Mesoporous Silica Nanoparticles: Types, Synthesis, Role in the Treatment of Alzheimer’s Disease, and Other Applications
Abstract
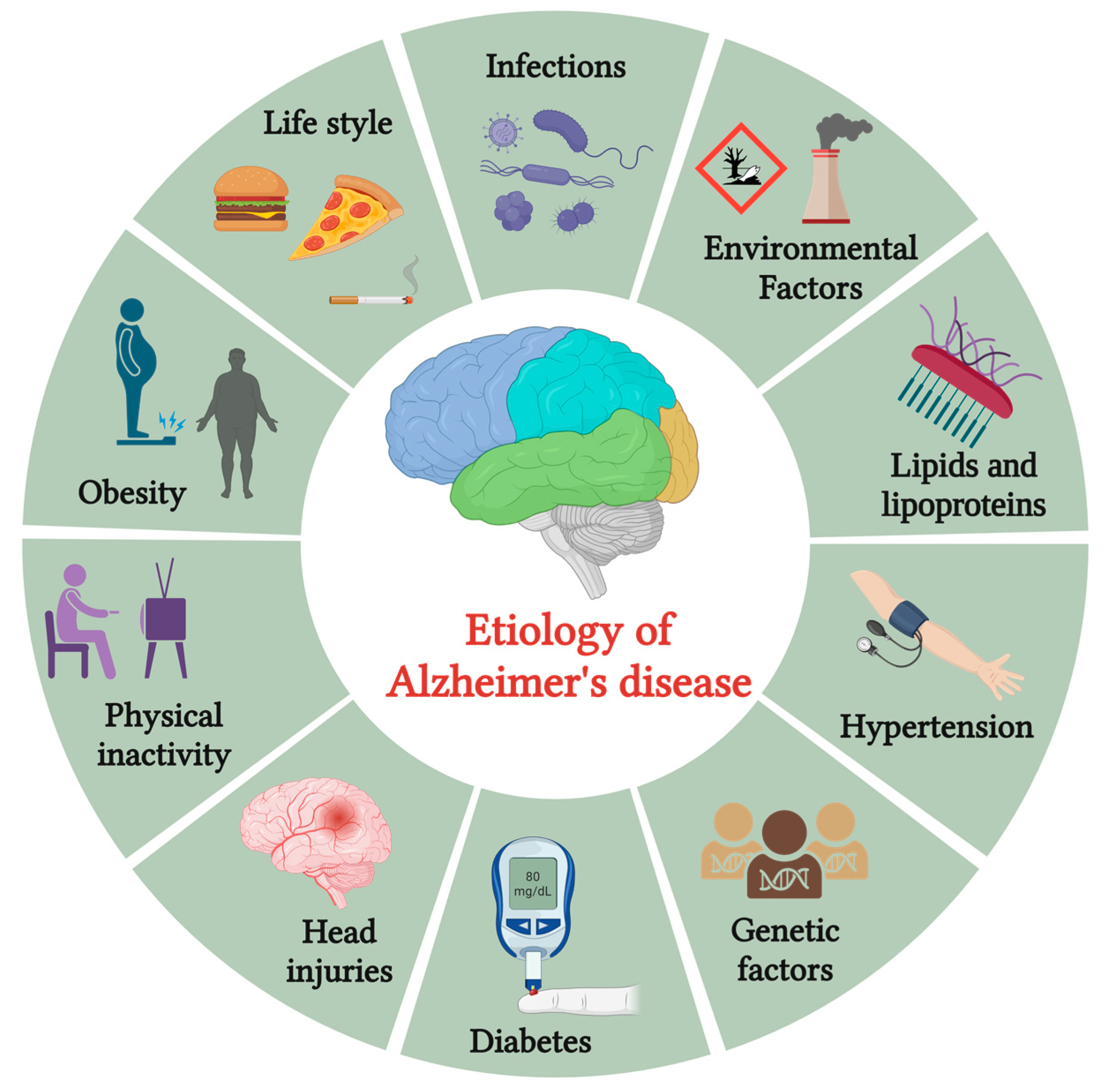
:1. Introduction
2. Pathology of AD
3. Current Treatments and Their Limitations
4. Mesoporous Silica Nanoparticles (MSNs)
4.1. Types of MSNs
4.1.1. Physical Stimuli-Responsive MSNs
4.1.2. Chemical Stimuli-Responsive MSNs
4.1.3. Biological Stimuli-Responsive MSNs
4.2. Synthesis/Fabrication Methods
4.2.1. Sol-Gel Method
4.2.2. Soft Templating Process
4.2.3. Hard Templating Process
5. MSNs for the Treatment of AD
6. Representative Applications of MSNs in the Medical Sector
6.1. Wound Healing and Tissue Regeneration
6.2. Imaging and Diagnostic Application
6.3. Biocatalyst
7. Advantages and Limitations
8. Future Perspective
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, S.; Ahmad, R.; Khare, S.K. Alzheimer’s Disease and Its Treatment by Different Approaches: A Review. Eur. J. Med. Chem. 2021, 216, 113320. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, G.; Dolciotti, C.; Picchi, L.; Bonuccelli, U. Alzheimer and His Disease: A Brief History. Neurol. Sci. 2011, 32, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-P.; Wu, K.-C.; Lin, C.-Y.; Chern, Y. Emerging roles of dysregulated adenosine homeostasis in brain disorders with a specific focus on neurodegenerative diseases. J. Biomed. Sci. 2021, 28, 70. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Altieri, M.; Garramone, F.; Santangelo, G. Functional autonomy in dementia of the Alzheimer’s type, mild cognitive impairment, and healthy aging: A meta-analysis. Neurol. Sci. 2021, 42, 1773–1783. [Google Scholar] [CrossRef]
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef]
- Richard, A.A. Risk Factors for Alzheimer’s Disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar]
- Anand, P.; Singh, B. A Review on Cholinesterase Inhibitors for Alzheimer’s Disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Shityakov, S.; Skorb, E.V.; Förster, C.Y.; Dandekar, T. Scaffold Searching of FDA and EMA-Approved Drugs Identifies Lead Candidates for Drug Repurposing in Alzheimer’s Disease. Front. Chem. 2021, 9, 736509. [Google Scholar] [CrossRef]
- Zemek, F.; Drtinova, L.; Nepovimova, E.; Sepsova, V.; Korabecny, J.; Klimes, J.; Kuca, K. Outcomes of Alzheimer’s Disease Therapy with Acetylcholinesterase Inhibitors and Memantine. Expert Opin. Drug Saf. 2014, 13, 759–774. [Google Scholar]
- Hasan, I.; Guo, B.; Zhang, J.; Chang, C. Advances in Antioxidant Nanomedicines for Imaging and Therapy of Alzheimer’s Disease. Antioxid. Redox Signal. 2023. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, D.; Wang, J.; Huang, H.; Li, R.; Wu, C.; Deng, Y.; Hu, G.; Guo, B. Recent advances in small molecular near-infrared fluorescence probes for a targeted diagnosis of the Alzheimer disease. Analyst 2022, 147, 4701–4723. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; Vallet-Regí, M. Mesoporous Silica Nanoparticles as Carriers for Therapeutic Biomolecules. Pharmaceutics 2020, 12, 432. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Sabio, R.M.; Meneguin, A.B.; Ribeiro, T.C.; Silva, R.R.; Chorilli, M. New Insights Towards Mesoporous Silica Nanoparticles as A Technological Platform for Chemotherapeutic Drugs Delivery. Int. J. Pharm. 2019, 564, 379–409. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta, S.; Hussein, M.; Nasser, H.A.; Ali, A.A.A. Multidisciplinary Role of Mesoporous Silica Nanoparticles in Brain Regeneration and Cancers: From Crossing the Blood–Brain Barrier to Treatment. Part. Part. Syst. Charact. 2019, 36, 1900195. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Lombard, J.; Pomara, N. Pathophysiology of Alzheimer’s Disease. Neuroimaging Clin. N. Am. 2005, 15, 727–753. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fukushima, T.; Shimizu, E.; Okada, S.I.; Komatsu, N.; Okamura, N.; Koike, K.; Koizumi, H.; Kumakiri, C.; Imai, K.; et al. Possible Role of D-Serine in the Pathophysiology of Alzheimer’s Disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 385–388. [Google Scholar] [CrossRef]
- Chouliaras, L.; Rutten, B.P.; Kenis, G.; Peerbooms, O.; Visser, P.J.; Verhey, F.; van Os, J.; Steinbusch, H.W.; van den Hove, D.L. Epigenetic Regulation in the Pathophysiology of Alzheimer’s Disease. Prog. Neurobiol. 2010, 90, 498–510. [Google Scholar] [CrossRef]
- Nagy, Z.; Esiri, M.M.; Smith, A.D. The Cell Division Cycle and The Pathophysiology of Alzheimer’s Disease. Neuroscience 1998, 87, 731–739. [Google Scholar] [PubMed]
- Thal, D.R.; Tomé, S.O. The Central Role of Tau in Alzheimer’s Disease: From Neurofibrillary Tangle Maturation to the Induction of Cell Death. Brain Res. Bull. 2022, 190, 204–217. [Google Scholar] [CrossRef]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Thirumalai, D.; Palaniappan, B. Role of Tau Protein in Alzheimer’s Disease: The Prime Pathological Player. Int. J. Biol. Macromol. 2020, 163, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Stomrud, E.; Insel, P.S.; Leuzy, A.; Johansson, P.M.; Smith, R.; Ismail, Z.; Janelidze, S.; Palmqvist, S.; van Westen, D.; et al. Mild Behavioral Impairment and Its Relation to Tau Pathology in Preclinical Alzheimer’s Disease. Transl. Psychiatry 2021, 11, 76. [Google Scholar] [CrossRef]
- Cheng, Z.; Dai, L.; Wu, Y.; Cao, Y.; Chai, X.; Wang, P.; Liu, C.; Ni, M.; Gao, F.; Wang, Q.; et al. Correlation of Blood-Brain Barrier Leakage with Cerebral Small Vessel Disease Including Cerebral Microbleeds in Alzheimer’s Disease. Front. Neurol. 2023, 14, 1077860. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current Understanding of Alzheimer’s Disease Diagnosis and Treatment. F1000Research 2018, 7, F1000 Faculty Rev-1161. [Google Scholar] [CrossRef]
- Mohammad, D.; Chan, P.; Bradley, J.; Lanctôt, K.; Herrmann, N. Acetylcholinesterase Inhibitors for Treating Dementia Symptoms-A Safety Evaluation. Expert Opin. Drug Saf. 2017, 16, 1009–1019. [Google Scholar] [CrossRef]
- Osada, T.; Watanabe, N.; Asano, N.; Adachi, Y.; Yamamura, K. Adverse Drug Events Affecting Medication Persistence with Rivastigmine Patch Application. Patient Prefer. Adherence 2018, 12, 1247–1252. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments in Alzheimer disease: An update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef]
- Mishra, S.K.; Hidau, M.K.; Rai, S. Memantine Treatment Exerts An Antidepressant-Like Effect By Preventing Hippocampal Mitochondrial Dysfunction And Memory Impairment Via Upregulation of CREB/BDNF Signaling in the Rat Model of Chronic Unpredictable Stress-Induced Depression. Neurochem. Int. 2021, 142, 104932. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.; Guan, C.; Qiu, L.; Zhang, L.; Zhang, S.; Zhou, H.; Du, H.; Li, C.; Wu, Y.; et al. Effects of Membrane Transport Activity and Cell Metabolism on the Unbound Drug Concentrations in the Skeletal Muscle and Liver of Drugs: A Microdialysis Study in Rats. Pharmacol. Res. Perspect. 2021, 9, e00879. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.C.; Wang, Y.T.; Ren, J. Basic Information about Memantine and Its Treatment of Alzheimer’s Disease and Other Clinical Applications. Ibrain 2023, 9, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments for Alzheimer’s Disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Wongchitrat, P.; Govitrapong, P. A Synopsis of Multitarget Potential Therapeutic Effects of Huperzine A in Diverse Pathologies–Emphasis on Alzheimer’s Disease Pathogenesis. Neurochem. Res. 2022, 47, 1166–1182. [Google Scholar] [CrossRef]
- Roy, N.M.; Al-Harthi, L.; Sampat, N.; Al-Mujaini, R.; Mahadevan, S.; Al Adawi, S.; Essa, M.M.; Al Subhi, L.; Al-Balushi, B.; Qoronfleh, M.W. Impact of Vitamin D on Neurocognitive Function In Dementia, Depression, Schizophrenia and ADHD. Front. Biosci. Landmark 2020, 26, 566–611. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Nguyen, T.H.; Nguyen, D.H. Development, and in vitro Evaluation of Liposomes Using Soy Lecithin to Encapsulate Paclitaxel. Int. J. Biomater. 2017, 2017, 8234712. [Google Scholar] [CrossRef]
- Nguyen, C.K.; Tran, N.Q.; Nguyen, T.P.; Nguyen, D.H. Biocompatible Nanomaterials Based on Dendrimers, Hydrogels, and Hydrogel Nanocomposites for Use in Biomedicine. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 015001. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Patel, R.J.; Pandey, P.; Patel, A.A.; Prajapati, B.G.; Alexander, A.; Pandya, V.; Trivedi, N.; Shah, S.; Patel, V. Ordered mesoporous silica nanocarriers: An innovative paradigm and a promising therapeutic efficient carrier for delivery of drugs. J. Drug Deliv. Sci. Technol. 2023, 82, 104306. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Towards Multifunctional, Targeted Drug Delivery Systems Using Mesoporous Silica Nanoparticles-Opportunities & Challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar]
- Hoang Thi, T.T.; Cao, V.D.; Nguyen, T.N.Q.; Hoang, D.T.; Ngo, V.C.; Nguyen, D.H. Functionalized Mesoporous Silica Nanoparticles and Biomedical Applications. Mater. Sci. Eng. C 2019, 99, 631–656. [Google Scholar] [CrossRef]
- Guo, F.; Li, G.; Ma, S.; Zhou, H.; Chen, X. Multi-responsive Nanocarriers Based on Β-CD-PNIPAM Star Polymer Coated MSN-SS-Fc Composite Particles. Polymers 2019, 11, 1716. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yu, X.; Ruan, Z.; Zhu, M.; Zhu, Y.; Hanagata, N.J.M. Magnetic Mesoporous Silica Nanoparticles Coated with Thermo-Responsive Copolymer for Potential Chemo-and Magnetic Hyperthermia Therapy. Microporous Mesoporous Mater. 2018, 256, 1–9. [Google Scholar] [CrossRef]
- Taghizadeh, B.; Taranejoo, S.; Monemian, S.A.; Salehi Moghaddam, Z.; Daliri, K.; Derakhshankhah, H.; Derakhshani, Z. Classification of Stimuli-Responsive Polymers as Anticancer Drug Delivery Systems. Drug Deliv. 2015, 22, 145–155. [Google Scholar] [CrossRef]
- Lopes, J.R.; Santos, G.; Barata, P.; Oliveira, R.; Lopes, C.M. Physical and Chemical Stimuli-Responsive Drug Delivery Systems: Targeted Delivery and Main Routes of Administration. Curr. Pharm. Des. 2013, 19, 7169–7184. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Shi, H.; Dong, G.; Zhou, M.; Wang, T.; Xin, H. Thermo-responsive Mesoporous Silica/Lipid Bilayer Hybrid Nanoparticles for Doxorubicin on-Demand Delivery and Reduced Premature Release. Colloids Surf. B Biointerfaces 2017, 160, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shang, H.; Lai, S.; Di, Y.; Sun, X.; Qiao, N.; Han, L.; Zhao, Z.; Lu, Y. Preparation and Evaluation of Controllable Drug Delivery System: A Light Responsive Nanosphere Based on Β-Cyclodextrin/Mesoporous Silica. Chin. J. Chem. Eng. 2023, 62, 159–167. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, F.; Xu, N.; Yao, Q.; Wang, R.; Xie, X.; Zhang, F.; He, Y.; Shao, D.; Dong, W.F.; et al. Red-light-triggered Self-Destructive Mesoporous Silica Nanoparticles for Cascade-Amplifying Chemo-Photodynamic Therapy Favoring Antitumor Immune Responses. Biomaterials 2022, 281, 121368. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive Polymers and Their Applications in Nanomedicine. Biointerphases 2012, 7, 9. [Google Scholar] [CrossRef]
- Jia, C.; Wu, H.; Luo, K.; Hao, W.; Wang, S.; Huang, M. Magnetic Silica Nanosystems with NIR-Responsive and Redox Reaction Capacity for Drug Delivery and Tumor Therapy. Front. Chem. 2020, 8, 567652. [Google Scholar] [CrossRef]
- Entzian, K.; Aigner, A. Drug Delivery by Ultrasound-Responsive Nanocarriers for Cancer Treatment. Pharmaceutics 2021, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chen, Y.; Tu, J.; Liufu, C.; Yu, J.; Yuan, Z.; Gong, X.; Chen, Z. Ultrasound Responsive Magnetic Mesoporous Silica Nanoparticle-Loaded Microbubbles for Efficient Gene Delivery. ACS Biomater. Sci. Eng. 2020, 6, 2904–2912. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Shin, H.H.; Choi, H.W.; Lim, J.H.; Mo, S.J.; Ahrberg, C.D.; Lee, J.M.; Chung, B.G. Electro-responsive Hydrogel-Based Microfluidic Actuator Platform for Photothermal Therapy. Lab Chip 2020, 20, 3354–3364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A Study of Chitosan Hydrogel with Embedded Mesoporous Silica Nanoparticles Loaded By Ibuprofen as a Dual Stimuli-Responsive Drug Release System for Surface Coating of Titanium Implants. Colloids Surf. B Biointerfaces 2014, 123, 657–663. [Google Scholar] [CrossRef]
- García-Fernández, A.; Lozano-Torres, B.; Blandez, J.F.; Monreal-Trigo, J.; Soto, J.; Collazos-Castro, J.E.; Alcañiz, M.; Marcos, M.D.; Sancenón, F.; Martínez-Máñez, R. Electro-responsive Films Containing Voltage-Responsive Gated Mesoporous Silica Nanoparticles Grafted onto PEDOT-based Conducting Polymer. J. Control. Release 2020, 323, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, Q.; Song, J.; Li, S.; Li, X.L.; Kang, B.; Chen, H.Y.; Xu, J.J. Photothermally Triggered Copper Payload Release for Cuproptosis-Promoted Cancer Synergistic Therapy. Angew. Chem. 2023, 62, e202213922. [Google Scholar] [CrossRef]
- Mo, R.; Sun, Q.; Xue, J.; Li, N.; Li, W.; Zhang, C.; Ping, Q. Multistage pH-Responsive Liposomes for Mitochondrial-Targeted Anticancer Drug Delivery. Adv. Mater. 2012, 24, 3659–3665. [Google Scholar] [CrossRef]
- Pan, Q.S.; Chen, T.T.; Nie, C.P.; Yi, J.T.; Liu, C.; Hu, Y.L.; Chu, X. In Situ Synthesis of Ultrathin ZIF-8 Film-Coated MSNs for Co-delivering Bcl2 siRNA and Doxorubicin to Enhance Chemotherapeutic Efficacy in Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10, 33070–33077. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Bharathiraja, S.; Manivasagan, P.; Lee, K.D.; Oh, J. Synthesis of Surface Capped Mesoporous Silica Nanoparticles for pH-Stimuli Responsive Drug Delivery Applications. Medchemcomm 2017, 8, 1797–1805. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Mackiewicz, M.; Yazdimamaghani, M. Recent Advances in the Redox-Responsive Drug Delivery Nanoplatforms: A Chemical Structure and Physical Property Perspective. Mater. Sci. Eng. C 2021, 118, 111536. [Google Scholar] [CrossRef]
- Cui, Y.; Dong, H.; Cai, X.; Wang, D.; Li, Y. Mesoporous Silica Nanoparticles Capped with Disulfide-Linked PEG Gatekeepers for Glutathione-Mediated Controlled Release. ACS Appl. Mater. Interfaces 2012, 4, 3177–3183. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, L.; Chang, Y.; Cao, Y.; Chen, C.; Wang, D. pH and Redox Dual-Responsive MSN-S-S-CS as a Drug Delivery System in Cancer Therapy. Materials 2020, 13, 1279. [Google Scholar] [CrossRef]
- Geng, J.; Li, M.; Wu, L.; Chen, C.; Qu, X. Mesoporous Silica Nanoparticle-Based H2O2 Responsive Controlled-Release System Used for Alzheimer’s Disease Treatment. Adv. Healthc. Mater. 2012, 1, 332–336. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Zhang, Y.; Zhou, J.; Sun, S.; Liu, Y. H2O2-Responsive Mesoporous Silica Nanoparticles Integrated with Microneedle Patches for the Glucose-Monitored Transdermal Delivery of Insulin. J. Mater. Chem. B 2017, 5, 8200–8208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Luo, G.F.; Zhu, J.Y.; Xu, X.D.; Zeng, X.; Cheng, D.B.; Li, Y.M.; Wu, Y.; Zhang, X.Z.; Zhuo, R.X.; et al. Enzyme-induced and Tumor-Targeted Drug Delivery System Based on Multifunctional Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 9078–9087. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Hao, Y.; Yuan, Z.; Tao, B.; Chen, M.; Lin, C.; Liu, P.; Cai, K. A Dual-Functional Implant with an Enzyme-Responsive Effect for Bacterial Infection Therapy and Tissue Regeneration. Biomater. Sci. 2020, 8, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Jiang, T.; DiSanto, R.; Tai, W.; Gu, Z. ATP-Triggered Anticancer Drug Delivery. Nat. Commun. 2014, 5, 3364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tozzi, F.; Chen, J.; Fan, F.; Xia, L.; Wang, J.; Gao, G.; Zhang, A.; Xia, X.; Brasher, H.; et al. Intracellular ATP levels are a Pivotal Determinant of Chemoresistance in Colon Cancer Cells. Cancer Res. 2012, 72, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Shah, B.P.; Zhang, Y.; Yang, L.; Lee, K.B. Real-Time Monitoring of ATP-Responsive Drug Release Using Mesoporous-Silica-Coated Multicolor Upconversion Nanoparticles. ACS Nano 2015, 9, 5234–5245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, N.; Guo, W.; Xia, F.; Jiang, L. Highly Efficient Gating of Solid-State Nanochannels by DNA Super Sandwich Structure Containing ATP Aptamers: A Nanofluidic IMPLICATION Logic Device. J. Am. Chem. Soc. 2012, 134, 15395–15401. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, C.; Wei, Z.; Yang, W.; Ge, W.; Qu, X.; Si, W.; Wang, W.; Mou, X.; Dong, X. TME-Triggered MnSiO3@Met@GOx Nanosystem for ATP Dual-Inhibited Starvation/Chemodynamic Synergistic Therapy. Biomaterials 2022, 287, 121682. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Anderson, D.G. Smart Approaches to Glucose-Responsive Drug Delivery. J. Drug Target. 2015, 23, 651–655. [Google Scholar] [CrossRef]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Yan, L.; Wang, X.; Lin, S.; Zeng, Q. Glucose-Responsive Polyelectrolyte Complexes Based on Dendritic Mesoporous Silica for Oral Insulin Delivery. AAPS PharmSciTech 2021, 22, 226. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.-W.; Chrzanowski, W. Silica-Based Mesoporous Nanoparticles for Controlled Drug Delivery. J. Tissue Eng. 2013, 4, 2041731413503357. [Google Scholar] [CrossRef] [PubMed]
- Porrang, S.; Rahemi, N.; Davaran, S.; Mahdavi, M.; Hassanzadeh, B. Preparation and In-Vitro Evaluation of Mesoporous Biogenic Silica Nanoparticles Obtained From Rice and Wheat Husk as a Biocompatible Carrier for Anti-Cancer Drug Delivery. Eur. J. Pharm. Sci. 2021, 163, 105866. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Geng, T.; Md, A.; Banerjee, P.; Wang, B. Novel Scheme for Rapid Synthesis of Hollow Mesoporous Silica Nanoparticles (HMSNs) and Their Application as an Efficient Delivery Carrier for Oral Bioavailability Improvement of Poorly Water-Soluble BCS Type II Drugs. Colloids Surf. B Biointerfaces 2019, 176, 185–193. [Google Scholar] [CrossRef]
- Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Hosseini, M. Mesoporous silica nanoparticles: Synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chem. Eng. J. 2019, 359, 684–705. [Google Scholar] [CrossRef]
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis Methods of Mesoporous Silica Materials. Mater. Today Proc. 2017, 4, 350–357. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Shen, D.; Wu, F.; Pleixats, R.; Pan, J. Functionalized Silica Nanoparticles: Classification, Synthetic Approaches and Recent Advances in Adsorption Applications. Nanoscale 2021, 13, 15998–16016. [Google Scholar] [CrossRef]
- Galabova, B.B. Mesoporous Silica Nanoparticles: Synthesis, Functionalization, Drug Loading and Release-A Review. Trop. J. Pharm. Res. 2021, 20, 1091–1100. [Google Scholar] [CrossRef]
- Khoeini, M.; Najafi, A.; Rastegar, H.; Amani, M. Improvement of Hollow Mesoporous Silica Nanoparticles Synthesis By Hard-Templating Method via CTAB Surfactant. Ceram. Int. 2019, 45, 12700–12707. [Google Scholar] [CrossRef]
- Pérez-Garnes, M.; Gutiérrez-Salmerón, M.; Morales, V.; Chocarro-Calvo, A.; Sanz, R.; García-Jiménez, C.; García-Muñoz, R.A. Engineering Hollow Mesoporous Silica Nanoparticles to Increase Cytotoxicity. Mater. Sci. Eng. C 2020, 112, 110935. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.C.; Monteiro, A.R.; Silva, R.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: Crossing or circumventing the blood-brain barrier (BBB) to manage neurological disorders. Adv. Drug Deliv. Rev. 2022, 189, 114485. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chou, C.M.; Chang, T.Y.; Ting, H.; Dembélé, J.; Chu, Y.T.; Liu, T.P.; Changou, C.A.; Liu, C.W.; Chen, C.T. Bridging Size and Charge Effects of Mesoporous Silica Nanoparticles for Crossing the Blood-Brain Barrier. Front. Chem. 2022, 10, 931584. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, S.S.; Rathore, A.S.; Singh, S.P.; Mishra, G.; Awasthi, R.; Mishra, S.K.; Gautam, V.; Singh, S.K. Lipid-Coated MCM-41 Mesoporous Silica Nanoparticles Loaded with Berberine Improved Inhibition of Acetylcholine Esterase and Amyloid Formation. ACS Biomater. Sci. Eng. 2021, 7, 3737–3753. [Google Scholar] [CrossRef]
- Ribeiro, T.D.; Sábio, R.M.; Luiz, M.T.; de Souza, L.C.; Fonseca-Santos, B.; Cides da Silva, L.C.; Fantini, M.C.; Planeta, C.D.; Chorilli, M. Curcumin-Loaded Mesoporous Silica Nanoparticles Dispersed in Thermo-Responsive Hydrogel as Potential Alzheimer Disease Therapy. Pharmaceutics 2022, 14, 1976. [Google Scholar] [CrossRef]
- Xu, L.; Guo, M.; Hung, C.T.; Shi, X.L.; Yuan, Y.; Zhang, X.; Jin, R.H.; Li, W.; Dong, Q.; Zhao, D. Chiral Skeletons of Mesoporous Silica Nanospheres to Mitigate Alzheimer’s β-Amyloid Aggregation. J. Am. Chem. Soc. 2023, 145, 7810–7819. [Google Scholar] [CrossRef]
- Liu, N.; Yang, C.; Liang, X.; Cao, K.; Xie, J.; Luo, Q.; Luo, H. Mesoporous Silica Nanoparticle-Encapsulated Bifidobacterium Attenuates Brain Aβ Burden and Improves Olfactory Dysfunction of APP/PS1 Mice by Nasal Delivery. J. Nanobiotechnology 2022, 20, 439. [Google Scholar] [CrossRef]
- Swar, S.; Máková, V.; Stibor, I. Effectiveness of Diverse Mesoporous Silica Nanoparticles as Potent Vehicles for the Drug L-DOPA. Materials 2019, 12, 3202. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Sharma, A.K.; Rani, S.; Mishra, G.; Kandasamy, G.; Patra, A.K.; Rana, M.; Sharma, A.K.; Yadav, A.K.; Gupta, U. MCM-41 Nanoparticles for Brain Delivery: Better Choline-Esterase and Amyloid Formation Inhibition with Improved Kinetics. ACS Biomater. Sci. Eng. 2018, 4, 2860–2869. [Google Scholar] [CrossRef]
- Halevas, E.; Mavroidi, B.; Nday, C.M.; Tang, J.; Smith, G.C.; Boukos, N.; Litsardakis, G.; Pelecanou, M.; Salifoglou, A. Modified magnetic core-shell mesoporous silica nano-formulations with encapsulated quercetin exhibit anti-amyloid and antioxidant activity. J. Inorg. Biochem. 2020, 213, 111271. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; He, C. Mesoporous Silica Nanoparticles for Tissue-Engineering Applications. Wiley Interdiscip. Rev. Nanomed. 2019, 11, e1573. [Google Scholar] [CrossRef]
- Chen, W.; Tsai, P.H.; Hung, Y.; Chiou, S.H.; Mou, C.Y. Nonviral cell labeling and differentiation agent for induced pluripotent stem cells based on mesoporous silica nanoparticles. ACS Nano 2013, 7, 8423–8440. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.; Zhang, P.; Cai, L.; Yang, X.; Chen, Y.; Jing, Y.; Kong, J.; Yang, X.; Sun, F.L. Sustained Delivery Growth Factors with Polyethyleneimine-Modified Nanoparticles Promote Embryonic Stem Cells Differentiation and Liver Regeneration. Adv. Sci. 2016, 3, 1500393. [Google Scholar] [CrossRef]
- Farshbaf, M.; Salehi, R.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Davaran, S. pH- and thermo-sensitive MTX-loaded magnetic nanocomposites: Synthesis, characterization, and in vitro studies on A549 lung cancer cell and MR imaging. Drug Dev. Ind. Pharm. 2018, 44, 452–462. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, Z.; Lu, M.; Shao, D.; Yue, J.; Yang, D.; Zheng, X.; Li, M.; He, K.; Zhang, M.; et al. Shape-controlled magnetic mesoporous silica nanoparticles for magnetically-mediated suicide gene therapy of hepatocellular carcinoma. Biomaterials 2018, 154, 147–157. [Google Scholar] [CrossRef]
- Song, G.; Cheng, N.; Zhang, J.; Huang, H.; Yuan, Y.; He, X.; Luo, Y.; Huang, K. Nanoscale cerium oxide: Synthesis, biocatalytic mechanism, and applications. Catalysts 2021, 11, 1123. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Jokar, F.; Hadian, P.; Ma’mani, L.; Gharaghani, S.; Fereidoonnezhad, M.; Salekdeh, G.H. An efficient nano-biocatalyst for lignocellulosic biomass hydrolysis: Xylanase immobilization on organically modified biogenic mesoporous silica nanoparticles. Int. J. Biol. Macromol. 2020, 164, 3462–3473. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, Y.; Lin, V.S.; Slowing, I.I.; Trewyn, B.G. Luciferase and luciferin co-immobilized mesoporous silica nanoparticle materials for intracellular biocatalysis. J. Am. Chem. Soc. 2011, 133, 18554–18557. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, Z.; Chen, Z.; Ren, J.; Qu, X. Mesoporous silica-encapsulated gold nanoparticles as artificial enzymes for self-activated cascade catalysis. Biomaterials 2013, 34, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhang, H.; Liu, C.G.; Kanubaddi, K.R.; Lee, C.H.; Wang, S.B.; Cui, W.; Santos, H.A.; Lin, K.; Chen, A.Z. Metal species–encapsulated mesoporous silica nanoparticles: Current advancements and latest breakthroughs. Adv. Funct. Mater. 2019, 29, 1902652. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Chan, H.N.; Liu, G.; Wang, Z.; Li, H.W.; Wong, M.S. Amyloid-β Oligomer-Targeted Gadolinium-Based NIR/MR Dual-Modal Theranostic Nanoprobe for Alzheimer’s Disease. Adv. Funct. Mater. 2020, 30, 1909529. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xie, R.; Burger, J.C.; Tong, Y.; Gong, S. Overcoming the Blood-Brain Barrier for Gene Therapy via Systemic Administration of GSH-Responsive Silica Nanocapsules. Adv. Mater. 2023, 35, e2208018. [Google Scholar] [CrossRef]
- Li, T.; Geng, Y.; Zhang, H.; Wang, J.; Feng, Y.; Chen, Z.; Xie, X.; Qin, X.; Li, S.; Wu, C.; et al. A versatile nanoplatform for synergistic chemo-photothermal therapy and multimodal imaging against breast cancer. Expert Opin. Drug Deliv. 2020, 17, 725–733, published correction appears in Expert Opin. Drug. Deliv. 2022, 19, i. [Google Scholar] [CrossRef]
- Cortés, H.; Alcalá-Alcalá, S.; Caballero-Florán, I.H.; Bernal-Chávez, S.A.; Ávalos-Fuentes, A.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Floran, B.; et al. A Reevaluation of Chitosan-Decorated Nanoparticles to Cross the Blood-Brain Barrier. Membranes 2020, 10, 212. [Google Scholar] [CrossRef]
- Rastegari, E.; Hsiao, Y.J.; Lai, W.Y.; Lai, Y.H.; Yang, T.C.; Chen, S.J.; Huang, P.I.; Chiou, S.H.; Mou, C.Y.; Chien, Y. An update on mesoporous silica nanoparticle applications in nanomedicine. Pharmaceutics 2021, 13, 1067. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, K.; Liu, J.; Sun, G.; Yin, Q.; Lu, L. Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials 2015, 60, 111–120. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, J.; Xu, X. Emerging and innovative theranostic approaches for mesoporous silica nanoparticles in hepatocellular carcinoma: Current status and advances. Front. Bioeng. Biotechnol. 2020, 8, 184. [Google Scholar] [CrossRef]
- Robertson, J.D.; Rizzello, L.; Avila-Olias, M.; Gaitzsch, J.; Contini, C.; Magoń, M.S.; Renshaw, S.A.; Battaglia, G. Purification of Nanoparticles by Size and Shape. Sci. Rep. 2016, 6, 27494. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Han, Y.-H.; Xia, H.-Y.; Wang, S.-B.; Chen, A.-Z. Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications. J. Nanobiotechnology 2022, 20, 126. [Google Scholar] [CrossRef]
- Garcia-Fernandez, A.; Sancenon, F.; Martinez-Manez, R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2021, 177, 113953. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Vallet-Regí, M. Mesoporous silica nanoparticles as theranostic antitumoral nanomedicines. Pharmaceutics 2020, 12, 957. [Google Scholar] [CrossRef] [PubMed]
- Florensa, M.; Llenas, M.; Medina-Gutiérrez, E.; Sandoval, S.; Tobías-Rossell, G. Key parameters for the rational design, synthesis, and functionalization of biocompatible mesoporous silica nanoparticles. Pharmaceutics 2022, 14, 2703. [Google Scholar] [CrossRef]
- Gao, J.; Fan, K.; Jin, Y.; Zhao, L.; Wang, Q.; Tang, Y.; Xu, H.; Liu, Z.; Wang, S.; Lin, J. PEGylated lipid bilayer coated mesoporous silica nanoparticles co-delivery of paclitaxel and curcumin leads to increased tumor site drug accumulation and reduced tumor burden. Eur. J. Pharm. Sci. 2019, 140, 105070. [Google Scholar] [CrossRef]
- Fernandes, N.B.; Nayak, Y.; Garg, S.; Nayak, U.Y. Multifunctional engineered mesoporous silica/inorganic material hybrid nanoparticles: Theranostic perspectives. Coord. Chem. Rev. 2023, 478, 214977. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef]










| MSNs/ Subtype | Drug Used | Method of Preparation | Physiochemical Parameters | Outcomes | Ref. |
|---|---|---|---|---|---|
| MCM-41 | BBR | Passive approach | Entrapment efficiency: 75.21 ± 1.59% Particle size: 143 ± 11 nm Polydispersity index: 0.30 ± 1.28 Zeta potential: 8.7 ± 1.9 mV | MSNs-BBR-L showed AChE inhibitory activity. MSNs-BBR-L significantly reduced the BACE-1 level in vivo. | [87] |
| MCM and SBA, PHTS, MCF | L-Dopa | Soft templating approach | MCM-41(S): Particle size: 200 to 900 nm Surface area: 880 m2/g Pore volume: 0.72 cm3/g Loading capacity: 5.1 ± 0.3% MCM-41(HO): Particle size: 400 to 600 nm Surface area: 1120 m2/g Pore volume: 0.75 cm3/g Loading capacity: 5.3 ± 0.5% MCM-48: Particle size: >1000 nm Surface area: 470 m2/g Pore volume: 0.77 cm3/g Loading capacity: 4.9 ± 0.3% PHTS: Particle size: >1000 nm Surface area: 940 m2/g Pore volume: 0.83 cm3/g Loading capacity: 4.2 ± 0.6% MCF: Particle size: >1000 nm Surface area: 760 m2/g Pore volume: 1.88 cm3/g Loading capacity: 5.6 ± 0.2% SBA-15: Particle size: 300 to 500 nm Surface area: 1020 m2/g Pore volume: 1.17 cm3/g Loading capacity: 5.9 ± 0.3% | SBA-15 with elevated surface area and substantial pore volume was developed. SBA-15 exhibited the most significant drug release. | [91] |
| MCM-41 | Rivastigmine | Solvent equilibrium approach | Entrapment efficiency: 89 ± 1.45% Particle size: 145 ± 0.5 nm Zeta potential: −38.9 ± 1.4 mV | Delivered rivastigmine (127 fold) effectively to the brain in vivo. The bioavailability of the drug was increased (12.3 fold). The efficiency of encapsulated drugs was higher than naked drugs. Choline-esterase and amyloid formation inhibition activity. | [92] |
| -- | Quercetin | Sol-gel approach | Entrapment efficiency: 71.65% Pore size: 169.97 Å Pore volume: 0.75 cm3/g Surface charge −34 ± 0.55 mV Surface area: 109.34 m2/g | Drug-loaded nanocarriers showed enhanced stability and solubility of the drug. Anti-amyloid and antioxidant activity. | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivamaruthi, B.S.; Kapoor, D.U.; Kukkar, R.R.; Gaur, M.; Elossaily, G.M.; Prajapati, B.G.; Chaiyasut, C. Mesoporous Silica Nanoparticles: Types, Synthesis, Role in the Treatment of Alzheimer’s Disease, and Other Applications. Pharmaceutics 2023, 15, 2666. https://doi.org/10.3390/pharmaceutics15122666
Sivamaruthi BS, Kapoor DU, Kukkar RR, Gaur M, Elossaily GM, Prajapati BG, Chaiyasut C. Mesoporous Silica Nanoparticles: Types, Synthesis, Role in the Treatment of Alzheimer’s Disease, and Other Applications. Pharmaceutics. 2023; 15(12):2666. https://doi.org/10.3390/pharmaceutics15122666
Chicago/Turabian StyleSivamaruthi, Bhagavathi Sundaram, Devesh U. Kapoor, Rajiv R. Kukkar, Mansi Gaur, Gehan M. Elossaily, Bhupendra G. Prajapati, and Chaiyavat Chaiyasut. 2023. "Mesoporous Silica Nanoparticles: Types, Synthesis, Role in the Treatment of Alzheimer’s Disease, and Other Applications" Pharmaceutics 15, no. 12: 2666. https://doi.org/10.3390/pharmaceutics15122666
APA StyleSivamaruthi, B. S., Kapoor, D. U., Kukkar, R. R., Gaur, M., Elossaily, G. M., Prajapati, B. G., & Chaiyasut, C. (2023). Mesoporous Silica Nanoparticles: Types, Synthesis, Role in the Treatment of Alzheimer’s Disease, and Other Applications. Pharmaceutics, 15(12), 2666. https://doi.org/10.3390/pharmaceutics15122666











