Electrospun Scaffolds of Polylactic Acid, Collagen, and Amorphous Calcium Phosphate for Bone Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amorphous Calcium Phosphate
2.3. HAP and ACP Characterization
2.4. Electrospun Scaffold Fabrication
2.5. Scaffold Characterization
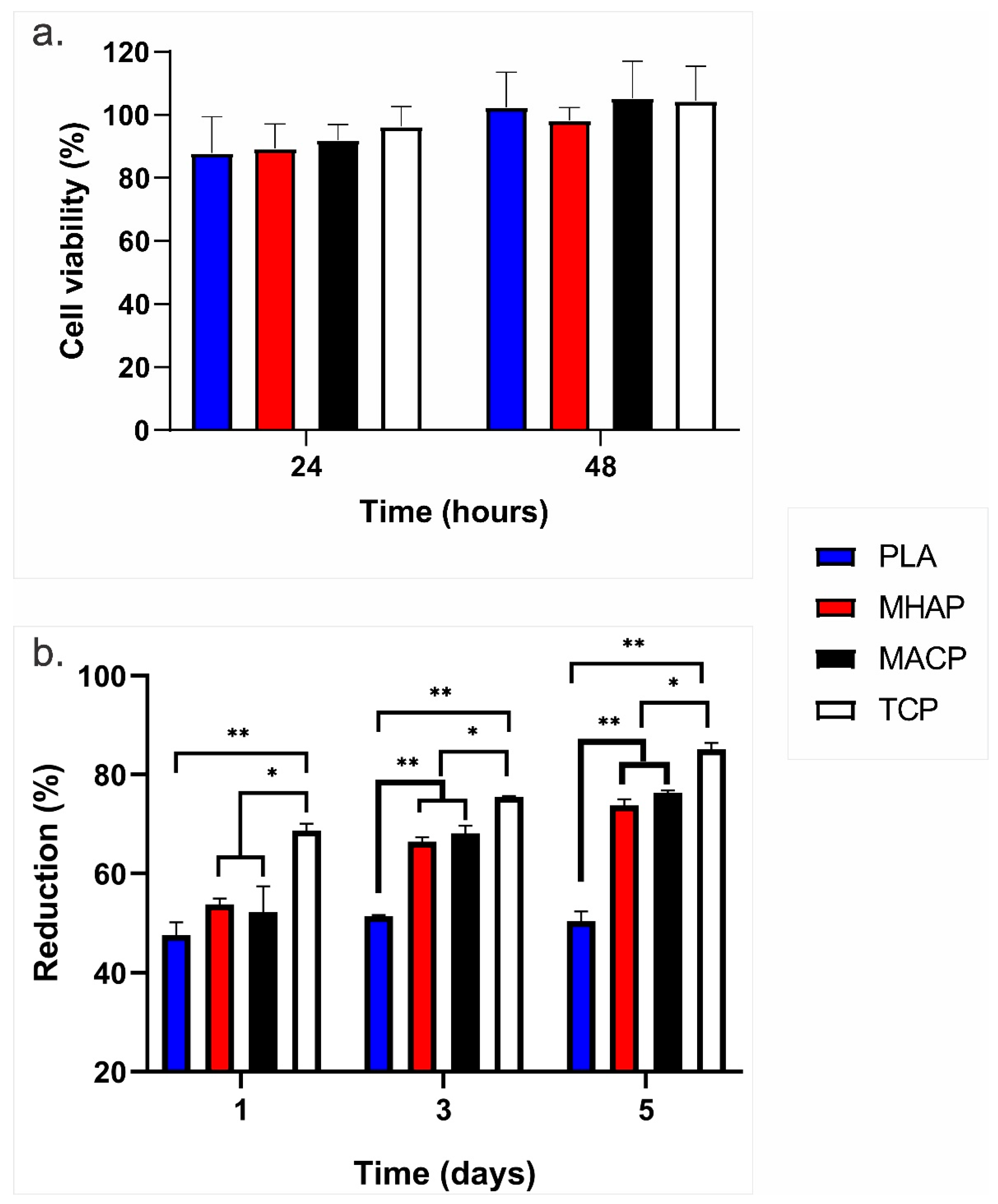
2.6. Cytotoxicity Assay
2.7. Cell Adhesion and Proliferation
2.8. Secretion of Growth Factors
2.9. Osteogenic Differentiation
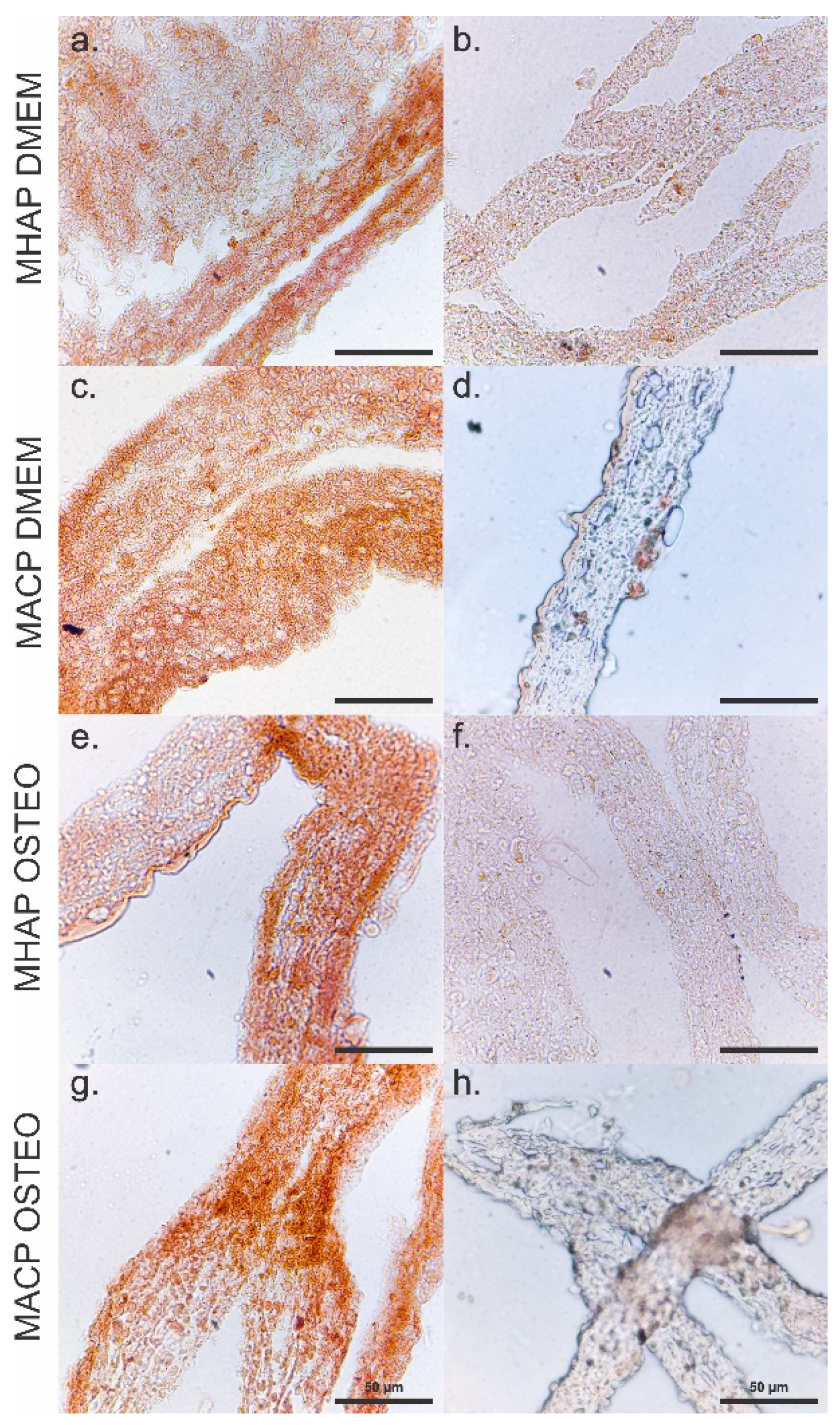
2.10. Immunohistochemical Analysis
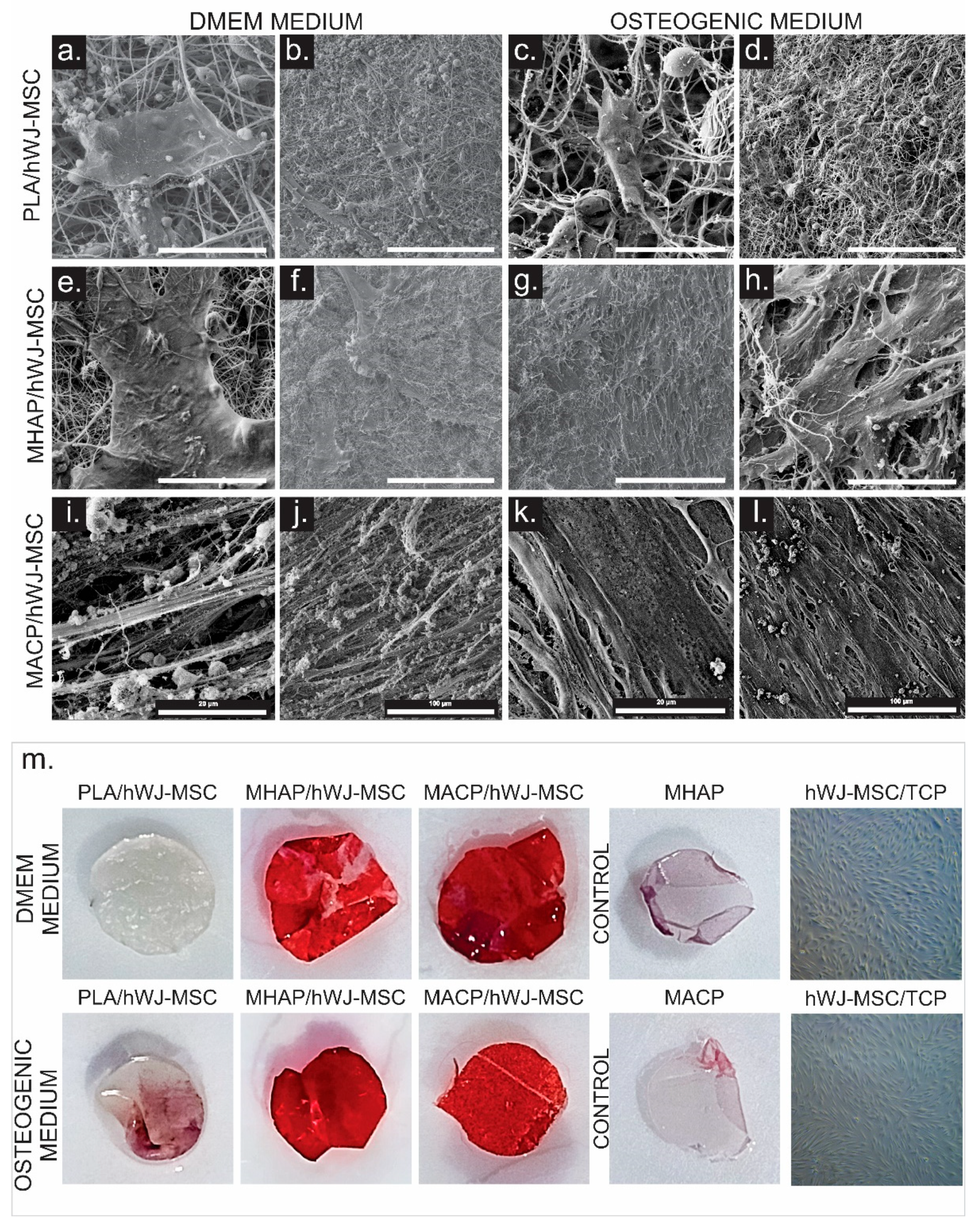
2.11. SEM Imaging of Cells
2.12. Statistical Analysis
3. Results
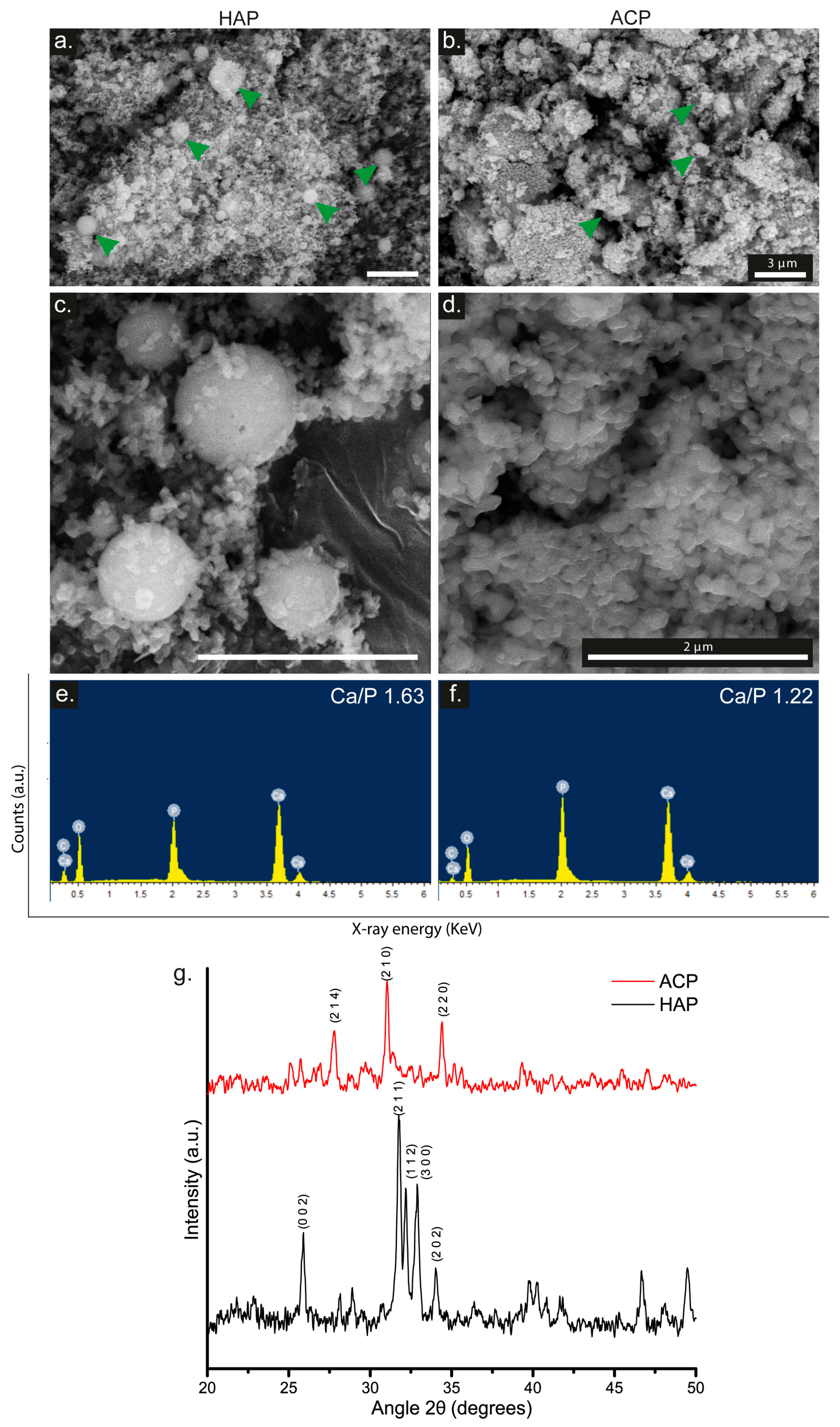
3.1. HAP and ACP Characterization
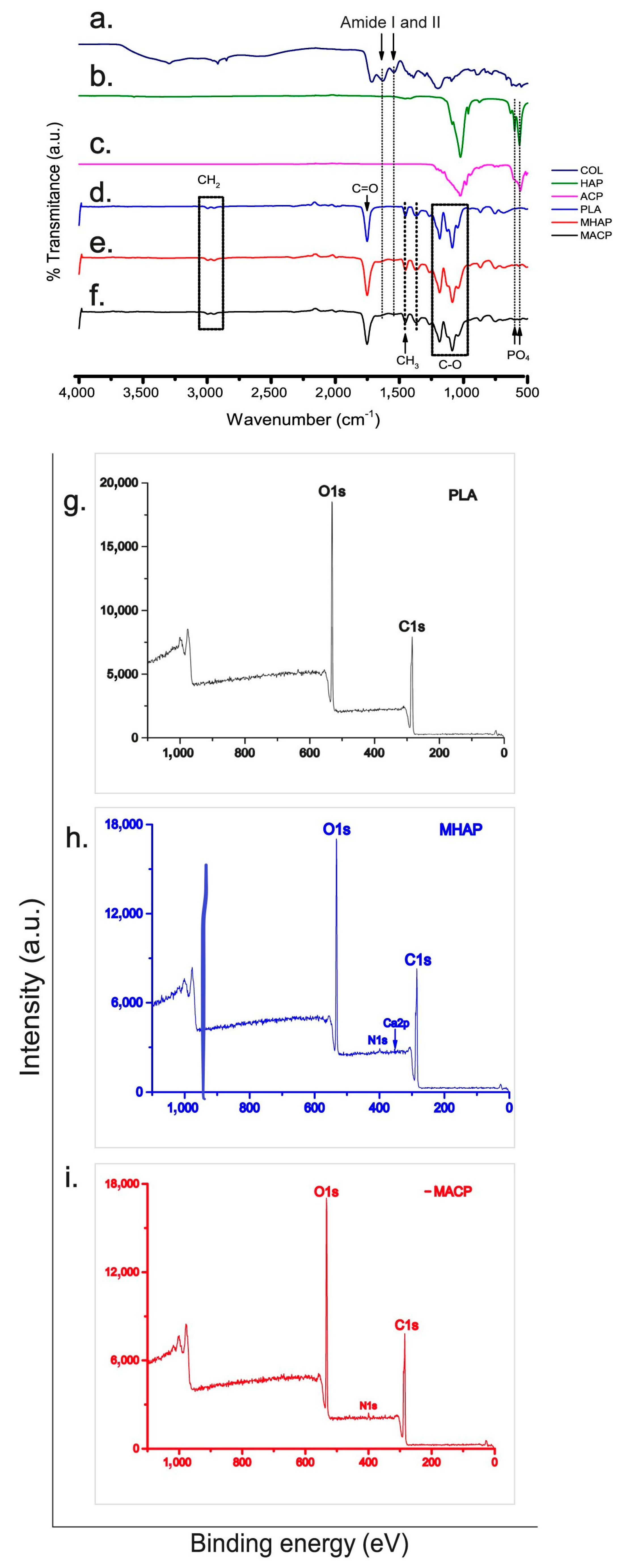
3.2. FTIR-ATR of Electrospun Scaffolds
3.3. XPS
3.4. SEM
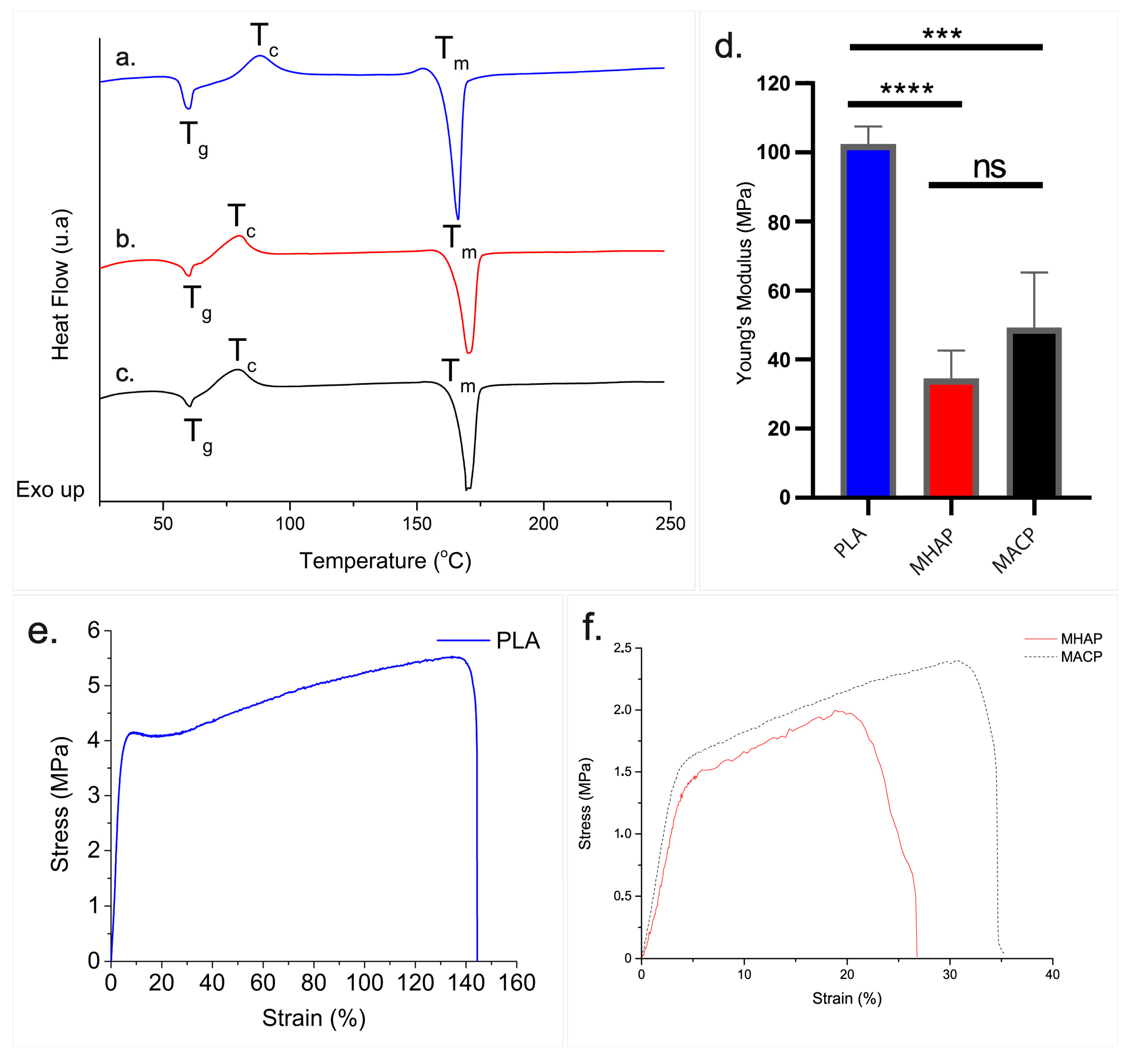
3.5. DSC
3.6. Young’s Modulus of Scaffolds
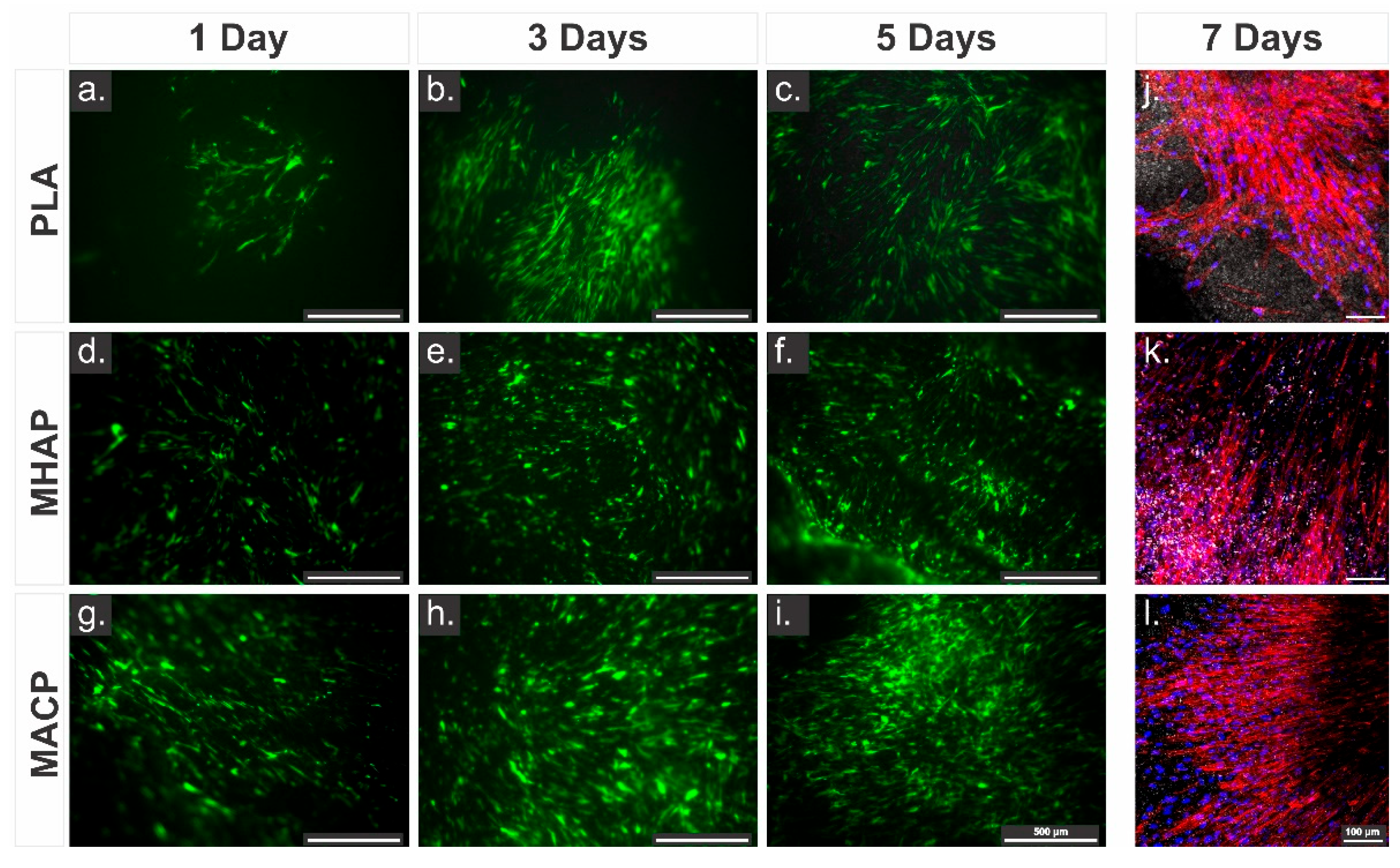
3.7. Adhesion and Proliferation Cellular
3.8. Quantification of Growth Factors
3.9. Calcium Phosphate Compounds-Containing Scaffolds Induce the Osteogenic Differentiation of hWJ-MSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7, 485383. [Google Scholar] [CrossRef]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing Application of Mesenchymal Stem Cell-Based Bone Tissue Regeneration. Bioact. Mater. 2021, 6, 666–683. [Google Scholar] [CrossRef]
- Chandra, P.K.; Soker, S.; Atala, A. Tissue Engineering: Current Status and Future Perspectives. Princ. Tissue Eng. 2020, 1–35. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Fusco, A.; Contaldo, M.; Maleki, H.; Azimi, B.; Ismaeilimoghadam, S.; Danti, S. Poly (Lactic Acid)-Based Electrospun Fibrous Structures for Biomedical Applications. Appl. Sci. 2022, 12, 3192. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, C.; Bai, H.; Zhang, J.; Wang, Z.; Li, Z.; Zhao, X.; Wang, J.; Liu, H. Functionalization of Biomimetic Mineralized Collagen for Bone Tissue Engineering. Mater. Today Bio. 2023, 20, 100660. [Google Scholar] [CrossRef] [PubMed]
- Edén, M. Structure and Formation of Amorphous Calcium Phosphate and Its Role as Surface Layer of Nanocrystalline Apatite: Implications for Bone Mineralization. Materialia 2021, 17, 101107. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Shaheen, M.Y. Nanocrystalline Hydroxyapatite in Periodontal Bone Regeneration: A Systematic Review. Saudi Dent. J. 2022, 34, 647–660. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing Methods for Making Porous Bioactive Glass-Based Scaffolds—A State-of-the-Art Review. Int. J. Appl. Ceram. Technol. 2019, 16, 1762–1796. [Google Scholar] [CrossRef]
- Espitia-Quiroz, L.C.; Fernández-Orjuela, A.L.; Anaya-Sampayo, L.M.; Acosta-Gómez, A.P.; Sequeda-Castañeda, L.G.; Gutiérrez-Prieto, S.J.; Roa-Molina, N.S.; García-Robayo, D.A. Viability and Adhesion of Periodontal Ligament Fibroblasts on a Hydroxyapatite Scaffold Combined with Collagen, Polylactic Acid–Polyglycolic Acid Copolymer and Platelet-Rich Fibrin: A Preclinical Pilot Study. Dent. J. 2022, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Garkal, A.; Kulkarni, D.; Musale, S.; Mehta, T.; Giram, P. Electrospinning Nanofiber Technology: A Multifaceted Paradigm in Biomedical Applications. New J. Chem. 2021, 45, 21508–21533. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Cheng, D.; Xu, S.; Du, C.; Xie, L.; Zhao, W. Applications of Electrospun Scaffolds with Enlarged Pores in Tissue Engineering. Biomater. Sci. 2022, 10, 1423–1447. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ramakrishna, S.; Liu, X. Electrospinning and Emerging Healthcare and Medicine Possibilities. APL Bioeng. 2020, 4, 030901. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef] [PubMed]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef]
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite Scaffolds Exhibit in Vitro Immunological Inertness and Promote Robust Osteogenic Differentiation of Human Mesenchymal Stem Cells without Osteogenic Stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Santhakumar, S.; Oyane, A.; Nakamura, M.; Koga, K.; Miyata, S.; Muratsubaki, K.; Miyaji, H. In Situ Precipitation of Amorphous Calcium Phosphate Nanoparticles within 3D Porous Collagen Sponges for Bone Tissue Engineering. Mater. Sci. Eng. C 2020, 116, 111194. [Google Scholar] [CrossRef]
- Hoeher, A.J.; Mergelsberg, S.T.; Borkiewicz, O.J.; Michel, F.M. Impacts of Initial Ca/P on Amorphous Calcium Phosphate. Cryst. Growth Des. 2021, 21, 3736–3745. [Google Scholar] [CrossRef]
- Bahremandi-Toloue, E.; Mohammadalizadeh, Z.; Mukherjee, S.; Karbasi, S. Incorporation of Inorganic Bioceramics into Electrospun Scaffolds for Tissue Engineering Applications: A Review. Ceram. Int. 2022, 48, 8803–8837. [Google Scholar] [CrossRef]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Healthc. Mater. 2020, 9, 2000707. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Z.; Tian, F.; Chen, S.; Lei, L.; Jiang, T.; Feng, Q.; Fan, Y. Sustained Delivery of Calcium and Orthophosphate Ions from Amorphous Calcium Phosphate and Poly (L-Lactic Acid)-Based Electrospinning Nanofibrous Scaffold. Sci. Rep. 2017, 7, 45655. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, F.; Zhu, Y.-J.; Cui, T.; Liu, X.-Y. Amorphous Calcium Phosphate/Poly (D, L-Lactic Acid) Composite Nanofibers: Electrospinning Preparation and Biomineralization. J. Colloid Interface Sci. 2011, 359, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, Q.-W.; Sun, T.-W.; Chen, F.; Qi, C.; Wu, J.; Cai, Z.-Y.; Qian, Q.-R.; Zhu, Y.-J. Amorphous Calcium Phosphate, Hydroxyapatite and Poly (D, L-Lactic Acid) Composite Nanofibers: Electrospinning Preparation, Mineralization and in Vivo Bone Defect Repair. Colloids Surfaces B Biointerfaces 2015, 136, 27–36. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Wang, Z.; Tong, H.; Ma, L.; Zhang, Y.; Shan, F.; Meng, Y.; Yuan, Z. Comparative Analysis of Human Mesenchymal Stem Cells from Fetal-Bone Marrow, Adipose Tissue, and Warton’s Jelly as Sources of Cell Immunomodulatory Therapy. Hum. Vaccines Immunother. 2016, 12, 85–96. [Google Scholar] [CrossRef]
- Manochantr, S.; U-pratya, Y.; Kheolamai, P.; Rojphisan, S.; Chayosumrit, M.; Tantrawatpan, C.; Supokawej, A.; Issaragrisil, S. Immunosuppressive Properties of Mesenchymal Stromal Cells Derived from Amnion, Placenta, W Harton’s Jelly and Umbilical Cord. Intern. Med. J. 2013, 43, 430–439. [Google Scholar] [CrossRef]
- Liu, S.; Jia, Y.; Yuan, M.; Guo, W.; Huang, J.; Zhao, B.; Peng, J.; Xu, W.; Lu, S.; Guo, Q. Repair of Osteochondral Defects Using Human Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells in a Rabbit Model. Biomed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Abidi, S.S.A.; Murtaza, Q. Synthesis and Characterization of Nano-Hydroxyapatite Powder Using Wet Chemical Precipitation Reaction. J. Mater. Sci. Technol. 2014, 30, 307–310. [Google Scholar] [CrossRef]
- Akkouch, A.; Zhang, Z.; Rouabhia, M. A Novel Collagen/Hydroxyapatite/Poly(Lactide-Co-ε-Caprolactone) Biodegradable and Bioactive 3D Porous Scaffold for Bone Regeneration. J. Biomed. Mater. Res. A 2011, 96, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pachón, E.; Vera-Graziano, R.; Montiel, R.; Cardenas-Aguazaco, W.; Ochica, A.F.; Gómez-Pachón, E.; Vera-Graziano, R.; Montiel, R.; Cardenas-Aguazaco, W.; Ochica, A.F. Effects of Heat Treatment and the Method of Collection on the Crystal Structure of the Poly Lactic Acid Electrospinned Nanofibers. Ingeniare. Rev. Chil. Ing. 2019, 27, 586–599. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/36406.html (accessed on 2 October 2023).
- Miguez, M.; Sabarots, M.G.; Cid, M.P.; Salvatierra, N.A.; Comín, R. Fabrication and Characterization of Gelatin/Calcium Phosphate Electrospun Composite Scaffold for Bone Tissue Engineering. Fibers Polym. 2022, 23, 1915–1923. [Google Scholar] [CrossRef]
- Lee, A.; Jain, A. Measuring Cell-Viability by Resazurin (Alamarblue®) Assay Using Photopette® Cell. Appl. note, Tip Biosyst. Cell Viability Life Sci. 2017. Available online: http//tipbiosystems.com/tbs/wpcontent/uploads/2017/06/AN016-Cell-Viability-using-Resazurin-Dye_2017_05_29.pdf (accessed on 15 May 2020).
- Lopresti, F.; Carfì Pavia, F.; Vitrano, I.; Kersaudy-Kerhoas, M.; Brucato, V.; La Carrubba, V. Effect of Hydroxyapatite Concentration and Size on Morpho-Mechanical Properties of PLA-Based Randomly Oriented and Aligned Electrospun Nanofibrous Mats. J. Mech. Behav. Biomed. Mater. 2020, 101, 103449. [Google Scholar] [CrossRef]
- Munir, M.U.; Salman, S.; Ihsan, A.; Elsaman, T. Synthesis, Characterization, Functionalization and Bio-Applications of Hydroxyapatite Nanomaterials: An Overview. Int. J. Nanomed. 2022, 17, 1903. [Google Scholar] [CrossRef]
- Padmanabhan, V.P.; Kulandaivelu, R.; Panneer, D.S.; Vivekananthan, S.; Sagadevan, S.; Lett, J.A. Microwave Synthesis of Hydroxyapatite Encumbered with Ascorbic Acid Intended for Drug Leaching Studies. Mater. Res. Innov. 2019, 24, 171–178. [Google Scholar] [CrossRef]
- Xidaki, D.; Agrafioti, P.; Diomatari, D.; Kaminari, A.; Tsalavoutas-Psarras, E.; Alexiou, P.; Psycharis, V.; Tsilibary, E.C.; Silvestros, S.; Sagnou, M. Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and in Vitro Studies. Materials 2018, 11, 595. [Google Scholar] [CrossRef]
- Xie, Q.; Guo, G.; Lu, W.; Sun, C.; Zhou, J.; Zheng, Y.; Shan, G.; Bao, Y.; Pan, P. Polymorphic Homocrystallization and Phase Behavior of High-Molecular-Weight Poly (L-Lactic Acid)/Poly (D-Lactic Acid) Racemic Mixture with Intentionally Enhanced Stereocomplexation Ability via Miscible Blending. Polymer 2020, 201, 122597. [Google Scholar] [CrossRef]
- Gieroba, B.; Przekora, A.; Kalisz, G.; Kazimierczak, P.; Song, C.L.; Wojcik, M.; Ginalska, G.; Kazarian, S.G.; Sroka-Bartnicka, A. Collagen Maturity and Mineralization in Mesenchymal Stem Cells Cultured on the Hydroxyapatite-Based Bone Scaffold Analyzed by ATR-FTIR Spectroscopic Imaging. Mater. Sci. Eng. C 2021, 119, 111634. [Google Scholar] [CrossRef]
- Apalangya, V.A.; Rangari, V.K.; Tiimob, B.J.; Jeelani, S.; Samuel, T. Eggshell Based Nano-Engineered Hydroxyapatite and Poly(Lactic) Acid Electrospun Fibers as Potential Tissue Scaffold. Int. J. Biomater. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Orsini, T.; Morra, M.; Iviglia, G.; Valbonetti, L. Biomimetic Surfaces Coated with Covalently Immobilized Collagen Type I: An X-Ray Photoelectron Spectroscopy, Atomic Force Microscopy, Micro-CT and Histomorphometrical Study in Rabbits. Int. J. Mol. Sci. 2019, 20, 724. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, Y.; Zhang, A.; Zhang, H. Characterization and Physical Properties of Electrospun Gelatin Nanofibrous Films by Incorporation of Nano-Hydroxyapatite. Food Hydrocoll. 2020, 103, 105640. [Google Scholar] [CrossRef]
- Demri, B.; Muster, D. XPS Study of Some Calcium Compounds. J. Mater. Process. Technol. 1995, 55, 311–314. [Google Scholar] [CrossRef]
- Murphy, B.; Baez, J.; Morris, M.A. Characterising Hydroxyapatite Deposited from Solution onto Novel Substrates: Growth Mechanism and Physical Properties. Nanomaterials 2023, 13, 2483. [Google Scholar] [CrossRef] [PubMed]
- Leonés, A.; Peponi, L.; Lieblich, M.; Benavente, R.; Fiori, S. In Vitro Degradation of Plasticized PLA Electrospun Fiber Mats: Morphological, Thermal and Crystalline Evolution. Polymers 2020, 12, 2975. [Google Scholar] [CrossRef]
- Vidović, E.; Faraguna, F.; Jukić, A. Influence of Inorganic Fillers on PLA Crystallinity and Thermal Properties. J. Therm. Anal. Calorim. 2017, 127, 371–380. [Google Scholar] [CrossRef]
- Seraji, A.A.; Goharpey, F.; Khademzadeh Yeganeh, J. Highly Crystallized and Tough Polylactic Acid through Addition of Surface Modified Cellulose Nanocrystals. J. Appl. Polym. Sci. 2022, 139, e52871. [Google Scholar] [CrossRef]
- Dao, T.T.-T.; Nguyen, C.T.-H.; Vu, N.B.; Le, H.T.-N.; Nguyen, P.D.-N.; Van Pham, P. Evaluation of Proliferation and Osteogenic Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Porous Scaffolds. In Proceedings of the Tissue Engineering and Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2019; pp. 207–220. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef]
- Jenkins, T.L.; Little, D. Synthetic Scaffolds for Musculoskeletal Tissue Engineering: Cellular Responses to Fiber Parameters. NPJ Regen. Med. 2019, 4, 15. [Google Scholar] [CrossRef]
- Posa, F.; Di Benedetto, A.; Ravagnan, G.; Cavalcanti-Adam, E.A.; Lo Muzio, L.; Percoco, G.; Mori, G. Bioengineering Bone Tissue with 3D Printed Scaffolds in the Presence of Oligostilbenes. Materials 2020, 13, 4471. [Google Scholar] [CrossRef]
- Lv, Y.; Sang, X.; Tian, Z.; Jiang, S.; Li, C.; Guo, Q.; Wang, C.; Hu, P.; Liu, Y. Electrospun Hydroxyapatite Loaded L-Polylactic Acid Aligned Nanofibrous Membrane Patch for Rotator Cuff Repair. Int. J. Biol. Macromol. 2022, 217, 180–187. [Google Scholar] [CrossRef]
- Sanosh, K.P.; Chu, M.C.; Balakrishnan, A.; Kim, T.N.; Cho, S.J. Preparation and Characterization of Nano-Hydroxyapatite Powder Using Sol-Gel Technique. Bull. Mater. Sci. 2009, 32, 465–470. [Google Scholar] [CrossRef]
- Alrebaish, A.S.; Wilson, O.C., Jr. Mesocrystal Aggregation of Biological Apatite Nanocrystals. Med. Devices Sens. 2021, 4, e10155. [Google Scholar] [CrossRef]
- Boukha, Z.; Yeste, M.P.; Cauqui, M.Á.; Gonzalez-Velasco, J.R. Influence of Ca/P Ratio on the Catalytic Performance of Ni/Hydroxyapatite Samples in Dry Reforming of Methane. Appl. Catal. A Gen. 2019, 580, 34–45. [Google Scholar] [CrossRef]
- Bakan, F. A Systematic Study of the Effect of PH on the Initialization of Ca-Deficient Hydroxyapatite to β-TCP Nanoparticles. Materials 2019, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Vecstaudza, J.; Locs, J. Novel Preparation Route of Stable Amorphous Calcium Phosphate Nanoparticles with High Specific Surface Area. J. Alloys Compd. 2017, 700, 215–222. [Google Scholar] [CrossRef]
- Dorozhkin, S. V Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of Amorphous Calcium Phosphate to Bone-like Apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef]
- Barbosa, F.; Ferreira, F.C.; Silva, J.C. Piezoelectric Electrospun Fibrous Scaffolds for Bone, Articular Cartilage and Osteochondral Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 2907. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; Gómez-Lazaro, B.; López-Saucedo, F.; Vera-Graziano, R.; Bucio, E.; Mendizábal, E. Electrospun Scaffolds for Tissue Engineering: A Review. Macromol 2023, 3, 524–553. [Google Scholar] [CrossRef]
- Chen, W.; Zhong, C.; Li, S.; Wen, D.; Zhou, D.; Shao, J.; Chen, S.; Hou, H.; Xiang, S. The Crystallization Behavior of Poly (D-Lactic Acid)/Poly (L-Lactic Acid) Asymmetric Blends: Effect of Morphology of Stereocomplex Crystals on the Formation of Homochiral Crystals. J. Polym. Environ. 2023, 1–12. [Google Scholar] [CrossRef]
- Georgiopoulos, P.; Christopoulos, A.; Koutsoumpis, S.; Kontou, E. The Effect of Surface Treatment on the Performance of Flax/Biodegradable Composites. Compos. Part B Eng. 2016, 106, 88–98. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.P.; Feldmann, M. Impact Modified PLA-Hydroxyapatite Composites–Thermo-Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2018, 107, 326–333. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Lieblich, M.; Saldaña, L.; González-Carrasco, J.L.; Benavente, R. In Vitro Degradation of Biodegradable Polylactic Acid/Mg Composites: Influence of Nature and Crystalline Degree of the Polymeric Matrix. Materialia 2019, 6, 100270. [Google Scholar] [CrossRef]
- Leonés, A.; Salaris, V.; Mujica-Garcia, A.; Arrieta, M.P.; Lopez, D.; Lieblich, M.; Kenny, J.M.; Peponi, L. PLA Electrospun Fibers Reinforced with Organic and Inorganic Nanoparticles: A Comparative Study. Molecules 2021, 26, 4925. [Google Scholar] [CrossRef] [PubMed]
- Moncayo Donoso, M.Á. Desarrollo de Prototipos de Soportes Tridimensionales a Base de Colágeno Tipo I e Hidroxiapatita Para Regeneración Ósea. Ph.D. Thesis, Universidad de Málaga, Málaga, Spain, 2022. Available online: https://repositorio.unal.edu.co/handle/unal/77380 (accessed on 2 October 2023).
- Jeong, D.; Jang, J.; Lee, D.Y. Electrospun Poly (D, L-Lactic Acid)/Gelatin Membrane Using Green Solvent for Absorbable Periodontal Tissue Regeneration. J. Korean Cryst. Growth Cryst. Technol 2023, 33, 104–109. [Google Scholar]
- Chowdhury, S.R.; Mh Busra, M.F.; Lokanathan, Y.; Ng, M.H.; Law, J.X.; Cletus, U.C.; Binti Haji Idrus, R. Collagen Type I: A Versatile Biomaterial. Adv. Exp. Med. Biol. 2018, 1077, 389–414. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Notodihardjo, S.C.; Morimoto, N.; Kakudo, N.; Mitsui, T.; Le, T.M.; Tabata, Y.; Kusumoto, K. Comparison of the Efficacy of Cryopreserved Human Platelet Lysate and Refrigerated Lyophilized Human Platelet Lysate for Wound Healing. Regen. Ther. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Choi, H.; Jeong, B.C.; Hur, S.W.; Kim, J.W.; Lee, K.B.; Koh, J.T. The Angiopoietin-1 Variant COMP-Ang1 Enhances BMP2-Induced Bone Regeneration with Recruiting Pericytes in Critical Sized Calvarial Defects. PLoS ONE 2015, 10, e0140502. [Google Scholar] [CrossRef]
- Toosi, S.; Behravan, J. Osteogenesis and Bone Remodeling: A Focus on Growth Factors and Bioactive Peptides. Biofactors 2020, 46, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, X.; Shen, Y.; Meng, L. Cell–Scaffold Interactions in Tissue Engineering for Oral and Craniofacial Reconstruction. Bioact. Mater. 2023, 23, 16–44. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Synergistic Effect of Extracellularly Supplemented Osteopontin and Osteocalcin on Stem Cell Proliferation, Osteogenic Differentiation, and Angiogenic Properties. J. Cell. Biochem. 2019, 120, 6555–6569. [Google Scholar] [CrossRef] [PubMed]


| Nomenclature | Calcium Phosphate | PLA (mg/mL) | COL (mg/mL) | CaP (mg/mL) |
|---|---|---|---|---|
| MHAP | Hydroxyapatite | 100 | 10 | 10 |
| MACP | Amorphous | 100 | 10 | 10 |
| PLA | N/A | 100 | - | - |
| Scaffold | Tg (°C) | ΔHg (J/°C) | Tc (°C) | ΔHc (J/°C) | Tm (°C) | ΔHm (J/°C) |
|---|---|---|---|---|---|---|
| MHAP | 60.29 | 2.62 | 79.88 | 10.80 | 170.16 | 35.28 |
| MACP | 60.59 | 4.14 | 78.64 | 11.76 | 169.42 | 35.04 |
| PLA | 59.47 | 6.91 | 87.76 | 13.79 | 166.16 | 38.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas-Aguazaco, W.; Camacho, B.; Gómez-Pachón, E.Y.; Lara-Bertrand, A.L.; Silva-Cote, I. Electrospun Scaffolds of Polylactic Acid, Collagen, and Amorphous Calcium Phosphate for Bone Repair. Pharmaceutics 2023, 15, 2529. https://doi.org/10.3390/pharmaceutics15112529
Cárdenas-Aguazaco W, Camacho B, Gómez-Pachón EY, Lara-Bertrand AL, Silva-Cote I. Electrospun Scaffolds of Polylactic Acid, Collagen, and Amorphous Calcium Phosphate for Bone Repair. Pharmaceutics. 2023; 15(11):2529. https://doi.org/10.3390/pharmaceutics15112529
Chicago/Turabian StyleCárdenas-Aguazaco, William, Bernardo Camacho, Edwin Yesid Gómez-Pachón, Adriana Lorena Lara-Bertrand, and Ingrid Silva-Cote. 2023. "Electrospun Scaffolds of Polylactic Acid, Collagen, and Amorphous Calcium Phosphate for Bone Repair" Pharmaceutics 15, no. 11: 2529. https://doi.org/10.3390/pharmaceutics15112529
APA StyleCárdenas-Aguazaco, W., Camacho, B., Gómez-Pachón, E. Y., Lara-Bertrand, A. L., & Silva-Cote, I. (2023). Electrospun Scaffolds of Polylactic Acid, Collagen, and Amorphous Calcium Phosphate for Bone Repair. Pharmaceutics, 15(11), 2529. https://doi.org/10.3390/pharmaceutics15112529






