In Vivo Evaluation of a Gastro-Resistant Enprotect® Capsule under Postprandial Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Materials
2.2. Study Participants
2.3. Experimental Design
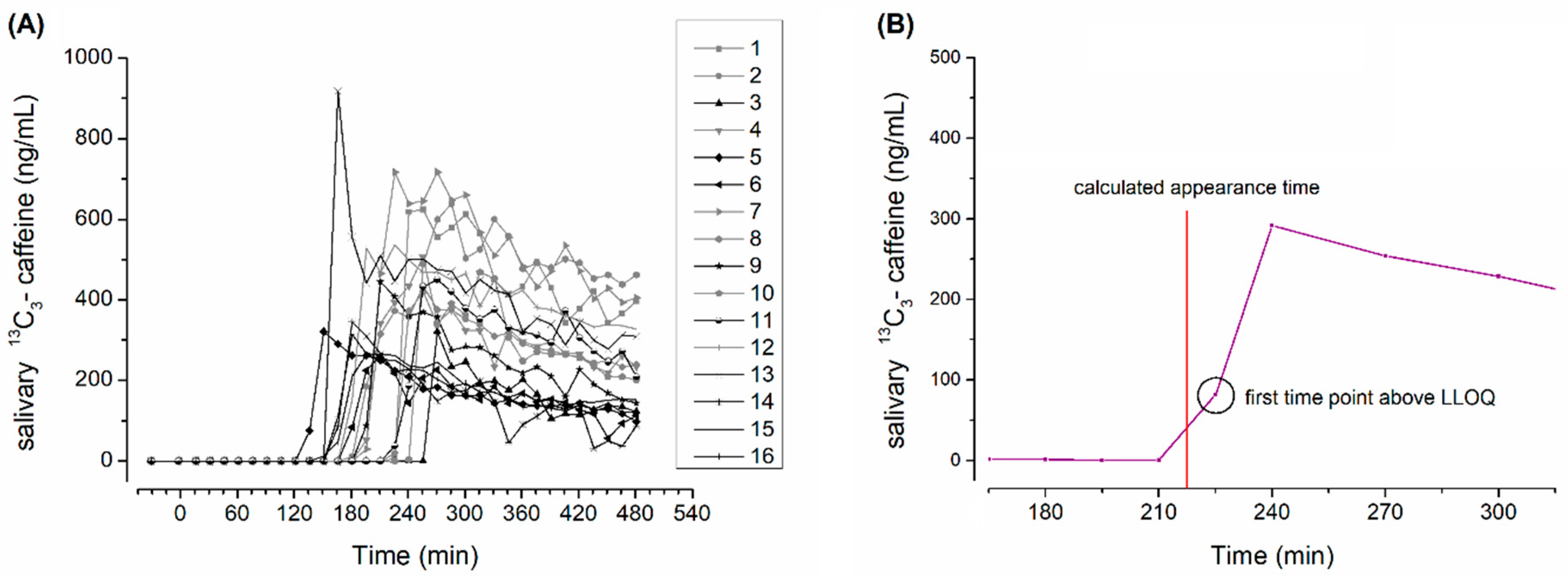
2.4. Salivary Sample Preparation and Evaluation of Caffeine Pharmacokinetics
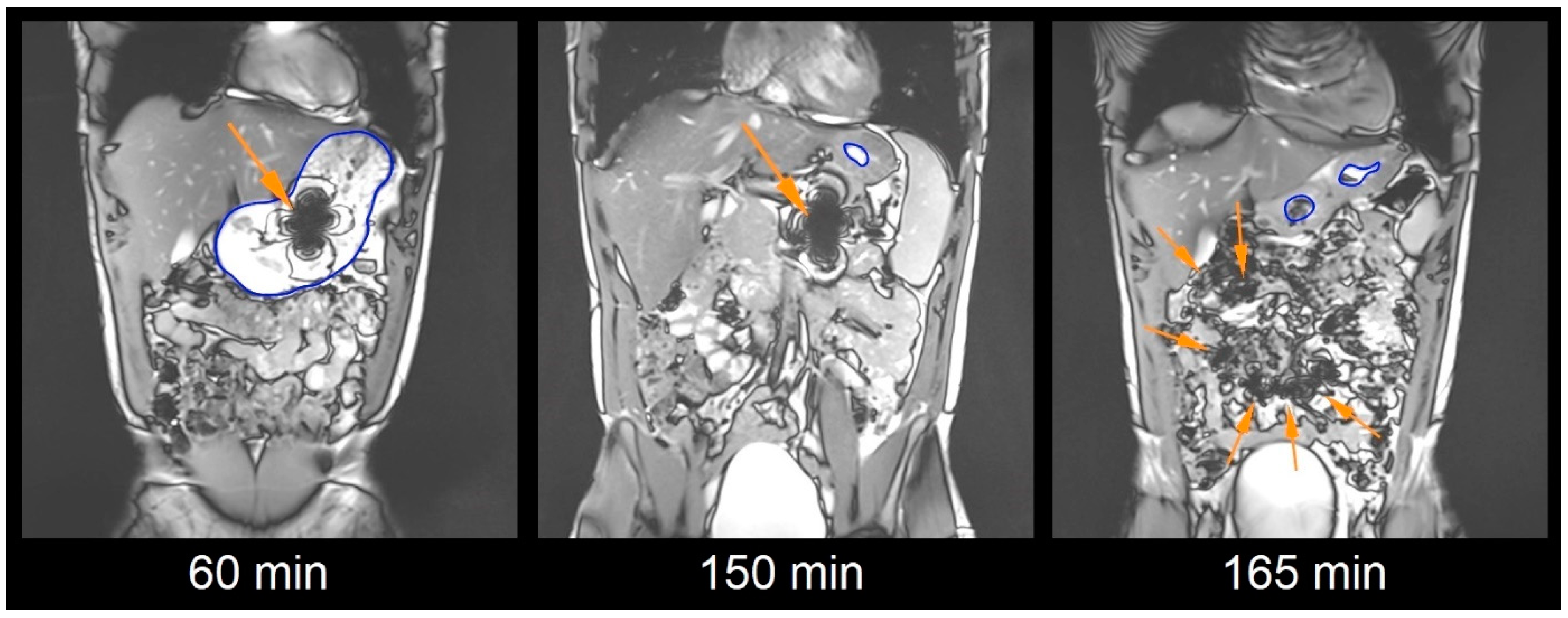
2.5. Magnetic Resonance Imaging Sequences
2.6. Image Analysis
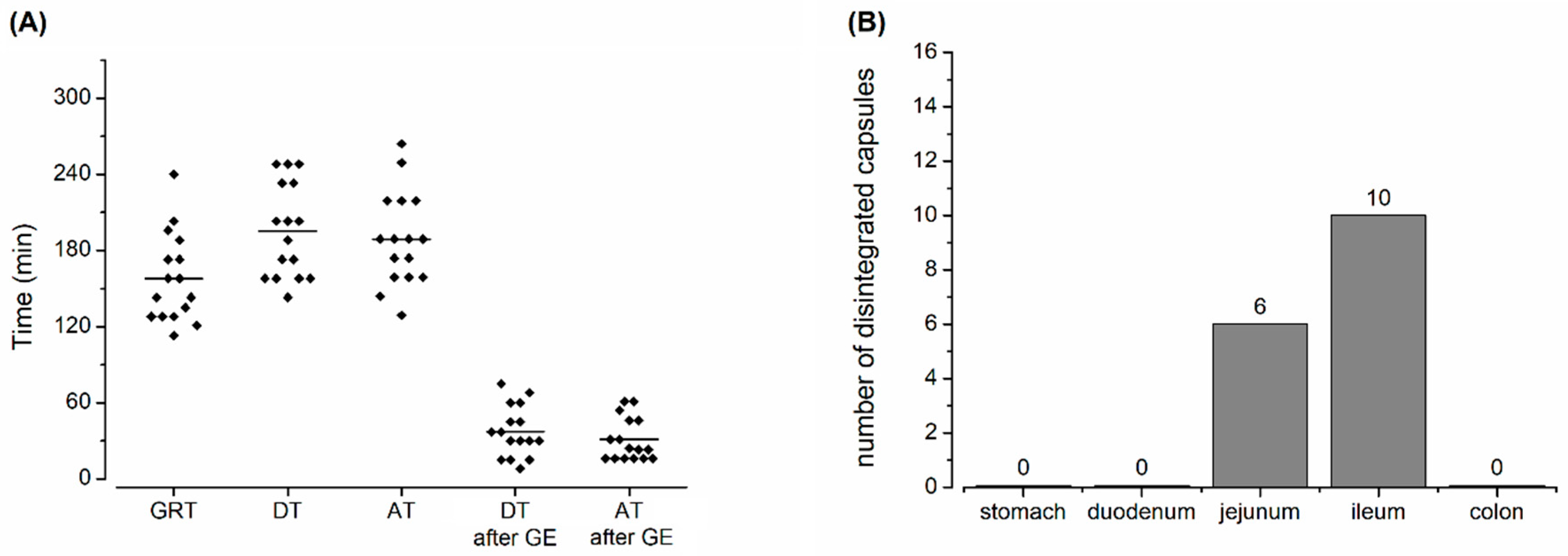
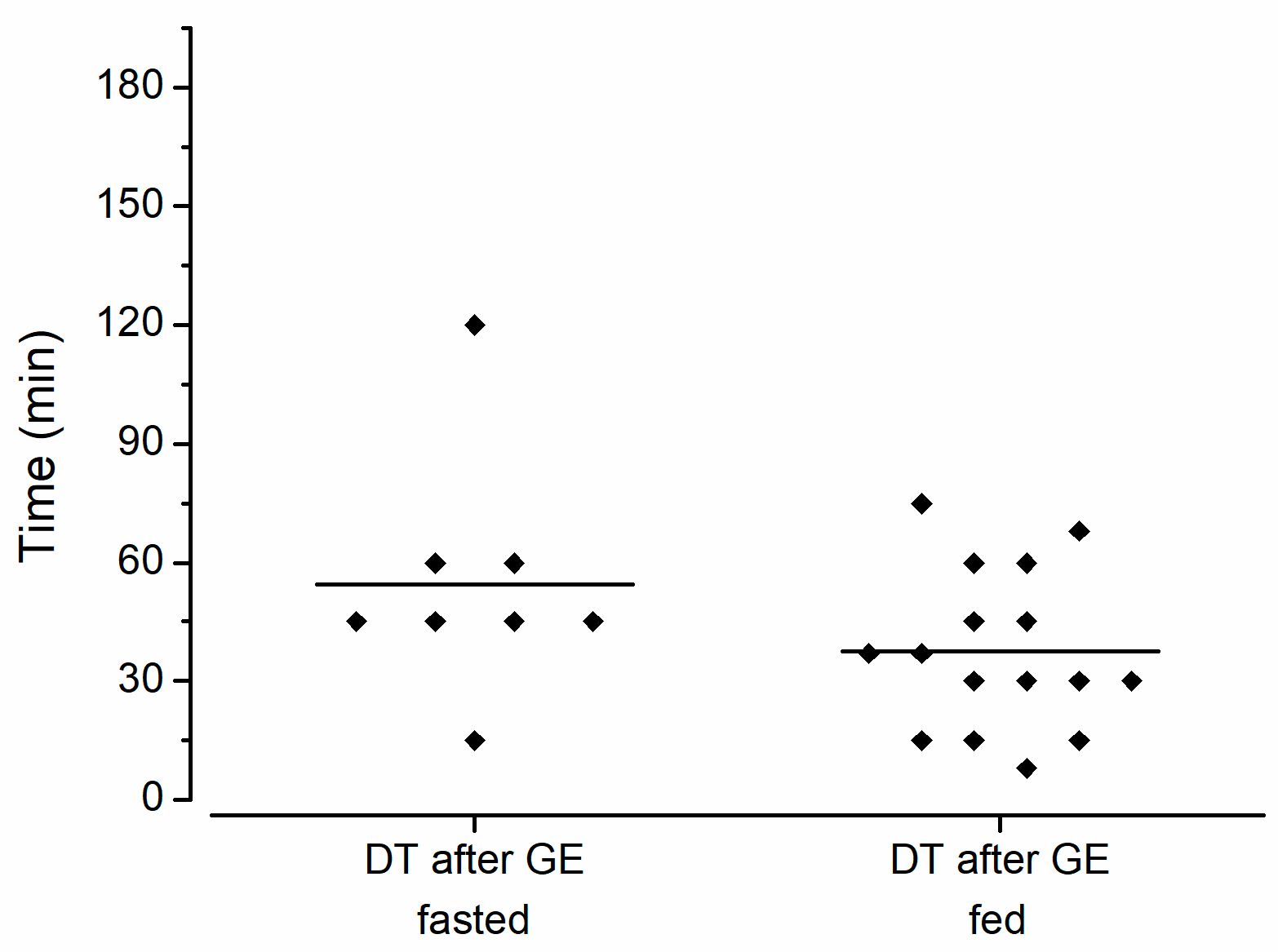
2.7. Capsule Evaluation Criteria
2.8. Statistical Analysis
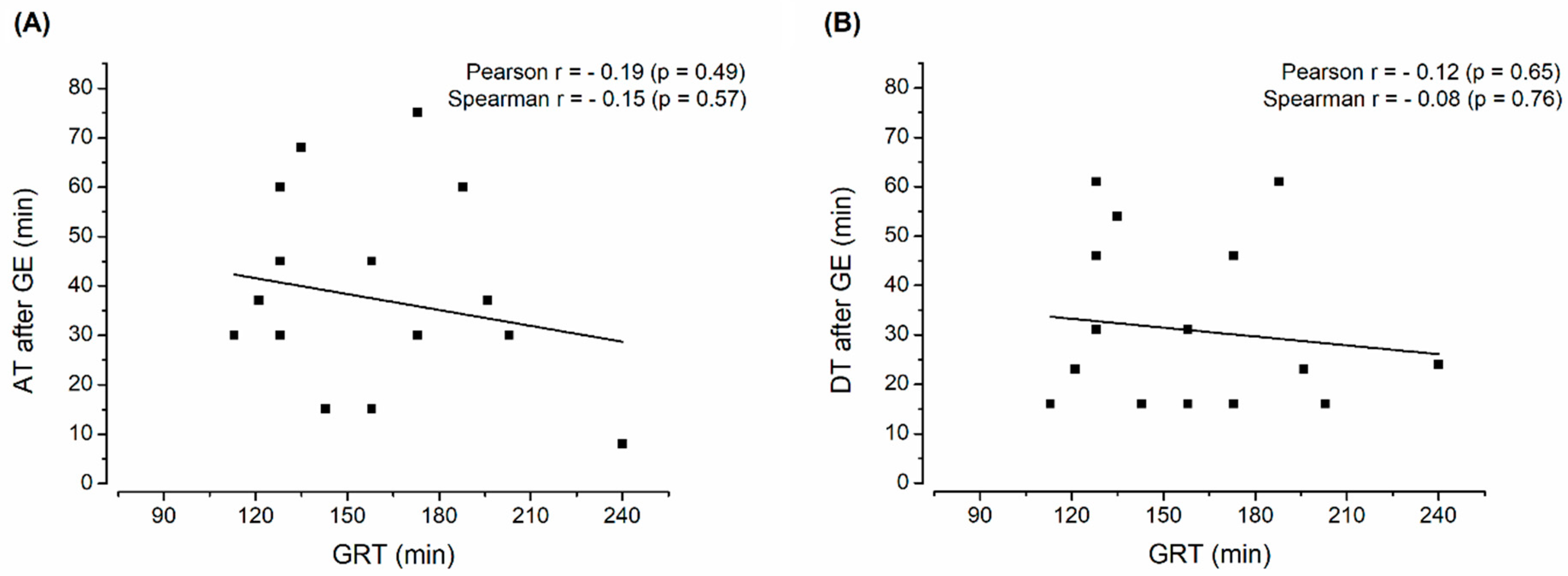
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jannin, V.; Duysburgh, C.; Gonzalez, V.; Govaert, M.; Agisson, M.; Marzorati, M.; Madit, N. In vitro evaluation of the gastrointestinal delivery of acid-sensitive pancrelipase in a next generation enteric capsule using an exocrine pancreatic insufficiency disease model. Int. J. Pharm. 2023, 630, 122441. [Google Scholar] [CrossRef] [PubMed]
- Rump, A.; Kromrey, M.; Scheuch, E.; Jannin, V.; Rehenbrock, L.; Tzvetkov, M.V.; Weitschies, W.; Grimm, M. In Vivo Evaluation of a Gastro-Resistant HPMC-Based “Next Generation Enteric” Capsule. Pharmaceutics 2022, 14, 1999. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Schneider, F.; Jedamzik, P.; Sager, M.; Kühn, J.-P.; Siegmund, W.; Weitschies, W. Navigating the human gastrointestinal tract for oral drug delivery: Uncharted waters and new frontiers. Adv. Drug Deliv. Rev. 2016, 101, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Steingoetter, A.; Weishaupt, D.; Kunz, P.; Mäder, K.; Lengsfeld, H.; Thumshirn, M.; Boesiger, P.; Fried, M.; Schwizer, W. Magnetic Resonance Imaging for the in Vivo Evaluation of Gastric-Retentive Tablets. Pharm. Res. 2003, 20, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Knörgen, M.; Spielmann, R.P.; Abdalla, A.; Metz, H.; Mäder, K. Non-invasive MRI detection of individual pellets in the human stomach. Eur. J. Pharm. Biopharm. 2010, 74, 120–125. [Google Scholar] [CrossRef]
- Sager, M.; Grimm, M.; Aude, P.; Schick, P.; Merdivan, S.; Hasan, M.; Kromrey, M.-L.; Sivert, A.; Benameur, H.; Koziolek, M.; et al. In vivo characterization of enTRinsicTM drug delivery technology capsule after intake in fed state: A cross-validation approach using salivary tracer technique in comparison to MRI. J. Control. Release 2019, 313, 24–32. [Google Scholar] [CrossRef]
- Rump, A.; Weiss, F.N.; Schulz, L.; Kromrey, M.-L.; Scheuch, E.; Tzvetkov, M.V.; White, T.; Durkee, S.; Judge, K.W.; Jannin, V.; et al. The Effect of Capsule-in-Capsule Combinations on In Vivo Disintegration in Human Volunteers: A Combined Imaging and Salivary Tracer Study. Pharmaceutics 2021, 13, 2002. [Google Scholar] [CrossRef]
- Sager, M.; Schick, P.; Mischek, M.; Schulze, C.; Hasan, M.; Kromrey, M.-L.; Benameur, H.; Wendler, M.; Tzvetkov, M.V.; Weitschies, W.; et al. Comparison of In Vitro and In Vivo Results Using the GastroDuo and the Salivary Tracer Technique: Immediate Release Dosage Forms under Fasting Conditions. Pharmaceutics 2019, 11, 659. [Google Scholar] [CrossRef]
- Wilding, I.R.; Hardy, J.G.; Sparrow, R.A.; Davis, S.S.; Daly, P.B.; English, J.R. In Vivo Evaluation of Enteric-Coated Naproxen Tablets Using Gamma Scintigraphy. Pharm. Res. 1992, 9, 1436–1441. [Google Scholar] [CrossRef]
- Vertzoni, M.; Augustijns, P.; Grimm, M.; Koziolek, M.; Lemmens, G.; Parrott, N.; Pentafragka, C.; Reppas, C.; Rubbens, J.; Van Den Abeele, J.; et al. Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: An UNGAP review. Eur. J. Pharm. Sci. 2019, 134, 153–175. [Google Scholar] [CrossRef]
- Olivares-Morales, A.; Ghosh, A.; Aarons, L.; Rostami-Hodjegan, A. Development of a Novel Simplified PBPK Absorption Model to Explain the Higher Relative Bioavailability of the OROS® Formulation of Oxybutynin. AAPS J. 2016, 18, 1532–1549. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.; Gershkovich, P.; Hoad, C.L.; Calladine, M.; Spiller, R.C.; Stolnik, S.; Marciani, L. Application of In Vivo MRI Imaging to Track a Coated Capsule and Its Disintegration in the Gastrointestinal Tract in Human Volunteers. Pharmaceutics 2022, 14, 270. [Google Scholar] [CrossRef]
- Hahn, T.; Kozerke, S.; Schwizer, W.; Fried, M.; Boesiger, P.; Steingoetter, A. Visualization and quantification of intestinal transit and motor function by real-time tracking of 19F labeled capsules in humans. Magn. Reson. Med. 2011, 66, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.; Garbacz, G.; Neumann, M.; Weitschies, W. Simulating the postprandial stomach: Physiological considerations for dissolution and release testing. Mol. Pharm. 2013, 10, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.; Grimm, M.; Garbacz, G.; Kühn, J.-P.; Weitschies, W. Intragastric Volume Changes after Intake of a High-Caloric, High-Fat Standard Breakfast in Healthy Human Subjects Investigated by MRI. Mol. Pharm. 2014, 11, 1632–1639. [Google Scholar] [CrossRef]
- Koziolek, M.; Schneider, F.; Grimm, M.; Modeβ, C.; Seekamp, A.; Roustom, T.; Siegmund, W.; Weitschies, W. Intragastric pH and pressure profiles after intake of the high-caloric, high-fat meal as used for food effect studies. J. Control. Release 2015, 220, 71–78. [Google Scholar] [CrossRef]
- Chavan, R.B.; Rathi, S.; Jyothi, V.G.S.S.; Shastri, N.R. Cellulose based polymers in development of amorphous solid dispersions. Asian J. Pharm. Sci. 2019, 14, 248–264. [Google Scholar] [CrossRef]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M.; et al. The mechanisms of pharmacokinetic food-drug interactions—A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef]
- Klein, S.; Butler, J.; Hempenstall, J.M.; Reppas, C.; Dressman, J.B.; Butler, J.; Hempenstall, J.M.; Reppas, C. Media to simulate the postprandial stomach I. Matching the physicochemical characteristics of standard breakfasts. J. Pharm. Pharmacol. 2004, 56, 605–610. [Google Scholar] [CrossRef]
- Koziolek, M.; Carrière, F.; Porter, C.J.H.H. Lipids in the Stomach—Implications for the Evaluation of Food Effects on Oral Drug Absorption. Pharm. Res. 2018, 35, 55. [Google Scholar] [CrossRef]
- Kalantzi, L.; Goumas, K.; Kalioras, V.; Abrahamsson, B.; Dressman, J.B.; Reppas, C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 2006, 23, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Grimm, M.; Koziolek, M.; Modeß, C.; Dokter, A.; Roustom, T.; Siegmund, W.; Weitschies, W. Resolving the physiological conditions in bioavailability and bioequivalence studies: Comparison of fasted and fed state. Eur. J. Pharm. Biopharm. 2016, 108, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Fadda, H.; McConnell, E.; Short, M.B.; Basit, A.W. Meal-Induced Acceleration of Tablet Transit Through the Human Small Intestine. Pharm. Res. 2009, 26, 356–360. [Google Scholar] [CrossRef] [PubMed]
| Ingredient | Producer/Distributor |
|---|---|
| Capsugel® Enprotect® capsules size 0 | Capsugel France SAS, Lonza group, Colmar, France |
| Black iron oxide E172 | Caesar & Loretz GmbH, Hilden, Germany |
| 13C3-labelled caffeine | SIGMA-ALDRICH CHEMIE GmbH, Schnelldorf, Germany |
| Croscarmellose, Ph.Eur. | JRS Pharma GmbH & Co. KG, Rosenberg, Germany |
| Mannitol, Ph.Eur. | Fagron GmbH & Co. KG, Barsbüttel, Germany |
| Silicon dioxide, Ph.Eur. | Fagron GmbH & Co. KG, Barsbüttel, Germany |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimm, M.; Rump, A.; Kromrey, M.-L.; Morof, F.; Dumont, C.; Jannin, V.; Tzvetkov, M.V.; Weitschies, W. In Vivo Evaluation of a Gastro-Resistant Enprotect® Capsule under Postprandial Conditions. Pharmaceutics 2023, 15, 2576. https://doi.org/10.3390/pharmaceutics15112576
Grimm M, Rump A, Kromrey M-L, Morof F, Dumont C, Jannin V, Tzvetkov MV, Weitschies W. In Vivo Evaluation of a Gastro-Resistant Enprotect® Capsule under Postprandial Conditions. Pharmaceutics. 2023; 15(11):2576. https://doi.org/10.3390/pharmaceutics15112576
Chicago/Turabian StyleGrimm, Michael, Adrian Rump, Marie-Luise Kromrey, Felix Morof, Camille Dumont, Vincent Jannin, Mladen Vassilev Tzvetkov, and Werner Weitschies. 2023. "In Vivo Evaluation of a Gastro-Resistant Enprotect® Capsule under Postprandial Conditions" Pharmaceutics 15, no. 11: 2576. https://doi.org/10.3390/pharmaceutics15112576
APA StyleGrimm, M., Rump, A., Kromrey, M.-L., Morof, F., Dumont, C., Jannin, V., Tzvetkov, M. V., & Weitschies, W. (2023). In Vivo Evaluation of a Gastro-Resistant Enprotect® Capsule under Postprandial Conditions. Pharmaceutics, 15(11), 2576. https://doi.org/10.3390/pharmaceutics15112576






