Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives
Abstract
:1. Introduction
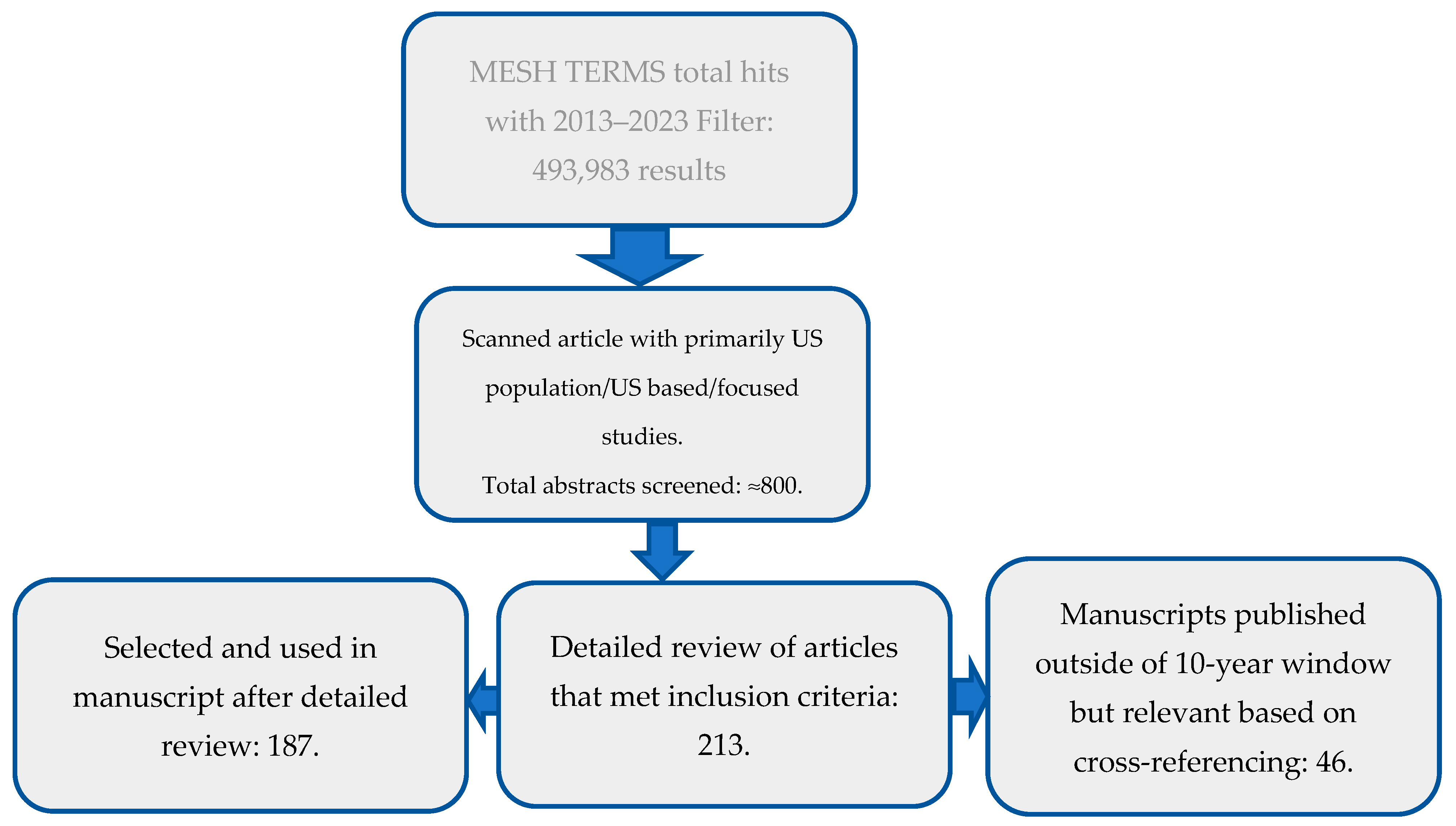
2. Search Method
3. Results and Discussion
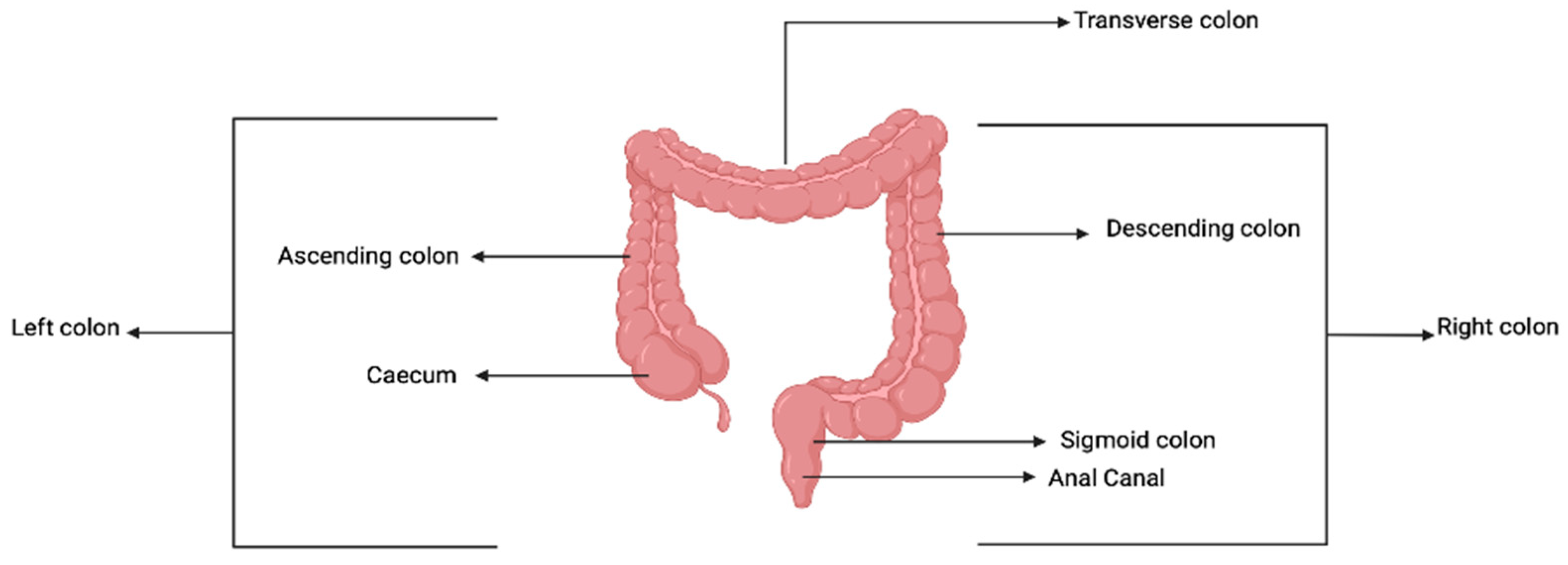
3.1. The Colon and Peculiarities of Colorectal Cancer
3.2. CRC Development Processes
3.3. H. pylori and Colorectal Cancer

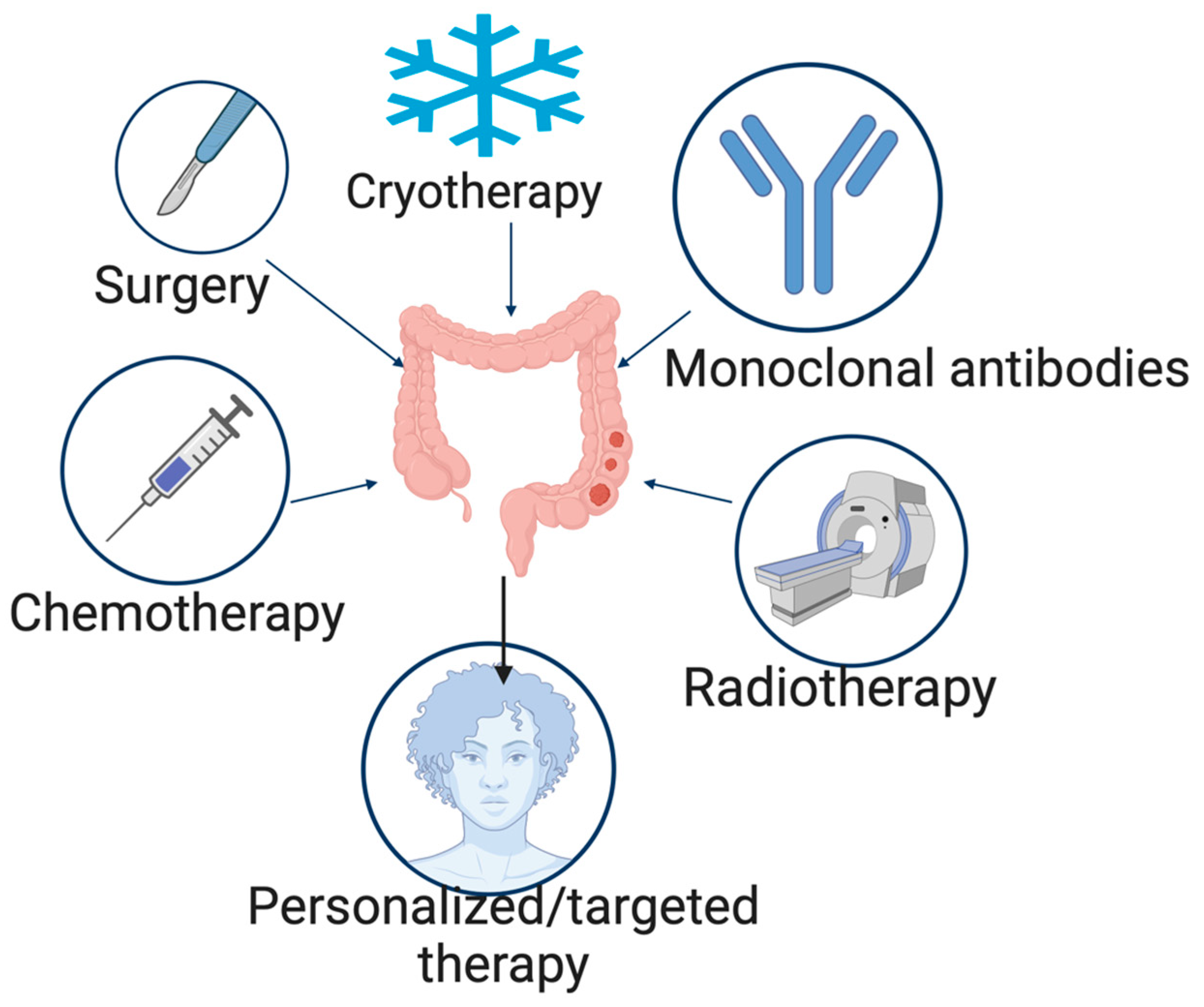
4. Treatment of Colorectal Cancer
4.1. Surgery
4.2. Cryotherapy
4.3. Radiation Therapy
4.4. Chemotherapy
4.5. Monoclonal Antibodies
4.6. Side Effects and Complications of CRC Treatments
5. Colon-Specific Drug Delivery Systems
5.1. Approaches for Colon-Specific Drug Delivery
5.1.1. PH Approach
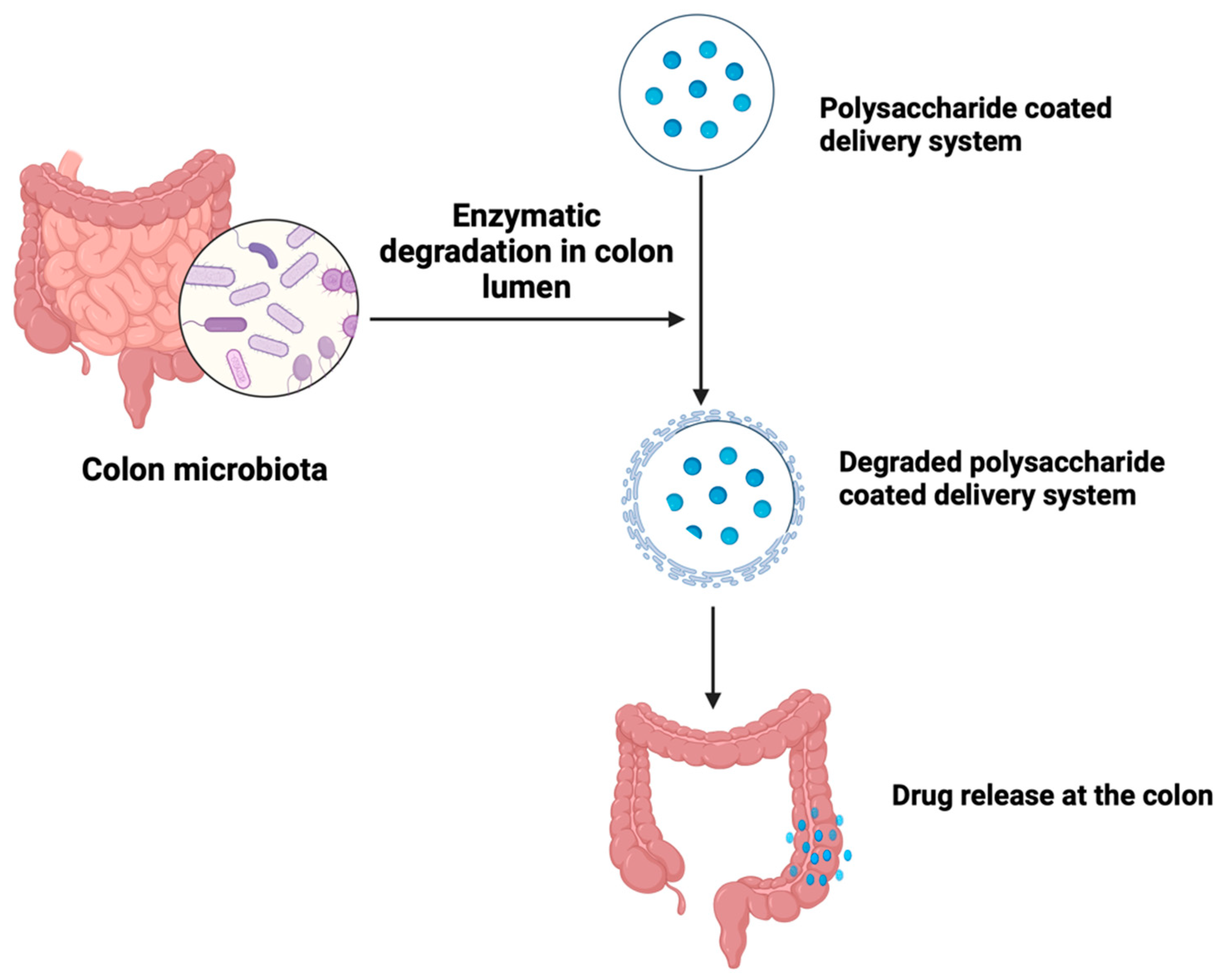
5.1.2. Microbiota-Based Drug Delivery
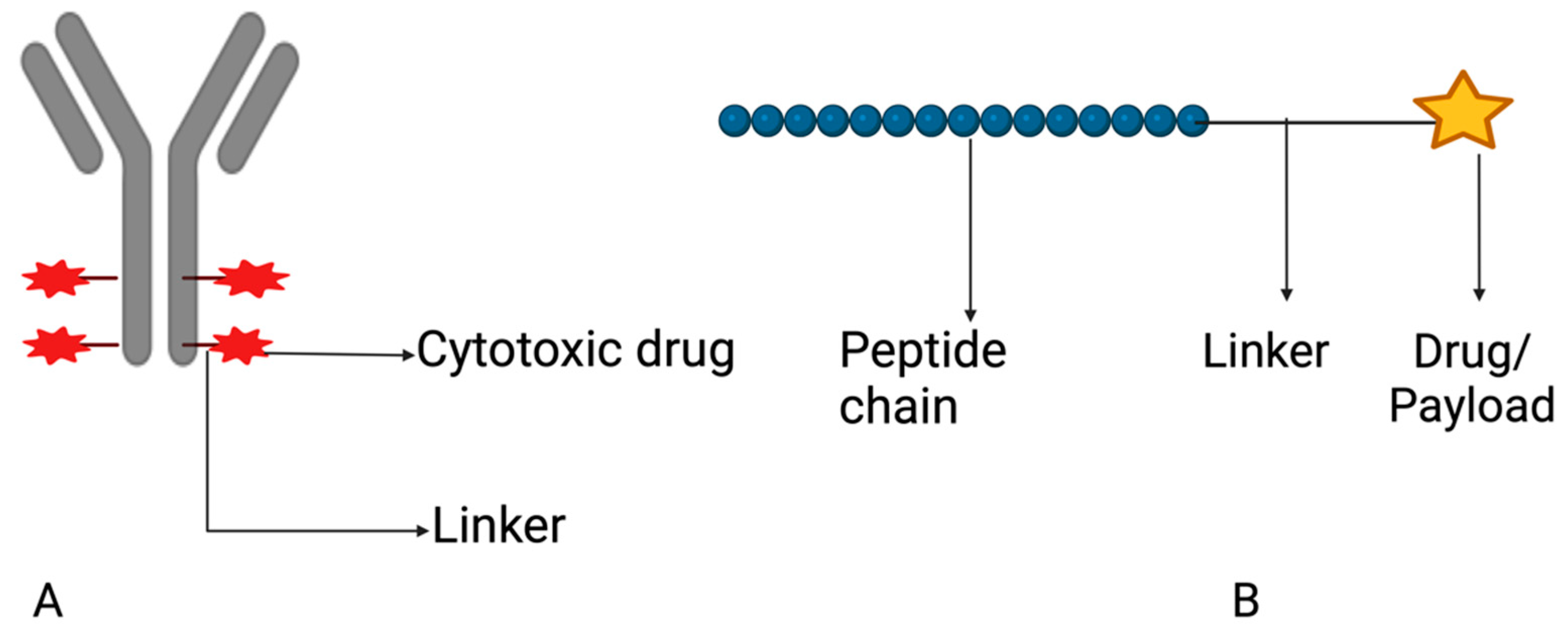
5.1.3. Drug Conjugates
| Targets | Ligands | References |
|---|---|---|
| Carcinoembryonic antigen | Antibody | Tiernan et al., 2013 [145] |
| Folate receptor alpha (FRα) | Folic acid | Noe et al., 2022 [146] |
| Human epidermal growth factor 2 (HER-2) | Peptide | Suwaidan et al., 2022 [147] |
| Hyaluronic acid receptor | Hyaluronic acid | Mansoori et al., 2020 [148] |
| CD44 receptor | Hyaluronic acid | Tabasi et al., 2021 [149] |
| Drug Conjugate | Target | Phase of Study | Clinical Trial Identifier | Sponsor |
|---|---|---|---|---|
| Disitamab vedotin and Tisleizumab | HER-2 | II | NCT05493683 | The First Affiliated Hospital with Nanjing Medical University (Nanjing, China) |
| M9140 | Carcinoembryonic antigen | I | NCT05464030 | EMD Serono Research & Development Institute, Inc. (Darmstadt, Germany) |
| CBP-1019 | Unknown | I, II | NCT05830097 | Coherent Biopharma (Hefei) Co., Ltd. (Hefei, China) |
| BDC-1001 (Nivolumab) | HER-2 | I, II | NCT04278144 | Bolt Biotherapeutics Inc. (Redwood City, CA, USA) |
| ELU001 | (FRα) | I, II | NCT05001282 | Elucida Oncology (Monmouth Junction, NJ, USA) |
| Datopotamab deruxtecan | Unknown | II | NCT05489211 | AstraZeneca (Minato City, Janpan) |
| SBT6050 (Cemiplimab) | HER-2 | I, IB | NCT04460456 | Silverback Therapeutics (Seattle, WA, USA) |
| TORL-3-600 | Unknown | I | NCT05948826 | TORL Biotherapeutics, LLC (Albuquerque, NM, USA) |
5.1.4. Vesicular Systems
Liposomes
Niosomes
Polymeric Nanoparticles
6. Prospects: Individualized Therapy and Drugs in Clinical Trials
7. Impact of Circadian Rhythm on CRD Drug Delivery and Efficacy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Wang, Q.; Wu, K.; Sun, Z.; Tang, Z.; Zhang, B. Global, Regional, and National Burden of Colorectal Cancer and Attributable Risk Factors, from 1990 to 2019: Updated Results from the Global Burden of Disease Study 2019. Available online: https://www.researchsquare.com/article/rs-1478628/v1 (accessed on 23 October 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. Available online: https://pubmed.ncbi.nlm.nih.gov/33538338/ (accessed on 23 October 2023). [CrossRef]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomized controlled trials and observational studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. World Health Organization. Available online: https://www.who.int/publications-detail-redirect/9789240001299 (accessed on 17 August 2023).
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society (ACS). Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2022; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html (accessed on 26 May 2023).
- Lebwohl, B.; Capiak, K.; Neugut, A.I.; Kastrinos, F. Risk of colorectal adenomas and advanced neoplasia in Hispanic, black and white patients undergoing screening colonoscopy. Aliment. Pharmacol. Ther. 2012, 35, 1467–1473. [Google Scholar] [CrossRef]
- Coughlin, S.S. Social determinants of colorectal cancer risk, stage, and survival: A systematic review. Int. J. Color. Dis. 2020, 35, 985–995. [Google Scholar] [CrossRef]
- Engin, O.; Kilinc, G.; Salimoglu, S. Trends, risk factors, and preventions in colorectal cancer. Colon. Polyps Color. Cancer 2020, 213–233. [Google Scholar] [CrossRef]
- Li, Y.; Lauriola, M.; Kim, D.; Francesconi, M.; D’Uva, G.; Shibata, D.; Malafa, M.P.; Yeatman, T.J.; Coppola, D.; Solmi, R.; et al. Adenomatous polyposis coli (APC) regulates MIR17-92 cluster through β-catenin pathway in colorectal cancer. Oncogene 2016, 35, 4558–4568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shay, J.W. Multiple roles of APC and its therapeutic implications in colorectal cancer. JNCI J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef]
- Zhou, J.J. MicroRNA regulation network in colorectal cancer metastasis. World J. Biol. Chem. 2014, 5, 301. [Google Scholar] [CrossRef]
- Tian, Y.; Kharazmi, E.; Sundquist, K.; Sundquist, J.; Brenner, H.; Fallah, M. Familial colorectal cancer risk in half siblings and siblings: Nationwide cohort study. BMJ 2019, 364, l803. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Hadi, M.A. Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Sandborn, W.J.; Gupta, S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management Colorectal Cancer in Inflammatory Bowel Disease. Cancer Prev. Res. 2016, 9, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Pancione, M.; Remo, A.; Colantuoni, V. Genetic and epigenetic events generate multiple pathways in colorectal cancer progression. Pathol. Res. Int. 2012, 2012, 509348. [Google Scholar] [CrossRef]
- Ziapour, P.; Ataee, R.; Shadifar, M.; Vaillancourt, C.; Ahmadi, A.; Jafari-Sabet, M.; Ataee, A. New intracellular and molecular aspects in pathophysiology of colorectal cancer. Gastroenterol. Hepatol. Bed Bench 2011, 4, 43–52. [Google Scholar]
- Gupta, R.; Sinha, S.; Paul, R.N. The impact of microsatellite stability status in colorectal cancer. Curr. Probl. Cancer 2018, 42, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.H.; Zhang, W.; Sood, A.K. Chromosomal instability in tumor initiation and development. Cancer Res. 2019, 79, 3995–4002. [Google Scholar] [CrossRef]
- Cisterna, B.A.; Kamaly, N.; Choi, W.I.; Tavakkoli, A.; Farokhzad, O.C.; Vilos, C. Targeted nanoparticles for colorectal cancer. Nanomedicine 2016, 11, 2443–2456. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Engler, J.A.; Collawn, J.F.; Moore, B.A. Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur. J. Biochem. 2001, 268, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Bickel, U. Transferrin receptor mediated brain uptake during ischemia and reperfusion. J. Pharm. Pharm. Sci. 2013, 16, 541. [Google Scholar] [CrossRef]
- Abegg, D.; Gasparini, G.; Hoch, D.G.; Shuster, A. Strained Cyclic Disulfides Enable Cellular Uptake by Reacting with the Transferrin Receptor. 2016. Available online: https://pubs.acs.org/doi/suppl/10.1021/jacs.6b09643/suppl_file/ja6b09643_si_001.pdf (accessed on 17 August 2023).
- Rapoport, S.M. Uptake and fate of iron: The transferrin receptor. In The Reticulocyte; CRC Press: Boca Raton, FL, USA, 2020; pp. 145–154. [Google Scholar] [CrossRef]
- Xue, X.; Kim, H. IDDF2022-ABS-0024 Transferrin Receptor-Mediated Iron Uptake Is Essential for Colon Tumorigenesis. Basic. Gastroenterol. 2022, 71, A32. Available online: https://gut.bmj.com/content/71/Suppl_2/A32.1 (accessed on 17 August 2023).
- Ren, J.; Zhang, P.; Li, Z.; Zhang, X.; Shen, D.; Chen, P.; Mao, C. Association of screening status, polygenic risk score and environmental risk factors with colorectal cancer incidence and mortality risks. Int. J. Cancer 2023, 152, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Krul, M.F.; Elferink, M.A.G.; Kok, N.F.M.; Dekker, E.; Lansdorp-Vogelaar, I.; Meijer, G.A.; Nagtegaal, I.D.; Breekveldt, E.C.H.; Ruers, T.J.M.; van Leerdam, M.E.; et al. Initial impact of national CRC screening on incidence and advanced colorectal cancer. Clin. Gastroenterol. Hepatol. 2023, 21, 797–807. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A.; Sharma, V.; Kumar, S.; Kumar, A.; Deo, S.; Pathy, S.; Shukla, N.K.; Pramanik, R.; Raina, V.; et al. Long-Term Survivors of Metastatic Colorectal Cancer: A Tertiary Care Centre Experience. South. Asian J. Cancer 2021, 10, 87–91. [Google Scholar] [CrossRef]
- Sharma, R. Global, regional, national burden of breast cancer in 185 countries: Evidence from Globocan 2018. Breast Cancer Res. Treat. 2021, 187, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Kaminski, M.F. Effect of colonoscopy screening on risks of colorectal cancer and related death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef]
- Diedrich, L.; Brinkmann, M.; Dreier, M.; Rossol, S.; Schramm, W.; Krauth, C. Is there a place for sigmoidoscopy in colorectal cancer screening? A systematic review and critical appraisal of cost-effectiveness models. PLoS ONE 2023, 18, e0290353. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Ransohoff, D.F. In adults, sigmoidoscopy screening invitation reduced CRC incidence and related mortality at 15 y. Ann. Intern. Med. 2023, 176, JC19. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, A.; Lee, Y.Y. Colon and pelvic floor anatomy and physiology. Clin. Basic. Neurogastroenterol. Motil. 2020, 113–126. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Camilleri, M. Physiology of the colon and its measurement. In Shackelford’s Surgery of the Alimentary Tract; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1676–1688. [Google Scholar]
- Iacopetta, B. Are there two sides to colorectal cancer? Int. J. Cancer 2002, 101, 403–408. [Google Scholar] [CrossRef]
- Gervaz, P.; Bucher, P.; Morel, P. Two colons-two cancers: Paradigm shift and clinical implications. J. Surg. Oncol. 2004, 88, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Santi, I.; Weires, M.; Thomsen, H.; Sundquist, J.; Bermejo, J.L. Tumor location and patient characteristics of colon and rectal adenocarcinomas in relation to survival and TNM classes. BMC Cancer 2010, 10, 688. [Google Scholar] [CrossRef]
- Price, T.J.; Beeke, C.; Ullah, S.; Padbury, R.; Maddern, G.; Roder, D.; Karapetis, C. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 2015, 12, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, S.; Tejpar, S.; Gibbs, P.; Thiebach, L.; Lenz, H.J. Understanding the role of primary tumour localization in colorectal cancer treatment and outcomes. Eur. J. Cancer 2017, 84, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Shin, U.S.; Ki, Y.J.; Kim, Y.B.; Moon, S.M.; Sung, S.J. Is the location of the tumor another prognostic factor for patients with colon cancer? Ann. Coloproctol. 2017, 33, 210. [Google Scholar] [CrossRef]
- Cappell, M.S. From colonic polyps to colon cancer: Pathophysiology, clinical presentation, and diagnosis. Clin. Lab. Med. 2005, 25, 135–177. [Google Scholar] [CrossRef]
- Loupakis, F.; Yang, D.; Yau, L.; Feng, S.; Cremolini, C.; Zhang, W.; Lenz, H.J. Primary tumor location as a prognostic factor in metastatic colorectal cancer. JNCI J. Natl. Cancer Inst. 2015, 107, dju427. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Malietzis, G.; Askari, A.; Bernardo, D.; Al-Hassi, H.O.; Clark, S.K. Is right-sided colon cancer different to left-sided colorectal cancer? –a systematic review. Eur. J. Surg. Oncol. 2015, 41, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Hochster, H.; Shitara, K.; Mayer, R.; Ohtsu, A.; Falcone, A.; Tabernero, J. Pooled safety analysis from phase III studies of trifluridine/tipiracil in patients with metastatic gastric or gastroesophageal junction cancer and metastatic colorectal cancer. ESMO Open 2022, 7, 100633. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Colangelo, T.; Polcaro, G.; Muccillo, L.; D’Agostino, G.; Rosato, V.; Ziccardi, P. Colantuoni, Friend or foe?: The tumor microenvironment dilemma in colorectal cancer. Biochim. Et. Biophys. Acta (BBA)-Rev. Cancer 2017, 1867, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cirri, P.; Chiarugi, P. Cancer associated fibroblasts: The dark side of the coin. Am. J. Cancer Res. 2011, 1, 482. [Google Scholar] [PubMed]
- Hawinkels LJ, A.C.; Paauwe, M.; Verspaget, H.W.; Wiercinska, E.; Van Der Zon, J.M.; Van Der Ploeg, K.; Sier CF, M. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene 2014, 33, 97–107. [Google Scholar] [CrossRef]
- Paulsson, J.; Micke, P. Prognostic relevance of cancer-associated fibroblasts in human cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2014; Volume 25, pp. 61–68. [Google Scholar]
- Robinwarren, J. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 321, 1273–1275. [Google Scholar] [CrossRef]
- Chmiela, M.; Karwowska, Z.; Gonciarz, W.; Allushi, B.; Stączek, P. Host pathogen interactions in helicobacter pylori related gastric cancer. World J. Gastroenterol. 2017, 23, 1521. [Google Scholar] [CrossRef]
- Suerbauma, S.; Achtmanb, M. Helicobacter pylori: Recombination, population structure and human migrations. Int. J. Med. Microbiol. 2004, 294, 133–139. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Wang, F.; Chang, D.; Han, B.; You, D.Y. Meta-analysis of different test indicators: Helicobacter pylori infection and the risk of colorectal cancer. Int. J. Color. Dis. 2008, 23, 875–882. [Google Scholar] [CrossRef]
- Zuo, Y.; Jing, Z.; Bie, M.; Xu, C.; Hao, X.; Wang, B. Association between Helicobacter pylori infection and the risk of colorectal cancer: A systematic review and meta-analysis. Medicine 2020, 99, 37. [Google Scholar] [CrossRef]
- Zhang, Y.; Hoffmeister, M.; Weck, M.N.; Chang-Claude, J.; Brenner, H. Helicobacter pylori infection and colorectal cancer risk: Evidence from a large population-based case-control study in Germany. Am. J. Epidemiol. 2012, 175, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Larrea-Baz, N.; Michel, A.; Romero, B.; Pérez-Gómez, B.; Moreno, V.; Martín, V.; Dierssen-Sotos, T.; Jiménez-Moleón, J.J.; Castilla, J.; Tardón, A.; et al. Helicobacter pylori Antibody Reactivities and Colorectal Cancer Risk in a Case-control Study in Spain. Front. Microbiol. 2017, 8, 888. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.; Varga, M.G.; Blot, W.J.; Teras, L.; Visvanathan, K.; Le Marchand, L.; Epplein, M. Serologic response to Helicobacter pylori proteins associated with risk of colorectal cancer among diverse populations in the United States. Gastroenterology 2019, 156, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sokic-Milutinovic, A.; Alempijevic, T.; Milosavljevic, T. Role of Helicobacter pylori infection in gastric carcinogenesis: Current knowledge and future directions. World J. Gastroenterol. 2015, 21, 11654. [Google Scholar] [CrossRef]
- Takahashi-Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular anatomy and pathogenic actions of helicobacter pylori CagA that underpin gastric carcinogenesis. Cell. Mol. Immunol. 2019, 17, 50–63. [Google Scholar] [CrossRef]
- World Health Organization. WHO Publishes List of Bacteria for which New Antibiotics Are Urgently Needed. World Health Organization. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 17 August 2023).
- Srisuphanunt, M.; Wilairatana, P.; Kooltheat, N.; Duangchan, T.; Katzenmeier, G.; Rose, J.B. Molecular mechanisms of antibiotic resistance and novel treatment strategies for helicobacter pylori infections. Trop. Med. Infect. Dis. 2023, 8, 163. [Google Scholar] [CrossRef]
- Cardos, A.I.; Maghiar, A.; Zaha, D.C.; Pop, O.; Fritea, L.; Miere, F.; Cavalu, S. Evolution of diagnostic methods for helicobacter pylori infections: From traditional tests to high technology, advanced sensitivity and discrimination tools. Diagnostics 2022, 12, 508. [Google Scholar] [CrossRef]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Muro, K.; Ajioka, Y.; Hashiguchi, Y.; Ito, Y.; Saito, Y. Japanese Society for Cancer of the Colon and Rectum Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 1–34. [Google Scholar] [CrossRef]
- Dorudi, S.; Steele, R.J.; McArdle, C.S. Surgery for colorectal cancer. Br. Med. Bull. 2002, 64, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.D.; Felder, S.I.; Bhama, A.R.; Hawkins, A.T.; Langenfeld, S.J.; Shaffer, V.O.; Paquette, I.M. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of colon cancer. Dis. Colon. Rectum 2022, 65, 148–177. [Google Scholar] [CrossRef] [PubMed]
- Pak, H.; Maghsoudi, L.H.; Soltanian, A.; Gholami, F. Surgical complications in colorectal cancer patients. Ann. Med. Surg. 2020, 55, 13–18. [Google Scholar] [CrossRef]
- Gomez Ruiz, M.; Lainez Escribano, M.; Cagigas Fernandez, C.; Cristobal Poch, L.; Santarrufina Martinez, S. Robotic surgery for colorectal cancer. Ann. Gastroenterol. Surg. 2020, 4, 646–651. [Google Scholar] [CrossRef]
- Theodorescu, D. Cancer cryotherapy: Evolution and biology. Rev. Urol. 2004, 6 (Suppl. S4), S9. [Google Scholar]
- Jiang, X.; Ji, Z.; Lei, X.; Liu, C.; Yuan, F. Suitable T stage for cryosurgery to spare the anus in patients with low rectal cancer. Cryobiology 2023, 111, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Bageacu, S.; Kaczmarek, D.; Lacroix, M.; Dubois, J.; Forest, J.; Porcheron, J. Cryosurgery for resectable and unresectable hepatic metastases from colorectal cancer. Eur. J. Surg. Oncol. 2007, 33, 590–596. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Menon, H.; Ramapriyan, R.; Cushman, T.R.; Verma, V.; Kim, H.H.; Schoenhals, J.E.; Welsh, J.W. Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front. Immunol. 2019, 10, 193. [Google Scholar] [CrossRef]
- Haddock, M.G. Intraoperative radiation therapy for colon and rectal cancers: A clinical review. Radiat. Oncol. 2017, 12, 11. [Google Scholar] [CrossRef]
- Potemin, S.; Kübler, J.; Uvarov, I.; Wenz, F.; Giordano, F. Intraoperative radiotherapy as an immediate adjuvant treatment of rectal cancer due to limited access to external-beam radiotherapy. Radiat. Oncol. 2020, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Tofthagen, C. Surviving chemotherapy for colon cancer and living with the consequences. J. Palliat. Med. 2010, 13, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Ades, S. Adjuvant chemotherapy for colon cancer in the elderly: Moving from evidence to practice. Oncology 2009, 23, 162. [Google Scholar]
- Dickens, E.; Ahmed, S. Principles of cancer treatment by chemotherapy. Surgery 2018, 36, 134–138. [Google Scholar]
- Bhosle, J.; Hall, G. Principles of cancer treatment by chemotherapy. Surgery 2009, 27, 173–177. [Google Scholar]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J. Benefits and drawbacks of the use of oral fluoropyrimidines as single-agent therapy in advanced colorectal cancer. Clin. Color. Cancer 2005, 5, S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Ashwanikumar, N.; Kumar, N.A.; Nair, S.A.; Kumar, G.V. Methacrylic-based nanogels for the pH-sensitive delivery of 5-fluorouracil in the colon. Int. J. Nanomed. 2012, 7, 5769–5779. [Google Scholar]
- Dhawale, S.C.; Bankar, A.S.; Patro, M.N. Formulation and evaluation porous microspheres of 5-fluorouracil for colon targeting. Int. J. Pharm. Tech. Res. 2010, 2, 1112–1118. [Google Scholar]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J.; Kim, S.; LoRusso, P.; Li, J. Pharmacometabolomics reveals irinotecan mechanism of action in cancer patients. J. Clin. Pharmacol. 2019, 59, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Reyhanoglu, G.; Smith, T. Irinotecan in StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Miele, L.; Liguori, A.; Marrone, G.; Biolato, M.; Araneo, C.; Vaccaro, F.G.; Grieco, A. Fatty liver and drugs: The two sides of the same coin. Eur. Rev. Med. Pharmacol. Sci. 2017, 21 (Suppl. S1), 86–94. [Google Scholar] [PubMed]
- Milano, G.; Innocenti, F.; Minami, H. Liposomal irinotecan (Onivyde): Exemplifying the benefits of nanotherapeutic drugs. Cancer Sci. 2022, 113, 2224–2231. [Google Scholar] [CrossRef]
- Machover, D. Treatment of advanced colorectal and gastric adenocarcinomas with 5-FU combined with high-dose folinic acid: A pilot study. Cancer Treat. Rep. 1982, 66, 1803–1807. [Google Scholar]
- Thirion, P.; Michiels, S.; Pignon, J.P.; Buyse, M.; Braud, A.C.; Carlson, R.W.; O’Connell, M.; Sargent, P.; Piedbois, P. Meta-Analysis Group in Cancer Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: An updated meta-analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 3766–3775. [Google Scholar] [CrossRef]
- Ibrahim, B.; Mady, O.Y.; Tambuwala, M.M.; Haggag, Y.A. pH-sensitive nanoparticles containing 5-fluorouracil and leucovorin as an improved anti-cancer option for colon cancer. Nanomedicine 2022, 17, 367–381. [Google Scholar] [CrossRef]
- Veronese, M.L.; O’Dwyer, P.J. Monoclonal antibodies in the treatment of colorectal cancer. Eur. J. Cancer 2004, 40, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Cohen, R.B.; Meropol, N.J. Targeting signal transduction pathways in colorectal cancer—More than skin deep. J. Clin. Oncol. 2005, 23, 5374–5385. [Google Scholar] [CrossRef]
- Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 4368–4380. [Google Scholar] [CrossRef]
- Grünwald, V.; Hidalgo, M. Developing inhibitors of the epidermal growth factor receptor for cancer treatment. J. Natl. Cancer Inst. 2003, 95, 851–867. [Google Scholar] [CrossRef]
- Aprile, G.; Rihawi, K.; De Carlo, E.; Sonis, S.T. Treatment-related gastrointestinal toxicities and advanced colorectal or pancreatic cancer: A critical update. World J. Gastroenterol. 2015, 21, 11793–11803. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, C.; Wang, F.; Li, P.; Hu, Z.; Shan, Y.; Zhang, J. Development of 5-fluorouracil derivatives as anticancer agents. Curr. Med. Chem. 2011, 18, 4538–4556. [Google Scholar] [CrossRef]
- Kim, J.H. Chemotherapy for colorectal cancer in the elderly. World J. Gastroenterol. 2015, 21, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Shitara, K.; Mizusawa, J.; Hamaguchi, T.; Shida, D.; Komori, K.; Ikeda, S.; Ojima, H.; Ike, H.; Shiomi, A.; et al. Primary tumor resection plus chemotherapy versus chemotherapy alone for colorectal cancer patients with asymptomatic, synchronous unresectable metastases (JCOG1007; ipacs): A randomized clinical trial. J. Clin. Oncol. 2021, 39, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, S.; Huang, G. Design and application of Oral Colon Administration System. J. Enzym. Inhib. Med. Chem. 2019, 34, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Otte, A.; Park, K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J. Control. Release 2022, 342, 53–65. [Google Scholar] [CrossRef]
- Williams, T.M.; Sable, R.; Singh, S.; Vicente, M.G.; Jois, S.D. Peptide ligands for targeting the extracellular domain of EGFR: Comparison between linear and cyclic peptides. Chem. Biol. Drug Des. 2017, 91, 605–619. [Google Scholar] [CrossRef]
- Islam, M.S.; Junod, S.L.; Zhang, S.; Buuh, Z.Y.; Guan, Y.; Zhao, M.; Kaneria, K.H.; Kafley, P.; Cohen, C.; Maloney, R.; et al. Unprotected peptide macrocyclization and stapling via a fluorine-thiol displacement reaction. Nat. Commun. 2022, 13, 350. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. 2020, 590, 119912. [Google Scholar] [CrossRef]
- Bahrami, A.; Khazaei, M.; Hassanian, S.M.; ShahidSales, S.; Joudi-Mashhad, M.; Maftouh, M.; Jazayeri, M.H.; Parizade, M.R.; Ferns, G.A.; Avan, A. Targeting the tumor microenvironment as a potential therapeutic approach in colorectal cancer: Rational and progress. J. Cell. Physiol. 2018, 233, 2928–2936. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Bhatt, N.; Patel, K.R.; Patel, N.M.; Patel, M.R. Colon targeted drug delivery system: A review system. J. Pharm. Sci. Biosci. Res. 2011, 1, 37–49. [Google Scholar]
- Kumar, M.; Ali, A.; Kaldhone, P.; Shirode, A.; Kadam, V.J. Report on pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Res. 2010, 3, 157–159. [Google Scholar]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-targeted oral drug delivery systems: Design trends and approaches. AAPS Pharm. SciTech 2015, 16, 731–741. [Google Scholar] [CrossRef]
- Yoshida, T.; Lai, T.C.; Kwon, G.S.; Sako, K. pH-and ion-sensitive polymers for drug delivery. Expert. Opin. Drug Deliv. 2013, 10, 1497–1513. [Google Scholar] [CrossRef]
- Newton AM, J.; Prabakaran, L.; Jayaveera, K. Pectin-HPMC E15LV vs. pH sensitive polymer coating films for delayed drug delivery to colon: A comparison of two dissolution models to assess colonic targeting performance in-vitro. Int. J. Appl. Res. Nat. Prod. 2012, 5, 1–16. [Google Scholar]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Naik, J.B.; Waghulde, M.R. Development of vildagliptin loaded Eudragit® microspheres by screening design: In vitro evaluation. J. Pharm. Investig. 2018, 48, 627–637. [Google Scholar] [CrossRef]
- Mahkam, M.; Abbaszad Rafi, A.; Mohammadzadeh Gheshlaghi, L. Preparation of novel pH-sensitive nanocomposites based on ionic-liquid modified montmorillonite for colon specific drug delivery system. Polym. Compos. 2016, 37, 182–187. [Google Scholar] [CrossRef]
- Lamprecht, A.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Design of pH-sensitive microspheres for the colonic delivery of the immunosuppressive drug tacrolimus. Eur. J. Pharm. Biopharm. 2004, 58, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv. Drug Deliv. Rev. 2019, 146, 248–266. [Google Scholar] [CrossRef]
- Prajakta, D.; Ratnesh, J.; Chandan, K.; Suresh, S.; Grace, S.; Meera, V.; Vandana, P. Curcumin-loaded pH-sensitive nanoparticles for the treatment of colon cancer. J. Biomed. Nanotechnol. 2009, 5, 445–455. [Google Scholar] [CrossRef]
- Naeem, M.; Awan, U.A.; Subhan, F.; Cao, J.; Hlaing, S.P.; Lee, J.; Yoo, J.W. Advances in colon-targeted nano-drug delivery systems: Challenges and solutions. Arch. Pharmacal. Res. 2020, 43, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gan, X.; Chen, Y. A novel pH-sensitive hydrogels for potential colon-specific drug delivery: Characterization and in vitro release studies. Starch-Stärke 2011, 63, 503–511. [Google Scholar] [CrossRef]
- Mamidi, N.; Velasco Delgadillo, R.; Gonzáles Ortiz, A.; Barrera, E.V. Carbon. nano-onions reinforced multilayered thin film system for stimuli-responsive drug release. Pharmaceutics 2020, 12, 1208. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing γ-Cyclodextrin as Drug Carrier for High-Performance PH-Triggered Drug Release. Pharmaceutics 2021, 14, 291. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8064464/ (accessed on 14 August 2023).
- Mamidi, N.; Delgadillo, R.M. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf. B Biointerfaces 2021, 204, 1118–1119. [Google Scholar] [CrossRef]
- Garrett, W.S. The gut microbiota and colon cancer. Science 2019, 364, 1133–1135. [Google Scholar] [CrossRef]
- Shukla, R.K.; Tiwari, A. Carbohydrate polymers: Applications and recent advances in delivering drugs to the colon. Carbohydr. Polym. 2012, 88, 399–416. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gupta, A.B.; Kumar, R.; Yadav, J.S.; Kumar, B. Mucoadhesive polymers: Means of improving the mucoadhesive properties of drug delivery system. J. Chem. Pharm. Res. 2010, 2, 418–432. [Google Scholar]
- Zhu, J.; Zhong, L.; Chen, W.; Song, Y.; Qian, Z.; Cao, X.; Chen, W. Preparation and characterization of pectin/chitosan beads containing porous starch embedded with doxorubicin hydrochloride: A novel and simple colon targeted drug delivery system. Food Hydrocoll. 2019, 95, 562–570. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Meng, Q.; Zhong, S.; Xu, L.; Wang, J.; Zhang, Z.; Gao, Y.; Cui, X. Review on design strategies and considerations of polysaccharide-based smart drug delivery systems for cancer therapy. Carbohydr. Polym. 2022, 279, 119013. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Shukla, N.; Mahajan, S. Polysaccharides in colon specific drug delivery. J. Transl. Sci. 2015, 1, 3–11. [Google Scholar]
- Wen, Y.; Oh, J.K. Recent strategies to develop polysaccharide-based nanomaterials for biomedical applications. Macromol. Rapid Commun. 2014, 35, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Prudhviraj, G.; Vaidya, Y.; Singh, S.K.; Yadav, A.K.; Kaur, P.; Gulati, M.; Gowthamarajan, K. Effect of co-administration of probiotics with polysaccharide-based colon targeted delivery systems to optimize site specific drug release. Eur. J. Pharm. Biopharm. 2015, 97, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Si, X.Y.; Merlin, D.; Xiao, B. Recent advances in orally administered cell-specific nanotherapeutics for inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7718. [Google Scholar] [CrossRef]
- Alas, M.; Saghaeidehkordi, A.; Kaur, K. Peptide–drug conjugates with different linkers for cancer therapy. J. Med. Chem. 2020, 64, 216–232. [Google Scholar] [CrossRef]
- Candelaria, P.V.; Leoh, L.S.; Penichet, M.L.; Daniels-Wells, T.R. Antibodies targeting the transferrin receptor 1 (tfr1) as direct anti-cancer agents. Front. Immunol. 2021, 12, 607692. [Google Scholar] [CrossRef]
- Tesauro, D.; Accardo, A.; Diaferia, C.; Milano, V.; Guillon, J.; Ronga, L.; Rossi, F. Peptide-based drug-delivery systems in biotechnological applications: Recent advances and perspectives. Molecules 2019, 24, 351. [Google Scholar] [CrossRef]
- Ghosh, D.; Peng, X.; Leal, J.; Mohanty, R.P. Peptides as drug delivery vehicles across biological barriers. J. Pharm. Investig. 2018, 48, 89–111. [Google Scholar] [CrossRef]
- Jiang, Z.; Guan, J.; Qian, J.; Zhan, C. Peptide ligand-mediated targeted drug delivery of nanomedicines. Biomater. Sci. 2019, 7, 461–471. [Google Scholar] [CrossRef]
- Al-azzawi, S.; Masheta, D. Designing a drug delivery system for improved tumor treatment and targeting by functionalization of a cell-penetrating peptide. J. Pharm. Investig. 2019, 49, 643–654. [Google Scholar] [CrossRef]
- Ren, Y.; Mu, Y.; Song, Y.; Xie, J.; Yu, H.; Gao, S.; Lu, W. A new peptide ligand for colon cancer targeted delivery of micelles. Drug Deliv. 2016, 23, 1763–1772. [Google Scholar] [CrossRef]
- Guo, F.; Ouyang, T.; Peng, T.; Zhang, X.; Xie, B.; Yang, X.; Zhong, H. Enhanced oral absorption of insulin using colon-specific nanoparticles co-modified with amphiphilic chitosan derivatives and cell-penetrating peptides. Biomater. Sci. 2019, 7, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Langel, Ü. Methods for CPP functionalization. CPP Cell-Penetrating Pept. 2019, 83–156. [Google Scholar] [CrossRef]
- Tiernan, J.P.; Perry, S.L.; Verghese, E.T.; West, N.P.; Yeluri, S.; Jayne, D.G.; Hughes, T.A. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br. J. Cancer 2013, 108, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Noe, O.; Ngo, N.; Lin, L.; Stanbery, L.; Creeden, J.F.; Hamouda, D.; Nemunaitis, J. Folate Receptor as a Biomarker and Therapeutic Target in Solid Tumors. Curr. Probl. Cancer 2022, 47, 100917. [Google Scholar]
- Suwaidan, A.A.; Lau, D.K.; Chau, I. HER2 targeted therapy in colorectal cancer: New horizons. Cancer Treat. Rev. 2022, 105, 102363. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Abedi-Gaballu, F.; Abbaspour, S.; Ghasabi, M.; Yekta, R.; Baradaran, B. Hyaluronic acid-decorated liposomal nanoparticles for targeted delivery of 5-fluorouracil into HT-29 colorectal cancer cells. J. Cell. Physiol. 2020, 235, 6817–6830. [Google Scholar] [CrossRef]
- Tabasi, H.; Mosavian, M.H.; Sabouri, Z.; Khazaei, M.; Darroudi, M. pH-responsive and CD44-targeting by Fe3O4/MSNs-NH2 nanocarriers for oxaliplatin loading and colon cancer treatment. Inorg. Chem. Commun. 2021, 125, 108430. [Google Scholar] [CrossRef]
- Kadam, R.S.; Bourne, D.W.; Kompella, U.B. Nano-advantage in enhanced drug delivery with biodegradable nanoparticles: Contribution of reduced clearance. Drug Metab. Dispos. 2012, 40, 1380–1388. [Google Scholar] [CrossRef]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Lohrasbi-Nejad, A.; Nematollahi, M.H. A new formulation of hydrophobin-coated niosome as a drug carrier to cancer cells. Mater. Sci. Eng. 2020, 113, 110975. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Chu, B.S.; Yaakob, H. Niosomal drug delivery systems: Formulation, preparation and applications. World Appl. Sci. J. 2014, 32, 1671–1685. [Google Scholar]
- Sebaaly, C.; Greige-Gerges, H.; Stainmesse, S.; Fessi, H.; Charcosset, C. Effect of composition, hydrogenation of phospholipids and lyophilization on the characteristics of eugenol-loaded liposomes prepared by ethanol injection method. Food Biosci. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, characterization and applications of liposomes: State of the art. J. Colloid. Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Niu, M.; Lu, Y.; Hovgaard, L.; Guan, P.; Tan, Y.; Lian, R.; Qi, J.; Wu, W. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: The effect of cholate type, particle size and administered dose. Eur. J. Pharm. Biopharm. 2012, 81, 265–272. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Li, T.; Deng, Y. Modulation of the physicochemical state of interior agents to prepare controlled release liposomes. Colloids Surf. B Biointerfaces 2009, 69, 232–238. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef]
- Khuntawee, W.; Amornloetwattana, R.; Vongsangnak, W.; Namdee, K.; Yata, T.; Karttunen, M.; Wong-Ekkabut, J. In silico and in vitro design of cordycepin encapsulation in liposomes for colon cancer treatment. RSC Adv. 2021, 11, 8475–8484. [Google Scholar] [CrossRef]
- Xiong, M.; Lei, Q.; You, X.; Gao, T.; Song, X.; Xia, Y.; Yu, L. Mannosylated liposomes improve therapeutic effects of paclitaxel in colon cancer models. J. Microencapsul. 2017, 34, 513–521. [Google Scholar] [CrossRef]
- Yasamineh, S.; Yasamineh, P.; Ghafouri Kalajahi, H.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Hossein Kheirkhah, A.; Taghizadeh, M.; Yazdani, Y.; et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Varshosaz, J.; Taymouri, S.; Pardakhty, A.; Asadi-Shekaari, M.; Babaee, A. Niosomes of ascorbic acid and α-tocopherol in the cerebral ischemia-reperfusion model in male rats. BioMed Res. Int. 2014, 2014, 816103. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Kumar, Y.A.; Hemanth, A.; Anitha, M. Preparation and evaluation of niosomes containing aceclofenac. Dig. J. Nanomater. Bios 2010, 5, 249–254. [Google Scholar]
- Shakya, V.; Bansal, B.K. Niosomes: A novel trend in drug delivery. Int. J. Res. Dev. Pharm. Life Sci. 2014, 3, 1036–1041. [Google Scholar]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Witika, B.A.; Bassey, K.E.; Demana, P.H.; Siwe-Noundou, X.; Poka, M.S. Current advances in specialised niosomal drug delivery: Manufacture, characterization and drug delivery applications. Int. J. Mol. Sci. 2022, 23, 9668. [Google Scholar] [CrossRef]
- El-Ridy, M.S.; Badawi, A.A.; Safar, M.M.; Mohsen, A.M. Niosomes as a novel pharmaceutical formulation encapsulating the hepatoprotective drug silymarin. Int. J. Pharm. Pharm. Sci. 2012, 4, 549–559. [Google Scholar]
- Umbarkar, M.G. Niosome as a novel pharmaceutical drug delivery: A brief review highlighting formulation, types, composition and application. Indian. J. Pharm. Educ. Res. 2021, 55, s11–s28. [Google Scholar] [CrossRef]
- Ugorji, O.L.; Umeh ON, C.; Agubata, C.O.; Adah, D.; Obitte, N.C.; Chukwu, A. The effect of noisome preparation methods in encapsulating 5-fluorouracil and real time cell assay against HCT-116 colon cancer cell line. Heliyon 2022, 8, e12369. [Google Scholar] [CrossRef]
- El-Far, S.W.; Abo El-Enin, H.A.; Abdou, E.M.; Nafea, O.E.; Abdelmonem, R. Targeting colorectal cancer cells with niosomes systems loaded with two anticancer drugs models; comparative in vitro and anticancer studies. Pharmaceuticals 2022, 15, 816. [Google Scholar] [CrossRef]
- Prabhu, R.H.; Patravale, V.B.; Joshi, M.D. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomed. 2015, 10, 1001–1018. [Google Scholar] [CrossRef]
- Lamprecht, A. Nanomedicines in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 195–204. [Google Scholar] [CrossRef]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Cancer Nanotechnol. Methods Protoc. 2010, 624, 25–37. [Google Scholar]
- Ejigah, V.; Owoseni, O.; Bataille-Backer, P.; Ogundipe, O.D.; Fisusi, F.A.; Adesina, S.K. Approaches to improve macromolecule and nanoparticle accumulation in the tumor microenvironment by the enhanced permeability and retention effect. Polymers 2022, 14, 2601. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Taieb, J.; Prager, G.W.; Ciardiello, F.; Fakih, M.; Leger, C.; Fougeray, R.; Amellal, N.; van Cutsem, E. Trifluridine/tipiracil plus bevacizumab for third-line management of metastatic colorectal cancer: SUNLIGHT study design. Future Oncol. 2021, 17, 1977–1985. [Google Scholar] [CrossRef]
- Prager, G.W.; Taieb, J.; Fakih, M.; Ciardiello, F.; Van Cutsem, E.; Elez, E.; Tabernero, J. Trifluridine–tipiracil and bevacizumab in refractory metastatic colorectal cancer. N. Engl. J. Med. 2023, 388, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Terazawa, T.; Masuishi, T.; Nakamura, M.; Watanabe, J.; Ojima, H.; Makiyama, A.; Kotaka, M.; Hara, H.; Kagawa, Y.; et al. Trifluridine/tipiracil+ bevacizumab (BEV) vs. fluoropyrimidine-irinotecan+ BEV as second-line therapy for metastatic colorectal cancer: A randomised noninferiority trial. Br. J. Cancer 2023, 128, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Lonardi, S.; Garcia-Carbonero, R.; Elez, E.; Yoshino, T.; Sobrero, A.; Yao, J.; García-Alfonso, P.; Kocsis, J.; Cubillo Gracian, A.; et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (fresco-2): An International, multicentre, randomised, double-blind, phase 3 study. Lancet 2023, 402, 41–53. [Google Scholar] [CrossRef]
- Shafi, A.A.; Knudsen, K.E. Cancer and the circadian clock. Cancer Res. 2019, 79, 3806–3814. [Google Scholar] [CrossRef]
- Kinouchi, K.; Sassone-Corsi, P. Metabolic rivalry: Circadian homeostasis and tumorigenesis. Nat. Rev. Cancer 2020, 20, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Zhang, X.; Tang, Q. New insights into cancer chronotherapies. Front. Pharmacol. 2021, 12, 741295. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, Q.; Liu, Y.; Zhu, Z.; Wang, L.; Wang, H.; Li, K. Efficacy and safety of chronomodulated chemotherapy for patients with metastatic colorectal cancer: A systematic review and meta-analysis. Asia-Pac. J. Clin. Oncol. 2016, 13, e171–e178. [Google Scholar] [CrossRef]
- Nassar, A.; Abdelhamid, A.; Ramsay, G.; Bekheit, M. Chronomodulated Administration of Chemotherapy in Advanced Colorectal Cancer: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e36522. [Google Scholar] [CrossRef] [PubMed]


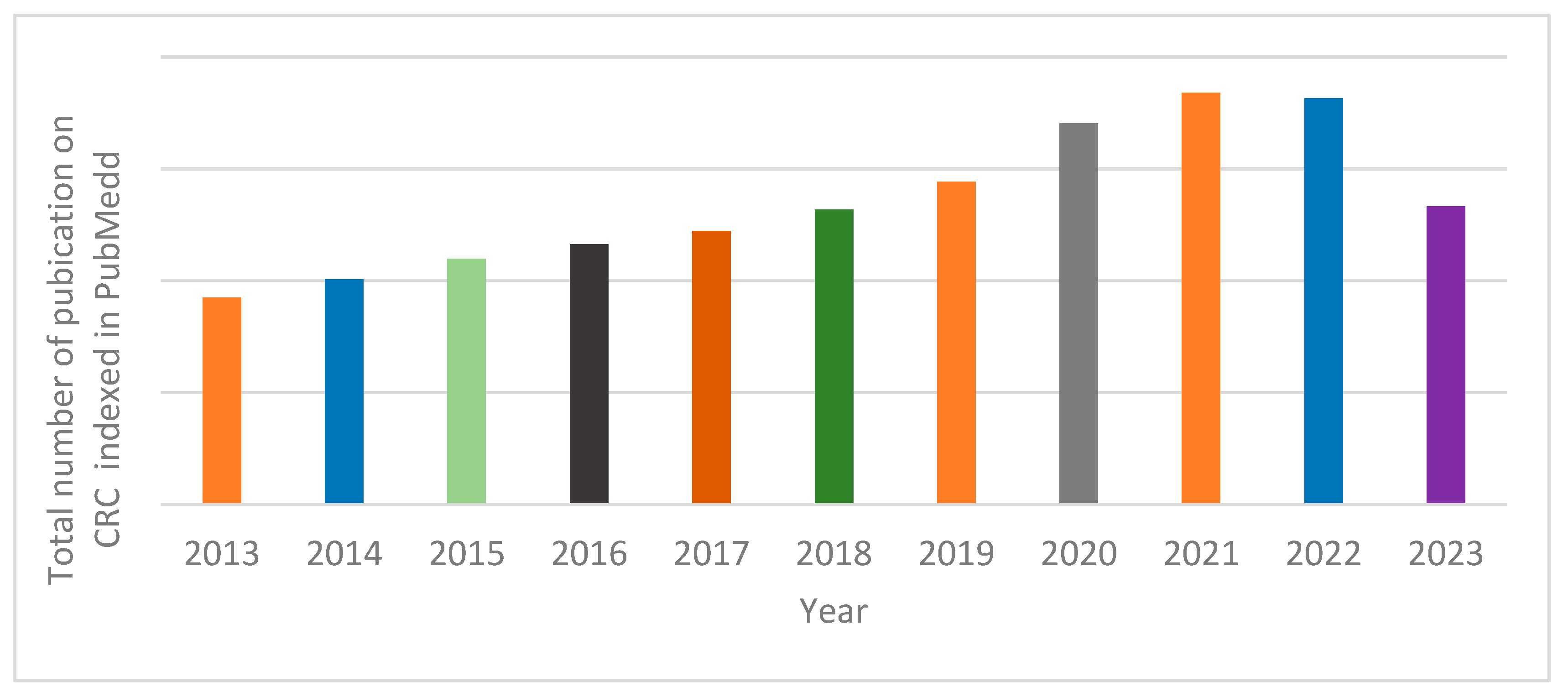





| Vesicular System | Payload | Phase of Study | Clinical Trial Identifier | Sponsor |
|---|---|---|---|---|
| Liposome | Irinotecan and Bevacizumab | I | NCT05854498 | University of Wisconsin (Madison, WI, USA) |
| Irinotecan-based FOLFIRI combined with Bevacizumab | II | NCT05969899 | Fudan University (Shanghai, China) | |
| Fluorouracil and Irinotecan and Leucovorin | I, II | NCT03337087 | Academic and Community Cancer Research United (Rochester, MN, USA) | |
| Polymeric nanoparticle | Cetuximab | I | NCT03774680 | Al-Azhar University (Cairo, Egypt) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adebayo, A.S.; Agbaje, K.; Adesina, S.K.; Olajubutu, O. Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives. Pharmaceutics 2023, 15, 2620. https://doi.org/10.3390/pharmaceutics15112620
Adebayo AS, Agbaje K, Adesina SK, Olajubutu O. Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives. Pharmaceutics. 2023; 15(11):2620. https://doi.org/10.3390/pharmaceutics15112620
Chicago/Turabian StyleAdebayo, Amusa S., Kafilat Agbaje, Simeon K. Adesina, and Oluwabukunmi Olajubutu. 2023. "Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives" Pharmaceutics 15, no. 11: 2620. https://doi.org/10.3390/pharmaceutics15112620
APA StyleAdebayo, A. S., Agbaje, K., Adesina, S. K., & Olajubutu, O. (2023). Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives. Pharmaceutics, 15(11), 2620. https://doi.org/10.3390/pharmaceutics15112620





