Polysaccharide-Based Nanogels to Overcome Mucus, Skin, Cornea, and Blood-Brain Barriers: A Review
Abstract
:1. Introduction
2. Polysaccharide-Based Nanogels and Recent Advancement
3. Polysaccharide-Based NGs to Overcome the Mucus Gel Barrier
3.1. Characteristics of Mucus Gel Barrier
3.2. Strategies to Overcome the Mucus Gel Barrier
3.2.1. Muco-Adhesive
3.2.2. Mucus Penetration
4. Polysaccharide-Based NGs to Overcome the Skin Barrier
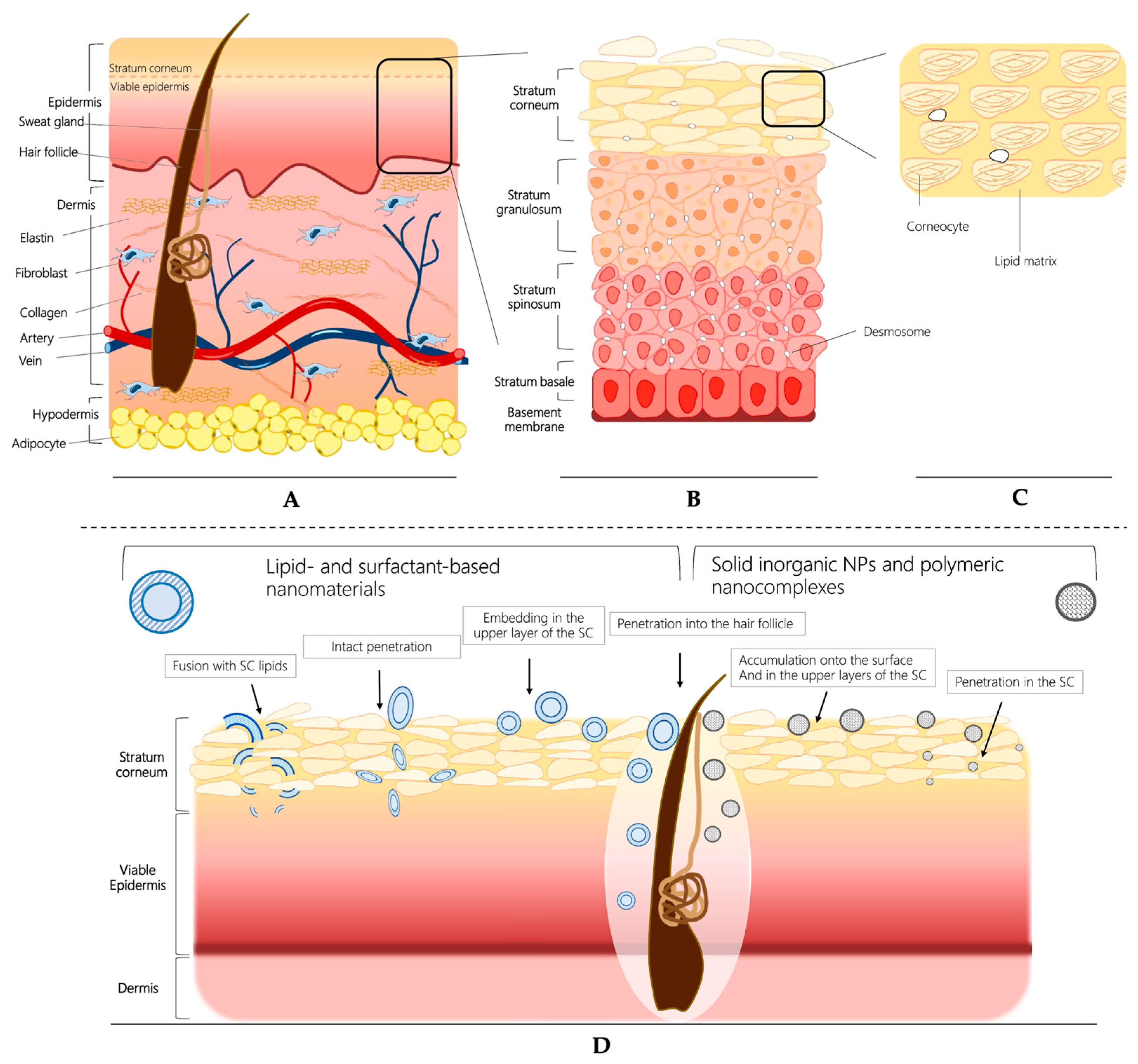
4.1. Characteristics of Skin Barrier
4.2. Strategies to Overcome the Skin Barrier
4.2.1. Enhancement of Permeation and Targeted Delivery
4.2.2. pH-Responsive Nanogel Systems
4.2.3. Thermo-Responsive Nanogel Systems
| Author | Type of Nanogel | Function and Mechanism | Size/Zeta Potential | Loaded Cargo | Type of Study | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Chatterjee et al. | Chitosan, hyaluronic acid | Dual-responsive (pH/temperature) | −17 mV | Cortex Moutan | In vitro | 2021 | [91] |
| Liu et al. | Hyaluronic acid | Enhance permeation | 122 ± 34 nm | Tranexamic acid | In vitro In vivo | 2021 | [83] |
| Kim et al. | Hyaluronic acid, β-glucan | Enhance permeation/uptake of dendritic cells | 100–300 nm −7 mV | / | In vitro Ex vivo | 2021 | [84] |
| Pan et al. | Sugarcane bagasse cellulose | Multi-responsive (pH/temperature/redox) | / | Doxorubicin | In vitro | 2021 | [98] |
| Shaikh et al. | Starch polymer, Carbopol | Enhance permeation and skin retention | 369.1–745.4 nm | Luliconazole | In vitro Ex vivo | 2020 | [78] |
| Montanari et al. | Hyaluronic acid | Enhance permeation, CD44 targeting | / | Antibiotics | In vitro Ex vivo | 2020 | [82] |
| Ghaeini-H et al. | Chondroitin sulfate | Dual-responsive (pH/temperature) | 181 nm −8.6 mV | Antimicrobial peptide | In vitro | 2020 | [90] |
| Sahu et al. | Chitosan | pH response, Enhance permeation | 100–180 nm +43.15 mV | 5-Fluorouracil | In vitro In vivo | 2019 | [86,87] |
| Abnoos et al. | Chitosan-sodium alginate | Enhance permeation | Around 80 nm | Pirfenidone | In vitro Ex vivo | 2018 | [80] |
| Giulbudagian et al. | β-cyclodextrin | Thermo-responsive, enhance permeation | / | Dexamethasone | In vitro Ex vivo | 2018 | [96] |
| Panonnummal et al. | Chitin | Enhanced permeation | 132 ± 14 nm | Clobetasol | In vitro In vivo | 2017 | [76] |
| Carmona-Moran et al. | Gellan | Thermo-responsive, enhance permeation | / | Diclofenac | In vitro | 2016 | [97] |
| Al-Kassas et al. | Chitosan, poloxamer, Carbopol | Enhance adhesive, permeation, prolonged drug release | less than 500 nm >30 mV | Propranolol | Ex vivo | 2016 | [77] |
| Jana et al. | Chitosan, Carbopol 940 | Enhance permeation | 352.90 nm −22.10 mV | Aceclofenac | Ex vivo In vivo | 2014 | [75] |
5. Polysaccharide-Based NGs to Overcome the Ocular Delivery Barrier
5.1. Characteristics of the Ocular Barrier
5.2. Strategies for Enhancing Ocular Drug Delivery
5.2.1. Enhance the Muco-Adhesion and Permeability
5.2.2. Stimuli-Responsive in situ Gelling Systems
| Author | Type of Nanogel | Function and Mechanism | Size/Zeta Potential | Loaded Cargo | Type of Study | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Buosi et al. | Chitosan | ARPE-19 cell targeting | ~140 nm, 32 ± 2 mV | Resveratrol | In vitro | 2020 | [107] |
| Laradji et al. | Hyaluronic acid | Redox-responsive release, penetration | ~80 nm, −7.56~−2.24 mV | Fluorescein | In vitro In vivo | 2021 | [108] |
| Zheng et al. | Catechol modified quaternized chitosan | Thermosensitive, tissue adhesive | / | / | In vitro In vivo | 2020 | [110] |
| Chaharband et al. | Chitosan, hyaluronic acid | Overcoming vitreous and retina barriers | / | siRNA | In vitro | 2020 | [112] |
| Silva et al. | Chitosan, hyaluronic acid | Enhanced muco-adhesion and permeation | ≤300 nm, +30 mV | Erythropoietin | In vitro Ex vivo | 2020 | [113] |
| Lavikainen et al. | Gellan | Enhanced muco-adhesion and permeation | / | / | In vitro | 2021 | [116] |
| Nagai et al. | Methylcellulose | Prolonged pre-corneal and pre-conjunctival contact time | ~93 nm | Tranilast | In vitro In vivo | 2020 | [119] |
6. Polysaccharide-Based NGs to Overcome the Blood-Brain Barrier (BBB)
6.1. Characteristic of BBB
6.2. Strategies for Overcoming the BBB
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cairns, R.; Papandreou, I.; Denko, N. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol. Cancer Res. 2006, 4, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wanat, K. Biological barriers, and the influence of protein binding on the passage of drugs across them. Mol. Biol. Rep. 2020, 47, 3221–3231. [Google Scholar] [CrossRef] [PubMed]
- Koppa Raghu, P.; Bansal, K.K.; Thakor, P.; Bhavana, V.; Madan, J.; Rosenholm, J.M.; Mehra, N.K. Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route. Pharmaceuticals 2020, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, D.; Peppas, N.A. Advanced engineered nanoparticulate platforms to address key biological barriers for delivering chemotherapeutic agents to target sites. Adv. Drug Deliv. Rev. 2020, 167, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.I.; Sandeman, S.R. Nano carriers for drug transport across the blood-brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Wu, H.Q.; Wang, C.C. Biodegradable Smart Nanogels: A New Platform for Targeting Drug Delivery and Biomedical Diagnostics. Langmuir 2016, 32, 6211–6225. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Blanco, E.R.O.; Gugliotta, L.M.; Alvarez Igarzabal, C.I.; Calderón, M. Crossing biological barriers with nanogels to improve drug delivery performance. J. Control. Release 2019, 307, 221–246. [Google Scholar] [CrossRef]
- Hasnain, S.M.; Hasnain, M.S.; Nayak, A.K. Natural polysaccharides: Sources and extraction methodologies. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–14. [Google Scholar] [CrossRef]
- Zhong, H.; Gao, X.; Cheng, C.; Liu, C.; Wang, Q.; Han, X. The Structural Characteristics of Seaweed Polysaccharides and Their Application in Gel Drug Delivery Systems. Mar. Drugs 2020, 18, 658. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Y.-J.; Sun, P.-L.; Zhang, F.-M.; Linhardt, R.J.; Zhang, A.-Q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shen, M.; Hong, Y.; Ye, H.; Huang, L.; Xie, J. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr. Polym. 2020, 229, 115436. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Meng Du, J.; Jang, M.-S.; Mo, X.W.; Sun, X.S.; Lee, D.S.; Lee, J.H.; Fu, Y. CD44-targeted and enzyme-responsive photo-cross-linked nanogels with enhanced stability for in vivo protein delivery. Biomacromolecules 2021, 22, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, Y.; Wang, L.; Liang, Z.; Li, D.; Xu, X.; Chen, Y.; Yang, X.; Zhang, H.; Niu, H. Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioact. Mater. 2021, 6, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Rojekar, S.; Bachra, Y.; Varma, R.S.; Andra, S.; Balu, S.; Pardeshi, C.V.; Patel, P.J.; Patel, H.M.; Paiva-Santos, A.C.; et al. Polysaccharide-based nanogels for biomedical applications: A comprehensive review. J. Drug Deliv. Sci. Technol. 2023, 84, 104447. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef] [PubMed]
- Modi, D.M.; Modi, A.D. Polysaccharide-based nanogels and ocular drug delivery: The emerging nanocarrier for crossing blood retinal barrier. Carbohydr. Polym. Technol. Appl. 2023, 6, 100331. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Moghaddam, Z.H.; Tavakol, M. Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater. Sci. Eng. C 2019, 102, 391–404. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H. Recent progress of polysaccharide-based hydrogel interfaces for wound healing and tissue engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Rial-Hermida, M.I.; Rey-Rico, A.; Blanco-Fernandez, B.; Carballo-Pedrares, N.; Byrne, E.M.; Mano, J.F. Recent progress on polysaccharide-based hydrogels for controlled delivery of therapeutic biomolecules. ACS Biomater. Sci. Eng. 2021, 7, 4102–4127. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef] [PubMed]
- Zoratto, N.; Montanari, E.; Viola, M.; Wang, J.; Coviello, T.; Di Meo, C.; Matricardi, P. Strategies to load therapeutics into polysaccharide-based nanogels with a focus on microfluidics: A review. Carbohydr. Polym. 2021, 266, 118119. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Michel, R.; Poirot, R.; Dakir, M.; Sancey, L.; Ravaine, V.; Auzély-Velty, R. Dynamic covalent chemistry enables reconfigurable all-polysaccharide nanogels. Macromol. Rapid Commun. 2020, 41, 2000213. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhong, S.; Xu, L.; Wang, J.; Zhang, Z.; Gao, Y.; Cui, X. Review on design strategies and considerations of polysaccharide-based smart drug delivery systems for cancer therapy. Carbohydr. Polym. 2022, 279, 119013. [Google Scholar] [CrossRef]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Yao, X.; Zhang, Y.; Wu, W.; Jiang, X. Hyaluronic acid nanogels with enzyme-sensitive cross-linking group for drug delivery. J. Control. Release 2015, 205, 206–217. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Yu, B.; Hu, H.; Xu, F.-J. Polysaccharide-based tumor microenvironment-responsive drug delivery systems for cancer therapy. J. Control. Release 2023, 362, 19–43. [Google Scholar] [CrossRef]
- Meng, Y.; Qiu, C.; Li, X.; McClements, D.J.; Sang, S.; Jiao, A.; Jin, Z. Polysaccharide-based nano-delivery systems for encapsulation, delivery, and pH-responsive release of bioactive ingredients. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, D.; Harada, N.; Shiku, H.; Akiyoshi, K. Self-assembled polysaccharide nanogel delivery system for overcoming tumor immune resistance. J. Control. Release 2022, 347, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jing, X.; Ma, X.; Feng, Y.; Hu, H. Versatile Types of Polysaccharide-Based Drug Delivery Systems: From Strategic Design to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9159. [Google Scholar] [CrossRef] [PubMed]
- Alioghli Ziaei, A.; Erfan-Niya, H.; Fathi, M.; Amiryaghoubi, N. In situ forming alginate/gelatin hybrid hydrogels containing doxorubicin loaded chitosan/AuNPs nanogels for the local therapy of breast cancer. Int. J. Biol. Macromol. 2023, 246, 125640. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the blood-brain barrier: Advances in nanoparticle technology for drug delivery in neuro-oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, S.; Wu, F.; Li, D.; Zhang, X.; Chen, W.; Xing, B. Rational design of nanogels for overcoming the biological barriers in various administration routes. Angew. Chem. Int. Ed. 2021, 60, 14760–14778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, H.; Wang, X.; Yu, B.; Cong, H.; Shen, Y. Polysaccharide-based nanocarriers for efficient transvascular drug delivery. J. Control. Release 2023, 354, 167–187. [Google Scholar] [CrossRef]
- Pearson, J.P.; Chater, P.I.; Wilcox, M.D. The properties of the mucus barrier, a unique gel–how can nanoparticles cross it? Ther. Deliv. 2016, 7, 229–244. [Google Scholar] [CrossRef]
- Boegh, M.; Nielsen, H.M. Mucus as a barrier to drug delivery–understanding and mimicking the barrier properties. Basic Clin. Pharmacol. Toxicol. 2015, 116, 179–186. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, C.; Liu, L.; Zhao, L.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; Pan, B.; Tang, X. Progress and prospects of polysaccharide-based nanocarriers for oral delivery of proteins/peptides. Carbohydr. Polym. 2023, 312, 120838. [Google Scholar] [CrossRef] [PubMed]
- Murgia, X.; Loretz, B.; Hartwig, O.; Hittinger, M.; Lehr, C.-M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 2018, 124, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Bassi, P.; Kaur, G. Bioadhesive vaginal drug delivery of nystatin using a derivatized polymer: Development and characterization. Eur. J. Pharm. Biopharm. 2015, 96, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Sun, G.; Wang, Z.; Cheng, X.; Park, H.; Cha, D.; Kong, M.; Chen, X. Transport mechanism of doxorubicin loaded chitosan based nanogels across intestinal epithelium. Eur. J. Pharm. Biopharm. 2014, 87, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Li, J.; Kong, M.; Liu, Y.; Cheng, X.J.; Li, Y.; Park, H.J.; Chen, X.G. Surface charge effect on mucoadhesion of chitosan based nanogels for local anti-colorectal cancer drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 439–447. [Google Scholar] [CrossRef]
- Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.T.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Tauser, R.G.; Lupascu, D.; Giusca, S.E.; Caruntu, I.-D.; Profire, L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A food perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Zoratto, N.; Forcina, L.; Matassa, R.; Mosca, L.; Familiari, G.; Musarò, A.; Mattei, M.; Coviello, T.; Di Meo, C.; Matricardi, P. Hyaluronan-cholesterol nanogels for the enhancement of the ocular delivery of therapeutics. Pharmaceutics 2021, 13, 1781. [Google Scholar] [CrossRef]
- Valente, S.A.; Silva, L.M.; Lopes, G.R.; Sarmento, B.; Coimbra, M.A.; Passos, C.P. Polysaccharide-based formulations as potential carriers for pulmonary delivery—A review of their properties and fates. Carbohydr. Polym. 2022, 277, 118784. [Google Scholar] [CrossRef]
- Bandi, S.P.; Bhatnagar, S.; Venuganti, V.V.K. Advanced materials for drug delivery across mucosal barriers. Acta Biomater. 2021, 119, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Lanthaler, M.; Laffleur, F.; Huck, C.W.; Bernkop-Schnürch, A. Thiolated chitosan micelles: Highly mucoadhesive drug carriers. Carbohydr. Polym. 2017, 167, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Hu, G.; Zhao, K. Mannose-anchored quaternized chitosan/thiolated carboxymethyl chitosan composite NPs as mucoadhesive carrier for drug delivery. Carbohydr. Polym. 2022, 283, 119174. [Google Scholar] [CrossRef] [PubMed]
- Guaresti, O.; Maiz–Fernández, S.; Palomares, T.; Alonso–Varona, A.; Eceiza, A.; Pérez–Álvarez, L.; Gabilondo, N. Dual charged folate labelled chitosan nanogels with enhanced mucoadhesion capacity for targeted drug delivery. Eur. Polym. J. 2020, 134, 109847. [Google Scholar] [CrossRef]
- Nowak, J.; Laffleur, F.; Bernkop-Schnürch, A. Preactivated hyaluronic acid: A potential mucoadhesive polymer for vaginal delivery. Int. J. Pharm. 2015, 478, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Shtenberg, Y.; Goldfeder, M.; Schroeder, A.; Bianco-Peled, H. Alginate modified with maleimide-terminated PEG as drug carriers with enhanced mucoadhesion. Carbohydr. Polym. 2017, 175, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Dünnhaupt, S.; Kammona, O.; Waldner, C.; Kiparissides, C.; Bernkop-Schnürch, A. Nano-carrier systems: Strategies to overcome the mucus gel barrier. Eur. J. Pharm. Biopharm. 2015, 96, 447–453. [Google Scholar] [CrossRef]
- Teng, Z.; Meng, L.-Y.; Yang, J.-K.; He, Z.; Chen, X.-G.; Liu, Y. Bridging nanoplatform and vaccine delivery, a landscape of strategy to enhance nasal immunity. J. Control. Release 2022, 351, 456–475. [Google Scholar] [CrossRef]
- Tian, H.; He, Z.; Sun, C.; Yang, C.; Zhao, P.; Liu, L.; Leong, K.W.; Mao, H.Q.; Liu, Z.; Chen, Y. Uniform core–shell nanoparticles with thiolated hyaluronic acid coating to enhance oral delivery of insulin. Adv. Healthc. Mater. 2018, 7, 1800285. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Wang, J.; Sun, B.; Rewatkar, P.; Popat, A.; Fu, C.; Peng, H.; Xu, Z.P.; Li, L. Enhanced Mucosal Transport of Polysaccharide–Calcium Phosphate Nanocomposites for Oral Vaccination. ACS Appl. Bio Mater. 2021, 4, 7865–7878. [Google Scholar] [CrossRef]
- Casettari, L.; Vllasaliu, D.; Castagnino, E.; Stolnik, S.; Howdle, S.; Illum, L. PEGylated chitosan derivatives: Synthesis, characterizations and pharmaceutical applications. Prog. Polym. Sci. 2012, 37, 659–685. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Filippov, S.K.; Maji, S.; Glassner, M.; Cegłowski, M.; Hoogenboom, R.; King, S.; Lau, W.M.; Khutoryanskiy, V.V. Mucus-penetrating nanoparticles based on chitosan grafted with various non-ionic polymers: Synthesis, structural characterisation and diffusion studies. J. Colloid Interface Sci. 2022, 626, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Zhang, T.; Sun, J.; Liu, X.; Ren, G.; Kou, L.; Zhang, Y.; Han, X.; Ding, W.; Ai, X.; et al. Enhanced oral delivery of paclitaxel using acetylcysteine functionalized chitosan-vitamin E succinate nanomicelles based on a mucus bioadhesion and penetration mechanism. Mol. Pharm. 2013, 10, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Honeywell-Nguyen, P.L.; Gooris, G.S.; Ponec, M. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 2003, 42, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. Viewp. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Brandner, J.; Zorn-Kruppa, M.; Yoshida, T.; Moll, I.; Beck, L.; De Benedetto, A. Epidermal tight junctions in health and disease. Tissue Barriers 2015, 3, e974451. [Google Scholar] [CrossRef]
- Murphy, M.; Kerr, P.; Grant-Kels, J.M. The histopathologic spectrum of psoriasis. Clin. Dermatol. 2007, 25, 524–528. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef]
- Ting, W.W.; Vest, C.D.; Sontheimer, R.D. Review of traditional and novel modalities that enhance the permeability of local therapeutics across the stratum corneum. Int. J. Dermatol. 2004, 43, 538–547. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Chitin and chitosan in drug delivery. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 175–239. [Google Scholar]
- Jana, S.; Manna, S.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Carbopol gel containing chitosan-egg albumin nanoparticles for transdermal aceclofenac delivery. Colloids Surf. B Biointerfaces 2014, 114, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Panonnummal, R.; Jayakumar, R.; Sabitha, M. Comparative anti-psoriatic efficacy studies of clobetasol loaded chitin nanogel and marketed cream. Eur. J. Pharm. Sci. 2017, 96, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassas, R.; Wen, J.; Cheng, A.E.-M.; Kim, A.M.-J.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Kale, M.A. Formulation and molecular docking simulation study of luliconazole nanosuspension–based nanogel for transdermal drug delivery using modified polymer. Mater. Today Chem. 2020, 18, 100364. [Google Scholar] [CrossRef]
- Henrique Marcondes Sari, M.; Mota Ferreira, L.; Cruz, L. The use of natural gums to produce nano-based hydrogels and films for topical application. Int. J. Pharm. 2022, 626, 122166. [Google Scholar] [CrossRef]
- Abnoos, M.; Mohseni, M.; Mousavi, S.A.J.; Ashtari, K.; Ilka, R.; Mehravi, B. Chitosan-alginate nano-carrier for transdermal delivery of pirfenidone in idiopathic pulmonary fibrosis. Int. J. Biol. Macromol. 2018, 118, 1319–1325. [Google Scholar] [CrossRef]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic acid-mediated drug delivery system targeting for inflammatory skin diseases: A mini review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Montanari, E.; Mancini, P.; Galli, F.; Varani, M.; Santino, I.; Coviello, T.; Mosca, L.; Matricardi, P.; Rancan, F.; Di Meo, C. Biodistribution and intracellular localization of hyaluronan and its nanogels. A strategy to target intracellular S. aureus in persistent skin infections. J. Control. Release 2020, 326, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Zhu, T.; Wu, X.; Yu, W.; Zhu, J.; Shang, Y.; Lin, X.; Zhao, T. Targeting delivery and minimizing epidermal diffusion of tranexamic acid by hyaluronic acid-coated liposome nanogels for topical hyperpigmentation treatment. Drug Deliv. 2021, 28, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.; Ki, C.S. Modular formation of hyaluronic acid/β-glucan hybrid nanogels for topical dermal delivery targeting skin dendritic cells. Carbohydr. Polym. 2021, 252, 117132. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Sonzogni, A.S.; Calderon, M. Can dermal delivery of therapeutics be improved using thermoresponsive nanogels? Future Med. 2019, 14, 2891–2895. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels For Topical Chemotherapy of Aggressive Melanoma. Colloids Surf. B Biointerfaces 2019, 174, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Jain, S.; Sau, S.; Iyer, A.K. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: In vitro and ex vivo studies. J. Control. Release 2017, 253, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Urias, A.; Zapata-Gonzalez, I.; Licea-Claverie, A.; Licea-Navarro, A.F.; Bernaldez-Sarabia, J.; Cervantes-Luevano, K. Cationic versus anionic core-shell nanogels for transport of cisplatin to lung cancer cells. Colloids Surf. B Biointerfaces 2019, 182, 110365. [Google Scholar] [CrossRef] [PubMed]
- Divya, G.; Panonnummal, R.; Gupta, S.; Jayakumar, R.; Sabitha, M. Acitretin and aloe-emodin loaded chitin nanogel for the treatment of psoriasis. Eur. J. Pharm. Biopharm. 2016, 107, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Ghaeini-Hesaroeiye, S.; Boddohi, S.; Vasheghani-Farahani, E. Dual responsive chondroitin sulfate based nanogel for antimicrobial peptide delivery. Int. J. Biol. Macromol. 2020, 143, 297–304. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L.; Siu, W.S.; Kan, C.-W.; Leung, P.-C.; Wanxue, C.; Chiou, J.-C. Influence of pH-responsive compounds synthesized from chitosan and hyaluronic acid on dual-responsive (pH/temperature) hydrogel drug delivery systems of Cortex Moutan. Int. J. Biol. Macromol. 2021, 168, 163–174. [Google Scholar] [CrossRef]
- Vogt, A.; Wischke, C.; Neffe, A.T.; Ma, N.; Alexiev, U.; Lendlein, A. Nanocarriers for drug delivery into and through the skin —Do existing technologies match clinical challenges? J. Control. Release 2016, 242, 3–15. [Google Scholar] [CrossRef]
- Sahle, F.F.; Giulbudagian, M.; Bergueiro, J.; Lademann, J.; Calderón, M. Dendritic polyglycerol and N-isopropylacrylamide based thermoresponsive nanogels as smart carriers for controlled delivery of drugs through the hair follicle. Nanoscale 2017, 9, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Asadian-Birjand, M.; Dogan, S.; Graf, C.; Cuellar, L.; Lommatzsch, S.; Blume-Peytavi, U.; Calderón, M.; Vogt, A. Effects of thermoresponsivity and softness on skin penetration and cellular uptake of polyglycerol-based nanogels. J. Control. Release 2016, 228, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Zavgorodnya, O.; Carmona-Moran, C.A.; Kozlovskaya, V.; Liu, F.; Wick, T.M.; Kharlampieva, E. Temperature-responsive nanogel multilayers of poly(N-vinylcaprolactam) for topical drug delivery. J. Colloid Interface Sci. 2017, 506, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Giulbudagian, M.; Hönzke, S.; Bergueiro, J.; Işık, D.; Schumacher, F.; Saeidpour, S.; Lohan, S.; Meinke, M.; Teutloff, C.; Schäfer-Korting, M. Enhanced topical delivery of dexamethasone by β-cyclodextrin decorated thermoresponsive nanogels. Nanoscale 2018, 10, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Moran, C.A.; Zavgorodnya, O.; Penman, A.D.; Kharlampieva, E.; Bridges, S.L.; Hergenrother, R.W.; Singh, J.A.; Wick, T.M. Development of gellan gum containing formulations for transdermal drug delivery: Component evaluation and controlled drug release using temperature responsive nanogels. Int. J. Pharm. 2016, 509, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, J.; Yang, K.; Cai, P.; Xiao, H. Novel multi-responsive and sugarcane bagasse cellulose-based nanogels for controllable release of doxorubicin hydrochloride. Mater. Sci. Eng. C 2021, 118, 111357. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-based ocular drug delivery systems: Recent advances and future prospects. J. Nanobiotechnol. 2023, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Ansong, M.; Pal, D. Prodrugs and nanomicelles to overcome ocular barriers for drug penetration. Expert Opin. Drug Metab. Toxicol. 2020, 16, 885–906. [Google Scholar] [CrossRef]
- Dave, R.S.; Goostrey, T.C.; Ziolkowska, M.; Czerny-Holownia, S.; Hoare, T.; Sheardown, H. Ocular drug delivery to the anterior segment using nanocarriers: A mucoadhesive/mucopenetrative perspective. J. Control. Release 2021, 336, 71–88. [Google Scholar] [CrossRef]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The impact of oxidative stress on blood-retinal barrier physiology in age-related macular degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lockwood, A. Topical ocular drug delivery systems: Innovations for an unmet need. Exp. Eye Res. 2022, 218, 109006. [Google Scholar] [CrossRef] [PubMed]
- Gause, S.; Hsu, K.-H.; Shafor, C.; Dixon, P.; Powell, K.C.; Chauhan, A. Mechanistic modeling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses. Adv. Colloid Interface Sci. 2016, 233, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.-L.; Dinu-Pîrvu, C.-E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Buosi, F.S.; Alaimo, A.; Di Santo, M.C.; Elías, F.; García Liñares, G.; Acebedo, S.L.; Castañeda Cataña, M.A.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol encapsulation in high molecular weight chitosan-based nanogels for applications in ocular treatments: Impact on human ARPE-19 culture cells. Int. J. Biol. Macromol. 2020, 165, 804–821. [Google Scholar] [CrossRef] [PubMed]
- Laradji, A.M.; Kolesnikov, A.V.; Karakoçak, B.B.; Kefalov, V.J.; Ravi, N. Redox-responsive hyaluronic acid-based nanogels for the topical delivery of the visual chromophore to retinal photoreceptors. ACS Omega 2021, 6, 6172–6184. [Google Scholar] [CrossRef] [PubMed]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Bian, S.; Li, Z.; Zhang, Z.; Liu, Y.; Zhai, X.; Pan, H.; Zhao, X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826. [Google Scholar] [CrossRef]
- Hammi, N.; El Hankari, S.; Katir, N.; Marcotte, N.; Draoui, K.; Royer, S.; El Kadib, A. Polysaccharide templated biomimetic growth of hierarchically porous metal-organic frameworks. Microporous Mesoporous Mater. 2020, 306, 110429. [Google Scholar] [CrossRef]
- Chaharband, F.; Daftarian, N.; Kanavi, M.R.; Varshochian, R.; Hajiramezanali, M.; Norouzi, P.; Arefian, E.; Atyabi, F.; Dinarvand, R. Trimethyl chitosan-hyaluronic acid nano-polyplexes for intravitreal VEGFR-2 siRNA delivery: Formulation and in vivo efficacy evaluation. Nanomed. Nanotechnol. Biol. Med. 2020, 26, 102181. [Google Scholar] [CrossRef]
- Silva, B.; Marto, J.; Braz, B.S.; Delgado, E.; Almeida, A.J.; Gonçalves, L. New nanoparticles for topical ocular delivery of erythropoietin. Int. J. Pharm. 2020, 576, 119020. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sheshala, R.; Kok, Y.Y.; Ng, J.M.; Thakur, R.; Dua, K. In Situ Gelling Ophthalmic Drug Delivery System: An Overview and Its Applications. Recent Pat. Drug Deliv. Formul. 2015, 9, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Lavikainen, J.; Dauletbekova, M.; Toleutay, G.; Kaliva, M.; Chatzinikolaidou, M.; Kudaibergenov, S.E.; Tenkovtsev, A.; Khutoryanskiy, V.V.; Vamvakaki, M.; Aseyev, V. Poly (2-ethyl-2-oxazoline) grafted gellan gum for potential application in transmucosal drug delivery. Polym. Adv. Technol. 2021, 32, 2770–2780. [Google Scholar] [CrossRef]
- Karmakar, S.; Manna, S.; Kabiraj, S.; Jana, S. Recent progress in alginate-based carriers for ocular targeting of therapeutics. Food Hydrocoll. Health 2022, 2, 100071. [Google Scholar] [CrossRef]
- Al-Juboori, Z.A.; Mahdi, Z.H.; Alhamdany, A.T. Formulation and Evaluation of ocular in-Situ gelling system containing ciprofloxacin and Naproxen Sodium. Res. J. Pharm. Technol. 2021, 14, 91–95. [Google Scholar] [CrossRef]
- Nagai, N.; Minami, M.; Deguchi, S.; Otake, H.; Sasaki, H.; Yamamoto, N. An in situ gelling system based on methylcellulose and tranilast solid nanoparticles enhances ocular residence time and drug absorption into the cornea and conjunctiva. Front. Bioeng. Biotechnol. 2020, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, A.; Giri, T.K. Polysaccharide as renewable responsive biopolymer for in situ gel in the delivery of drug through ocular route. Int. J. Biol. Macromol. 2020, 150, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, P.; Kumar, M.; Pathak, K. Norfloxacin Loaded pH Triggered Nanoparticulate in-situ Gel for Extraocular Bacterial Infections: Optimization, Ocular Irritancy and Corneal Toxicity. Iran J. Pharm. Res. 2016, 15, 3–22. [Google Scholar]
- Liu, R.; Sun, L.; Fang, S.; Wang, S.; Chen, J.; Xiao, X.; Liu, C. Thermosensitive in situ nanogel as ophthalmic delivery system of curcumin: Development, characterization, in vitro permeation and in vivo pharmacokinetic studies. Pharm. Dev. Technol. 2016, 21, 576–582. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Jiang, J.; Li, C.; Zhang, H.; Zhang, X.; Jiang, T.; Wang, L.; Wang, Y.; Feng, L. Micro-Nanocarriers Based Drug Delivery Technology for Blood-Brain Barrier Crossing and Brain Tumor Targeting Therapy. Small 2022, 18, 2203678. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.S.; Bu, Y.; Lo, A.C.-Y.; Chan, T.C.-Y.; So, K.F.; Lai, J.S.-M. A systematic review of potential therapeutic use of Lycium barbarum polysaccharides in disease. BioMed Res. Int. 2019, 2019, 4615745. [Google Scholar] [CrossRef] [PubMed]
- Cortés, H.; Alcalá-Alcalá, S.; Caballero-Florán, I.H.; Bernal-Chávez, S.A.; Ávalos-Fuentes, A.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Floran, B.; et al. A Reevaluation of Chitosan-Decorated Nanoparticles to Cross the Blood-Brain Barrier. Membranes 2020, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Caprifico, A.E.; Foot, P.J.S.; Polycarpou, E.; Calabrese, G. Overcoming the Blood-Brain Barrier: Functionalised Chitosan Nanocarriers. Pharmaceutics 2020, 12, 1013. [Google Scholar] [CrossRef]
- Curcio, M.; Cirillo, G.; Rouaen, J.R.C.; Saletta, F.; Nicoletta, F.P.; Vittorio, O.; Iemma, F. Natural Polysaccharide Carriers in Brain Delivery: Challenge and Perspective. Pharmaceutics 2020, 12, 1183. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Atluri, V.; Raymond, A.; Kaushik, A.; Parira, T.; Huang, Z.; Durygin, A.; Tomitaka, A.; Nikkhah-Moshaie, R.; Vashist, A. Development of multifunctional biopolymeric auto-fluorescent micro-and nanogels as a platform for biomedical applications. Front. Bioeng. Biotechnol. 2020, 8, 315. [Google Scholar] [CrossRef]
- Azadi, A.; Hamidi, M.; Rouini, M.-R. Methotrexate-loaded chitosan nanogels as ‘Trojan Horses’ for drug delivery to brain: Preparation and in vitro/in vivo characterization. Int. J. Biol. Macromol. 2013, 62, 523–530. [Google Scholar] [CrossRef]
- Pourtalebi Jahromi, L.; Moghaddam Panah, F.; Azadi, A.; Ashrafi, H. A mechanistic investigation on methotrexate-loaded chitosan-based hydrogel nanoparticles intended for CNS drug delivery: Trojan horse effect or not? Int. J. Biol. Macromol. 2019, 125, 785–790. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Lalatsa, A.; Górecki, D.C.; Barbu, E. Engineering butylglyceryl-modified polysaccharides towards nanomedicines for brain drug delivery. Carbohydr. Polym. 2020, 236, 116060. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.G.M.; et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.; Rasal, N.; Sonawane, R.; Sekar, M.; Kokare, C. Nose-to-brain delivery of teriflunomide-loaded lipid-based carbopol-gellan gum nanogel for glioma: Pharmacological and in vitro cytotoxicity studies. Int. J. Biol. Macromol. 2021, 167, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Ourani-Pourdashti, S.; Mirzaei, E.; Heidari, R.; Ashrafi, H.; Azadi, A. Preparation and evaluation of niosomal chitosan-based in situ gel formulation for direct nose-to-brain methotrexate delivery. Int. J. Biol. Macromol. 2022, 213, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gu, Z.; An, H.; Chen, C.; Chen, J.; Cui, R.; Chen, S.; Chen, W.; Chen, X.; Chen, X.; et al. Precise nanomedicine for intelligent therapy of cancer. Sci. China Chem. 2018, 61, 1503–1552. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, C.; Yong, T.; Li, Z.; Gan, L.; Yang, X. Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem. Soc. Rev. 2020, 49, 2273–2290. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, Y.; Zeng, H.; Wang, C.; Xu, C.; Wang, Q.; Wang, H.; Li, S.; Chen, J.; Xiao, C. Mechano-boosting nanomedicine antitumour efficacy by blocking the reticuloendothelial system with stiff nanogels. Nat. Commun. 2023, 14, 1437. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, Z.; Cui, J. Modulation of Colloidal Particle Stiffness for the Exploration of Bio–Nano Interactions. Langmuir 2022, 38, 6780–6785. [Google Scholar] [CrossRef]



| Author | Type of Nanogel | Function and Mechanism | Size/Zeta Potential | Loaded Cargo | Year | Ref. |
|---|---|---|---|---|---|---|
| Mahmood et al. | Thiolated chitosan | Mucosal adhesion | 26.30 ± 26.86 nm 0.03 mV | Curcumin | 2017 | [53] |
| Jin et al. | Thiolated chitosan | Mucosal adhesion | 196.72 ± 0.45 nm 17.12 ± 0.50 mV | Bovine serum albumin | 2022 | [54] |
| Guaresti et al. | Thiolated chitosan | Mucosal adhesion | 5–12 nm 36 ± 4 to −7 ± 1 mV | / | 2020 | [55] |
| Shtenberg et al. | PEG modified alginate | Mucosal adhesion | / | Ibuprofen sodium | 2017 | [57] |
| Tian et al. | Thiolated hyaluronic acid | Mucus penetration | Average 100 nm −26.2 ± 1.0 mV | INS | 2018 | [61] |
| Cao et al. | Chitosan | Mucus penetrationMuco-adhesive | / | Oral Vaccination | 2021 | [63] |
| Ways et al. | Chitosan | Mucus penetrating | 145 ± 21 nm 15.0 ± 0.3 mV | / | 2022 | [65] |
| Author | Type of Nanogel | Function and Mechanism | Size/Zeta Potential | Loaded Cargo | Type of Study | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Vashist et al. | Chitosan, hydroxyethyl cellulose | Auto-fluorescence, promote penetration | / | Bioactives | In vitro | 2020 | [130] |
| Pourtalebi Jahromi et al. | Chitosan | Enhanced pernetration | <200 nm, 22.8 ± 6.55 mV | Methotrexate | In vitro | 2019 | [132] |
| Bostanudin et al. | Guar, pullulan, chitosan | Enhanced permeation | 120–200 nm, −23~−32 mV | Doxorubicin | In vitro | 2020 | [133] |
| Gadhave et al. | Gellan, Carbopol | Enhanced permeation, in situ gel, nose-to-brain delivery | 117.80 nm, −21.86 mV | Teriflunomide | In vitro | 2021 | [135] |
| Ourani-Pourdashti et al. | Chitosan, Poloxamer 407 | Temperature-sensitive in situ gel, nose-to-brain delivery | 130.5 nm, −38.5 mV | Methotrexate | In vitro In vivo | 2022 | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Viola, M.; Migliorini, C.; Paoletti, L.; Arpicco, S.; Di Meo, C.; Matricardi, P. Polysaccharide-Based Nanogels to Overcome Mucus, Skin, Cornea, and Blood-Brain Barriers: A Review. Pharmaceutics 2023, 15, 2508. https://doi.org/10.3390/pharmaceutics15102508
Wang J, Viola M, Migliorini C, Paoletti L, Arpicco S, Di Meo C, Matricardi P. Polysaccharide-Based Nanogels to Overcome Mucus, Skin, Cornea, and Blood-Brain Barriers: A Review. Pharmaceutics. 2023; 15(10):2508. https://doi.org/10.3390/pharmaceutics15102508
Chicago/Turabian StyleWang, Ju, Marco Viola, Claudia Migliorini, Luca Paoletti, Silvia Arpicco, Chiara Di Meo, and Pietro Matricardi. 2023. "Polysaccharide-Based Nanogels to Overcome Mucus, Skin, Cornea, and Blood-Brain Barriers: A Review" Pharmaceutics 15, no. 10: 2508. https://doi.org/10.3390/pharmaceutics15102508





