Novel Transethosomal Gel Containing Miconazole Nitrate; Development, Characterization, and Enhanced Antifungal Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MCNR-Loaded Transethosomes
2.3. In Vitro Characterization of MCNR-Loaded Transethosomal Formulations
2.3.1. Entrapment Efficiency (%EE)
2.3.2. Particle size, Polydispersity Index (PDI), and Zeta Potential
2.4. Optimum Formula Selection
2.5. In Vitro Characterization of Optimum MCNR Formulation
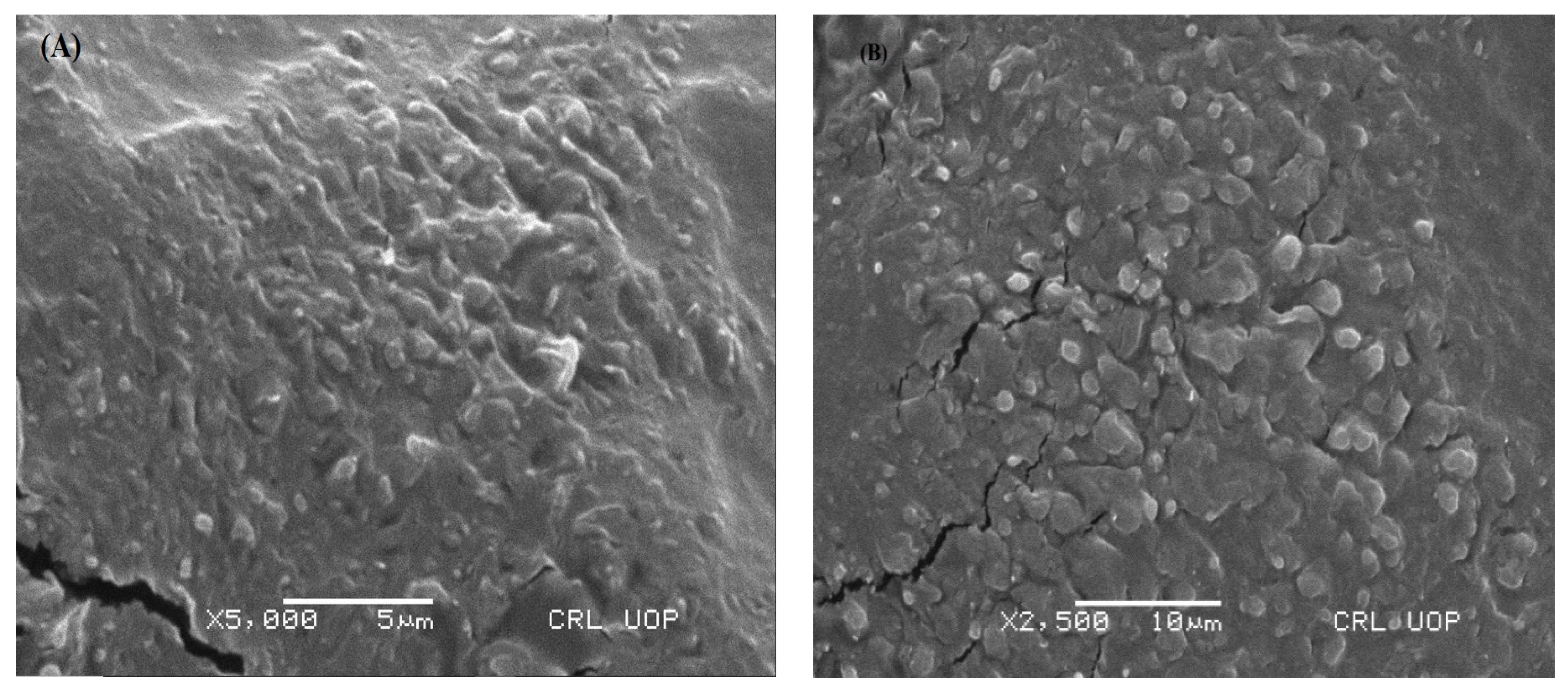
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
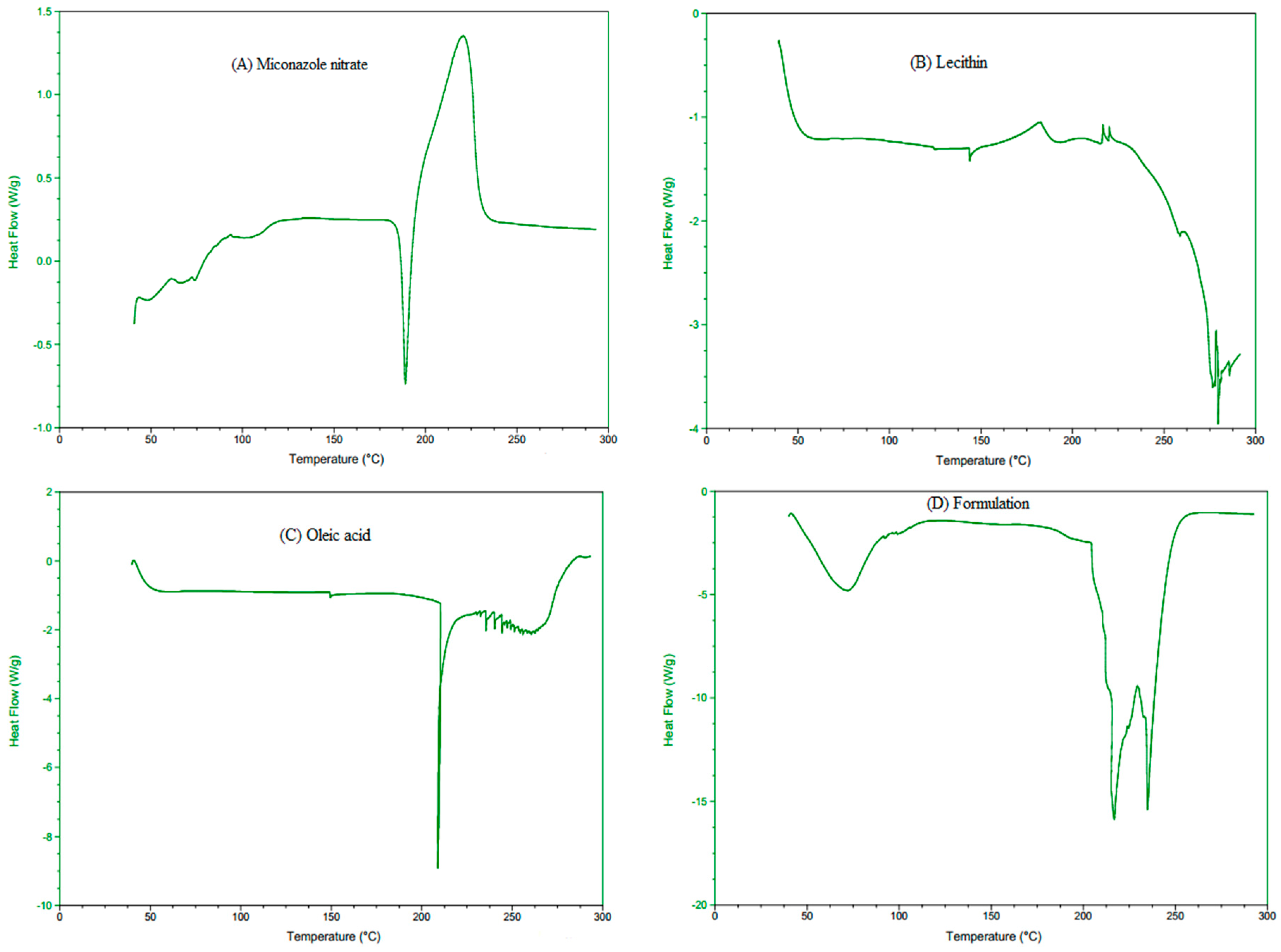
2.5.3. Differential Scanning Calorimetry (DSC) Analysis
2.5.4. Stability Studies of Optimized MCNR Formulation
2.6. Preparation of Transethosomal Gel
2.7. Evaluation of Gels
2.7.1. Organoleptic Investigation and Stability Studies
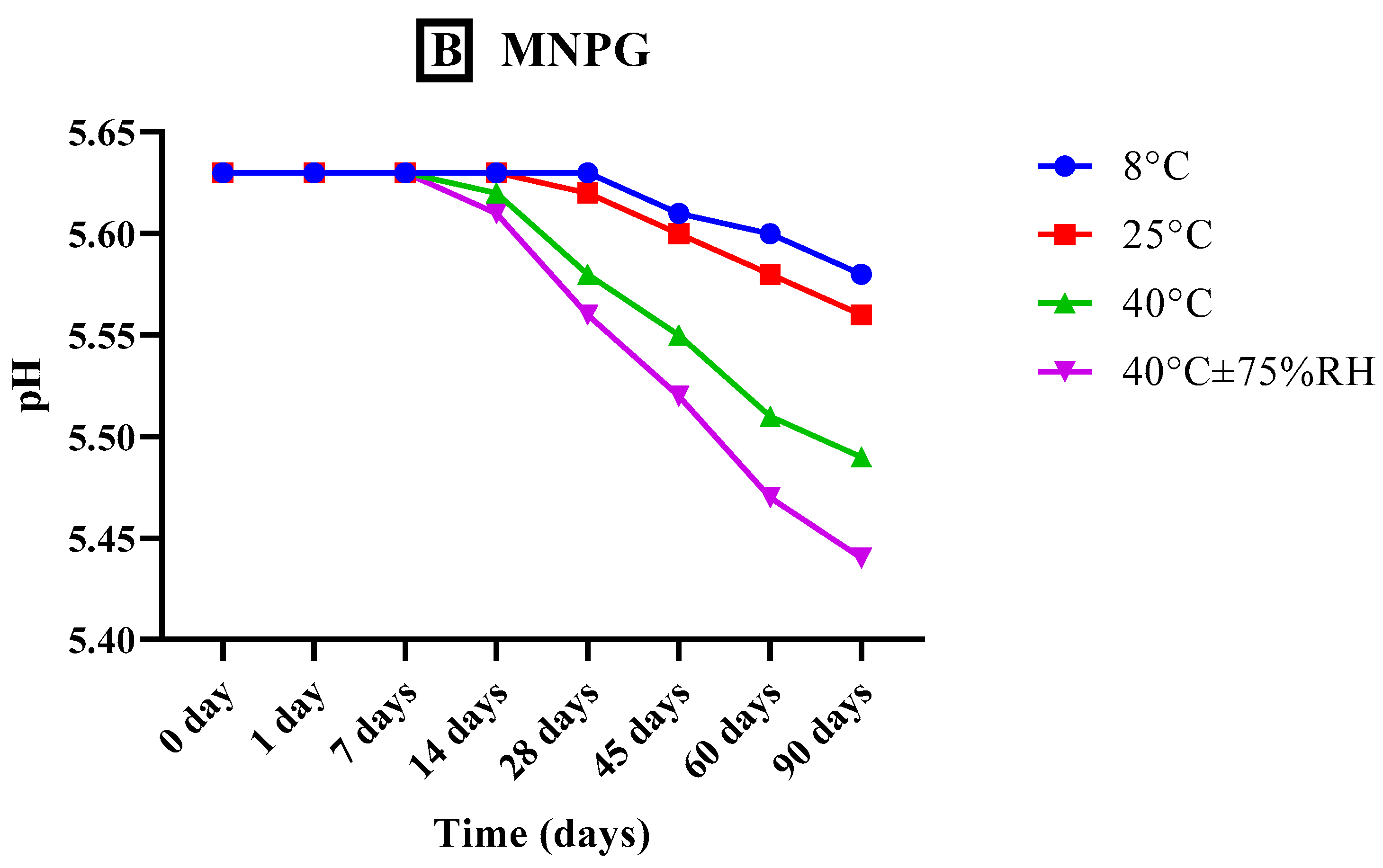
2.7.2. Analysis of pH
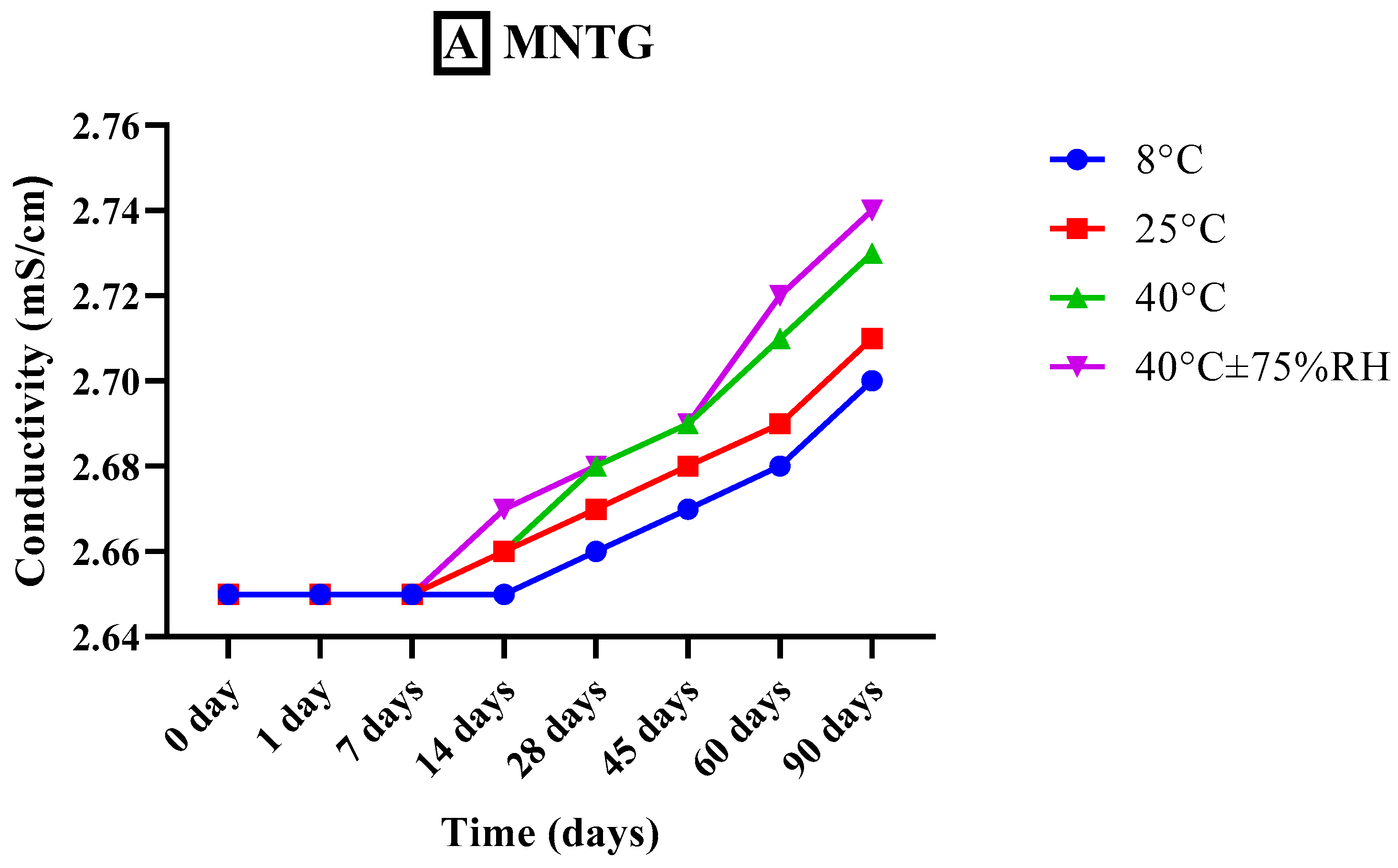
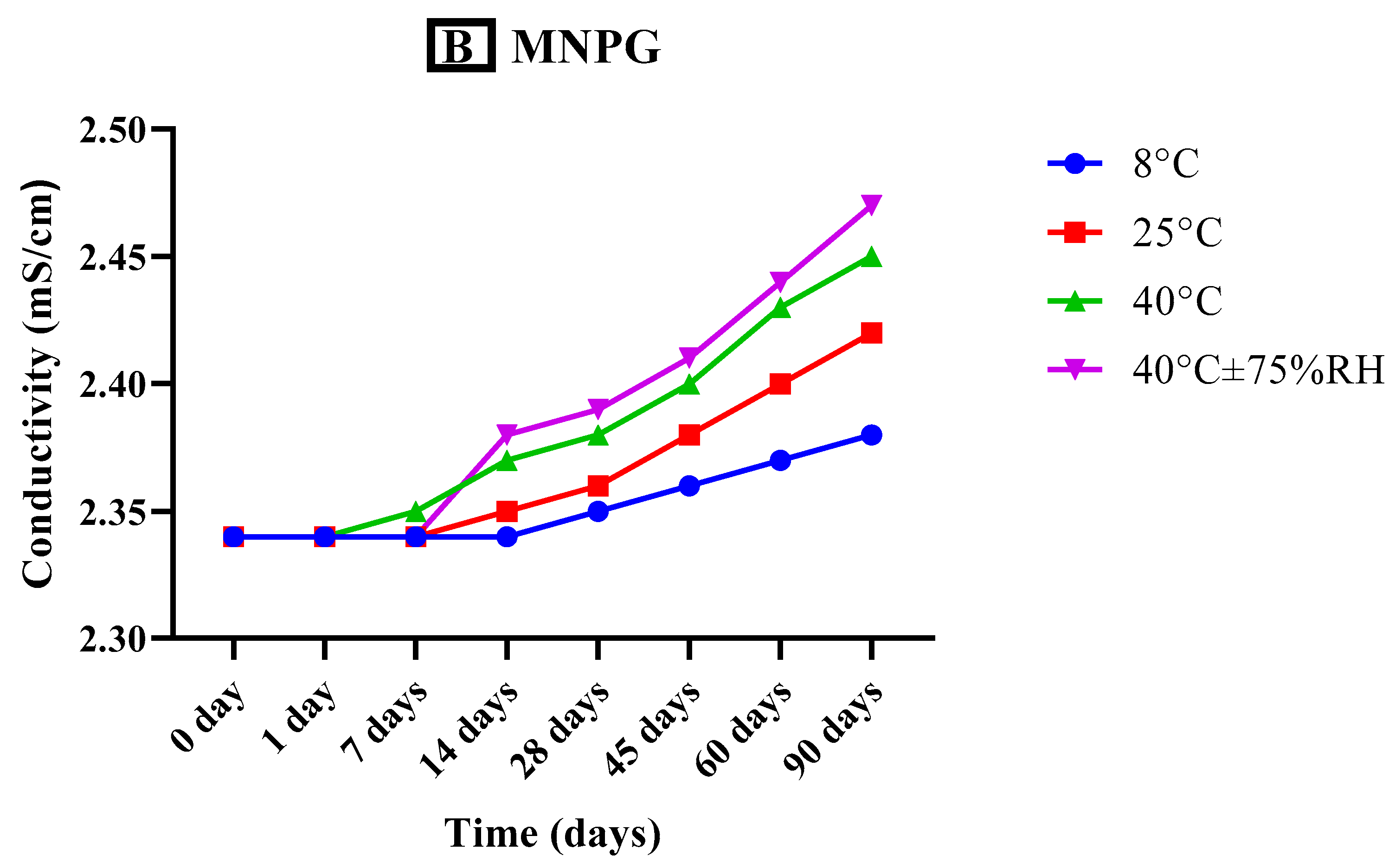
2.7.3. Measurement of Conductivity
2.7.4. Viscosity Analysis
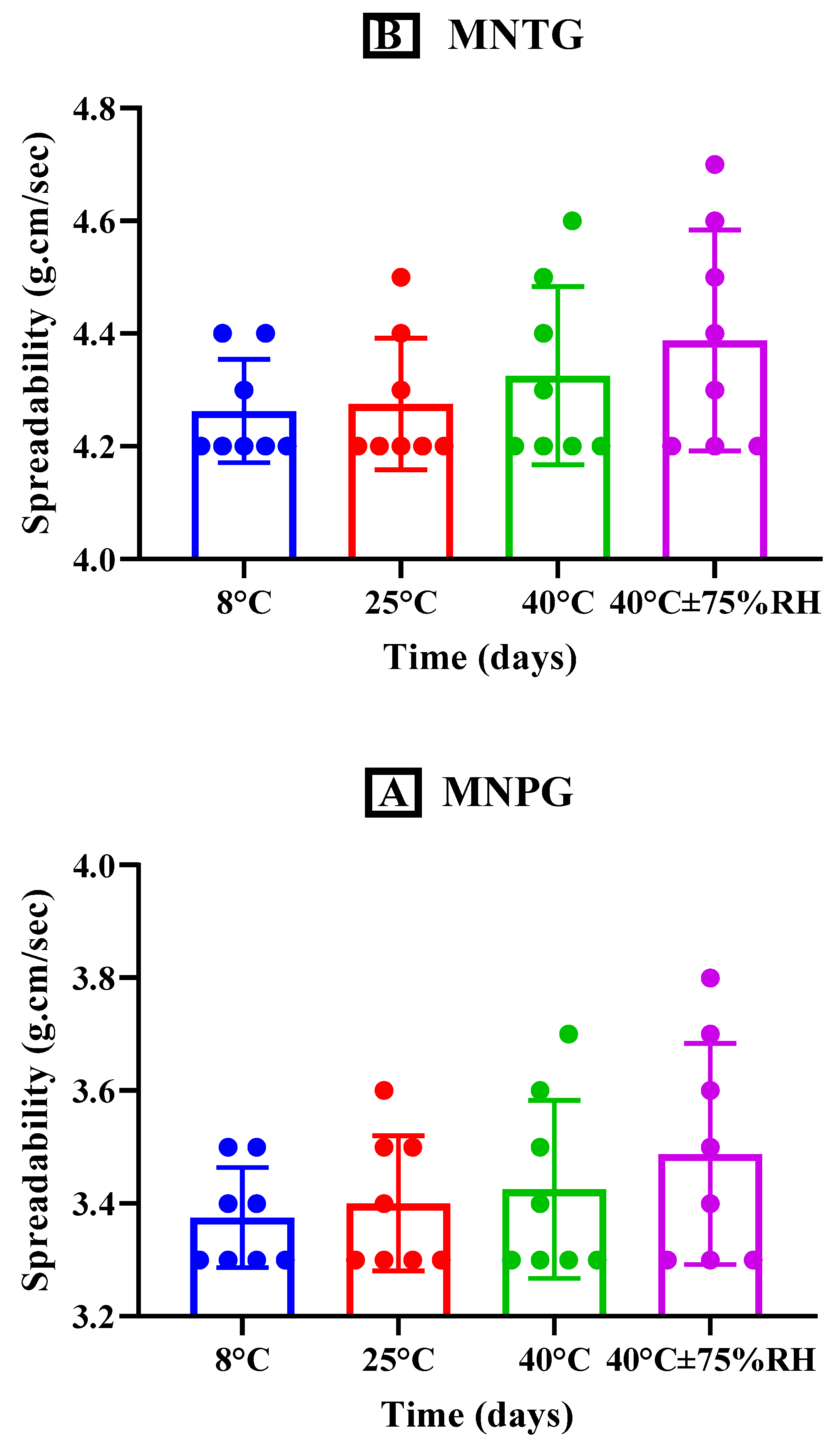
2.7.5. Spreadability
2.8. In Vitro Release Study and Drug Release Kinetics
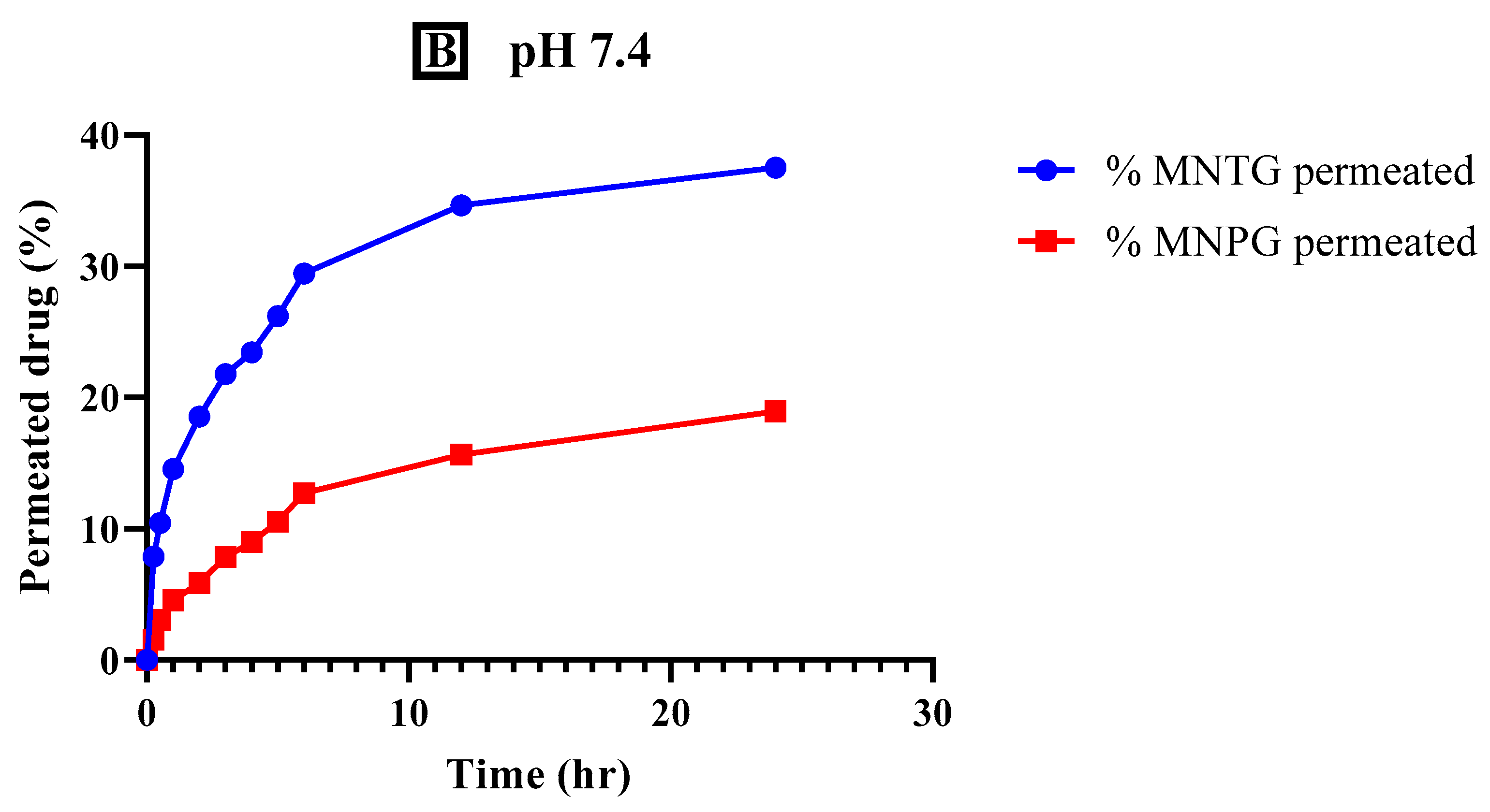
2.9. Ex Vivo Permeation
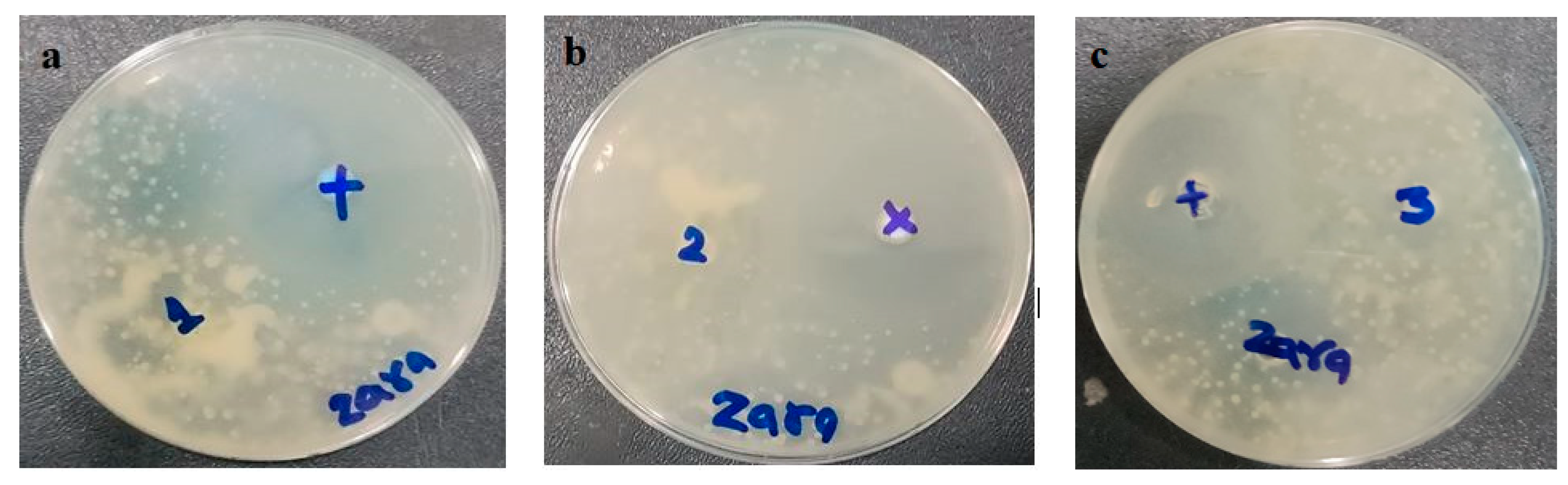
2.10. In Vitro Antifungal Activity
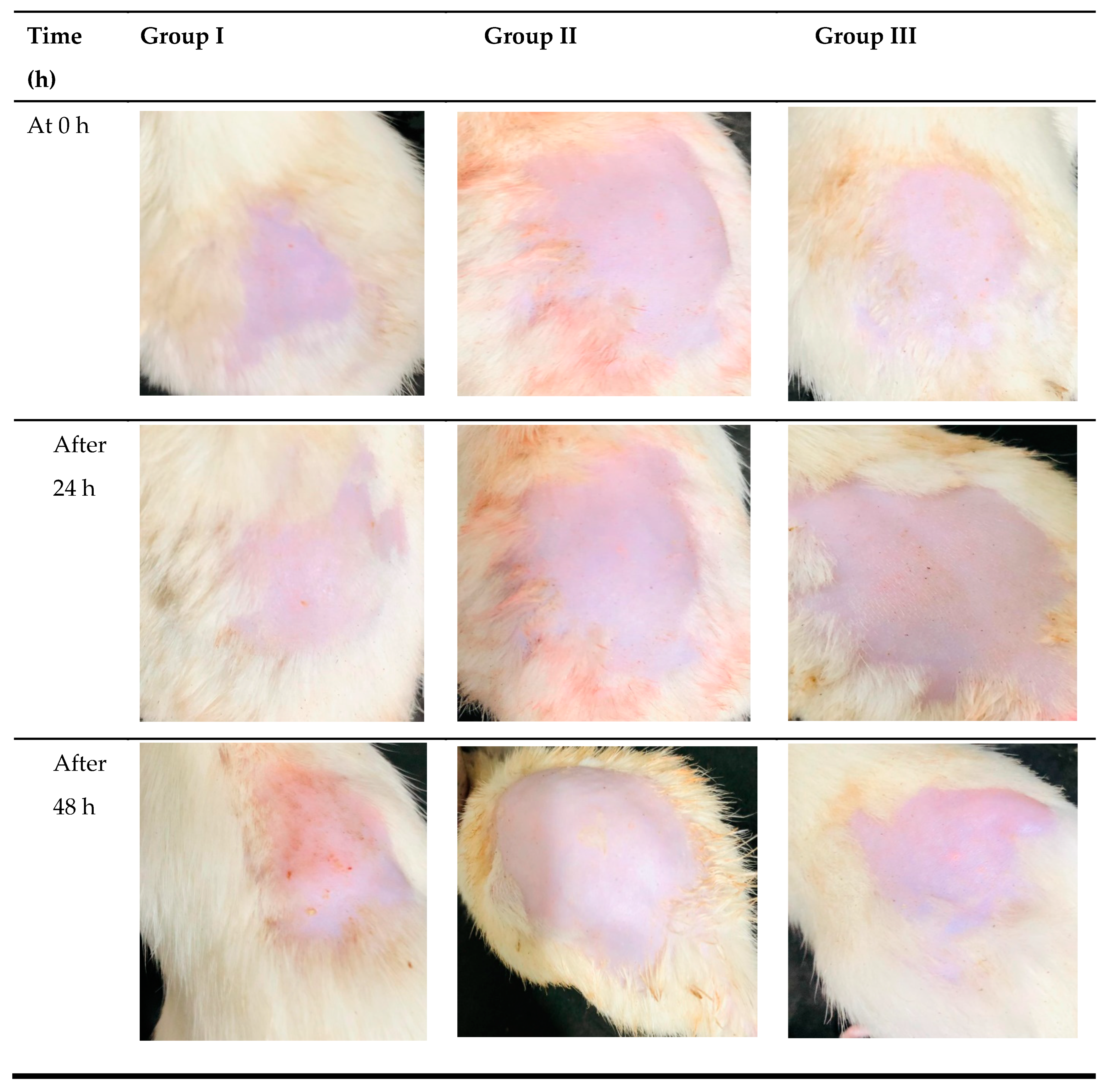
2.11. In Vivo Skin Irritation Study
2.12. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Characterization of MCNR-Loaded Transethosomes
3.1.1. Entrapment Efficiency
3.1.2. Determination of Particle Size
3.1.3. Determination of PDI
3.1.4. Determination of Zeta Potential
3.2. Optimum Formula Selection
3.3. In Vitro Characterization of Optimum MCNR Formulation
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
3.3.3. Differential Scanning Calorimetry (DSC) Analysis
3.4. Stability Studies of Optimized Transethosomes Formulation
3.5. Evaluation of Gels
3.5.1. Organoleptic Investigation and Stability Studies
3.5.2. Analysis of pH
3.5.3. Measurement of Conductivity
3.5.4. Viscosity Analysis
3.5.5. Spreadability
3.6. In Vitro Release Study
Kinetic Models for Drug Release Characterization
3.7. Ex Vivo Permeation Study
3.8. In Vitro Antifungal Activity
3.9. In Vivo Skin Irritation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shakeel, F.; Shafiq, S.; Haq, N.; Alanazi, F.K.; Alsarra, I.A. Nanoemulsions as potential vehicles for transdermal and dermal delivery of hydrophobic compounds: An overview. Expert. Opin. Drug Deliv. 2012, 9, 953–974. [Google Scholar] [CrossRef] [PubMed]
- Bonina, F.; Montenegro, L. Vehicle effects on in vitro heparin release and skin penetration from different gels. Int. J. Pharm. 1994, 102, 19–24. [Google Scholar] [CrossRef]
- Mulani, H.; Bhise, K.S. QbD Approach in the formulation and evaluation of Miconazole Nitrate loaded ethosomal cream-o-gel. Int. Res. J. Pharm. Sci. 2017, 8, 1–13. [Google Scholar]
- Pandit, J.; Garg, M.; Jain, N.K. Miconazole nitrate bearing ultraflexible liposomes for the treatment of fungal infection. J. Liposome Res. 2014, 24, 163–169. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Güng, S. New formulation strategies in topical antifungal therapy. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 56–65. [Google Scholar]
- Qushawy, M.; Nasr, A.; Abd-Alhaseeb, M.; Swidan, S. Design, optimization and characterization of a transfersomal gel using miconazole nitrate for the treatment of candida skin infections. Pharmaceutics 2018, 10, 26. [Google Scholar] [CrossRef]
- Mendes, A.; Silva, A.C.; Catita, J.A.M.; Cerqueira, F.; Gabriel, C.; Lopes, C. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: Improving antifungal activity. Colloids Surf. B Biointerfaces 2013, 111, 755–763. [Google Scholar] [CrossRef]
- Aljaeid, B.M.; Hosny, K.M. Miconazole-loaded solid lipid nanoparticles: Formulation and evaluation of a novel formula with high bioavailability and antifungal activity. Int. J. Nanomed. 2016, 11, 441–447. [Google Scholar] [CrossRef]
- Shah, R.M.; Eldridge, D.S.; Palombo, E.A.; Harding, I.H. Biopharmaceutics. Microwave-assisted microemulsion technique for production of miconazole nitrate-and econazole nitrate-loaded solid lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 141–150. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Zheng, F.; Zhang, X.; Gao, J.; Liang, W. In vitro percutaneous permeation and skin accumulation of finasteride using vesicular ethosomal carriers. AAPS PharmSciTech 2008, 9, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Mittal, A.; Chauhan, N.; Alam, S. Role of surfactants as penetration enhancer in transdermal drug delivery system. J. Mol. Pharm. Org. Process Res. 2014, 2, 2–7. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Ubaid, M.; Ilyas, S.; Mir, S.; Khan, A.K.; Rashid, R.; Khan, M.Z.; Kanwal, Z.G.; Nawaz, A.; Shah, A.; Murtaza, G. Formulation and in vitro evaluation of carbopol 934-based modified clotrimazole gel for topical application. An. Acad. Bras. Ciênc. 2016, 88, 2303–2317. [Google Scholar] [CrossRef]
- Jana, S.; Manna, S.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Carbopol gel containing chitosan-egg albumin nanoparticles for transdermal aceclofenac delivery. Colloids Surf. B Biointerfaces 2014, 114, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Lu, R. Mechanism and application of chitosan and its derivatives in promoting permeation in transdermal drug delivery systems: A review. Pharmaceuticals 2022, 15, 459. [Google Scholar] [CrossRef]
- Guo, F.; Wang, J.; Ma, M.; Tan, F.; Li, N. Skin targeted lipid vesicles as novel nano-carrier of ketoconazole: Characterization, in vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2015, 26, 1–13. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; da Costa, T.G.; de Lima, R.; Fraceto, L.F.; de Araujo, D.R. Using Chitosan-Coated Polymeric Nanoparticles-Thermosensitive Hydrogels in association with Limonene as Skin Drug Delivery Strategy. BioMed Res. Int. 2022, 2022, 9165443. [Google Scholar] [CrossRef]
- Akhtar, N.; Akhtar, N. Development of stable tocopherol succinate-loaded ethosomes to enhance transdermal permeation: In vitro and in vivo characterizations. J. Cosmet. Dermatol. 2022, 21, 4942–4955. [Google Scholar] [CrossRef]
- Salim, M.W.; Shabbir, K.; Yousaf, A.M.; Choi, H.-G.; Khan, G.M. Preparation, in-vitro and in-vivo evaluation of Rifampicin and Vancomycin Co-loaded transfersomal gel for the treatment of cutaneous leishmaniasis. J. Drug Deliv. Sci. 2020, 60, 101996. [Google Scholar] [CrossRef]
- Chen, J.-G.; Liu, Y.-F.; Gao, T.-W. Preparation and anti-inflammatory activity of triptolide ethosomes in an erythema model. J. Liposome Res. 2010, 20, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ghourab, M.; Gad, S.; Qushawy, M. The application of Plackett-Burman design and response surface methodology for optimization of formulation variables to produce Piroxicam niosomes. Int. J. Drug Dev. Res. 2013, 5, 121–130. [Google Scholar]
- Ahmed, T.A. Preparation of transfersomes encapsulating sildenafil aimed for transdermal drug delivery: Plackett–Burman design and characterization. J. Liposome Res. 2015, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H. Transfersomes as a transdermal drug delivery system for enhancement the antifungal activity of nystatin. Liposome Nanotechnol. 2013, 5, 390. [Google Scholar]
- Castro, N.R.; Cristal dos Santos, C.P.; de Campos, V.E.B.; Cardoso, V.; Vermelho, A.B.; dos Santos, E.P.; Mansur, C.R.E. Development of hybrid vesicular nanosystems composed of lipids and chitosan for octyl methoxycinnamate encapsulation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125476. [Google Scholar] [CrossRef]
- Sharif, A.; Akhtar, N. Fabrication of niosomes containing N-acetyl glucosamine: In-vitro and ex-vivo characterizations. Pak. J. Pharm. Sci. 2020, 33, 1321–1326. [Google Scholar]
- Mahgoub, E.S. Clinical trial with clotrimazole cream (Bay b 5097) in dermatophytosis and onychomycosis. Mycopathologia 1975, 56, 149–152. [Google Scholar] [CrossRef]
- Khan, P.; Akhtar, N. Phytochemical investigations and development of ethosomal gel with Brassica oleraceae L. (Brassicaceae) extract: An innovative nano approach towards cosmetic and pharmaceutical industry. Ind. Crop. Prod. 2022, 183, 114905. [Google Scholar] [CrossRef]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; Almeida, J.R.G. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. Int. J. Pharm. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.M.; Abdelbary, G.A.; Basha, M.; Awad, G.E.; El-Hashemy, H.A. Design and evaluation of proniosomes as a carrier for ocular delivery of lomefloxacin HCl. J. Liposome Res. 2017, 27, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Iizhar, S.A.; Syed, I.A.; Satar, R.; Ansari, S.A. In vitro assessment of pharmaceutical potential of ethosomes entrapped with terbinafine hydrochloride. J. Adv. Res. 2016, 7, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Al-Maghrabi, P.M.; Khafagy, E.-S.; Ghorab, M.M.; Gad, S.J.C.; Biointerfaces, S.B. Influence of formulation variables on miconazole nitrate–loaded lipid based nanocarrier for topical delivery. Colloids Surf. B Biointerfaces 2020, 193, 111046. [Google Scholar] [CrossRef]
- Girhepunje, K.; Pal, R.; Gevariya, H.; Behera, A.; Thirumoorthy, N. Ethosomes: A novel vesicular carrier for enhanced dermal delivery of ciclopiroxolamine. Der Pharm. Lett. 2010, 2, 360–367. [Google Scholar]
- James, O.; Sunday, A.B. Evaluation of acute dermal irritation and wound contraction by Gymnema sylvestre and Datura metel extracts in rats. Am. J. Biomed. 2014, 2, 83–88. [Google Scholar] [CrossRef]
- Adegbenro, O.O.; Opeyemi, O.T. Development and estimation of anti-inflammatory activity of topical etoricoxib emulgel by carrageenan induced paw oedema method. Univers. J. Pharm. Res. 2019, 4, 23–28. [Google Scholar] [CrossRef]
- Zuang, V.; Alonso, M.-A.; Botham, P.A.; Eskes, C.; Fentem, J.; Liebsch, M.; van de Sandt, J.J. 3.2. Skin Irritation and Corrosion. Altern. Lab. Anim. 2005, 33, 35–46. [Google Scholar] [CrossRef]
- El-Shenawy, A.A.; Abdelhafez, W.A.; Ismail, A.; Kassem, A.A. Formulation and characterization of nanosized ethosomal formulations of antigout model drug (febuxostat) prepared by cold method: In vitro/ex vivo and in vivo assessment. AAPS PharmSciTech 2020, 21, 31. [Google Scholar] [CrossRef]
- Shatalebi, M.; Mostafavi, S.; Moghaddas, A. Niosome as a drug carrier for topical delivery of N-acetyl glucosamine. Res. Pharm. Sci. 2010, 5, 107. [Google Scholar] [PubMed]
- He, J.; Shi, H.; Huang, S.; Han, L.; Zhang, W.; Zhong, Q. Core-shell nanoencapsulation of α-tocopherol by blending sodium oleate and rebaudioside A: Preparation, characterization, and antioxidant activity. Molecules 2018, 23, 3183. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.-L.; Gu, W.; Lu, S.-S.; Chen, Z.-P.; Cai, B.-C. Skin permeation behavior of elastic liposomes: Role of formulation ingredients. Expert. Opin. Drug Deliv. 2013, 10, 845–856. [Google Scholar] [CrossRef]
- Verma, S.; Utreja, P. Transethosomes of econazole nitrate for transdermal delivery: Development, in-vitro characterization, and ex-vivo assessment. Pharm. Nanotechnol. 2018, 6, 171–179. [Google Scholar] [CrossRef]
- Iyer, S.S.; Barr, W.H.; Karnes, H.T. Profiling in vitro drug release from subcutaneous implants: A review of current status and potential implications on drug product development. Biopharm. Drug Dispos. 2006, 27, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Ainbinder, D.; Touitou, E. Testosterone ethosomes for enhanced transdermal delivery. Drug Deliv. 2005, 12, 297–303. [Google Scholar] [CrossRef]
- Khan, D.H.; Bashir, S.; Figueiredo, P.; Santos, H.A.; Khan, M.I.; Peltonen, L. Process optimization of ecological probe sonication technique for production of rifampicin loaded niosomes. J. Drug Deliv. Sci. 2019, 50, 27–33. [Google Scholar] [CrossRef]
- Thang, L.; Hanh, N.; Duong, D.Q. Study on cause-effect relations and optimization of exemestane-loaded nanostructured lipid carriers. Int. J. Pharm. Sci. 2017, 9, 68–74. [Google Scholar] [CrossRef]
- Vasanth, S.; Dubey, A.; Ravi, G.S.; Lewis, S.A.; Ghate, V.M.; El-Zahaby, S.A.; Hebbar, S. Development and investigation of vitamin C-enriched adapalene-loaded transfersome gel: A collegial approach for the treatment of acne vulgaris. AAPS PharmSciTech 2020, 21, 61. [Google Scholar] [CrossRef]
- Jain, S.; Jain, P.; Umamaheshwari, R.; Jain, N.K. Transfersomes—A novel vesicular carrier for enhanced transdermal delivery: Development, characterization, and performance evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. [Google Scholar] [CrossRef]
- Thatipamula, R.; Palem, C.; Gannu, R.; Mudragada, S.; Yamsani, M. Tehran University of Medical Sciences. Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. Daru J. Pharm. Sci. 2011, 19, 23. [Google Scholar]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef]
- Andleeb, M.; Shoaib Khan, H.M.; Daniyal, M. Development, characterization and stability evaluation of topical gel loaded with ethosomes containing Achillea millefolium L. extract. Front. Pharmacol. 2021, 12, 603227. [Google Scholar] [CrossRef] [PubMed]
- Limsuwan, T.; Boonme, P.; Khongkow, P.; Amnuaikit, T. Ethosomes of phenylethyl resorcinol as vesicular delivery system for skin lightening applications. BioMed Res. Int. 2017, 2017, 8310979. [Google Scholar] [CrossRef]
- Raghavendra, P.; Pullaiah, T. Advances in Cell and Molecular Diagnostics; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Bodade, S.S.; Shaikh, K.S.; Kamble, M.S.; Chaudhari, P.D. A study on ethosomes as mode for transdermal delivery of an antidiabetic drug. Drug Deliv. 2013, 20, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Bhardwaj, A.; Vij, M.; Bajpai, P.; Goutam, N.; Kumar, L. Oleic acid vesicles: A new approach for topical delivery of antifungal agent. Artif. Cells Nanomed. Biotechnol. 2014, 42, 95–101. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: Design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Assi, R.A.; Khan, N.A.K. Transethosomal gels as carriers for the transdermal delivery of colchicine: Statistical optimization, characterization, and ex vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 795–813. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Pathak, K. Cavamax W7 composite ethosomal gel of clotrimazole for improved topical delivery: Development and comparison with ethosomal gel. AAPS PharmSciTech 2012, 13, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Arshad, W.; Khan, H.M.S.; Akhtar, N.; Mohammad, I.S. Polymeric emulgel carrying Cinnamomum tamala extract: Promising delivery system for potential topical applications. Braz. J. Pharm. Sci. 2020, 56, e18318. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N. Stability of a cosmetic multiple emulsion loaded with green tea extract. Sci. World J. 2013, 2013, 153695. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.; Campos, P.M. Rheological behavior and the SPF of sunscreens. Int. J. Pharm. 2003, 250, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Nanda, S.; Sharma, G.; Katare, O. Systematically optimized coenzyme q10-loaded novel proniosomal formulation for treatment of photo-induced aging in mice: Characterization, biocompatibility studies, biochemical estimations and anti-aging evaluation. J. Drug Target. 2016, 24, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Barbi, M.S.; Sarmento, V.H.V.; Chiavacci, L.A.; Netto, F.M.; Gremião, M.P. Pharmacology. Surfactant systems for nasal zidovudine delivery: Structural, rheological and mucoadhesive properties. J. Pharm. Pharmacol. 2010, 62, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Want, M.Y.; Islammudin, M.; Chouhan, G.; Ozbak, H.A.; Hemeg, H.A.; Chattopadhyay, A.P.; Afrin, F. Nanoliposomal artemisinin for the treatment of murine visceral leishmaniasis. Int. J. Nanomed. 2017, 12, 2189–2204. [Google Scholar] [CrossRef]
- Shirsand, S.; Para, M.; Nagendrakumar, D.; Kanani, K.; Keerthy, D. Formulation and evaluation of Ketoconazole niosomal gel drug delivery system. Int. J. Pharm. Investig. 2012, 2, 201. [Google Scholar] [CrossRef]
- Hamed, R.; Al Baraghthi, T.; Alkilani, A.Z.; Abu-Huwaij, R. Correlation between rheological properties and in vitro drug release from penetration enhancer-loaded Carbopol® gels. J. Pharm. Innov. 2016, 11, 339–351. [Google Scholar] [CrossRef]
- Elmoslemany, R.M.; Abdallah, O.Y.; El-Khordagui, L.K.; Khalafallah, N.M. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: Comparison with conventional liposomes. AAPS PharmSciTech 2012, 13, 723–731. [Google Scholar] [CrossRef]





| Code | Drug (mg) | Lecithin: OA (mg) | Ethanol (v/v%) | PBS (pH 5.5) (v/v%) | Hydration Volume (mL) |
|---|---|---|---|---|---|
| MT-1 | 100 | 75:25 | 20 | 80 | 10 |
| MT-2 | 100 | 85:15 | 20 | 80 | 10 |
| MT-3 | 100 | 95:05 | 20 | 80 | 10 |
| MT-4 | 100 | 75:25 | 30 | 70 | 10 |
| MT-5 | 100 | 85:15 | 30 | 70 | 10 |
| MT-6 | 100 | 95:05 | 30 | 70 | 10 |
| MT-7 | 100 | 75:25 | 40 | 60 | 10 |
| MT-8 | 100 | 85:15 | 40 | 60 | 10 |
| MT-9 | 100 | 95:05 | 40 | 60 | 10 |
| Code | Entrapment Efficiency (%EE) | Particle Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|---|
| MT-1 | 74.45 ± 1.15 | 343.7± 1.67 | 0.315 ± 0.01 | −15.5 ± 0.21 |
| MT-2 | 78.05 ± 0.34 | 376.5 ± 1.45 | 0.349 ± 0.06 | −20. ± 0.14 |
| MT-3 | 86.31 ± 2.17 | 380.8 ± 1.21 | 0.426 ± 0.02 | −28.2 ± 0.52 |
| MT-4 | 79.43 ± 1.67 | 202.5 ± 1.76 | 0.390 ± 0.04 | −14.5 ± 0.27 |
| MT-5 | 85.90 ± 2.17 | 288.4 ± 2.87 | 0.346 ± 0.08 | −16.3 ± 0.37 |
| MT-6 | 87.61 ± 1.74 | 310.0 ± 2.45 | 0.274 ± 0.09 | −21.6 ± 0.62 |
| MT-7 | 83.98 ± 1.12 | 174.6 ± 1.26 | 0.192 ± 0.03 | −16.7 ± 0.14 |
| MT-8 | 89.93 ± 1.32 | 139.3 ± 1.14 | 0.188 ± 0.05 | −18.1 ± 0.10 |
| MT-9 | 88.87 ± 2.42 | 196.0 ± 1.03 | 0.391 ± 0.07 | −18.5 ± 0.65 |
| Parameter | Time (days) | 4 °C | 25 °C |
|---|---|---|---|
| pH | 0 | 5.54 ± 0.13 | 5.54 ± 0.13 |
| 45 | 5.52 ± 0.04 | 5.51 ± 0.24 | |
| 90 | 5.51 ± 0.11 | 5.47 ± 0.19 | |
| Color | 0 | Mw | Mw |
| 45 | Mw | Mw | |
| 90 | Mw | Ly | |
| % EE | 0 | 89.93 ± 1.32% | 89.93 ± 1.32% |
| 45 | 87.52 ± 0.14% | 85.39 ± 1.25% | |
| 90 | 84.79 ± 1.28% | 80.14 ± 2.11% | |
| Particle size (nm) | 0 | 139.3 ± 1.14 | 139.3 ± 1.14 |
| 45 | 1.43 ± 2.16 | 1.48 ± 1.47 | |
| 90 | 146.1 ± 0.05 | 159.4 ± 1.21 |
| Parameters | Temp. | Fresh | 24 h | 7 Days | 14 Days | 28 Days | 45 Days | 60 Days | 90 Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PG | TG | PG | TG | PG | TG | PG | TG | PG | TG | PG | TG | PG | TG | PG | TG | ||
| Color | 8 °C | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE |
| 25 °C | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | |
| 40 °C | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | OWT | WTE | LY | |
| 40 °C ± 75% RH | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | WTE | OWT | WTE | LY | |
| Odor | 8 °C | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 25 °C | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 40 °C | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 40 °C ± 75% RH | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Liquefaction | 8 °C | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 25 °C | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 40 °C | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 40 °C ± 75% RH | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Appearance | 8 °C | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY |
| 25 °C | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | |
| 40 °C | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | |
| 40 °C ± 75% RH | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | TRP | MKY | |
| Formulation | Zero-Order | First Order | Higuchi | Korsmeyer–Peppas | |
|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | N | |
| MCNR-TEs | 0.0170 | 0.7556 | 0.8917 | 0.9795 | 0.353 |
| MNTG | −0.0907 | 0.5041 | 0.8470 | 0.9572 | 0.338 |
| pH 5.5 | |||
|---|---|---|---|
| Formulations | Flux (µg/cm2/h) | Permeability Coefficient (cm/h) | Enhancement Ratio |
| MNTG | 26.01 | 0.0953 | 1.50 |
| MNPG | 17.34 | 0.0781 | |
| pH 7.4 | |||
| MNTG | 3.17 | 0.0319 | 0.47 |
| MNPG | 6.64 | 0.0112 | |
| Sample | Named | Zone of Inhibition (mm) | |
|---|---|---|---|
| * Positive Control | Test Samples | ||
| MNPG | 1 | 30 ± 0.02 mm | 11 ± 0.05 mm |
| MNTG | 2 | 28 ± 0.03 mm | 20.5 ± 0.02 mm |
| Marketed cream | 3 | 30 ± 0.03 mm | 16 ± 0.01 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, Z.; Jamshaid, T.; Sajid-ur-Rehman, M.; Jamshaid, U.; Gad, H.A. Novel Transethosomal Gel Containing Miconazole Nitrate; Development, Characterization, and Enhanced Antifungal Activity. Pharmaceutics 2023, 15, 2537. https://doi.org/10.3390/pharmaceutics15112537
Asghar Z, Jamshaid T, Sajid-ur-Rehman M, Jamshaid U, Gad HA. Novel Transethosomal Gel Containing Miconazole Nitrate; Development, Characterization, and Enhanced Antifungal Activity. Pharmaceutics. 2023; 15(11):2537. https://doi.org/10.3390/pharmaceutics15112537
Chicago/Turabian StyleAsghar, Zara, Talha Jamshaid, Muhammad Sajid-ur-Rehman, Usama Jamshaid, and Heba A. Gad. 2023. "Novel Transethosomal Gel Containing Miconazole Nitrate; Development, Characterization, and Enhanced Antifungal Activity" Pharmaceutics 15, no. 11: 2537. https://doi.org/10.3390/pharmaceutics15112537





