Interaction of Graphene Oxide Particles and Dendrimers with Human Breast Cancer Cells by Real-Time Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. MCF-7 Cell Culture
2.3. Generation of MCF-7 Cell Spheroids
2.4. Treatment with Graphene Oxide and Dendrimers
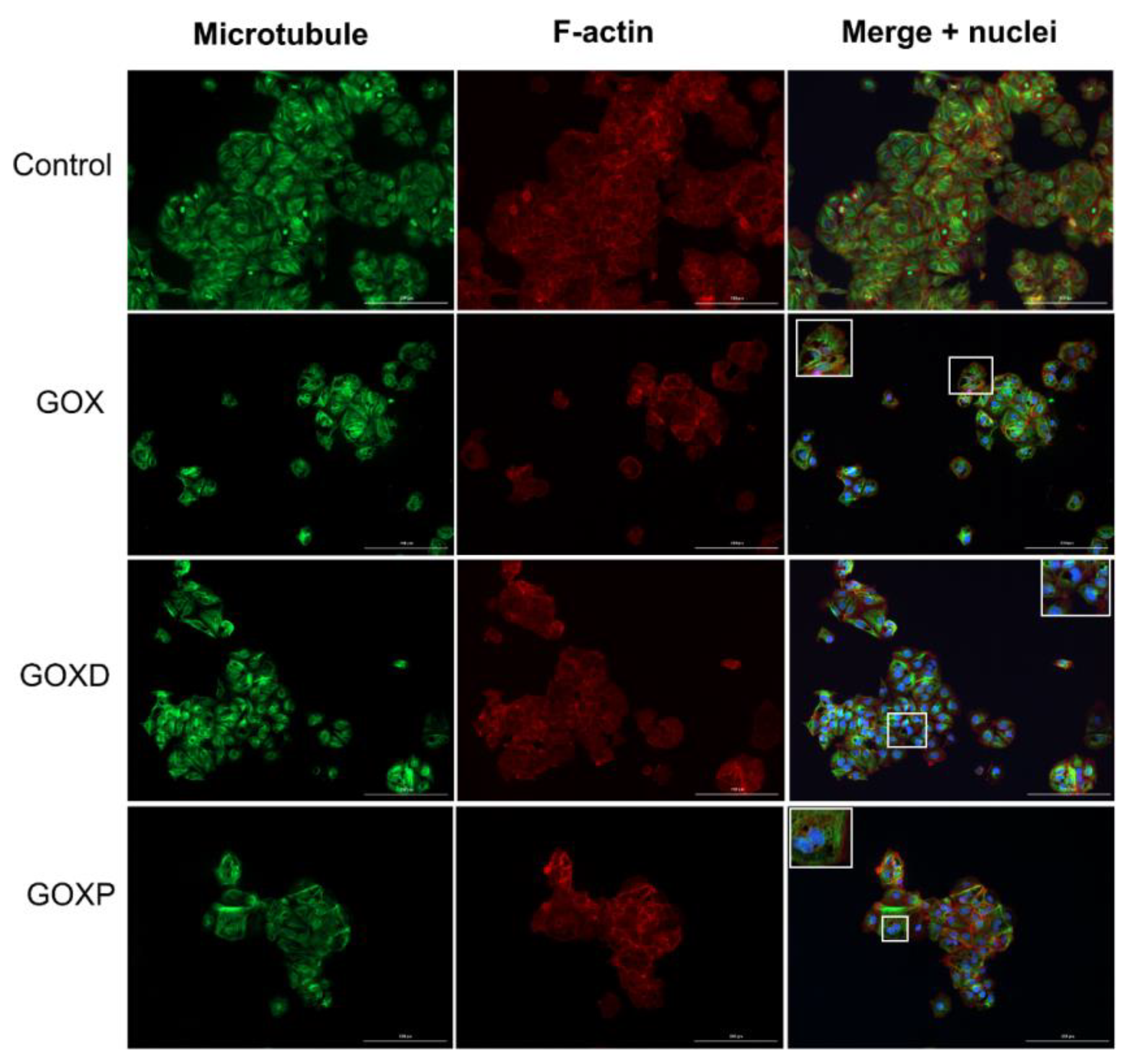
2.5. Immunofluorescence
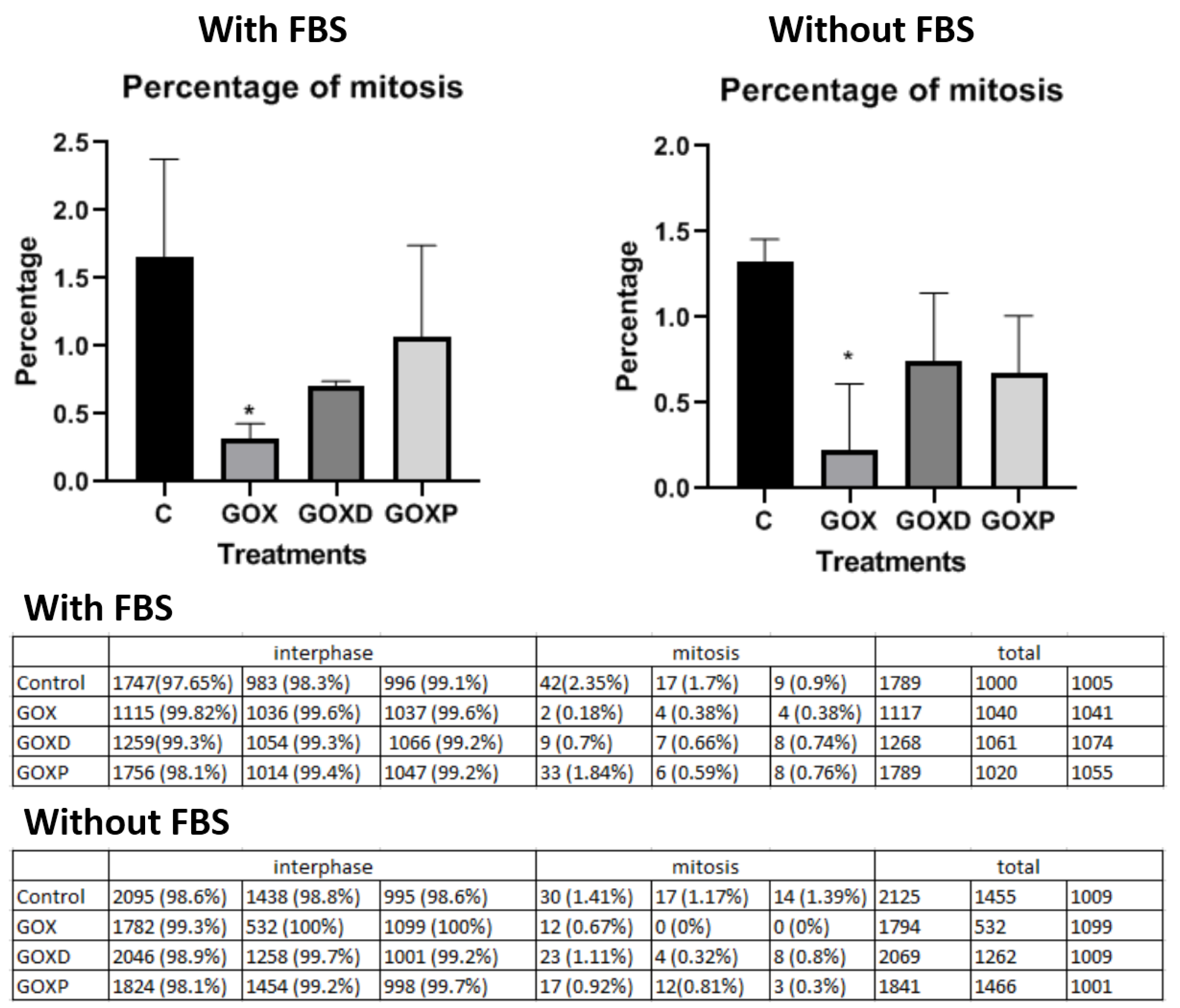
2.6. Mitosis Quantification
2.7. Real-Time Microscopy Cell Culture Monolayer and 3D Spheroids
2.8. Cellular Exposition to Fluorescent Graphene in the Presence of Internalization Inhibitors
3. Results
3.1. The Presence of FBS in the Culture Medium Provided the Formation of Material Aggregates Avoiding the Formation of Large Particle Clusters
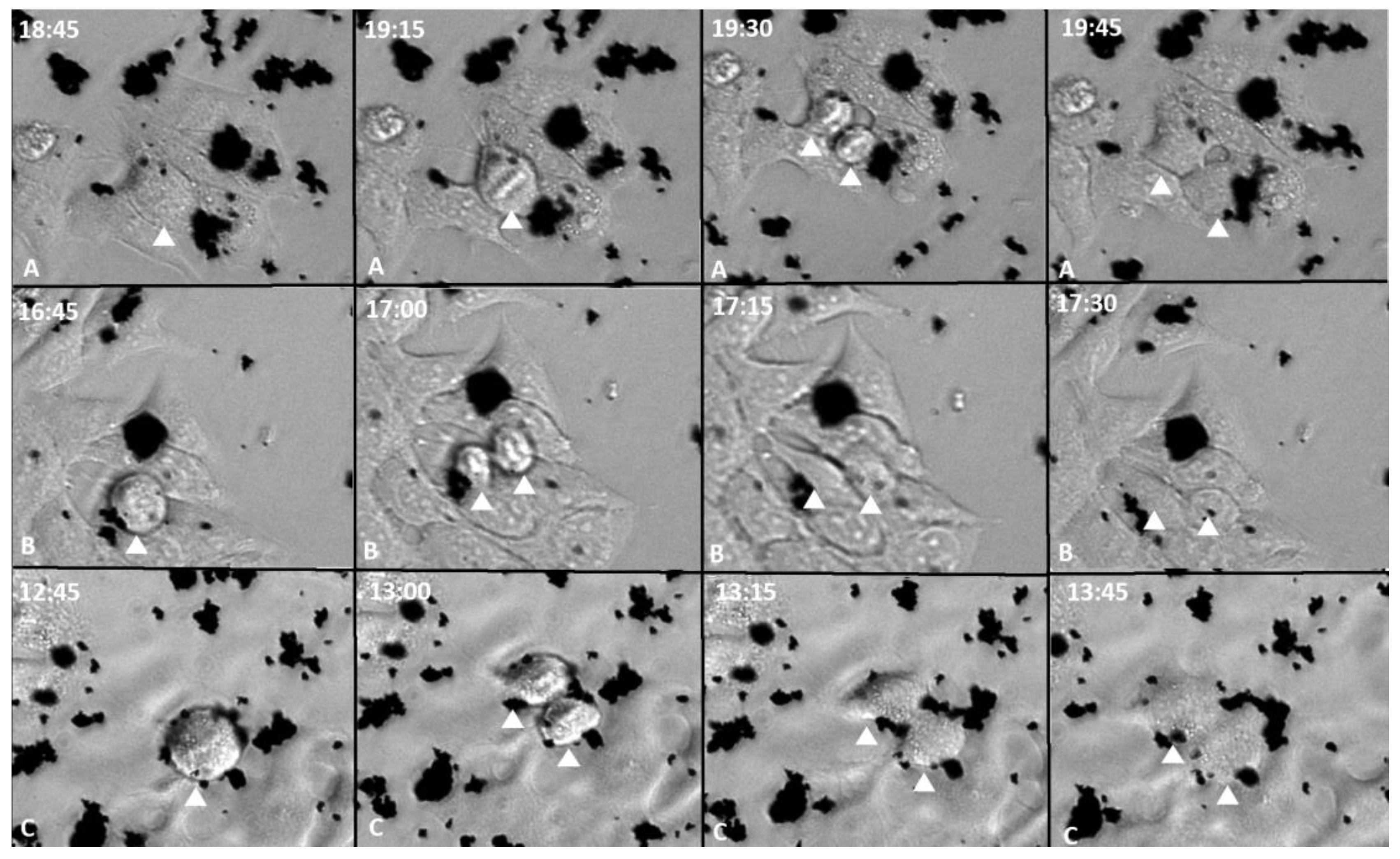
3.2. During Cell Division GOX, GOXD and GOXP Particles Were Redistributed to the Daughter Cells
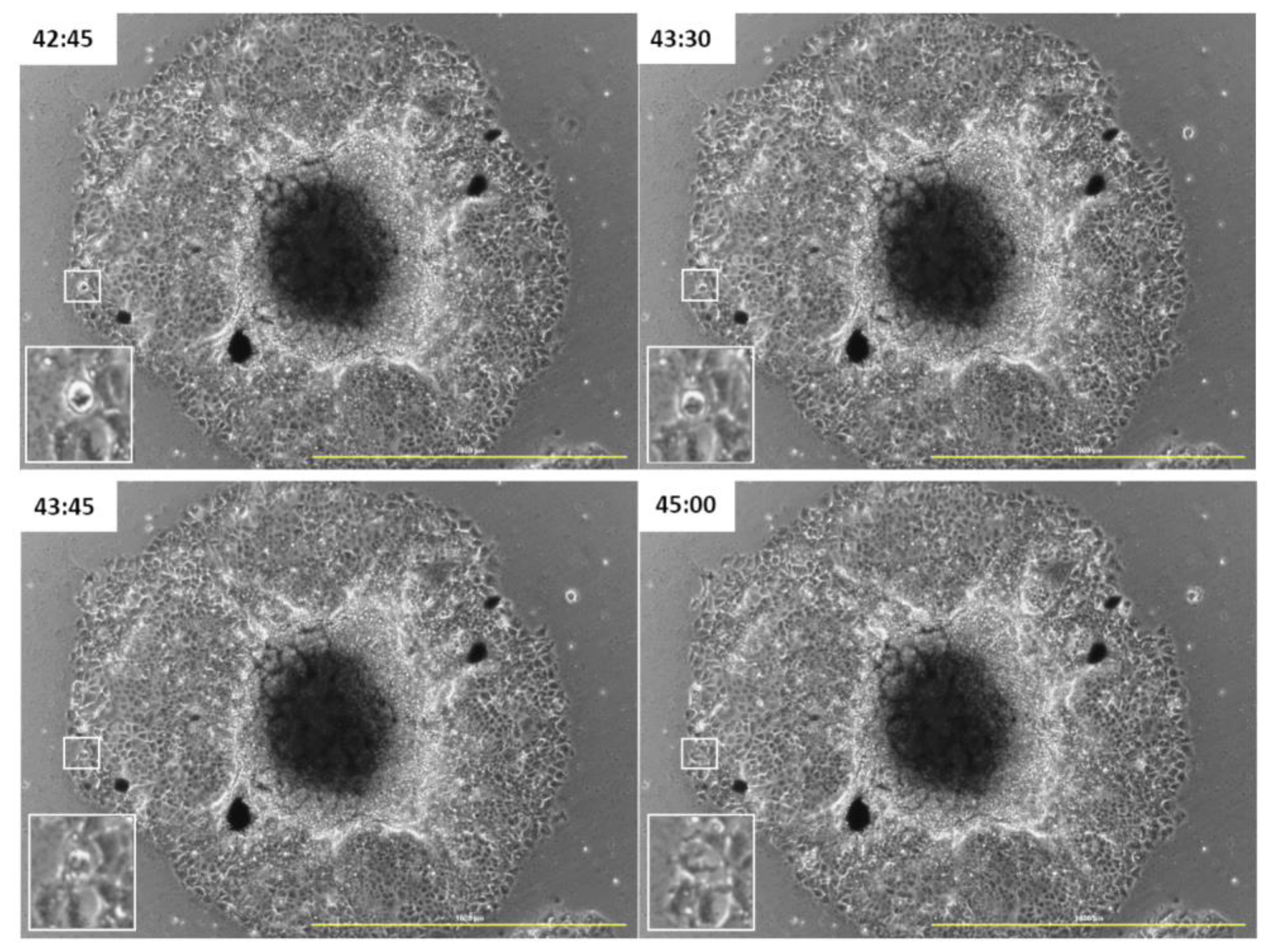
3.3. GOXP Is also Redistributed in Cells Migrating from the Spheroid
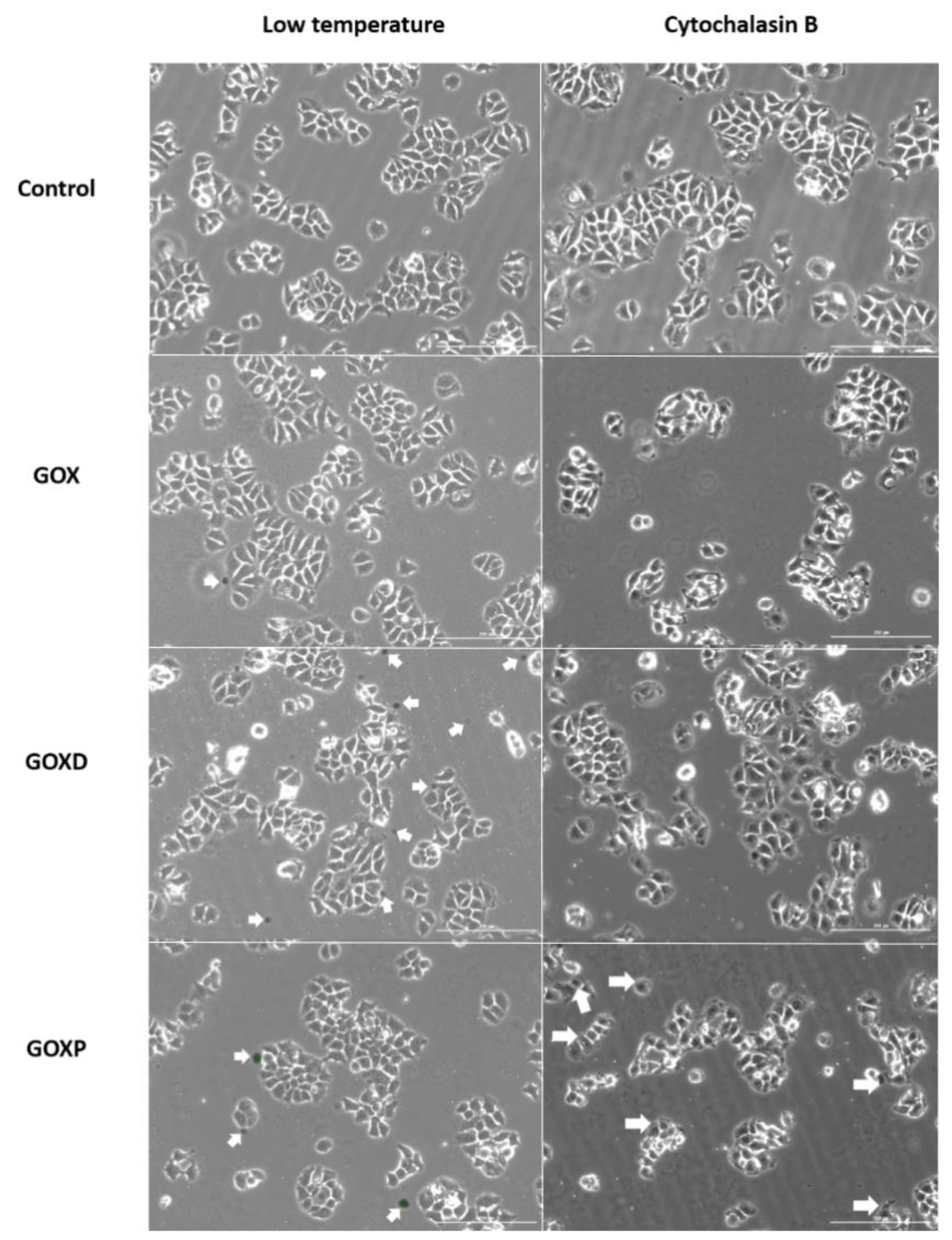
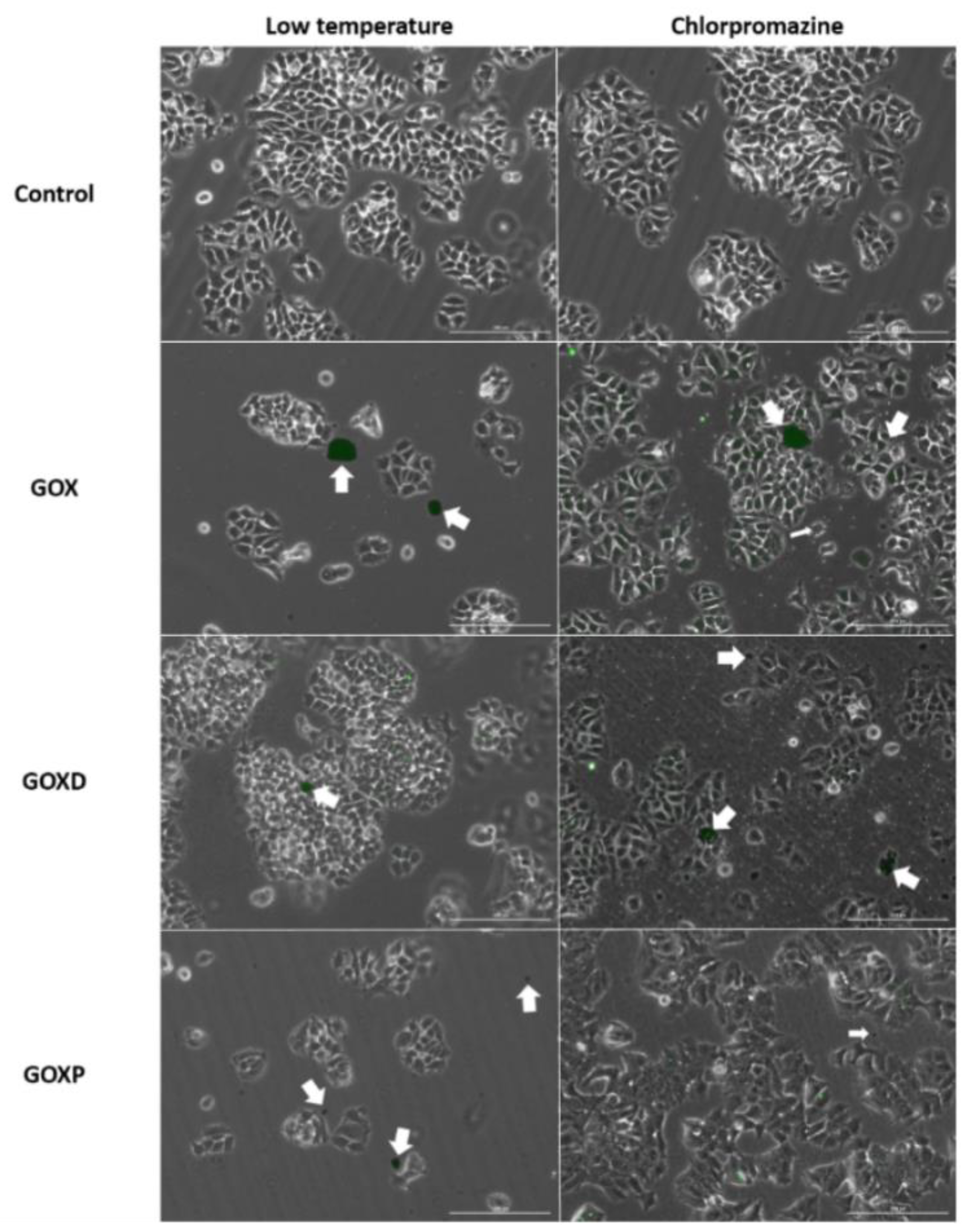
3.4. GOXP Internalization Is Carried out by Clathrin-Mediated Endocytosis, While GOX and GOXD Are Internalized by Macropinocytosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Balaji, A.; Yang, S.; Wang, J.; Zhang, J. Graphene Oxide-Based Nanostructured DNA Sensor. Biosensors 2019, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-M.; Zheng, K.-W.; Wang, R.-L.; Yue, S.-F.; Chen, J.; Zhao, Z.-W.; Song, F.; Su, Y.; Ma, Q. Functionalization and optimization-strategy of graphene oxide-based nanomaterials for gene and drug delivery. Am. J. Transl. Res. 2020, 12, 1515–1534. [Google Scholar] [PubMed]
- Li, J.; Zeng, H.; Zeng, Z.; Zeng, Y.; Xie, T. Promising Graphene-Based Nanomaterials and Their Biomedical Applications and Potential Risks: A Comprehensive Review. ACS Biomater. Sci. Eng. 2021, 7, 5363–5396. [Google Scholar] [CrossRef]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Ferreira, E.I.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef]
- Li, H.; Papadakis, R. Click Chemistry Enabling Covalent and Non-Covalent Modifications of Graphene with (Poly)saccharides. Polymers 2021, 13, 142. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, P.; Lin, J.; Chen, K.; Shen, J. Recent advances in access to overcome cancer drug resistance by nanocarrier drug delivery system. Cancer Drug Resist. 2023, 6, 390–415. [Google Scholar] [CrossRef]
- Rahman, S.; Kumar, V.; Kumar, A.; Abdullah, T.S.; Rather, I.A.; Jan, A.T. Molecular Perspective of Nanoparticle Mediated Therapeutic Targeting in Breast Cancer: An Odyssey of Endoplasmic Reticulum Unfolded Protein Response (UPRER) and Beyond. Biomedicines 2021, 9, 635. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Asgari, S.; Hosseini, S.H.; Akhlaghi, M. Codelivery of Hydrophobic and Hydrophilic Drugs by Graphene-Decorated Magnetic Dendrimers. Langmuir 2018, 34, 15304–15318. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Huang, A.-T.; Kao, C.-L.; Peng, L. Poly(amidoamine) dendrimers: Covalent and supramolecular synthesis. Mater. Today Chem. 2019, 13, 34–48. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Tintaru, A.; Peng, L. Self-assembling supramolecular dendrimers for biomedical applications: Lessons learned from poly(amidoamine) dendrimers. Acc. Chem. Res. 2020, 53, 2936–2949. [Google Scholar] [CrossRef]
- Marichal, L.; Giraudon--Colas, G.; Cousin, F.; Thill, A.; Labarre, J.; Boulard, Y.; Aude, J.-C.; Pin, S.; Renault, J.P. Protein–nanoparticle interactions: What are the protein–corona thickness and organization? Langmuir 2019, 35, 10831–10837. [Google Scholar] [CrossRef] [PubMed]
- Vianello, F.; Cecconello, A.; Magro, M. Toward the Specificity of Bare Nanomaterial Surfaces for Protein Corona Formation. Int. J. Mol. Sci. 2021, 22, 7625. [Google Scholar] [CrossRef]
- Simon, J.; Müller, L.K.; Kokkinopoulou, M.; Lieberwirth, I.; Morsbach, S.; Landfester, K.; Mailänder, V. Exploiting the biomolecular corona: Pre-coating of nanoparticles enables controlled cellular interactions. Nanoscale 2018, 10, 10731–10739. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.R.; Neves, M.G.P.M.S.; Trindade, T. Functionalization of Graphene Oxide with Porphyrins: Synthetic Routes and Biological Applications. ChemPlusChem 2020, 85, 1857–1880. [Google Scholar] [CrossRef]
- Vacchi, I.A.; Guo, S.; Raya, J.; Bianco, A.; Ménard-Moyon, C. Strategies for the Controlled Covalent Double Functionalization of Graphene Oxide. Chem. A Eur. J. 2020, 26, 6591–6598. [Google Scholar] [CrossRef]
- Jayaram, D.T.; Pustulka, S.M.; Mannino, R.G.; Lam, W.A.; Payne, C.K. Protein corona in response to flow: Effect on protein concentration and structure. Biophys. J. 2018, 115, 209–216. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M.; Ding, J. The interaction between nanoparticles-protein corona complex and cells and its toxic effect on cells. Chemosphere 2020, 245, 125624. [Google Scholar] [CrossRef]
- Pinals, R.L.; Yang, D.; Rosenberg, D.J.; Chaudhary, T.; Crothers, A.R.; Iavarone, A.T.; Hammel, M.; Landry, M.P. Quantitative Protein Corona Composition and Dynamics on Carbon Nanotubes in Biological Environments. Angew. Chem. Int. Ed. 2020, 59, 23668–23677. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Åberg, C.; Piattelli, V.; Montizaan, D.; Salvati, A. Sources of variability in nanoparticle uptake by cells. Nanoscale 2021, 13, 17530–17546. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Varma, S.; Dey, S.; Palanisamy, D. Cellular Uptake Pathways of Nanoparticles: Process of Endocytosis and Factors Affecting their Fate. Curr. Pharm. Biotechnol. 2022, 23, 679–706. [Google Scholar] [CrossRef]
- Ribeiro, B.F.M.; Souza, M.M.; Fernandes, D.S.; Carmo, D.R.D.; Machado-Santelli, G.M. Graphene oxide-based nanomaterial interaction with human breast cancer cells. J. Biomed. Mater. Res. Part A 2020, 108, 863–870. [Google Scholar] [CrossRef]
- Fernandes, D.S.; Bonfim, K.S.; Carmo, D.R.D. Silver Hexacyanoferrate (III) on a Hybrid Graphene Oxide/PAMAM Dendrimer Surface and Application as an Electrocatalyst in the Detection of Isoniazid. Electroanalysis 2018, 30, 1107–1116. [Google Scholar] [CrossRef]
- Carmo, D.R.D.; Fernandes, D.S. Hybrid graphene oxide/DAB-Am-16 dendrimer: Preparation, characterization chemical reactivity and their electrocatalytic detection of l-Dopamine. Solid State Sci. 2017, 71, 33–41. [Google Scholar] [CrossRef]
- Wu, B.; Xiao, L.; Zhang, M.; Yang, C.; Li, Q.; Li, G.; He, Q.; Liu, J. Facile synthesis of dendritic-like CeO2/rGO composite and application for detection of uric acid and tryptophan simultaneously. J. Solid State Chem. 2021, 296, 122023. [Google Scholar] [CrossRef]
- Bussy, C.; Kostarelos, K. Culture media critically influence graphene oxide effects on plasma membranes. Chem 2017, 2, 322–323. [Google Scholar] [CrossRef]
- Sun, C.; Wakefield, D.L.; Han, Y.; Muller, D.A.; Holowka, D.A.; Baird, B.A.; Dichtel, W.R. Graphene oxide nanosheets stimulate ruffling and shedding of mammalian cell plasma membranes. Chem 2016, 1, 273–286. [Google Scholar] [CrossRef]
- Sima, L.E.; Chiritoiu, G.; Negut, I.; Grumezescu, V.; Orobeti, S.; Munteanu, C.V.A.; Sima, F.; Axente, E. Functionalized Graphene Oxide Thin Films for Anti-tumor Drug Delivery to Melanoma Cells. Front. Chem. 2020, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.A.; Mohanta, Z.; Srivastava, C.; Atreya, H.S. Modulation of protein–graphene oxide interactions with varying degrees of oxidation. Nanoscale Adv. 2020, 2, 1904–1912. [Google Scholar] [CrossRef]
- Franqui, L.S.; De Farias, M.A.; Portugal, R.V.; Costa, C.A.; Domingues, R.R.; Filho, A.G.S.; Coluci, V.R.; Leme, A.F.; Martinez, D.S.T. Interaction of graphene oxide with cell culture medium: Evaluation the fetal bovine serum protein corona formation towards in vitro nanotoxicity assessments and nanobiointeractions. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 363–377. [Google Scholar] [CrossRef]
- Partikel, K.; Partikel, K.; Korte, R.; Korte, R.; Mulac, D.; Mulac, D.; Humpf, H.-U.; Humpf, H.-U.; Langer, K.; Langer, K. Serum type and concentration both affect the protein-corona composition of PLGA nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Gil, P.; De Aberasturi, D.J.; Wulf, V.; Pelaz, B.; Del Pino, P.; Zhao, Y.; De La Fuente, J.M.; De Larramendi, I.R.; Rojo, T.; Liang, X.-J.; et al. The Challenge To Relate the Physicochemical Properties of Colloidal Nanoparticles to Their Cytotoxicity. Acc. Chem. Res. 2013, 46, 743–749. [Google Scholar] [CrossRef]
- Wang, F.; Yu, L.; Monopoli, M.P.; Sandin, P.; Mahon, E.; Salvati, A.; Dawson, K.A. The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, B.; Żuchowska, A.; Brzózka, Z. Graphene oxide internalization into mammalian cells—A review. Colloids Surf. B Biointerfaces 2023, 221, 112998. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Eom, H.-J.; Choi, J. A systems toxicology approach to the surface functionality control of graphene–cell interactions. Biomaterials 2014, 35, 1109–1127. [Google Scholar] [CrossRef]
- Huang, J.; Zong, C.; Shen, H.; Liu, M.; Chen, B.; Ren, B.; Zhang, Z. Mechanism of cellular uptake of graphene oxide studied by surface-enhanced raman spectroscopy. Small 2012, 8, 2577–2584. [Google Scholar] [CrossRef]
- Bina, A.; Raissi, H.; Hashemzadeh, H.; Farzad, F. Conjugation of a smart polymer to doxorubicin through a pH-responsive bond for targeted drug delivery and improving drug loading on graphene oxide. RSC Adv. 2021, 11, 18809–18817. [Google Scholar] [CrossRef]
- Sebak, A.A.; Gomaa, I.E.O.; ElMeshad, A.N.; Farag, M.H.; Breitinger, U.; Breitinger, H.-G.; AbdelKader, M.H. Distinct Proteins in Protein Corona of Nanoparticles Represent a Promising Venue for Endogenous Targeting—Part I: In vitro Release and Intracellular Uptake Perspective. Int. J. Nanomed. 2020, 15, 8845–8862. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Amaral, J.B.D.; Rezende-Teixeira, P.; Freitas, V.M.; Machado-Santelli, G.M. MCF-7 cells as a three-dimensional model for the study of human breast cancer. Tissue Eng. Part C Methods 2011, 17, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug delivery to solid tumors: The predictive value of the multicellular tumor spheroid model for nanomedicine screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Stenzel, M.H. Multicellular tumor spheroids (MCTS) as a 3D in vitro evaluation tool of nanoparticles. Small 2018, 14, e1702858. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, B.F.M.; Chaves, J.B.; De Souza, M.M.; Keppler, A.F.; Do Carmo, D.R.; Machado-Santelli, G.M. Interaction of Graphene Oxide Particles and Dendrimers with Human Breast Cancer Cells by Real-Time Microscopy. Pharmaceutics 2023, 15, 2655. https://doi.org/10.3390/pharmaceutics15122655
Ribeiro BFM, Chaves JB, De Souza MM, Keppler AF, Do Carmo DR, Machado-Santelli GM. Interaction of Graphene Oxide Particles and Dendrimers with Human Breast Cancer Cells by Real-Time Microscopy. Pharmaceutics. 2023; 15(12):2655. https://doi.org/10.3390/pharmaceutics15122655
Chicago/Turabian StyleRibeiro, Beatriz Fumelli Monti, Julyane Batista Chaves, Marcelo Medina De Souza, Artur Franz Keppler, Devaney Ribeiro Do Carmo, and Gláucia M. Machado-Santelli. 2023. "Interaction of Graphene Oxide Particles and Dendrimers with Human Breast Cancer Cells by Real-Time Microscopy" Pharmaceutics 15, no. 12: 2655. https://doi.org/10.3390/pharmaceutics15122655
APA StyleRibeiro, B. F. M., Chaves, J. B., De Souza, M. M., Keppler, A. F., Do Carmo, D. R., & Machado-Santelli, G. M. (2023). Interaction of Graphene Oxide Particles and Dendrimers with Human Breast Cancer Cells by Real-Time Microscopy. Pharmaceutics, 15(12), 2655. https://doi.org/10.3390/pharmaceutics15122655





