Combination of Nanodelivery Systems and Constituents Derived from Novel Foods: A Comprehensive Review
Abstract
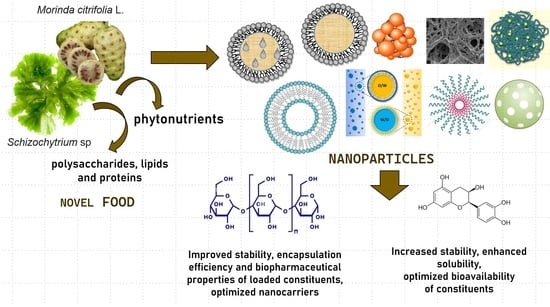
:1. Introduction
2. Novel Food Materials for Nanoparticle Production
2.1. Polymeric Nanoparticles
2.2. Lipid-Based Nanoparticles
2.3. Extracellular Vesicles
2.4. Nanoemulsion Systems
2.5. Protein-Based Nanoparticles
3. Compound Derived from Novel Foods Embedded into Nanocarriers
| Novel Food | Nutraceutical Compounds | Activity | Type of Nanoparticle | Results | Reference |
|---|---|---|---|---|---|
| Angelica gigas Nakai (AGN) dried root | Pyranocoumarins (e.g., decursin and decursinol angelate) | Antioxidant | HPMC as biopolymer + acetic acid as plasticizer for final solid formulation | Better extraction of phenolic compounds and flavonoids from AGN using HPMC and plasticizer | [83] |
| Astaxanthin-rich oleoresin from Haematococcus pluvialis algae | Astaxanthin | Antioxidant, anti-cancer, and cardiovascular disease prevention agent | Nanoemulsions, liposomes, SLNs, chitosan-based and PLGA-based nanoparticles | Improved bioavailability | [81] |
| Antarctic krill oil from Euphausia Superba | Omega-3 fatty acids (mainly docosahexaenoic and eicosapentaenoic acids) and astaxanthin | Reduces blood lipid, sugar levels, and the risk of atherosclerosis, slows down nerve aging, relieves the symptoms of depression, and anti-inflammatory | NLC | Protection of polyunsaturated fatty acids from oxidation, light, and temperature | [84] |
| Antrodia camphorata (or cinnamonea) | Triterpenoids | Anti-diabetic | Silica/chitosan | Demonstrate anti-diabetic properties on rat model and protection of testicular dysfunction | [85] |
| Bovine lactoferrin | Bovine lactoferrin | Anti-viral against SARS-CoV-2 | Phosphatidil coline liposome | Reduction of the infection of about 80% on the lung cell line, and a higher anti-viral effect than non-liposomal lactoferrin. Protection from gastrointestinal conditions after oral administration | [86] |
| Cranberry extract powder from Vaccinium macrocarpon | Polyphenols (mainly proanthocyanidins) | Antioxidant | Liposomes containing bile salt in the double layer to inhibit enzymes in the gastrointestinal tract | Protection of the liver by the reduction of the level of antioxidant enzymes | [87] |
| Antimicrobial | Nanoemulsion with green tea catechins | Synergic effect against E. coli for urinary infection treatment | [88] | ||
| Antioxidant | Chitosan/carrageenan NPs | Prevention of their degradation in the digestive medium | [89] | ||
| Antimicrobial | Chitosan NPs | Interaction with bacteria reducing adherence to intestinal tissue | [90] | ||
| Echinacea angustifolia extract from cell cultures | Echinacoside and polyphenols | Antimicrobial | Alginate/chitosan nanoparticles | The encapsulated extract displayed a higher biofilm inhibition and up to a 32-fold lower MIC compared to the free extract against Staphylococcus aureus | [91] |
| Niosome | The encapsulated extract showed up to a 16-fold greater antibacterial activity against Klebsiella pneumoniae compared to the free extract | [92] | |||
| Egg membrane hydrolysate proteins | Collagen, elastin, glycosaminoglycans | Antioxidant, anti-inflammatory against IL-8 in intestinal epithelial cells | pH sensitive chitosan/fucoidan NPs | Protection from intestinal degradation, increased antioxidant activity, and mucoadhesion that increase local delivery | [22] |
| Epigallocatechin-3-gallate as a purified extract from green tea leaves (Camellia sinensis) | Epigallocatechin-3-gallate | Antioxidant, anti-inflammatory | Chitosan-based NPs | Controlled release in the intestinal environment and intestinal protection of epigallocatechin-3-gallate (preserved antioxidant activity) | [93] |
| Iron hydroxide adipate tartrate | Iron deficiency such as anemia | NPs of iron hydroxide adipate tartrate is insoluble in GI tract | Slowly ferrous iron release avoiding ROS production | [94,95,96] | |
| Lonicera caerulea berries | Anthocyanins | Antioxidant and antimicrobial | PLGA and carboxymethyl chitosan NPs | Improved therapeutic efficiency | [80] |
| Lycopene (LYC) produced with a synthetic process or extracted from Blakeslea trispora (red guava), tomato peels, or oleoresin from tomatoes | - | Antioxidant | Polyelectrolyte complexes with sodium caseinate and TLH-3, an acidic polysaccharide | Protection from oxidation and controlled release in the gastrointestinal tract | [97] |
| - | Antioxidant, anti-inflammatory | Liposome with lecithin and cholesterol | Increased level of LYC in serum and brain compared with free LYC. Reduction in ROS and inflammation | [98] | |
| Antioxidant, anti-tumoral (prostate cancer) | Self-emulsifying system with coconut oil and sorbitan monostearate | Confirm the antioxidant activity without side effects. Ability to reach prostate tissue | [99] | ||
| - | Antioxidant for liver disease | Chitosan-based NPs | Hepatoprotection with reduction in IL and TNF-alpha | [100] | |
| - | Antioxidant | Catechin NPs coated with chitosan | Gastric protection and higher plasma concentration | [101] | |
| - | Antioxidant, anti-tumoral (breast cancer) | Whey protein isolate NPs | High plasma concentration after oral administration | [102] | |
| Magnolia bark extract (Magnolia officinalis) | Magnolol and honokyol | Antioxidant, anti-inflammatory, anti-cancer, antidepressant, and for the treatment of ulcers | Mixed micelles and nanosuspensions | Increased permeation of magnolol in Caco-2 cell lines and in vivo gastrointestinal absorption | [103] |
| Amorphous solid dispersion using HPMC acetate succinate | Increased bioavailability with antioxidant activity and gut protection | [104] | |||
| Protein NPs into chitosan/alginate hydrogel MPs | Release in the colon; uptake and anti-inflammatory effect on epithelial and macrophage colon cells | [105] | |||
| Micelles with Pluronic F127 and L61 or with copolymers (Soluplus, Solutol, and D-alpha-toco-pheryl PEG 1000 succinate) | pH-dependent release in the intestine; bioavailability 3-fold greater than raw product | [106,107] | |||
| Noni fruit juice Noni fruit juice powder Noni fruit puree and concentrate (Morinda citrifolia) | Anthraquinones (damnacanthal) and polyphenols | Anti-cancer | PLGA-PEG nanocapsules | Higher activity in cell growth inhibition, compared to non-encapsulated damnacanthal | Reviewed by [79] |
| Phytosterols/phytostanols | Anti-cancer | SLNs loading several phytosterols using different glycerides | Increased solubility (bioavailability) and better hypocaloric properties | [108] | |
| Anti-cancer (breast cancer) | Chitosan/alginate NP functionalized with folate for breast cancer-targeting delivering β-sitosterol | Protection to enzymes and hydrolysis; prolonged release; good permeation across intestinal cells; and high toxicity against cancer cells | [109,110] | ||
| LDL cholesterol- lowering properties | Nanoporous starch aerogel impregnated with phytosterol from soybeans | Increased bioavailability from 3 to 35%; reduction of crystallinity | [111,112] | ||
| Cholesterol-lowering properties | Soy protein vehicles delivering several phytosterols | Reduction of cholesterol level and better bioavailability | [113] | ||
| - | Cholesterol-lowering properties | Sodium caseinate/pectin coacervate | Protection in the gastrointestinal tract; hydrophobic bond sodium caseinate reduce crystallinity and increase bioaccessibility after digestion | [114] | |
| Cholesterol-lowering properties | Soybean protein/pectin coacervate delivering stigmasterols | Stability at different pH values with an increased stability to the stomach environment; release in intestinal fluids | [115] | ||
| Cholesterol-lowering properties | Zein/pectin coacervate delivering stigmasterols | Pectin creates a gel around zein/phytosterol | [116] | ||
| Taxifolin-rich extract from the wood of Dahurian Larch (Larix gmelinii) | Taxifolin | Antioxidant | Micelles, liposomes, polymeric NPs, and hybrid systems | Improved stability, permeability, and systemic availability of quercitin, a taxifolin analogue | Reviewed by [117] |
| Trans-resveratrol produced with a synthetic process | Antimicrobial, antioxidant, anti-aging | SLNs, liposomes, dendrimers, polymeric NPs | Increased bioavailability and permeability. Increased concentration in the brain compared to free resveratrol | Reviewed by [75] | |
| Vitamin K2 (menaquinone) produced with a synthetic or microbiological process | - | Fat-soluble vitamin deficiencies in pancreatic-insufficient CF patients | Liposomes containing a blend of vitamins + vit. K2 | Increased level of other vitamins in plasma in comparison with the same supplements without vitamin K2 (trial in cystic fibrosis patients) | [118] |
| - | Bone support, promotes heart health and helps boost immunity | Liposomes loaded with vit. D3/K2 and coated with chitosan with mucoadhesive- properties | High encapsulation and controlled release in situ | [119] | |
| Zeaxanthin produced with synthesis | Zeaxanthin | Antioxidant, cardiovascular disease prevention agent | Liposomes, nanoemulsions, polymeric NPs, and polymer–lipid hybrid NPs | Increased bioavailability and stability of lutein, the isomer of zeaxanthin | Reviewed by [120] |
4. Nanoceutical Application in the Food Sector: Safety Issues and Regulations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Department of Economic and Social Affairs. World Population Prospects 2022—Summary of Results; Department of Economic and Social Affairs: New York, NY, USA, 2022. [Google Scholar]
- Food and Agriculture Organization. The Future of Food and Agriculture—Alternative Pathways to 2050; Summary Version; FAO: Rome, Italy, 2018. [Google Scholar]
- European Commission Union. List of Novel Foods; European Commission Union: Brussels, Belgium, 2017.
- Vapnek, J.; Purnhagen, K.; Hillel, B. Regulatory and Legislative Framework for Novel Foods. In Food Formulation: Novel Ingredients and Processing Techniques; Pathania, S., Brijesh, K.T., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; Volume 1, pp. 285–308. [Google Scholar]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for Biorelated Polymers and Applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M.; Zuccari, G. Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks. Polymers 2021, 13, 2262. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Y.; Jia, N.; Qiao, H.; Zhu, M.; Meng, Q.; Lu, Q.; Zu, Y. Synthesis and Evaluation of a Novel Water-Soluble High Se-Enriched Astragalus Polysaccharide Nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1438–1448. [Google Scholar] [CrossRef]
- Pang, G.; Chen, C.; Liu, Y.; Jiang, T.; Yu, H.; Wu, Y.; Wang, Y.; Wang, F.J.; Liu, Z.; Zhang, L.W. Bioactive Polysaccharide Nanoparticles Improve Radiation-Induced Abscopal Effect through Manipulation of Dendritic Cells. ACS Appl. Mater. Interfaces 2019, 11, 42661–42670. [Google Scholar] [CrossRef]
- Susa, F.; Bucca, G.; Limongi, T.; Cauda, V.; Pisano, R. Enhancing the Preservation of Liposomes: The Role of Cryoprotectants, Lipid Formulations and Freezing Approaches. Cryobiology 2021, 98, 46–56. [Google Scholar] [CrossRef]
- Kuznetcova, D.V.; Linder, M.; Jeandel, C.; Paris, C.; Desor, F.; Baranenko, D.A.; Nadtochii, L.A.; Arab-Tehrany, E.; Yen, F.T. Nanoliposomes and Nanoemulsions Based on Chia Seed Lipids: Preparation and Characterization. Int. J. Mol. Sci. 2020, 21, 9079. [Google Scholar] [CrossRef]
- Mane, S.; Pathan, E.; Tupe, S.; Deshmukh, S.; Kale, D.; Ghormade, V.; Chaudhari, B.; Deshpande, M. Isolation and Characterization of Chitosans from Different Fungi with Special Emphasis on Zygomycetous Dimorphic Fungus Benjaminiella poitrasii: Evaluation of Its Chitosan Nanoparticles for the Inhibition of Human Pathogenic Fungi. Biomacromolecules 2022, 23, 808–815. [Google Scholar] [CrossRef]
- Wu, J.; Niu, Y.; Jiao, Y.; Chen, Q. Fungal Chitosan from Agaricus bisporus (Lange) Sing. Chaidam Increased the Stability and Antioxidant Activity of Liposomes Modified with Biosurfactants and Loading Betulinic Acid. Int. J. Biol. Macromol. 2019, 123, 291–299. [Google Scholar] [CrossRef]
- Sharma, R.; Kuche, K.; Thakor, P.; Bhavana, V.; Srivastava, S.; Mehra, N.K.; Jain, S. Chondroitin Sulfate: Emerging Biomaterial for Biopharmaceutical Purpose and Tissue Engineering. Carbohydr. Polym. 2022, 286, 119305. [Google Scholar] [CrossRef]
- Amhare, A.F.; Lei, J.; Deng, H.; Lv, Y.; Han, J.; Zhang, L. Biomedical Application of Chondroitin Sulfate with Nanoparticles in Drug Delivery Systems: Systematic Review. J. Drug Target 2021, 29, 259–268. [Google Scholar] [PubMed]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; Bronze, M.d.R. Hyaluronic Acid and Chondroitin Sulfate from Marine and Terrestrial Sources: Extraction and Purification Methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef]
- Edelman, R.; Engelberg, S.; Fahoum, L.; Meyron-Holtz, E.G.; Livney, Y.D. Potato Protein- Based Carriers for Enhancing Bioavailability of Astaxanthin. Food Hydrocoll. 2019, 96, 72–80. [Google Scholar] [CrossRef]
- Lan, M.; Fu, Y.; Dai, H.; Ma, L.; Yu, Y.; Zhu, H.; Wang, H.; Zhang, Y. Encapsulation of β-Carotene by Self-Assembly of Rapeseed Meal-Derived Peptides: Factor Optimization and Structural Characterization. LWT 2021, 138, 110456. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Pinteala, M.; Simionescu, N. Dextran Formulations as Effective Delivery Systems of Therapeutic Agents. Molecules 2023, 28, 1086. [Google Scholar] [PubMed]
- John, J.E.; Tytler, B.A.; Habila, J.; Apeji, Y.E.; Olayemi, O.; Isimi, C.Y. Cross-Linking with Multifunctional Excipients and Its Effect on the Physicochemical Properties and Release Profile of Ibuprofen-Loaded Digitaria exilis Starch Nanoparticles. J. Res. Pharm. 2022, 26, 1190–1201. [Google Scholar] [CrossRef]
- Jia, J.; Liu, G.; Guo, Z.-X.; Yu, J.; Duan, Y. Preparation and Characterization of Soluble Eggshell Membrane Protein/PLGA Electrospun Nanofibers for Guided Tissue Regeneration Membrane. J. Nanomater. 2012, 2012, 282736. [Google Scholar] [CrossRef]
- Lee, M.-C.; Huang, Y.-C. Soluble Eggshell Membrane Protein-Loaded Chitosan/Fucoidan Nanoparticles for Treatment of Defective Intestinal Epithelial Cells. Int. J. Biol. Macromol. 2019, 131, 949–958. [Google Scholar] [CrossRef]
- Chai, Z.; Li, Y.; Liu, F.; Du, B.; Jiao, T.; Zhang, C.; Leng, X. Outer Eggshell Membrane as Delivery Vehicle for Polysaccharide/Protein Microcapsules Incorporated with Vitamin E. J. Agric. Food Chem. 2013, 61, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.V.; Gasilova, E.R.; Skorik, Y.A. Nano-Sized Fucoidan Interpolyelectrolyte Complexes: Recent Advances in Design and Prospects for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 2615. [Google Scholar]
- Saral, A.M.; Mathew, M.S.F. Excipient Profile and Future Possibilities of Fucoidan: A Review. Int. J. Pharm. Res. 2020, 12, 3786–3791. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.-Y.; Chen, L.; Li, Q.-J.; Shen, Y.-Z.; Jin, L.; Zhang, X.; Chen, P.-C.; Wu, M.-J.; Choi, J.; et al. Different Extraction Methods Bring about Distinct Physicochemical Properties and Antioxidant Activities of Sargassum Fusiforme Fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J. Guar Gum-Based Nanoformulations: Implications for Improving Drug Delivery. Int. J. Biol. Macromol. 2023, 229, 476–485. [Google Scholar] [CrossRef]
- Hadidi, M.; Rostamabadi, H.; Moreno, A.; Jafari, S.M. Nanoencapsulation of Essential Oils from Industrial Hemp (Cannabis sativa L.) by-Products into Alfalfa Protein Nanoparticles. Food Chem. 2022, 386, 132765. [Google Scholar] [CrossRef] [PubMed]
- Koralegedara, I.D.; Hettiarachchi, C.A.; Prasantha, B.D.R.; Wimalasiri, K.M.S. Synthesis of Nano-Scale Biopolymer Particles from Legume Protein Isolates and Carrageenan. Food Technol. Biotechnol. 2020, 58, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Mirmohammad Meiguni, M.S.; Salami, M.; Rezaei, K.; Ghaffari, S.B.; Aliyari, M.A.; Emam-Djomeh, Z.; Barazandegan, Y.; Gruen, I. Curcumin-Loaded Complex Coacervate Made of Mung Bean Protein Isolate and Succinylated Chitosan as a Novel Medium for Curcumin Encapsulation. J. Food Sci. 2022, 87, 4930–4944. [Google Scholar] [CrossRef]
- Ma, M.; Liu, X.; Ma, C.; Guo, R.; Zhang, X.; Zhang, Z.; Ren, X. Enhancing the Antitumor Immunosurveillance of PD-L1-Targeted Gene Therapy for Metastatic Melanoma Using Cationized Panax notoginseng Polysaccharide. Int. J. Biol. Macromol. 2023, 226, 1309–1318. [Google Scholar] [CrossRef]
- Simmons, J.; Nickels, J.D.; Michalski, M.; Grossutti, M.; Shamana, H.; Stanley, C.B.; Schwan, A.L.; Katsaras, J.; Dutcher, J.R. Structure, Hydration, and Interactions of Native and Hydrophobically Modified Phytoglycogen Nanoparticles. Biomacromolecules 2020, 21, 4053–4062. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Jiang, H.; Guan, X.; Yang, C.; Ngai, T. Chitosan-Coated Phytoglycogen for Preparation of Biocompatible Pickering Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128861. [Google Scholar] [CrossRef]
- Xue, J.; Li, Z.; Duan, H.; He, J.; Luo, Y. Chemically Modified Phytoglycogen: Physicochemical Characterizations and Applications to Encapsulate Curcumin. Colloids Surf. B Biointerfaces 2021, 205, 111829. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, J.; Wusigale; Wang, T.; Hu, Q.; Luo, Y. Carboxymethylation of Phytoglycogen and Its Interactions with Caseinate for the Preparation of Nanocomplex. Food Hydrocoll. 2020, 100, 105390. [Google Scholar] [CrossRef]
- Ma, Y.; Adibnia, V.; Mitrache, M.; Halimi, I.; Walker, G.C.; Kumacheva, E. Stimulus-Responsive Nanoconjugates Derived from Phytoglycogen Nanoparticles. Biomacromolecules 2021, 23, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and Physicochemical Properties of Octenyl Succinic Anhydride Modified Starches: A Review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of MRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Poklar Ulrih, N. Comparative Effects of Cholesterol and β-Sitosterol on the Liposome Membrane Characteristics. Eur. J. Lipid Sci. Technol. 2018, 120, 1800039. [Google Scholar] [CrossRef]
- Bernardo, J.; Videira, R.A.; Valentão, P.; Veiga, F.; Andrade, P.B. Extraction of Phospholipid-Rich Fractions from Egg Yolk and Development of Liposomes Entrapping a Dietary Polyphenol with Neuroactive Potential. Food Chem. Toxicol. 2019, 133, 110749. [Google Scholar] [CrossRef]
- Srihera, N.; Li, Y.; Zhang, T.-T.; Wang, Y.-M.; Yanagita, T.; Waiprib, Y.; Xue, C.-H. Preparation and Characterization of Astaxanthin-Loaded Liposomes Stabilized by Sea Cucumber Sulfated Sterols Instead of Cholesterol. J. Oleo Sci. 2022, 71, ess21233. [Google Scholar] [CrossRef]
- Yuba, E.; Osaki, T.; Ono, M.; Park, S.; Harada, A.; Yamashita, M.; Azuma, K.; Tsuka, T.; Ito, N.; Imagawa, T.; et al. Bleomycin-Loaded PH-Sensitive Polymer–Lipid-Incorporated Liposomes for Cancer Chemotherapy. Polymers 2018, 10, 74. [Google Scholar] [CrossRef]
- Le, N.T.T.; Du Cao, V.; Nguyen, T.N.Q.; Le, T.T.H.; Tran, T.T.; Hoang Thi, T.T. Soy Lecithin-Derived Liposomal Delivery Systems: Surface Modification and Current Applications. Int. J. Mol. Sci. 2019, 20, 4706. [Google Scholar] [CrossRef]
- Cansell, M.; Nacka, F.; Combe, N. Marine Lipid-Based Liposomes Increase In Vivo FA Bioavailability. Lipids 2003, 38, 551–559. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.X.; Zhang, T.; He, C.; He, R.; Ju, X.; Wu, X.Y. In Situ Proapoptotic Peptide-Generating Rapeseed Protein-Based Nanocomplexes Synergize Chemotherapy for Cathepsin-B Overexpressing Breast Cancer. ACS Appl. Mater. Interfaces 2018, 10, 41056–41069. [Google Scholar] [CrossRef] [PubMed]
- Echeverri, J.D.; Alhajj, M.J.; Montero, N.; Yarce, C.J.; Barrera-Ocampo, A.; Salamanca, C.H. Study of in Vitro and in Vivo Carbamazepine Release from Coarse and Nanometric Pharmaceutical Emulsions Obtained via Ultra-High-Pressure Homogenization. Pharmaceuticals 2020, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Dąbek, P.; Witkowski, A.; Monedeiro, F.; Pomastowski, P.; Buszewski, B.; Nowak, I. Lipid Constituents of Diatoms (Halamphora) as Components for Production of Lipid Nanoparticles. Pharmaceutics 2022, 14, 1171. [Google Scholar] [CrossRef]
- Kumar, H.; Gehlaut, A.K.; Gupta, H.; Gaur, A.; Park, J.W. Development of Copper Loaded Nanoparticles Hydrogel Made from Waste Biomass (Sugarcane Bagasse) for Special Medical Application. In Proceedings of the Key Engineering Materials; Trans Tech Publications Ltd.: Stafa, Switzerland, 2020; Volume 847, pp. 102–107. [Google Scholar]
- Okagu, O.D.; Verma, O.; McClements, D.J.; Udenigwe, C.C. Utilization of Insect Proteins to Formulate Nutraceutical Delivery Systems: Encapsulation and Release of Curcumin Using Mealworm Protein-Chitosan Nano-Complexes. Int. J. Biol. Macromol. 2020, 151, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, S.; Santonicola, P.; Paterna, A.; Rao, E.; Raccosta, S.; Romancino, D.P.; Noto, R.; Touzet, N.; Manno, M.; Di Schiavi, E.; et al. Extracellular Vesicles from Microalgae: Uptake Studies in Human Cells and Caenorhabditis Elegans. Front. Bioeng. Biotechnol. 2022, 10, 830189. [Google Scholar] [CrossRef]
- Adamo, G.; Fierli, D.; Romancino, D.P.; Picciotto, S.; Barone, M.E.; Aranyos, A.; Božič, D.; Morsbach, S.; Raccosta, S.; Stanly, C.; et al. Nanoalgosomes: Introducing Extracellular Vesicles Produced by Microalgae. J. Extracell. Vesicles 2021, 10, e12081. [Google Scholar] [CrossRef]
- Zhang, M.; Kim, J.A.; Huang, A.Y.-C. Optimizing Tumor Microenvironment for Cancer Immunotherapy: β-Glucan-Based Nanoparticles. Front. Immunol. 2018, 9, 341. [Google Scholar] [CrossRef]
- Huang, J.; Wu, C.; Tang, S.; Zhou, P.; Deng, J.; Zhang, Z.; Wang, Y.; Wang, Z. Chiral Active β-Glucan Nanoparticles for Synergistic Delivery of Doxorubicin and Immune Potentiation. Int. J. Nanomed. 2020, 15, 5083–5095. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can Fungi Compete with Marine Sources for Chitosan Production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Wang, W.; Du, Y.; Qiu, Y.; Wang, X.; Hu, Y.; Yang, J.; Cai, J.; Kennedy, J.F. A New Green Technology for Direct Production of Low Molecular Weight Chitosan. Carbohydr. Polym. 2008, 74, 127–132. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.H.; El-Tras, W.F.; Elguindy, N.M.; Opwis, K. Antimicrobial Textile Treated with Chitosan from Aspergillus niger Mycelial Waste. Int. J. Biol. Macromol. 2011, 49, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, Y.; Wang, Y.; Gao, X. Astragalus membranaceus Extract Promotes Neovascularisation by VEGF Pathway in Rat Model of Ischemic Injury. Pharmazie 2011, 66, 144–150. [Google Scholar] [PubMed]
- Ko, J.K.-S.; Chik, C.W.-S. The Protective Action of Radix Astragalus membranaceus against Hapten-Induced Colitis through Modulation of Cytokines. Cytokine 2009, 47, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-J.; Xia, Q.; Wang, J.-J.; Wang, P.-P. Molecular Weight and Monosaccharide Composition of Astragalus Polysaccharides. Molecules 2008, 13, 2408–2415. [Google Scholar] [CrossRef]
- Tiippana, E.; Hamunen, K.; Heiskanen, T.; Nieminen, T.; Kalso, E.; Kontinen, V.K. New Approach for Treatment of Prolonged Postoperative Pain: APS Out-Patient Clinic. Scand. J. Pain 2016, 12, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yixi, B.; Waikei, L.; Wenwen, L.; Fang, L.; Xuan, Z.; Jing, L.; Hongping, W. Immunoregulatory and Anti-Tumor Effects of Polysaccharopeptide and Astragalus polysaccharides on Tumor-Bearing Mice. Immunopharmacol. Immunotoxicol. 2008, 30, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Chondroitin Sulfate Safety and Quality. Molecules 2019, 24, 1447. [Google Scholar] [CrossRef]
- Besednova, N.N.; Zaporozhets, T.S.; Kuznetsova, T.A.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Ermakova, S.P. Extracts and Marine Algae Polysaccharides in Therapy and Prevention of Inflammatory Diseases of the Intestine. Mar. Drugs 2020, 18, 289. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Y. Particulate Structure of Phytoglycogen Nanoparticles Probed Using Amyloglucosidase. Carbohydr. Polym. 2011, 83, 1665–1671. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Matsuoka, M.; Imai, H.; Izutsu, K.; Sakai-Kato, K. Detection of Material-Derived Differences in the Stiffness of Egg Yolk Phosphatidylcholine-Containing Liposomes Using Atomic Force Microscopy. Chem. Phys. Lipids 2020, 233, 104992. [Google Scholar] [CrossRef] [PubMed]
- Kondratowicz, A.; Weiss, M.; Juzwa, W.; Majchrzycki, Ł.; Lewandowicz, G. Characteristics of Liposomes Derived from Egg Yolk. Open Chem. 2019, 17, 763–778. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Lin, D.; Xu, D.; Li, S.; Huang, J.; Weng, S.; Lin, Z.; Zheng, Y.; Yao, H.; et al. Proliposomes for Oral Delivery of Total Biflavonoids Extract from Selaginella Doederleinii: Formulation Development, Optimization, and in Vitro-in Vivo Characterization. Int. J. Nanomed. 2019, 14, 6691–6706. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, W. Micro/Nano Emulsion Delivery Systems: Effects of Potato Protein/Chitosan Complex on the Stability, Oxidizability, Digestibility and β—Carotene Release Characteristics of the Emulsion. Innov. Food Sci. Emerg. Technol. 2022, 77, 102980. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Islam, F.; Amer Ali, Y.; Imran, A.; Afzaal, M.; Zahra, S.M.; Fatima, M.; Saeed, F.; Usman, I.; Shehzadi, U.; Mehta, S.; et al. Vegetable Proteins as Encapsulating Agents: Recent Updates and Future Perspectives. Food Sci. Nutr. 2023, 11, 1705–1717. [Google Scholar] [CrossRef]
- Yi-Shen, Z.; Shuai, S.; FitzGerald, R. Mung Bean Proteins and Peptides: Nutritional, Functional and Bioactive Properties. Food Nutr. Res. 2018, 62, 1290. [Google Scholar] [CrossRef]
- Hadidi, M.; Khaksar, F.B.; Pagan, J.; Ibarz, A. Application of Ultrasound-Ultrafiltration-Assisted Alkaline Isoelectric Precipitation (UUAAIP) Technique for Producing Alfalfa Protein Isolate for Human Consumption: Optimization, Comparison, Physicochemical, and Functional Properties. Food Res. Int. 2020, 130, 108907. [Google Scholar] [CrossRef]
- Bohara, R.A.; Tabassum, N.; Singh, M.P.; Gigli, G.; Ragusa, A.; Leporatti, S. Recent Overview of Resveratrol’s Beneficial Effects and Its Nano-Delivery Systems. Molecules 2022, 27, 5154. [Google Scholar] [CrossRef]
- Sahadevan, R.; Singh, S.; Binoy, A.; Sadhukhan, S. Chemico-Biological Aspects of (−)-Epigallocatechin-3-Gallate (EGCG) to Improve Its Stability, Bioavailability and Membrane Permeability: Current Status and Future Prospects. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Scheepens, A.; Tan, K.; Paxton, J.W. Improving the Oral Bioavailability of Beneficial Polyphenols through Designed Synergies. Genes Nutr. 2010, 5, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Choiri, Z.; Danar Dono, N.; Ariyadi, B.; Hanim, C.; Martien, R.; Zuprizal. Effect of Nano-Encapsulation of Noni (Morinda citrifolia) Fruit Extract on Jejunal Morphology and Microbial Populations in Laying Hens. Pak. J. Nutr. 2017, 17, 34–38. [Google Scholar] [CrossRef]
- Rahman Mohd, M.; Ariff, T.M.; Mohamad, N.; Zubaidi, A.; Latif, A.; Norsani, W.M.; Nik, W.; Mohamed, A.; Fahim, I.; Suffian, M. Development of Biodegradable Sustained-Release Damnacanthal Nanocapsules for Potential Application in In-Vitro Breast Cancer Studies. Pak. J. Pharm. Sci. 2019, 32, 2155–2162. [Google Scholar]
- Negreanu-Pirjol, B.-S.; Oprea, O.C.; Negreanu-Pirjol, T.; Roncea, F.N.; Prelipcean, A.-M.; Craciunescu, O.; Iosageanu, A.; Artem, V.; Ranca, A.; Motelica, L.; et al. Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants 2023, 12, 951. [Google Scholar] [CrossRef]
- Abdol Wahab, N.R.; Meor Mohd Affandi, M.M.R.; Fakurazi, S.; Alias, E.; Hassan, H. Nanocarrier System: State-of-the-Art in Oral Delivery of Astaxanthin. Antioxidants 2022, 11, 1676. [Google Scholar] [CrossRef]
- Yamauchi, K.; Wakabayashi, H.; Shin, K.; Takase, M. Bovine Lactoferrin: Benefits and Mechanism of Action against Infections. Biochem. Cell Biol. 2006, 84, 291–296. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kang, W.S.; Lim, J.D.; Park, C.H. Bio-Fortification of Angelica gigas Nakai Nano-Powder Using Bio-Polymer by Hot Melt Extrusion to Enhance the Bioaccessibility and Functionality of Nutraceutical Compounds. Pharmaceuticals 2020, 13, 3. [Google Scholar] [CrossRef]
- Lin, Y.; Yin, W.; Li, Y.; Liu, G. Influence of Different Solid Lipids on the Properties of a Novel Nanostructured Lipid Carrier Containing Antarctic Krill Oil. Int. J. Food Sci. Technol. 2022, 57, 2886–2895. [Google Scholar] [CrossRef]
- Sudirman, S.; Hsu, Y.H.; Johnson, A.; Tsou, D.; Kong, Z.L. Amelioration Effects of Nanoencapsulated Triterpenoids from Petri Dish-Cultured Antrodia cinnamomea on Reproductive Function of Diabetic Male Rats. Int. J. Nanomed. 2018, 13, 5059–5073. [Google Scholar] [CrossRef]
- Andreu, S.; Ripa, I.; Bello-Morales, R.; López-Guerrero, J.A. Liposomal Lactoferrin Exerts Antiviral Activity against HCoV-229E and SARS-CoV-2 Pseudoviruses In Vitro. Viruses 2023, 15, 972. [Google Scholar] [CrossRef]
- Soliman, S.M.; Mosallam, S.; Mamdouh, M.A.; Hussein, M.A.; El-Halim, S.M.A. Design and Optimization of Cranberry Extract Loaded Bile Salt Augmented Liposomes for Targeting of MCP-1/STAT3/VEGF Signaling Pathway in DMN-Intoxicated Liver in Rats. Drug Deliv. 2022, 29, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Gabrani, R.; Dang, S. Nanoemulsions of Green Tea Catechins and Other Natural Compounds for the Treatment of Urinary Tract Infection: Antibacterial Analysis. Adv. Pharm. Bull. 2019, 9, 401–408. [Google Scholar] [CrossRef]
- Jeong, S.J.; Lee, J.S.; Lee, H.G. Nanoencapsulation of Synergistic Antioxidant Fruit and Vegetable Concentrates and Their Stability during in Vitro Digestion. J. Sci. Food Agric. 2020, 100, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Proanthocyanidin-Chitosan Composite Nanoparticles Prevent Bacterial Invasion and Colonization of Gut Epithelial Cells by Extra-Intestinal Pathogenic Escherichia coli. Int. J. Biol. Macromol. 2019, 135, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Taghiloo, S.; Ghajari, G.; Zand, Z.; Kabiri-Samani, S.; Kabiri, H.; Rajaei, N.; Piri-Gharaghie, T. Designing Alginate/Chitosan Nanoparticles Containing Echinacea Angustifolia: A Novel Candidate for Combating Multidrug-Resistant Staphylococcus Aureus. Chem. Biodivers. 2023, 20, e202201008. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Mirzaie, A.; Zabet, N.; Moammeri, A.; Mansoori-Kermani, A.; Akbarzadeh, I.; Eshrati Yeganeh, F.; Chitgarzadeh, A.; Bagheri Kashtali, A.; Ren, Q. Enhanced Antibacterial Activity of Echinacea angustifolia Extract against Multidrug-Resistant Klebsiella pneumoniae through Niosome Encapsulation. Nanomaterials 2021, 11, 1573. [Google Scholar] [CrossRef]
- Tyagi, T.; Garlapati, P.K.; Yadav, P.; Naika, M.; Mallya, A.; Kandangath Raghavan, A. Development of Nano-Encapsulated Green Tea Catechins: Studies on Optimization, Characterization, Release Dynamics, and in-Vitro Toxicity. J. Food Biochem. 2021, 45, e13951. [Google Scholar] [CrossRef]
- Rodríguez Pescador, A.; Gutiérrez Romero, L.; Blanco-González, E.; Montes-Bayón, M.; Sierra, L.M. Intracellular Biotransformation of Ultrasmall Iron Oxide Nanoparticles and Their Effect in Cultured Human Cells and in Drosophila Larvae In Vivo. Int. J. Mol. Sci. 2022, 23, 8788. [Google Scholar] [CrossRef]
- Helman, S.L.; Wilkins, S.J.; McKeating, D.R.; Perkins, A.V.; Cuffe, J.S.M.; Hartel, G.; Faria, N.; Powell, J.J.; Anderson, G.J.; Frazer, D.M. A Novel Ferritin-Core Analog Is a Safe and Effective Alternative to Oral Ferrous Iron for Treating Iron Deficiency during Pregnancy in Mice. J. Nutr. 2022, 152, 714–722. [Google Scholar] [CrossRef]
- Pereira, D.I.A.; Mohammed, N.I.; Ofordile, O.; Camara, F.; Baldeh, B.; Mendy, T.; Sanyang, C.; Jallow, A.T.; Hossain, I.; Wason, J.; et al. A Novel Nano-Iron Supplement to Safely Combat Iron Deficiency and Anaemia in Young Children: The IHAT-GUT Double-Blind, Randomised, Placebo-Controlled Trial Protocol. Gates Open Res. 2018, 2, 48. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, Y.; Xiang, M.; Wu, F.; Sun, M.; Du, X.F.; Chen, L. Biocompatible Polyelectrolyte Complex Nanoparticles for Lycopene Encapsulation Attenuate Oxidative Stress-Induced Cell Damage. Front. Nutr. 2022, 9, 902208. [Google Scholar] [CrossRef]
- Zhao, Y.; Xin, Z.; Li, N.; Chang, S.; Chen, Y.; Geng, L.; Chang, H.; Shi, H.; Chang, Y.-Z. Nano-Liposomes of Lycopene Reduces Ischemic Brain Damage in Rodents by Regulating Iron Metabolism. Free Radic. Biol. Med. 2018, 124, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.G.; Barros, A.L.A.N.; Cabral, W.F.; Moreira, D.C.; da Silva, I.G.M.; Silva-Carvalho, A.; de Almeida, M.P.; Albuquerque, L.F.F.; dos Santos, R.C.; Ana, A.K.; et al. Promising Self-Emulsifying Drug Delivery System Loaded with Lycopene from Red Guava (Psidium Guajava L.): In Vivo Toxicity, Biodistribution and Cytotoxicity on DU-145 Prostate Cancer Cells. Cancer Nanotechnol. 2021, 12, 30. [Google Scholar] [CrossRef]
- Al-Eisa, R.A. Synergistic Antioxidant Capacity of Chitosan Nanoparticles and Lycopene against Aging Hepatotoxicity Induced by D-Galactose in Male Rats. Int. J. Pharmacol. 2018, 14, 811–825. [Google Scholar] [CrossRef]
- Li, W.; Yalcin, M.; Lin, Q.; Ardawi, M.-S.M.; Mousa, S.A. Self-Assembly of Green Tea Catechin Derivatives in Nanoparticles for Oral Lycopene Delivery. J. Control. Release 2017, 248, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sharma, G.; Ghoshal, G.; Kesharwani, P.; Singh, B.; Shivhare, U.S.; Katare, O.P. Lycopene Loaded Whey Protein Isolate Nanoparticles: An Innovative Endeavor for Enhanced Bioavailability of Lycopene and Anti-Cancer Activity. Int. J. Pharm. 2018, 546, 97–105. [Google Scholar] [CrossRef]
- Li, G.; Lu, Y.; Fan, Y.; Ning, Q.; Li, W. Enhanced Oral Bioavailability of Magnolol via Mixed Micelles and Nanosuspensions Based on Soluplus ® -Poloxamer 188. Drug Deliv. 2020, 27, 1010–1017. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, S.; Hao, Y.; Fan, K.; Wang, L.; Zhao, X.; He, X. Amorphous Solid Dispersion Preparation via Coprecipitation Improves the Dissolution, Oral Bioavailability, and Intestinal Health Enhancement Properties of Magnolol. Poult. Sci. 2023, 102, 102676. [Google Scholar] [CrossRef]
- Wang, X.; Gu, H.; Zhang, H.; Xian, J.; Li, J.; Fu, C.; Zhang, C.; Zhang, J. Oral Core–Shell Nanoparticles Embedded in Hydrogel Microspheres for the Efficient Site-Specific Delivery of Magnolol and Enhanced Antiulcerative Colitis Therapy. ACS Appl. Mater. Interfaces 2021, 13, 33948–33961. [Google Scholar] [CrossRef]
- Ding, P.; Shen, H.; Wang, J.; Ju, J. Improved Oral Bioavailability of Magnolol by Using a Binary Mixed Micelle System. Artif. Cells Nanomed. Biotechnol. 2018, 46, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, S.; Ding, P.; Wang, L.; Ju, J.; Liang, G. Enhancement of Oral Bioavailability of Magnolol by Encapsulation in Mixed Micelles Containing Pluronic F127 and L61. J. Pharm. Pharmacol. 2018, 70, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.J.; Ma, C.G.; Hu, Y.Y.; Bai, G.; Song, Z.J.; Cao, X.Q. Solid Lipid Nanoparticles for Phytosterols Delivery: The Acyl Chain Number of the Glyceride Matrix Affects the Arrangement, Stability, and Release. Food Chem. 2022, 394. [Google Scholar] [CrossRef]
- Afzal, O.; Akhter, M.H.; Ahmad, I.; Muzammil, K.; Dawria, A.; Zeyaullah, M.; Altamimi, A.S.A.; Khalilullah, H.; Mir Najib Ullah, S.N.; Rahman, M.A.; et al. A β–Sitosterol Encapsulated Biocompatible Alginate/Chitosan Polymer Nanocomposite for the Treatment of Breast Cancer. Pharmaceutics 2022, 14, 1711. [Google Scholar] [CrossRef]
- Karim, S.; Akhter, M.H.; Burzangi, A.S.; Alkreathy, H.; Alharthy, B.; Kotta, S.; Md, S.; Rashid, M.A.; Afzal, O.; Altamimi, A.S.A.; et al. Phytosterol-Loaded Surface-Tailored Bioactive-Polymer Nanoparticles for Cancer Treatment: Optimization, In Vitro Cell Viability, Antioxidant Activity, and Stability Studies. Gels 2022, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Ubeyitogullari, A.; Moreau, R.; Rose, D.J.; Zhang, J.; Ciftci, O.N. Enhancing the Bioaccessibility of Phytosterols Using Nanoporous Corn and Wheat Starch Bioaerogels. Eur. J. Lipid Sci. Technol. 2019, 121, 1700229. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Moreau, R.; Rose, D.J.; Ciftci, O.N. In Vitro Bioaccessibility of Low-Crystallinity Phytosterol Nanoparticles Generated Using Nanoporous Starch Bioaerogels. J. Food Sci. 2019, 84, 1812–1819. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Chen, F.; Zhang, S.; Li, L.; Ban, Z. Soy Proteins as Vehicles for Enhanced Bioaccessibility and Cholesterol-Lowering Activity of Phytosterols. J. Sci. Food Agric. 2023, 103, 205–212. [Google Scholar] [CrossRef]
- Gan, C.; Liu, Q.; Zhang, Y.; Shi, T.; He, W.S.; Jia, C. A Novel Phytosterols Delivery System Based on Sodium Caseinate-Pectin Soluble Complexes: Improving Stability and Bioaccessibility. Food Hydrocoll. 2022, 124, 107295. [Google Scholar] [CrossRef]
- Feng, S.; Yan, J.; Wang, D.; Jiang, L.; Sun, P.; Xiang, N.; Shao, P. Preparation and Characterization of Soybean Protein Isolate/Pectin-Based Phytosterol Nanodispersions and Their Stability in Simulated Digestion. Food Res. Int. 2021, 143, 110237. [Google Scholar] [CrossRef]
- Feng, S.; Wang, D.; Gan, L.; Shao, P.; Jiang, L.; Sun, P. Preparation and Characterization of Zein/Pectin-Based Phytosterol Nanodispersions and Kinetic Study of Phytosterol Release during Simulated Digestion in Vitro. LWT 2020, 128, 109446. [Google Scholar] [CrossRef]
- Rathod, S.; Arya, S.; Kanike, S.; Shah, S.A.; Bahadur, P.; Tiwari, S. Advances on Nanoformulation Approaches for Delivering Plant-Derived Antioxidants: A Case of Quercetin. Int. J. Pharm. 2022, 625, 122093. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.K.; Krzyzanowska-Jankowska, P.; Drzymała-Czyz, S.; Gozdzik-Spychalska, J.; Wojsyk-Banaszak, I.; Skorupa, W.; Sapiejka, E.; Miskiewicz-Chotnicka, A.; Brylak, J.; Zielinska-Psuja, B.; et al. Fat-Soluble Vitamins in Standard vs. Liposomal Form Enriched with Vitamin K2 in Cystic Fibrosis: A Randomized Multi-Center Trial. J. Clin. Med. 2022, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, A.; Bochicchio, S.; Lamberti, G.; Bertoncin, P.; Janssens, B.; Barba, A.A. Micronutrients Encapsulation in Enhanced Nanoliposomal Carriers by a Novel Preparative Technology. RSC Adv. 2019, 9, 19800–19812. [Google Scholar] [CrossRef] [PubMed]
- Algan, A.H.; Gungor-Ak, A.; Karatas, A. Nanoscale Delivery Systems of Lutein: An Updated Review from a Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1852. [Google Scholar] [CrossRef]
- Vitulo, M.; Gnodi, E.; Meneveri, R.; Barisani, D. Interactions between Nanoparticles and Intestine. Int. J. Mol. Sci. 2022, 23, 4339. [Google Scholar] [CrossRef]
- Ladaycia, A.; Passirani, C.; Lepeltier, E. Microbiota and Nanoparticles: Description and Interactions. Eur. J. Pharm. Biopharm. 2021, 169, 220–240. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is Nano Safe in Foods? Establishing the Factors Impacting the Gastrointestinal Fate and Toxicity of Organic and Inorganic Food-Grade Nanoparticles. NPJ Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef]
- Manocha, S.; Dhiman, S.; Grewal, A.S.; Guarve, K. Nanotechnology: An Approach to Overcome Bioavailability Challenges of Nutraceuticals. J. Drug Deliv. Sci. Technol. 2022, 72, 103418. [Google Scholar] [CrossRef]
- Chai, J.; Jiang, P.; Wang, P.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Zhao, L.; Norde, W.; et al. The Intelligent Delivery Systems for Bioactive Compounds in Foods: Physicochemical and Physiological Conditions, Absorption Mechanisms, Obstacles and Responsive Strategies. Trends Food Sci. Technol. 2018, 78, 144–154. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions a New Circular Economy Action Plan for a Cleaner and More Competitive Europe. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583933814386&uri=COM:2020:98:FIN (accessed on 3 November 2023).
- More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on Technical Requirements for Regulated Food and Feed Product Applications to Establish the Presence of Small Particles Including Nanoparticles. EFSA J. 2021, 19, e06769. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Hougaard Bennekou, S.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on Risk Assessment of Nanomaterials to Be Applied in the Food and Feed Chain: Human and Animal Health. EFSA J. 2021, 19, e06768. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans, R.; Castenmiller, J.; Chaudhry, Q.; Cubadda, F.; Daskaleros, T.; Franz, R.; Gott, D.; Mast, J.; Mortensen, A.; Oomen, A.G.; et al. Regulatory Safety Assessment of Nanoparticles for the Food Chain in Europe. Trends Food Sci. Technol. 2023, 134, 98–111. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Bremer-Hoffmann, S. Main Trends of Immune Effects Triggered by Nanomedicines in Preclinical Studies. Int. J. Nanomed. 2018, 13, 5419–5431. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A Successful Tool to Increase Solubility, Stability and Optimise Bioefficacy of Natural Constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef]


| Material(s) from Novel Foods | Type of Carrier | References |
|---|---|---|
| Astragalus membranaceus root extract | Polysaccharide nanoparticles | [8,9] |
| Cellobiose | Cryoprotectant for liposomes | [10] |
| Chia seed oil from Salvia hispanica L. | Liposomes and nanoemulsions | [11] |
| Chitosan extracted from fungi (Aspergillus niger; Agaricus bisporus) | Chitosan nanoparticles | [8,12,13] |
| Chondroitin sulphate (synthetic) | Polysaccharide nanoparticles | [14,15,16] |
| Coagulated potato proteins | Protein-based nanoparticles | [17,18] |
| Dextran from Leuconostoc mesenteroides | Polysaccharide nanoparticles | Reviewed by [19] |
| Digitaria exilis | Polysaccharide nanoparticles | [20] |
| Eggshell membrane protein hydrolysate | Protein-based nanoparticles | [21,22,23] |
| Fucoidan extracted from the seaweed Fucus vesiculosus and Undaria pinnatifida | Polysaccharide nanoparticles | [24,25,26] |
| Guar gum | Polysaccharide nanoparticles | [27] |
| Lucerne leaf extract from Medicago sativa | Protein-based nanoparticles | [28] |
| Mung bean seed proteins from Vigna radiata | Protein-based nanoparticles | [29,30] |
| Panax notoginseng root extract | Polysaccharide nanoparticles | [31] |
| Phytoglycogen | Polysaccharide nanoparticles Polyelectrolyte complex | [32,33,34,35,36,37] |
| Phytosterols | Solid lipid nanoparticles Liposomes | [38,39] |
| Phospholipids from egg yolk | Liposomes | [40,41,42] |
| Phosphatidylserine from soya and fish phospholipids | Liposomes | [43,44] |
| Rapeseed protein from Brassica napus L. and Brassica rapa L. | Protein-based nanoparticles | [18,45] |
| Sacha inchi seed oil from Plukenetia volubilis | Nanoemulsions | [46] |
| Schizochytrium sp. oil | Nanostructure lipid nanoparticles | [47] |
| Sugar cane fiber | Polysaccharide nanoparticles | [48] |
| Tenebrio molitor L. | Protein-based nanoparticles | [49] |
| Tetraselmis chuii microalgae | Extracellular vesicles | [50,51] |
| Trehalose | Cryoprotectant for liposomes | [10] |
| Yeast β-glucan | Polysaccharide nanoparticles | [52,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truzzi, E.; Bertelli, D.; Bilia, A.R.; Vanti, G.; Maretti, E.; Leo, E. Combination of Nanodelivery Systems and Constituents Derived from Novel Foods: A Comprehensive Review. Pharmaceutics 2023, 15, 2614. https://doi.org/10.3390/pharmaceutics15112614
Truzzi E, Bertelli D, Bilia AR, Vanti G, Maretti E, Leo E. Combination of Nanodelivery Systems and Constituents Derived from Novel Foods: A Comprehensive Review. Pharmaceutics. 2023; 15(11):2614. https://doi.org/10.3390/pharmaceutics15112614
Chicago/Turabian StyleTruzzi, Eleonora, Davide Bertelli, Anna Rita Bilia, Giulia Vanti, Eleonora Maretti, and Eliana Leo. 2023. "Combination of Nanodelivery Systems and Constituents Derived from Novel Foods: A Comprehensive Review" Pharmaceutics 15, no. 11: 2614. https://doi.org/10.3390/pharmaceutics15112614
APA StyleTruzzi, E., Bertelli, D., Bilia, A. R., Vanti, G., Maretti, E., & Leo, E. (2023). Combination of Nanodelivery Systems and Constituents Derived from Novel Foods: A Comprehensive Review. Pharmaceutics, 15(11), 2614. https://doi.org/10.3390/pharmaceutics15112614