Fish Scale Gelatin Nanofibers with Helichrysum italicum and Lavandula latifolia Essential Oils for Bioactive Wound-Healing Dressings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fish Scale Gelatin Preparation
2.2.2. Essential Oil Emulsions Preparation
2.2.3. Preparation of Fish Scale Gelatin Nanofibers without and with Essential Oil Emulsions
2.3. Investigation Methods
2.3.1. Characterization of Fish Scales and Fish Scale Gelatins
2.3.2. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy (SEM–EDX) Analysis
2.3.3. Antimicrobial Activity
Antimicrobial Activity and Minimum Inhibitory Concentrations of Essential Oils
Determination of Microbial Contamination of Fish Scale Gelatin Nanofibers with and without Essential Oil Emulsions
2.3.4. Cytotoxicity Evaluation for Fish Scale Gelatin Nanofibers with and without Essential Oil Emulsions
2.3.5. The “Scratch-Test” on Human Dermal Fibroblasts
2.3.6. Statistical Analysis
3. Results and Discussions
3.1. Physical–Chemical Characteristics of Fish Scale Gelatins
3.2. Scanning Electron Microscopy–Energy Dispersive-X ray (SEM–EDX)
3.3. Antimicrobial Properties of Essential Oils
3.4. Antimicrobial Properties of Fish Scale Gelatin Nanofibers
3.5. Evaluation of the Cytotoxic Effect of Fish Scale Gelatin Nanofibers and Helichrysum Italicum and Lavandula Latifolia Essential Oils with Fish Scale Gelatin Nanofibers on Human Dermal Fibroblast Cells
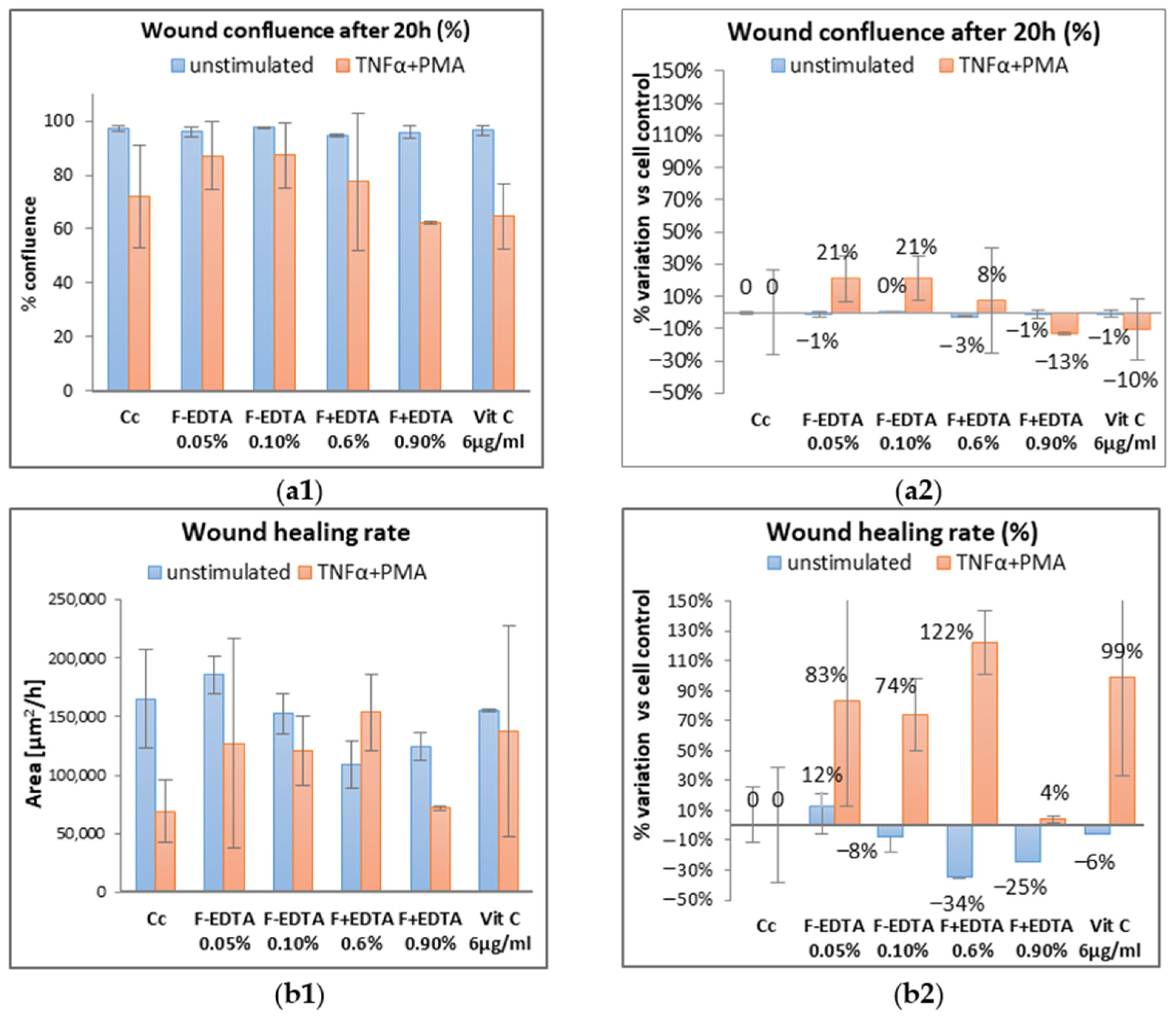
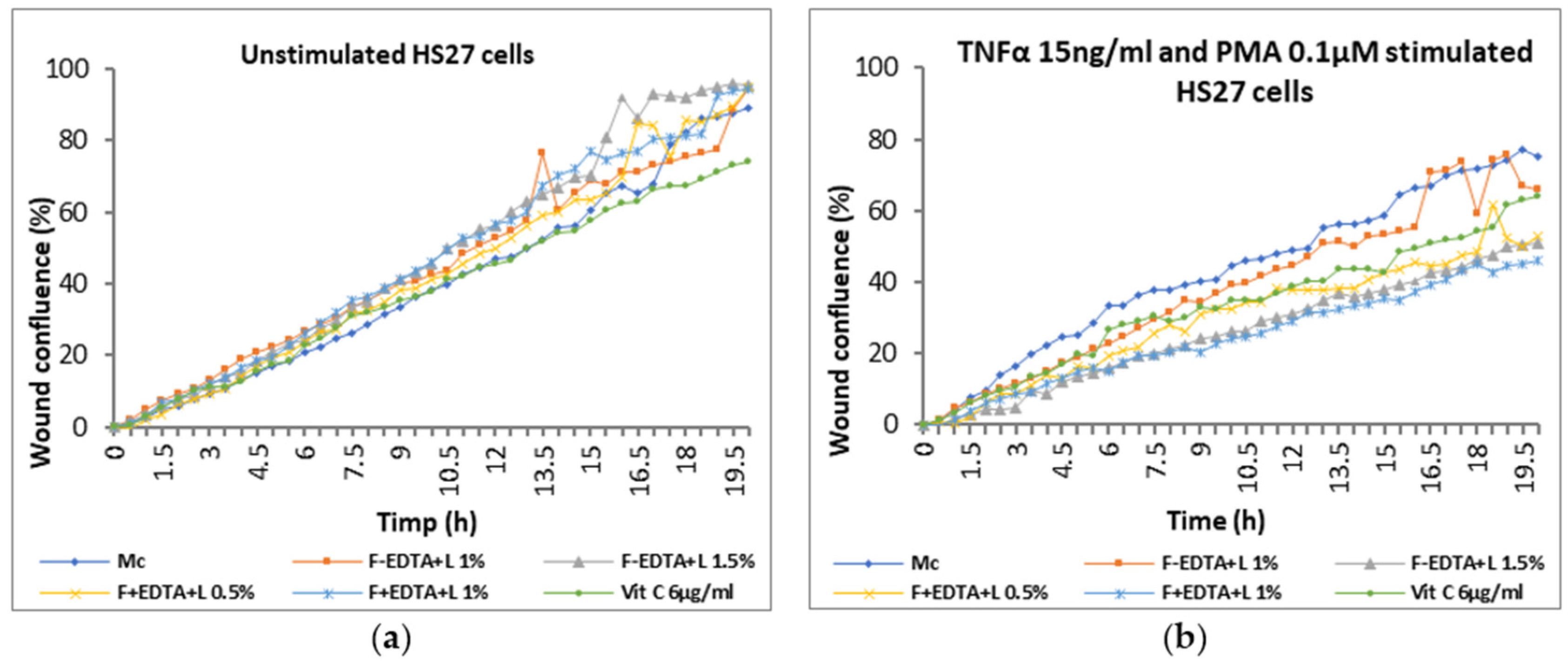
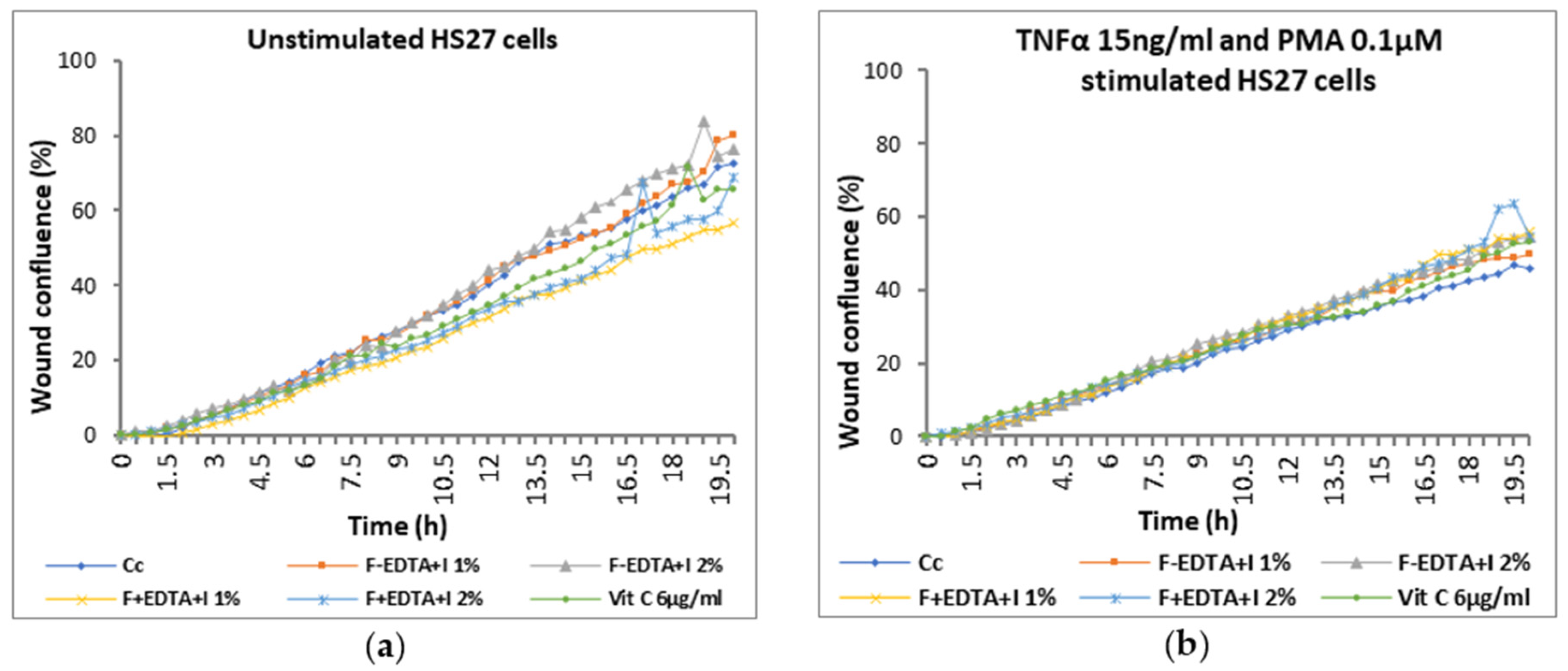
3.6. The “Scratch-Test” on Human Dermal Fibroblasts
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Behrens, A.M.; Casey, B.J.; Sikorski, M.J.; Wu, K.L.; Tutak, W.; Sandler, A.D.; Kofinas, P. In Situ deposition of PLGA nanofibers via solution blow spinning. ACS Macro Lett. 2014, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P.; Kharaziha, M.; Nikkhah, M.; Shin, S.R.; Jin, J.; He, S.; Sun, W.; Zhong, C.; Dokmeci, M.R.; Khademhosseini, A.; et al. Chitin nanofiber micropatterned flexible substrates for tissue engineering. J. Mater. Chem. B 2013, 34, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zou, W.; Su, Y.; Zhu, Y.; Jiang, H.; Shen, J.; Li, C. Activated nitrogen-doped carbon nanofibers with hierarchical pore as efficient oxygen reduction reaction catalyst for microbial fuel cells. J. Power Sources 2014, 266, 36–42. [Google Scholar] [CrossRef]
- Liao, Y.; Li, X.G.; Hoek, E.M.V.; Kaner, R.B. Carbon nanotube/polyaniline nanofiber ultrafiltration membranes. J. Mater. Chem. A 2013, 1, 15390–15396. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; He, X.; Zhang, W.; Zhang, X.; Lu, C. In Situ synthesis of MnO2 coated cellulose nanofibers hybrid for effective removal of methylene blue. Carbohydr. Polym. 2014, 110, 302–308. [Google Scholar] [CrossRef]
- Ayad, M.M.; Salahuddin, N.A.; Minisy, I.M.; Amer, W.A. Chitosan/polyaniline nanofibers coating on the quartz crystal microbalance electrode for gas sensing. Sens. Actuators B 2014, 202, 144–153. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Zhang, Y.; Zhang, D.; Tang, Z.; Chen, X.; Wang, C. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int. J. Biol. Macromol. 2014, 68, 92–97. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Kenry; Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; and Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Wound dressings: Current advances and future directions. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Le, T.T.N.; Nguyen, T.; Lea, H.N.T.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Mrkolj, V.S. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Persano, L.; Camoseo, A.; Tekme, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Jiffrin, R.; Razak, S.I.A.; Jamaludin, M.I.; Hamzah, A.S.A.; Mazian, M.A.; Jaya, M.A.T.; Nasrullah, M.Z.; Majrashi, M.; Theyab, A.; Aldarmahi, A.A.; et al. Electrospun Nanofiber Composites for Drug Delivery: A Review on Current Progresses. Polymers 2022, 14, 3725. [Google Scholar] [CrossRef]
- Dolgin, J.; Hanumantharao, S.N.; Farias, S.; Simon, C.G., Jr.; Rao, S. Mechanical Properties and Morphological Alterations in Fiber-Based Scaffolds Affecting Tissue Engineering Outcomes. Fibers 2023, 11, 39. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef]
- Ababzadeh, S.; Farzin, A.; Goodarzi, A.; Karimi, R.; Farahani, M.S.; Farsani, M.E.; Gharibzad, K.; Zahiri, M.; Ai, J. High porous electrospun poly(ε-caprolactone)/gelatin/MgO scaffolds preseeded with endometrial stem cells promote tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2020, 108, 2961–2970. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of skin regeneration through co-axial electrospun fibersloaded with basic fibroblast growth factor. Adv. Compos. Hybrid. Mater. 2022, 5, 1111–1125. [Google Scholar] [CrossRef]
- Goudarzi, Z.M.; Behzad, T.; Ghasemi-Mobarakeh, L.; Kharaziha, M. An investigation into influence of acetylated cellulose nanofibers on properties of PCL/Gelatin electrospun nanofibrous scaffold for soft tissue engineering. Polymer 2021, 213, 123313. [Google Scholar] [CrossRef]
- Chandika, P.; Oh, G.-W.; Heo, S.-Y.; Kim, S.-C.; Kim, T.-H.; Kim, M.-S.; Jung, W.-K. Electrospun porous bilayer nano-fibrousfish collagen/PCL bio-composite scaffolds with covalently cross-linked chitooligosaccharides for full-thickness wound-healing applications. Mater. Sci. Eng. C 2021, 121, 111871. [Google Scholar] [CrossRef]
- Li, P.; Ruan, L.; Wang, R.; Liu, T.; Song, G.; Gao, X.; Jiang, G.; Liu, X. Electrospun Scaffold of Collagen and Polycaprolactone Containing ZnO Quantum Dots for Skin Wound Regeneration. J. Bionic Eng. 2021, 18, 1378–1390. [Google Scholar] [CrossRef]
- Takarina, N.D.; Fanani, A.A. Characterization of Chitin and Chitosan Synthesized from Red Snapper (Lutjanus sp.) Scale’s Waste. AIP Conf. Proc. 2017, 1862, 030108. [Google Scholar] [CrossRef]
- Sockalingam, K.; Abdullah, H.Z. Extraction and Characterization of Gelatin Biopolymer from Black Tilapia (Oreochromis Mossambicus) Scales. Malays. J. Microsc. 2014, 10, 132–137. [Google Scholar]
- Salindeho, N.; Mokolensang, F.J.; Manu, L.; Nurpudji Astuti Taslim, N.A.; Fahrul Nurkolis, F.; Gunawan, W.B.; Yusuf, M.; Mayulu, N.; Tsopmo, A. Fish scale rich in functional compounds and peptides: A potential nutraceutical to overcome undernutrition. Front. Nutr. 2022, 9, 1072370. [Google Scholar] [CrossRef]
- Battaglia, S. The Complete Guide to Aromatherapy; Brisbane: Perfect Potion; The International Centre of Holistic Aromatherapy: Zillmere, Australia, 2004.
- Wildwood, C. The Encyclopedia of Aromatherapy; US edition; Healing Arts Press: Rochester, NY, USA, 1996. [Google Scholar]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Viegas, D.A.; Palmeira-de-Oliveira, A.; Salgueiro, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Helichrysumitalicum: From traditional use to scientific data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef]
- Mihai (Bășa), D.-C.; Lia-Maria Dițu, L.-M.; Gheorghe, I.; Banu, O.; Georgescu, M.; Mihăescu, G. Investigation of the Antibiofilm Activity of Some Spices and Medicinal Plants Essential Oils. Biointerface Res. Appl. Chem. 2023, 13, 301. [Google Scholar] [CrossRef]
- Mesic, A.; Mahmutović-Dizdarević, I.; Tahirović, E.; Durmišević, I.; Eminovic, I.; Jerković-Mujkić, A.; Bešta-Gajević, R. Evaluation of toxicological and antimicrobial activity of lavender and immortelle essential oils. Drug Chem. Toxicol. 2019, 44, 190–197. [Google Scholar] [CrossRef]
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; Di Salle, A.; Germann, N. Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review. Appl. Sci. 2021, 11, 1713. [Google Scholar] [CrossRef]
- Souza do Nascimento, A.; Tamiasso, R.S.S.; Morais, S.F.M.; Gnatta, J.R.; Turrini, R.N.T.; Calache, A.L.S.; Poveda, V.B. Essential oils for healing and/or preventing infection of surgical wounds: A systematic review. Rev. Esc. Enferm. USP 2022, 56, e20210442. [Google Scholar] [CrossRef]
- Akagündüz, Y.; Mosquera, M.; Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Sea bream bones and scales as a source of gelatin and ACE inhibitory peptides. LWT-Food Sci. Technol. 2014, 55, 579–585. [Google Scholar] [CrossRef]
- He, J.; Zhang, J.; Xu, Y.; Ma, Y.; Guo, X. The Structural and Functional Differences between Three Species of Fish Scale Gelatin and Pigskin Gelatin. Foods 2022, 11, 3960. [Google Scholar] [CrossRef]
- Jakhar, J.K.; Kumar, A.; Vardia, H.K. Extraction of Gelatin from Skin and Scale of Indian Major Carps. Environ. Ecol. 2016, 34, 1513–1518. [Google Scholar]
- SR EN ISO 4684:2006. Piele. Analize Chimice. Determinarea Materiilor Volatile. Available online: https://magazin.asro.ro/ro/standard/112366 (accessed on 1 June 2023).
- SR EN ISO 4047:2002. Piei Finite. Determinarea Cenuşii Totale Sulfatate şi a Cenuşii Sulfatate Insolubilă în Apă. Available online: https://magazin.asro.ro/ro/standard/820 (accessed on 1 June 2023).
- SR EN ISO 5397:1996. Leather—Determination of Nitrogen Content and “Hide Substance”—Titrimetric Method. Available online: https://www.iso.org/standard/11439.html (accessed on 1 June 2023).
- SR EN 27888:1997. Calitatea Apei. Determinarea Conductivităţii Electrice. Available online: https://magazin.asro.ro/ro/standard/22665 (accessed on 1 June 2023).
- STAS 8619/3-90. PH-Metrie. Determinarea Electrometrică a pH-ului Soluţiilor Apoase. Available online: https://magazin.asro.ro/ro/standard/21072 (accessed on 1 June 2023).
- Official Procedure of the Gelatin Manufacturers Institute of America, Inc. Gmia Standard Methods for the Testing of Edible Gelatin. Available online: http://www.gelatin-gmia.com/2016 (accessed on 24 September 2023).
- CellTiter 96® AQueous One Solution Cell Proliferation Assay. CytoTox 96® Non-Radioactive Cytotoxicity Assay. Available online: http://www.promega.ro (accessed on 15 July 2023).
- Silva, R.S.G.; Bandeira, S.F.; Pinto, L.A.A. Characteristics and chemical composition of skin gelatin from cobia (Rhacycentron canadum). LWT-Food Sci. Technol. 2014, 57, 580–585. [Google Scholar] [CrossRef]
- Le Corre-Bordes, D.; Hofman, K.; Hall, B. Guide to electrospinning denaturated whole chain collagen from hoki fish using benign sovents. Int. J. Biol. Mol. 2018, 112, 1289–1296. [Google Scholar]
- Nurilmala, M.; Suryamarevita, H.; Hizbullah, H.H.; Jacoeb, A.M.; Ochiai, Y. Fish skin as a biomaterial for halal collagen and gelatin. Saudi J. Biol. Sci. 2022, 29, 1100–1110. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołegowska, B. The Antibacterial Activity of Lavender Essential Oil Alone and in Combination with Octenidine Dihydrochloride against MRSA Strains. Molecules 2020, 25, 95. [Google Scholar] [CrossRef]
- Balázs, V.L.; Filep, R.; Répás, F.; Kerekes, E.; Szabó, P.; Kocsis, B.; Böszörményi, A.; Krisch, J.; Horváth, G. Immortelle (Helichrysum italicum (Roth) G. Don) Essential Oil Showed Antibacterial and Biofilm Inhibitory Activity against Respiratory Tract Pathogens. Molecules 2022, 27, 5518. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Buton, N.; Badea, M.L.; Marutescu, L. Antimicrobial activity of new materials based on lavender and basil essential oils and hydroxyapatite. Nanomaterials 2018, 8, 291. [Google Scholar] [CrossRef]
- De Rapper, S.; Viljoen, A.; van Vuuren, S. The in vitro antimicrobial effects of Lavandula angustifolia essential oil in combination with conventional antimicrobial agents. Evid. Based Complement. Alternat. Med. 2016, 2016, 2752739. [Google Scholar] [CrossRef]
- Mori, H.-M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef]
- Voincheti, V.; Giraud-Robert, A.-M. Utilisation de l’huile essentielle d’helichryse italienne et de l’huile vegerale de rose musque apres intervention de chirurgie plastique reparatrice et esthetique. Phytotherapie 2007, 2, 67–72. [Google Scholar] [CrossRef]
- Djihane, B.; Wafa, N.; Elkhamssa, S.; Pedro, D.H.J.; Maria, A.E.; Mihoub, Z.M. Chemical constituents of Helichrysum italicum (Roth) G. Don essential oil and their antimicrobial activity against Gram-positive and Gram-negative bacteria, filamentous fungi and Candida albicans. Saudi Pharm. J. 2017, 25, 780–787. [Google Scholar] [CrossRef]
- Olariu, L.; Dumitriu, B.G.; Gaidau, C.; Stanca, M.; Tanase, L.M.; Ene, M.D.; Stanculescu, I.-R.; Tablet, C. Bioactive Low Molecular Weight Keratin Hydrolysates for Improving Skin Wound Healing. Polymers 2022, 14, 1125. [Google Scholar] [CrossRef]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Smith, S.M.; Wunder, M.B.; Norris, D.A.; Shellman, Y.G. A Simple Protocol for Using a LDH-Based Cytotoxicity Assay to Assess the Effects of Death and Growth Inhibition at the Same Time. PLoS ONE 2011, 6, e26908. [Google Scholar] [CrossRef]
- Sardareh, E.A.; Shahzeidi, M.; Ardestani, M.T.S.; Mousavi-Khattat, M.; Zarepour, A.; Zarrabi, A. Antimicrobial Activity of Blow Spun PLA/Gelatin Nanofibers Containing Green Synthesized Silver Nanoparticles against Wound Infection-Causing Bacteria. Bioengineering 2022, 9, 518. [Google Scholar] [CrossRef]
- Nezamoleslami, S.; Fattahi, A.; Nemati, H.; Bagrezaie, F.; Pourmanouchehri, Z.; Kiaie, S.H. Electrospun sandwich-structured of polycaprolactone/gelatin-based nanofibers with controlled release of ceftazidime for wound dressing. Int. J. Biol. Macromol. 2023, 236, 123819. [Google Scholar] [CrossRef]
- Ali, I.H.; Ouf, A.; Elshishiny, F.; Taskin, M.B.; Song, J.; Dong, M.; Chen, M.; Siam, R.; Mamdouh, W. Antimicrobial and Wound-Healing Activities of Graphene-Reinforced Electrospun Chitosan/Gelatin Nanofibrous Nanocomposite Scaffolds. ACS Omega 2022, 7, 1838–1850. [Google Scholar] [CrossRef]
- Chi, H.Y.; Chang, N.Y.; Li, C.; Chan, V.; Hsieh, J.H.; Tsai, Y.H.; Lin, T.C. Fabrication of Gelatin Nanofibers by Electrospinning-Mixture of Gelatin and Polyvinyl Alcohol. Polymers 2022, 14, 2610. [Google Scholar] [CrossRef]
- Kwak, H.W.; Shin, M.; Lee, J.Y.; Yun, H.; Song, D.W.; Yang, Y.; Shin, B.S.; Park, Y.H.; Lee, K.H. Fabrication of an ultrafine fish gelatin nanofibrous web from an aqueous solution by electrospinning. Int. J. Biol. Macromol. 2017, 102, 1092–1103. [Google Scholar] [CrossRef]
- Masae, R.; Kazuyoshi, K.; Emi, K.; Hiromasa, T.; Keiko, I.; Yoshimichi, I.; Ryoko, M.; Masahiro, T. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar]
- Damascena, H.L.; Silveira, W.A.A.; Castro, M.S.; Fontes, W. Neutrophil Activated by the Famous and Potent PMA (Phorbol Myristate Acetate). Cells 2022, 11, 2889. [Google Scholar] [CrossRef]
- Weeks, B.S.; Fu, R.; Zaidi, M. Vitamin C Promotes Wound Healing: The Use of In Vitro Scratch Assays to Assess Re-Epithelialization. In Cell Physiology—Annual Volume 2023; IntechOpen: London, UK, 2023. [Google Scholar]
- Chaitrakoonthong, T.; Ampornaramveth, R.; Kamolratanakul, P. Rinsing with L-Ascorbic Acid Exhibits Concentration-Dependent Effects on Human Gingival Fibroblast In Vitro Wound Healing Behavior. Int. J. Dent. 2020, 2020, 4706418. [Google Scholar] [CrossRef]
- Beishenaliev, A.; Lim, S.S.; Tshai, K.Y.; Khiew, P.S.; Sghayyar, H.N.M.; Loh, H.-S. Fabrication and preliminary in vitro evaluation of ultraviolet crosslinked electrospun fish scale gelatin nanofibrous scaffolds. J. Mater. Sci. Mater. Med. 2019, 30, 62. [Google Scholar] [CrossRef]
- Chester, D.; Marrow, E.A.; Daniele, M.A.; Brown, A.C. Wound Healing and the Host Response in Regenerative Engineering. Encycl. Biomed. Eng. 2019, 1, 707–718. [Google Scholar] [CrossRef]
- Yao, C.H.; Chen, K.Y.; Chen, Y.S.; Li, S.J.; Huang, C.H. Lithospermi radix extract-containing bilayer nanofiber scaffold for promoting wound healing in a rat model. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 850–858. [Google Scholar] [CrossRef]
- Gomes, S.R.; Rodrigues, G.; Martins, G.G.; Roberto, M.A.; Mafra, M.; Henriques, C.M.R.; Silva, J.C. In Vitro and in vivo evaluation of electrospun nanofibers of PCL, chitosan and gelatin: A comparative study. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 348–358. [Google Scholar] [CrossRef]
- Sghayyar, H.N.M.; Lim, S.S.; Ahmed, I.; Lai, J.Y.; Cheong, X.Y.; Chong, Z.W.; Lim, A.F.X.; Loh, H.-S. Fish biowaste gelatin coated phosphate-glass fibres for wound-healing application. Eur. Polym. J. 2020, 122, 109386. [Google Scholar] [CrossRef]
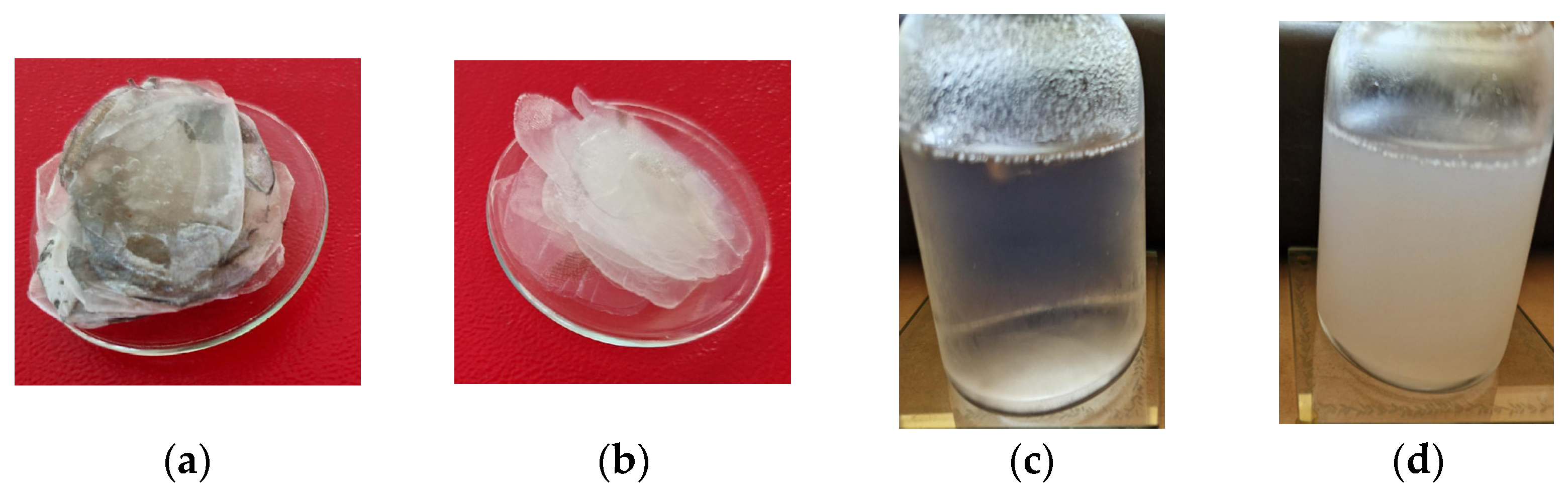
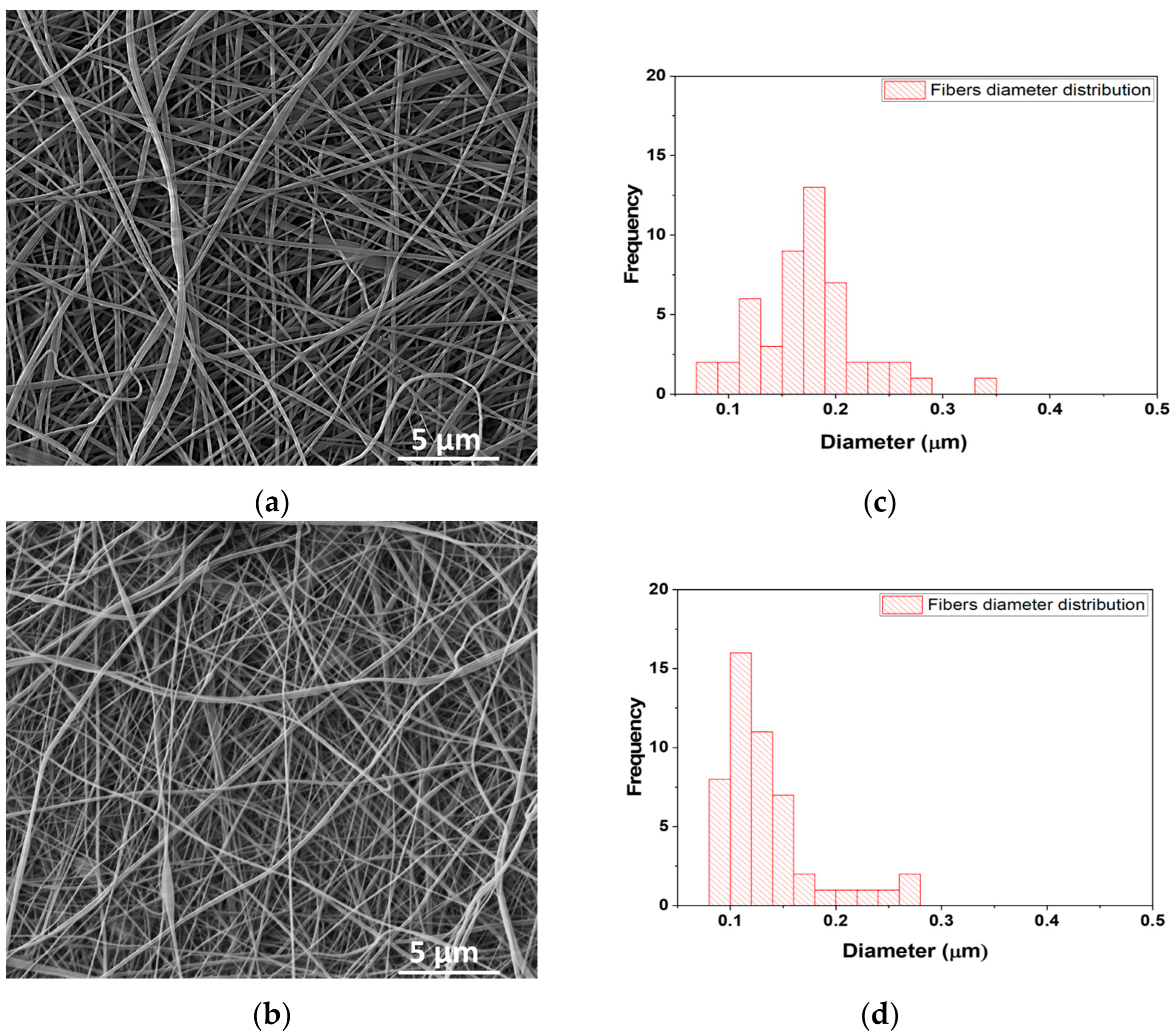
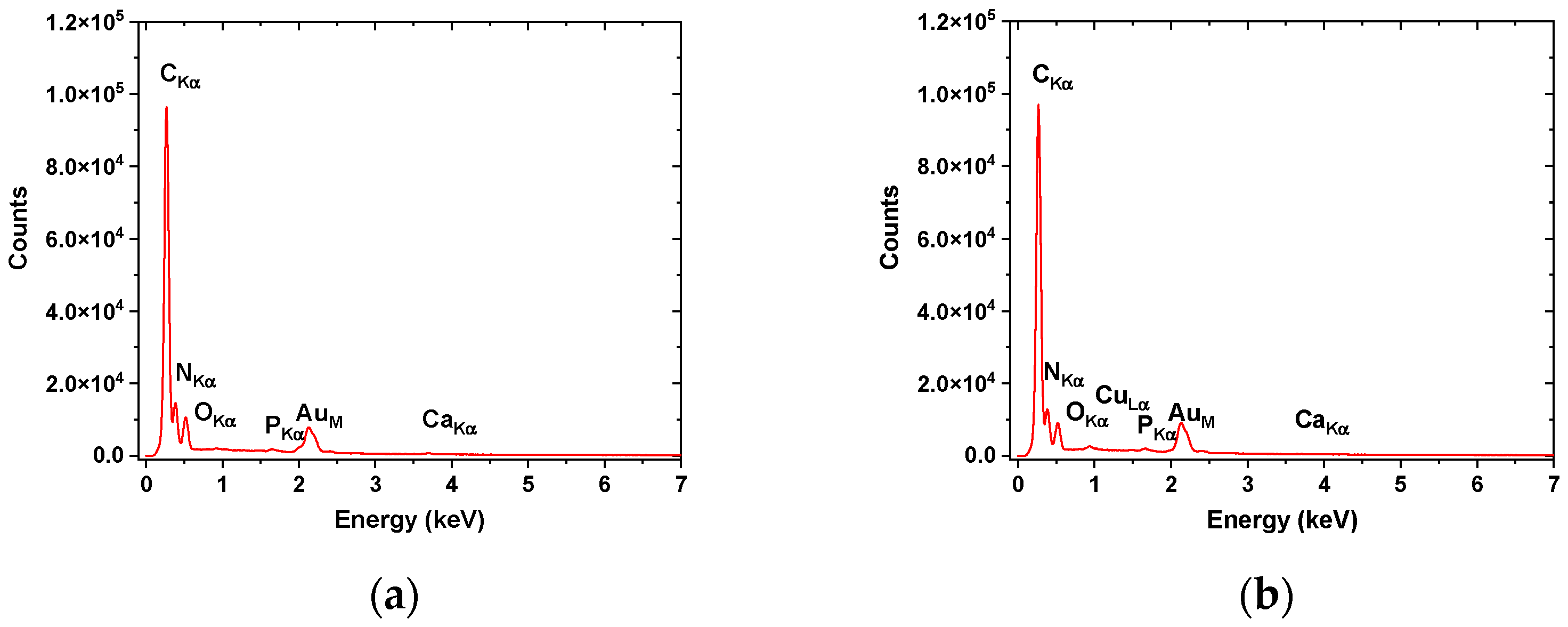




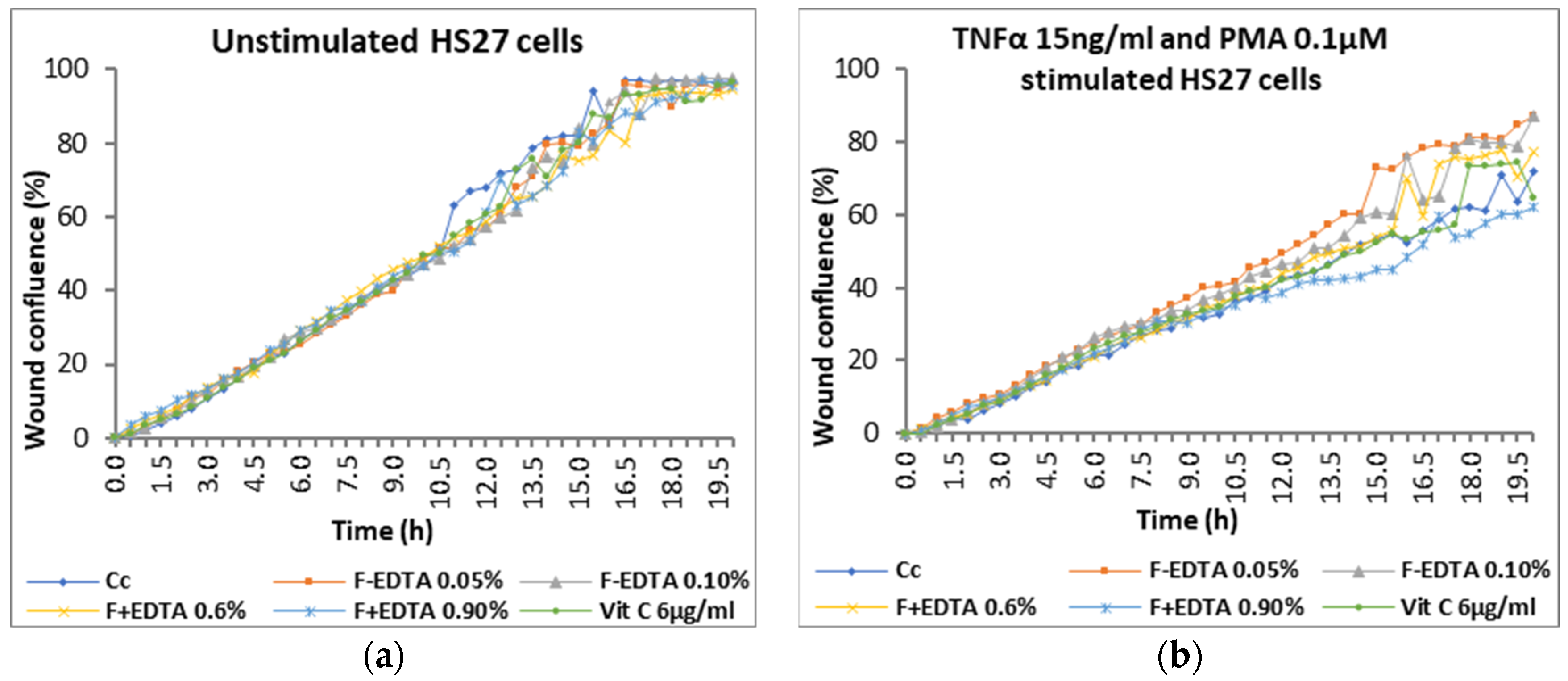


| Sample Abbreviation | Description |
|---|---|
| F-EDTA | Nanofibers of fish scale gelatin without EDTA |
| F+EDTA | Nanofibers of fish scale gelatin with EDTA |
| F-EDTA+L | Nanofibers of fish scale gelatin without EDTA, with lavender essential oil emulsion |
| F-EDTA+I | Nanofibers of fish scale gelatin without EDTA, with immortelle essential oil emulsion |
| F+EDTA+L | Nanofibers of fish scale gelatin with EDTA and lavender essential oil emulsion |
| F+EDTA+I | Nanofibers of fish scale gelatin with EDTA and immortelle essential oil emulsion |
| Parameters | F-EDTA | F+EDTA | F-EDTA+L | F-EDTA+I | F+EDTA+L | F+EDTA+I |
|---|---|---|---|---|---|---|
| Flow rate (mL/h) | 1.4 | 0.8 | 0.9/0.3 | 1.2/0.7 | 1.9/0.7 | 0.7/0.3 |
| Voltage supply (kV) | 22.15 | 22.11 | 23.60 | 23.60 | 23.60 | 23.60 |
| Collector distance (mm) | 120 | 120 | 120 | 120 | 120 | 120 |
| Characteristics | Gelatins Extracted from Fish Scales | |
|---|---|---|
| F-EDTA | F+EDTA | |
| Dry substance, % | 3.61 ± 0.35 | 4.82 ± 0.35 |
| Total ash, % | 0.02 ± 0.01 | nd |
| pH (1:10), pH units | 6.59 ± 0.10 | 4.91 ± 0.10 |
| Bloom test, g | 249.40 | 362.10 |
| Relaxation, % | 34.50 | 14.30 |
| Viscosity, CP | 3.35 ± 0.05 | 4.00 ± 0.08 |
| Conductivity, μS/cm | 344 ± 0.10 | 442 ± 0.10 |
| MW, mol/g | 292,008 | 346,816 |
| Polydispersity | 1.01 | 1.03 |
| Band No. | F-EDTA | F+EDTA | ||
|---|---|---|---|---|
| MW (kDa) | Band (%) | MW (kDa) | Band (%) | |
| 1 | 250 | 4.4 | 250 | 8.1 |
| 2 | 250 | 3.1 | 249.8 | 30.4 |
| 3 | 238.9 | 2.7 | 160.3 | 3.5 |
| 4 | 181.9 | 5.9 | 147.1 | 3.1 |
| 5 | 147.3 | 9.9 | 129.7 | 19.9 |
| 6 | 134.6 | 15.8 | 119.5 | 10.9 |
| 7 | 119.7 | 6.9 | 107.3 | 3.2 |
| 8 | 105.6 | 1.8 | 96.0 | 4.0 |
| 9 | 79.4 | 20.3 | 88.1 | 2.7 |
| 10 | 69.9 | 5.7 | ||
| 11 | 60.4 | 0.7 | ||
| 12 | 55.8 | 2.3 | ||
| 13 | 44.7 | 5.6 | ||
| 14 | 38.3 | 3.2 | ||
| 15 | 25.7 | 3.2 | ||
| 16 | 20.4 | 2.2 | ||
| 17 | 15 | 0.7 | ||
| 18 | 10 | 1.7 | ||
| 19 | 10 | 1 | ||
| 20 | 10 | 2.3 | ||
| Nanofibers | C | N | O | P | Ca | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wt% | At% | Wt% | At% | Wt% | At% | Wt% | At% | Wt% | At% | |
| F-EDTA | 45.03 | 58.87 | 24.49 | 27.45 | 11.48 | 11.27 | 1.15 | 0.58 | 1.03 | 0.40 |
| F+EDTA | 46.98 | 62.76 | 22.63 | 25.92 | 9.25 | 9.28 | 0.27 | 0.14 | 0.15 | 0.06 |
| Essential Oils/ Reference | Inhibition Zones, mm | ||
|---|---|---|---|
| E. coli | S. aureus | C. albicans | |
| Helichrysum italicum | 9.83 ± 0.76 | 31.50 ± 0.50 | 11.67 ± 0.58 |
| Lavandula latifolia | 13.50 ± 1.50 | 46.50 ± 0.50 | 17.33 ± 3.51 |
| Ampicillin | 8.00 ± 2.00 | 32.67 ± 2.52 | 21.32 ± 0.68 |
| Microorganisms | MIC, µL × mL−1 | ||
|---|---|---|---|
| Helichrysum italicum | Lavandula latifolia | Gentamicin | |
| E. coli | 1.56 ± 0.00 | 6.25 ± 0.00 | 0.70 ± 0.00 |
| S. aureus | 0.63 ± 0.00 | 2.60 ± 0.90 | 0.80 ± 0.00 |
| Fish Scale Gelatin Nanofibers and Essential Oils Emulsions | TAMC, CFU/g | TYMC, CFU/g |
|---|---|---|
| F-EDTA | 5500.00 ± 4.36 | 45.00 ± 3.00 |
| F-EDTA+L | 22.33 ± 9.29 | 14.66 ± 1.16 |
| F-EDTA+I | 31.33 ± 7.76 | 20.33 ± 1.53 |
| F+EDTA | 2300.00 ± 2.00 | 22.00 ± 1.73 |
| F+EDTA+L | 14.00 ± 2.00 | 8.33 ± 2.08 |
| F+EDTA+I | 21.33 ± 0.58 | 11.33 ± 2.52 |
| Lavandula latifolia essential oil emulsion (L) | 0 | 0 |
| Helichrysum italicum essential oil emulsion (I) | 0 | 0 |
| Fish scale Gelatin Nanofibers and Essential Oils Emulsions | Staphylococcus aureus | Escherichia coli | Candida albicans |
|---|---|---|---|
| F-EDTA | Positive | Positive | Negative |
| F- EDTA+L | Negative | Negative | Negative |
| F-EDTA+I | Negative | Negative | Negative |
| F+EDTA | Negative | Positive | Negative |
| F+EDTA+L | Negative | Negative | Negative |
| F+EDTA+I | Negative | Negative | Negative |
| Lavandula latifolia essential oil emulsion (L) | Negative | Negative | Negative |
| Helichrysum italicum essential oil emulsion (I) | Negative | Negative | Negative |
| Sample | Maximum Toxicity Dose, % (±SD) |
|---|---|
| F-EDTA | 1 ± 0.008 |
| F+EDTA | 1 ± 0.007 |
| F-EDTA+L | 2 ± 0.018 |
| F+EDTA+L | 1.5 ± 0.050 |
| F-EDTA+I | 4 ± 0.037 |
| F+EDTA+I | 4 ± 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaidau, C.; Râpă, M.; Stanca, M.; Tanase, M.-L.; Olariu, L.; Constantinescu, R.R.; Lazea-Stoyanova, A.; Alexe, C.-A.; Tudorache, M. Fish Scale Gelatin Nanofibers with Helichrysum italicum and Lavandula latifolia Essential Oils for Bioactive Wound-Healing Dressings. Pharmaceutics 2023, 15, 2692. https://doi.org/10.3390/pharmaceutics15122692
Gaidau C, Râpă M, Stanca M, Tanase M-L, Olariu L, Constantinescu RR, Lazea-Stoyanova A, Alexe C-A, Tudorache M. Fish Scale Gelatin Nanofibers with Helichrysum italicum and Lavandula latifolia Essential Oils for Bioactive Wound-Healing Dressings. Pharmaceutics. 2023; 15(12):2692. https://doi.org/10.3390/pharmaceutics15122692
Chicago/Turabian StyleGaidau, Carmen, Maria Râpă, Maria Stanca, Mariana-Luiza Tanase, Laura Olariu, Rodica Roxana Constantinescu, Andrada Lazea-Stoyanova, Cosmin-Andrei Alexe, and Madalina Tudorache. 2023. "Fish Scale Gelatin Nanofibers with Helichrysum italicum and Lavandula latifolia Essential Oils for Bioactive Wound-Healing Dressings" Pharmaceutics 15, no. 12: 2692. https://doi.org/10.3390/pharmaceutics15122692
APA StyleGaidau, C., Râpă, M., Stanca, M., Tanase, M.-L., Olariu, L., Constantinescu, R. R., Lazea-Stoyanova, A., Alexe, C.-A., & Tudorache, M. (2023). Fish Scale Gelatin Nanofibers with Helichrysum italicum and Lavandula latifolia Essential Oils for Bioactive Wound-Healing Dressings. Pharmaceutics, 15(12), 2692. https://doi.org/10.3390/pharmaceutics15122692











