Evidence of Reliable Gastro-Resistance of Novel Enteric Ready-to-Fill Capsules Simplifying Pharmaceutical Manufacturing
Abstract
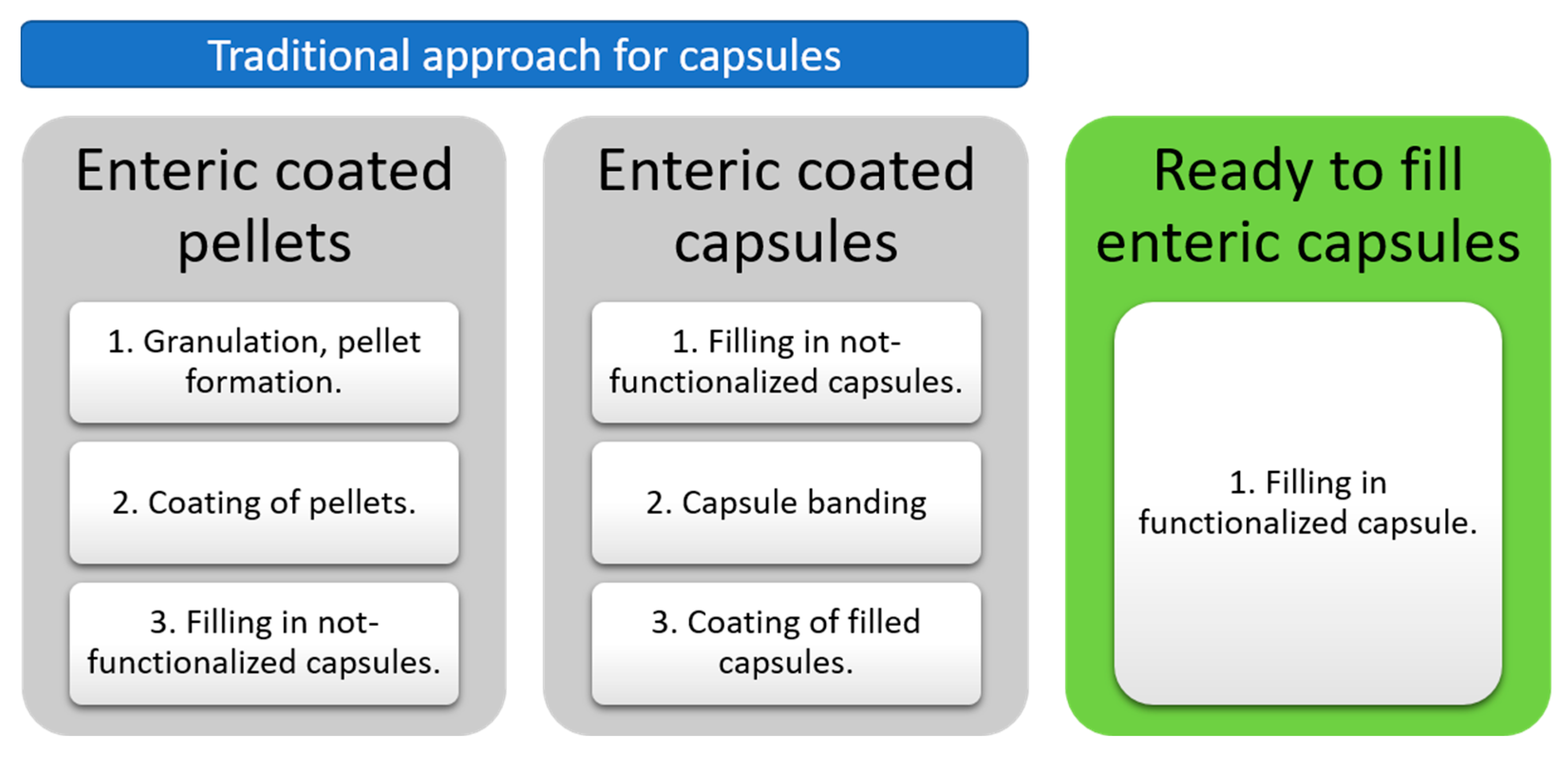
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Standards, Samples and Excipients
2.3. Equipment
2.4. Analytical Methodologies
2.4.1. Diclofenac Determination after Acid Stage Dissolution Test
2.4.2. Omeprazole Determination after the Acid Stage Dissolution Test
2.4.3. Acid Resistance Test Using Hydroxy Naphthol Blue
3. Results
3.1. Diclofenac Study Case
3.2. Omeprazole Study Case
3.3. Acid Resistance Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| GI | Gastrointestinal |
| HCl | Hydrochloric acid |
| HPLC | High-performance liquid chromatography |
| HPMC | Hydroxypropyl methylcellulose |
| ICH | International Council for Harmonization |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MCC | Microcrystalline cellulose |
| NaOH | Sodium hydroxide |
| NLT | Not less than |
| NMT | Not more than |
| Ph.Eur. | European Pharmacopoeia |
| PK | Pharmacokinetics |
| RNA | Ribonucleic acid |
| RT | Retention time |
| USP | United States Pharmacopoeia |
References
- Congressional Budget Office, U.S. Research and Development in the Pharmaceutical Industry. Available online: https://www.cbo.gov/publication/57126 (accessed on 31 May 2023).
- USP43-NF38 〈1151〉 Pharmaceutical Dosage Forms. Available online: https://online.uspnf.com/uspnf/document/1_GUID-431F93A9-1FEC-42AE-8556-AA5B604B2E36_8_en-US?source=SearchResults&highlight=1151 (accessed on 31 May 2023).
- Trenfield, S.J.; Basit, A.W. Modified drug release: Current strategies and novel technologies for oral drug delivery. In Nanotechnology for Oral Drug Delivery; UCL School of Pharmacy, University College London: London, UK, 2020; pp. 177–197. [Google Scholar]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef] [PubMed]
- Al-Gousous, J.; Tsume, Y.; Fu, M.; Salem, I.I.; Langguth, P. Unpredictable Performance of pH-Dependent Coatings Accentuates the Need for Improved Predictive in Vitro Test Systems. Mol. Pharm. 2017, 14, 4209–4219. [Google Scholar] [CrossRef] [PubMed]
- Mastropietro, D.; Park, K.; Omidian, H. Polymers in Oral Drug Delivery. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; Volume 4, pp. 430–444. [Google Scholar]
- Hoelzer, B.; Jain, V. Eudracap, Fuctional Ready-to-Fill Capsules. Available online: https://healthcare.evonik.com/en/drugdelivery/oral-drug-delivery/oral-excipients/eudracap-portfolio (accessed on 14 June 2023).
- Monografie Capsules. In European Pharmacopoeia 11.2; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2018; pp. 966–967.
- Hoag, S.W. Capsules Dosage Form. In Developing Solid Oral Dosage Forms; Elsevier: Amsterdam, The Netherlands, 2017; pp. 723–747. [Google Scholar]
- Baltoyiannis, G.; Christodoulos, N.; Mitsis, M.; Stephanou, D.; Ioannou, H.; Nousias, V.; Kappas, A.M. Experimental Medicine A Comparative Experimental Study of the Effects of Diclofenac and Ketoprofen on the Small-Bowel Mucosa of Canines; Springer: New York City, NY, USA, 2001; Volume 200, pp. 125–135. [Google Scholar]
- Gan, T.J. Diclofenac: An update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Hammami, M.M.; Hussein, R.F.; Alswayeh, R.; Alvi, S.N. Eight enteric-coated 50 mg diclofenac sodium tablet formulations marketed in Saudi Arabia: In vitro quality evaluation. BMC Res. Notes 2020, 13, 428. [Google Scholar] [CrossRef] [PubMed]
- Zakowiecki, D.; Frankiewicz, M.; Hess, T.; Cal, K.; Gajda, M.; Dabrowska, J.; Kubiak, B.; Paszkowska, J.; Wiater, M.; Hoc, D.; et al. Development of a biphasic-release multiple-unit pellet system with diclofenac sodium using novel calcium phosphate-based starter pellets. Pharmaceutics 2021, 13, 805. [Google Scholar] [CrossRef] [PubMed]
- Zakowiecki, D.; Szczepanska, M.; Hess, T.; Cal, K.; Mikolaszek, B.; Paszkowska, J.; Wiater, M.; Hoc, D.; Garbacz, G. Preparation of delayed-release multiparticulate formulations of diclofenac sodium and evaluation of their dissolution characteristics using biorelevant dissolution methods. J. Drug Deliv. Sci. Technol. 2020, 60, 101986. [Google Scholar] [CrossRef]
- Franc, A.; Vetchý, D.; Fülöpová, N. Commercially Available Enteric Empty Hard Capsules, Production Technology and Application. Pharmaceuticals 2022, 15, 1398. [Google Scholar] [CrossRef] [PubMed]
- Srebro, J.; Brniak, W.; Mendyk, A. Formulation of Dosage Forms with Proton Pump Inhibitors: State of the Art, Challenges and Future Perspectives. Pharmaceutics 2022, 14, 2043. [Google Scholar] [CrossRef] [PubMed]
- Olbe, L.; Carlsson, E.; Lindberg, P. A proton-pump inhibitor expedition: The case histories of omeprazole and esomeprazole. Nat. Rev. Drug Discov. 2003, 2, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.; Smith, A.; Ye, W.; Toler, D.Y.; Westenberger, B.J.; Lionberger, R.; Raw, A.; Yu, L.; Buhse, L.F. Generic omeprazole delayed-release capsules: In vitro performance evaluations. Drug Dev. Ind. Pharm. 2009, 35, 917–921. [Google Scholar] [CrossRef] [PubMed]
- USP43-NF38 Omeprazole Delayed-Release Capsules. Available online: https://online.uspnf.com/uspnf/document/1_GUID-71E87DD7-0164-42C0-8027-A9211B069968_1_en-US?source=QuickSearch&highlight=omeprazole (accessed on 31 May 2023).
- European Pharmacopoeia 11.2 Omeprazole. Available online: https://pheur.edqm.eu/app/11-2/content/11-2/0942E.htm?highlight=on&terms=omeprazole (accessed on 31 May 2023).
- Vanderhoff, B.T.; Tahboub, R.M.; Hershey, M.S. Pharmacological Profiles of PPIs; American Family Physian: Leawood, KS, USA, 2002; Volume 66, pp. 273–280. [Google Scholar]
- Ochoa, D.; Román, M.; Cabaleiro, T.; Saiz-Rodríguez, M.; Mejía, G.; Abad-Santos, F. Effect of food on the pharmacokinetics of omeprazole, pantoprazole and rabeprazole. BMC Pharmacol. Toxicol. 2020, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- USP43-NF38 〈711〉 Dissolution. Available online: https://online.uspnf.com/uspnf/document/1_GUID-AC788D41-90A2-4F36-A6E7-769954A9ED09_1_en-US?source=SearchResults&highlight=711 (accessed on 18 January 2023).
- 2.9.3. Dissolution Test for Solid Dosage Forms. In European Pharmacopoeia 11.2; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2022; pp. 348–355.
- USP43-NF38 Diclofenac Sodium Delayed-Release Tablets. Available online: https://online.uspnf.com/uspnf/document/1_GUID-4A290B3C-D12E-4A48-AEEF-79B9269DC606_1_en-US?source=QuickSearch&highlight=diclofenacdelayed (accessed on 31 May 2023).
- USP43-NF38 〈1226〉 Verification of Compendial Procedures. Available online: https://online.uspnf.com/uspnf/document/1_GUID-18A6E56B-8EA7-4B37-AB7D-82352B73A309_3_en-US?source=SearchResults&highlight=1226 (accessed on 1 June 2023).
- International Council for Harmonisation Validation of Analytical Procedures Q2(R2). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf (accessed on 25 October 2022).
- USP43-NF38 〈1225〉 Validation of Compendial Procedures. Available online: https://online.uspnf.com/uspnf/document/1_GUID-E2C6F9E8-EA71-4B72-A7BA-76ABD5E72964_4_en-US?source=QuickSearch&highlight=validation (accessed on 22 February 2022).
- USP43-NF38 〈701〉 Disintegration. Available online: https://online.uspnf.com/uspnf/document/1_GUID-6B930D76-6026-4693-844B-FB9C90728D4F_3_en-US?source=QuickSearch&highlight=701 (accessed on 21 June 2023).
- Koziolek, M.; Schneider, F.; Grimm, M.; Mode, C.; Seekamp, A.; Roustom, T.; Siegmund, W.; Weitschies, W. Intragastric pH and pressure profiles after intake of the high-caloric, high-fat meal as used for food effect studies. J. Control. Release 2015, 220, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Vertzoni, M.; Augustijns, P.; Grimm, M.; Koziolek, M.; Lemmens, G.; Parrott, N.; Pentafragka, C.; Reppas, C.; Rubbens, J.; Van Den Abeele, J.; et al. Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: An UNGAP review. Eur. J. Pharm. Sci. 2019, 134, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal Absorption Study: Challenges and Absorption Enhancement Strategies in Improving Oral Drug Delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H. Drug Absorption from the Colon In Situ. In Drug Absorption Studies; Springer US: Boston, MA, USA, 2008; pp. 77–88. [Google Scholar]




| Chromatographic Feature | Setup |
|---|---|
| Mobile phases | A: 10 mM ammonium acetate buffer pH 5.3 B: Acetonitrile |
| Gradient program | %A0min = 70, %A0.5min = 70, %A8.5min = 5, %A10min = 5, %A10.1min = 70 and %A14min = 70 |
| Flow | 0.3 mL/min |
| Injection volume | 1 µL |
| Time per injection | 14 min |
| Column temperature | 35 °C |
| Sample temperature | 20 °C |
| Wavelength | 280 nm |
| Chromatographic Feature | Setup |
|---|---|
| Mobile phases | A: 10 mM ammonium bicarbonate buffer pH 8.75 B: Acetonitrile |
| Gradient program | %A0min = 90, %A3min = 90, %A10min = 40, %A11min = 90 and %A15min = 90 |
| Flow | 1.9 mL/min |
| Injection volume | 5 µL |
| Time per injection | 15 min |
| Column temperature | 35 °C |
| Sample temperature | 20 °C |
| Wavelength | 305 nm |
| Chromatographic Feature | Setup |
|---|---|
| Mobile phases | A: Glycine 3g/L adjusted to pH 9.0 |
| Gradient program | %A0min = 88, %A20min = 40, %A21min = 88 and %A25min = 88 |
| Flow | 1.2 mL/min |
| Injection volume | 10 µL |
| Time per injection | 25 min |
| Column temperature | 20 °C |
| Sample temperature | 20 °C |
| Wavelength | 305 nm |
| Impurity | Acceptance Criteria, NMT (%) | EUDRACAP® NMT (%) | Diclovit®, NMT (%) |
|---|---|---|---|
| Oxindole | — | 0.00 | 0.00 |
| Diclofenac-related compound D (diclofenac-bromo analog) | — | 0.28 | 0.28 |
| Diclofenac-related compound A | 0.50 | 0.00 | 0.00 |
| Diclofenac-alcohol analog | — | 0.00 | 0.03 |
| Diclofenac-benzaldehyde analog | — | 0.00 | 0.01 |
| Any individual unspecified impurity | 0.50 | 0.15 | 0.28 |
| Total impurities | 1.50 | 0.44 * | 0.44 * |
| Impurity | Acceptance Criteria, NMT (%) | EUDRACAP® after 2 h, NMT (%) | EUDRACAP® after 4 h, NMT (%) | Losec®, after 2 h NMT (%) |
|---|---|---|---|---|
| Omeprazole-related compounds F and G | 0.5 | 0.02 | 0.22 | 0.67 |
| 5-Methoxy-1H-benzimidazole-2-thiol | 0.5 | 0.00 | 0.00 | 0.01 |
| Any other individual impurity | 0.5 | 0.13 | 0.13 | 0.29 |
| Total impurities | 2 | 0.22 * | 0.74 * | 1.51 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso Urich, J.A.; Fedorko, A.; Hölzer, B.; Khinast, J. Evidence of Reliable Gastro-Resistance of Novel Enteric Ready-to-Fill Capsules Simplifying Pharmaceutical Manufacturing. Pharmaceutics 2023, 15, 2592. https://doi.org/10.3390/pharmaceutics15112592
Afonso Urich JA, Fedorko A, Hölzer B, Khinast J. Evidence of Reliable Gastro-Resistance of Novel Enteric Ready-to-Fill Capsules Simplifying Pharmaceutical Manufacturing. Pharmaceutics. 2023; 15(11):2592. https://doi.org/10.3390/pharmaceutics15112592
Chicago/Turabian StyleAfonso Urich, Jesús Alberto, Anna Fedorko, Bettina Hölzer, and Johannes Khinast. 2023. "Evidence of Reliable Gastro-Resistance of Novel Enteric Ready-to-Fill Capsules Simplifying Pharmaceutical Manufacturing" Pharmaceutics 15, no. 11: 2592. https://doi.org/10.3390/pharmaceutics15112592
APA StyleAfonso Urich, J. A., Fedorko, A., Hölzer, B., & Khinast, J. (2023). Evidence of Reliable Gastro-Resistance of Novel Enteric Ready-to-Fill Capsules Simplifying Pharmaceutical Manufacturing. Pharmaceutics, 15(11), 2592. https://doi.org/10.3390/pharmaceutics15112592








