Longitudinal Trajectory Modeling to Assess Adherence to Sacubitril/Valsartan among Patients with Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
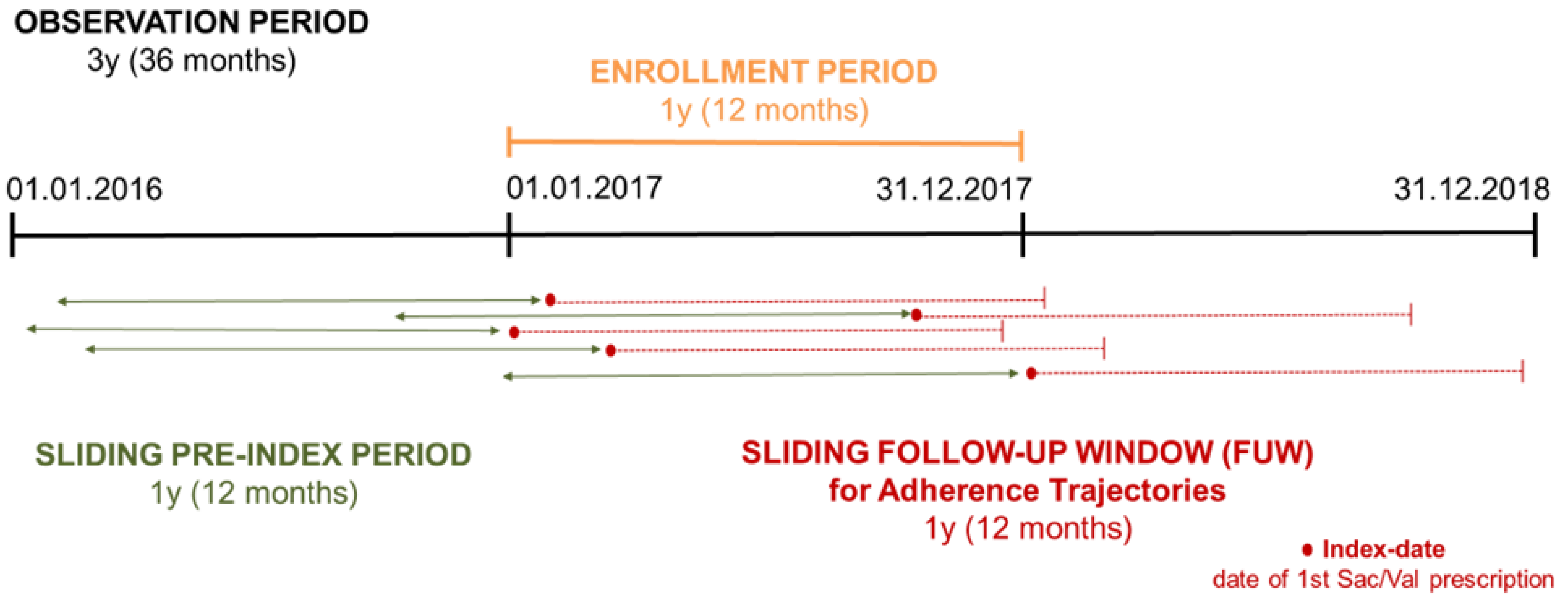
2.1. Study Design, Population, and Data Sources
2.2. Adherence Measurement
2.3. Sociodemographic and Clinical Covariates
2.4. Statistical Analysis
3. Results
3.1. Cohort Characteristics
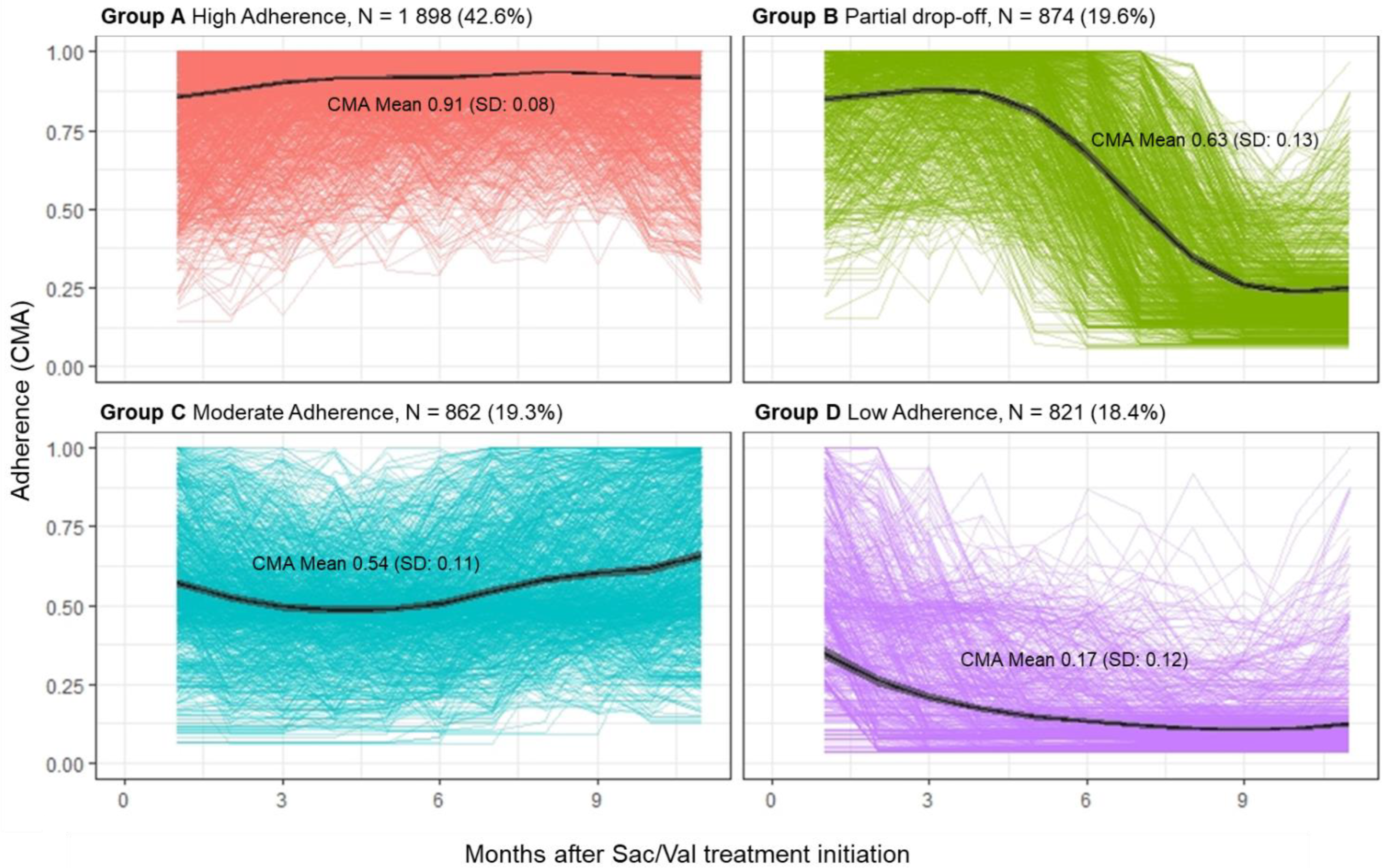
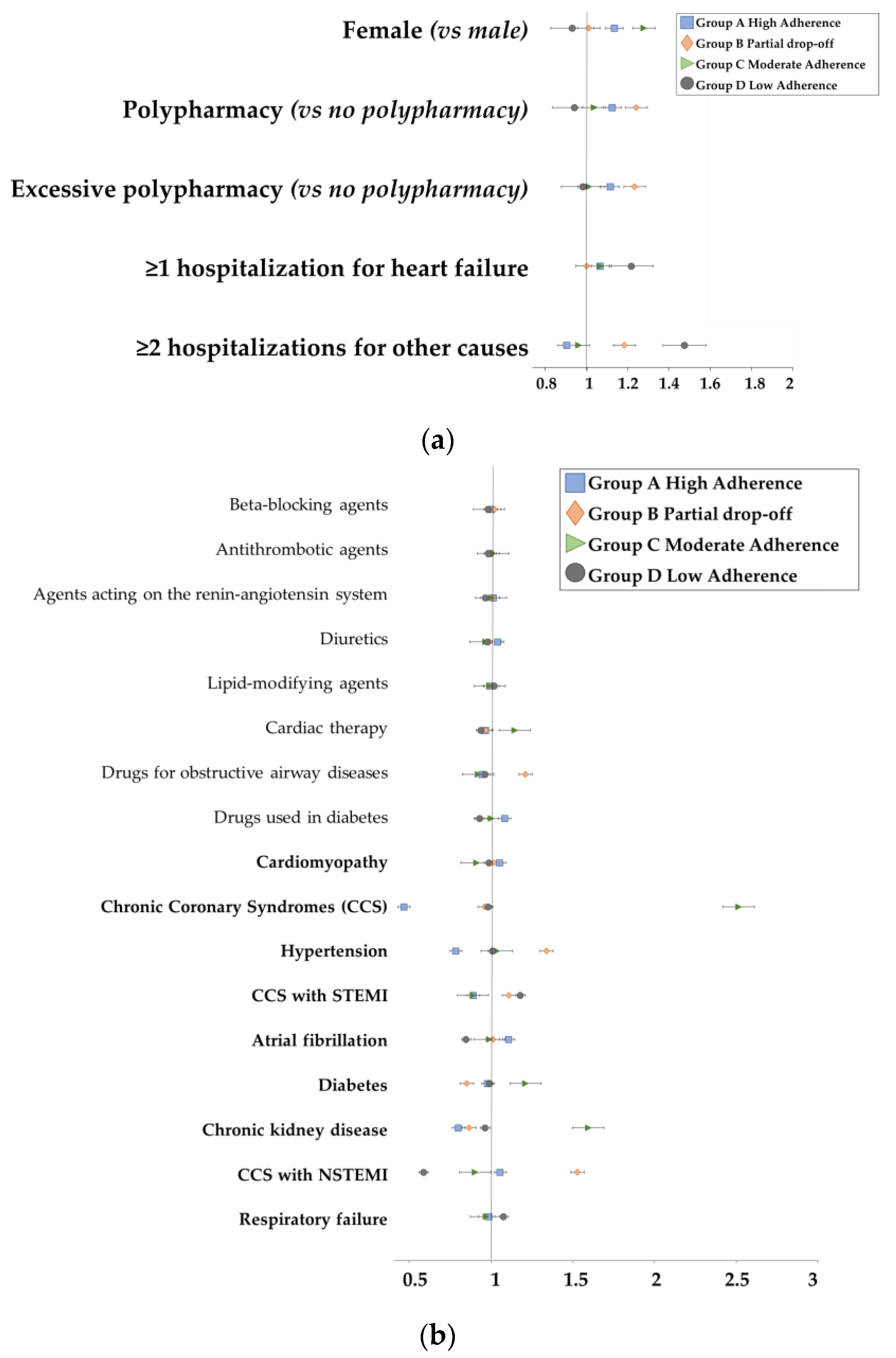
3.2. Adherence Measurement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, P.K.; DeVore, A.D.; Hellkamp, A.S.; Thomas, L.; Albert, N.M.; Butler, J.; Patterson, J.H.; Spertus, J.A.; Williams, F.B.; Duffy, C.I.; et al. Heart failure hospitalizationand guideline-directed prescribing patterns among heart failure with reduced ejection fraction patients. JACC Heart Fail. 2021, 9, 28–38. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–356. [Google Scholar]
- Butler, J.; Yang, M.; Manzi, M.A.; Hess, G.P.; Patel, M.J.; Rhodes, T.; Givertz, M.M. Clinical course of patients with worsening heart failure with reduced ejection fraction. J. Am. Coll. Cardiol. 2019, 73, 935–944. [Google Scholar] [CrossRef]
- Malik, A.; Gill, G.S.; Lodhi, F.K.; Tummala, L.S.; Singh, S.N.; Morgan, C.J.; Allman, R.M.; Fonarow, G.C.; Ahmed, A. Prior heart failure hospitalization and outcomes in patients with heart failure with preserved and reduced ejection fraction. Am. J. Med. 2020, 133, 84–94. [Google Scholar]
- European Medicine Agency. Entresto. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/entresto (accessed on 28 July 2023).
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 9931004. [Google Scholar] [CrossRef]
- Desai, A.S.; Vardeny, O.; Claggett, B.; McMurray, J.J.; Packer, M.; Swedberg, K.; Rouleau, J.L.; Zile, M.R.; Lefkowitz, M.; Shi, V.; et al. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: A secondary analysis of the PARADIGM-HF Trial. JAMA Cardiol. 2017, 2, 7985. [Google Scholar] [CrossRef]
- Vardeny, O.; Claggett, B.; Kachadourian, J.; Desai, A.S.; Packer, M.; Rouleau, J.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: The PARADIGM-HF trial. Eur. J. Heart Fail. 2019, 21, 337341. [Google Scholar] [CrossRef]
- Vardeny, O.; Claggett, B.; Kachadourian, J.; Pearson, S.M.; Desai, A.S.; Packer, M.; Rouleau, J.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; et al. Incidence; predictors; and outcomes associated with hypotensive episodes among heart failure patients receiving sacubitril/valsartan or enalapril: The PARADIGM-HF trial (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor with Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ. Heart Fail. 2018, 11, e004745. [Google Scholar]
- Bhatt, A.S.; Vaduganathan, M.; Solomon, S.D.; Schneeweiss, S.; Lauffenburger, J.C.; Desai, R.J. Sacubitril/valsartan use patterns among older adults with heart failure in clinical practice: A population-based cohort study of >25 000 Medicare beneficiaries. Eur. J. Heart Fail. 2022, 24, 1506–1515. [Google Scholar]
- Carnicelli, A.P.; Li, Z.; Greiner, M.A.; Lippmann, S.J.; Greene, S.J.; Mentz, R.J.; Hardy, N.C.; Blumer, V.; Shen, X.; Yancy, C.W.; et al. Sacubitril/Valsartan Adherence and Postdischarge Outcomes Among Patients Hospitalized for Heart Failure with Reduced Ejection Fraction. JACC Heart Fail. 2021, 9, 876–886. [Google Scholar]
- Mentz, R.J.; Lautsch, D.; Pulungan, Z.; Kim, S.; Hilkert, R.; Teigland, C.; Yang, M.; Djatche, L. Medication Trajectory and Treatment Patterns in Medicare Patients with Heart Failure and Reduced Ejection Fraction. J. Card. Fail. 2022, 28, 1349–1354. [Google Scholar] [CrossRef]
- Cutler, R.L.; Fernandez-Llimos, F.; Frommer, M.; Benrimoj, C.; Garcia-Cardenas, V. Economic impact of medication non-adherence by disease groups: A systematic review. BMJ Open. 2018, 8, e016982. [Google Scholar] [CrossRef]
- Galozy, A.; Nowaczyk, S.; Sant’Anna, A.; Ohlsson, M.; Lingman, M. Pitfalls of medication adherence approximation through EHR and pharmacy records: Definitions; data and computation. Int. J. Med. Inform. 2020, 136, 104092. [Google Scholar] [CrossRef]
- OECD. Using Routinely Collected Data to Inform Pharmaceutical Policies. Analytical Report for OECD and EU Countries. Available online: https://www.oecd.org/health/health-systems/Using-Routinely-Collected-Data-to-Inform-Pharmaceutical-Policies-Analytical-Report-2019.pdf (accessed on 14 October 2022).
- Elseviers, M.; Wettermark, B.; Almarsdóttir, A.B.; Andersen, M.; Benko, R.; Bennie, M.; Eriksson, I.; Godman, B.; Krska, J.; Poluzzi, E.; et al. Drug Utilization Research: Methods and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- De Geest, S.; Zullig, L.L.; Dunbar-Jacob, J.; Helmy, R.; Hughes, D.A.; Wilson, I.B.; Vrijens, B. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann. Intern. Med. 2018, 169, 30–35. [Google Scholar] [CrossRef]
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- Dima, A.; Allemann, S.; Dediu, D. AdhereR: An Open Science Approach to Estimating Adherence to Medications Using Electronic Healthcare Databases. Stud. Health Technol. Inform. 2019, 264, 1451–1452. [Google Scholar]
- Dima, A.L.; Dediu, D. Computation of adherence to medication and visualization of medication histories in R with AdhereR: Towards transparent and reproducible use of electronic healthcare data. PLoS ONE 2017, 12, e0174426. [Google Scholar] [CrossRef]
- Walsh, C.A.; Mucherino, S.; Orlando, V.; Bennett, K.E.; Menditto, E.; Cahir, C. Mapping the use of Group-Based Trajectory Modelling in medication adherence research: A scoping review protocol. HRB Open Res. 2020, 3, 25. [Google Scholar] [CrossRef]
- Hernandez, I.; He, M.; Chen, N.; Brooks, M.M.; Saba, S.; Gellad, W.F. Trajectories of Oral Anticoagulation Adherence Among Medicare Beneficiaries Newly Diagnosed with Atrial Fibrillation. J. Am. Heart Assoc. 2019, 8, e011427. [Google Scholar] [CrossRef]
- Ajrouche, A.; Estellat, C.; De Rycke, Y.; Tubach, F. Trajectories of Adherence to Low-Dose Aspirin Treatment Among the French Population. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 37–46. [Google Scholar] [CrossRef]
- Salmasi, S.; De Vera, M.A.; Safari, A.; Lynd, L.D.; Koehoorn, M.; Barry, A.R.; Andrade, J.G.; Deyell, M.W.; Rush, K.; Zhao, Y.; et al. Longitudinal Oral Anticoagulant Adherence Trajectories in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2021, 78, 2395–2404. [Google Scholar] [CrossRef]
- van Boven, J.F.M.; Koponen, M.; Lalic, S.; George, J.; Bell, J.S.; Hew, M.; Ilomaki, J. Trajectory Analyses of Adherence Patterns in a Real-Life Moderate to Severe Asthma Population. J. Allergy Clin. Immunol. Pract. 2020, 8, 1961–1969.e6. [Google Scholar] [CrossRef]
- Orlando, V.; Coscioni, E.; Guarino, I.; Mucherino, S.; Perrella, A.; Trama, U.; Limongelli, G.; Menditto, E. Drug-utilisation profiles and COVID-19. Sci. Rep. 2021, 11, 8913. [Google Scholar] [CrossRef]
- Orlando, V.; Mucherino, S.; Monetti, V.M.; Trama, U.; Menditto, E. Treatment patterns and medication adherence among newly diagnosed patients with migraine: A drug utilisation study. BMJ Open 2020, 10, e038972. [Google Scholar] [CrossRef]
- Guerrero-Fernández de Alba, I.; Orlando, V.; Monetti, V.M.; Mucherino, S.; Gimeno-Miguel, A.; Vaccaro, O.; Forjaz, M.J.; Poblador Plou, B.; Prados-Torres, A.; Riccardi, G.; et al. Comorbidity in an Older Population with Type-2 Diabetes Mellitus: Identification of the Characteristics and Healthcare Utilization of High-Cost Patients. Front. Pharmacol. 2020, 11, 586187. [Google Scholar] [CrossRef]
- Iolascon, G.; Gimigliano, F.; Orlando, V.; Capaldo, A.; Di Somma, C.; Menditto, E. Osteoporosis drugs in real-world clinical practice: An analysis of persistence. Aging Clin. Exp. Res. 2013, 25 (Suppl. 1), S137–S141. [Google Scholar] [CrossRef]
- Orlando, V.; Guerriero, F.; Putignano, D.; Monetti, V.M.; Tari, D.U.; Farina, G.; Illario, M.; Iaccarino, G.; Menditto, E. Prescription Patterns of Antidiabetic Treatment in the Elderly. Results from Southern Italy. Curr. Diabetes Rev. 2015, 12, 100–106. [Google Scholar] [CrossRef]
- Orlando, V.; Mucherino, S.; Guarino, I.; Guerriero, F.; Trama, U.; Menditto, E. Gender Differences in Medication Use: A Drug Utilization Study Based on Real World Data. Int. J. Environ. Res. Public. Health 2020, 17, 3926. [Google Scholar] [CrossRef]
- Orlando, V.; Monetti, V.M.; Moreno Juste, A.; Russo, V.; Mucherino, S.; Trama, U.; Guida, A.; Menditto, E. Drug Utilization Pattern of Antibiotics: The Role of Age; Sex and Municipalities in Determining Variation. Risk Manag. Healthc. Policy 2020, 13, 63–71. [Google Scholar] [CrossRef]
- Kolluri, S.; Lin, J.; Liu, R.; Zhang, Y.; Zhang, W. Machine Learning and Artificial Intelligence in Pharmaceutical Research and Development: A Review. AAPS J. 2022, 24, 19. [Google Scholar] [CrossRef]
- Ahmed, M.; Seraj, R.; Islam, S.M.S. The k-means Algorithm: A Comprehensive Survey and Performance Evaluation. Electronics 2020, 9, 1295. [Google Scholar] [CrossRef]
- Sinaga, K.P.; Miin-Shen, Y. Unsupervised K-means clustering algorithm. IEEE Access 2020, 8, 80716–80727. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, H. Research on K-Value Selection Method of K-Means Clustering Algorithm. J 2019, 2, 226–235. [Google Scholar] [CrossRef]
- Genolini, C.; Ecochard, R.; Benghezal, M.; Driss, T.; Andrieu, S.; Subtil, F. kmlShape: An Efficient Method to Cluster Longitudinal Data (Time-Series) According to Their Shapes. PLoS ONE 2016, 11, e0150738. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef]
- Cahill, T.J.; Kharbanda, R.K. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanisms; incidence and identification of patients at risk. World J. Cardiol. 2017, 9, 407–415. [Google Scholar] [CrossRef]
- Dargie, H. Heart failure post-myocardial infarction: A review of the issues. Heart 2005, 91 (Suppl. 2), ii3–ii6; discussion ii31; ii43–ii48. [Google Scholar] [CrossRef] [PubMed]
- Gerber, Y.; Weston, S.A.; Enriquez-Sarano, M.; Berardi, C.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Dunlay, S.M.; Roger, V.L. Mortality Associated with Heart Failure After Myocardial Infarction: A Contemporary Community Perspective. Circ. Heart Fail. 2016, 9, e002460. [Google Scholar] [CrossRef]
- Wolk, M.J.; Scheidt, S.; Killip, T. Heart failure complicating acute myocardial infarction. Circulation 1972, 45, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, J.P.; Claggett, B.; Seidelmann, S.B.; Seely, E.W.; Packer, M.; Zile, M.R.; Rouleau, J.L.; Swedberg, K.; Lefkowitz, M.; Shi, V.C.; et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: A post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017, 5, 333340. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall N = 4455 |
|---|---|
| Gender, N (%) | |
| Female | 1336 (30.0) |
| Male | 3119 (70.0) |
| Age, Mean (SD) | 69.1 ± 12.0 |
| Age groups, N (%) | |
| 0–25 y | 17 (0.4) |
| 26–50 y | 306 (6.9) |
| 51–75 y | 2653 (59.7) |
| over 76 y | 1469 (33) |
| Index Dosage, N (%) | |
| Low dosage (24 mg/26 mg) | 2941 (66) |
| Medium dosage (49 mg/51 mg) | 1306 (29.3) |
| High dosage (97 mg/103 mg) | 208 (4.7) |
| Polypharmacy, * N (%) | |
| No polypharmacy (1–4 drugs) | 1363 (30.6) |
| Polypharmacy (5–9 drugs) | 1410 (31.6) |
| Excessive polypharmacy (≥10 drugs) | 1654 (37.1) |
| ACCI score, Mean (SD) ** | 7.9 (6.2) |
| Hospital admission, * Mean (SD) | 1.7 (1.1) |
| Had ≥1 hospitalization for HF, N (%) | 1489 (33.4) |
| Had ≥2 hospitalizations for other causes, N (%) | 875 (19.6) |
| Medications HF-related, * N (%) | |
| Beta-blocking agents | 4080 (91.6) |
| Antithrombotic agents | 3900 (87.6) |
| Agents acting on the renin-angiotensin system | 3790 (85.1) |
| Diuretics | 3689 (82.8) |
| Other medications, * N (%) | |
| Lipid-modifying agents | 3155 (70.8) |
| Cardiac therapy | 2471 (55.5) |
| Drugs for obstructive airway diseases | 1977 (44.4) |
| Drugs used in diabetes | 1655 (37.2) |
| Cardiovascular comorbidities, * N (%) | |
| Cardiomyopathy | 422 (9.5) |
| CCS | 339 (7.6) |
| Hypertension | 221 (5) |
| CCS with STEMI | 179 (4) |
| CCS with NSTEMI | 164 (3.7) |
| Atrial fibrillation | 149 (3.3) |
| Other comorbidities, * N (%) | |
| Diabetes | 506 (11.4) |
| Chronic kidney disease | 206 (4.6) |
| Respiratory failure | 132 (3) |
| Patients’ Adherence Profiles | Group A High Adherence | Group B Partial Drop-Off * | Group C Moderate Adherence | Group D Low Adherence |
|---|---|---|---|---|
| N = 1898 | N = 874 | N = 862 | N = 821 | |
| CMA, Mean (SD) | 0.91 (0.08) | 0.63 (0.13) | 0.54 (0.11) | 0.17 (0.12) |
| Days on treatment, Median (IQR) | 322 (103) | 173.5 (93.5) | 157 (89) | 79.5 (57) |
| Switchers, ° N (%) | 261 (13.8) | 92 (10.5) | 202 (23.4) | 127 (15.5) |
| Switch after 1 month from index date § | 226 (86.6) | 20 (21.7) | 121 (59.9) | 53 (41.7) |
| Switch after 2 months from index date § | 28 (10.7) | 7 (7.6) | 58 (28.7) | 40 (31.5) |
| Switch after 6 months from index date § | 7 (2.7) | 65 (70.7) | 23 (11.4) | 34 (26.8) |
| Group A High Adherence | Group B Partial Drop-Off | Group C Moderate Adherence | Group D Low Adherence | p-Value | |
|---|---|---|---|---|---|
| Total, ° N (%) | 1898 (42.6) | 874 (19.6) | 862 (19.3) | 821 (18.4) | |
| Age, Mean (SD) | 69.0 (11.3) | 69.1 (11.8) | 69.3 (11.7) | 69.2 (13.8) | 0.003 |
| Sex, N (%) | 0.003 | ||||
| Female | 549 (28.9) | 270 (30.9) | 228 (26.5) | 289 (35.2) | |
| Male | 1349 (71.1) | 604 (69.1) | 634 (73.5) | 532 (64.8) | |
| Polypharmacy, * N (%) | 0.001 | ||||
| No polypharmacy (1–4 drugs) | 561 (29.6) | 248 (28.4) | 270 (31.3) | 284 (34.6) | |
| Polypharmacy (5–9 drugs) | 617 (32.5) | 269 (30.8) | 291 (33.8) | 233 (28.4) | |
| Excessive polypharmacy (≥10 drugs) | 715 (37.7) | 351 (40.2) | 298 (34.6) | 290 (35.3) | |
| ACCI score, Mean (SD) ** | 7.5 (5.5) | 7.6 (5.9) | 8.2 (6.1) | 9.0 (7.9) | 0.001 |
| Hospital admission, * Mean (SD) | 1.6 (1.0) | 1.7 (1.1) | 1.7 (1.1) | 1.9 (1.3) | 0.001 |
| ≥1 hospitalization for HF, N (%) | 637 (33.6) | 278 (31.8) | 293 (34) | 281 (34.2) | 0.001 |
| ≥2 hospitalizations for other causes, N (%) | 931 (49.1) | 426 (48.7) | 179 (20.8) | 185 (22.5) | 0.005 |
| Medications HF-related, * N (%) | |||||
| Beta-blocking agents | 1682 (88.6) | 770 (88.1) | 767 (89) | 686 (83.6) | <0.001 |
| Antithrombotic agents | 1599 (84.2) | 740 (84.7) | 732 (84.9) | 662 (80.6) | <0.001 |
| Agents acting on the renin-angiotensin system | 1587 (83.6) | 737 (84.3) | 701 (81.3) | 600 (73.1) | <0.001 |
| Diuretics | 1538 (81) | 692 (79.2) | 687 (79.7) | 614 (74.8) | <0.001 |
| Other medications, * N (%) | |||||
| Lipid-modifying agents | 1335 (70.3) | 599 (68.5) | 572 (66.4) | 512 (62.4) | <0.001 |
| Cardiac therapy | 1021 (53.8) | 436 (49.9) | 501 (58.1) | 406 (49.5) | <0.001 |
| Drugs for obstructive airway diseases | 788 (41.5) | 349 (39.9) | 369 (42.8) | 386 (47) | <0.001 |
| Drugs used in diabetes | 689 (36.3) | 321 (36.7) | 275 (31.9) | 298 (36.3) | <0.001 |
| Cardiovascular comorbidities, * N (%) | |||||
| Cardiomyopathy | 189 (10) | 70 (8) | 90 (10.4) | 73 (8.9) | <0.001 |
| CCS | 157 (8.3) | 62 (7.1) | 70 (8.1) | 50 (6.1) | <0.001 |
| Hypertension | 79 (4.2) | 47 (5.4) | 43 (5) | 52 (6.3) | 0.001 |
| CCS with NSTEMI | 69 (3.6) | 42 (4.8) | 28 (3.2) | 25 (3) | 0.001 |
| CCS with STEMI | 66 (3.5) | 37 (4.2) | 36 (4.2) | 40 (4.9) | <0.001 |
| Atrial fibrillation | 65 (3.4) | 22 (2.5) | 30 (3.5) | 32 (3.9) | <0.001 |
| Other comorbidities, * N (%) | |||||
| Diabetes | 203 (10.7) | 90 (10.3) | 105 (12.2) | 108 (13.2) | 0.001 |
| Chronic kidney disease | 74 (3.9) | 41 (4.7) | 48 (5.6) | 43 (5.2) | 0.002 |
| Respiratory failure | 45 (2.4) | 28 (3.2) | 25 (2.9) | 34 (4.1) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mucherino, S.; Dima, A.L.; Coscioni, E.; Vassallo, M.G.; Orlando, V.; Menditto, E. Longitudinal Trajectory Modeling to Assess Adherence to Sacubitril/Valsartan among Patients with Heart Failure. Pharmaceutics 2023, 15, 2568. https://doi.org/10.3390/pharmaceutics15112568
Mucherino S, Dima AL, Coscioni E, Vassallo MG, Orlando V, Menditto E. Longitudinal Trajectory Modeling to Assess Adherence to Sacubitril/Valsartan among Patients with Heart Failure. Pharmaceutics. 2023; 15(11):2568. https://doi.org/10.3390/pharmaceutics15112568
Chicago/Turabian StyleMucherino, Sara, Alexandra Lelia Dima, Enrico Coscioni, Maria Giovanna Vassallo, Valentina Orlando, and Enrica Menditto. 2023. "Longitudinal Trajectory Modeling to Assess Adherence to Sacubitril/Valsartan among Patients with Heart Failure" Pharmaceutics 15, no. 11: 2568. https://doi.org/10.3390/pharmaceutics15112568
APA StyleMucherino, S., Dima, A. L., Coscioni, E., Vassallo, M. G., Orlando, V., & Menditto, E. (2023). Longitudinal Trajectory Modeling to Assess Adherence to Sacubitril/Valsartan among Patients with Heart Failure. Pharmaceutics, 15(11), 2568. https://doi.org/10.3390/pharmaceutics15112568







