99mTc-Labeled Cyclic Peptide Targeting PD-L1 as a Novel Nuclear Imaging Probe
Abstract
:1. Introduction
2. Materials and Methods
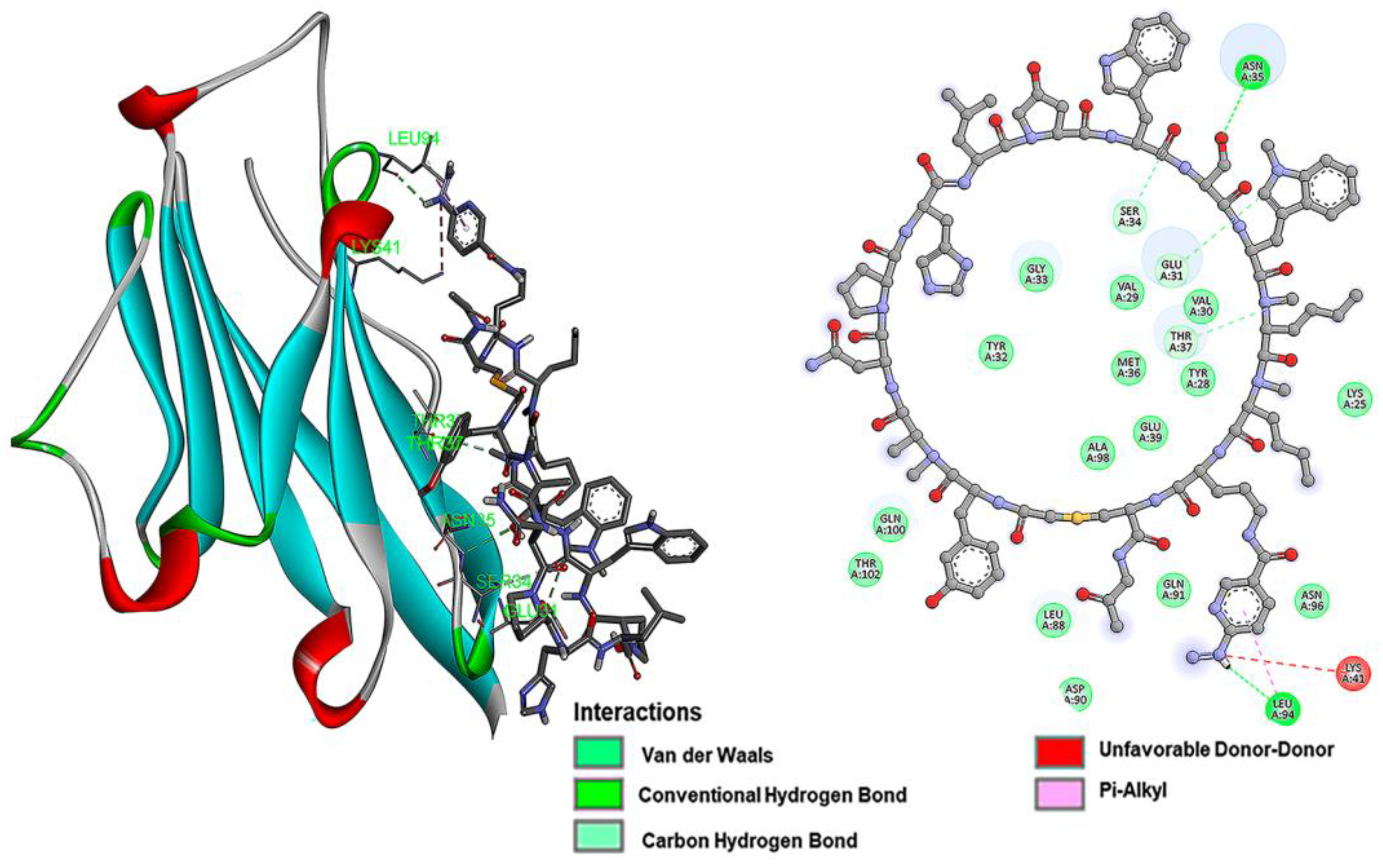
2.1. Docking
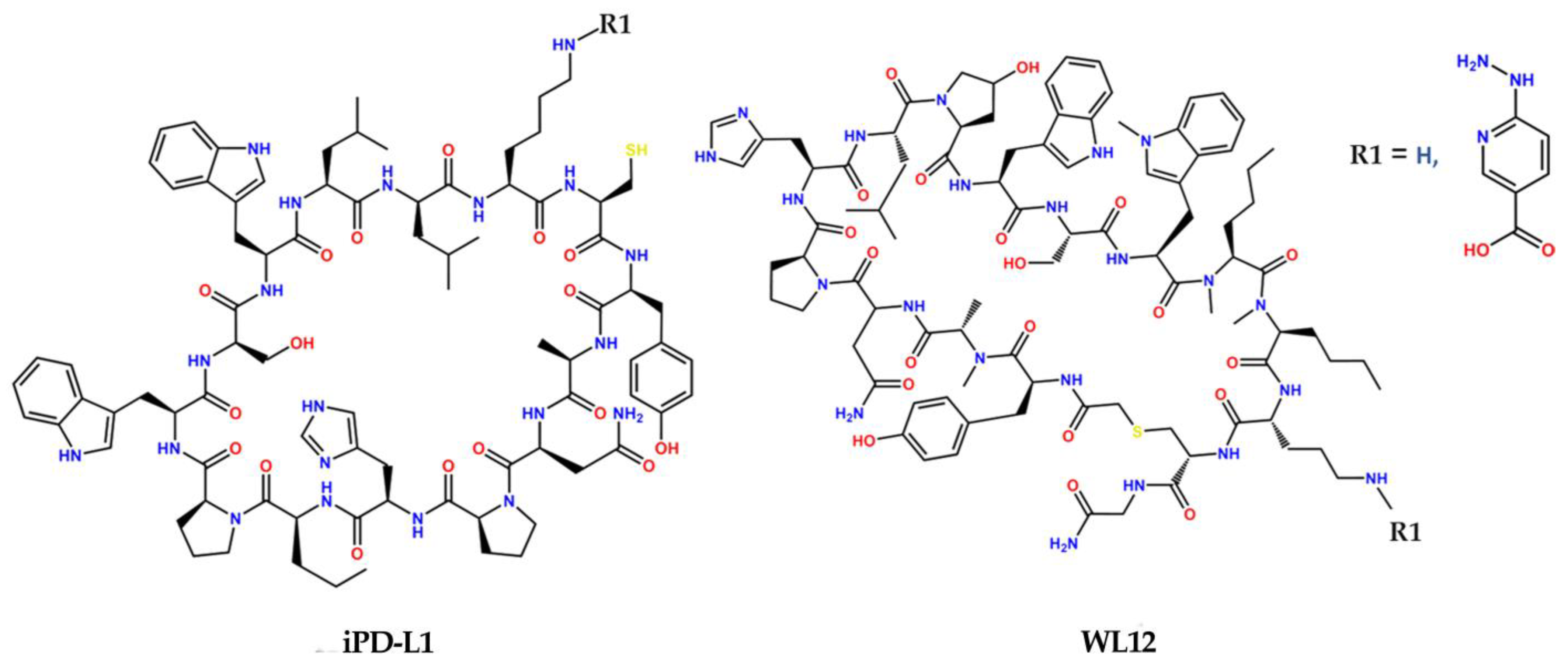
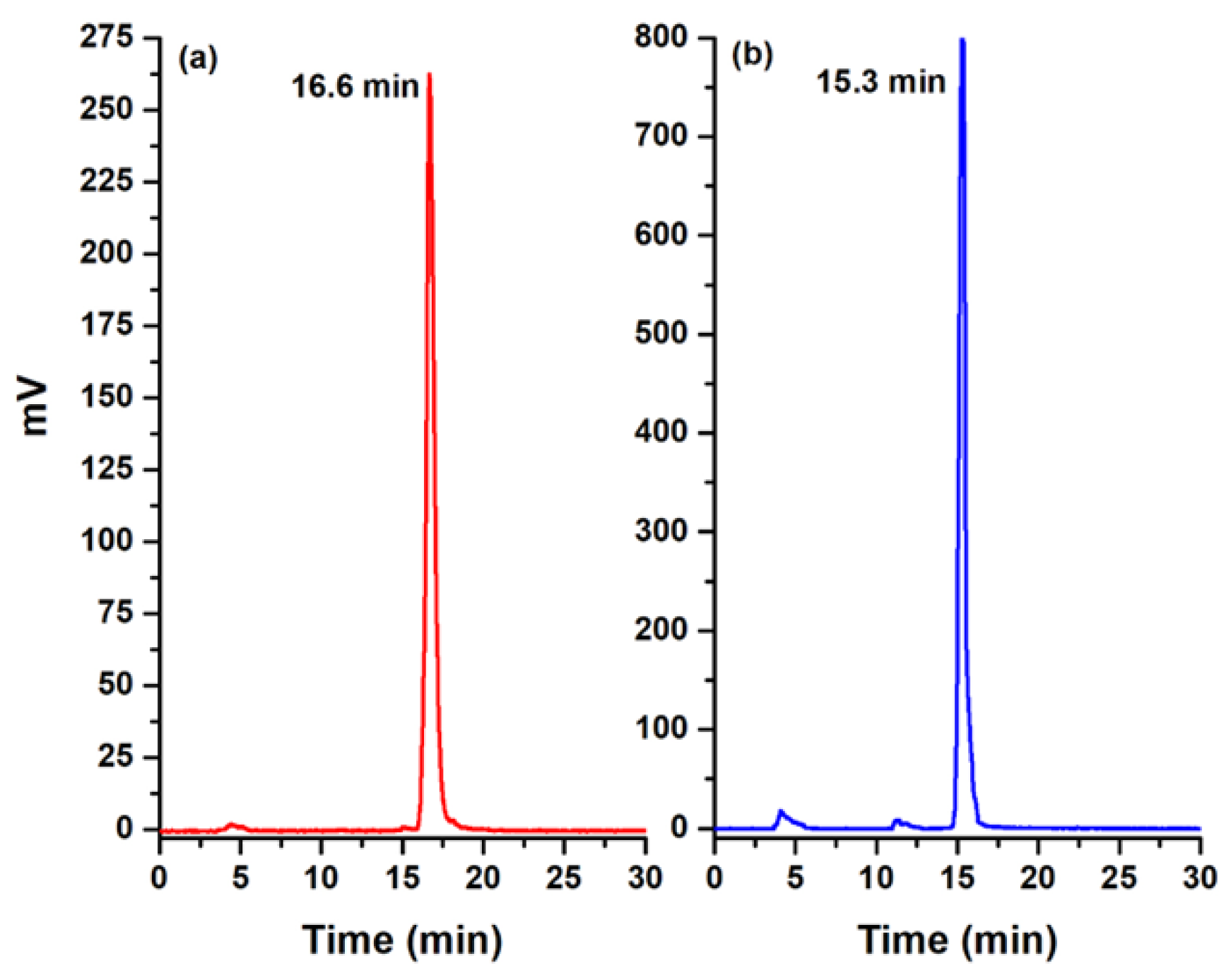
2.2. Synthesis and Chemical Characterization
2.3. Radiolabeling
2.4. Quality Control
2.5. Stability
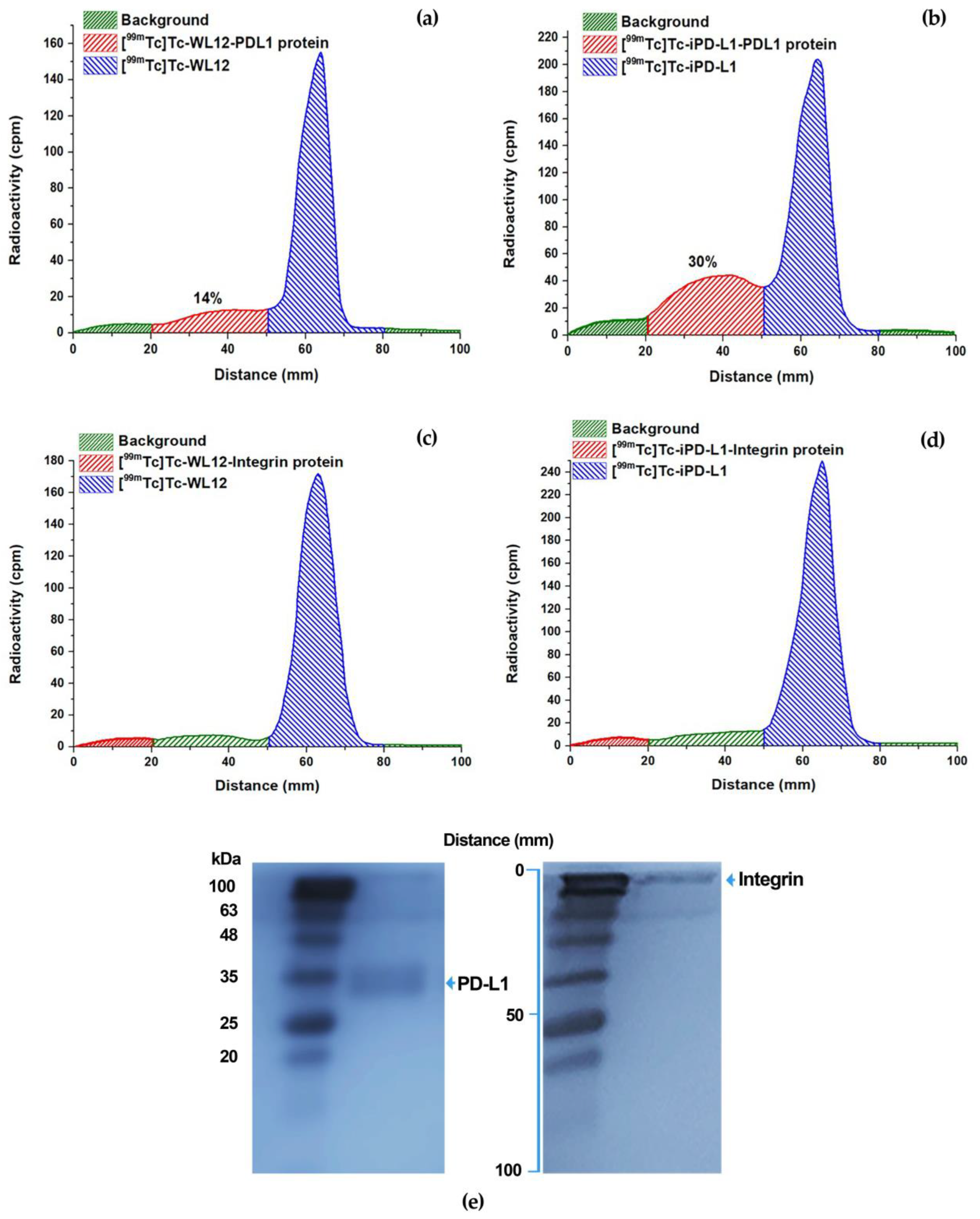
2.6. Molecular Recognition by the PD-L1 Protein: Radio-SDS-PAGE
2.7. Immunofluorescence
2.8. Cellular Uptake and Internalization
2.9. Biodistribution
2.10. Clinical Imaging
3. Results
3.1. Docking
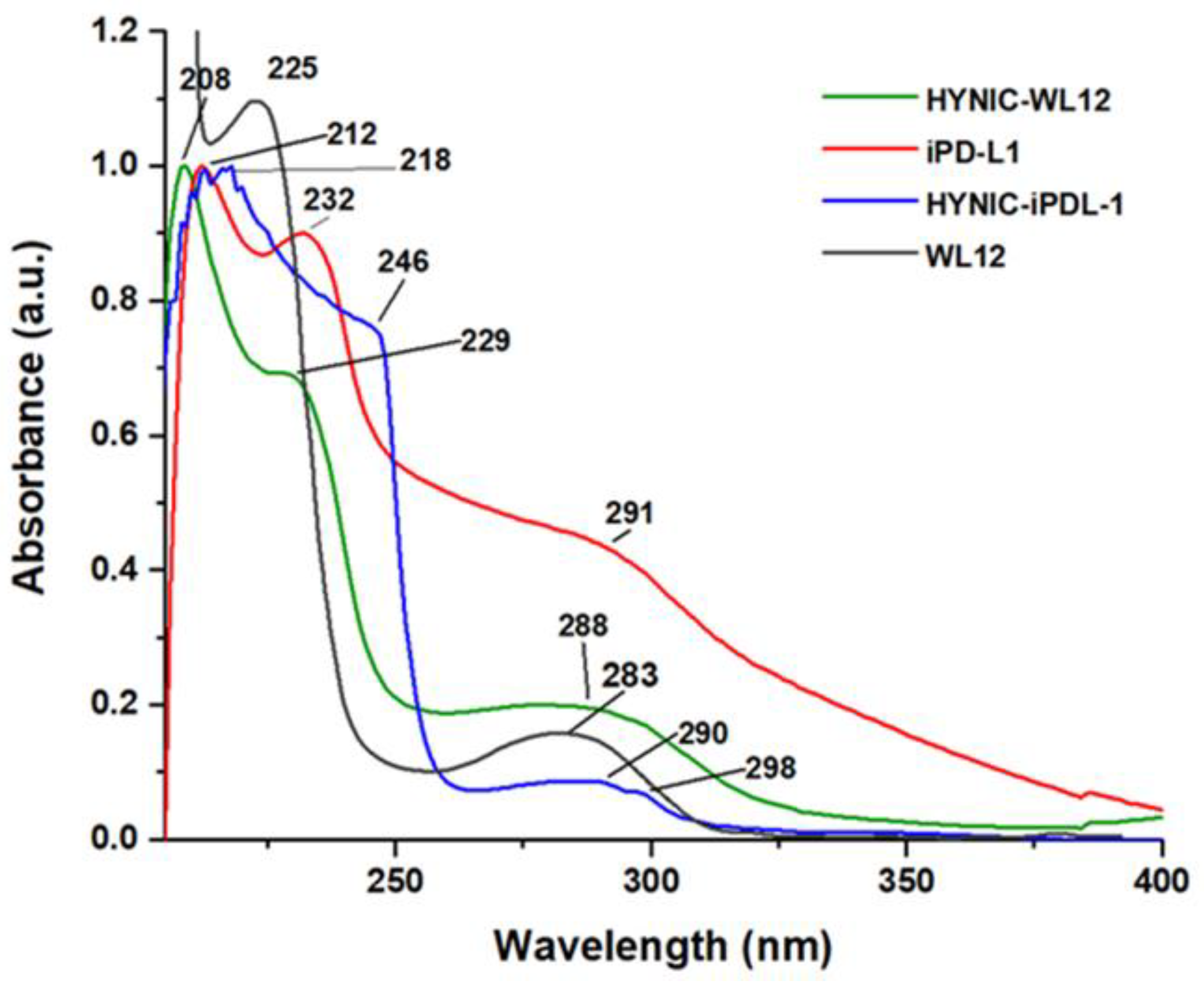
3.2. Synthesis and IR, Mass, and UV–Vis Spectroscopy Analyses
3.3. Radiolabeling
3.4. Stability
3.5. Molecular Recognition by the PD-L1 Protein: Radio-SDS-PAGE
3.6. Specific Cellular Uptake and Internalization
3.7. Biodistribution
3.8. Clinical Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Sosman, J.A.; Atkins, M.B.; Leming, P.D. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non–small cell lung cancer treated with nivolumab. JAMA Oncol. 2019, 5, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.C.; Sosman, J.A.; Sznol, M.; Gordon, M.S.; Hollebecque, A.; Hamid, O.; McDermott, D.F.; Delord, J.-P.; Rhee, I.P.; Mokatrin, A. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC). J. Clin. Oncol. 2013, 31, 15. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020. [Google Scholar] [CrossRef]
- Weber, J.; Minor, D.; D’Angelo, S.; Hodi, F.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.; Miller, W.; Grob, J. A phase 3 randomized, open-label study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) versus investigator’s choice chemotherapy (ICC) in patients with advanced melanoma after prior anti-CTLA-4 therapy. Ann. Oncol. 2014, 25, v1. [Google Scholar] [CrossRef]
- Krutzek, F.; Kopka, K.; Stadlbauer, S. Development of Radiotracers for Imaging of the PD-1/PD-L1 Axis. Pharmaceuticals 2022, 15, 747. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Rubins, D.; Bennacef, I.; Holahan, M.; Haley, H.; Purcell, M.; Gantert, L.; Hseih, S.; Judo, M. PET/CT imaging of 89 Zr-N-sucDf-Pembrolizumab in healthy cynomolgus monkeys. Mol. Imaging Biol. 2021, 23, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jiang, J.; Yang, X.; Liu, T.; Ding, J.; Nimmagadda, S.; Pomper, M.G.; Zhu, H.; Zhao, J.; Yang, Z. First-in-Humans evaluation of a PD-L1–binding peptide PET radiotracer in non–small cell lung cancer patients. J. Nucl. Med. 2022, 63, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Guzik, K.; Tomala, M.; Muszak, D.; Konieczny, M.; Hec, A.; Błaszkiewicz, U.; Pustuła, M.; Butera, R.; Dömling, A.; Holak, T.A. Development of the inhibitors that target the PD-1/PD-L1 interaction—A brief look at progress on small molecules, peptides and macrocycles. Molecules 2019, 24, 2071. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, Q.; Liu, Z.; Chen, Y.; Feng, F.; Sun, H. Peptide-based and small synthetic molecule inhibitors on PD-1/PD-L1 pathway: A new choice for immunotherapy? Eur. J. Med. Chem. 2019, 161, 378–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, X.; Guo, C.; Zhang, K.; Xu, J.; Li, Z.; Jiang, S. Development of inhibitors of the programmed cell death-1/programmed cell death-ligand 1 signaling pathway. J. Med. Chem. 2018, 62, 1715–1730. [Google Scholar] [CrossRef] [PubMed]
- Huck, B.R.; Kötzner, L.; Urbahns, K. Small molecules drive big improvements in immuno-oncology therapies. Angew. Chem. Int. Ed. 2018, 57, 4412–4428. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Benítez, D.; Luna-Gutiérrez, M.; Ferro-Flores, G.; Ocampo-García, B.; Santos-Cuevas, C.; Bravo-Villegas, G.; Morales-Ávila, E.; Cruz-Nova, P.; Díaz-Nieto, L.; García-Quiroz, J. Design, Synthesis and Preclinical Assessment of 99mTc-iFAP for In Vivo Fibroblast Activation Protein (FAP) Imaging. Molecules 2022, 27, 264. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Flores, G.; Luna-Gutiérrez, M.; Ocampo-García, B.; Santos-Cuevas, C.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Orocio-Rodríguez, E.; Davanzo, J.; García-Pérez, F.O. Clinical translation of a PSMA inhibitor for 99mTc-based SPECT. Nucl. Med. Biol. 2017, 48, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Armenta, P.; Santos-Cuevas, C.; Soto-Andonaegui, J.; Villanueva-Pérez, R.M.; González-Díaz, J.I.; García-Pérez, F.O.; Arrellano-Zarate, A.; Luna-Gutiérrez, M.; Azorín-Vega, E.; Ocampo-García, B. 99m Tc-CXCR4-L for imaging of the chemokine-4 receptor associated with brain tumor invasiveness: Biokinetics, radiation dosimetry, and proof of concept in humans. Contrast Media Mol. Imaging 2020, 2020, 2525037. [Google Scholar] [CrossRef] [PubMed]
- Salud, S.D. Farmacopea de los Estados Unidos Mexicanos. 2021 Pharmacopoeia of the United Mexican States (Thirteenth Edition), Mexico. 2021. Available online: https://www.farmacopea.org.mx/publicaciones-detalle.php?m=3&pid=12 (accessed on 31 July 2023).
- Iman, M.; Saadabadi, A.; Davood, A. Molecular docking analysis and molecular dynamics simulation study of ameltolide analogous as a sodium channel blocker. Turk. J. Chem. 2015, 39, 306–316. [Google Scholar] [CrossRef]
- De Silva, R.A.; Kumar, D.; Lisok, A.; Chatterjee, S.; Wharram, B.; Venkateswara Rao, K.; Mease, R.; Dannals, R.F.; Pomper, M.G.; Nimmagadda, S. Peptide-based 68Ga-PET radiotracer for imaging PD-L1 expression in cancer. Mol. Pharm. 2018, 15, 3946–3952. [Google Scholar] [CrossRef]
- Hughes, D.J.; Chand, G.; Johnson, J.; Bailey, D.; Adamson, K.; Goh, V.; Cook, G.J. Inter-rater and intra-rater agreement of [99mTc]-labelled NM-01, a single-domain programmed death-ligand 1 (PD-L1) antibody, using quantitative SPECT/CT in non-small cell lung cancer. EJNMMI Res. 2023, 13, 51. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Li, N.; Wang, S.; Zhou, S.; Li, R.; Yang, H.; Li, W.; Qu, J. Noninvasive Imaging of Tumor PD-L1 Expression Using [99mTc] Tc-Labeled KN035 with SPECT/CT. Mol. Pharm. 2022, 20, 690–700. [Google Scholar] [CrossRef]
- Hughes, D.J.; Chand, G.; Goh, V.; Cook, G.J. Inter-and intraobserver agreement of the quantitative assessment of [99m Tc]-labelled anti-programmed death-ligand 1 (PD-L1) SPECT/CT in non-small cell lung cancer. EJNMMI Res. 2020, 10, 145. [Google Scholar] [CrossRef] [PubMed]







| Inhibitor Peptide | Affinity (kcal/mol) | Inhibition Constant (µM) | Binding Site |
|---|---|---|---|
| iPD-L1 | −6.7 | 12.26 | Lys25, Lys41, Leu94, Leu27, Phe42, Val23. Glu39, Lys124, Pro24, Thr22, Val44 |
| HYNIC-iPD-L1 | −7.2 | 5.27 | Gln107, Arg125, Ala132, Leu106, Val111, Ile126, Glu60, Ala109, Met5, Thr127, Arg113, Lys129, Tyr112 |
| WL12 | −5.1 | 82.51 | Ser34, Glu39, Glu31, Asn35 |
| HYNIC-WL12 | −6.0 | 39.95 | Leu94, Leu88, Glu31, Gln100, Thr102, Ser34 |
| Adenopathy/Lesion | [18F]FDG Uptake | [99mTc]Tc-iPD-L1 Uptake |
|---|---|---|
| Mediastinal adenopathies level 4R, 8 and 10L up to 12 mm | ✓ | × |
| Left retrocrural adenopathy of 9 mm | ✓ | × |
| Liver with heterogeneous appearance due to the presence of poorly defined hypodense images distributed in an allotropic manner, the largest in segment 7 | ✓ | × |
| Retroperitoneal paraaortic and intercavoaortic adenopathies of 14 mm | ✓ | × |
| Adenopathies of the common, internal, external, and obturator iliac chains of left predominance up to 26 mm | ✓ | × |
| Left inguinal adenopathies forming clusters of 29 mm | ✓ | † |
| Left femoral adenopathies of 12 mm | ✓ | † |
| Left popliteal hollow adenopathies up to 20 mm | ✓ | † |
| Adenopathies in the entire intramuscular tract of the left lower extremity up to 14 mm | ✓ | † |
| A lesion with an exophytic component with poorly defined oval edges with heterogeneous soft tissue density due to the presence of air corpuscles in its interior, located in the heel region with extension to the medial region of the left foot measuring 67 × 35 mm | ✓ | † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro-Flores, G.; Ocampo-García, B.; Cruz-Nova, P.; Luna-Gutiérrez, M.; Bravo-Villegas, G.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Michel-Sánchez, E.; García-Pérez, O.; Lara-Almazán, N.; et al. 99mTc-Labeled Cyclic Peptide Targeting PD-L1 as a Novel Nuclear Imaging Probe. Pharmaceutics 2023, 15, 2662. https://doi.org/10.3390/pharmaceutics15122662
Ferro-Flores G, Ocampo-García B, Cruz-Nova P, Luna-Gutiérrez M, Bravo-Villegas G, Azorín-Vega E, Jiménez-Mancilla N, Michel-Sánchez E, García-Pérez O, Lara-Almazán N, et al. 99mTc-Labeled Cyclic Peptide Targeting PD-L1 as a Novel Nuclear Imaging Probe. Pharmaceutics. 2023; 15(12):2662. https://doi.org/10.3390/pharmaceutics15122662
Chicago/Turabian StyleFerro-Flores, Guillermina, Blanca Ocampo-García, Pedro Cruz-Nova, Myrna Luna-Gutiérrez, Gerardo Bravo-Villegas, Erika Azorín-Vega, Nallely Jiménez-Mancilla, Emiliano Michel-Sánchez, Osvaldo García-Pérez, Nancy Lara-Almazán, and et al. 2023. "99mTc-Labeled Cyclic Peptide Targeting PD-L1 as a Novel Nuclear Imaging Probe" Pharmaceutics 15, no. 12: 2662. https://doi.org/10.3390/pharmaceutics15122662
APA StyleFerro-Flores, G., Ocampo-García, B., Cruz-Nova, P., Luna-Gutiérrez, M., Bravo-Villegas, G., Azorín-Vega, E., Jiménez-Mancilla, N., Michel-Sánchez, E., García-Pérez, O., Lara-Almazán, N., & Santos-Cuevas, C. (2023). 99mTc-Labeled Cyclic Peptide Targeting PD-L1 as a Novel Nuclear Imaging Probe. Pharmaceutics, 15(12), 2662. https://doi.org/10.3390/pharmaceutics15122662










