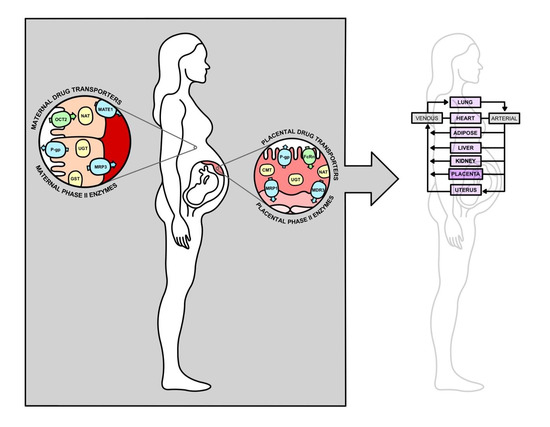
A Literature Review of Changes in Phase II Drug-Metabolizing Enzyme and Drug Transporter Expression during Pregnancy
Abstract
:1. Introduction
2. Placenta
2.1. Placental Anatomy
2.2. Placental Drug Transport
3. Drug Transporters
3.1. ATP-Binding Cassette Superfamily
3.1.1. Multidrug Resistance Protein (MDR) Family
3.1.2. Multidrug Resistance-Associated Protein (MRP) Family
3.1.3. Breast Cancer Resistance Protein (BCRP)
3.2. Solute Carrier Superfamily
3.2.1. Organic Anion-Transporting Polypeptide (OATP) Family
3.2.2. Organic Cation Transporter (OCT) Family
3.2.3. Organic Cation/Carnitine Transporter (OCTN) Family
3.2.4. Organic Anion Transporter (OAT) Family
3.2.5. Concentrative Nucleoside Transporter (CNT) Family
3.2.6. Equilibrative Nucleoside Transporter (ENT) Family
3.2.7. Multidrug and Toxin Extrusion (MATE) Family
3.3. Neonatal Fc Receptor (FcRn)
4. Phase II Enzymes
4.1. Methyltransferase (MT) Superfamily
4.2. Glutathione S-Transferase (GST) Superfamily
4.3. N-Acetyltransferase (NAT) Superfamily
4.4. Sulfotransferase (SULT) Superfamily
4.5. UDP-Glucuronosyltransferase (UGT) Superfamily
5. Materials and Methods
6. Results
7. Discussion
7.1. Effects of 17β-Estradiol and Progesterone on Drug Metabolism and Transport
7.2. Effects of Gestational Age on Phase II Enzyme Expression or Activity
7.2.1. Maternal Phase II Enzyme Expression or Activity
7.2.2. Placental Phase II Enzyme Expression or Activity
7.3. Effects of Gestational Age on Drug Transporter Expression or Activity
7.3.1. Maternal Drug Transporter Expression or Activity
7.3.2. Placental Drug Transporter Expression or Activity
7.4. Limitations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feghali, M.; Venkataramanan, R.; Caritis, S. Pharmacokinetics of Drugs in Pregnancy. Semin. Perinatol. 2015, 39, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Isoherranen, N.; Thummel, K.E. Drug Metabolism and Transport during Pregnancy: How Does Drug Disposition Change during Pregnancy and What Are the Mechanisms that Cause Such Changes? Drug Metab. Dispos. 2013, 41, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Lupattelli, A.; Spigset, O.; Twigg, M.J.; Zagorodnikova, K.; Mårdby, A.C.; Moretti, M.E.; Drozd, M.; Panchaud, A.; Hämeen-Anttila, K.; Rieutord, A.; et al. Medication Use in Pregnancy: A Cross-Sectional, Multinational Web-Based Study. BMJ Open 2014, 4, e004365. [Google Scholar] [CrossRef] [PubMed]
- Caritis, S.N.; Venkataramanan, R. Obstetrical, Fetal, and Lactation Pharmacology—A Crisis That Can No Longer Be Ignored. Am. J. Obstet. Gynecol. 2021, 225, 10–20. [Google Scholar] [CrossRef]
- Cole, S.; Coppola, P.; Kerwash, E.; Nooney, J.; Lam, S.P. Pharmacokinetic Characterization to Enable Medicine Use in Pregnancy, the Potential Role of Physiologically-Based Pharmacokinetic Modeling: A Regulatory Perspective. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 547–549. [Google Scholar] [CrossRef]
- Coppola, P.; Kerwash, E.; Cole, S. The Use of Pregnancy Physiologically Based Pharmacokinetic Modeling for Renally Cleared Drugs. J. Clin. Pharmacol. 2022, 62 (Suppl. S1), S129–S139. [Google Scholar] [CrossRef]
- Coppola, P.; Kerwash, E.; Cole, S. Physiologically Based Pharmacokinetics Model in Pregnancy: A Regulatory Perspective on Model Evaluation. Front. Pediatr. 2021, 9, 687978. [Google Scholar] [CrossRef] [PubMed]
- Tasnif, Y.; Morado, J.; Hebert, M.F. Pregnancy-Related Pharmacokinetic Changes. Clin. Pharmacol. Ther. 2016, 100, 53–62. [Google Scholar] [CrossRef]
- Anderson, G.D. Pregnancy-Induced Changes in Pharmacokinetics: A Mechanistic-Based Approach. Clin. Pharmacokinet. 2005, 44, 989–1008. [Google Scholar] [CrossRef]
- Myllynen, P.; Immonen, E.; Kummu, M.; Vähäkangas, K. Developmental Expression of Drug Metabolizing Enzymes and Transporter Proteins in Human Placenta and Fetal Tissues. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1483–1499. [Google Scholar] [CrossRef]
- Dallmann, A.; Liu, X.I.; Burckart, G.J.; van den Anker, J. Drug Transporters Expressed in the Human Placenta and Models for Studying Maternal-Fetal Drug Transfer. J. Clin. Pharmacol. 2019, 59 (Suppl. S1), S70–S81. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Ceckova, M. Regulation of Drug Transporter Expression and Function in the Placenta. Expert Opin. Drug Metab. Toxicol. 2015, 11, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Kozlosky, D.; Barrett, E.; Aleksunes, L.M. Regulation of Placental Efflux Transporters during Pregnancy Complications. Drug Metab. Dispos. 2022, 50, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.A.; Vaidya, S.S.; St-Pierre, M.V.; Mikheev, A.M.; Desino, K.E.; Nyandege, A.N.; Audus, K.L.; Unadkat, J.D.; Gerk, P.M. Placental ABC Transporters: Biological Impact and Pharmaceutical Significance. Pharm. Res. 2016, 33, 2847–2878. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Markert, U.R. Overview of Drug Transporters in Human Placenta. Int. J. Mol. Sci. 2021, 22, 13149. [Google Scholar] [CrossRef]
- Staud, F.; Cerveny, L.; Ceckova, M. Pharmacotherapy in Pregnancy; Effect of ABC and SLC Transporters on Drug Transport across the Placenta and Fetal Drug Exposure. J. Drug Target. 2012, 20, 736–763. [Google Scholar] [CrossRef]
- Myllynen, P.; Pasanen, M.; Vähäkangas, K. The Fate and Effects of Xenobiotics in Human Placenta. Expert Opin. Drug Metab. Toxicol. 2007, 3, 331–346. [Google Scholar] [CrossRef]
- Ushigome, F.; Takanaga, H.; Matsuo, H.; Yanai, S.; Tsukimori, K.; Nakano, H.; Uchiumi, T.; Nakamura, T.; Kuwano, M.; Ohtani, H.; et al. Human Placental Transport of Vinblastine, Vincristine, Digoxin and Progesterone: Contribution of P-Glycoprotein. Eur. J. Pharmacol. 2000, 408, 1–10. [Google Scholar] [CrossRef]
- Kozłowska-Rup, D.; Czekaj, P.; Plewka, D.; Sikora, J. Immunolocalization of ABC Drug Transporters in Human Placenta from Normal and Gestational Diabetic Pregnancies. Ginekol. Pol. 2014, 85, 410–419. [Google Scholar] [CrossRef]
- Nagashige, M.; Ushigome, F.; Koyabu, N.; Hirata, K.; Kawabuchi, M.; Hirakawa, T.; Satoh, S.; Tsukimori, K.; Nakano, H.; Uchiumi, T.; et al. Basal Membrane Localization of MRP1 in Human Placental Trophoblast. Placenta 2003, 24, 951–958. [Google Scholar] [CrossRef]
- Evseenko, D.A.; Paxton, J.W.; Keelan, J.A. Independent Regulation of Apical and Basolateral Drug Transporter Expression and Function in Placental Trophoblasts by Cytokines, Steroids, and Growth Factors. Drug Metab. Dispos. 2007, 35, 595–601. [Google Scholar] [CrossRef]
- Meyer zu Schwabedissen, H.E.; Jedlitschky, G.; Gratz, M.; Haenisch, S.; Linnemann, K.; Fusch, C.; Cascorbi, I.; Kroemer, H.K. Variable Expression of MRP2 (ABCC2) in Human Placenta: Influence of Gestational Age and Cellular Differentiation. Drug Metab. Dispos. 2005, 33, 896–904. [Google Scholar] [CrossRef]
- Gedeon, C.; Behravan, J.; Koren, G.; Piquette-Miller, M. Transport of Glyburide by Placental ABC Transporters: Implications in Fetal Drug Exposure. Placenta 2006, 27, 1096–1102. [Google Scholar] [CrossRef]
- Kojovic, D.; Ghoneim, R.H.; Serghides, L.; Piquette-Miller, M. Role of HIV and Antiretroviral Therapy on the Expression of Placental Transporters in Women with HIV. AAPS J. 2020, 22, 138. [Google Scholar] [CrossRef]
- Meyer Zu Schwabedissen, H.E.; Grube, M.; Heydrich, B.; Linnemann, K.; Fusch, C.; Kroemer, H.K.; Jedlitschky, G. Expression, Localization, and Function of MRP5 (ABCC5), a Transporter for Cyclic Nucleotides, in Human Placenta and Cultured Human Trophoblasts: Effects of Gestational Age and Cellular Differentiation. Am. J. Pathol. 2005, 166, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gedeon, C.; Anger, G.; Piquette-Miller, M.; Koren, G. Breast Cancer Resistance Protein: Mediating the Trans-placental Transfer of Glyburide across the Human Placenta. Placenta 2008, 29, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Fokina, V.M.; Patrikeeva, S.; Wang, X.M.; Noguchi, S.; Tomi, M.; König, J.; Ahmed, M.S.; Nanovskaya, T. Role of Uptake Transporters OAT4, OATP2A1, and OATP1A2 in Human Placental Bio-disposition of Pravastatin. J. Pharm. Sci. 2022, 111, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, Z.; Dong, M.; Zhu, X.; Wang, H.; Wang, Z. Alteration in Placental Expression of Bile Acids Transporters OATP1A2, OATP1B1, OATP1B3 in Intrahepatic Cholestasis of Pregnancy. Arch. Gynecol. Obstet. 2012, 285, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Reuther, S.; Meyer Zu Schwabedissen, H.; Köck, K.; Draber, K.; Ritter, C.A.; Fusch, C.; Jedlitschky, G.; Kroemer, H.K. Organic Anion Transporting Polypeptide 2B1 and Breast Cancer Resistance Protein Interact in the Transepithelial Transport of Steroid Sulfates in Human Placenta. Drug Metab. Dispos. 2007, 35, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sata, R.; Ohtani, H.; Tsujimoto, M.; Murakami, H.; Koyabu, N.; Nakamura, T.; Uchiumi, T.; Kuwano, M.; Nagata, H.; Tsukimori, K.; et al. Functional Analysis of Organic Cation Transporter 3 Expressed in Human Placenta. J. Pharmacol. Exp. Ther. 2005, 315, 888–895. [Google Scholar] [CrossRef]
- Grube, M.; Meyer Zu Schwabedissen, H.; Draber, K.; Präger, D.; Möritz, K.U.; Linnemann, K.; Fusch, C.; Jedlitschky, G.; Kroemer, H.K. Expression, Localization, and Function of the Carnitine Transporter Octn2 (Slc22a5) in Human Placenta. Drug Metab. Dispos. 2005, 33, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rytting, E.; Audus, K.L. Novel Organic Cation Transporter 2-Mediated Carnitine Uptake in Placental Choriocarcinoma (BeWo) Cells. J. Pharmacol. Exp. Ther. 2005, 312, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Nishimura, T.; Fujibayashi, A.; Maruyama, T.; Tomi, M.; Nakashima, E. Organic Anion Transporter 4-Mediated Transport of Olmesartan at Basal Plasma Membrane of Human Placental Barrier. J. Pharm. Sci. 2015, 104, 3128–3135. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, R.; Bakken, A.H.; Hudkins, K.L.; Lai, Y.; Casado, F.J.; Pastor-Anglada, M.; Tse, C.M.; Hayashi, J.; Unadkat, J.D. In Situ Hybridization and Immunolocalization of Concentrative and Equilibrative Nucleoside Transporters in the Human Intestine, Liver, Kidneys, and Placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1809–R1822. [Google Scholar] [CrossRef]
- Griffiths, M.; Yao, S.Y.; Abidi, F.; Phillips, S.E.; Cass, C.E.; Young, J.D.; Baldwin, S.A. Molecular Cloning and Characterization of a Nitrobenzylthioinosine-Insensitive (Ei) Equilibrative Nucleoside Transporter from Human Placenta. Biochem. J. 1997, 328 Pt 3, 739–743. [Google Scholar] [CrossRef]
- Landor, M.; Rubinstein, A.; Kim, A.; Calvelli, T.; Mizrachi, Y. Receptor-Mediated Maternofetal Transfer of Immunoglobulins. Inhibition of Transport of Anti-HIV-1 Immunoglobulin by Generic Immunoglobulins in the In Vitro Perfused Placenta. Int. Arch. Allergy Immunol. 1998, 115, 203–209. [Google Scholar] [CrossRef]
- Ho, R.H.; Kim, R.B. Transporters and Drug Therapy: Implications for Drug Disposition and Disease. Clin. Pharmacol. Ther. 2005, 78, 260–277. [Google Scholar] [CrossRef]
- Petzinger, E.; Geyer, J. Drug Transporters in Pharmacokinetics. Naunyn Schmiedeberg’s Arch. Pharmacol. 2006, 372, 465–475. [Google Scholar] [CrossRef]
- Mölsä, M.; Heikkinen, T.; Hakkola, J.; Hakala, K.; Wallerman, O.; Wadelius, M.; Wadelius, C.; Laine, K. Functional Role of P-Glycoprotein in the Human Blood-Placental Barrier. Clin. Pharmacol. Ther. 2005, 78, 123–131. [Google Scholar] [CrossRef]
- Imperio, G.E.; Javam, M.; Lye, P.; Constantinof, A.; Dunk, C.E.; Reis, F.M.; Lye, S.J.; Gibb, W.; Matthews, S.G.; Ortiga-Carvalho, T.M.; et al. Gestational Age-Dependent Gene Expression Profiling of ATP-Binding Cassette Transporters in the Healthy Human Placenta. J. Cell. Mol. Med. 2019, 23, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Manautou, J.E. Regulation of Hepatic ABCC Transporters by Xenobiotics and in Disease States. Drug Metab. Rev. 2010, 42, 482–538. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, H.; Sugimoto, Y.; Sugiyama, Y. ABCG2 Transports Sulfated Conjugates of Steroids and Xenobiotics. J. Biol. Chem. 2003, 278, 22644–22649. [Google Scholar] [CrossRef]
- Blazquez, A.G.; Briz, O.; Romero, M.R.; Rosales, R.; Monte, M.J.; Vaquero, J.; Macias, R.I.; Cassio, D.; Marin, J.J. Characterization of the Role of ABCG2 as a Bile Acid Transporter in Liver and Placenta. Mol. Pharmacol. 2012, 81, 273–283. [Google Scholar] [CrossRef]
- Liu, X. SLC Family Transporters. Adv. Exp. Med. Biol. 2019, 1141, 101–202. [Google Scholar] [CrossRef]
- Nigam, S.K. The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 663–687. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Dai, B.; Bai, M.; Lu, S.; Lin, N.; Zhou, H.; Jiang, H. Bilirubin Reduces the Uptake of Estrogen Precursors and the Followed Synthesis of Estradiol in Human Placental Syncytiotrophoblasts via Inhibition and Downregulation of Organic Anion Transporter 4. Drug Metab. Dispos. 2022, 50, 341–350. [Google Scholar] [CrossRef]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A Guide to Plasma Membrane Solute Carrier Proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef]
- Young, J.D.; Yao, S.Y.; Baldwin, J.M.; Cass, C.E.; Baldwin, S.A. The Human Concentrative and Equilibrative Nucleoside Transporter Families, SLC28 and SLC29. Mol. Asp. Med. 2013, 34, 529–547. [Google Scholar] [CrossRef]
- Ahmadimoghaddam, D.; Zemankova, L.; Nachtigal, P.; Dolezelova, E.; Neumanova, Z.; Cerveny, L.; Ceckova, M.; Kacerovský, M.; Micuda, S.; Staud, F. Organic Cation Transporter 3 (OCT3/SLC22A3) and Multidrug and Toxin Extrusion 1 (MATE1/SLC47A1) Transporter in the Placenta and Fetal Tissues: Expression Profile and Fetus Protective Role at Different Stages of Gestation. Biol. Reprod. 2013, 88, 55. [Google Scholar] [CrossRef]
- Tegenge, M.A.; Mahmood, I.; Struble, E.B.; Sauna, Z. Pharmacokinetics of Antibodies during Pregnancy: General Pharmacokinetics and Pregnancy Related Physiological Changes (Part 1). Int. Immunopharmacol. 2023, 117, 109914. [Google Scholar] [CrossRef]
- Ellinger, I.; Schwab, M.; Stefanescu, A.; Hunziker, W.; Fuchs, R. IgG Transport across Trophoblast-Derived BeWo Cells: A Model System to Study IgG Transport in the Placenta. Eur. J. Immunol. 1999, 29, 733–744. [Google Scholar] [CrossRef]
- Einarsdottir, H.K.; Stapleton, N.M.; Scherjon, S.; Andersen, J.T.; Rispens, T.; van der Schoot, C.E.; Vidarsson, G. On the Perplexingly Low Rate of Transport of IgG2 across the Human Placenta. PLoS ONE 2014, 9, e108319. [Google Scholar] [CrossRef]
- Seow, C.H.; Leung, Y.; Vande Casteele, N.; Ehteshami Afshar, E.; Tanyingoh, D.; Bindra, G.; Stewart, M.J.; Beck, P.L.; Kaplan, G.G.; Ghosh, S.; et al. The Effects of Pregnancy on the Pharmacokinetics of Infliximab and Adalimumab in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2017, 45, 1329–1338. [Google Scholar] [CrossRef]
- Paik, M.K.; Hwang, B.D.; Lim, K. Human Placenta S-Adenosylmethionine: Protein Carboxyl O-Methyltransferase (Protein Methylase II). Purification and Characterization. Int. J. Biochem. 1988, 20, 1107–1112. [Google Scholar] [CrossRef]
- Zhu, B.T.; Wu, K.Y.; Wang, P.; Cai, M.X.; Conney, A.H. O-methylation of Catechol Estrogens by Human Placental Catechol-O-Methyltransferase: Interindividual Differences in Sensitivity to Heat Inactivation and to Inhibition by Dietary Polyphenols. Drug Metab. Dispos. 2010, 38, 1892–1899. [Google Scholar] [CrossRef]
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II Drug Metabolizing Enzymes. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2010, 154, 103–116. [Google Scholar] [CrossRef]
- Datta, K.; Roy, S.K.; Mitra, A.K.; Kulkarni, A.P. Glutathione S-Transferase Mediated Detoxification and Bioactivation of Xenobiotics during Early Human Pregnancy. Early Hum. Dev. 1994, 37, 167–174. [Google Scholar] [CrossRef]
- Hayes, J.D.; Strange, R.C. Glutathione S-Transferase Polymorphisms and Their Biological Consequences. Pharmacology 2000, 61, 154–166. [Google Scholar] [CrossRef]
- Derewlany, L.O.; Knie, B.; Koren, G. Arylamine N-Acetyltransferase Activity of the Human Placenta. J. Pharmacol. Exp. Ther. 1994, 269, 756–760. [Google Scholar]
- Minchin, R.F.; Hanna, P.E.; Dupret, J.M.; Wagner, C.R.; Rodrigues-Lima, F.; Butcher, N.J. Arylamine N-Acetyltransferase I. Int. J. Biochem. Cell Biol. 2007, 39, 1999–2005. [Google Scholar] [CrossRef]
- Bernier, F.; Lopez Solache, I.; Labrie, F.; Luu-The, V. Cloning and Expression of cDNA Encoding Human Placental Estrogen Sulfotransferase. Mol. Cell. Endocrinol. 1994, 99, R11–R15. [Google Scholar] [CrossRef]
- Kiang, T.K.; Ensom, M.H.; Chang, T.K. UDP-Glucuronosyltransferases and Clinical Drug-Drug Interactions. Pharmacol. Ther. 2005, 106, 97–132. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.C.; Ganley, N.A.; Tingle, M.D.; Blumenstein, M.; Marvin, K.W.; Paxton, J.W.; Mitchell, M.D.; Keelan, J.A. UDP-Glucuronosyltransferase Activity, Expression and Cellular Localization in Human Placenta at Term. Biochem. Pharmacol. 2002, 63, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Office on Women’s Health. Stages of Pregnancy. Available online: https://www.womenshealth.gov/pregnancy/youre-pregnant-now-what/stages-pregnancy# (accessed on 1 June 2023).
- Chen, H.; Yang, K.; Choi, S.; Fischer, J.H.; Jeong, H. Up-regulation of UDP-Glucuronosyltransferase (UGT) 1A4 by 17Beta-Estradiol: A Potential Mechanism of Increased Lamotrigine Elimination in Pregnancy. Drug Metab. Dispos. 2009, 37, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.D.; Lee, I.J.; Voulalas, P.J.; Eddington, N.D. Estradiol and Progesterone-Mediated Regulation of P-gp in P-gp Overexpressing Cells (NCI-ADR-RES) and Placental Cells (JAR). Mol. Pharm. 2009, 6, 1816–1825. [Google Scholar] [CrossRef]
- Song, X.; Vasilenko, A.; Chen, Y.; Valanejad, L.; Verma, R.; Yan, B.; Deng, R. Transcriptional Dynamics of Bile Salt Export Pump during Pregnancy: Mechanisms and Implications in Intrahepatic Cholestasis of Pregnancy. Hepatology 2014, 60, 1993–2007. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.Z.; Zhang, X.W.; Ding, Y.L.; Yang, M.Y. miR-148a-Mediated Estrogen-Induced Cholestasis in Intrahepatic Cholestasis of Pregnancy: Role of PXR/MRP3. PLoS ONE 2017, 12, e0178702. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Gupta, A.; Vethanayagam, R.R.; Zhang, Y.; Unadkat, J.D.; Mao, Q. Regulation of BCRP/ABCG2 Expression by Progesterone and 17Beta-Estradiol in Human Placental BeWo Cells. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E798–E807. [Google Scholar] [CrossRef]
- Wang, H.; Lee, E.W.; Zhou, L.; Leung, P.C.; Ross, D.D.; Unadkat, J.D.; Mao, Q. Progesterone Receptor (PR) Isoforms PRA and PRB Differentially Regulate Expression of the Breast Cancer Resistance Protein in Human Placental Choriocarcinoma BeWo Cells. Mol. Pharmacol. 2008, 73, 845–854. [Google Scholar] [CrossRef]
- Zhou, F.; Hong, M.; You, G. Regulation of Human Organic Anion Transporter 4 by Progesterone and Protein Kinase C in Human Placental BeWo Cells. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E57–E61. [Google Scholar] [CrossRef]
- Ma, Z.; Lu, S.; Sun, D.; Bai, M.; Jiang, T.; Lin, N.; Zhou, H.; Zeng, S.; Jiang, H. Roles of Organic Anion Transporter 2 and Equilibrative Nucleoside Transporter 1 in Hepatic Disposition and Antiviral Activity of Entecavir during Non-Pregnancy and Pregnancy. Br. J. Pharmacol. 2019, 176, 3236–3249. [Google Scholar] [CrossRef]
- Zusterzeel, P.L.; Knapen, M.F.; Roes, E.M.; Steegers-Theunissen, R.P.; Peters, W.H.; Merkus, H.M.; Steegers, E.A. Glutathione S-Transferase Alpha Levels in Epileptic and Healthy Women Preconceptionally and Throughout Pregnancy. Gynecol. Obstet. Investig. 1999, 48, 89–92. [Google Scholar] [CrossRef]
- Knapen, M.F.; Mulder, T.P.; Bisseling, J.G.; Penders, R.H.; Peters, W.H.; Steegers, E.A. Plasma Glutathione S-Transferase Alpha 1-1: A More Sensitive Marker for Hepatocellular Damage than Serum Alanine Aminotransferase in Hypertensive Disorders of Pregnancy. Am. J. Obstet. Gynecol. 1998, 178, 161–165. [Google Scholar] [CrossRef]
- Han, L.W.; Ryu, R.J.; Cusumano, M.; Easterling, T.R.; Phillips, B.R.; Risler, L.J.; Shen, D.D.; Hebert, M.F. Effect of N-Acetyltransferase 2 Genotype on the Pharmacokinetics of Hydralazine during Pregnancy. J. Clin. Pharmacol. 2019, 59, 1678–1689. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Kotegawa, T.; Matsuki, S.; Tanaka, Y.; Ishii, Y.; Kodama, Y.; Kuranari, M.; Miyakawa, I.; Nakano, S. The Effect of Pregnancy on Cytochrome P4501A2, Xanthine Oxidase, and N-Acetyltransferase Activities in Humans. Clin. Pharmacol. Ther. 2001, 70, 121–125. [Google Scholar] [CrossRef]
- Zhang, H.; Bastian, J.R.; Zhao, W.; Chen, H.; Shaik, I.H.; Chaphekar, N.; Caritis, S.N.; Venkataramanan, R. Pregnancy Alters CYP- and UGT-Mediated Metabolism of Buprenorphine. Ther. Drug Monit. 2020, 42, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Hebert, M.F.; Easterling, T.R.; Kirby, B.; Carr, D.B.; Buchanan, M.L.; Rutherford, T.; Thummel, K.E.; Fishbein, D.P.; Unadkat, J.D. Effects of Pregnancy on CYP3A and P-Glycoprotein Activities as Measured by Disposition of Midazolam and Digoxin: A University of Washington Specialized Center of Research Study. Clin. Pharmacol. Ther. 2008, 84, 248–253. [Google Scholar] [CrossRef]
- de Lima Moreira, F.; Melli, P.; Marques, M.P.; Rocha, A.; Nardotto, G.H.B.; Duarte, G.; Lanchote, V.L. P-Glycoprotein and Organic Anion Transporter Polypeptide 1B/Breast Cancer Resistance Protein Drug Transporter Activity in Pregnant Women Living with HIV. J. Clin. Pharmacol. 2023, 63, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.; Saura, R.; Forestier, F.; Farinotti, R. P-Glycoprotein Expression of the Human Placenta during Pregnancy. Placenta 2005, 26, 268–270. [Google Scholar] [CrossRef]
- Sun, M.; Kingdom, J.; Baczyk, D.; Lye, S.J.; Matthews, S.G.; Gibb, W. Expression of the Multidrug Resistance P-Glycoprotein, (ABCB1 Glycoprotein) in the Human Placenta Decreases with Advancing Gestation. Placenta 2006, 27, 602–609. [Google Scholar] [CrossRef]
- Anoshchenko, O.; Prasad, B.; Neradugomma, N.K.; Wang, J.; Mao, Q.; Unadkat, J.D. Gestational Age-Dependent Abundance of Human Placental Transporters as Determined by Quantitative Targeted Proteomics. Drug Metab. Dispos. 2020, 48, 735–741. [Google Scholar] [CrossRef]
- Scott, H.; Martinelli, L.M.; Grynspan, D.; Bloise, E.; Connor, K.L. Preterm Birth Associates with Increased Placental Expression of MDR Transporters Irrespective of Prepregnancy BMI. J. Clin. Endocrinol. Metab. 2022, 107, 1140–1158. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Weerasekera, N.; Hitchins, M.; Boyd, C.A.; Johnston, D.G.; Williamson, C. Semi Quantitative Expression Analysis of MDR3, FIC1, BSEP, OATP-A, OATP-C, OATP-D, OATP-E and NTCP Gene Transcripts in 1st and 3rd Trimester Human Placenta. Placenta 2003, 24, 39–44. [Google Scholar] [CrossRef]
- Petrovic, V.; Kojovic, D.; Cressman, A.; Piquette-Miller, M. Maternal Bacterial Infections Impact Expression of Drug Transporters in Human Placenta. Int. Immunopharmacol. 2015, 26, 349–356. [Google Scholar] [CrossRef]
- Yeboah, D.; Sun, M.; Kingdom, J.; Baczyk, D.; Lye, S.J.; Matthews, S.G.; Gibb, W. Expression of Breast Cancer Resistance Protein (BCRP/ABCG2) in Human Placenta Throughout Gestation and at Term before and after Labor. Can. J. Physiol. Pharmacol. 2006, 84, 1251–1258. [Google Scholar] [CrossRef]
- Sieppi, E.; Vähäkangas, K.; Rautio, A.; Ietta, F.; Paulesu, L.; Myllynen, P. The Xenoestrogens, Bisphenol A and Para-Nonylphenol, Decrease the Expression of the ABCG2 Transporter Protein in Human Term Placental Explant Cultures. Mol. Cell. Endocrinol. 2016, 429, 41–49. [Google Scholar] [CrossRef]
- Nabekura, T.; Kawasaki, T.; Kamiya, Y.; Uwai, Y. Effects of Antiviral Drugs on Organic Anion Transport in Human Placental BeWo Cells. Antimicrob. Agents Chemother. 2015, 59, 7666–7670. [Google Scholar] [CrossRef] [PubMed]
- Bergagnini-Kolev, M.C.; Hebert, M.F.; Easterling, T.R.; Lin, Y.S. Pregnancy Increases the Renal Secretion of N(1)-methylnicotinamide, an Endogenous Probe for Renal Cation Transporters, in Patients Prescribed Metformin. Drug Metab. Dispos. 2017, 45, 325–329. [Google Scholar] [CrossRef]
- Lee, N.; Hebert, M.F.; Prasad, B.; Easterling, T.R.; Kelly, E.J.; Unadkat, J.D.; Wang, J. Effect of Gestational Age on mRNA and Protein Expression of Polyspecific Organic Cation Transporters during Pregnancy. Drug Metab. Dispos. 2013, 41, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ladumor, M.K.; Unadkat, J.D. Prediction of Pregnancy-Induced Changes in Secretory and Total Renal Clearance of Drugs Transported by Organic Anion Transporters. Drug Metab. Dispos. 2021, 49, 929–937. [Google Scholar] [CrossRef]
- Jiraskova, L.; Cerveny, L.; Karbanova, S.; Ptackova, Z.; Staud, F. Expression of Concentrative Nucleoside Transporters (SLC28A) in the Human Placenta: Effects of Gestation Age and Prototype Differentiation-Affecting Agents. Mol. Pharm. 2018, 15, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Cerveny, L.; Ptackova, Z.; Ceckova, M.; Karahoda, R.; Karbanova, S.; Jiraskova, L.; Greenwood, S.L.; Glazier, J.D.; Staud, F. Equilibrative Nucleoside Transporter 1 (ENT1, SLC29A1) Facilitates Transfer of the Antiretroviral Drug Abacavir across the Placenta. Drug Metab. Dispos. 2018, 46, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Palfi, M.; Selbing, A. Placental Transport of Maternal Immunoglobulin G. Am. J. Reprod. Immunol. 1998, 39, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.E.; Barman, S.M.; Brooks, H.L.; Yuan, J.J. Reproductive Development & Function of the Female Reproductive System. In Ganong’s Review of Medical Physiology; McGraw Hill: New York, NY, USA, 2019. [Google Scholar]
- Watts, D.H.; Stek, A.; Best, B.M.; Wang, J.; Capparelli, E.V.; Cressey, T.R.; Aweeka, F.; Lizak, P.; Kreitchmann, R.; Burchett, S.K.; et al. Raltegravir Pharmacokinetics during Pregnancy. J. Acquir. Immune Defic. Syndr. 2014, 67, 375–381. [Google Scholar] [CrossRef]
- Berezowska, M.; Sharma, P.; Reddy, V.P.; Coppola, P. Minireview. 2023; submitted. [Google Scholar]
- van Hoogdalem, M.W.; Wexelblatt, S.L.; Akinbi, H.T.; Vinks, A.A.; Mizuno, T. A Review of Pregnancy-Induced Changes in Opioid Pharmacokinetics, Placental Transfer, and Fetal Exposure: Towards Fetomaternal Physiologically-Based Pharmacokinetic Modeling to Improve the Treatment of Neonatal Opioid Withdrawal Syndrome. Pharmacol. Ther. 2022, 234, 108045. [Google Scholar] [CrossRef]
- Kunze, A.; Huwyler, J.; Camenisch, G.; Poller, B. Prediction of Organic Anion-Transporting Polypeptide 1B1- and 1B3-Mediated Hepatic Uptake of Statins Based on Transporter Protein Expression and Activity Data. Drug Metab. Dispos. 2014, 42, 1514–1521. [Google Scholar] [CrossRef]
- Kitamura, S.; Maeda, K.; Wang, Y.; Sugiyama, Y. Involvement of Multiple Transporters in the Hepatobiliary Transport of Rosuvastatin. Drug Metab. Dispos. 2008, 36, 2014–2023. [Google Scholar] [CrossRef]
- Mathialagan, S.; Feng, B.; Rodrigues, A.D.; Varma, M.V.S. Drug-Drug Interactions Involving Renal OCT2/MATE Transporters: Clinical Risk Assessment May Require Endogenous Biomarker-Informed Approach. Clin. Pharmacol. Ther. 2021, 110, 855–859. [Google Scholar] [CrossRef]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific Organic Cation Transporters: Structure, Function, Physiological Roles, and Biopharmaceutical Implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.Z.; Flood Nichols, S.K.; Ahmed, M.; Clark, S.; Hankins, G.D.; Caritis, S.; Venkataramanan, R.; Haas, D.; Quinney, S.K.; Haneline, L.S.; et al. Effects of Pregnancy on the Pharmacokinetics of Metformin. Drug Metab. Dispos. 2020, 48, 264–271. [Google Scholar] [CrossRef] [PubMed]


| pregnancy | MRP2 | OCT3 | glucuronosyltransferase |
| pregnant | MRP3 | OCTN1 | UGT |
| placenta | MRP4 | OCTN2 | UGT1A1 |
| placental | MRP5 | OAT1 | UGT1A4 |
| transporter(s) | MRP6 | OAT2 | UGT1A9 |
| transport | breast cancer resistance protein | OAT3 | UGT2B4 |
| P-glycoprotein | BCRP | CNT1 | UGT2B7 |
| P-gp | OATP1B1 | ENT1 | UGT2B15 |
| MDR1 | OATP1B3 | ENT2 | sulfotransferase |
| MDR3 | OATP2B1 | MATE1 | N-acetyltransferase |
| bile salt export pump | OATP4A1 | MATE2 | S-transferase |
| BSEP | OCT1 | PEPT1 | methyltransferase |
| MRP1 | OCT2 | PEPT2 |
| nutrient | folate | sodium |
| mineral | metal | sulfate |
| amino acid(s) | cadmium | sulphate |
| taurine | calcium | zinc |
| creatine | chloride | choline |
| glucose | chromium | norepinephrine |
| fatty acid(s) | copper | serotonin |
| lipid | iron | monoamine |
| cholesterol | magnesium | thyroid |
| vitamin | phosphate | polymorphism(s) |
| thiamine | potassium | |
| riboflavin | selenium |
| Effect of 17β-Estradiol | Effect of Progesterone | |||||||
|---|---|---|---|---|---|---|---|---|
| Enzyme or Transporter | Location | mRNA | Protein | Activity | mRNA | Protein | Activity | Citations |
| Phase II Enzyme | ||||||||
| UGT1A4 | liver | ↑ | - | ↑ | - | - | - | [66] |
| Drug Transporter | ||||||||
| P-gp | placenta | ↑ | ↑ | ↑ | ↔ | ↑ | ↓ | [18,21,67] |
| MDR3 | placenta | ↑ | ↑ | - | - | - | - | [21] |
| BSEP | liver | ↓ | ↓ | - | - | - | - | [68] |
| MRP1 | placenta | - | - | substrate | ↑ | ↔ | - | [21,24] |
| MRP3 | liver | ↑? | ↑? | - | - | - | - | [69] |
| BCRP | placenta | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ | [21,70,71] |
| OAT4 | placenta | - | - | ↔? | - | - | ↔? | [72] |
| ENT1 | liver | - | - | ↓? | - | - | ↓? | [73] |
| Expression or Activity in the Pregnant Woman | Expression or Activity in the Placenta | |||||||
|---|---|---|---|---|---|---|---|---|
| Enzyme | Trimester 1 | Trimester 2 | Trimester 3 | Postpartum | Trimester 1 | Trimester 2 | Trimester 3 | Citations |
| CMT | - | - | - | - | ↑ | peak activity | ↓ | [54] |
| GSTA | ↔ | ↔ | ↔ | ↑ | ↓ | - | - | [57,74,75] |
| GST-μ | - | - | - | - | ↔ | - | - | [57] |
| GST-π | - | - | - | - | ↔ | - | - | [57] |
| NAT1 | - | - | - | - | - | - | ↑ | [59] |
| NAT2 | ↓ | ↑ to baseline | ↔ | ↔ | - | - | - | [76,77] |
| UGT1A1 * | ↑ | ↑ | peak activity | ↓ to baseline | - | - | - | [78] |
| UGT1A3 * | ↑ | ↑ | peak activity | ↓ to baseline | - | - | - | [78] |
| UGT1A4 | ↑? | ↑? | ↑? | - | - | - | - | [66] |
| UGT2B7 * | ↑ | ↑ | peak activity | ↓ to baseline | - | - | - | [78] |
| Expression or Activity in the Pregnant Woman | Expression or Activity in the Placenta | |||||||
|---|---|---|---|---|---|---|---|---|
| Transporter | Trimester 1 (T1) | Trimester 2 (T2) | Trimester 3 (T3) | Postpartum | Trimester 1 (T1) | Trimester 2 (T2) | Trimester 3 (T3) | Citations |
| P-gp | - | - | - | ↓ to baseline (renal) ↔ (intestinal) | peak activity | ↓ ↔ | ↓ | [79,80,81,82,83,84] |
| MDR3 | - | - | - | - | - | - | ↓ ↑ from T1 | [40,85] |
| BSEP | ↓? | ↓? | ↓? | - | - | - | ↓ from T1 | [40,68,85] |
| MRP1 | - | - | - | - | ↔? | ↔? | ↔? | [21] |
| MRP2 | - | - | - | - | - | ↑ | ↓ ↑ | [22,40] |
| MRP3 | ↑? | ↑? | ↑? | - | - | - | ↑ from T1 | [40,69] |
| MRP5 | - | - | - | - | - | ↓ during T2 | ↓ | [25] |
| BCRP * | - | - | ↓ | ↑ to baseline | ↔ | ↔ | ↓ ↑ | [80,84,86,87,88] |
| OATP1A2 | - | - | - | - | - | - | ↓ from T1 | [85] |
| OATP1B1 * | - | - | ↓ | ↑ to baseline | - | - | - | [80] |
| OATP1B3 * | - | - | ↓ | ↑ to baseline | - | - | - | [80] |
| OATP2B1 | - | - | - | - | - | ↓ | ↓ ↑ | [83,86,89] |
| OATP3A1 | - | - | - | - | - | - | ↓ from T1 | [85] |
| OCT2 ^ | - | ↑ | ↓ ↑ | ↓ | - | - | ↓ from T1 | [49,90] |
| OCT3 | - | - | - | - | - | ↑ | ↓ ↑ from T1 | [49,83,91] |
| OCTN2 | - | - | - | - | - | - | ↔ during T3 | [31] |
| OAT1 | - | peak activity | ↓ | ↓ to baseline | - | - | - | [92] |
| OAT2 | - | - | peak activity | ↓ to baseline | - | - | - | [92] |
| OAT3 | peak activity | ↓ | ↓ | ↓ to baseline | - | - | - | [92] |
| OAT4 | - | - | - | - | - | - | ↑ | [83] |
| CNT2 | - | - | - | - | - | - | ↑ from T1 | [93] |
| CNT3 | - | - | - | - | - | - | ↑ from T1 | [93] |
| ENT1 | ↓? | ↓? | ↓? | - | - | - | ↔ from T1 | [94] |
| ENT2 | - | - | - | - | - | - | ↔ from T1 | [94] |
| MATE1 ^ | - | ↑ | ↓ ↑ | ↓ | - | - | - | [90] |
| MATE2 ^ | - | ↑ | ↓ ↑ | ↓ | - | - | - | [90] |
| FcRn | - | - | - | - | - | ↑? | ↑? | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, C.; Bertagnolli, L.N.; Boulton, D.W.; Coppola, P. A Literature Review of Changes in Phase II Drug-Metabolizing Enzyme and Drug Transporter Expression during Pregnancy. Pharmaceutics 2023, 15, 2624. https://doi.org/10.3390/pharmaceutics15112624
Gong C, Bertagnolli LN, Boulton DW, Coppola P. A Literature Review of Changes in Phase II Drug-Metabolizing Enzyme and Drug Transporter Expression during Pregnancy. Pharmaceutics. 2023; 15(11):2624. https://doi.org/10.3390/pharmaceutics15112624
Chicago/Turabian StyleGong, Christine, Lynn N. Bertagnolli, David W. Boulton, and Paola Coppola. 2023. "A Literature Review of Changes in Phase II Drug-Metabolizing Enzyme and Drug Transporter Expression during Pregnancy" Pharmaceutics 15, no. 11: 2624. https://doi.org/10.3390/pharmaceutics15112624
APA StyleGong, C., Bertagnolli, L. N., Boulton, D. W., & Coppola, P. (2023). A Literature Review of Changes in Phase II Drug-Metabolizing Enzyme and Drug Transporter Expression during Pregnancy. Pharmaceutics, 15(11), 2624. https://doi.org/10.3390/pharmaceutics15112624