Smart Responsive and Controlled-Release Hydrogels for Chronic Wound Treatment
Abstract
:1. Introduction
2. Chronic Wound Characteristics and Hydrogel Design
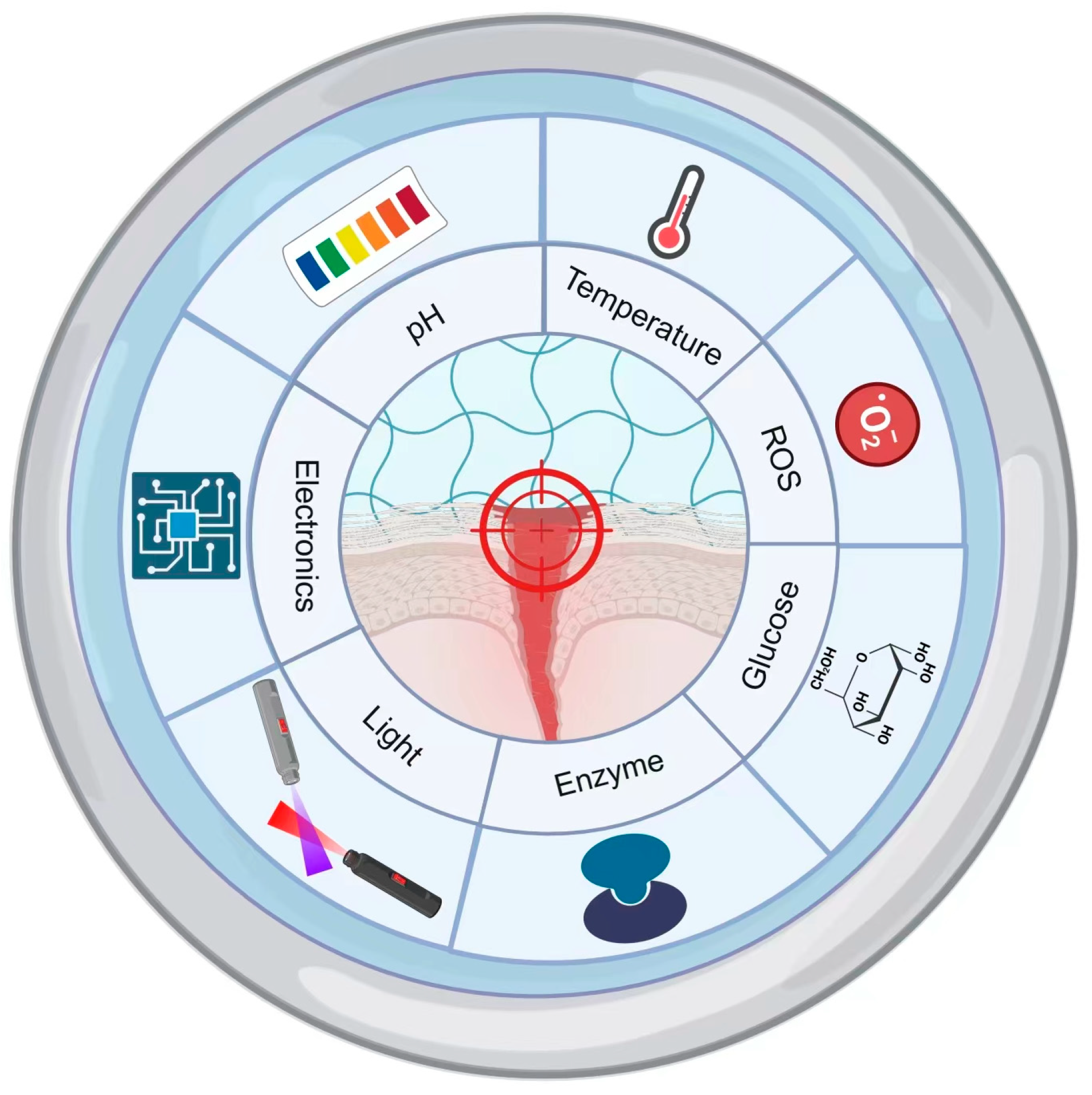
3. Smart Responsive Hydrogel Design
3.1. pH-Responsive Gels
3.1.1. pH-Responsive Design
3.1.2. Schiff Bases
3.1.3. Boronic Ester Bonds
3.1.4. pKa
3.1.5. Electrostatic Interactions
3.2. Thermoresponsive Gels

3.3. ROS-Responsive Gels
3.4. Glucose-Responsive Gels
3.5. Enzyme-Responsive Gels
3.6. Photo-Responsive Gels
3.7. Electro-Responsive Gels
4. Discussion and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair. Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Graves, N.; Phillips, C.J.; Harding, K. A Narrative Review of the Epidemiology and Economics of Chronic Wounds. Br. J. Dermatol. 2022, 187, 141–148. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef]
- Huang, F.; Lu, X.; Yang, Y.; Yang, Y.; Li, Y.; Kuai, L.; Li, B.; Dong, H.; Shi, J. Microenvironment-Based Diabetic Foot Ulcer Nanomedicine. Adv. Sci. 2023, 10, e2203308. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, L.; Huang, L.; Zhang, Y.; Tong, M.; Pan, H.; Shangguan, J.; Yao, Q.; Xu, S.; Xu, H. In Situ Hydrogel Capturing Nitric Oxide Microbubbles Accelerates the Healing of Diabetic Foot. J. Control. Release 2022, 350, 93–106. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Noor, S.; Khan, R.U.; Ahmad, J. Understanding Diabetic Foot Infection and Its Management. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 149–156. [Google Scholar] [CrossRef]
- Everett, E.; Mathioudakis, N. Update on Management of Diabetic Foot Ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound Healing and Treating Wounds. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Dixon, D.; Edmonds, M. Managing Diabetic Foot Ulcers: Pharmacotherapy for Wound Healing. Drugs 2020, 81, 29–56. [Google Scholar] [CrossRef] [PubMed]
- McColl, D.; Cartlidge, B.; Connolly, P.J.I. Real-Time Monitoring of Moisture Levels in Wound Dressings in Vitro: An Experimental Study. Int. J. Surg. 2007, 5, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S.J.C. Sodium Alginate Hydrogel Containing Platelet-Rich Plasma for Wound Healing. Colloids Surf. B Biointerfaces 2023, 222, 113096. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.E.; Foster, D.S.; Longaker, M.T. Management of Chronic Wounds-2018. JAMA 2018, 320, 1481–1482. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Tian, S.; Shi, Y.; Yang, J.; Li, H.; Tang, H.; Yang, W. Preparation and Evaluation of a Novel Alginate-Arginine-Zinc Ion Hydrogel Film for Skin Wound Healing. Carbohydr. Polym. 2023, 311, 120757. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, Z.; Liu, Y.; Zhang, W.; Geng, L.; Ni, T. Incorporation of Ros-Responsive Substance P-Loaded Zeolite Imidazolate Framework-8 Nanoparticles into a Ca2+-Cross-Linked Alginate/Pectin Hydrogel for Wound Dressing Applications. Int. J. Nanomed. 2020, 15, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Mirbagheri, M.S.; Akhavan-Mahdavi, S.; Hasan, A.; Kharazmi, M.S.; Jafari, S.M. Chitosan-Based Electrospun Nanofibers for Diabetic Foot Ulcer Management; Recent Advances. Carbohydr. Polym. 2023, 313, 120512. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. Ph/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing Via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef]
- Maver, T.; Gradisnik, L.; Smrke, D.M.; Stana Kleinschek, K.; Maver, U. Systematic Evaluation of a Diclofenac-Loaded Carboxymethyl Cellulose-Based Wound Dressing and Its Release Performance with Changing Ph and Temperature. AAPS PharmSciTech 2019, 20, 29. [Google Scholar] [CrossRef]
- Pan, N.; Qin, J.; Feng, P.; Li, Z.; Song, B. Color-Changing Smart Fibrous Materials for Naked Eye Real-Time Monitoring of Wound Ph. J. Mater. Chem. B 2019, 7, 2626–2633. [Google Scholar] [CrossRef]
- Haidari, H.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Bacteria-Activated Dual Ph- and Temperature-Responsive Hydrogel for Targeted Elimination of Infection and Improved Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 51744–51762. [Google Scholar] [CrossRef]
- Agrawal, K.; Sarda, A.; Shrotriya, R.; Bachhav, M.; Puri, V.; Nataraj, G. Acetic Acid Dressings: Finding the Holy Grail for Infected Wound Management. Indian J. Plast. Surg. 2017, 50, 273–280. [Google Scholar] [CrossRef]
- Wlaschek, M.; Singh, K.; Sindrilaru, A.; Crisan, D.; Scharffetter-Kochanek, K. Iron and Iron-Dependent Reactive Oxygen Species in the Regulation of Macrophages and Fibroblasts in Non-Healing Chronic Wounds. Free Radic. Biol. Med. 2019, 133, 262–275. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef]
- Aitcheson, S.M.; Frentiu, F.D.; Hurn, S.E.; Edwards, K.; Murray, R.Z. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules 2021, 26, 4917. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Xu, Y.; Niu, Y. Application of a Cascaded Nanozyme in Infected Wound Recovery of Diabetic Mice. ACS Biomater. Sci. Eng. 2022, 8, 1522–1531. [Google Scholar] [CrossRef]
- Chang, M.; Nguyen, T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (Gelma) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Fu, M.; Zhao, Y.; Wang, Y.; Li, Y.; Wu, M.; Liu, Q.; Hou, Z.; Lu, Z.; Wu, K.; Guo, J. On-Demand Removable Self-Healing and Ph-Responsive Europium-Releasing Adhesive Dressing Enables Inflammatory Microenvironment Modulation and Angiogenesis for Diabetic Wound Healing. Small 2023, 19, e2205489. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Liu, Y.; Hua, S.; Meng, F.; Ma, Q.; Kong, L.; Pan, S.; Che, Y. Injectable, Self-Healable and Antibacterial Multi-Responsive Tunicate Cellulose Nanocrystals Strengthened Supramolecular Hydrogels for Wound Dressings. Int. J. Biol. Macromol. 2023, 240, 124365. [Google Scholar] [CrossRef]
- Kang, X.; Guan, P.; Xiao, C.; Liu, C.; Guan, Y.; Lin, Y.; Tian, Y.; Ren, K.; Huang, Y.; Fu, R.; et al. Injectable Intrinsic Photothermal Hydrogel Bioadhesive with on-Demand Removability for Wound Closure and Mrsa-Infected Wound Healing. Adv. Healthc. Mater. 2023, 12, e2203306. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Peng, S.; Yao, Y.; Xie, J.; Li, S.; Tu, C.; Gao, C. A Nanofibrous Membrane Loaded with Doxycycline and Printed with Conductive Hydrogel Strips Promotes Diabetic Wound Healing in Vivo. Acta Biomater. 2022, 152, 60–73. [Google Scholar] [CrossRef]
- Lei, H.; Fan, D. A Combination Therapy Using Electrical Stimulation and Adaptive, Conductive Hydrogels Loaded with Self-Assembled Nanogels Incorporating Short Interfering Rna Promotes the Repair of Diabetic Chronic Wounds. Adv. Sci. 2022, 9, 2201425. [Google Scholar] [CrossRef]
- Andrade-Vivero, P.; Fernandez-Gabriel, E.; Alvarez-Lorenzo, C.; Concheiro, A. Improving the Loading and Release of Nsaids from Phema Hydrogels by Copolymerization with Functionalized Monomers. J. Pharm. Sci. 2007, 96, 802–813. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Q.; Qin, H.; Guan, Y.; Shen, D.; Hua, Y.; Tang, Y.; Xu, J. A Temperature-Responsive Copolymer Hydrogel in Controlled Drug. Macromolecules 2006, 39, 6584–6589. [Google Scholar] [CrossRef]
- Yan, X.; Fang, W.W.; Xue, J.; Sun, T.C.; Dong, L.; Zha, Z.; Qian, H.; Song, Y.H.; Zhang, M.; Gong, X.; et al. Thermoresponsive in Situ Forming Hydrogel with Sol-Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus Aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Zhengda, Z.; Weizhong, Y.; Shuying, G.; Tianfu, R.; Jie, R. Click Chemistry and Its Growing Applications in Biomedical Field. Prog. Chem. 2010, 22, 417–426. [Google Scholar]
- van Dijk, M.; van Nostrum, C.; Hennink, W.; Rijkers, D.; Liskamp, R.J.B. Synthesis and Characterization of Enzymatically Biodegradable Peg and Peptide-Based Hydrogels Prepared by Click Chemistry. Biomacromolecules 2010, 11, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Qizhi, Y.; Jia, L.; Yulin, J. Application of Click Chemistry in Biomedical Polymers. Prog. Chem. 2010, 22, 2377–2387. [Google Scholar]
- Hennink, W.E.; van Nostrum, C.F. Novel Crosslinking Methods to Design Hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click Hydrogels, Microgels and Nanogels: Emerging Platforms for Drug Delivery and Tissue Engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer Nanogels: A Versatile Nanoscopic Drug Delivery Platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-Responsive Polymeric Nanogels as Smart Drug Delivery Systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, Y.; Pang, L.; Xu, T.; Yu, B.; Cong, H.; Shen, Y. Wound Microenvironment-Responsive Protein Hydrogel Drug-Loaded System with Accelerating Healing and Antibacterial Property. ACS Appl. Mater. Interfaces 2022, 14, 10187–10199. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Tennikova, T. Tunable Hydrogels: Smart Materials for Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Nagahama, K.; Mori, Y.; Ohya, Y.; Ouchi, T.J.B. Biodegradable Nanogel Formation of Polylactide-Grafted Dextran Copolymer in Dilute Aqueous Solution and Enhancement of Its Stability by Stereocomplexation. Biomacromolecules 2007, 8, 2135–2141. [Google Scholar] [CrossRef]
- Qu, X.; Yang, Z. Synthesis of Ph Responsible Drug Delivery Systems by the Inclusion of a Dynamic Covalent Bond, Benzoic-Imine. Acta Polym. Sin. 2011, 11, 1118–1124. [Google Scholar] [CrossRef]
- Lin, X.; Mao, Y.; Li, P.; Bai, Y.; Chen, T.; Wu, K.; Chen, D.; Yang, H.; Yang, L. Ultra-Conformable Ionic Skin with Multi-Modal Sensing, Broad-Spectrum Antimicrobial and Regenerative Capabilities for Smart and Expedited Wound Care. Adv. Sci. 2021, 8, 2004627. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.W.; Li, Y.; Kim, J. Transparent and Semi-Interpenetrating Network P(Vinyl Alcohol)- P(Acrylic Acid) Hydrogels: Ph Responsive and Electroactive Application. Int. J. Smart Nano Mater. 2017, 8, 80–94. [Google Scholar] [CrossRef]
- Niu, Z.; Xie, M.; Wei, Z.; Guo, Y.; Han, M.; Ding, Y.; Huang, J.; Zheng, K.; Zhang, Y.; Song, Y.; et al. In Situ Structure Transformation of a Sprayed Gel for Ph-Ultrasensitive Nano-Catalytic Antibacterial Therapy. Adv. Healthc. Mater. 2023, 12, e2202441. [Google Scholar] [CrossRef]
- Jia, Y.; Yan, X.; Li, J. Schiff Base Mediated Dipeptide Assembly toward Nanoarchitectonics. Angew. Chem. Int. Ed. 2022, 61, e202207752. [Google Scholar] [CrossRef]
- Fang, W.; Yang, L.; Chen, Y.; Hu, Q. Bioinspired Multifunctional Injectable Hydrogel for Hemostasis and Infected Wound Management. Acta Biomater. 2023, 161, 50–66. [Google Scholar] [CrossRef]
- Senkała, S.; Małecki, J.; Vasylieva, M.; Łabuz, A.; Nosek, K.; Piwowarczyk, K.; Czyż, J.; Schab-Balcerzak, E.; Janeczek, H.; Korzec, M. Hydrolysis of Schiff Bases with Phenyl-Ethynyl-Phenyl System: The Importance for Biological and Physicochemical Studies. J. Photochem. Photobiol. B Biol. 2020, 212, 112020. [Google Scholar] [CrossRef]
- Barbosa, H.; Attjioui, M.; Leitão, A.; Moerschbacher, B.; Cavalheiro, É.J. Characterization, Solubility and Biological Activity of Amphihilic Biopolymeric Schiff Bases Synthesized Using Chitosans. Carbohydr. Polym. 2019, 220, 1–11. [Google Scholar] [CrossRef]
- Adrover, M.; Vilanova, B.; Muñoz, F.; Donoso, J. Unexpected Isomeric Equilibrium in Pyridoxamine Schiff Bases. Bioorg. Chem. 2009, 37, 26–32. [Google Scholar] [CrossRef]
- Masson, C.; Garinot, M.; Mignet, N.; Wetzer, B.; Mailhe, P.; Scherman, D.; Bessodes, M. Ph-Sensitive Peg Lipids Containing Orthoester Linkers: New Potential Tools for Nonviral Gene Delivery. J. Control. Release 2004, 99, 423–434. [Google Scholar] [CrossRef]
- Sonawane, H.; Vibhute, B.; Aghav, B.; Deore, J.; Patil, S. Versatile Applications of Transition Metal Incorporating Quinoline Schiff Base Metal Complexes: An Overview. Eur. J. Med. Chem. 2023, 258, 115549. [Google Scholar] [CrossRef]
- Xin, F. Synthesis and Antibacterial Studies of Asymmetric Di-Schiff Bases Master; Qingdao University of Sciense & Technology: Qingdao, China, 2012. [Google Scholar]
- Wei, Z. Synthesis, Characterization and Biological Activities of Transition Metal Complexes Derived from 2-Hydroxy-1-Naphthaldehyde Schiff Base Ligands. Master’s Thesis, Guangxi Normal University, Guilin, China, 2020. [Google Scholar]
- Qian, L. Synthesis and Biological Activity of Schiff Bases and Its Metalcomplexes. Master’s Thesis, South-Central University for Nationlities, Wuhan, China, 2013. [Google Scholar]
- Dutta, B.; Halder, S. Schiff Base Compounds as Fluorimetric Ph Sensor: A Review. Anal. Methods 2022, 14, 2132–2146. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-Modifications and Applications: Opportunities Galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Song, F.; Kong, Y.; Shao, C.; Cheng, Y.; Lu, J.; Tao, Y.; Du, J.; Wang, H. Chitosan-Based Multifunctional Flexible Hemostatic Bio-Hydrogel. Acta Biomater. 2021, 136, 170–183. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-Based Hydrogel Wound Dressing: From Mechanism to Applications, a Review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef]
- Pathak, K.; Misra, S.K.; Sehgal, A.; Singh, S.; Bungau, S.; Najda, A.; Gruszecki, R.; Behl, T. Biomedical Applications of Quaternized Chitosan. Polymers 2021, 13, 2514. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and Conductive Injectable Hydrogels Based on Quaternized Chitosan-Graft-Polyaniline/Oxidized Dextran for Tissue Engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-Crosslinked Mussel-Inspired Smart Hydrogels with Enhanced Antibacterial and Angiogenic Properties for Chronic Infected Diabetic Wound Treatment Via Ph-Responsive Quick Cargo Release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Liu, X.; Dou, G.; Li, Z.; Wang, X.; Jin, R.; Liu, Y.; Kuang, H.; Huang, X.; Yang, X.; Yang, X.; et al. Hybrid Biomaterial Initiates Refractory Wound Healing Via Inducing Transiently Heightened Inflammatory Responses. Adv. Sci. 2022, 9, e2105650. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, S.; Starnes, T.; Becker, F.; Lam, P.; Huttenlocher, A. Redox and Src Family Kinase Signaling Control Leukocyte Wound Attraction and Neutrophil Reverse Migration. J. Cell Biol. 2014, 207, 589–598. [Google Scholar] [CrossRef]
- Ma, M.; Zhong, Y.; Jiang, X. Thermosensitive and Ph-Responsive Tannin-Containing Hydroxypropyl Chitin Hydrogel with Long-Lasting Antibacterial Activity for Wound Healing. Carbohydr. Polym. 2020, 236, 116096. [Google Scholar] [CrossRef]
- Wang, G.; Meng, X.; Wang, P.; Wang, X.; Liu, G.; Wang, D.; Fan, C. A Catechol Bioadhesive for Rapid Hemostasis and Healing of Traumatic Internal Organs and Major Arteries. Biomaterials 2022, 291, 121908. [Google Scholar] [CrossRef]
- Xu, J.; Strandman, S.; Zhu, J.; Barralet, J.; Cerruti, M. Genipin-Crosslinked Catechol-Chitosan Mucoadhesive Hydrogels for Buccal Drug Delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Destefani, C.A.; Motta, L.C.; Costa, R.A.; Macrino, C.J.; Bassane, J.F.P.; Filho, J.F.A.; Silva, E.M.; Greco, S.J.; Carneiro, M.T.W.D.; Endringer, D.C.; et al. Evaluation of Acute Toxicity of Europium–Organic Complex Applied as a Luminescent Marker for the Visual Identification of Gunshot Residue. Microchem. J. 2016, 124, 195–200. [Google Scholar] [CrossRef]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological Evaluations of Rare Earths and Their Health Impacts to Workers: A Literature Review. Saf. Health Work. 2013, 4, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, H.; Cai, J.; Wang, M.; Zhou, X.; Ren, L. Poly(Pentahydropyrimidine)-Based Hybrid Hydrogel with Synergistic Antibacterial and Pro-Angiogenic Ability for the Therapy of Diabetic Foot Ulcers. Adv. Funct. Mater. 2023. early view. [Google Scholar]
- Zhou, P.; Wu, S.; Hegazy, M.; Li, H.; Xu, X.; Lu, H.; Huang, X. Engineered Borate Ester Conjugated Protein-Polymer Nanoconjugates for Ph-Responsive Drug Delivery. Mater. Sci. Eng. C 2019, 104, 109914. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Zheng, D.; Wu, X.; Luo, W.; Yuan, C.; Dai, L. Dynamic Boronate Ester Bond Manipulating Catechol Groups for the Design of Functional Adhesive Polymers. Acta Polym. Sin. 2022, 53, 796–811. [Google Scholar]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-Responsive Injectable Hydrogels Encapsulating Drug-Loaded Micelles for on-Demand Antimicrobial Activity and Accelerated Wound Healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Cui, T.; Yu, J.; Wang, C.F.; Chen, S.; Li, Q.; Guo, K.; Qing, R.; Wang, G.; Ren, J. Micro-Gel Ensembles for Accelerated Healing of Chronic Wound Via Ph Regulation. Adv. Sci. 2022, 9, e2201254. [Google Scholar] [CrossRef]
- Moreno, A.; Lligadas, G.; Adamson, J.; Maurya, D.; Percec, V. Assembling Complex Macromolecules and Self-Organizations of Biological Relevance with Cu(I)-Catalyzed Azide-Alkyne, Thio-Bromo, and Termini Double “Click” Reactions. Polymers 2023, 15, 1075. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.; Moorhouse, A.J. The Growing Applications of Click Chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Li, Q.; Li, G.; Fan, L.; Yu, Y.; Liu, J. Click Reaction Triggered Turn-on Fluorescence Strategy for Highly Sensitive and Selective Determination of Steroid Hormones in Food Samples. Food Chem. 2022, 374, 131565. [Google Scholar] [CrossRef]
- Taiariol, L.; Chaix, C.; Farre, C.; Moreau, E.J. Click and Bioorthogonal Chemistry: The Future of Active Targeting of Nanoparticles for Nanomedicines? Chem. Rev. 2022, 122, 340–384. [Google Scholar] [CrossRef]
- Parker, C.; Pratt, M.J.C. Click Chemistry in Proteomic Investigations. Cell 2020, 180, 605–632. [Google Scholar] [CrossRef]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive Polysaccharides and Their Thermoreversible Physical Hydrogel Networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef]
- Fu, H.; Xue, K.; Zhang, Y.; Xiao, M.; Wu, K.; Shi, L.; Zhu, C. Thermoresponsive Hydrogel-Enabled Thermostatic Photothermal Therapy for Enhanced Healing of Bacteria-Infected Wounds. Adv. Sci. 2023, 10, e2206865. [Google Scholar] [CrossRef]
- Long, S.; Huang, J.; Xiong, J.; Liu, C.; Chen, F.; Shen, J.; Huang, Y.; Li, X.J.P. Designing Multistimuli-Responsive Anisotropic Bilayer Hydrogel Actuators by Integrating Lcst Phase Transition and Photochromic Isomerization. Polymers 2023, 15, 786. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Q.; Xu, Y.; Yang, M.; Wu, Q.; Wang, F.; Sun, P. Highly Bidirectional Bendable Actuator Engineered by Lcst-Ucst Bilayer Hydrogel with Enhanced Interface. ACS Appl. Mater. Interfaces 2020, 12, 55290–55298. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Z.; He, Y.; Lu, Q.; Chen, R.; Zhao, C.; Dong, D.; Sun, Y.; He, H. Dual Light-Responsive Cellulose Nanofibril-Based in Situ Hydrogel for Drug-Resistant Bacteria Infected Wound Healing. Carbohydr. Polym. 2022, 297, 120042. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Oxidative Stress in Normal and Impaired Wound Repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. Ros-Scavenging Hydrogel to Promote Healing of Bacteria Infected Diabetic Wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Long, L.; Hu, C.; Kong, Q.; Wang, Y. A Spatiotemporal Release Platform Based on Ph/Ros Stimuli-Responsive Hydrogel in Wound Repairing. J. Control. Release 2022, 341, 147–165. [Google Scholar] [CrossRef]
- Peng, Y.; He, D.; Ge, X.; Lu, Y.; Chai, Y.; Zhang, Y.; Mao, Z.; Luo, G.; Deng, J.; Zhang, Y. Construction of Heparin-Based Hydrogel Incorporated with Cu5.4o Ultrasmall Nanozymes for Wound Healing and Inflammation Inhibition. Bioact. Mater. 2021, 6, 3109–3124. [Google Scholar] [CrossRef]
- Ding, Q.; Sun, T.; Su, W.; Jing, X.; Ye, B.; Su, Y.; Zeng, L.; Qu, Y.; Yang, X.; Wu, Y.; et al. Bioinspired Multifunctional Black Phosphorus Hydrogel with Antibacterial and Antioxidant Properties: A Stepwise Countermeasure for Diabetic Skin Wound Healing. Adv. Healthc. Mater. 2022, 11, e2102791. [Google Scholar] [CrossRef]
- Shi, W.; Kong, Y.; Su, Y.; Kuss, M.A.; Jiang, X.; Li, X.; Xie, J.; Duan, B. Tannic Acid-Inspired, Self-Healing, and Dual Stimuli Responsive Dynamic Hydrogel with Potent Antibacterial and Anti-Oxidative Properties. J. Mater. Chem. B 2021, 9, 7182–7195. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, J.; Wang, S.; Wang, Q.; Teng, W. Dually Crosslinked Copper-Poly(Tannic Acid) Nanoparticles with Microenvironment-Responsiveness for Infected Wound Treatment. Adv. Healthc. Mater. 2023, 12, e2203063. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Yu, H.; Wang, L.; Huang, Y.; Lu, H.; Zhou, H.; Liu, Q. Multistage Ros-Responsive and Natural Polyphenol-Driven Prodrug Hydrogels for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 52643–52658. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wu, Y.; Li, W.; Wang, Y.; Kong, Q. Development of a Microenvironment-Responsive Hydrogel Promoting Chronically Infected Diabetic Wound Healing through Sequential Hemostatic, Antibacterial, and Angiogenic Activities. ACS Appl. Mater. Interfaces 2022, 14, 30480–30492. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.-Q.; Li, Z.-B.; Wu, Y.-L.; Li, D.-W. Research Advances in Smart Responsive-Hydrogel Dressings with Potential Clinical Diabetic Wound Healing Properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; Shi, Z.; Lin, L.; Li, Y.; Wang, M.; Pan, G.; Lei, Y.; Xue, L. Responsive Hydrogel-Based Microneedle Dressing for Diabetic Wound Healing. J. Mater. Chem. B 2022, 10, 3501–3511. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, W.; Xu, P.; Fu, X.; Yu, X.; Chen, L.; Leng, F.; Yu, C.; Yang, Z. Glucose-Responsive Multifunctional Metal-Organic Drug-Loaded Hydrogel for Diabetic Wound Healing. Acta Biomater. 2022, 140, 206–218. [Google Scholar] [CrossRef]
- Kawamura, A.; Hata, Y.; Miyata, T.; Uragami, T. Synthesis of Glucose-Responsive Bioconjugated Gel Particles Using Surfactant-Free Emulsion Polymerization. Colloids Surf. B Biointerfaces 2012, 99, 74–81. [Google Scholar] [CrossRef]
- Wimmer, R.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hämmerle, M.; Esk, C.; Bagley, J.; et al. Human Blood Vessel Organoids as A model Of diabetic Vasculopathy. Nature 2019, 565, 505–510. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Song, J.; Yang, J.; Du, Z.; Zhao, W.; Guo, H.; Wen, C.; Li, Q.; Sui, X.; et al. A Multifunctional Pro-Healing Zwitterionic Hydrogel for Simultaneous Optical Monitoring of Ph and Glucose in Diabetic Wound Treatment. Adv. Funct. Mater. 2019, 30, 1905493. [Google Scholar] [CrossRef]
- Tian, R.; Qiu, X.; Yuan, P.; Lei, K.; Wang, L.; Bai, Y.; Liu, S.; Chen, X. Fabrication of Self-Healing Hydrogels with on-Demand Antimicrobial Activity and Sustained Biomolecule Release for Infected Skin Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 17018–17027. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, S.; Wu, Z.; Li, Q.; Ren, S.; Chen, J.; Xu, X.; Wang, C.; Lu, C.; Yang, X.; et al. Adsc-Exo@Mmp-Peg Smart Hydrogel Promotes Diabetic Wound Healing by Optimizing Cellular Functions and Relieving Oxidative Stress. Mater. Today Bio 2022, 16, 100365. [Google Scholar] [CrossRef] [PubMed]
- Chandrawati, R. Enzyme-Responsive Polymer Hydrogels for Therapeutic Delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Che, Q.T.; Charoensri, K.; Seo, J.W.; Nguyen, M.H.; Jang, G.; Bae, H.; Park, H.J. Triple-Conjugated Photo-/Temperature-/Ph-Sensitive Chitosan with an Intelligent Response for Bioengineering Applications. Carbohydr. Polym. 2022, 298, 120066. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, B.; Li, M.; Wang, H.; Zhu, J.; Li, Q.; Gao, H.; Feng, Q.; Cao, X. Microenvironment Responsive Nanocomposite Hydrogel with Nir Photothermal Therapy, Vascularization and Anti-Inflammation for Diabetic Infected Wound Healing. Bioact. Mater. 2023, 26, 306–320. [Google Scholar] [CrossRef]
- Ouyang, J.; Ji, X.; Zhang, X.; Feng, C.; Tang, Z.; Kong, N.; Xie, A.; Wang, J.; Sui, X.; Deng, L.; et al. In Situ Sprayed Nir-Responsive, Analgesic Black Phosphorus-Based Gel for Diabetic Ulcer Treatment. Proc. Natl. Acad. Sci. USA 2020, 117, 28667–28677. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Cheng, G.; Guo, J.; Du, S.; Qiu, J.; Wang, C.; Li, C.; Yang, X.; Chen, T.; et al. Tailored Hydrogel Delivering Niobium Carbide Boosts Ros-Scavenging and Antimicrobial Activities for Diabetic Wound Healing. Small 2022, 18, e2201300. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with on-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]
- Liu, P.-Y.; Miao, Z.-H.; Li, K.; Yang, H.; Zhen, L.; Xu, C.-Y. Biocompatible Fe3+–Ta Coordination Complex with High Photothermal Conversion Efficiency for Ablation of Cancer Cells. Colloids Surf. B Biointerfaces 2018, 167, 183–190. [Google Scholar] [CrossRef]
- Pan, W.; Wu, B.; Nie, C.; Luo, T.; Song, Z.; Lv, J.; Tan, Y.; Liu, C.; Zhong, M.; Liao, T.; et al. Nir-Ii Responsive Nanohybrids Incorporating Thermosensitive Hydrogel as Sprayable Dressing for Multidrug-Resistant-Bacteria Infected Wound Management. ACS Nano 2023, 17, 11253–11267. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.; Xiang, G.; Jiang, T.; Zhao, X. Photodynamic Alginate Zn-Mof Thermosensitive Hydrogel for Accelerated Healing of Infected Wounds. ACS Appl. Mater. Interfaces 2023, 15, 22830–22842. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Fu, F.-S.; Chen, Q.-W.; Zhang, Y.; Zhang, X.-Z. Two-Pronged Microbe Delivery of Nitric Oxide and Oxygen for Diabetic Wound Healing. Nano Lett. 2023, 23, 5595–5602. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “Smart” Materials-Based near-Infrared Light-Responsive Drug Delivery Systems for Cancer Treatment: A Review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Wang, Y.; Han, F.; Shen, K.; Luo, L.; Li, Y.; Jia, Y.; Zhang, J.; Cai, W.; et al. Exosome/Metformin-Loaded Self-Healing Conductive Hydrogel Rescues Microvascular Dysfunction and Promotes Chronic Diabetic Wound Healing by Inhibiting Mitochondrial Fission. Bioact. Mater. 2023, 26, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, A.Y.; Morozova, E.A.; Ryabkin, D.I.; Fayzullin, A.; Tarasenko, S.V.; Molodykh, V.V.; Pyankov, E.S.; Savelyev, M.S.; Sorokina, E.A.; Rogalsky, A.Y.; et al. Reconstruction of Soft Biological Tissues Using Laser Soldering Technology with Temperature Control and Biopolymer Nanocomposites. Bioengineering 2022, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Popovich, K.D.; Vagner, S.A.; Murashko, D.T.; Ten, G.N.; Ryabkin, D.I.; Savelyev, M.S.; Kitsyuk, E.P.; Gerasimenko, E.A.; Edelbekova, P.; Konovalov, A.N.; et al. Stability and Thrombogenicity Analysis of Collagen/Carbon Nanotube Nanocomposite Coatings Using a Reversible Microfluidic Device. Membranes 2023, 13, 403. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, G.; Ruan, H.; Chen, K.; Cai, Z.; Lu, G.; Li, R.; Deng, L.; Cai, M.; Cui, W. Viacapturing Magnesium Ions Microfluidic Hydrogel Microspheres for Promoting Cancellous Bone Regeneration. ACS Nano 2021, 15, 13041–13054. [Google Scholar] [CrossRef]
- Tang, P.; Han, L.; Li, P.; Jia, Z.; Wang, K.; Zhang, H.; Tan, H.; Guo, T.; Lu, X. Mussel-Inspired Electroactive and Antioxidative Scaffolds with Incorporation of Polydopamine-Reduced Graphene Oxide for Enhancing Skin Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 7703–7714. [Google Scholar] [CrossRef]
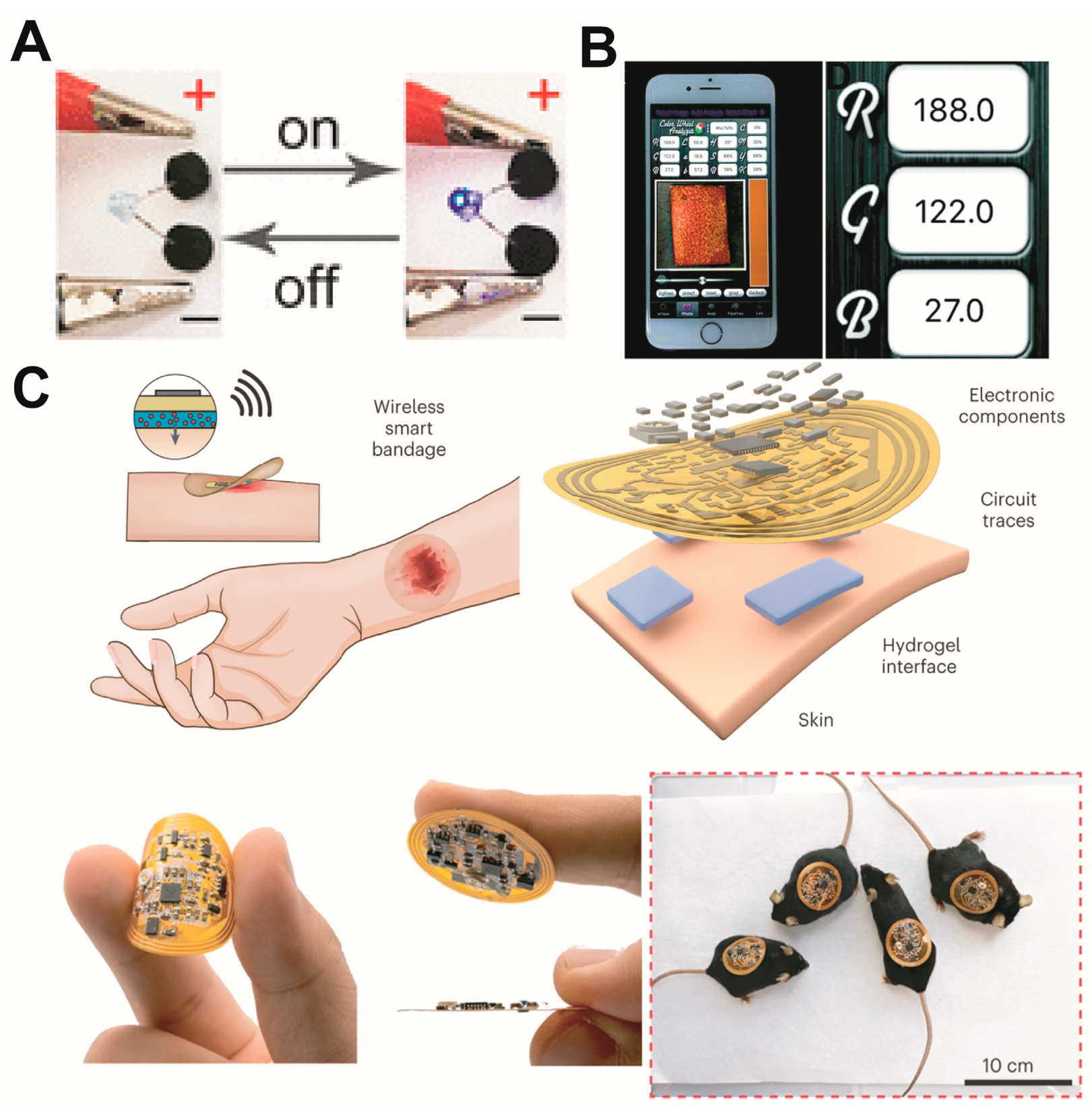
- Jiang, Y.; Trotsyuk, A.A.; Niu, S.; Henn, D.; Chen, K.; Shih, C.C.; Larson, M.R.; Mermin-Bunnell, A.M.; Mittal, S.; Lai, J.C.; et al. Wireless, Closed-Loop, Smart Bandage with Integrated Sensors and Stimulators for Advanced Wound Care and Accelerated Healing. Nat. Biotechnol. 2023, 41, 652–662. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.X.; Tang, S.; Chen, Y.; Lan, L.M.; Li, S.; Xiong, M.; Hu, X.; Liu, Y.H.; Sun, J.; et al. A Boron-Based Probe Driven Theranostic Hydrogel Dressing for Visual Monitoring and Matching Chronic Wounds Healing. Adv. Funct. Mater. 2023. early view. [Google Scholar]
- Zhao, Y.; Wang, D.; Qian, T.; Zhang, J.; Li, Z.; Gong, Q.; Ren, X.; Zhao, Y. Biomimetic Nanozyme-Decorated Hydrogels with H2o2-Activated Oxygenation for Modulating Immune Microenvironment in Diabetic Wound. ACS Nano 2023, 17, 16854–16869. [Google Scholar] [CrossRef]
- Yang, J.; Jin, X.; Liu, W.; Wang, W. A Programmable Oxygenation Device Facilitates Oxygen Generation and Replenishment to Promote Wound Healing. Adv. Mater. 2023. early view. [Google Scholar]
- Wang, L.; Chen, G.; Fan, L.; Chen, H.; Zhao, Y.; Lu, L.; Shang, L. Biomimetic Enzyme Cascade Structural Color Hydrogel Microparticles for Diabetic Wound Healing Management. Adv. Sci. 2023, 10, 2206900. [Google Scholar] [CrossRef]
- Haghighat Bayan, M.A.; Dias, Y.J.; Rinoldi, C.; Nakielski, P.; Rybak, D.; Truong, Y.B.; Yarin, A.L.; Pierini, F. Near-Infrared light Activated Core-Shell Electrospun Nanofibers Decorated with Photoactive Plasmonic Nanoparticles for on-Demand Smart Drug Delivery Applications. J. Polym. Sci. 2023, 61, 521–533. [Google Scholar] [CrossRef]
- da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jin, H.; Li, L.; Zhang, X.; Zheng, C.; Gao, X.; Yang, Y.; Sun, B. An Injectable, Activated Neutrophil-Derived Exosome Mimetics/Extracellular Matrix Hybrid Hydrogel with Antibacterial Activity and Wound Healing Promotion Effect for Diabetic Wound Therapy. J. Nanobiotechnol. 2023, 21, 308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Niu, X.; Su, T.; Huang, X.; Lu, F.; Chang, Q. Direct Three-Dimensional Printed Egg White Hydrogel Wound Dressing Promotes Wound Healing with Hitching Adipose Stem Cells. Front. Bioeng. Biotechnol. 2022, 10, 930551. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liu, W.; Wei, W.; Zhao, Y.; Zhuang, P.; Wang, X.; Wang, Y.; Hu, Y.; Dai, H. Photothermal Hydrogel Encapsulating Intelligently Bacteria-Capturing Bio-Mof for Infectious Wound Healing. ACS Nano 2022, 16, 19491–19508. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Wen, T.-K.; Dai, N.-T.; Hsu, S.-H. Cryogel/Hydrogel Biomaterials and Acupuncture Combined to Promote Diabetic Skin Wound Healing through Immunomodulation. Biomaterials 2021, 269, 120608. [Google Scholar] [CrossRef]
- Ribeiro, M.P.; Morgado, P.I.; Miguel, S.P.; Coutinho, P.; Correia, I.J. Dextran-Based Hydrogel Containing Chitosan Microparticles Loaded with Growth Factors to Be Used in Wound Healing. Mater. Sci. Eng. C 2013, 33, 2958–2966. [Google Scholar] [CrossRef]
- Beninatto, R.; Barbera, C.; De Lucchi, O.; Borsato, G.; Serena, E.; Guarise, C.; Pavan, M.; Luni, C.; Martewicz, S.; Galesso, D.; et al. Photocrosslinked Hydrogels from Coumarin Derivatives of Hyaluronic Acid for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 96, 625–634. [Google Scholar] [CrossRef]


| Type of Responsiveness | Materials | Cargo | Responsive Unit | Release Behaviour Study | Effect | Reference |
|---|---|---|---|---|---|---|
| pH-Responsive | Polycaprolactone | Curcumin | Curcumin conjugated effect and reciprocal isomer | × | Wound monitoring | [19] |
| Carboxymethyl-CS, Sodium alginate | Mn2+ | MnSiO3 | √ | Antibacterial | [51] | |
| PEG, Acetylenedicarboxylic acid | BSA | Electrostatic interaction | √ | Proliferation and differentiation of fibroblasts, collagen accumulation, epidermal layer stacking | [45] | |
| Catechol-QCS, Dibenzaldehyde-PEG | / | Schiff base | √ | Antibacterial, antioxidant, haemostasis | [53] | |
| CS quaternary ammonium salt, Oxidised dextran-DA | Ag NPs, DFO | Schiff base | √ | Antibacterial, angiogenesis | [69] | |
| FPBA-CS | SiO2-Fas Ligand | Schiff base | √ | Activating immune response | [70] | |
| Hydroxypropyl acrylate, Acrylic acid, CMCS | Mg2+ | -COOH, -NH2 | × | Angiogenesis, proliferation and migration of fibroblasts, polarization of macrophages | [81] | |
| Guar gum, Polyvinyl alcohol | Borax-TA | Borate ester bonds | √ | Antibacterial, wound monitoring | [126] | |
| ROS-Responsive | Sodium alginate, Pectin, PEG-thioketal | Substance P | Thioketal | √ | Proliferation of human dermal fibroblasts, ↑inflammation-related genes in macrophages | [15] |
| Polyvinyl alcohol, N1-(4-boronobenzyl)-N3-(4-boronophenyl)-N1,N1,N3,N3-tetramethylpropane-1, 3-diaminium (TPA) | Mupirocin, Granulocyte-macrophage Colony-stimulating factor | TPA | √ | Scavenge ROS, ↓pro-inflammatory cytokines, ↑M2 phenotype macrophages, angiogenesis, production of collagen | [93] | |
| TA, Polyvinyl alcohol, PBA-modified polyphosphazene | / | Phenylborate ester bond | × | Anti-inflammatory, scavenge ROS, antibacterial Re-epithelialization, ↑granulation tissue | [99] | |
| Sodium hyaluronate, polyvinyl alcohol | MnCoO@PDA/CPH | Catalase-mimic nanozyme | × | Anti-inflammatory, re-epithelialization ↑collagen deposition, angiogenesis | [127] | |
| Glucose-Responsive | Gelatine methacrylate, 4-(2-acrylamidoethylcarbamoyl)-3-Fluorophenylboronic acid | G-insulin | Borate ester bonds | √ | Anti-inflammatory, ↑collagen deposition, glucose control | [102] |
| N,N-Diethylaminoethyl methacrylate, PEG-dimethacrylate, 2-glucosyloxyethyl methacrylate, Modified-ConA | Con A | Reversible cross-link | × | Delivery system | [104] | |
| Enzyme-Responsive | Hyaluronic acid | Fe3+ | Hyaluronidase | √ | Antibacterial, angiogenesis | [107] |
| Four-armed PEG, PEG-sulfhydryl group | Exosome | Matrix metalloproteinase | √ | Antioxidant, proliferation and migration of cells | [108] | |
| Photo-Responsive | Poly (ethylene glycol) diglycidyl ether, 1,8-octanediamine, 3-perfluorohexyl-1,2-epoxypropane Gelatine | / | PDA | √ | Improvement of hypoxia | [128] |
| Electro-Responsive | QCS grafted polyaniline, Oxidised dextran | / | Polyaniline | × | Antibacterial, proliferation of cells | [67] |
| 4-Arm-PEG-Thiol, Carbon nanotubes (CNTs) | Exosome Metformin | CNTs | √ | Proliferation of cells, angiogenesis | [120] | |
| PDA-reduced-graphene, oxide (pGO), CS, Silk fibroin | PGO | PGO | √ | Antioxidant | [124] | |
| Electro-, ROS-Responsive | Polyvinyl alcohol, Human-like collagen, TA | TA, siRNA | Borate ester bond, electric stimulation | √ | ↓ROS and MMP-9, ↑polarization of macrophages, production of collagen, angiogenesis | [33] |
| Electro-, pH-Responsive | Polyacrylamide, Starch | Drug | -OH, hydrogen bond | √ | Antibacterial, ↑collagen deposition, angiogenesis, epidermis formation | [49] |
| Electro-, Thermo-Responsive | QCS grafted polyaniline, Benzaldehyde group-PEG-co-poly(glycerol sebacate) | / | Polyaniline, -NH2, quaternary ammonium groups | × | Antibacterial, antioxidant | [68] |
| Poly-(3,4-thylenedioxythiophene), Polystyrene sulfonate, NIPAM, Acrylamide | / | LCST, ↓interfacial impedance | × | Activation of monocyte and macrophage cell, monitoring wound | [125] | |
| pH, ROS-Responsive | Alginic acid sodium salt, HA, 3-aminophenyl boronic acid | Amikacin, Naproxen | Boronic ester bond, micelle | √ | Antibacterial, anti-inflammatory | [80] |
| PBA-grafted oxidised dextran, Caffeic acid-grafted ε-poly-lysine | Mangiferin Diclofenac sodium | Boronic ester bonds, Schiff base, micelle | √ | Antibacterial, anti-inflammatory antioxidant, angiogenesis | [94] | |
| HA-PBA, TA | Ag NPs | Boronic ester bond | √ | Antibacterial, antioxidant | [97] | |
| Aminated gelatine, Oxidised dextran | ZnO NPs Paeoniflorin | Schiff base Thiol group | √ | Haemostatic, antibacterial, angiogenic | [100] | |
| pH, Glucose-Responsive | (Dihydrocaffeic acid and l-Arg)-CS, (PBA and benzaldehyde bifunctional PEG)-co-p(glycerol sebacic acid) | Metformin PBA (Reduced-GO) | Schiff base, Phenylboronic ester bond | √ | Antibacterial antioxidant, haemostasis | [17] |
| 4,5-Imidazoledicarboxylic acid | Zn2+, DFO, Glucose oxidase (GOX) | GOX Metal–organic hydrogels | √ | Antibacterial, angiogenesis glucose control | [103] | |
| Hyaluronic acid methacryloyl, Acrylic acid | GOX Copper peroxide | pKa | √ | Antibacterial, angiogenic | [129] | |
| pH, Thermo-, Responsive | F127, Oxidative-HA, Poly-ε-l-lysine | Exosome | Schiff base, LCST | √ | Angiogenesis, re-epithelization, collagen deposition | [6] |
| CMC-Na | Diclofenac sodium | -COOH | √ | Anti-inflammatory | [18] | |
| NIPAM, AA | Ag NPs | pKa, LCST | √ | Antibacterial | [20] | |
| Hydroxypropyl chitin, TA-Fe3+ | / | TA-Fe3+ | √ | Antibacterial | [72] | |
| Thermo-, Photo-Responsive | F127, Hydroxypropyl methyl cellulose, Cellulose nanofibril | Prussian blue | PTT PDT (Protoporphyrin IX) | √ | Antibacterial, haemostasis | [91] |
| 4-Octyl itaconate, PEG, GelMA | Black phosphorus | PTT PDT | √ | Antibacterial, antioxidant, angiogenesis | [96] | |
| Fibrinogen, Thrombin | Black phosphorus (BP) Lidocaine | PTT | √ | Pain relief, antibacterial anti-inflammatory, angiogenesis | [112] | |
| PLGA PEG PLGA | Niobium carbide | PTT | × | ROS-scavenging antibacterial, haemostasis | [113] | |
| PLGA PEG PLGA | Imipenem@Au liposome | PTT | √ | Antibacterial, haemostasis anti-inflammatory | [116] | |
| Alginate, F127 | Zn2+ | PDT (Ce6) | √ | Antibacterial, collagen deposition, re-epithelialization | [117] | |
| pH, Photo-Responsive | Poly(pentahydropyrimidine), TA-Fe3+, Polyvinyl alcohol | / | Schiff base, Phenylborate ester bond, PTT (TA−Fe3+) | √ | Antibacterial, angiogenesis | [77] |
| Protocatechualdehyde-Fe3+, QCS | / | Catechol−Fe3+ Schiff base, PTT | × | Antibacterial, haemostatic | [114] | |
| Electro-, ROS, Photo-Responsive | GelMA, [2-(acryloyloxy) ethyl]-Trimethylammonium-chloride (Bio-IL), | Doxycycline | Bio-IL Thioketal | √ | ↑M2 phenotype macrophages, collagen deposition, antioxidant, angiogenesis, re-epithelialization | [32] |
| pH, Thermo-, Photo-Responsive | TA-Europium | / | Metal–phenolic coordination bonds | √ | Antibacterial antioxidant, angiogenesis | [29] |
| Phenylazo-F127, QCS -graft-cyclo-dextrin, PDA coated tunicate cellulose | Curcumin | Schiff base Host-guest interaction of cyclodextrin and azobenzene | √ | Antibacterial, haemostasis | [30] | |
| Gelatine, TA quinone, Borax | / | Boronic ester bond, Schiff base, Polyphenol, PTT (Quinone group) | √ | Antibacterial, haemostasis | [31] | |
| Hydroxybutyl-CS, Methacrylic anhydride, Succinic anhydride | / | Succinyl groups Photoinitiator (LAP) | × | Delivery systems | [110] | |
| pH, Thermo-, Glucose, Photo-Responsive | HA-PBA, Gelatine, Hyaluronic acid | Metformin @Cu-PDA NPs | Boronic ester bonds PTT | √ | Antibacterial, anti-inflammatory, angiogenesis, deposition of ECM and collagen | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Dou, Z.; Zhang, Y.; Li, F.; Xing, B.; Hu, Z.; Li, X.; Liu, Z.; Yang, W.; Liu, Z. Smart Responsive and Controlled-Release Hydrogels for Chronic Wound Treatment. Pharmaceutics 2023, 15, 2735. https://doi.org/10.3390/pharmaceutics15122735
Jia X, Dou Z, Zhang Y, Li F, Xing B, Hu Z, Li X, Liu Z, Yang W, Liu Z. Smart Responsive and Controlled-Release Hydrogels for Chronic Wound Treatment. Pharmaceutics. 2023; 15(12):2735. https://doi.org/10.3390/pharmaceutics15122735
Chicago/Turabian StyleJia, Xintao, Zixuan Dou, Ying Zhang, Fanqin Li, Bin Xing, Zheming Hu, Xin Li, Zhongyan Liu, Wenzhuo Yang, and Zhidong Liu. 2023. "Smart Responsive and Controlled-Release Hydrogels for Chronic Wound Treatment" Pharmaceutics 15, no. 12: 2735. https://doi.org/10.3390/pharmaceutics15122735
APA StyleJia, X., Dou, Z., Zhang, Y., Li, F., Xing, B., Hu, Z., Li, X., Liu, Z., Yang, W., & Liu, Z. (2023). Smart Responsive and Controlled-Release Hydrogels for Chronic Wound Treatment. Pharmaceutics, 15(12), 2735. https://doi.org/10.3390/pharmaceutics15122735






