A Systematic Evaluation of Curcumin Concentrations and Blue Light Parameters towards Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Curcumin Powder
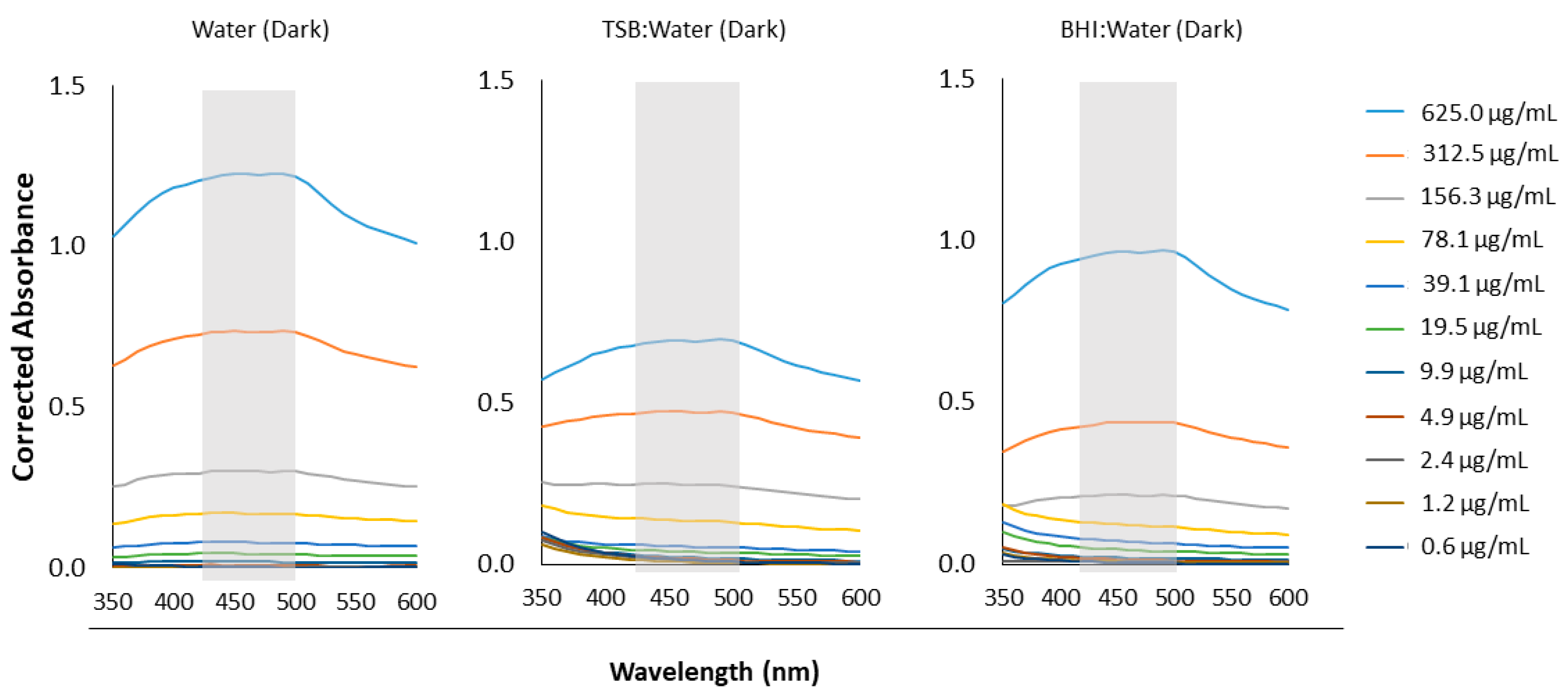
2.2. Molar Absorptivity Coefficients of Aqueous Curcumin Solutions
2.3. Quantifying ROS Release Following aPDT
2.3.1. Singlet Oxygen
2.3.2. Superoxide Anions
2.4. Bacterial Strain and Growth Conditions
2.5. Minimum Inhibitory Concentration (MIC) Following aPDT
2.6. Minimum Bactericidal or Fungicidal Concentration (MBC or MFC) Following aPDT
2.7. Statistical Analysis
3. Results
3.1. Confirming Curcumin Powder Structure and Chemistry
3.2. Light Absorbance by Curcumin
3.3. ROS Release Following aPDT
3.4. Minimum Bactericidal or Fungicidal Concentration (MBC or MFC) Following aPDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Oral Health Status Report; WHO: Geneva, Switzerland, 2022; Volume 57, ISBN 9789240061484. [Google Scholar]
- Bernabé, E.; Marcenes, W. Income Inequality and Tooth Loss in the United States. J. Dent. Res. 2011, 90, 724–729. [Google Scholar] [CrossRef]
- Koo, H.; Bowen, W.H. Candida Albicans and Streptococcus Mutans: A Potential Synergistic Alliance to Cause Virulent Tooth Decay in Children. Future Microbiol. 2014, 9, 1295–1297. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic Relationship between Streptococcus Mutans and Candida Albicans Synergizes Virulence of Plaque Biofilms In Vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus Mutans, Candida Albicans, and the Human Mouth: A Sticky Situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef]
- Pereira, D.F.A.; Seneviratne, C.J.; Koga-Ito, C.Y.; Samaranayake, L.P. Is the Oral Fungal Pathogen Candida Albicans a Cariogen? Oral Dis. 2018, 24, 518–526. [Google Scholar] [CrossRef]
- Hagihara, Y.; Kaminishi, H.; Cho, T.; Tanaka, M.; Kaita, H. Short communication degradation of human dentine collagen by an enzyme produced by the yeast Candida albicans. Arch. Oral Biol. 1988, 33, 617–619. [Google Scholar] [CrossRef]
- Kaminishi, H.; Hagihara, Y.; Hayashi, S.; Cho, T. Isolation and Characteristics of Collagenolytic Enzyme Produced by Candida Albicans. Infect. Immun. 1986, 53, 312–316. [Google Scholar] [CrossRef]
- Aoba, T.; Fejerskov, O. Dental Fluorosis: Chemistry and Biology. Crit. Rev. Oral Biol. Med. 2002, 13, 155–170. [Google Scholar] [CrossRef]
- Fekrazad, R.; Khoei, F.; Hakimiha, N.; Bahador, A. Photoelimination of Streptococcus Mutans with Two Methods of Photodynamic and Photothermal Therapy. Photodiagn. Photodyn. Ther. 2013, 10, 626–631. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial Photodynamic Therapy: Overview of a Promising Approach to Fight Antibiotic-Resistant Bacterial Infections. J. Clin. Transl. Res. 2015, 1, 140. [Google Scholar] [CrossRef]
- Biel, M.A.; Pedigo, L.; Gibbs, A.; Loebel, N. Photodynamic Therapy of Antibiotic-Resistant Biofilms in a Maxillary Sinus Model. Int. Forum Allergy Rhinol. 2013, 3, 468–473. [Google Scholar] [CrossRef]
- Carrera, E.T.; Dias, H.B.; Corbi, S.C.T.; Marcantonio, R.A.C.; Bernardi, A.C.A.; Bagnato, V.S.; Hamblin, M.R.; Rastelli, A.N.S. The Application of Antimicrobial Photodynamic Therapy (APDT) in Dentistry: A Critical Review. Laser Phys. 2016, 26, 123001. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Liu, Y.C.; Sun, H.; Guo, D.S. Type I Photodynamic Therapy by Organic–Inorganic Hybrid Materials: From Strategies to Applications. Coord. Chem. Rev. 2019, 395, 46–62. [Google Scholar] [CrossRef]
- Thompson, S.A.; Aggarwal, A.; Singh, S.; Adam, A.P.; Tome, J.P.C.; Drain, C.M. Compromising the Plasma Membrane as a Secondary Target in Photodynamic Therapy-Induced Necrosis. Bioorg. Med. Chem. 2018, 26, 5224–5228. [Google Scholar] [CrossRef]
- Paschoal, M.A.; Tonon, C.C.; Spolidório, D.M.P.; Bagnato, V.S.; Giusti, J.S.M.; Santos-Pinto, L. Photodynamic Potential of Curcumin and Blue LED against Streptococcus Mutans in a Planktonic Culture. Photodiagn. Photodyn. Ther. 2013, 10, 313–319. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Lin, H.; Zhou, Y. Curcumin as a Promising Antibacterial Agent: Effects on Metabolism and Biofilm Formation in S. mutans. BioMed Res. Int. 2018, 2018, 4508709. [Google Scholar] [CrossRef]
- Comeau, P.; Panariello, B.; Duarte, S.; Manso, A. Impact of Curcumin Loading on the Physicochemical, Mechanical and Antimicrobial Properties of a Methacrylate-Based Experimental Dental Resin. Sci. Rep. 2022, 12, 18691. [Google Scholar] [CrossRef]
- Manoil, D.; Filieri, A.; Gameiro, C.; Lange, N.; Schrenzel, J.; Wataha, J.C.; Bouillaguet, S. Flow Cytometric Assessment of Streptococcus Mutans Viability after Exposure to Blue Light-Activated Curcumin. Photodiagn. Photodyn. Ther. 2014, 11, 372–379. [Google Scholar] [CrossRef]
- Li, B.; Pan, T.; Lin, H.; Zhou, Y. The Enhancing Antibiofilm Activity of Curcumin on Streptococcus Mutans Strains from Severe Early Childhood Caries. BMC Microbiol. 2020, 20, 286. [Google Scholar] [CrossRef]
- dos Santos, D.D.L.; Besegato, J.F.; DeMelo, P.B.G.; Oshiro Junior, J.A.; Chorilli, M.; Deng, D.; Bagnato, V.S.; Rastelli, A.N.d.S. Curcumin-Loaded Pluronicâ F-127 Micelles as a Drug Delivery System for Curcumin-Mediated Photodynamic Therapy for Oral Application. Photochem. Photobiol. Sci. 2021, 97, 1072–1088. [Google Scholar] [CrossRef]
- Sakima, V.T.; Barbugli, P.A.; Cerri, P.S.; Chorilli, M.; Carmello, J.C.; Pavarina, A.C.; De Oliveira Mima, E.G. Antimicrobial Photodynamic Therapy Mediated by Curcumin-Loaded Polymeric Nanoparticles in a Murine Model of Oral Candidiasis. Molecules 2018, 23, 2075. [Google Scholar] [CrossRef]
- Paolillo, F.R.; Rodrigues, P.G.S.; Bagnato, V.S.; Alves, F.; Pires, L.; Corazza, A.V. The Effect of Combined Curcumin-Mediated Photodynamic Therapy and Artificial Skin on Staphylococcus Aureus–Infected Wounds in Rats. Lasers Med. Sci. 2021, 36, 1219–1226. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.D.; Brunetti, I.L.; Costa, C.A.D.S.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the Photodynamic Effects of Curcumin against Candida Albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.Y.; Corke, H. Antimicrobial and Anticancer Applications and Related Mechanisms of Curcumin-Mediated Photodynamic Treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar] [CrossRef]
- Marques Meccatti, V.; De Souza Moura, L.; Guerra Pinto, J.; Ferreira-Strixino, J.; Abu Hasna, A.; Alves Figueiredo-Godoi, L.M.; Campos Junqueira, J.; Marcucci, M.C.; Paula Ramos, L.D.; Carvalho, C.A.T.; et al. Curcuma Longa L. Extract and Photodynamic Therapy Are Effective against Candida Spp. and Do Not Show Toxicity In Vivo. Int. J. Dent. 2022, 2022, 5837864. [Google Scholar] [CrossRef]
- Cusicanqui Méndez, D.A.; Gutierres, E.; José Dionisio, E.; Afonso Rabelo Buzalaf, M.; Cardoso Oliveira, R.; Andrade Moreira Machado, M.A.; Cruvinel, T. Curcumin-Mediated Antimicrobial Photodynamic Therapy Reduces the Viability and Vitality of Infected Dentin Caries Microcosms. Photodiagn. Photodyn. Ther. 2018, 24, 102–108. [Google Scholar] [CrossRef]
- Kurien, B.T.; Singh, A.; Matsumoto, H.; Scofield, R.H. Improving the Solubility and Pharmacological Efficacy of Curcumin by Heat Treatment. Assay Drug Dev. Technol. 2007, 5, 567–576. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, S.M.; Jeong, S.H.; Chung, K.H.; Kim, B.-I. Antibacterial Photodynamic Therapy with Curcumin and Curcuma Xanthorrhiza Extract against Streptococcus Mutans. Photodiagn. Photodyn. Ther. 2017, 20, 116–119. [Google Scholar] [CrossRef]
- Rolim, J.P.M.L.; De-Melo, M.A.S.; Guedes, S.F.; Albuquerque-Filho, F.B.; De Souza, J.R.; Nogueira, N.A.P.; Zanin, I.C.J.; Rodrigues, L.K.A. The Antimicrobial Activity of Photodynamic Therapy against Streptococcus Mutans Using Different Photosensitizers. J. Photochem. Photobiol. B 2012, 106, 40–46. [Google Scholar] [CrossRef]
- Sabino, C.P.; Wainwright, M.; dos Anjos, C.; Sellera, F.P.; Baptista, M.S.; Lincopan, N.; Ribeiro, M.S. Inactivation Kinetics and Lethal Dose Analysis of Antimicrobial Blue Light and Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2019, 28, 186–191. [Google Scholar] [CrossRef]
- Nagata, J.Y.; Hioka, N.; Kimura, E.; Batistela, V.R.; Terada, R.S.S.; Graciano, A.X.; Baesso, M.L.; Hayacibara, M.F. Antibacterial Photodynamic Therapy for Dental Caries: Evaluation of the Photosensitizers Used and Light Source Properties. Photodiagn. Photodyn. Ther. 2012, 9, 122–131. [Google Scholar] [CrossRef]
- Kajfasz, J.K.; Ganguly, T.; Hardin, E.L.; Abranches, J.; Lemos, J.A. Transcriptome Responses of Streptococcus Mutans to Peroxide Stress: Identification of Novel Antioxidant Pathways Regulated by Spx. Sci. Rep. 2017, 7, 16018. [Google Scholar] [CrossRef]
- Comeau, P.; Burgess, J.; Qomi, N.R.; Lee, A.; Manso, A. The Antimicrobial, Physical, and Chemical Properties of a Riboflavin-Loaded Dental Resin Intended for Antimicrobial Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2022, 40, 103124. [Google Scholar] [CrossRef]
- Igarashi, N.; Onoue, S.; Tsuda, Y. Photoreactivity of Amino Acids: Tryptophan-Induced Photochemical Events via Reactive Oxygen Species Generation. Anal. Sci. 2007, 23, 943–948. [Google Scholar] [CrossRef]
- Tonon, C.C.; Paschoal, M.A.; Correia, M.; Spolidório, D.M.; Bagnato, V.S.; Giusti, J.S.; Santos-Pinto, L. Comparative Effects of Photodynamic Therapy Mediated by Curcumin on Standard and Clinical Isolate of Streptococcus Mutans. J. Contemp. Dent. Pract. 2015, 16, 1–6. [Google Scholar] [CrossRef]
- Reis, A.C.M.; Regis, W.F.M.; Rodrigues, L.K.A. Scientific Evidence in Antimicrobial Photodynamic Therapy: An Alternative Approach for Reducing Cariogenic Bacteria. Photodiagn. Photodyn. Ther. 2019, 26, 179–189. [Google Scholar] [CrossRef]
- Erez, Y.; Simkovitch, R.; Shomer, S.; Gepshtein, R.; Huppert, D. Effect of Acid on the Ultraviolet-Visible Absorption and Emission Properties of Curcumin. J. Phys. Chem. A 2014, 118, 872–884. [Google Scholar] [CrossRef]
- Phillips, G.O.; Rickards, T. Photodegradation of Carbohydrates. Part IV. Direct Photolysis of D-Glucose in Aqueous Solution. J. Chem. Soc. B 1969, 455–461. [Google Scholar] [CrossRef]
- Akilov, O.E.; O’Riordan, K.; Kosaka, S.; Hasan, T. Photodynamic Therapy against Intracellular Pathogens: Problems and Potentials. Med. Laser Appl. 2006, 21, 251–260. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, Photochemistry and Photobiology of Curcumin: Studies from Organic Solutions, Bio-Mimetics and Living Cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Foster, T.H.; Murant, R.S.; Bryant, R.G.; Knox, R.S.; Gibson, L.; Hilf, R. Oxygen Consumption and Diffusion Effects in Photodynamic Therapy. Radiat. Res. 1991, 126, 296–303. [Google Scholar] [CrossRef]
- Le-Tan, H.; Jaeger, H. Impact of Cell Disintegration Techniques on Curcumin Recovery. Food Eng. Rev. 2022, 14, 655–672. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 39, pp. 113–204. [Google Scholar]
- Wang, Y.; Lu, Z.; Lv, F.; Bie, X. Study on Microencapsulation of Curcumin Pigments by Spray Drying. Eur. Food Res. Technol. 2009, 229, 391–396. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chang, K.C.; Chen, L.Y.; Hu, A. Low-Dose Blue Light Irradiation Enhances the Antimicrobial Activities of Curcumin against Propionibacterium Acnes. J. Photochem. Photobiol. B 2018, 189, 21–28. [Google Scholar] [CrossRef]
- Cava-Roda, R.M.; Taboada-Rodríguez, A.; Valverde-Franco, M.T.; Marín-Iniesta, F. Antimicrobial Activity of Vanillin and Mixtures with Cinnamon and Clove Essential Oils in Controlling Listeria Monocytogenes and Escherichia Coli O157:H7 in Milk. Food Bioprocess Technol. 2012, 5, 2120–2131. [Google Scholar] [CrossRef]
- Santosh Kumar, S.; Ghosh, A.; Devasagayam, T.P.A.; Chauhan, P.S. Effect of Vanillin on Methylene Blue plus Light-Induced Single-Strand Breaks in Plasmid PBR322 DNA. Mutat. Res./Genet. Toxicol. Environ. Mutagenes. 2000, 469, 207–214. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and Diseases: Role in Metabolism and Energy Supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Q.; Wang, W.; Shao, J.; Huang, W.; Dong, X. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Small 2021, 17, 2006742. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The Photodegradation of Porphyrins in Cells Can Be Used To Estimate the Lifetime of Singlet Oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Niedre, M.; Patterson, M.S.; Wilson, B.C. Direct Near-infrared Luminescence Detection of Singlet Oxygen Generated by Photodynamic Therapy in Cells In Vitro and Tissues In Vivo. Photochem. Photobiol. 2002, 75, 382–391. [Google Scholar] [CrossRef]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.C.; Ogilby, P.R. Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef]
- Dysart, J.S.; Patterson, M.S. Characterization of Photofrin Photobleaching for Singlet Oxygen Dose Estimation during Photodynamic Therapy of MLL Cells In Vitro. Phys. Med. Biol. 2005, 50, 2597–2616. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Spatially Resolved Cellular Responses to Singlet Oxygen. Photochem. Photobiol. 2006, 82, 1178. [Google Scholar] [CrossRef]
- Klis, F.M.; de Koster, C.G.; Brul, S. Cell Wall-Related Bionumbers and Bioestimates of Saccharomyces Cerevisiae and Candida Albicans. Eukaryot. Cell 2014, 13, 2–9. [Google Scholar] [CrossRef]
- Liu, T.; Liu, J.; Liu, J.; Yang, R.; Lu, X.; He, X.; Shi, W.; Guo, L. Interspecies Interactions Between Streptococcus Mutans and Streptococcus Agalactiae In Vitro. Front. Cell. Infect. Microbiol. 2020, 10, 344. [Google Scholar] [CrossRef]
- Misba, L.; Zaidi, S.; Khan, A.U. Efficacy of Photodynamic Therapy against Streptococcus Mutans Biofilm: Role of Singlet Oxygen. J. Photochem. Photobiol. B 2018, 183, 16–21. [Google Scholar] [CrossRef]
- De Melo, W.C.M.A.; Avci, P.; De Oliveira, M.N.; Gupta, A.; Vecchio, D.; Sadasivam, M.; Chandran, R.; Huang, Y.Y.; Yin, R.; Perussi, L.R.; et al. Photodynamic Inactivation of Biofilm: Taking a Lightly Colored Approach to Stubborn Infection. Expert Rev. Anti-Infect. Ther. 2013, 11, 669–693. [Google Scholar] [CrossRef]
- Soares, J.M.; Silva, K.O.O.; Inada, N.M.; Bagnato, V.S.; Blanco, K.C. Optimization for Microbial Incorporation and Efficiency of Photodynamic Therapy Using Variation on Curcumin Formulation. Photodiagn. Photodyn. Ther. 2020, 29, 101652. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef]
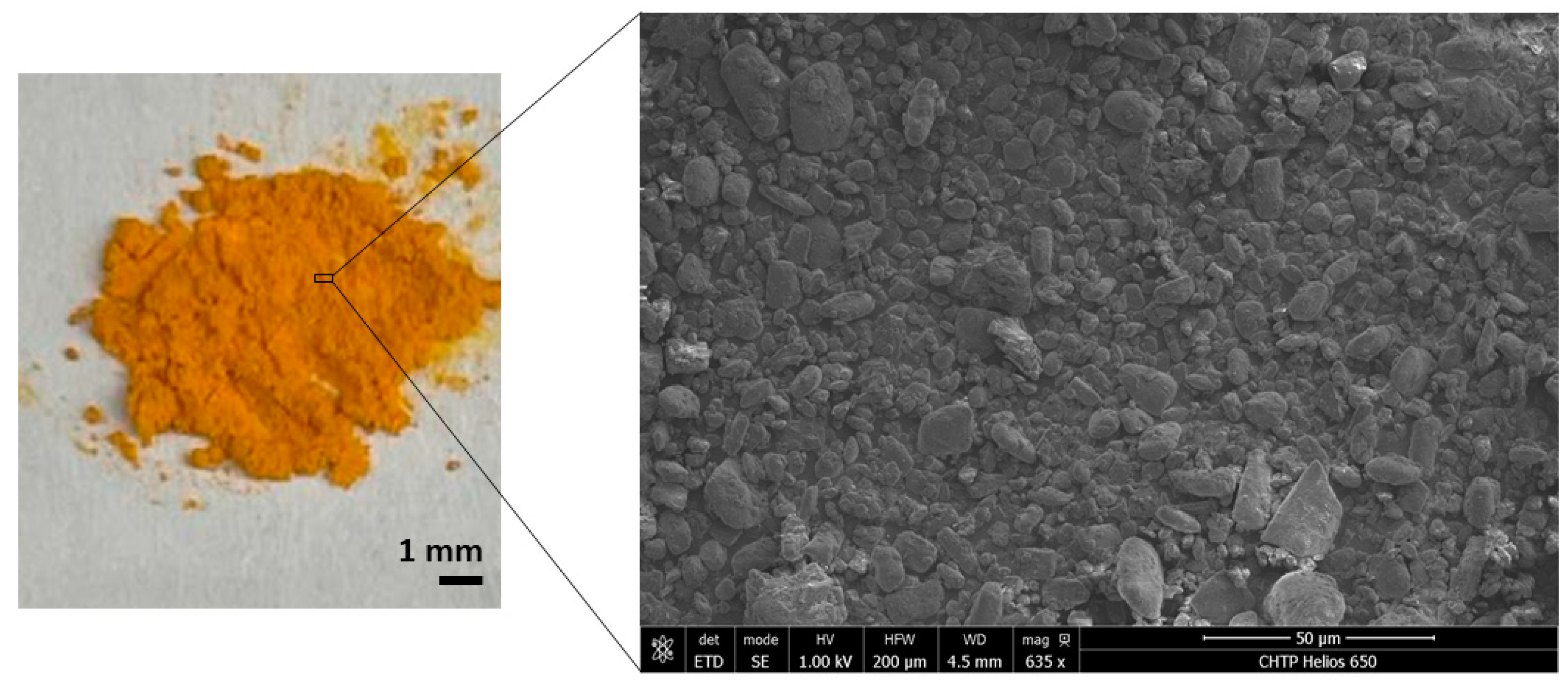




| No. | Mode | Segment 1 | Segment 2 | Segment 3 | Segment 4 | Segment 5 |
|---|---|---|---|---|---|---|
| 1 | Continuous | 1 min on | - | - | - | - |
| 2 | Fractional | 1 min on | 5 min off | 4 min on | - | - |
| 3 | Continuous | 5 min on | - | - | - | - |
| 4 | Fractional | 1 min on | 5 min off | 4 min on | 5 min off | 10 min on |
| 5 | Continuous | 15 min on | - | - | - | - |
| Light Treatment (Royal Blue LED, 56 mW/cm2) | ||||||
|---|---|---|---|---|---|---|
| Species | Test | Mode | 0 min | 1 min | 5 min | 15 min |
| C. albicans | MIC | Continuous | ND | ND | ND | 9.8 µg/mL |
| Fractional | ND | 19.5 µg/mL | ||||
| MFC | Continuous | ND | ND | ND | 9.8 µg/mL | |
| Fractional | ND | 625 µg/mL | ||||
| S. mutans | MIC | Continuous | ND | 39.1 µg/mL | 39.1 µg/mL | 19.5 µg/mL |
| Fractional | 19.5 µg/mL | 4.9 µg/mL | ||||
| MBC | Continuous | ND | 156.3 µg/mL | 78.1 µg/mL | 39.1 µg/mL | |
| Fractional | 39.1 µg/mL | 9.8 µg/mL | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comeau, P.; Manso, A. A Systematic Evaluation of Curcumin Concentrations and Blue Light Parameters towards Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms. Pharmaceutics 2023, 15, 2707. https://doi.org/10.3390/pharmaceutics15122707
Comeau P, Manso A. A Systematic Evaluation of Curcumin Concentrations and Blue Light Parameters towards Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms. Pharmaceutics. 2023; 15(12):2707. https://doi.org/10.3390/pharmaceutics15122707
Chicago/Turabian StyleComeau, Patricia, and Adriana Manso. 2023. "A Systematic Evaluation of Curcumin Concentrations and Blue Light Parameters towards Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms" Pharmaceutics 15, no. 12: 2707. https://doi.org/10.3390/pharmaceutics15122707
APA StyleComeau, P., & Manso, A. (2023). A Systematic Evaluation of Curcumin Concentrations and Blue Light Parameters towards Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms. Pharmaceutics, 15(12), 2707. https://doi.org/10.3390/pharmaceutics15122707





