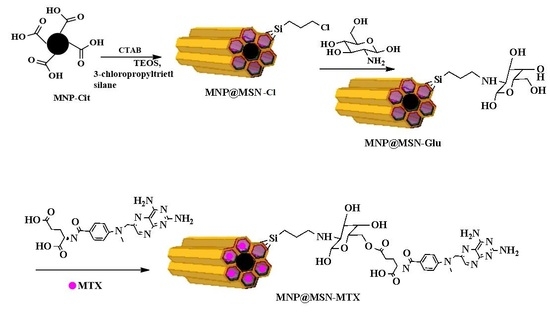
Glucosamine-Modified Mesoporous Silica-Coated Magnetic Nanoparticles: A “Raisin-Cake”-like Structure as an Efficient Theranostic Platform for Targeted Methotrexate Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Super-Paramagnetic Iron Oxide Nanoparticles (MNP)
2.3. Synthesis of Glucosamine-Modified Mesoporous Silica-Coated MNP (MNP@MSN-Glu)
2.4. Conjugation of MTX to MNP@MSN-Glu (MNP@MSN-MTX)
2.5. Rhodamine Conjugation to MNP@MSN-Glu (MNP@MSN-Rhod)
2.6. MTX Release Profile
2.7. Cell Lines and Cell Culture
2.8. MTT Assay
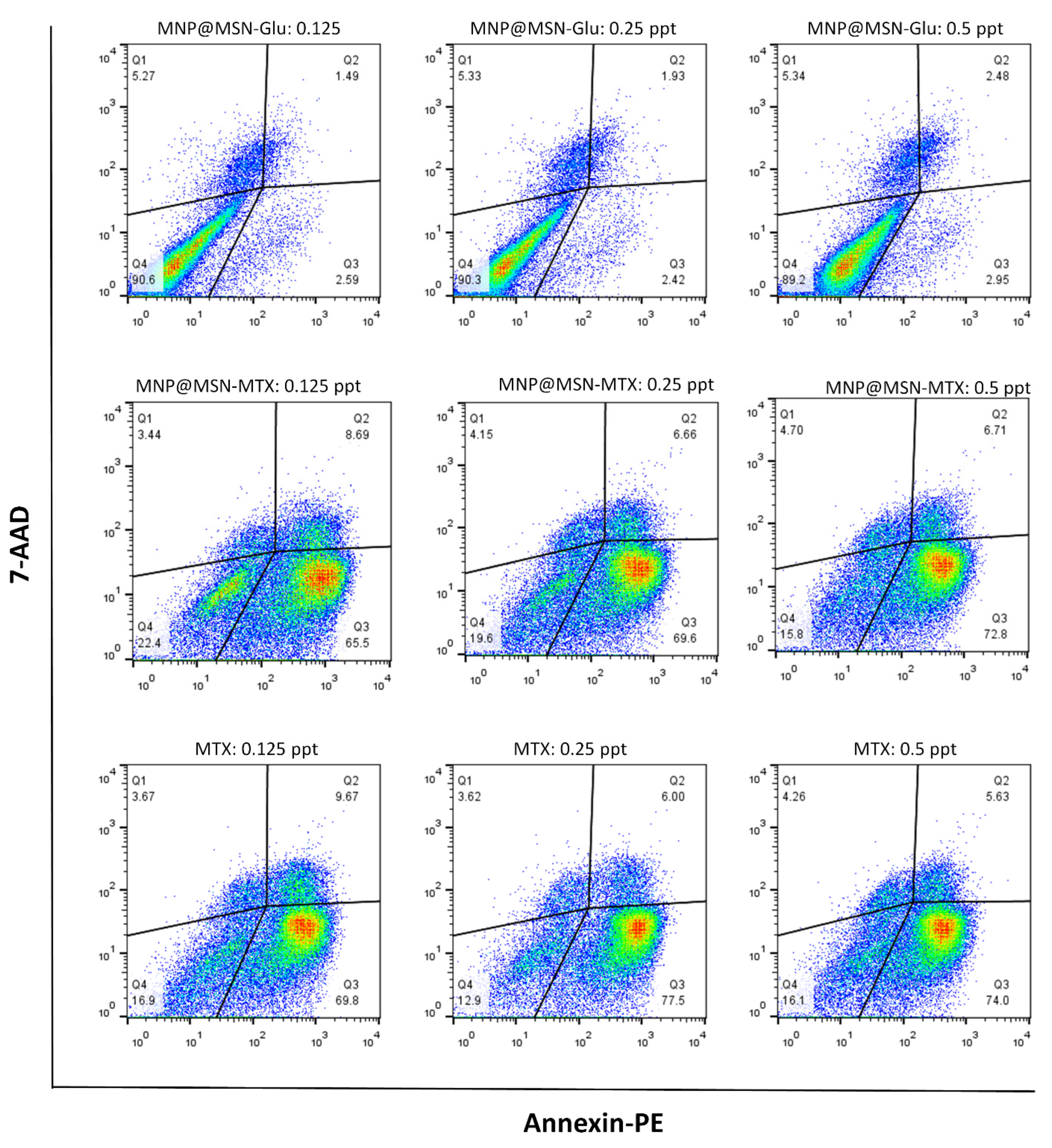
2.9. Apoptosis Assay
2.10. Drug Uptake by 4T1 Cells
2.11. Establishment of an Orthotopic Mouse 4T1 Breast Tumor Model
2.12. MRI Imaging
3. Results
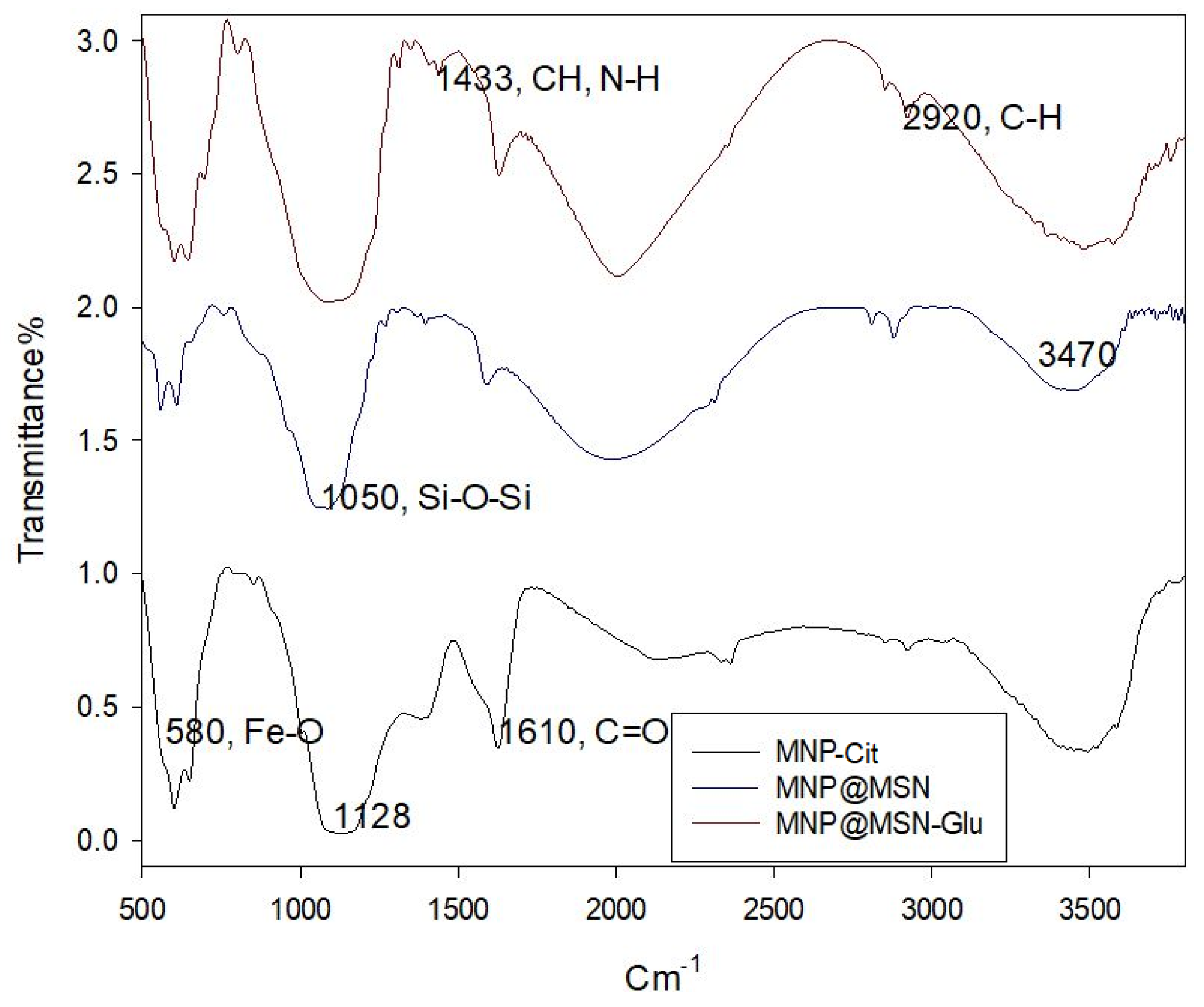
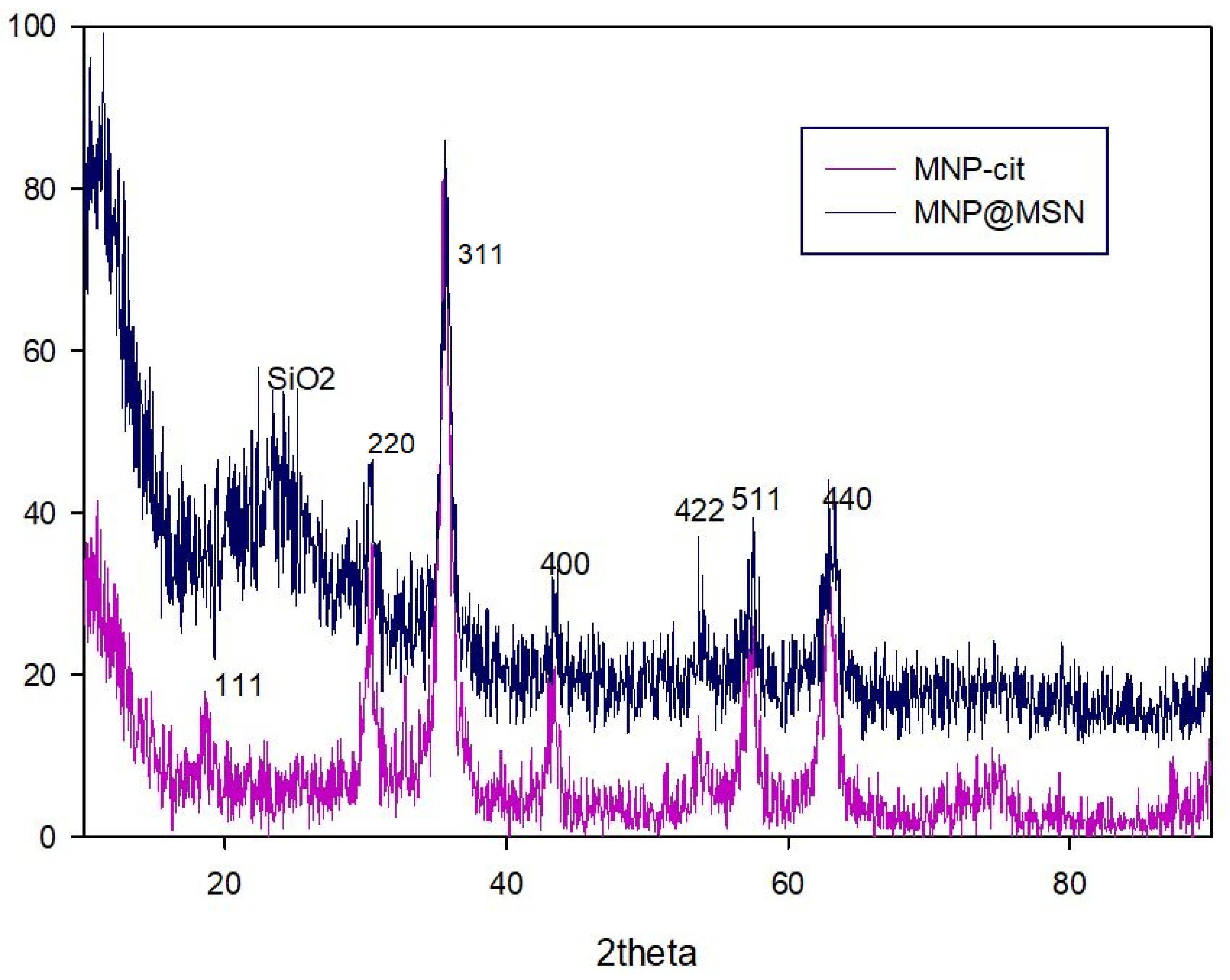
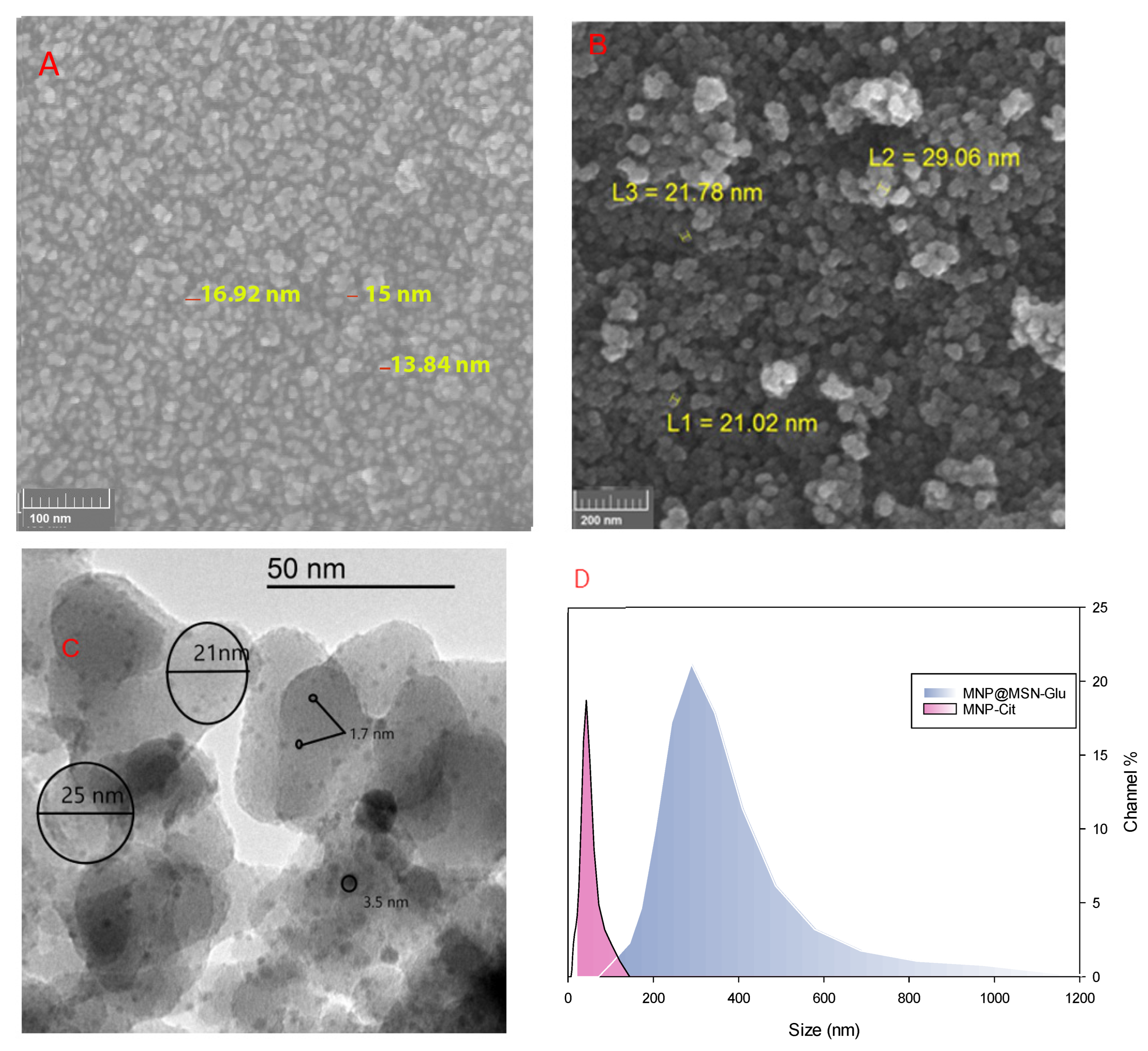
3.1. Synthesis and Characterization of MNP@MSN-MTX
3.2. MTX Conjugation and Release Profile
3.3. Cellular Uptake
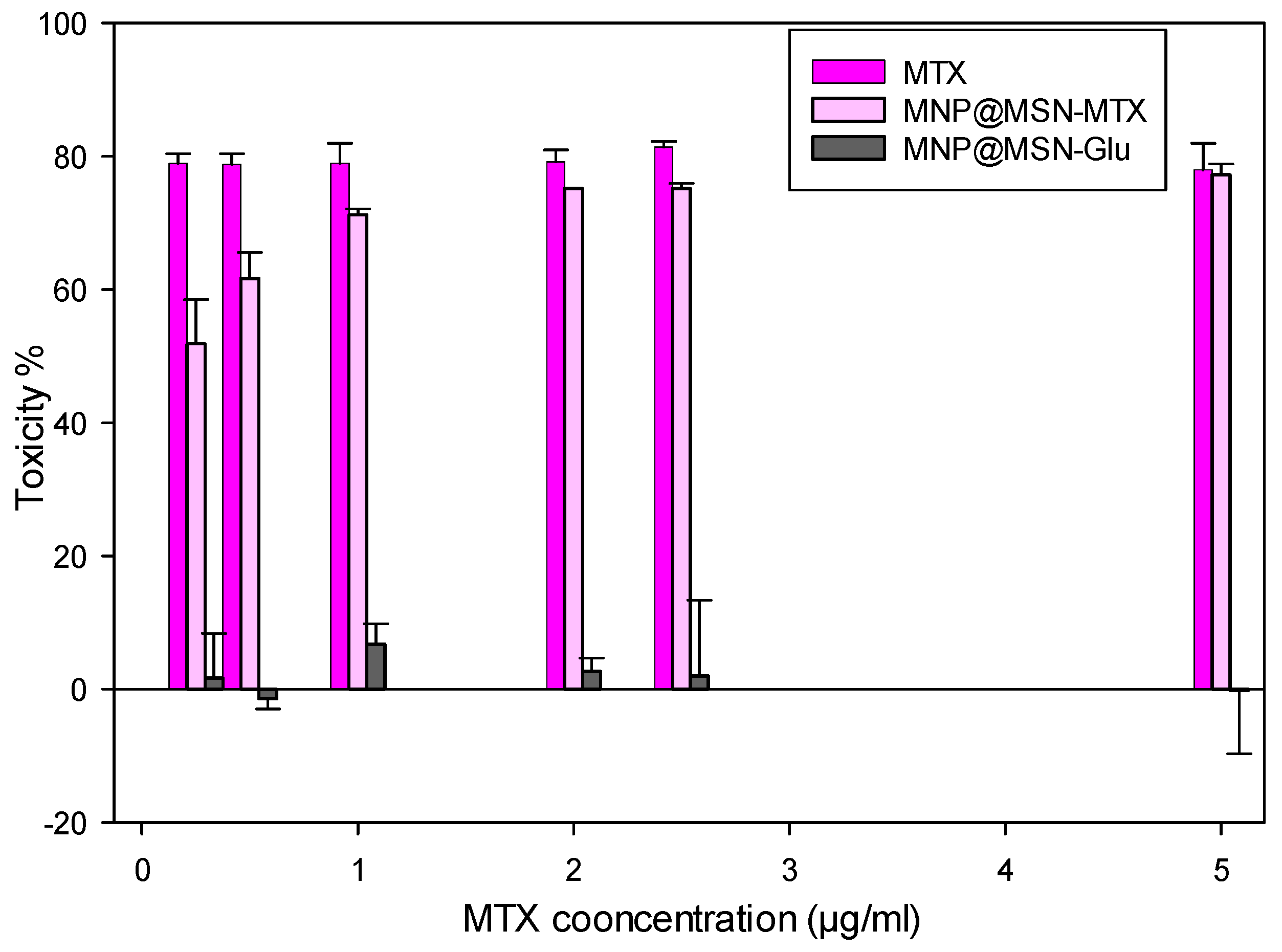
3.4. Cytotoxic Effect of MNP@MSN-Glu on 4T1 Cell Line
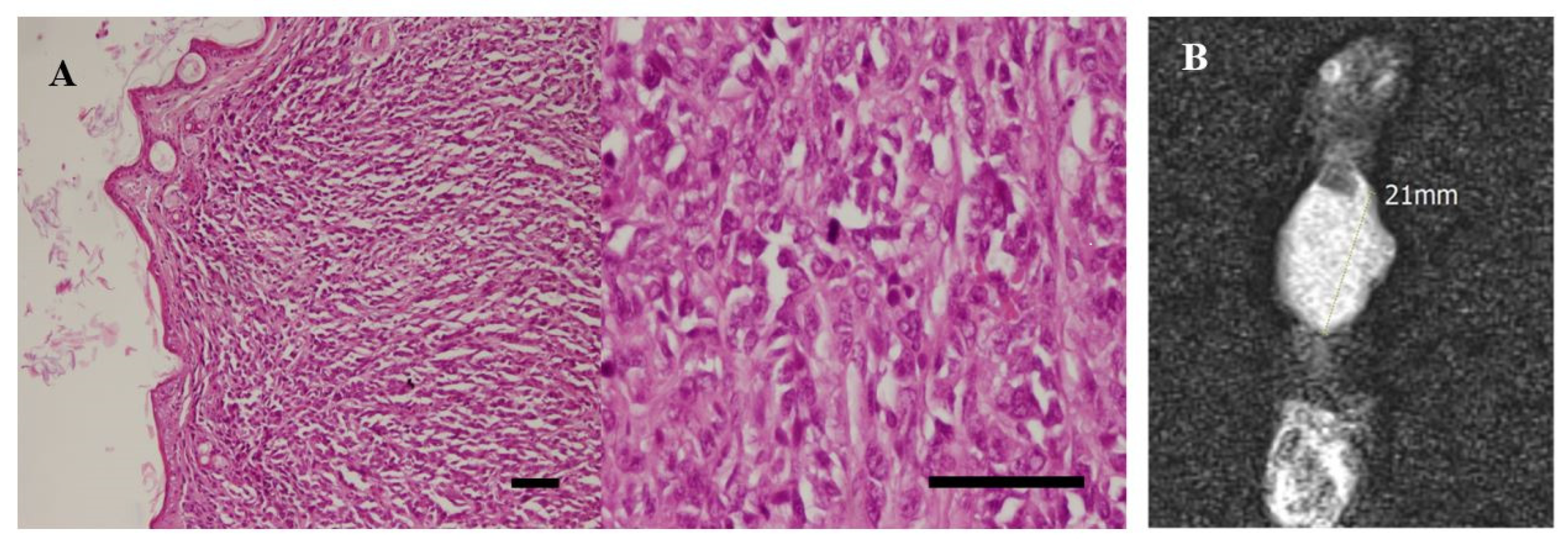
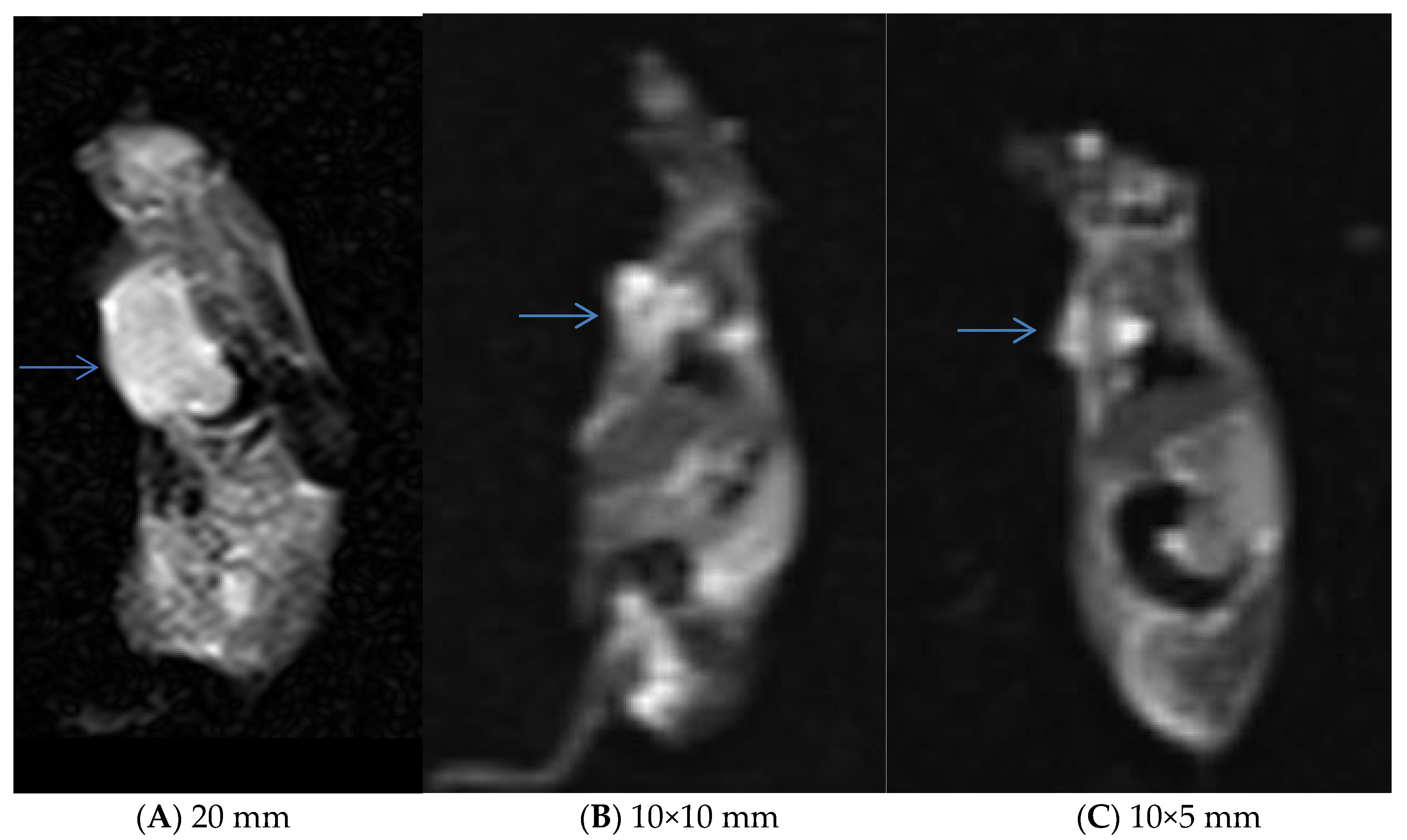
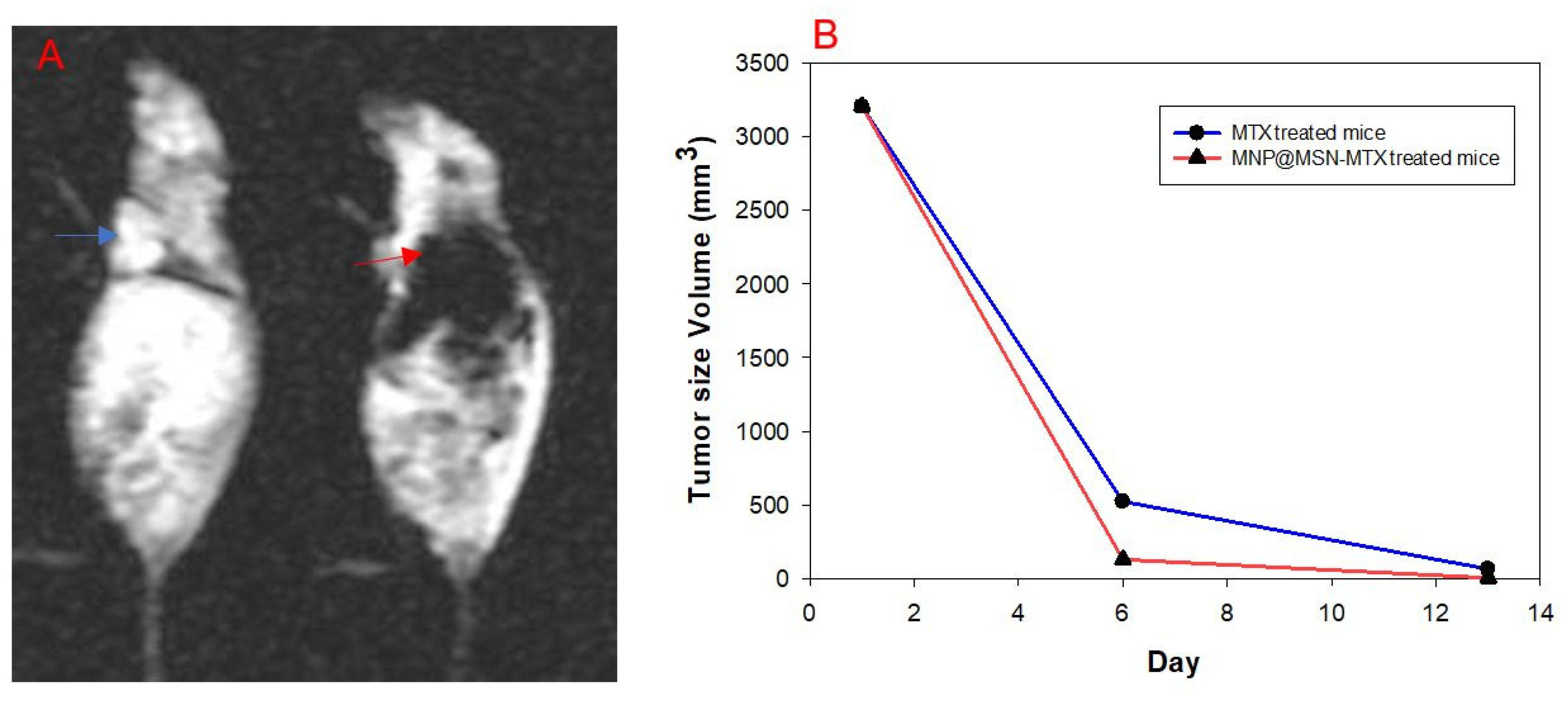
3.5. In Vivo Experiments and MRI Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghapour Asr, M.; Dayani, F.; Saedi Segherloo, F.; Kamedi, A.; Neill, A.O.; MacLoughlin, R.; Doroudian, M. Lipid Nanoparticles as Promising Carriers for mRNA Vaccines for Viral Lung Infections. Pharmaceutics 2023, 15, 1127. [Google Scholar] [CrossRef] [PubMed]
- Ajam-Hosseini, M.; Akhoondi, F.; Doroudian, M. Nano Based-Oncolytic viruses for Cancer therapy. Crit. Rev. Oncol./Hematol. 2023, 185, 103980. [Google Scholar] [CrossRef] [PubMed]
- Doroudian, M.; Armstrong, M.E.; Donnelly, S.C. Nano-Based Therapies for Acute and Chronic Lung Diseases. In Biotechnology Applied to Inflammatory Diseases: Cellular Mechanisms and Nanomedicine; Springer: Singapore, 2023; pp. 271–286. [Google Scholar]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Abdela Siraj, E. Targeted drug delivery—From magic bullet to nanomedicine: Principles, challenges, and future perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Doroudian, M.; Zanganeh, S.; Abbasgholinejad, E.; Donnelly, S.C. Nanomedicine in Lung Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2023, 11, 1144653. [Google Scholar] [CrossRef]
- Doroudian, M.; Azhdari, M.H.; Goodarzi, N.; O’Sullivan, D.; Donnelly, S.C. Smart nanotherapeutics and lung cancer. Pharmaceutics 2021, 13, 1972. [Google Scholar] [CrossRef]
- Doroudian, M.; O’Neill, A.; Mac Loughlin, R.; Prina-Mello, A.; Volkov, Y.; Donnelly, S.C. Nanotechnology in pulmonary medicine. Curr. Opin. Pharmacol. 2021, 56, 85–92. [Google Scholar] [CrossRef]
- Hapuarachchige, S.; Artemov, D. Theranostic pretargeting drug delivery and imaging platforms in cancer precision medicine. Front. Oncol. 2020, 10, 1131. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, S.; Akbarian, M.; Hoseini-Ghahfarokhi, M.; Moghoofei, M.; Doroudian, M. Physically stimulus-responsive nanoparticles for therapy and diagnosis. Front. Chem. 2022, 10, 952675. [Google Scholar] [CrossRef]
- Blamire, A. The technology of MRI—The next 10 years? Br. J. Radiol. 2008, 81, 601–617. [Google Scholar] [CrossRef]
- Hosseinkazemi, H.; Samani, S.; O’Neill, A.; Soezi, M.; Moghoofei, M.; Azhdari, M.H.; Aavani, F.; Nazbar, A.; Keshel, S.H.; Doroudian, M. Applications of iron oxide nanoparticles against breast cancer. J. Nanomater. 2022, 2022, 6493458. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 2018, 119, 957–1057. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic nanoparticles as MRI contrast agents. In Surface-Modified Nanobiomaterials for Electrochemical and Biomedicine Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 49–91. [Google Scholar]
- Doroudian, M.; O’Neill, A.; O’Reilly, C.; Tynan, A.; Mawhinney, L.; McElroy, A.; Webster, S.S.; MacLoughlin, R.; Volkov, Y.; EArmstrong, M.; et al. Aerosolized drug-loaded nanoparticles targeting migration inhibitory factors inhibit Pseudomonas aeruginosa-induced inflammation and biofilm formation. Nanomedicine 2020, 15, 2933–2953. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Huxford-Phillips, R.C.; Lin, W. Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem. Soc. Rev. 2012, 41, 2673–2685. [Google Scholar] [CrossRef]
- Ambrogio, M.W.; Thomas, C.R.; Zhao, Y.-L.; Zink, J.I.; Stoddart, J.F. Mechanized silica nanoparticles: A new frontier in theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Doroudian, M.; MacLoughlin, R.; Poynton, F.; Prina-Mello, A.; Donnelly, S.C. Nanotechnology based therapeutics for lung disease. Thorax 2019, 74, 965–976. [Google Scholar] [CrossRef]
- Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Hosseini, M. Mesoporous silica nanoparticles: Synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chem. Eng. J. 2019, 359, 684–705. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Zeng, J.; Huo, L.; Liu, K.; Wei, R.; Ni, K.; Gao, J. Recent advances in engineering iron oxide nanoparticles for effective magnetic resonance imaging. Bioact. Mater. 2022, 12, 214–245. [Google Scholar] [CrossRef]
- Abolmaali, S.S.; Tamaddon, A.M.; Dinarvand, R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother. Pharmacol. 2013, 71, 1115–1130. [Google Scholar] [CrossRef]
- Mukhtar, M.; Ezra Manicum, A.L.; Shojaei Barjouei, M.; Eshaghi Malekshah, R.; Behzadmehr, R.; Rahdar, A.; Ghotekar, S.; Baino, F. Nanocarriers for methotrexate delivery/codelivery in the frame of cancer diagnostics and treatment: A review. Front. Biomater. Sci. 2023, 2, 1200670. [Google Scholar] [CrossRef]
- Dehshahri, A.; Kumar, A.; Madamsetty, V.S.; Uzieliene, I.; Tavakol, S.; Azedi, F.; Fekri, H.S.; Zarrabi, A.; Mohammadinejad, R.; Thakur, V.K. New horizons in hydrogels for methotrexate delivery. Gels 2020, 7, 2. [Google Scholar] [CrossRef]
- Zhu, L.; Huo, Z.; Wang, L.; Tong, X.; Xiao, Y.; Ni, K. Targeted delivery of methotrexate to skeletal muscular tissue by thermosensitive magnetoliposomes. Int. J. Pharm. 2009, 370, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, F.; Ni, J. A novel method to prepare magnetite chitosan microspheres conjugated with methotrexate (MTX) for the controlled release of MTX as a magnetic targeting drug delivery system. Drug Deliv. 2009, 16, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Neoh, K.-G.; Wang, R.; Zong, B.-Y.; Tan, J.Y.; Kang, E.-T. Methotrexate-conjugated and hyperbranched polyglycerol-grafted Fe3O4 magnetic nanoparticles for targeted anticancer effects. Eur. J. Pharm. Sci. 2013, 48, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Attari, E.; Nosrati, H.; Danafar, H.; Kheiri Manjili, H. Methotrexate anticancer drug delivery to breast cancer cell lines by iron oxide magnetic based nanocarrier. J. Biomed. Mater. Res. Part A 2019, 107, 2492–2500. [Google Scholar] [CrossRef]
- Ou, L.; Zhang, Q.; Chang, Y.; Xia, N. Codelivery of Methotrexate and Nanohydroxyapatite with Polyethylene Glycol Polymers for Chemotherapy of Osteosarcoma. Micromachines 2023, 14, 757. [Google Scholar] [CrossRef]
- Carino, I.S.; Pasqua, L.; Testa, F.; Aiello, R.; Puoci, F.; Iemma, F.; Picci, N. Silica-based mesoporous materials as drug delivery system for methotrexate release. Drug Deliv. 2007, 14, 491–495. [Google Scholar] [CrossRef]
- Sapino, S.; Oliaro-Bosso, S.; Zonari, D.; Zattoni, A.; Ugazio, E. Mesoporous silica nanoparticles as a promising skin delivery system for methotrexate. Int. J. Pharm. 2017, 530, 239–248. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, S.; Mohammadi-Samani, S. Hydroxyl-modified magnetite nanoparticles as novel carrier for delivery of methotrexate. Int. J. Pharm. 2016, 504, 110–116. [Google Scholar] [CrossRef]
- Farzanfar, J.; Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Tayebi, L. Assessment of pH responsive delivery of methotrexate based on PHEMA-st-PEG-DA nanohydrogels. Macromol. Res. 2021, 29, 54–61. [Google Scholar] [CrossRef]
- Mazumder, A.; Assawapanumat, W.; Dwivedi, A.; Reabroi, S.; Chairoungdua, A.; Nasongkla, N. Glucose targeted therapy against liver hepatocellular carcinoma: In vivo study. J. Drug Deliv. Sci. Technol. 2019, 49, 502–512. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Zhao, C.; Wang, P.G.; Wang, X. Glucose-conjugated platinum(IV) complexes as tumor-targeting agents: Design, synthesis and biological evaluation. Bioorg. Med. Chem. 2019, 27, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Custódio, T.F.; Paulsen, P.A.; Frain, K.M.; Pedersen, B.P. Structural comparison of GLUT1 to GLUT3 reveal transport regulation mechanism in sugar porter family. Life Sci. Alliance 2021, 4, e202000858. [Google Scholar] [CrossRef]
- Zarkesh, K.; Heidari, R.; Iranpour, P.; Azarpira, N.; Ahmadi, F.; Mohammadi-Samani, S.; Farjadian, F. Theranostic Hyaluronan Coated EDTA Modified Magnetic Mesoporous Silica Nanoparticles for Targeted Delivery of Cisplatin. J. Drug Deliv. Sci. Technol. 2022, 77, 103903. [Google Scholar] [CrossRef]
- Heidari, R.; Sepahi, Z.; Mohammadi-Samani, S.; Tayebi, L.; Azarpira, N.; Doroudian, M.; Farjadian, F. Mesoporous silica application as an antidote of methotrexate and evaluation of the long-term oral administration: In vitro and in vivo study. J. Mater. Res. 2023, 38, 2930–2942. [Google Scholar] [CrossRef]
- Mohammadi, H.; Heidari, R.; Niknezhad, S.V.; Jamshidzadeh, A.; Farjadian, F. In vitro and in vivo evaluation of succinic acid-substituted mesoporous silica for ammonia adsorption: Potential application in the management of hepatic encephalopathy. Int. J. Nanomed. 2020, 15, 10085–10098. [Google Scholar] [CrossRef]
- Taqanaki, E.R.; Heidari, R.; Monfared, M.; Tayebi, L.; Azadi, A.; Farjadian, F. EDTA-modified mesoporous silica as supra adsorbent of copper ions with novel approach as an antidote agent in copper toxicity. Int. J. Nanomed. 2019, 14, 7781–7792. [Google Scholar] [CrossRef]
- Farjadian, F.; Moradi, S.; Hosseini, M. Thin chitosan films containing super-paramagnetic nanoparticles with contrasting capability in magnetic resonance imaging. J. Mater. Sci. Mater. Med. 2017, 28, 47. [Google Scholar] [CrossRef]
- Keshavarz, H.; Khavandi, A.; Alamolhoda, S.; Naimi-Jamal, M.R. pH-Sensitive magnetite mesoporous silica nanocomposites for controlled drug delivery and hyperthermia. RSC Adv. 2020, 10, 39008–39016. [Google Scholar] [CrossRef]
- Markides, H.; Rotherham, M.; El Haj, A. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 2012, 614094. [Google Scholar] [CrossRef]
- Shao, D.; Lu, M.M.; Zhao, Y.W.; Zhang, F.; Tan, Y.F.; Zheng, X.; Pan, Y.; Xiao, X.A.; Wang, Z.; Dong, W.F.; et al. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 2017, 49, 531–540. [Google Scholar] [CrossRef] [PubMed]





| Sample | Nitrogen% | Carbon% | Hydrogen% |
|---|---|---|---|
| MNP@MSN-Glu | 0.43 | 15.96 | 4.63 |
| MNP@MSN-MTX | 1.45 | 15.06 | 3.43 |
| Sample | Surface Area (m2/g) (BET Calculation) | Pore Volume (cm3/g) Single Point ads/des | Pore Width (nm) (BJH Calculation) |
|---|---|---|---|
| MNP@MSN | 644 | 0.58 | 3.6 |
| MNP@MSN-Glu | 310 | 0.22 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farjadian, F.; Faghih, Z.; Fakhimi, M.; Iranpour, P.; Mohammadi-Samani, S.; Doroudian, M. Glucosamine-Modified Mesoporous Silica-Coated Magnetic Nanoparticles: A “Raisin-Cake”-like Structure as an Efficient Theranostic Platform for Targeted Methotrexate Delivery. Pharmaceutics 2023, 15, 2491. https://doi.org/10.3390/pharmaceutics15102491
Farjadian F, Faghih Z, Fakhimi M, Iranpour P, Mohammadi-Samani S, Doroudian M. Glucosamine-Modified Mesoporous Silica-Coated Magnetic Nanoparticles: A “Raisin-Cake”-like Structure as an Efficient Theranostic Platform for Targeted Methotrexate Delivery. Pharmaceutics. 2023; 15(10):2491. https://doi.org/10.3390/pharmaceutics15102491
Chicago/Turabian StyleFarjadian, Fatemeh, Zahra Faghih, Maryam Fakhimi, Pooya Iranpour, Soliman Mohammadi-Samani, and Mohammad Doroudian. 2023. "Glucosamine-Modified Mesoporous Silica-Coated Magnetic Nanoparticles: A “Raisin-Cake”-like Structure as an Efficient Theranostic Platform for Targeted Methotrexate Delivery" Pharmaceutics 15, no. 10: 2491. https://doi.org/10.3390/pharmaceutics15102491
APA StyleFarjadian, F., Faghih, Z., Fakhimi, M., Iranpour, P., Mohammadi-Samani, S., & Doroudian, M. (2023). Glucosamine-Modified Mesoporous Silica-Coated Magnetic Nanoparticles: A “Raisin-Cake”-like Structure as an Efficient Theranostic Platform for Targeted Methotrexate Delivery. Pharmaceutics, 15(10), 2491. https://doi.org/10.3390/pharmaceutics15102491