Preparation and Primary Bioactivity Evaluation of Novel Water-Soluble Curcumin-Loaded Polymeric Micelles Fabricated with Chitooligosaccharides and Pluronic F-68
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimization of Conditions of COSPFCU Fabrication
2.3. Characterization of CU-Loaded Polymeric Micelles
2.4. X-ray Diffraction (XRD)
2.5. Spectroscopy Studies
2.6. In Vitro Curcumin Release Assay
2.7. DPPH (2,2-Diphenyl-2-Picrylhydrazyl Hydrate) Scavenging Assay
2.8. Cell Line Study
2.8.1. Cytotoxicity
2.8.2. Inhibition of Nitric Oxide Production in LPS-Stimulated RAW 264.7 Cells
3. Results and Discussion
3.1. Fabrication of COSPFCU Polymeric Micelles
3.2. Characterization of COSPFCU Polymeric Micelles
3.3. X-ray Diffraction Analysis (XRD)
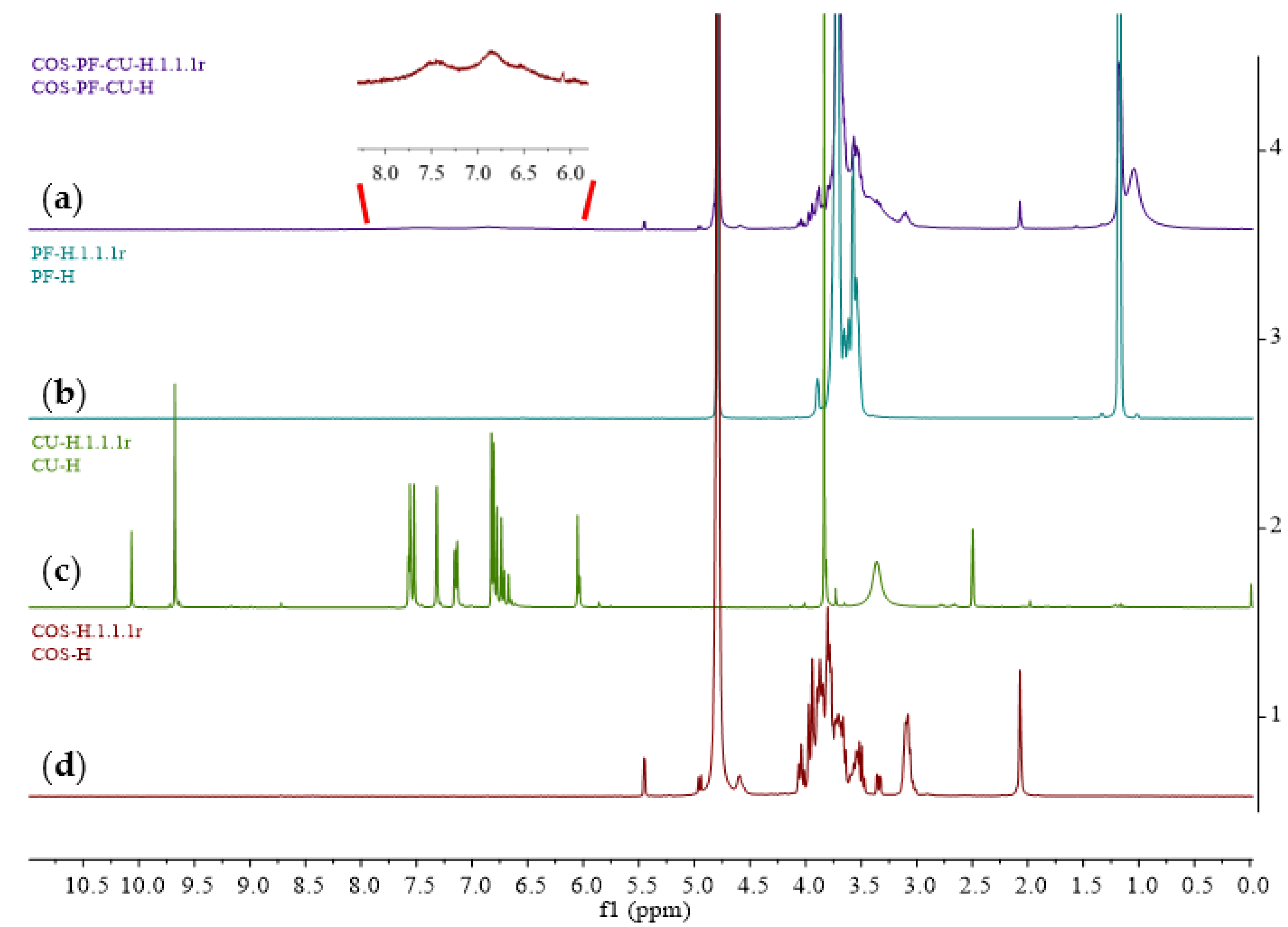
3.4. Spectroscopy Analyses
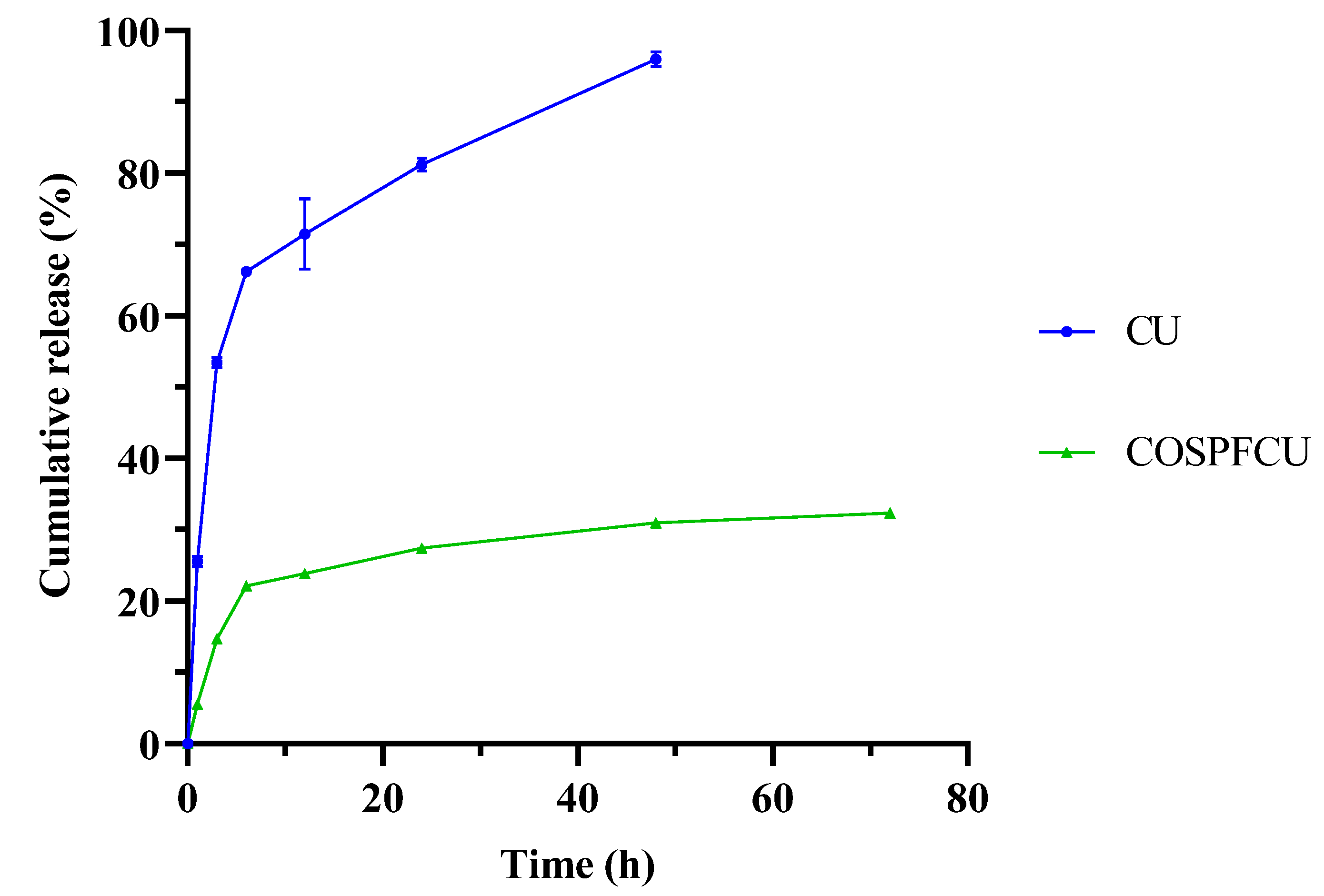
3.5. In Vitro Release Assay
3.6. DPPH Scavenging Activity of COSPFCU
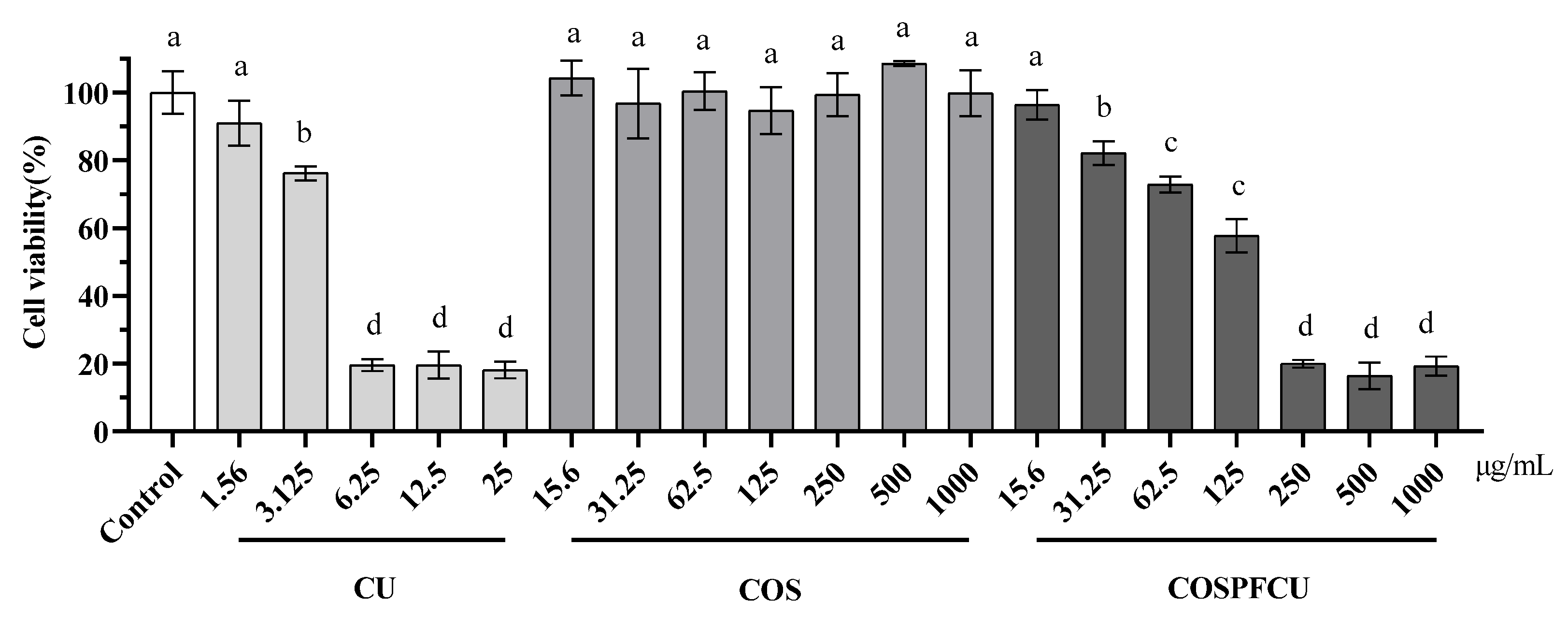
3.7. Cytotoxicity Test
3.8. Anti-Inflammatory Regulation by Inhibition of NO Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.N.; Chandra, A.; Nair, M.G. Novel bioactivities of Curcuma longa constituents. J. Nat. Prod. 1998, 61, 542–545. [Google Scholar] [PubMed]
- Araujo, C.; Leon, L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [PubMed]
- Infante, K.; Chowdhury, R.; Nimmanapalli, R.; Reddy, G. Antimicrobial activity of curcumin against food-borne pathogens. VRI Bio. Med. Chem 2014, 2, 12. [Google Scholar]
- Negi, P.; Jayaprakasha, G.; Jagan Mohan Rao, L.; Sakariah, K. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J. Agric. Food Chem. 1999, 47, 4297–4300. [Google Scholar] [PubMed]
- Ungphaiboon, S.; Supavita, T.; Singchangchai, P.; Sungkarak, S.; Rattanasuwan, P.; Itharat, A. Study on antioxidant and antimicrobial activities of turmeric clear liquid soap for wound treatment of HIV patients. Songklanakarin J. Sci. Technol. 2005, 27, 269–578. [Google Scholar]
- Martins, C.; Da Silva, D.; Neres, A.; Magalhaes, T.; Watanabe, G.; Modolo, L.; Sabino, A.; De Fátima, A.; De Resende, M. Curcumin as a promising antifungal of clinical interest. J. Antimicrob. Chemother. 2009, 63, 337–339. [Google Scholar]
- Kim, M.-K.; Choi, G.-J.; Lee, H.-S. Fungicidal property of Curcuma longa L. rhizome-derived curcumin against phytopathogenic fungi in a greenhouse. J. Agric. Food Chem. 2003, 51, 1578–1581. [Google Scholar]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2007; pp. 105–125. [Google Scholar]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Rao, T.S.; Basu, N.; Siddiqui, H. Anti-inflammatory activity of curcumin analogues. Indian J. Med. Res. 2013, 137, 01021. [Google Scholar]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2017, 7, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Sa, G.; Das, T. Anti cancer effects of curcumin: Cycle of life and death. Cell Div. 2008, 3, 14. [Google Scholar] [CrossRef]
- Basile, V.; Ferrari, E.; Lazzari, S.; Belluti, S.; Pignedoli, F.; Imbriano, C. Curcumin derivatives: Molecular basis of their anti-cancer activity. Biochem. Pharmacol. 2009, 78, 1305–1315. [Google Scholar] [CrossRef]
- Kaminaga, Y.; Nagatsu, A.; Akiyama, T.; Sugimoto, N.; Yamazaki, T.; Maitani, T.; Mizukami, H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003, 555, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, G. A critical appraisal of solubility enhancement techniques of polyphenols. J. Pharm. 2014, 2014, 180845. [Google Scholar] [CrossRef]
- Rana, V.; Sharma, R. Recent advances in development of nano drug delivery. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 93–131. [Google Scholar]
- Akbar, M.U.; Zia, K.M.; Nazir, A.; Iqbal, J.; Ejaz, S.A.; Akash, M.S.H. Pluronic-based mixed polymeric micelles enhance the therapeutic potential of curcumin. AAPS PharmSciTech 2018, 19, 2719–2739. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Sosnik, A. Poly (ethylene oxide)–poly (propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Shaarani, S.; Hamid, S.S.; Kaus, N.H.M. The influence of pluronic F68 and F127 nanocarrier on physicochemical properties, in vitro release, and antiproliferative activity of thymoquinone drug. Pharmacogn. Res. 2017, 9, 12. [Google Scholar]
- Singla, P.; Singh, O.; Sharma, S.; Betlem, K.; Aswal, V.K.; Peeters, M.; Mahajan, R.K. Temperature-Dependent Solubilization of the Hydrophobic Antiepileptic Drug Lamotrigine in Different Pluronic Micelles—A Spectroscopic, Heat Transfer Method, Small-Angle Neutron Scattering, Dynamic Light Scattering, and in Vitro Release Study. ACS Omega 2019, 4, 11251–11262. [Google Scholar] [CrossRef]
- Shimabayashi, S.; Ichimori, A.; Hino, T. Interaction and complex formation of pluronic polymers with ionic surfactants. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2001; Volume 132, pp. 133–136. [Google Scholar]
- Popovici, C.; Popa, M.; Sunel, V.; Atanase, L.I.; Ichim, D.L. Drug Delivery Systems Based on Pluronic Micelles with Antimicrobial Activity. Polymers 2022, 14, 3007. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Ben Henda, M.; Ghaouar, N.; Gharbi, A. Rheological Properties and Reverse Micelles Conditions of PEO-PPO-PEO Pluronic F68: Effects of Temperature and Solvent Mixtures. J. Polym. 2013, 2013, 768653. [Google Scholar] [CrossRef]
- Le, T.M.P.; Dang, T.M.L.; La, T.H.; Le, T.H.; Le, Q.H. Preparation of curcumin-loaded pluronic F127/chitosan nanoparticles for cancer therapy. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 025001. [Google Scholar]
- Laokuldilok, T.; Potivas, T.; Kanha, N.; Surawang, S.; Seesuriyachan, P.; Wangtueai, S.; Phimolsiripol, Y.; Regenstein, J.M. Physicochemical, antioxidant, and antimicrobial properties of chitooligosaccharides produced using three different enzyme treatments. Food Biosci. 2017, 18, 28–33. [Google Scholar] [CrossRef]
- Mourya, V.; Inamdar, N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Foster, L.J.R.; Ho, S.; Hook, J.; Basuki, M.; Marcal, H. Chitosan as a biomaterial: Influence of degree of deacetylation on its physiochemical, material and biological properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef]
- Miguez, N.; Kidibule, P.; Santos-Moriano, P.; Ballesteros, A.O.; Fernandez-Lobato, M.; Plou, F.J. Enzymatic synthesis and characterization of different families of chitooligosaccharides and their bioactive properties. Appl. Sci. 2021, 11, 3212. [Google Scholar] [CrossRef]
- Cao, R.; Li, X.; Zhou, Z.; Zhao, Z. Synthesis and biophysical analysis of Naringin-Chitooligosaccharide complex. Nat. Prod. Res. 2021, 35, 305–311. [Google Scholar] [CrossRef]
- Cao, R.; Ma, Q.; Fu, Y.; Zhou, Z.; Zhao, X. Preparation, Evaluation and Characterization of Rutin-Chitooligosaccharide Complex. Plant Foods Hum. Nutr. 2019, 74, 328–333. [Google Scholar] [CrossRef]
- Waiprib, Y.; Ingrungruengluet, P.; Worawattanamateekul, W. Nanoparticles Based on Chondroitin Sulfate from Tuna Heads and Chitooligosaccharides for Enhanced Water Solubility and Sustained Release of Curcumin. Polymers 2023, 15, 834. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Jin, Y.; Bao, W.; Gao, H.; Xu, M.; Wang, D.; Wang, X.; Yao, P.; Liu, L. In vitro characterization and in vivo evaluation of nanostructured lipid curcumin carriers for intragastric administration. Int. J. Nanomed. 2012, 7, 5395. [Google Scholar] [CrossRef]
- Srihera, N.; Li, Y.; Zhang, T.T.; Wang, Y.M.; Yanagita, T.; Waiprib, Y.; Xue, C.H. Preparation and Characterization of Astaxanthin-loaded Liposomes Stabilized by Sea Cucumber Sulfated Sterols Instead of Cholesterol. J. Oleo Sci. 2022, 71, 401–410. [Google Scholar] [CrossRef]
- Colaco, M.; Roquito, T.; Costa, J.P.; Cruz, M.T.; Borges, O. The Effect of Curcumin-Loaded Glucan Nanoparticles on Immune Cells: Size as a Critical Quality Attribute. Pharmaceutics 2023, 15, 623. [Google Scholar] [CrossRef]
- Nahar, P.P.; Slitt, A.L.; Seeram, N.P. Anti-inflammatory effects of novel standardized solid lipid curcumin formulations. J. Med. Food 2015, 18, 786–792. [Google Scholar] [CrossRef]
- Anand, S.; Sowbhagya, R.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Almalik, A.I.; Ahmad, W.; Tripathi, T.; Elderdery, A.Y. Polyphenols and Their Nanoformulations: Protective Effects against Human Diseases. Life 2022, 12, 1639. [Google Scholar] [CrossRef] [PubMed]
- Croy, S.; Kwon, G. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bao, H.; Li, L. Role of PPO–PEO–PPO triblock copolymers in phase transitions of a PEO–PPO–PEO triblock copolymer in aqueous solution. Eur. Polym. J. 2015, 71, 423–439. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J. Sci. Food Agric. 2018, 98, 2422–2427. [Google Scholar] [CrossRef]
- Vaidya, F.U.; Sharma, R.; Shaikh, S.; Ray, D.; Aswal, V.K.; Pathak, C. Pluronic micelles encapsulated curcumin manifests apoptotic cell death and inhibits pro-inflammatory cytokines in human breast adenocarcinoma cells. Cancer Rep. 2019, 2, e1133. [Google Scholar] [CrossRef] [PubMed]
- Bockuviene, A.; Sereikaite, J. Preparation and characterisation of novel water-soluble beta-carotene-chitooligosaccharides complexes. Carbohydr. Polym. 2019, 225, 115226. [Google Scholar] [CrossRef]
- Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of Crystalline Structural Differences Between alpha- and beta-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities. Polymers 2019, 11, 2010. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.F.G.; Francisco, D.S.; Ferreira, A.P.G.; Cavalheiro, E.T.G. A new look towards the thermal decomposition of chitins and chitosans with different degrees of deacetylation by coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, S.; Ma, M.; Xu, Y.; Wang, D. Delivery of curcumin by shellac encapsulation: Stability, bioaccessibility, freeze-dried redispersibility, and solubilization. Food Chem. X 2022, 15, 100431. [Google Scholar] [CrossRef]
- Enumo, A., Jr.; Argenta, D.F.; Bazzo, G.C.; Caon, T.; Stulzer, H.K.; Parize, A.L. Development of curcumin-loaded chitosan/pluronic membranes for wound healing applications. Int. J. Biol. Macromol. 2020, 163, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Kasoju, N.; Goswami, P.; Bora, U. Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J. Biomater. Appl. 2011, 25, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Rho, S.-J.; Kim, Y.-R. Solubility, stability, and bioaccessibility improvement of curcumin encapsulated using 4-α-glucanotransferase-modified rice starch with reversible pH-induced aggregation property. Food Hydrocoll. 2019, 95, 19–32. [Google Scholar] [CrossRef]
- Ejaz, S.; Ejaz, S.; Shahid, R.; Noor, T.; Shabbir, S.; Imran, M. Chitosan-curcumin complexation to develop functionalized nanosystems with enhanced antimicrobial activity against hetero-resistant gastric pathogen. Int. J. Biol. Macromol. 2022, 204, 540–554. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, J.H.; Hong, Y.S. Development of chitosan/poly-gamma-glutamic acid/pluronic/curcumin nanoparticles in chitosan dressings for wound regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 105, 81–90. [Google Scholar] [CrossRef]
- Nebrisi, E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, M.R.; Leite, F.R.; Spolidorio, L.C.; Kirkwood, K.L.; Rossa, C., Jr. Curcumin abrogates LPS-induced pro-inflammatory cytokines in RAW 264.7 macrophages. Evidence for novel mechanisms involving SOCS-1, -3 and p38 MAPK. Arch. Oral Biol. 2013, 58, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Yang, C.; Yang, R.; Gu, M.; Hao, J.; Wang, S.; Li, C. Chitooligosaccharides Derivatives Protect ARPE-19 Cells against Acrolein-Induced Oxidative Injury. Ma.r Drugs 2023, 21, 137. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingrungruengluet, P.; Wang, D.; Li, X.; Yang, C.; Waiprib, Y.; Li, C. Preparation and Primary Bioactivity Evaluation of Novel Water-Soluble Curcumin-Loaded Polymeric Micelles Fabricated with Chitooligosaccharides and Pluronic F-68. Pharmaceutics 2023, 15, 2497. https://doi.org/10.3390/pharmaceutics15102497
Ingrungruengluet P, Wang D, Li X, Yang C, Waiprib Y, Li C. Preparation and Primary Bioactivity Evaluation of Novel Water-Soluble Curcumin-Loaded Polymeric Micelles Fabricated with Chitooligosaccharides and Pluronic F-68. Pharmaceutics. 2023; 15(10):2497. https://doi.org/10.3390/pharmaceutics15102497
Chicago/Turabian StyleIngrungruengluet, Pattarachat, Dingfu Wang, Xin Li, Cheng Yang, Yaowapha Waiprib, and Chunxia Li. 2023. "Preparation and Primary Bioactivity Evaluation of Novel Water-Soluble Curcumin-Loaded Polymeric Micelles Fabricated with Chitooligosaccharides and Pluronic F-68" Pharmaceutics 15, no. 10: 2497. https://doi.org/10.3390/pharmaceutics15102497
APA StyleIngrungruengluet, P., Wang, D., Li, X., Yang, C., Waiprib, Y., & Li, C. (2023). Preparation and Primary Bioactivity Evaluation of Novel Water-Soluble Curcumin-Loaded Polymeric Micelles Fabricated with Chitooligosaccharides and Pluronic F-68. Pharmaceutics, 15(10), 2497. https://doi.org/10.3390/pharmaceutics15102497







