Nanoemulsion-Based Orodispersible Film Formulation of Guava Leaf Oil for Inhibition of Oral Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoemulsions
2.3. Droplet Size Determination
2.4. Zeta Potential Assesment
2.5. Fabrication of ODF Containing GLO Nanoemulsions
2.6. pH Measurements
2.7. Evaluation of Weight Variation
2.8. Evaluation of Thickness Uniformity
2.9. Moisture Absorption Assessment
2.10. Determination of Disintegration Time
2.11. Analysis of Mechanical Properties
2.12. Characterization of Infrared Spectroscopy
2.13. Scanning Electron Microscopy (SEM)
2.14. Quatification of β-Caryophyllene
2.15. Cytotoxicity Test
2.16. Stability Test
2.17. Colony Formation Assay
2.18. Cell Metastasis Assay
2.19. Apoptosis Assay
2.19.1. Analysis of Nuclear Fragmentation
2.19.2. Flow Cytometry Assay
2.20. Statistical Analysis
3. Results and Discussion
3.1. Droplet Size and Zeta Potential of GLO Nanoemulsions
3.2. pH
3.3. Average Weight and Film Thickness
3.4. Moisture Absorption
3.5. Disintegration Time
3.6. Mechanical Strength of the OFDs
3.7. FTIR
3.8. SEM
3.9. β-Caryophyllene Content
3.10. MTT Assay
3.11. Assessment of ODF Stability
3.12. Colony Formation Assay
3.13. Determination of Antimetastasis Activity
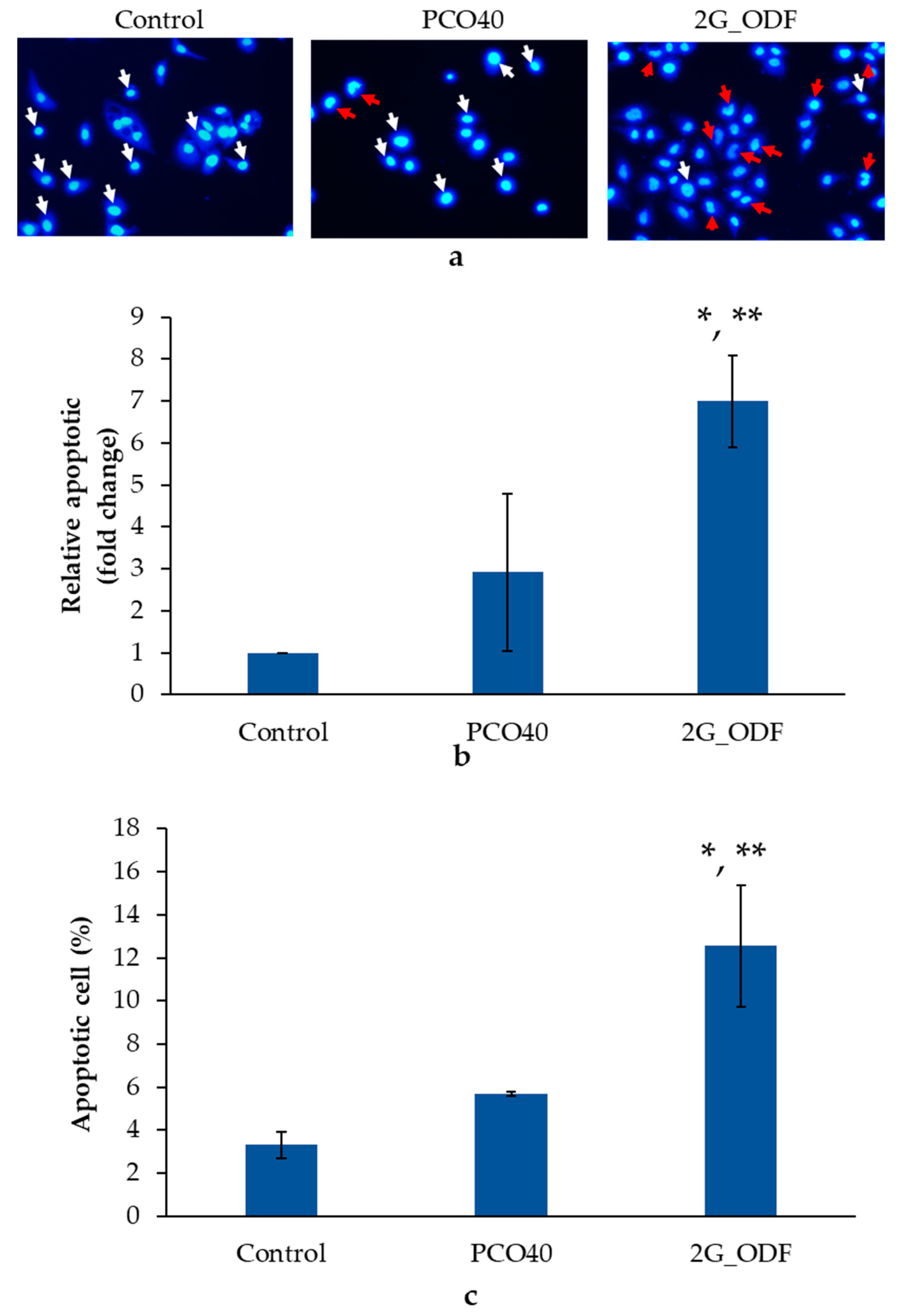
3.14. Apoptosis Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral. Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral cancer and precancer: A narrative review on the relevance of early diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef]
- Yu, M.Z.; Wu, M.M.; Chien, H.T.; Liao, C.T.; Su, M.J.; Huang, S.F.; Yeh, C.C. Risk prediction models for patients with head and neck cancer among the taiwanese population. Cancers 2022, 14, 5338. [Google Scholar] [CrossRef]
- Cardona-Mendoza, A.; Olivares-Niño, G.; Díaz-Báez, D.; Lafaurie, G.I.; Perdomo, S.J. Chemopreventive and anti-tumor potential of natural products in oral cancer. Nutr. Cancer 2022, 74, 779–795. [Google Scholar] [CrossRef]
- Lok, B.; Babu, D.; Tabana, Y.; Dahham, S.S.; Adam, M.A.A.; Barakat, K.; Sandai, D. The anticancer potential of Psidium guajava (Guava) extracts. Life 2023, 13, 346. [Google Scholar] [CrossRef]
- Jassal, K.; Kaushal, S. Phytochemical and antioxidant screening of guava (Psidium guajava) leaf essential oil. Agric. Res. J. 2019, 56, 528. [Google Scholar] [CrossRef]
- Chaturvedi, T.; Singh, S.; Nishad, I.; Kumar, A.; Tiwari, N.; Tandon, S.; Saikia, D.; Verma, R.S. Chemical composition and antimicrobial activity of the essential oil of senescent leaves of guava (Psidium guajava L.). Nat. Prod. Res. 2021, 35, 1393–1397. [Google Scholar] [CrossRef]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef]
- Mandal, A.K.; Paudel, S.; Pandey, A.; Yadav, P.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.H.; Khalilullah, H.; Verma, A. Guava leaf essential oil as a potent antioxidant and anticancer agent: Validated through experimental and computational study. Antioxidants 2022, 11, 2204. [Google Scholar] [CrossRef]
- Ramachandhiran, C.S.D.; Murali, R.; Babukumar, S.; Vinothkumar, V. β-Caryophyllene promotes oxidative stress and apoptosis in KB cells through activation of mitochondrial-mediated pathway—An in-vitro and in-silico study. Arch. Physiol. Biochem. 2019, 128, 148–162. [Google Scholar] [CrossRef]
- Ferlak, J.; Guzenda, W.; Osmałek, T. Orodispersible films—Current state of the art, limitations, advances and future perspectives. Pharmaceutics 2023, 15, 361. [Google Scholar] [CrossRef]
- Centkowska, K.; Ławrecka, E.; Sznitowska, M. Technology of orodispersible polymer films with micronized loratadine-influence of different drug loadings on film properties. Pharmaceutics 2020, 12, 250. [Google Scholar] [CrossRef]
- Cupone, I.E.; Sansone, A.; Marra, F.; Giori, A.M.; Jannini, E.A. Orodispersible Film (ODF) platform based on maltodextrin for therapeutical applications. Pharmaceutics 2022, 14, 2011. [Google Scholar] [CrossRef]
- Pacheco, M.S.; Barbieri, D.; da Silva, C.F.; de Moraes, M.A. A review on orally disintegrating films (ODFs) made from natural polymers such as pullulan, maltodextrin, starch, and others. Int. J. Biol. Macromol. 2021, 178, 504–513. [Google Scholar] [CrossRef]
- Harini, K.; Janani, K.; Teja, K.V.; Mohan, C.; Sukumar, M. Formulation and evaluation of oral disintegrating films using a natural ingredient against Streptococcus mutans. J. Conserv. Dent. 2022, 25, 128–134. [Google Scholar]
- Lordello, V.B.; Meneguin, A.B.; de Annunzio, S.R.; Taranto, M.P.; Chorilli, M.; Fontana, C.R.; Cavallini, D.C.U. Orodispersible film loaded with enterococcus faecium crl183 presents anti-candida albicans biofilm activity In Vitro. Pharmaceutics 2021, 13, 998. [Google Scholar] [CrossRef]
- Ee, S.L.; Duan, X.; Liew, J.; Nguyen, Q.D. Droplet size and stability of nano-emulsions produced by the temperature phase inversion method. J. Chem. Eng. 2008, 140, 626–631. [Google Scholar] [CrossRef]
- Wangjit, K.; Limmatvapirat, C.; Nattapulwat, N.; Sutananta, W.; Limmatvapirat, S. Factors affecting formation of nanoemulsions containing modified coconut oil and spearmint oil. Asian J. Pharm. 2016, 11, 227–228. [Google Scholar] [CrossRef]
- Kong, I.; Degraeve, P.; Pui, L.P. Polysaccharide-based edible films incorporated with essential oil nanoemulsions: Physico-chemical, mechanical properties and its application in food preservation—A Review. Foods 2022, 11, 555. [Google Scholar] [CrossRef]
- Weerapol, Y.; Manmuan, S.; Chaothanaphat, N.; Okonogi, S.; Limmatvapirat, C.; Limmatvapirat, S.; Tubtimsri, S. Impact of fixed oil on ostwald ripening of anti-oral cancer nanoemulsions loaded with Amomum kravanh essential oil. Pharmaceutics 2022, 14, 938. [Google Scholar] [CrossRef]
- Tubtimsri, S.; Limmatvapirat, C.; Limsirichaikul, S.; Akkaramongkolporn, P.; Piriyaprasarth, S.; Patomchaiviwat, V.; Limmatvapirat, S. Incorporation of fixed oils into spearmint oil-loaded nanoemulsions and their influence on characteristic and cytotoxic properties against human oral cancer cells. J. Drug Deliv. Sci. Technol. 2021, 63, 102443. [Google Scholar] [CrossRef]
- Arya, A.; Ph, A.C.; Sharma, V.; Pathak, K. Fast dissolving oral films: An innovative drug delivery system and dosage form. Int. J. Chemtech Res. 2010, 2, 576–583. [Google Scholar]
- Lei, J.; Wang, Q.; Li, G.; Li, Y.; Zhang, P.; Xu, G. β-Caryophyllene from chilli pepper inhibits the proliferation of non-small cell lung cancer cells by affecting miR-659-3p-Targeted Sphingosine Kinase 1 (SphK1). Int. J. Gen. Med. 2021, 14, 9599–9613. [Google Scholar] [CrossRef]
- Sousa, J.P.B.; Brancalion, A.P.S.; Souza, A.B.; Turatti, I.C.C.; Ambrósio, S.R.; Furtado, N.A.J.C.; Lopes, N.P.; Bastos, J.K. Validation of a gas chromatographic method to quantify sesquiterpenes in copaiba oils. J. Pharm. Biomed. Anal. 2011, 54, 653–659. [Google Scholar] [CrossRef]
- Cavanagh, R.J.; Smith, P.A.; Stolnik, S. Exposure to a nonionic surfactant induces a response akin to heat-shock apoptosis in intestinal epithelial cells: Implications for excipients safety. Mol. Pharm. 2019, 16, 618–631. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physical properties and antimicrobial efficacy of thyme oil nanoemulsions: Influence of ripening inhibitors. J. Agric. Food Chem. 2012, 60, 12056–12063. [Google Scholar] [CrossRef]
- El-Ahmady, S.H.; Ashour, M.L.; Wink, M. Chemical composition and anti-inflammatory activity of the essential oils of Psidium guajava fruits and leaves. J. Essent. Oil Res. 2013, 25, 475–481. [Google Scholar] [CrossRef]
- McClements, D.J.; Henson, L.; Popplewell, L.M.; Decker, E.A.; Choi, S.J. Inhibition of Ostwald ripening in model beverage emulsions by addition of poorly water soluble triglyceride oils. J. Food Sci. 2012, 77, C33–C38. [Google Scholar] [CrossRef]
- Loke, C.; Lee, J.; Sander, S.; Mei, L.; Farella, M. Factors affecting intra-oral pH—A review. J. Oral Rehabil. 2016, 43, 778–785. [Google Scholar] [CrossRef]
- Lussi, A.; Schlueter, N.; Rakhmatullina, E.; Ganss, C. Dental Erosion—An overview with emphasis on chemical and histopathological aspects. Caries Res. 2011, 45 (Suppl. S1), 2–12. [Google Scholar] [CrossRef]
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert. Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, C.; Xie, Y.; Mei, J.; Xie, J. Effect of Melissa officinalis L. Essential oil nanoemulsions on structure and properties of carboxymethyl chitosan/locust bean gum composite films. Membranes 2022, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Elshafeey, A.H.; El-Dahmy, R.M. Formulation and development of oral fast-dissolving films loaded with nanosuspension to augment paroxetine bioavailability: In Vitro Characterization, Ex Vivo permeation, and pharmacokinetic evaluation in healthy human volunteers. Pharmaceutics 2021, 13, 1869. [Google Scholar] [CrossRef] [PubMed]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Fasihi, H.; Fazilati, M.; Hashemi, M.; Noshirvani, N. Novel carboxymethyl cellulose-polyvinyl alcohol blend films stabilized by Pickering emulsion incorporation method. Carbohydr. Polym. 2017, 167, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Nurdianti, L.; Rusdiana, T.; Sopyan, I.; Putriana, N.A.; Aiman, H.R.; Fajria, T.R. Characteristic comparison of an intraoral thin film containing astaxanthin nanoemulsion using sodium alginate and gelatin polymers. Turk. J. Pharm. Sci. 2021, 18, 289–295. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Martins, H.H.A.; Manrich, A.; Otoni, C.G.; Carvalho, E.E.N.; Piccoli, R.H.; Oliveira, J.E.; Pinheiro, A.C.M.; Mattoso, L.H.C. Correlating emulsion characteristics with the properties of active starch films loaded with lemongrass essential oil. Food Hydrocoll. 2020, 100, 105428. [Google Scholar] [CrossRef]
- Zúñiga, R.N.; Skurtys, O.; Osorio, F.; Aguilera, J.M.; Pedreschi, F. Physical properties of emulsion-based hydroxypropyl methylcellulose films: Effect of their microstructure. Carbohydr. Polym. 2012, 90, 1147–1158. [Google Scholar] [CrossRef]
- Saranti, T.F.D.S.; Melo, P.T.S.; Cerqueira, M.A.; Aouada, F.A.; de Moura, M.R. Performance of gelatin films reinforced with cloisite Na+ and black pepper essential oil loaded nanoemulsion. Polymers 2021, 13, 4298. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B 2019, 177, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.A.Q.; Debeaufort, F.; Callegarin, F.; Voilley, A. Lipid hydrophobicity, physical state and distribution effects on the properties of emulsion-based edible films. J. Membr. Sci. 2000, 180, 37–46. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Y. Physicochemical and antioxidant properties based on fish sarcoplasmic protein/chitosan composite films containing ginger essential oil nanoemulsion. Food Bioprocess Tech. 2021, 14, 151–163. [Google Scholar] [CrossRef]
- Cupone, I.E.; Dellera, E.; Marra, F.; Giori, A.M. Development and characterization of an orodispersible film for vitamin D3 supplementation. Molecules 2020, 25, 5851. [Google Scholar] [CrossRef] [PubMed]
- Canga, I.; Vita, P.; Oliveira, A.I.; Castro, M.Á.; Pinho, C. In vitro cytotoxic activity of african plants: A review. Molecules 2022, 27, 4989. [Google Scholar] [CrossRef]
- Braga, T.; Dores, R.; Ramos, C.; Evangelista, F.C.; Tinoco, L.; Varotti, F.; Carvalho, M.; Sabino, A. Antioxidant, antibacterial and antitumor activity of ethanolic extract of the Psidium guajava leaves. Am. J. Plant Sci. 2014, 5, 3492–3500. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Holanda e silva, K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environmental. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Park, M.; Kim, D.; Ko, S.; Kim, A.; Mo, K.; Yoon, H. Breast Cancer Metastasis: Mechanisms and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 6806. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Amiel, E.; Ofir, R.; Dudai, N.; Soloway, E.; Rabinsky, T.; Rachmilevitch, S. Caryophyllene, a compound isolated from the biblical balm of gilead (Commiphora gileadensis), is a selective apoptosis inducer for tumor cell lines. Evid. Based Complement. Alternat. Med. 2012, 2012, 872394. [Google Scholar] [CrossRef]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Synergistic interaction of β-caryophyllene with aromadendrene oxide 2 and phytol induces apoptosis on skin epidermoid cancer cells. Phytomed. Int. J. Phytother. Phytopharm. 2018, 47, 121–134. [Google Scholar] [CrossRef] [PubMed]


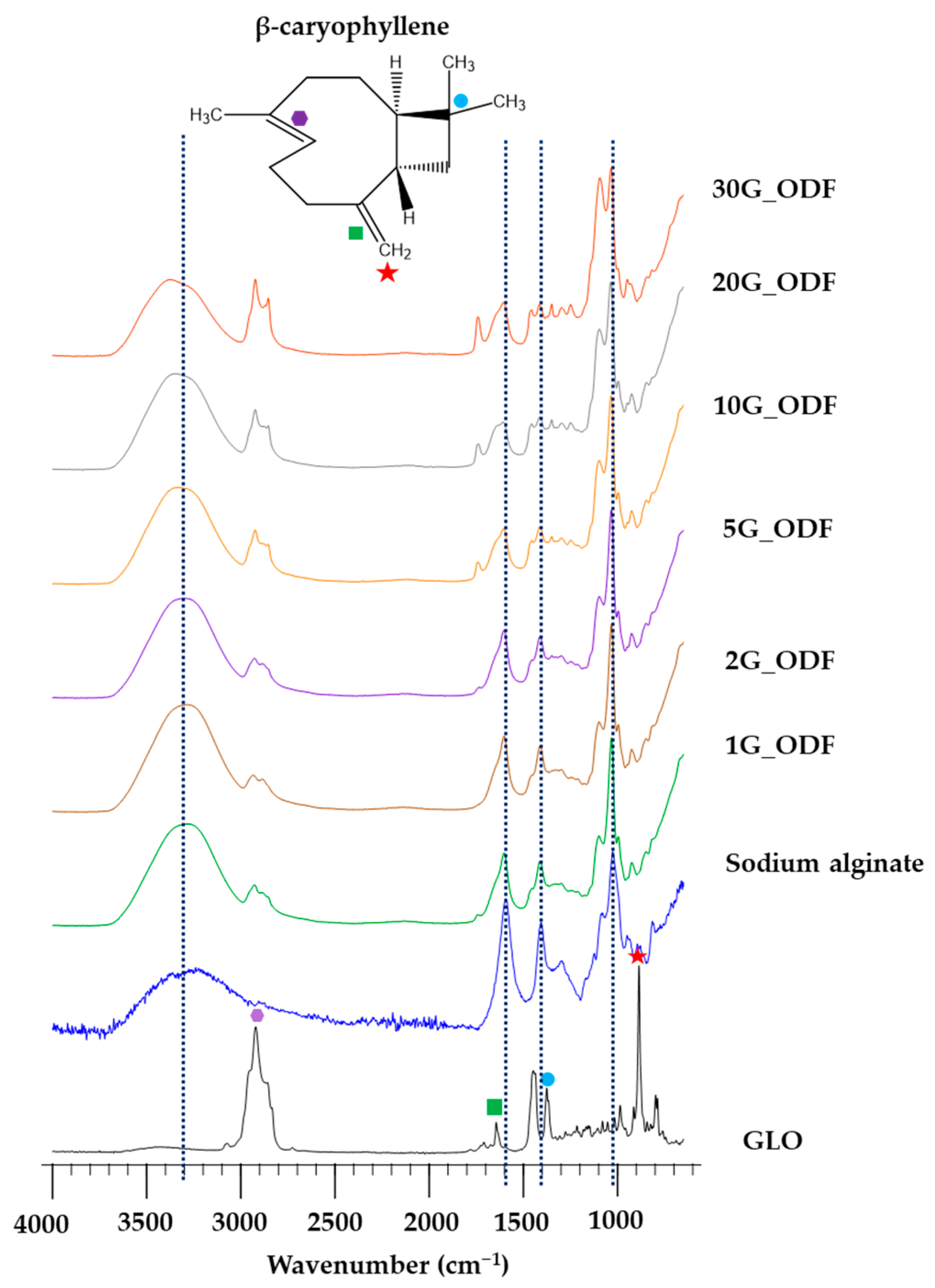



| Samples | pH | Weight (µg) | Thickness (µm) | Moisture Absorption (%) | Disintegration Time (s) |
|---|---|---|---|---|---|
| Sodium alginate film | 7.11 ± 0.03 | 6.8 ± 1.5 | 59.0 ± 9.0 | 57.56 ± 5.00 | 18.11 ± 0.60 |
| 1G_ODF | 6.24 ± 0.21 | 18.3 ± 2.8 | 41.3 ± 9.0 | 49.29 ± 6.18 | 12.75 ± 2.60 |
| 2G_ODF | 6.06 ± 0.08 | 21.4 ± 3.0 | 44.0 ± 14.0 | 29.63 ± 5.71 | 15.25 ± 1.13 |
| 5G_ODF | 5.91 ± 0.08 | 25.1 ± 3.0 | 60.7 ± 6.0 | 23.93 ± 7.69 | 17.00 ± 1.66 |
| 10G_ODF | 5.84 ± 0.03 | 29.2 ± 5.2 | 77.0 ± 13.0 | 21.11 ± 2.51 | 18.83 ± 4.22 |
| 20G_ODF | 5.61 ± 0.09 | 37.5 ± 4.4 | 82.5 ± 3.5 | 15.93 ± 5.55 | 21.35 ± 5.86 |
| 30G_ODF | 5.46 ± 0.01 | 46.5 ± 7.8 | 121.0 ± 9.0 | 10.65 ± 1.20 | 22.75 ± 4.50 |
| Sample | β-Caryophyllene Content (µg/g) |
|---|---|
| GLO | 176,950.98 ± 239.55 |
| 2G_ODF | 129.51 ± 3.58 |
| 5G_ODF | 220.82 ± 12.04 |
| 10G_ODF | 309.58 ± 15.92 |
| Samples | IC50 |
|---|---|
| β-caryophyllene | 3.73 ± 0.58 µg/mL |
| GLO | 11.65 ± 2.61 µg/mL |
| 2G_ODF | 62.49 ± 6.22 mg/mL |
| 5G_ODF | 24.11 ± 1.22 mg/mL |
| 10G_ODF | 15.29 ± 2.04 mg/mL |
| Formulation | Tensile Strength (N/mm2) | β-Caryophyllene Content (µg/g) | IC50 (mg/mL) |
|---|---|---|---|
| 2G_ODF at initial | 0.67 ± 0.15 | 129.51 ± 3.58 | 62.49 ± 6.22 |
| 2G_ODF after storage at 25 °C for 1 year | 0.60 ± 0.24 | 113.05 ± 4.45 | 65.53 ± 5.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerapol, Y.; Manmuan, S.; Chuenbarn, T.; Limmatvapirat, S.; Tubtimsri, S. Nanoemulsion-Based Orodispersible Film Formulation of Guava Leaf Oil for Inhibition of Oral Cancer Cells. Pharmaceutics 2023, 15, 2631. https://doi.org/10.3390/pharmaceutics15112631
Weerapol Y, Manmuan S, Chuenbarn T, Limmatvapirat S, Tubtimsri S. Nanoemulsion-Based Orodispersible Film Formulation of Guava Leaf Oil for Inhibition of Oral Cancer Cells. Pharmaceutics. 2023; 15(11):2631. https://doi.org/10.3390/pharmaceutics15112631
Chicago/Turabian StyleWeerapol, Yotsanan, Suwisit Manmuan, Tiraniti Chuenbarn, Sontaya Limmatvapirat, and Sukannika Tubtimsri. 2023. "Nanoemulsion-Based Orodispersible Film Formulation of Guava Leaf Oil for Inhibition of Oral Cancer Cells" Pharmaceutics 15, no. 11: 2631. https://doi.org/10.3390/pharmaceutics15112631





