Nano-Based Drug Delivery Systems: Potential Developments in the Therapy of Metastatic Osteosarcoma—A Narrative Review
Abstract
1. Introduction
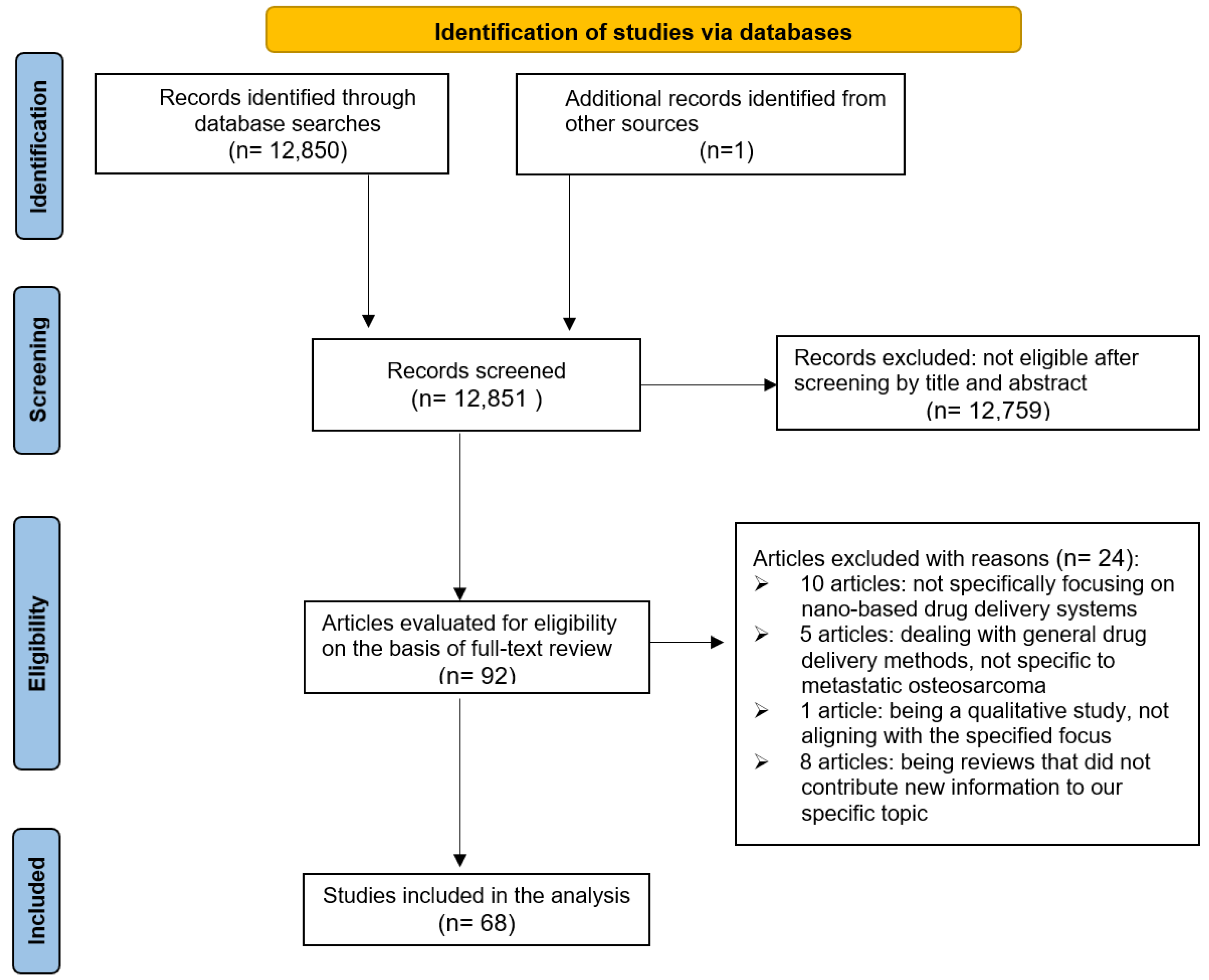
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Article Selection
2.4. Characterization of Articles
2.5. Evaluation of Methodological Quality
3. Results
3.1. Mechanism of MOSA
3.1.1. Genomic Aberrations
3.1.2. Epigenetic Variations
3.1.3. Metabolic Reprogramming
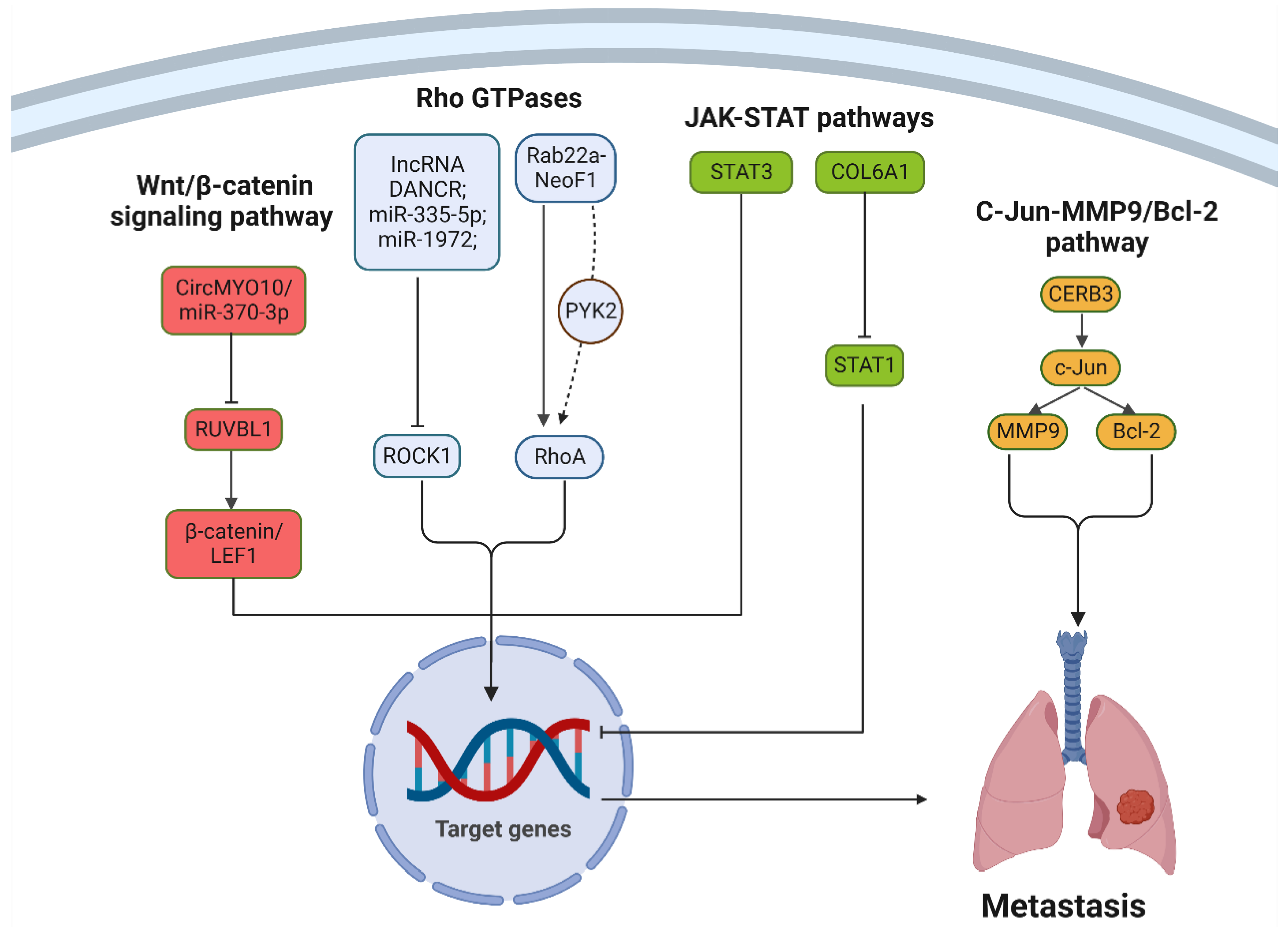
3.1.4. Dysregulated Signaling Pathways
3.1.5. Transcription Factor
3.1.6. Tumor Microenvironment
3.2. Conventional Treatments for MOSA
3.2.1. Chemotherapy
3.2.2. Radiation Therapy
3.2.3. Surgery
3.2.4. Targeted Therapies
| Treatment Approach | Key Content | Typical Indications | Advantages | Disadvantages |
|---|---|---|---|---|
| Chemotherapy | Combination of agents like methotrexate, cisplatin, and doxorubicin | Aggressive tumors; prepping for surgery | Shrink tumors; prevent metastatic spread | Non-selective; damage normal cells |
| Radiation Therapy | Advanced techniques like IMRT, SBRT, and proton therapy | Inoperable tumors; control of metastatic site tumors | Target and shrink tumors; minimize damage to healthy tissue | Osteosarcoma cells exhibit radioresistance; risk of long-term side effects |
| Surgery | Limb-sparing surgery or amputation; metastasectomy | Tumor is operable; metastases are limited and resectable | Completely remove tumor; limb-sparing techniques preserve function | Risk of complications, especially in the presence of multiple metastatic sites |
| Targeted Therapies | Agents targeting PD-1/PD-L1 pathway; angiogenesis inhibitors | Patients with specific molecular anomalies in osteosarcoma cells | More selective; tailored to individual patient’s tumor | Achieving precise delivery is challenging; potential severe side effects if not targeted correctly |
3.3. Nanoscale Drug Delivery Systems for the Treatment of MOSA
3.3.1. Polypeptide Nanogel
3.3.2. Nanomicelles
3.3.3. Graphene Quantum Dots
3.3.4. Liposomes
3.3.5. Polysaccharide Derivative
3.3.6. Protein-Based Nanoparticles
3.3.7. Redox-Responsive Polymersomes
3.3.8. Hyaluronate-Based Nanoparticles
3.3.9. Graphene Oxide Nanoparticles
3.3.10. Poly(lactic-co-glycolic acid)
3.3.11. Chitosan Nanoparticle
| Nanomaterial | Loaded Moiety | Tested Models | Outcomes Reported | References |
|---|---|---|---|---|
| Polypeptide nanogel | Shikonin | In vitro: U-2OS, MG-63, and Saos-2 OS cells In vivo: BALB/c nude mice bearing U-2OS subcutaneous xenograft | In vitro: enhanced cytotoxicity and uptake in OS cells In vivo: notably inhibited tumor growth with reduced side effects | [22] |
| Nanomicelles | [223Ra] RaCl2 | In vitro: Saos-2 OS cells In vivo: none | In vitro: 20% increased tumor-targeting efficiency vs. pure radium dichloride In vivo: none | [109] |
| DOX | In vitro: 143B OS cells In vivo: 143B xenografts in nude mice | In vitro: selective inhibition of 143B OS cells through necroptosis In vivo: excellent antitumor efficacy, inhibited pulmonary metastasis with low systemic toxicity | [113] | |
| Graphene quantum dots | 2-deoxy-d-glucose | In vitro: 143B OS cells and hFOB 1.19 cells In vivo: orthotopic OS-bearing nude mice | In vitro: selectively targeted and sensitized 143B OS cells to X-ray radiation, reduced migration and invasion capacity In vivo: significantly inhibited primary OS growth, reduced the risk of metastasis | [117] |
| Liposomes | IL-2 cDNA | In vitro: none In vivo: dogs with spontaneous pulmonary metastatic tumors | In vitro: none In vivo: significant antitumor activity, inhibition of pulmonary metastasis, and low systemic toxicity | [21] |
| Edelfosine | In vitro: MNNG-HOS and 143B OS cells In vivo: OS-induced mouse models using HOS and 143B cells | In vitro: effectively inhibited OS cell growth In vivo: remarkable antitumor efficacy, suppression of lung metastasis, and minimal systemic toxicity | [119] | |
| Polysaccharide derivative | AEG-1 siRNA | In vitro: U-2OS cells In vivo: U-2OS cells tumor-bearing mice models | In vitro: inhibition of cell proliferation and invasion In vivo: outstanding anti-tumor efficacy, inhibited pulmonary metastasis with low systemic toxicity | [122] |
| Protein-based nanoparticles | ALD | In vitro: 143B OS cells In vivo: orthotopic and subcutaneous 143B OS mouse models, as well as models for cardiac injury and lung metastasis | In vitro: significantly inhibited the proliferation of human osteosarcoma cells (143B) In vivo: a strong inhibitory effect on pulmonary tumor metastasis with mitigating cardiotoxicity associated with ALD | [126] |
| Redox-responsive polymersomes | DOX | In vitro: 143B OS cells In vivo: mouse model of orthotopic 143B OS, relapsed OS and PDX OS | In vitro: induced apoptosis and hindered 143B OS cell proliferation In vivo: significantly inhibited tumor growth and lung metastasis, while sparing systemic toxicity | [130] |
| Hyaluronate-based nanoparticles | DOX,CDDP, R848 | In vitro: OS cell lines, moDCs, and human primary T cells In vivo: 143B tumor-bearing mice model | In vitro: effectively delivered drugs and siRNA to OS cells, promoting T cell activation In vivo: significantly inhibited tumor growth and prolonged mice survival | [136] |
| Graphene oxide nanoparticles | Ginsenoside Rg3, ICG | In vitro: Saos-2 and MG-63 OS cells In vivo: MG-63 tumor-bearing BALB/c nude mice | In vitro: enhanced cytotoxicity in OS cells In vivo: significantly suppressed tumor growth in MG-63 tumor mice | [139] |
| Poly(lactic-co-glycolic acid) | castalin | In vitro: 143B OS cells In vivo: LM8 xenografted mice | In vitro: reduced cell viability in 143B cells and induced apoptosis effectively In vivo: showing a more pronounced reduction in tumor volume | [148] |
| Chitosan nanoparticle | DOX, Dz13 | In vitro: Saos-2 cells In vivo: Saos-2 tumor growth in mice | In vitro: successfully reduced Saos-2 viability by up to 80% and maintained their cell-kill efficiency In vivo: inhibited Saos-2 tumor growth and spread in mice more effectively | [16] |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.A.; Lim, J.; Jin, H.Y.; Park, M.; Park, H.J.; Park, J.W.; Kim, J.H.; Kang, H.G.; Won, Y.J. Osteosarcoma in Adolescents and Young Adults. Cells 2021, 10, 2684. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Han, J.; Yang, L.; Cai, Z.; Sun, W.; Hua, Y.; Xu, J. Immune Microenvironment in Osteosarcoma: Components, Therapeutic Strategies and Clinical Applications. Front. Immunol. 2022, 13, 907550. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.; Gebhardt, M.; Teot, L.; Gorlick, R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist 2004, 9, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Longhi, A.; Bertoni, F.; Briccoli, A.; Versari, M.; Pignotti, E.; Picci, P. Bone metastases in osteosarcoma patients treated with neoadjuvant or adjuvant chemotherapy: The Rizzoli experience in 52 patients. Acta Orthop. 2006, 77, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.J.; Geller, D.S.; Gorlick, R. Therapy for osteosarcoma: Where do we go from here? Paediatr. Drugs 2008, 10, 315–327. [Google Scholar] [CrossRef]
- Geller, D.S.; Gorlick, R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin. Adv. Hematol. Oncol. 2010, 8, 705–718. [Google Scholar]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [CrossRef]
- Evola, F.R.; Costarella, L.; Pavone, V.; Caff, G.; Cannavò, L.; Sessa, A.; Avondo, S.; Sessa, G. Biomarkers of Osteosarcoma, Chondrosarcoma, and Ewing Sarcoma. Front. Pharmacol. 2017, 8, 150. [Google Scholar] [CrossRef]
- Abu-Helil, B.; van der Weyden, L. Metastasis in the wild: Investigating metastasis in non-laboratory animals. Clin. Exp. Metastasis 2019, 36, 15–28. [Google Scholar] [CrossRef]
- Matus, M.F.; Ludueña, M.; Vilos, C.; Palomo, I.; Mariscal, M.M. Atomic-level characterization and cilostazol affinity of poly(lactic acid) nanoparticles conjugated with differentially charged hydrophilic molecules. Beilstein J. Nanotechnol. 2018, 9, 1328–1338. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hu, H.Z.; Qing, X.C.; Zhang, Z.C.; Shao, Z.W. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer 2020, 11, 69–82. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Wang, Z.; Du, Y.; Yuan, H.; Zhang, P.; Zhou, J.; Liu, F.; Li, C.; Hu, F. Specific tumor delivery of paclitaxel using glycolipid-like polymer micelles containing gold nanospheres. Biomaterials 2013, 34, 4510–4519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, X.; Huang, Y.; Zhang, Q.; Zhu, S.; Fu, W. Nanomaterial Technology and Soft Tissue Sarcomas. Front. Oncol. 2022, 12, 921983. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Dei Cas, M.; Cianciolo, S.; Fidilio, A.; Lazzara, F.; Paroni, R.; Pignatello, R.; Strettoi, E.; Ghidoni, R.; Drago, F.; et al. Novel ophthalmic formulation of myriocin: Implications in retinitis pigmentosa. Drug Deliv. 2019, 26, 237–243. [Google Scholar] [CrossRef]
- Chirio, D.; Sapino, S.; Chindamo, G.; Peira, E.; Vercelli, C.; Riganti, C.; Manzoli, M.; Gambino, G.; Re, G.; Gallarate, M. Doxorubicin-Loaded Lipid Nanoparticles Coated with Calcium Phosphate as a Potential Tool in Human and Canine Osteosarcoma Therapy. Pharmaceutics 2022, 14, 1362. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.L.; Friedhuber, A.M.; Dass, C.R. Co-nanoencapsulated doxorubicin and Dz13 control osteosarcoma progression in a murine model. J. Pharm. Pharmacol. 2013, 65, 35–43. [Google Scholar] [CrossRef]
- Henna, T.K.; Pramod, K. Graphene quantum dots redefine nanobiomedicine. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110651. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Dow, S.; Elmslie, R.; Kurzman, I.; MacEwen, G.; Pericle, F.; Liggitt, D. Phase I study of liposome-DNA complexes encoding the interleukin-2 gene in dogs with osteosarcoma lung metastases. Hum. Gene Ther. 2005, 16, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, T.; Xu, W.; Ding, J.; Yin, F.; Xu, J.; Sun, W.; Wang, H.; Sun, M.; Cai, Z.; et al. Sarcoma-Targeting Peptide-Decorated Polypeptide Nanogel Intracellularly Delivers Shikonin for Upregulated Osteosarcoma Necroptosis and Diminished Pulmonary Metastasis. Theranostics 2018, 8, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Brozek, J.L.; Akl, E.A.; Alonso-Coello, P.; Lang, D.; Jaeschke, R.; Williams, J.W.; Phillips, B.; Lelgemann, M.; Lethaby, A.; Bousquet, J.; et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 2009, 64, 669–677. [Google Scholar] [CrossRef] [PubMed]
- De Nonneville, A.; Salas, S.; Bertucci, F.; Sobinoff, A.P.; Adélaïde, J.; Guille, A.; Finetti, P.; Noble, J.R.; Churikov, D.; Chaffanet, M.; et al. TOP3A amplification and ATRX inactivation are mutually exclusive events in pediatric osteosarcomas using ALT. EMBO Mol. Med. 2022, 14, e15859. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Niu, X.; Wang, Z.; Song, C.L.; Huang, Z.; Chen, K.N.; Duan, J.; Bai, H.; Xu, J.; Zhao, J.; et al. Multiregion Sequencing Reveals the Genetic Heterogeneity and Evolutionary History of Osteosarcoma and Matched Pulmonary Metastases. Cancer Res. 2019, 79, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Chachad, D.; Zhang, Y.; Gencel-Augusto, J.; Sirito, M.; Pant, V.; Yang, P.; Sun, C.; Chau, G.; Qi, Y.; et al. Differential Gain-of-Function Activity of Three p53 Hotspot Mutants In Vivo. Cancer Res. 2022, 82, 1926–1936. [Google Scholar] [CrossRef]
- Liang, X.; Wang, X.; He, Y.; Wu, Y.; Zhong, L.; Liu, W.; Liao, D.; Kang, T. Acetylation dependent functions of Rab22a-NeoF1 Fusion Protein in Osteosarcoma. Theranostics 2020, 10, 7747–7757. [Google Scholar] [CrossRef]
- Zhong, L.; Liao, D.; Li, J.; Liu, W.; Wang, J.; Zeng, C.; Wang, X.; Cao, Z.; Zhang, R.; Li, M.; et al. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct. Target. Ther. 2021, 6, 59. [Google Scholar] [CrossRef]
- Namløs, H.M.; Skårn, M.; Ahmed, D.; Grad, I.; Andresen, K.; Kresse, S.H.; Munthe, E.; Serra, M.; Scotlandi, K.; Llombart-Bosch, A.; et al. miR-486-5p expression is regulated by DNA methylation in osteosarcoma. BMC Genom. 2022, 23, 142. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, W. DNMT3A Regulates miR-149 DNA Methylation to Activate NOTCH1/Hedgehog Pathway to Promote the Development of Junctional Osteosarcoma. Biomed. Res. Int. 2022, 2022, 3261213. [Google Scholar] [CrossRef]
- Yang, S.; Wang, B.; Liu, C.; Wang, Q.; Wang, R.; Su, W.; Zhu, Y.; Li, M.; Guo, Z.; Wu, X.; et al. THAP9-AS1 Promotes Tumorigenesis and Reduces ROS Generation through the JAK2/STAT3 Signaling Pathway by Increasing SOCS3 Promoter Methylation in Osteosarcoma. Oxid. Med. Cell Longev. 2021, 2021, 5620475. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.J.; Gu, W.X.; Duan, G.; Chen, H.L. Fat mass and obesity associated (FTO)-mediated N6-methyladenosine modification of Krüppel-like factor 3 (KLF3) promotes osteosarcoma progression. Bioengineered 2022, 13, 8038–8050. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, C.; Ju, Z.; Jiao, D.; Hu, D.; Qi, L.; Liu, S.; Wu, X.; Zhao, C. Krüppel-like factors in tumors: Key regulators and therapeutic avenues. Front. Oncol. 2023, 13, 1080720. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zeng, Z.; Sun, J.; Chen, Y.; Gao, X. A novel small-molecule antagonist enhances the sensitivity of osteosarcoma to cabozantinib in vitro and in vivo by targeting DNMT-1 correlated with disease severity in human patients. Pharmacol. Res. 2021, 173, 105869. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, X.; Liu, Y.; Liu, Y.; Sun, L.; Chen, F. PKM2, function and expression and regulation. Cell Biosci. 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Geng, M.; Ye, X.; Ji, Y.; Li, Y.; Zhang, X.; Xu, W. IRF7 inhibits the Warburg effect via transcriptional suppression of PKM2 in osteosarcoma. Int. J. Biol. Sci. 2022, 18, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, J.; Pan, X.; Zhang, Y.; Weng, Y.; Zhou, D.; He, S. LncRNA KCNQ1OT1 sponges miR-34c-5p to promote osteosarcoma growth via ALDOA enhanced aerobic glycolysis. Cell Death Dis. 2020, 11, 278. [Google Scholar] [CrossRef]
- Zhu, R.; Li, X.; Ma, Y. miR-23b-3p suppressing PGC1α promotes proliferation through reprogramming metabolism in osteosarcoma. Cell Death Dis. 2019, 10, 381. [Google Scholar] [CrossRef]
- Feng, Z.; Ou, Y.; Hao, L. The roles of glycolysis in osteosarcoma. Front. Pharmacol. 2022, 13, 950886. [Google Scholar] [CrossRef]
- Roy, J.; Dibaeinia, P.; Fan, T.M.; Sinha, S.; Das, A. Global analysis of osteosarcoma lipidomes reveal altered lipid profiles in metastatic versus nonmetastatic cells. J. Lipid Res. 2019, 60, 375–387. [Google Scholar] [CrossRef]
- Ren, L.; Ruiz-Rodado, V.; Dowdy, T.; Huang, S.; Issaq, S.H.; Beck, J.; Wang, H.; Tran Hoang, C.; Lita, A.; Larion, M.; et al. Glutaminase-1 (GLS1) inhibition limits metastatic progression in osteosarcoma. Cancer Metab. 2020, 8, 4. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Li, S.; Lin, W.; Wang, Z.; Wang, S.; Chen, W.; Shi, W.; Chen, T.; Zhou, H.; et al. Metabolic control of CD47 expression through LAT2-mediated amino acid uptake promotes tumor immune evasion. Nat. Commun. 2022, 13, 6308. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Zielenska, M.; Stein, G.S.; van Wijnen, A.J.; Squire, J.A. The Role of RUNX2 in Osteosarcoma Oncogenesis. Sarcoma 2011, 2011, 282745. [Google Scholar] [CrossRef]
- Vega, O.A.; Lucero, C.M.J.; Araya, H.F.; Jerez, S.; Tapia, J.C.; Antonelli, M.; Salazar-Onfray, F.; Las Heras, F.; Thaler, R.; Riester, S.M.; et al. Wnt/β-Catenin Signaling Activates Expression of the Bone-Related Transcription Factor RUNX2 in Select Human Osteosarcoma Cell Types. J. Cell. Biochem. 2017, 118, 3662–3674. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Cai, X.; Xu, T.; Wu, Y.; Wang, L.; Chen, P.; Su, C. LIMS2 is Downregulated in Osteosarcoma and Inhibits Cell Growth and Migration. J. Oncol. 2022, 2022, 4811260. [Google Scholar] [CrossRef]
- Dana, P.M.; Sadoughi, F.; Asemi, Z.; Yousefi, B. Molecular Signaling Pathways as Potential Therapeutic Targets in Osteosarcoma. Curr. Med. Chem. 2022, 29, 4436–4444. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xie, Z.; Chen, J.; Chen, J.; Ni, W.; Ma, Y.; Huang, K.; Wang, G.; Wang, J.; Ma, J.; et al. Circular RNA circTADA2A promotes osteosarcoma progression and metastasis by sponging miR-203a-3p and regulating CREB3 expression. Mol. Cancer 2019, 18, 73. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, L.; Xu, X.; Zhang, Z.; Ma, Y.; Hong, L.; Ma, J. Rho-GEF Trio regulates osteosarcoma progression and osteogenic differentiation through Rac1 and RhoA. Cell Death Dis. 2021, 12, 1148. [Google Scholar] [CrossRef]
- Du, X.; Ou, Y.; Zhang, M.; Li, K.; Huang, W.; Jiang, D. The mevalonate pathway promotes the metastasis of osteosarcoma by regulating YAP1 activity via RhoA. Genes Dis. 2022, 9, 741–752. [Google Scholar] [CrossRef]
- Xie, K.; Zhang, X.; Tao, Y. Rab22a-NeoF1: A promising target for osteosarcoma patients with lung metastasis. Signal Transduct. Target. Ther. 2020, 5, 161. [Google Scholar] [CrossRef]
- Zhan, F.; Deng, Q.; Chen, Z.; Xie, C.; Xiang, S.; Qiu, S.; Tian, L.; Wu, C.; Ou, Y.; Chen, J.; et al. SAR1A regulates the RhoA/YAP and autophagy signaling pathways to influence osteosarcoma invasion and metastasis. Cancer Sci. 2022, 113, 4104–4119. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Yang, X.; Lu, W.; Chen, Y.; Lin, Y.; Wang, J.; Lin, S.; Yun, J.P. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics 2021, 11, 1473–1492. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liao, S.; Bennett, S.; Tang, H.; Song, D.; Wood, D.; Zhan, X.; Xu, J. STAT3 and its targeting inhibitors in osteosarcoma. Cell Prolif. 2021, 54, e12974. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, Y.; Wang, G.; Feng, S.; Ge, X.; Ye, W.; Wang, Z.; Zhu, Y.; Cai, W.; Bai, J.; et al. TRIM22 inhibits osteosarcoma progression through destabilizing NRF2 and thus activation of ROS/AMPK/mTOR/autophagy signaling. Redox Biol. 2022, 53, 102344. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, G.; Liang, X.; Wang, W.; Wang, Z.; Liao, D.; Zhong, L.; Zhang, R.; Zeng, Y.X.; Wu, Y.; et al. Targeting the CK1α/CBX4 axis for metastasis in osteosarcoma. Nat. Commun. 2020, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M. RUNX Family in Hypoxic Microenvironment and Angiogenesis in Cancers. Cells 2022, 11, 3098. [Google Scholar] [CrossRef]
- Lamhamedi-Cherradi, S.E.; Mohiuddin, S.; Mishra, D.K.; Krishnan, S.; Velasco, A.R.; Vetter, A.M.; Pence, K.; McCall, D.; Truong, D.D.; Cuglievan, B.; et al. Transcriptional activators YAP/TAZ and AXL orchestrate dedifferentiation, cell fate, and metastasis in human osteosarcoma. Cancer Gene Ther. 2021, 28, 1325–1338. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the tumor stroma for cancer therapy. Mol. Cancer 2022, 21, 208. [Google Scholar] [CrossRef]
- Kirchhammer, N.; Trefny, M.P.; Auf der Maur, P.; Läubli, H.; Zippelius, A. Combination cancer immunotherapies: Emerging treatment strategies adapted to the tumor microenvironment. Sci. Transl. Med. 2022, 14, eabo3605. [Google Scholar] [CrossRef]
- Casanova, J.M.; Almeida, J.S.; Reith, J.D.; Sousa, L.M.; Fonseca, R.; Freitas-Tavares, P.; Santos-Rosa, M.; Rodrigues-Santos, P. Tumor-Infiltrating Lymphocytes and Cancer Markers in Osteosarcoma: Influence on Patient Survival. Cancers 2021, 13, 6075. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, L.; Wu, C.; Huang, L.; Ruan, Y.; Xue, W. Tumor-derived Exosomes Induced M2 Macrophage Polarization and Promoted the Metastasis of Osteosarcoma Cells Through Tim-3. Arch. Med. Res. 2021, 52, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Wu, X.F.; Gu, X.J.; Jiang, X.H. Exosomal miR-1228 From Cancer-Associated Fibroblasts Promotes Cell Migration and Invasion of Osteosarcoma by Directly Targeting SCAI. Oncol. Res. 2019, 27, 979–986. [Google Scholar] [CrossRef]
- Mazumdar, A.; Urdinez, J.; Boro, A.; Migliavacca, J.; Arlt, M.J.E.; Muff, R.; Fuchs, B.; Snedeker, J.G.; Gvozdenovic, A. Osteosarcoma-Derived Extracellular Vesicles Induce Lung Fibroblast Reprogramming. Int. J. Mol. Sci. 2020, 21, 5451. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Gomez-Salazar, M.; Tower, R.J.; Chang, L.; Morris, C.D.; McCarthy, E.F.; Ting, K.; Zhang, X.; James, A.W. NELL1 Regulates the Matrisome to Promote Osteosarcoma Progression. Cancer Res. 2022, 82, 2734–2747. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Schwenzer, A.; Rupp, T.; Murdamoothoo, D.; Vegliante, R.; Lefebvre, O.; Klein, A.; Hussenet, T.; Orend, G. Tenascin-C Promotes Tumor Cell Migration and Metastasis through Integrin α9β1-Mediated YAP Inhibition. Cancer Res. 2018, 78, 950–961. [Google Scholar] [CrossRef]
- Bacci, G.; Briccoli, A.; Ferrari, S.; Saeter, G.; Donati, D.; Longhi, A.; Manfrini, M.; Bertoni, F.; Rimondini, S.; Monti, C.; et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with synchronous lung metastases: Treatment with cisplatin, adriamycin and high dose of methotrexate and ifosfamide. Oncol. Rep. 2000, 7, 339–346. [Google Scholar] [CrossRef]
- Qi, B.; Yu, T.; Wang, C.; Wang, T.; Yao, J.; Zhang, X.; Deng, P.; Xia, Y.; Junger, W.G.; Sun, D. Shock wave-induced ATP release from osteosarcoma U2OS cells promotes cellular uptake and cytotoxicity of methotrexate. J. Exp. Clin. Cancer Res. 2016, 35, 161. [Google Scholar] [CrossRef]
- Scott, J.R.; Ward, D.A.; Crews, K.R.; Panetta, J.C.; Navid, F. Hypersensitivity reaction to high-dose methotrexate and successful rechallenge in a pediatric patient with osteosarcoma. Pediatr. Blood Cancer 2014, 61, 373–375. [Google Scholar] [CrossRef]
- Pastorino, U.; Palmerini, E.; Porcu, L.; Luksch, R.; Scanagatta, P.; Meazza, C.; Leuzzi, G.; Massimino, M.; Picci, P. Lung metastasectomy for osteosarcoma in children, adolescents, and young adults: Proof of permanent cure. Tumori 2023, 109, 79–85. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, S.; Yang, F.; Wang, L.; Zhu, S.; Qiu, B.; Li, S.; Deng, Z. Efficacy Comparison of Six Chemotherapeutic Combinations for Osteosarcoma and Ewing’s Sarcoma Treatment: A Network Meta-Analysis. J. Cell. Biochem. 2018, 119, 250–259. [Google Scholar] [CrossRef]
- Xie, L.; Xu, J.; Sun, X.; Li, X.; Liu, K.; Liang, X.; Zhou, Z.; Zhuang, H.; Sun, K.; Wu, Y.; et al. Apatinib plus ifosfamide and etoposide for relapsed or refractory osteosarcoma: A retrospective study in two centres. Oncol. Lett. 2021, 22, 552. [Google Scholar] [CrossRef] [PubMed]
- Marec-Berard, P.; Laurence, V.; Occean, B.V.; Ray-Coquard, I.; Linassier, C.; Corradini, N.; Collard, O.; Chaigneau, L.; Cupissol, D.; Kerbrat, P.; et al. Methotrexate-Etoposide-Ifosfamide Compared with Doxorubicin-Cisplatin-Ifosfamide Chemotherapy in Osteosarcoma Treatment, Patients Aged 18–25 Years. J. Adolesc. Young Adult Oncol. 2020, 9, 172–182. [Google Scholar] [CrossRef]
- Gaspar, N.; Hawkins, D.S.; Dirksen, U.; Lewis, I.J.; Ferrari, S.; Le Deley, M.C.; Kovar, H.; Grimer, R.; Whelan, J.; Claude, L.; et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J. Clin. Oncol. 2015, 33, 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Errani, C.; Angelini, A.; Mavrogenis, A.F. Current Treatment Considerations for Osteosarcoma Metastatic at Presentation. Orthopedics 2020, 43, e345–e358. [Google Scholar] [CrossRef]
- Ozaki, T.; Flege, S.; Liljenqvist, U.; Hillmann, A.; Delling, G.; Salzer-Kuntschik, M.; Jürgens, H.; Kotz, R.; Winkelmann, W.; Bielack, S.S. Osteosarcoma of the spine: Experience of the Cooperative Osteosarcoma Study Group. Cancer 2002, 94, 1069–1077. [Google Scholar] [CrossRef]
- Milner, R.J.; Dormehl, I.; Louw, W.K.; Croft, S. Targeted radiotherapy with Sm-153-EDTMP in nine cases of canine primary bone tumours. J. S Afr. Vet. Assoc. 1998, 69, 12–17. [Google Scholar] [CrossRef][Green Version]
- Franzius, C.; Bielack, S.; Flege, S.; Eckardt, J.; Sciuk, J.; Jürgens, H.; Schober, O. High-activity samarium-153-EDTMP therapy followed by autologous peripheral blood stem cell support in unresectable osteosarcoma. Nuklearmedizin 2001, 40, 215–220. [Google Scholar]
- Subbiah, V.; Anderson, P.M.; Kairemo, K.; Hess, K.; Huh, W.W.; Ravi, V.; Daw, N.C.; Somaiah, N.; Ludwig, J.A.; Benjamin, R.S.; et al. Alpha Particle Radium 223 Dichloride in High-risk Osteosarcoma: A Phase I Dose Escalation Trial. Clin. Cancer Res. 2019, 25, 3802–3810. [Google Scholar] [CrossRef]
- Anderson, P.M.; Scott, J.; Parsai, S.; Zahler, S.; Worley, S.; Shrikanthan, S.; Subbiah, V.; Murphy, E. 223-Radium for metastatic osteosarcoma: Combination therapy with other agents and external beam radiotherapy. ESMO Open 2020, 5, e000635. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Y.; Wu, Q.; Hao, D.; Li, D. Emodin Impairs Radioresistance of Human Osteosarcoma Cells by Suppressing Sonic Hedgehog Signaling. Med. Sci. Monit. 2017, 23, 5767–5773. [Google Scholar] [CrossRef]
- Qu, W.; Li, D.; Wang, Y.; Wu, Q.; Hao, D. Activation of Sonic Hedgehog Signaling Is Associated with Human Osteosarcoma Cells Radioresistance Characterized by Increased Proliferation, Migration, and Invasion. Med. Sci. Monit. 2018, 24, 3764–3771. [Google Scholar] [CrossRef]
- Li, H.X.; Meng, Q.P.; Liu, W.; Li, Y.G.; Zhang, H.M.; Bao, F.C.; Song, L.L.; Li, H.J. IMPDH2 mediate radioresistance and chemoresistance in osteosarcoma cells. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3038–3044. [Google Scholar]
- Han, Y.; Platonov, A.; Akhalaia, M.; Yun, Y.S.; Song, J.Y. Differential effect of gamma-radiation-induced heme oxygenase-1 activity in female and male C57BL/6 mice. J. Korean Med. Sci. 2005, 20, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Loh, A.H.; Navid, F.; Wang, C.; Bahrami, A.; Wu, J.; Neel, M.D.; Rao, B.N. Management of local recurrence of pediatric osteosarcoma following limb-sparing surgery. Ann. Surg. Oncol. 2014, 21, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Dang, P.; Bian, N.; Chen, X.; Yang, T.; Fan, Q.; Zhou, Y.; Zhao, T.; Wang, P. Is Limb Salvage With Microwave-induced Hyperthermia Better Than Amputation for Osteosarcoma of the Distal Tibia? Clin. Orthop. Relat. Res. 2017, 475, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Ayerza, M.A.; Farfalli, G.L.; Aponte-Tinao, L.; Muscolo, D.L. Does increased rate of limb-sparing surgery affect survival in osteosarcoma? Clin. Orthop. Relat. Res. 2010, 468, 2854–2859. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Heymann, M.F.; Stresing, V.; Mori, K.; Rédini, F.; Heymann, D. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers 2013, 5, 591–616. [Google Scholar] [CrossRef]
- Lussier, D.M.; O’Neill, L.; Nieves, L.M.; McAfee, M.S.; Holechek, S.A.; Collins, A.W.; Dickman, P.; Jacobsen, J.; Hingorani, P.; Blattman, J.N. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J. Immunother. 2015, 38, 96–106. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, J.; Lan, Y.; Yi, Q.; Liu, H. Targeting signaling pathways in osteosarcoma: Mechanisms and clinical studies. MedComm 2023, 4, e308. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nishimura, S.; Akagi, M. Characterization of PD-1/PD-L1 Immune Checkpoint Expression in Osteosarcoma. Diagnostics 2020, 10, 528. [Google Scholar] [CrossRef]
- Dhupkar, P.; Gordon, N.; Stewart, J.; Kleinerman, E.S. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018, 7, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Mei, J.; Gao, Y.S.; Ni, M.; Yao, B. Primary tumorectomy promotes angiogenesis and pulmonary metastasis in osteosarcoma-bearing nude mice. Acta Cir. Bras. 2013, 28, 190–194. [Google Scholar] [CrossRef]
- Qu, L.; Liu, B. Cyclooxygeanse-2 promotes metastasis in osteosarcoma. Cancer Cell Int. 2015, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, N.; Liao, S.; Rothzerg, E.; Yao, F.; Li, Y.; Wood, D.; Xu, J. Current research progress in targeted anti-angiogenesis therapy for osteosarcoma. Cell Prolif. 2021, 54, e13102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Liu, Q.; Tian, J.; He, H.B.; Luo, W. Angiogenesis Process in Osteosarcoma: An Updated Perspective of Pathophysiology and Therapeutics. Am. J. Med. Sci. 2019, 357, 280–288. [Google Scholar] [CrossRef]
- Jian, Y.K.; Zhu, H.Y.; Wu, X.L.; Li, B. Thrombospondin 1 Triggers Osteosarcoma Cell Metastasis and Tumor Angiogenesis. Oncol. Res. 2019, 27, 211–218. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, C.; Chen, M.; Yang, T.; Wang, X.; Gao, Y.; Wang, L.; Wen, Y.; Chen, G.; Wang, X.; et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics 2020, 10, 411–425. [Google Scholar] [CrossRef]
- Zeng, C.; Zhong, L.; Liu, W.; Zhang, Y.; Yu, X.; Wang, X.; Zhang, R.; Kang, T.; Liao, D. Targeting the Lysosomal Degradation of Rab22a-NeoF1 Fusion Protein for Osteosarcoma Lung Metastasis. Adv. Sci. 2023, 10, e2205483. [Google Scholar] [CrossRef]
- Lu, B.; Zou, C.; Yang, M.; He, Y.; He, J.; Zhang, C.; Chen, S.; Yu, J.; Liu, K.Y.; Cao, Q.; et al. Pharmacological Inhibition of Core Regulatory Circuitry Liquid-liquid Phase Separation Suppresses Metastasis and Chemoresistance in Osteosarcoma. Adv. Sci. 2021, 8, e2101895. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Kim, G.J.; Nie, S.; Shin, D.M. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Downing, G. Medical nanotechnology: Shortening clinical trials and regulatory pathways? BioDrugs 2005, 19, 203–210. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Rahdar, A. Functional Nanomaterials in Biomedicine: Current Uses and Potential Applications. ChemMedChem 2022, 17, e202200142. [Google Scholar] [CrossRef] [PubMed]
- Tehrani Fateh, S.; Moradi, L.; Kohan, E.; Hamblin, M.R.; Shiralizadeh Dezfuli, A. Comprehensive review on ultrasound-responsive theranostic nanomaterials: Mechanisms, structures and medical applications. Beilstein J. Nanotechnol. 2021, 12, 808–862. [Google Scholar] [CrossRef]
- Pascal, J.; Ashley, C.E.; Wang, Z.; Brocato, T.A.; Butner, J.D.; Carnes, E.C.; Koay, E.J.; Brinker, C.J.; Cristini, V. Mechanistic modeling identifies drug-uptake history as predictor of tumor drug resistance and nano-carrier-mediated response. ACS Nano 2013, 7, 11174–11182. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.M.; Azizov, S.; Elmasry, M.R.; Sharipov, M.; Lee, Y.I. Recent Advances in Nanomicelles Delivery Systems. Nanomaterials 2020, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, Y.; Chen, M.; Liu, G. Application of Nanomicelles in Enhancing Bioavailability and Biological Efficacy of Bioactive Nutrients. Polymers 2022, 14, 3278. [Google Scholar] [CrossRef]
- Bose, A.; Roy Burman, D.; Sikdar, B.; Patra, P. Nanomicelles: Types, properties and applications in drug delivery. IET Nanobiotechnol 2021, 15, 19–27. [Google Scholar] [CrossRef]
- Gote, V.; Ansong, M.; Pal, D. Prodrugs and nanomicelles to overcome ocular barriers for drug penetration. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 885–906. [Google Scholar] [CrossRef]
- Yang, Y.; Alencar, L.M.R.; Pijeira, M.S.O.; Batista, B.D.S.; França, A.R.S.; Rates, E.R.D.; Lima, R.C.; Gemini-Piperni, S.; Santos-Oliveira, R. [223Ra] RaCl2 nanomicelles showed potent effect against osteosarcoma: Targeted alpha therapy in the nanotechnology era. Drug Deliv. 2022, 29, 186–191. [Google Scholar] [CrossRef]
- Souza, B.; Ribeiro, E.; da Silva de Barros, A.O.; Pijeira, M.S.O.; Kenup-Hernandes, H.O.; Ricci-Junior, E.; Diniz Filho, J.F.S.; Dos Santos, C.C.; Alencar, L.M.R.; Attia, M.F.; et al. Nanomicelles of Radium Dichloride [223Ra] RaCl2 Co-Loaded with Radioactive Gold [(198)Au]Au Nanoparticles for Targeted Alpha-Beta Radionuclide Therapy of Osteosarcoma. Polymers 2022, 14, 1405. [Google Scholar] [CrossRef]
- Gemini-Piperni, S.; Ricci-Junior, E.; İlem-Özdemir, D.; da Silva Batista, B.; Alencar, L.M.R.; Rossi, A.M.; Santos-Oliveira, R. Nano-hydroxyapatite radiolabeled with radium dichloride 223Ra] RaCl2 for bone cancer targeted alpha therapy: In vitro assay and radiation effect on the nanostructure. Colloids Surf. B Biointerfaces 2023, 223, 113174. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Fears, A.; Summer, L.; Longtine, M.; Benabdallah, N.; Riddle, R.C.; Ulmert, D.; Michalski, J.; Wahl, R.L.; Chesner, D.; et al. Improved Radium-223 Therapy with Combination Epithelial Sodium Channel Blockade. J. Nucl. Med. 2021, 62, 1751–1758. [Google Scholar] [CrossRef]
- Yin, F.; Wang, Z.; Jiang, Y.; Zhang, T.; Wang, Z.; Hua, Y.; Song, Z.; Liu, J.; Xu, W.; Xu, J.; et al. Reduction-responsive polypeptide nanomedicines significantly inhibit progression of orthotopic osteosarcoma. Nanomedicine 2020, 23, 102085. [Google Scholar] [CrossRef]
- Iannazzo, D.; Celesti, C.; Espro, C. Recent Advances on Graphene Quantum Dots as Multifunctional Nanoplatforms for Cancer Treatment. Biotechnol. J. 2021, 16, e1900422. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2021, 33, e1904362. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, B.; Rao, Z.; Zhang, B.; Gong, J.R. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett. 2013, 13, 2436–2441. [Google Scholar] [CrossRef]
- Tung, F.I.; Zheng, L.J.; Hou, K.T.; Chiang, C.S.; Chen, M.H.; Liu, T.Y. One-stop radiotherapeutic targeting of primary and distant osteosarcoma to inhibit cancer progression and metastasis using 2DG-grafted graphene quantum dots. Nanoscale 2020, 12, 8809–8818. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- González-Fernández, Y.; Brown, H.K.; Patiño-García, A.; Heymann, D.; Blanco-Prieto, M.J. Oral administration of edelfosine encapsulated lipid nanoparticles causes regression of lung metastases in pre-clinical models of osteosarcoma. Cancer Lett. 2018, 430, 193–200. [Google Scholar] [CrossRef]
- Tang, J.; Cai, L.; Xu, C.; Sun, S.; Liu, Y.; Rosenecker, J.; Guan, S. Nanotechnologies in Delivery of DNA and mRNA Vaccines to the Nasal and Pulmonary Mucosa. Nanomaterials 2022, 12, 226. [Google Scholar] [CrossRef]
- Tabcheh, J.; Vergalli, J.; Davin-Régli, A.; Ghanem, N.; Pages, J.M.; Al-Bayssari, C.; Brunel, J.M. Rejuvenating the Activity of Usual Antibiotics on Resistant Gram-Negative Bacteria: Recent Issues and Perspectives. Int. J. Mol. Sci. 2023, 24, 1515. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pang, J.D.; Huang, L.L.; Wang, R.; Li, D.; Sun, K.; Wang, L.T.; Zhang, L.M. Nanoscale polysaccharide derivative as an AEG-1 siRNA carrier for effective osteosarcoma therapy. Int. J. Nanomed. 2018, 13, 857–875. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Yang, T.; Li, T.; Zhang, M.; Ke, H.; Ding, D.; Deng, Y.; Chen, H. Serum protein-based nanoparticles for cancer diagnosis and treatment. J. Control Release 2021, 329, 997–1022. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Pangua, C.; Reboredo, C.; Campión, R.; Morales-Gracia, J.; Irache, J.M. Protein-based nanoparticles for drug delivery purposes. Int. J. Pharm. 2020, 581, 119289. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Zhang, H.; Chen, J.; Sun, X.; Xu, J.; Ren, T.; Zhang, Y.; Ma, C.; Guo, W.; et al. Improving Bioavailability of Hydrophobic Prodrugs through Supramolecular Nanocarriers Based on Recombinant Proteins for Osteosarcoma Treatment. Angew. Chem. Int. Ed. Engl. 2021, 60, 11252–11256. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Z.; Huang, C.; Fan, F.; Liu, L.; Lu, L.; Wang, H.; Liu, Z.; Yang, J.; Wang, C.; et al. Folate-Targeted Redox-Responsive Polymersomes Loaded with Chemotherapeutic Drugs and Tariquidar to Overcome Drug Resistance. J. Biomed. Nanotechnol. 2018, 14, 1705–1718. [Google Scholar] [CrossRef]
- Ferrero, C.; Casas, M.; Caraballo, I. Redox-Responsive Polymersomes as Smart Doxorubicin Delivery Systems. Pharmaceutics 2022, 14, 1724. [Google Scholar] [CrossRef]
- Curcio, M.; Blanco-Fernandez, B.; Diaz-Gomez, L.; Concheiro, A.; Alvarez-Lorenzo, C. Hydrophobically Modified Keratin Vesicles for GSH-Responsive Intracellular Drug Release. Bioconjug Chem. 2015, 26, 1900–1907. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, R.; Yang, L.; Sha, Y.; Zhao, S.; Guo, J.; Chen, D.; Zhong, Z.; Meng, F. IL-11Rα-targeted nanostrategy empowers chemotherapy of relapsed and patient-derived osteosarcoma. J. Control Release 2022, 350, 460–470. [Google Scholar] [CrossRef]
- Sakurai, Y.; Harashima, H. Hyaluronan-modified nanoparticles for tumor-targeting. Expert. Opin. Drug Deliv. 2019, 16, 915–936. [Google Scholar] [CrossRef]
- Liu, K.; Huang, X. Synthesis of self-assembled hyaluronan based nanoparticles and their applications in targeted imaging and therapy. Carbohydr. Res. 2022, 511, 108500. [Google Scholar] [CrossRef]
- Jeannot, V.; Gauche, C.; Mazzaferro, S.; Couvet, M.; Vanwonterghem, L.; Henry, M.; Didier, C.; Vollaire, J.; Josserand, V.; Coll, J.L.; et al. Anti-tumor efficacy of hyaluronan-based nanoparticles for the co-delivery of drugs in lung cancer. J. Control Release 2018, 275, 117–128. [Google Scholar] [CrossRef]
- Seino, S.; Takada, Y.; Saika, S. [Effects of Benzalkonium Chloride in Ophthalmic Eyedrop Medications on Corneal Epithelium]. Yakugaku Zasshi 2021, 141, 35–39. [Google Scholar] [CrossRef]
- Kim, K.; Choi, H.; Choi, E.S.; Park, M.H.; Ryu, J.H. Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy. Pharmaceutics 2019, 11, 301. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, T.; Li, Z.; Luo, C.; Wu, Y.; Zhang, J.; Zhang, X.; Fan, W. Hyaluronate-Based Self-Stabilized Nanoparticles for Immunosuppression Reversion and Immunochemotherapy in Osteosarcoma Treatment. ACS Biomater. Sci. Eng. 2021, 7, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J. Control Release 2022, 350, 26–59. [Google Scholar] [CrossRef] [PubMed]
- Aikins, M.E.; Xu, C.; Moon, J.J. Engineered Nanoparticles for Cancer Vaccination and Immunotherapy. Acc. Chem. Res. 2020, 53, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Wang, Y.H.; Liu, G.F.; Wang, L.; Li, Y.; Guo, Z.Y.; Cheng, C. Graphene Oxide Nanoparticle-Loaded Ginsenoside Rg3 Improves Photodynamic Therapy in Inhibiting Malignant Progression and Stemness of Osteosarcoma. Front. Mol. Biosci. 2021, 8, 663089. [Google Scholar] [CrossRef] [PubMed]
- Häuser, M.; Langer, K.; Schönhoff, M. pH-Triggered release from surface-modified poly(lactic-co-glycolic acid) nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Mahdy, N.K.; Al Mulla, H.; ElMeshad, A.N.; Issa, M.Y.; Azzazy, H.M.E. PLGA/PEG Nanoparticles Loaded with Cyclodextrin-Peganum harmala Alkaloid Complex and Ascorbic Acid with Promising Antimicrobial Activities. Pharmaceutics 2022, 14, 142. [Google Scholar] [CrossRef]
- Lam, J.K.; Chow, M.Y.; Zhang, Y.; Leung, S.W. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef]
- Maher, R.; Moreno-Borrallo, A.; Jindal, D.; Mai, B.T.; Ruiz-Hernandez, E.; Harkin, A. Intranasal Polymeric and Lipid-Based Nanocarriers for CNS Drug Delivery. Pharmaceutics 2023, 15, 746. [Google Scholar] [CrossRef] [PubMed]
- Acharya, G.; Lee, C.H.; Lee, Y. Optimization of cardiovascular stent against restenosis: Factorial design-based statistical analysis of polymer coating conditions. PLoS ONE 2012, 7, e43100. [Google Scholar] [CrossRef] [PubMed]
- Lekshmy, M.; Dhanya, C.R.; Smrithi, J.S.; Sindhurani, J.A.; Vandanamthadathil, J.J.; Veettil, J.T.; Anila, L.; Lathakumari, V.S.; Nayar, A.M.; Madhavan, M. Peptide Vaccines as Therapeutic and Prophylactic Agents for Female-Specific Cancers: The Current Landscape. Pharmaceuticals 2023, 16, 1054. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, H.; Sun, H.T.; Cao, S. Cytotoxic Effects of Castalin Nanoparticles Against Osteosarcoma. Appl. Biochem. Biotechnol. 2023, 195, 5355–5364. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, V.C.; Viswanathan, G.; Baby, S. Insights Into the Molecular Aspects of Neuroprotective Bacoside A and Bacopaside I. Curr. Neuropharmacol. 2019, 17, 438–446. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, C. Application of single and cooperative different delivery systems for the treatment of intervertebral disc degeneration. Front. Bioeng. Biotechnol. 2022, 10, 1058251. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef]
- Matalqah, S.M.; Aiedeh, K.; Mhaidat, N.M.; Alzoubi, K.H.; Bustanji, Y.; Hamad, I. Chitosan Nanoparticles as a Novel Drug Delivery System: A Review Article. Curr. Drug Targets 2020, 21, 1613–1624. [Google Scholar] [CrossRef]
- Collado-González, M.; Esteban, M. Chitosan-nanoparticles effects on mucosal immunity: A systematic review. Fish. Shellfish. Immunol. 2022, 130, 1–8. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, P.; Hameed, A.; Orczyk, W. Chitosan and Chitosan Nanoparticles: Parameters Enhancing Antifungal Activity. Molecules 2023, 28, 2996. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, Y. Recent advances of chitosan-based nanoparticles for biomedical and biotechnological applications. Int. J. Biol. Macromol. 2022, 203, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Zaiki, Y.; Iskandar, A.; Wong, T.W. Functionalized chitosan for cancer nano drug delivery. Biotechnol. Adv. 2023, 67, 108200. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Shou, C.; He, M.; Xu, J.; Cheng, Y.; Yuan, Z.; Lan, M.; Zhao, Y.; Yang, Y.; Chen, X.; et al. A combination of LightOn gene expression system and tumor microenvironment-responsive nanoparticle delivery system for targeted breast cancer therapy. Acta Pharm. Sin. B 2020, 10, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; He, J.; Wang, X.; Wang, Y.; Wu, C.; Shi, M.; Jiang, H.; Wu, Z.; Liu, J.; Zhang, W. Glycoprotein Ib-regulated micro platelet ghost for biosafe distribution and photothermal oncotherapy. J. Control Release 2022, 351, 341–360. [Google Scholar] [CrossRef]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nanomicro Lett. 2020, 12, 142. [Google Scholar] [CrossRef]
- Maharjan, S.; Gautam, M.; Poudel, K.; Yong, C.S.; Ku, S.K.; Kim, J.O.; Byeon, J.H. Streamlined plug-in aerosol prototype for reconfigurable manufacture of nano-drug delivery systems. Biomaterials 2022, 284, 121511. [Google Scholar] [CrossRef]
- Gupta, R.; Chen, Y.; Xie, H. In vitro dissolution considerations associated with nano drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2021, 13, e1732. [Google Scholar] [CrossRef]
- Wang, Y.; Pi, C.; Feng, X.; Hou, Y.; Zhao, L.; Wei, Y. The Influence of Nanoparticle Properties on Oral Bioavailability of Drugs. Int. J. Nanomed. 2020, 15, 6295–6310. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Sun, M.; Tan, L.; Li, T.; Min, L. Nano-Based Drug Delivery Systems: Potential Developments in the Therapy of Metastatic Osteosarcoma—A Narrative Review. Pharmaceutics 2023, 15, 2717. https://doi.org/10.3390/pharmaceutics15122717
Luo Y, Sun M, Tan L, Li T, Min L. Nano-Based Drug Delivery Systems: Potential Developments in the Therapy of Metastatic Osteosarcoma—A Narrative Review. Pharmaceutics. 2023; 15(12):2717. https://doi.org/10.3390/pharmaceutics15122717
Chicago/Turabian StyleLuo, Yuanrui, Minghao Sun, Linyun Tan, Tao Li, and Li Min. 2023. "Nano-Based Drug Delivery Systems: Potential Developments in the Therapy of Metastatic Osteosarcoma—A Narrative Review" Pharmaceutics 15, no. 12: 2717. https://doi.org/10.3390/pharmaceutics15122717
APA StyleLuo, Y., Sun, M., Tan, L., Li, T., & Min, L. (2023). Nano-Based Drug Delivery Systems: Potential Developments in the Therapy of Metastatic Osteosarcoma—A Narrative Review. Pharmaceutics, 15(12), 2717. https://doi.org/10.3390/pharmaceutics15122717







