 2021, 151, 14–24. [Google Scholar] [CrossRef]
- Brendel, K.; Bekaii-Saab, T.; Boland, P.M.; Dayyani, F.; Dean, A.; Macarulla, T.; Maxwell, F.; Mody, K.; Pedret-Dunn, A.; Wainberg, Z.A. Population pharmacokinetics of liposomal irinotecan in patients with cancer and exposure–safety analyses in patients with metastatic pancreatic cancer. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 1550–1563. [Google Scholar] [CrossRef]
- Fishman, M.; Elsayed, Y.; Damjanov, N.; Steinberg, J.; Mahany, J.; Nieves, J.; Wanaski, S.; Dul, J.; Sherman, J. Phase I study of liposome entrapped paclitaxel (LEP-ETU) in patients with advanced cancer. J. Clin. Oncol. 2004, 22, 2110. [Google Scholar] [CrossRef]
- Slingerland, M.; Guchelaar, H.-J.; Rosing, H.; Scheulen, M.E.; van Warmerdam, L.J.; Beijnen, J.H.; Gelderblom, H. Bioequivalence of Liposome-Entrapped Paclitaxel Easy-To-Use (LEP-ETU) formulation and paclitaxel in polyethoxylated castor oil: A randomized, two-period crossover study in patients with advanced cancer. Clin. Ther. 2013, 35, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Papadopoulos, K.P.; Patnaik, A.; Rasco, D.W.; Martinez, D.; Wood, D.L.; Fielman, B.; Sharma, M.; Janisch, L.A.; Brown, B.D. Safety and Activity of DCR-MYC, a First-in-Class Dicer-Substrate Small Interfering RNA (DsiRNA) Targeting MYC, in a Phase I Study in Patients with Advanced Solid Tumors; American Society of Clinical Oncology: Alexandria, VA, USA, 2015. [Google Scholar]
- Chang, S.; Herse, Z.; Claxton, D.; Giffon, T.; Lewis, D.; Fairman, J. Immunotherapy of Acute Leukemia with Cationic Lipid DNA Complexes (JVRS-100); Wiley Online Library: Hoboken, NJ, USA, 2008. [Google Scholar]
- Celsion. Phase 3 Study of ThermoDox with Radiofrequency Ablation (RFA) in Treatment of Hepatocellular Carcinoma (HCC); National Library of Medicine: Bethesda, MD, USA, 2011. Available online: https://clinicaltrials.gov/ (accessed on 4 August 2023).
- Tuxen, M.K.; Cold, S.; Tange, U.B.; Balslev, E.; Nielsen, D.L. Phase II study of neoadjuvant pegylated liposomal doxorubicin and cyclophosphamide±trastuzumab followed by docetaxel in locally advanced breast cancer. Acta Oncol. 2014, 53, 1440–1445. [Google Scholar] [CrossRef]
- Koukouraki, K.S.; Giatromanolaki, A.; Kakolyris, S.; Georgoulias, V.; Velidaki, A.; Archimandritis, S.; Nikolaos, N. Karkavitsas, M.I. High intratumoral accumulation of stealth liposomal doxorubicin in sarcomas: Rationale for combination with radiotherapy. Acta Oncol. 2000, 39, 207–211. [Google Scholar]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef] [PubMed]




| Treatment | Advantages | Disadvantages |
|---|---|---|
| chemotherapy | cytostatic and cytotoxic abilities | acute side effects and high risk of recurrences |
| gene therapy | low risk of immunogenicity and high stability when loaded in NPs more direct inhibition of cell division of tumor cells | can cause changes to healthy genes due to off-targeting even at low doses, which can have generational effects |
| immunotherapy | fewer side effects because the therapy only targets the immune system immunological memory can reduce recurrence of cancer | effective for only certain cancers with high expressions of immunosuppressive proteins |
| photothermal therapy | strongly localized heating high tumor destruction efficiency using high temperatures within short periods of time | primarily used for superficial malignant tumors |
| photodynamic therapy | increased tissue penetration depth low light fluence rate increases selective apoptosis of tumor cells | tissue oxygenation is important for photodynamic effect photosensitivity reactions due to the use of PSs |
| magneto-thermal therapy | toxicity limited and side-effects reduced due to low doses of nanoparticles deep tissue penetration for treatment of malignant tumors | less control of local tumor temperature and greater damage to healthy tissue due to size of coils |
| ultrasound responsive therapy | deep tissue penetration with real-time imaging capability due to readily available ultrasound imaging systems | the different types of NPs/bubbles are limited due to the acoustic properties required |
| radiotherapy | lowers risk of recurrence and distant metastases due to deeper penetration of ionizing radiation | radiotoxicity due to the inclusion of RSs and ionizing radiation |
| Technique(s) | Penetration Depth | Advantages | Limitations |
|---|---|---|---|
| PET/SPECT | no limit | non-invasive, high sensitivity and can carry out quantitative analysis | exposure to ionizing radiation and relatively low spatial resolution |
| CT | no limit | high contrast and spatial resolution | relatively high dose of ionizing radiation and exposure to ionizing radiation |
| MRI | no limit | non-invasive and high spatial resolution | relatively low sensitivity, high cost and slow acquisition and post processing |
| OI | <1 cm | non-invasive, no harmful effect by non-ionizing radiation, multicolor capability, and fast acquisition and post processing | relatively low spatial resolution |
| USI and PAI | millimeters to centimeters | non-invasive, real time, low cost, and no harmful effect by non-ionizing radiation | limited spatial resolution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, D.A. Liposomes for Cancer Theranostics. Pharmaceutics 2023, 15, 2448. https://doi.org/10.3390/pharmaceutics15102448
Fernandes DA. Liposomes for Cancer Theranostics. Pharmaceutics. 2023; 15(10):2448. https://doi.org/10.3390/pharmaceutics15102448
Chicago/Turabian StyleFernandes, Donald A. 2023. "Liposomes for Cancer Theranostics" Pharmaceutics 15, no. 10: 2448. https://doi.org/10.3390/pharmaceutics15102448





