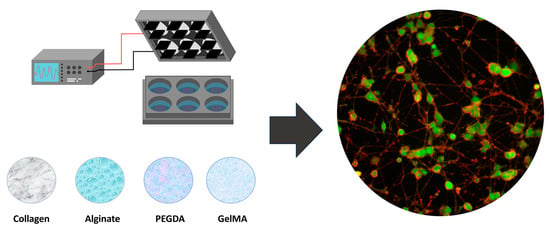
Effect of Electrical Stimulation on PC12 Cells Cultured in Different Hydrogels: Basis for the Development of Biomaterials in Peripheral Nerve Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. PC12 Cell Culture, Differentiation and Viability
2.3. Preparation of Hydrogels and Surface Modification of Culture Systems
2.4. Electrical Stimulation
2.5. AFM Measurements
2.6. Statistical Analysis
3. Results and Discussion
3.1. PC12 Cells Proliferation and Viability on Hydrogels
3.2. Differentiation of PC12 Cells in Different Hydrogels
3.3. Differentiation of PC12 Cells: Effect of Electrical Stimulation
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue engineering and regenerative medicine: Achievements, future, and sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.X.; Han, N.; Kou, Y.H.; Zhu, Q.T.; Liu, X.L.; Quan, D.P.; Chen, J.G.; Jiang, B.G. Tissue engineering for the repair of peripheral nerve injury. Neural Regen. Res. 2019, 14, 51–58. [Google Scholar] [PubMed]
- Carriel, V.; Alaminos, M.; Garzon, I.; Campos, A.; Cornelissen, M. Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 2014, 14, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for tissue engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Xiao, C.; Liu, B. Engineered hydrogels for peripheral nerve repair. Mater. Today Bio 2023, 20, 100668. [Google Scholar] [CrossRef]
- Silva, R.; Fabry, B.; Boccaccini, A.R. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials 2014, 35, 6727–6738. [Google Scholar] [CrossRef]
- Luo, L.; He, Y.; Jin, L.; Zhang, Y.; Guastaldi, F.P.; Albashari, A.A.; Hu, F.; Wang, X.; Wang, L.; Xiao, J.; et al. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact. Mater. 2021, 6, 638–654. [Google Scholar]
- Ma, F.; Xiao, Z.; Chen, B.; Hou, X.; Dai, J.; Xu, R. Linear ordered collagen scaffolds loaded with collagen-binding basic fibroblast growth factor facilitate recovery of sciatic nerve injury in rats. Tissue Eng. Part A 2014, 20, 1253–1262. [Google Scholar] [CrossRef]
- Bu, Y.; Xu, H.X.; Li, X.; Xu, W.J.; Yin, Y.X.; Dai, H.L.; Wang, X.B.; Huang, Z.J.; Xu, P.H. A conductive sodium alginate and carboxymethyl chitosan hydrogel doped with polypyrrole for peripheral nerve regeneration. RSC Adv. 2018, 8, 10806–10817. [Google Scholar] [CrossRef]
- Chapla, R.; Alhaj Abed, M.; West, J. Modulating functionalized poly(ethylene glycol) diacrylate hydrogel mechanical properties through competitive crosslinking mechanics for soft tissue applications. Polymers 2020, 12, 3000. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (gelma) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Faber, D.S.; Pereda, A.E. Two forms of electrical transmission between neurons. Front. Mol. Neurosci. 2018, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, Y.; Yue, H.; Fan, Y. Electrical stimulation promotes stem cell neural differentiation in tissue engineering. Stem Cells Int. 2021, 2021, 6697574. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng. Part A 2009, 15, 3605–3619. [Google Scholar] [CrossRef] [PubMed]
- Trueman, R.P.; Ahlawat, A.S.; Phillips, J.B. A shock to the (nervous) system: Bioelectricity within peripheral nerve tissue engineering. Tissue Eng. Part B Rev. 2022, 28, 1137–1150. [Google Scholar] [CrossRef]
- Yung, H.S.; Lai, K.H.; Chow, K.B.; Ip, N.Y.; Tsim, K.W.; Wong, Y.H.; Wu, Z.; Wise, H. Nerve growth factor-induced differentiation of pc12 cells is accompanied by elevated adenylyl cyclase activity. Neuro-Signals 2010, 18, 32–42. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. Pc12 cell line: Cell types, coating of culture vessels, differentiation and other culture conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of osteoblasts in injectable rgd-modified peg hydrogels for bone tissue engineering. Biomaterials 2002, 23, 4315–4323. [Google Scholar] [CrossRef]
- Distler, T.; Lauria, I.; Detsch, R.; Sauter, C.M.; Bendt, F.; Kapr, J.; Rutten, S.; Boccaccini, A.R.; Fritsche, E. Neuronal differentiation from induced pluripotent stem cell-derived neurospheres by the application of oxidized alginate-gelatin-laminin hydrogels. Biomedicines 2021, 9, 261. [Google Scholar] [CrossRef]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef]
- Ashok, A.; Tai, W.L.; Lennikov, A.; Chang, K.; Chen, J.; Li, B.; Cho, K.S.; Utheim, T.P.; Chen, D.F. Electrical stimulation alters DNA methylation and promotes neurite outgrowth. J. Cell. Biochem. 2023, 124, 1530–1545. [Google Scholar] [CrossRef]
- Zhang, Q.; Beirne, S.; Shu, K.; Esrafilzadeh, D.; Huang, X.F.; Wallace, G.G. Electrical stimulation with a conductive polymer promotes neurite outgrowth and synaptogenesis in primary cortical neurons in 3d. Sci. Rep. 2018, 8, 9855. [Google Scholar] [CrossRef]
- Hansma, P.; Cleveland, J.; Radmacher, M.; Walters, D.; Hillner, P.; Bezanilla, M.; Fritz, M.; Vie, D.; Hansma, H.; Prater, C.; et al. Tapping mode atomic force microscopy in liquids. Appl. Phys. Lett. 1994, 64, 1738–1740. [Google Scholar] [CrossRef]
- Hugel, T.; Seitz, M. The study of molecular interactions by afm force spectroscopy. Macromol. Rapid Commun. 2001, 22, 989–1016. [Google Scholar] [CrossRef]
- Hutter, J.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Dintwa, E.; Tijskens, E.; Ramon, H. On the accuracy of the hertz model to describe the normal contact of soft elastic spheres. Granul. Matter 2008, 10, 209–221. [Google Scholar] [CrossRef]
- Baldassarro, V.A.; Dolci, L.S.; Mangano, C.; Giardino, L.; Gualandi, C.; Focarete, M.L.; Calza, L. In vitro testing of biomaterials for neural repair: Focus on cellular systems and high-content analysis. BioResearch Open Access 2016, 5, 201–211. [Google Scholar] [CrossRef]
- McHugh, M.L. Multiple comparison analysis testing in anova. Biochem. Medica 2011, 21, 203–209. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Chua, P.; Lim, W.K. The strategic uses of collagen in adherent cell cultures. Cell Biol. Int. 2023, 47, 367–373. [Google Scholar] [CrossRef]
- Davis, K.A.; Gottipatti, A.; Peng, H.; Donahue, R.; Chelvarajan, L.; Cahall, C.; Tripathi, H.; Al-Darraji, A.; Ye, S.; Abdel-Latif, A.; et al. Gelatin coating enhances therapeutic cell adhesion to the infarcted myocardium via ecm binding. PLoS ONE 2022, 17, e0277561. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Alsberg, E. Photofunctionalization of alginate hydrogels to promote adhesion and proliferation of human mesenchymal stem cells. Tissue Eng. Part A 2013, 19, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Hahn, M.S.; Kim, I.; Nsiah, B.A.; West, J.L. Micropatterning of poly(ethylene glycol) diacrylate hydrogels with biomolecules to regulate and guide endothelial morphogenesis. Tissue Eng. Part A 2009, 15, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Qui, M.S.; Green, S.H. Pc12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged erk activity. Neuron 1992, 9, 705–717. [Google Scholar]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The evolution of polystyrene as a cell culture material. Tissue Eng. Part B Rev. 2018, 24, 359–372. [Google Scholar] [CrossRef]
- Zuniga-Aguilar, E.; Olayo, R.; Ramirez-Fernandez, O.; Morales, J.; Godinez, R. Nerve cells culture from lumbar spinal cord on surfaces modified by plasma pyrrole polymerization. J. Biomater. Sci. Polym. Ed. 2014, 25, 729–747. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Gao, X.; Wang, A.; Yang, Y.; Liu, S.; Yu, Z.; Song, G.B.; Zhao, H.C. Substrate stiffness affects neural network activity in an extracellular matrix proteins dependent manner. Colloids Surf. B Biointerfaces 2018, 170, 729–735. [Google Scholar] [CrossRef]
- Franze, K.; Janmey, P.A.; Guck, J. Mechanics in neuronal development and repair. Annu. Rev. Biomed. Eng. 2013, 15, 227–251. [Google Scholar] [CrossRef]
- Wu, Y.; Xiang, Y.; Fang, J.; Li, X.; Lin, Z.; Dai, G.; Yin, J.; Wei, P.; Zhang, D. The influence of the stiffness of gelma substrate on the outgrowth of pc12 cells. Biosci. Rep. 2019, 39, BSR20181748. [Google Scholar] [CrossRef]
- Athamneh, A.I.; Suter, D.M. Quantifying mechanical force in axonal growth and guidance. Front. Cell. Neurosci. 2015, 9, 359. [Google Scholar] [CrossRef]
- Gomez, T.M.; Letourneau, P.C. Actin dynamics in growth cone motility and navigation. J. Neurochem. 2014, 129, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness sensing by cells. Physiol. Rev. 2020, 100, 695–724. [Google Scholar] [CrossRef] [PubMed]
- Ogle, M.E.; Doron, G.; Levy, M.J.; Temenoff, J.S. Hydrogel culture surface stiffness modulates mesenchymal stromal cell secretome and alters senescence. Tissue Eng. Part A 2020, 26, 1259–1271. [Google Scholar] [CrossRef]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical aspects of cell culture substrates: Topography, roughness, and elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef] [PubMed]
- Covani, U.; Giacomelli, L.; Krajewski, A.; Ravaglioli, A.; Spotorno, L.; Loria, P.; Das, S.; Nicolini, C. Biomaterials for orthopedics: A roughness analysis by atomic force microscopy. J. Biomed. Mater. Res. Part A 2007, 82, 723–730. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth—facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Loesberg, W.A.; te Riet, J.; van Delft, F.C.; Schon, P.; Figdor, C.G.; Speller, S.; van Loon, J.J.; Walboomers, X.F.; Jansen, J.A. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials 2007, 28, 3944–3951. [Google Scholar] [CrossRef]
- Gonzalez-Meijome, J.M.; Lopez-Alemany, A.; Almeida, J.B.; Parafita, M.A. Surface afm microscopy of unworn and worn samples of silicone hydrogel contact lenses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 75–82. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Malamou, A. Atomic force microscopy nanoindentation method on collagen fibrils. Materials 2022, 15, 2477. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, L.; Xie, W.; Camacho, L.C.; Zhang, M.; Chu, Z.; Wei, Q.; Haag, R. Surface roughness and substrate stiffness synergize to drive cellular mechanoresponse. Nano Lett. 2020, 20, 748–757. [Google Scholar] [CrossRef]
- McNamara, M.; Pretzer, R.; Montazami, R.; Montazami, N. Shear at Fluid-Fluid Interfaces Affects the Surface Topologies of Alginate Microfibers. Clean Technol. 2019, 1, 265–272. [Google Scholar] [CrossRef]
- Munz, M. Microstructure and roughness of photopolymerized poly(ethylene glycol) diacrylate hydrogel as measured by atomic force microscopy in amplitude and frequency modulation mode. Appl. Surf. Sci. 2013, 279, 300–309. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, X.; Li, J.; Wu, Z.; Wang, Y.; Liu, F. The recent developments and applications of the traceless-staudinger reaction in chemical biology study. RSC Adv. 2015, 130, 107192–108066. [Google Scholar]
- Askari, F.; Zandi, M.; Shokrolahi, P.; Tabatabaei, M.H.; Hajirasoliha, E. Reduction in protein absorption on ophthalmic lenses by pegda bulk modification of silicone acrylate-based formulation. Prog. Biomater. 2019, 8, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Luo, L.J.; Ma, D.H. Effect of cross-linking density on the structures and properties of carbodiimide-treated gelatin matrices as limbal stem cell niches. Int. J. Mol. Sci. 2018, 19, 3294. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Wandiyanto, J.V.; Linklater, D.; Tharushi Perera, P.G.; Orlowska, A.; Truong, V.K.; Thissen, H.; Ghanaati, S.; Baulin, V.; Crawford, R.J.; Juodkazis, S.; et al. Pheochromocytoma (pc12) cell response on mechanobactericidal titanium surfaces. Materials 2018, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Simitzi, C.; Stratakis, E.; Fotakis, C.; Athanassakis, I.; Ranella, A. Microconical silicon structures influence ngf-induced pc12 cell morphology. J. Tissue Eng. Regen. Med. 2015, 9, 424–434. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef]
- Testore, D.; Zoso, A.; Kortaberria, G.; Sangermano, M.; Chiono, V. Electroconductive photo-curable pegda-gelatin/pedot:Pss hydrogels for prospective cardiac tissue engineering application. Front. Bioeng. Biotechnol. 2022, 10, 897575. [Google Scholar] [CrossRef]
- Agarwala, S. Electrically conducting hydrogels for health care: Concept, fabrication methods, and applications. Int. J. Bioprint. 2020, 6, 273. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Lim, J.H.; Lee, J.M.; Chung, B.G. Electro-responsive conductive blended hydrogel patch. Polymers 2023, 15, 2608. [Google Scholar] [CrossRef] [PubMed]
- Sirivisoot, S.; Pareta, R.; Harrison, B.S. Protocol and cell responses in three-dimensional conductive collagen gel scaffolds with conductive polymer nanofibres for tissue regeneration. Interface Focus 2014, 4, 20130050. [Google Scholar] [CrossRef] [PubMed]
- Kaklamani, G.; Kazaryan, D.; Bowen, J.; Iacovella, F.; Anastasiadis, S.H.; Deligeorgis, G. On the electrical conductivity of alginate hydrogels. Regen. Biomater. 2018, 5, 293–301. [Google Scholar] [CrossRef]
- Liang, Y.; Goh, J.C. Polypyrrole-incorporated conducting constructs for tissue engineering applications: A review. Bioelectricity 2020, 2, 101–119. [Google Scholar] [CrossRef]
- Cadena, M.; Ning, L.; King, A.; Hwang, B.; Jin, L.; Serpooshan, V.; Sloan, S.A. 3d bioprinting of neural tissues. Adv. Healthc. Mater. 2021, 10, e2001600. [Google Scholar] [CrossRef]
- Yang, S.; Jang, L.; Kim, S.; Yang, J.; Yang, K.; Cho, S.W.; Lee, J.Y. Polypyrrole/alginate hybrid hydrogels: Electrically conductive and soft biomaterials for human mesenchymal stem cell culture and potential neural tissue engineering applications. Macromol. Biosci. 2016, 16, 1653–1661. [Google Scholar] [CrossRef]
- Ma, H.; Yu, K.; Wang, H.; Liu, J.; Cheng, Y.Y.; Kang, Y.; Wang, H.; Zhang, J.; Song, K. Fabrication and detection of a novel hybrid conductive scaffold based on alginate/gelatin/carboxylated carbon nanotubes (alg/gel/mmwcnts) for neural tissue engineering. Tissue Cell 2023, 80, 101995. [Google Scholar] [CrossRef]
- Mardani, M.; Roshankhah, S.; Hashemibeni, B.; Salahshoor, M.; Naghsh, E.; Esfandiari, E. Induction of chondrogenic differentiation of human adipose-derived stem cells by low frequency electric field. Adv. Biomed. Res. 2016, 5, 97. [Google Scholar]
- Wang, Y.; Cui, H.; Wu, Z.; Wu, N.; Wang, Z.; Chen, X.; Wei, Y.; Zhang, P. Modulation of osteogenesis in mc3t3-e1 cells by different frequency electrical stimulation. PLoS ONE 2016, 11, e0154924. [Google Scholar] [CrossRef]
- Esfandiari, E.; Roshankhah, S.; Mardani, M.; Hashemibeni, B.; Naghsh, E.; Kazemi, M.; Salahshoor, M. The effect of high frequency electric field on enhancement of chondrogenesis in human adipose-derived stem cells. Iran. J. Basic Med. Sci. 2014, 17, 571–576. [Google Scholar] [PubMed]
- Dutta, S.D.; Ganguly, K.; Randhawa, A.; Patil, T.V.; Patel, D.K.; Lim, K.T. Electrically stimulated 3d bioprinting of gelatin-polypyrrole hydrogel with dynamic semi-ipn network induces osteogenesis via collective signaling and immunopolarization. Biomaterials 2023, 294, 121999. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Liu, E.W.; Lin, Y.J.; Ng, H.Y.; Lee, J.J.; Hsu, T.T. The synergistic effect of electrical stimulation and dermal fibroblast cells-laden 3d conductive hydrogel for full-thickness wound healing. Int. J. Mol. Sci. 2023, 24, 11698. [Google Scholar] [CrossRef] [PubMed]










| Collagen | GelMA | PEGDA | Alginate | |
|---|---|---|---|---|
| Ra (nm) | 11.4 | 1.3 | 1.2 | 1.6 |
| RMS (nm) | 14.61 | 1.6 | 1.6 | 2.0 |
| Young’s modulus (MPa) | 0.96 ± 0.11 | 0.59 ± 0.21 | 4.10 ± 0.67 | 0.53 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olguín, Y.; Selva, M.; Benavente, D.; Orellana, N.; Montenegro, I.; Madrid, A.; Jaramillo-Pinto, D.; Otero, M.C.; Corrales, T.P.; Acevedo, C.A. Effect of Electrical Stimulation on PC12 Cells Cultured in Different Hydrogels: Basis for the Development of Biomaterials in Peripheral Nerve Tissue Engineering. Pharmaceutics 2023, 15, 2760. https://doi.org/10.3390/pharmaceutics15122760
Olguín Y, Selva M, Benavente D, Orellana N, Montenegro I, Madrid A, Jaramillo-Pinto D, Otero MC, Corrales TP, Acevedo CA. Effect of Electrical Stimulation on PC12 Cells Cultured in Different Hydrogels: Basis for the Development of Biomaterials in Peripheral Nerve Tissue Engineering. Pharmaceutics. 2023; 15(12):2760. https://doi.org/10.3390/pharmaceutics15122760
Chicago/Turabian StyleOlguín, Yusser, Mónica Selva, Diego Benavente, Nicole Orellana, Ivan Montenegro, Alejandro Madrid, Diego Jaramillo-Pinto, María Carolina Otero, Tomas P. Corrales, and Cristian A. Acevedo. 2023. "Effect of Electrical Stimulation on PC12 Cells Cultured in Different Hydrogels: Basis for the Development of Biomaterials in Peripheral Nerve Tissue Engineering" Pharmaceutics 15, no. 12: 2760. https://doi.org/10.3390/pharmaceutics15122760
APA StyleOlguín, Y., Selva, M., Benavente, D., Orellana, N., Montenegro, I., Madrid, A., Jaramillo-Pinto, D., Otero, M. C., Corrales, T. P., & Acevedo, C. A. (2023). Effect of Electrical Stimulation on PC12 Cells Cultured in Different Hydrogels: Basis for the Development of Biomaterials in Peripheral Nerve Tissue Engineering. Pharmaceutics, 15(12), 2760. https://doi.org/10.3390/pharmaceutics15122760