Unveiling the Pain Relief Potential: Harnessing Analgesic Peptides from Animal Venoms
Abstract
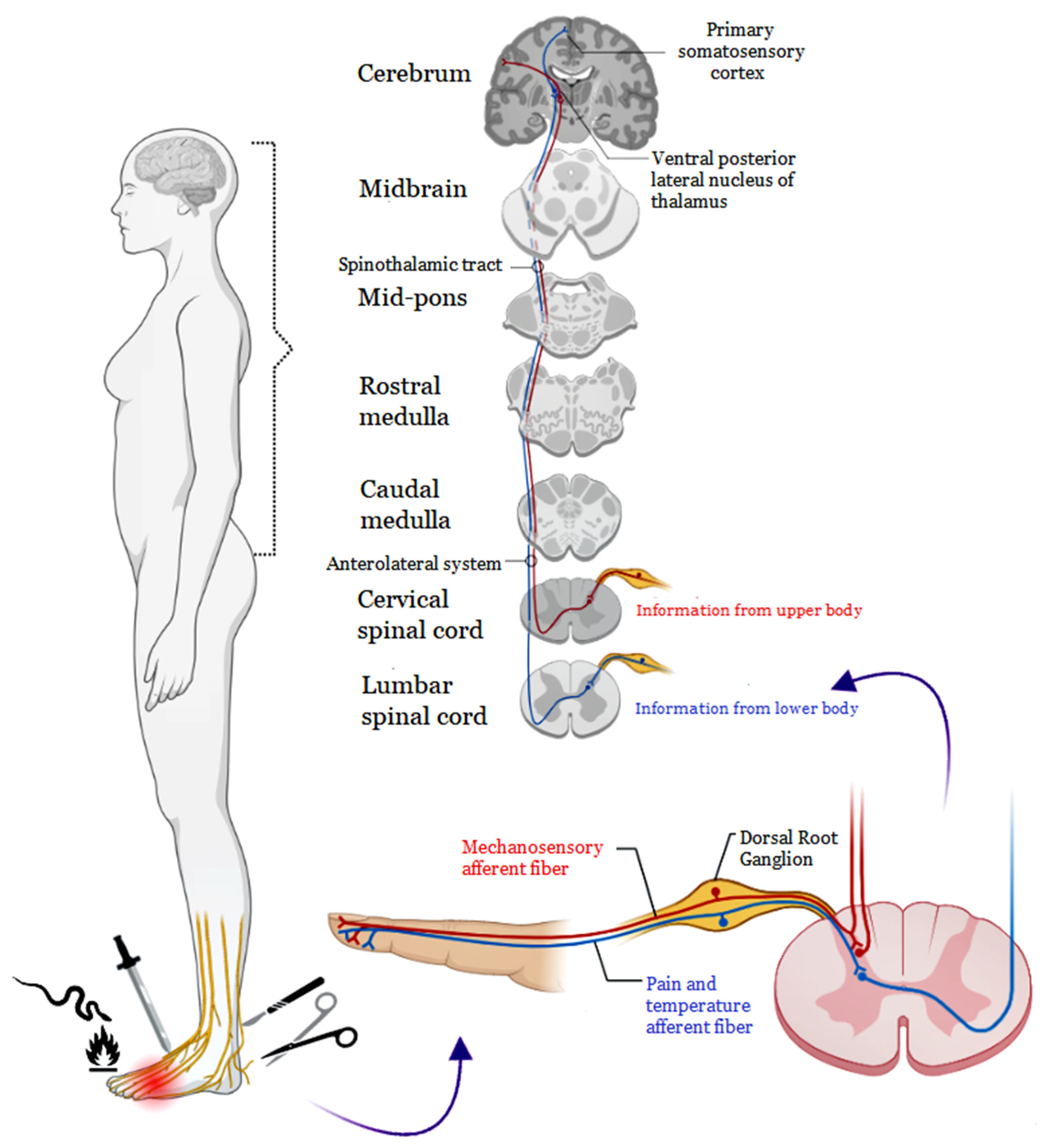
:1. Introduction
2. Pain Pathophysiology
2.1. Nociceptive Pain
2.2. Neuropathic Pain
2.3. Inflammatory Pain
3. Animal Venoms: Composition and Effects
4. Toxins Targeting Pain: Discovering Potential Analgesics
4.1. Snake Analgesic Peptides
4.2. Scorpion Analgesic Peptides
4.3. Spider Analgesic Peptides
| Toxin/ Molecule | Species | Production | Administration | Mechanism of Analgesia | Model | Ref. |
|---|---|---|---|---|---|---|
| PhTx3-4 | Phoneutria nigriventer | Purified from venom | Intrathecal (i.t.) injection | Reduction in glutamate levels in the cerebrospinal fluid | Persistent inflammatory pain and post-operative (plantar incision) nociception in mice | [92] |
| PnPP-19 | Phoneutria nigriventer | Synthesized | Subcutaneous (s.c.) injection | Both opioid and cannabinoid peripherical systems | Hyperalgesia induced by the administration of PGE2 | [94] |
| Tx3-5 | Phoneutria nigriventer | Purified from venom | Intrathecal (i.t.) injection | - | Postoperative (plantar incision), neuropathic (partial sciatic nerve ligation), and cancer-related pain (inoculation with melanoma cells) in animals | [105] |
| δ-CNTX-Pn1a | Phoneutria nigriventer | Purified from venom | Intrathecal (i.t.) injection | Opioid and cannabinoid systems | Hyperalgesia induced by the CCI model and hyperalgesia induced by the administration of PGE2 | [93] |
| PnPP-19 | Phoneutria nigriventer | Synthesized | Subcutaneous (s.c.) injection | Activation of NO-cGMP-KATP pathway | Hyperalgesia induced by the administration of PGE2 | [95] |
| PhKv | Phoneutria nigriventer | Purified from a PhTx3 fraction | Intrathecal (i.t.) injection | Inhibition of spinal AChE resulting in the activation of the muscarinic and nicotinic receptors | Chronic constriction injury model and after intraplantar injection of capsaicin | [97] |
| Phα1β | Phoneutria nigriventer | Purified from venom | Intrathecal (i.t.) or intraplantarly (i.pl.) injections | Selective antagonist of TRPA1 channels | Nocifensive responses evoked by reactive TRPA1 channel agonist, mechanical and cold hyperalgesia, neuropathic pain induced by the chemotherapeutic agent bortezomib | [106] |
| Pha1β | Phoneutria nigriventer | Purified from venom | Intrathecal (i.t.) injection | Reversibly inhibits the voltage-gated calcium channels (VGCC) | Nociception that was triggered by capsaicin, CCI model, and hyperalgesia was induced in the melanoma cancer pain model | [97] |
| Pha1β | Phoneutria nigriventer | Recombinant | Intrathecal (i.t.) injection | Reversibly inhibits the voltage-gated calcium channels (VGCC) | Nociception that was triggered by capsaicin, CCI model, and hyperalgesia was induced in the melanoma cancer pain model | [97] |
| PnTx4(5-5) | Phoneutria nigriventer | Purified from venom | Intraplantarly (i.pl.) and subcutaneous (s.c.) injection | The antinociceptive effect of PnTx4(5-5) can also be related to the glutamatergic system | Hyperalgesia induced by PGE2, carrageenan, and L-glutamate (L-Glu) | [107] |
| Phα1β | Phoneutria nigriventer | Recombinant | Intravenous (i.v.) injection | - | Pain was induced by the CCI model and paclitaxel-induced acute and chronic pain | [108] |
| CTK 01512-2 | Phoneutria nigriventer | Recombinant | Intrathecal (i.t.) injection | Reversibly, and not specifically a block of Cav 2.2 that affects the intracellular Ca2+ influx and the glutamate release | Mechanical and thermal hyperalgesia and cold allodynia | [109] |
| Phα1β | Phoneutria nigriventer | Purified from venom | Intrathecal (i.t.) injection | Blocker voltage-dependent calcium channels and the antagonism with the receptor CXCR4 | Diabetic neuropathic pain | [98] |
| PnAn13 | Phoneutria nigriventer | Synthetic peptide | Intrathecal (i.t.) injection | Opioid and cannabinoid systems | Hyperalgesia induced by PGE2 | [110] |
| Phα1β | Phoneutria nigriventer | - | Intrathecal (i.t.) injection | Blocker TRPA1 and Cav2.2 receptors | Postoperative (plantar incision) | [111] |
| Phα1β | Phoneutria nigriventer | Recombinant | Intravenous (i.v.) injection | High-voltage calcium channel inhibitors (HVCCs) and cationic channel antagonists of the potential transient receptor (TRPA1) | Hyperalgesia and mechanical allodynia induced by reserpine (fibromyalgia) | [112] |
| Cd1a | Ceratogyrus darlingi | Chemical synthesis | Intraplantar (i.pl.) injection | Nav/Cav channel inhibitor | Peripheral pain by NaV channels induced with α-scorpion toxin OD1 | [87] |
| JZTX-X | Chilobrachys jingzhao | Chemical synthesis | Intraplantar (i.pl.) injection | Kv4 channel inhibitor | Mechanical hypersensitivity | [100] |
| μ-TRTX-Ca2a | Cyriopagopus albostriatus | Purified from venom | Intrathecal (i.t.) and intraplantar (i.pl.) injection | Na v 1.7 channel inhibitor | Acute inflammation models induced by formalin and acetic acid and acute thermal pain models induced by hot plate | [101] |
| µ-TRTX-Ca1a | Cyriopagopus albostriatus | Purified from venom | Intraperitoneal (i.p.) injection | hNa v 1.7 channel inhibitor | Pain formalin-induced paw licking, hot plate test, and acetic acid-induced writhing | [102] |
| CyrTx-1a | Cyriopagopus schioedtei | Purified from venom | Intraperitoneal (i.p.) injection | hNa V 1.7 channel inhibitor | Thermal hyperalgesia | [103] |
| Df1a-NH2 | Davus fasciatus | Chemical synthesis | Intraplantar (i.pl.) injection | NaV and CaV3 channel inhibitor | Peripheral pain by NaV channels induced with α-scorpion toxin OD1 | [88] |
| Df1a-OH | Davus fasciatus | Chemical synthesis | Intraplantar (i.pl.) injection | NaV and CaV3 channel inhibitor | Peripheral pain by NaV channels induced with α-scorpion toxin OD1 | [88] |
| GpTx-1 and GpTx-1-71 | Grammostola porteni | Chemical synthesis | Intrathecal (i.t.) and Intracerebroventricular (i.c.v.) injection | Nav channel inhibitor | Tail-flick test, carrageenan- or complete Freund’s adjuvant (CFA)-induced inflammatory pain model, neuropathic pain model, mechanical allodynia, thermal hyperalgesia, writhing test, formalin test, tolerance evaluation, rotarod test, open field test, gastrointestinal transit test | [104] |
| HpTx3 | Heteropoda venatoria | Purified from venom | Intramuscular (im.i) and intraperitoneal (i.p.) injections | Nav1.7 channel inhibitor | Acute inflammation models induced by formalin and acetic acid, chronic inflammation pain models induced by complete Freund’s adjuvant, acute thermal pain models induced by hot plate, and chronic neuropathic pain models induced by spared nerve injury | [90] |
| μ-theraphotoxin-Pn3a | Pamphobeteus nigricolor | Purified from venom | Intraperitoneal (i.p.) injection | Nav1.7 channel inhibitor and synergy with opioids | Peripheral pain by NaV channels induced with α-scorpion toxin OD1 | [99] |
| ProTx-III | Thrixopelma pruriens | Recombinant | Intraplantar (i.pl.) injection | Nav1.7 channel inhibitor | Peripheral pain by NaV channels induced with α-scorpion toxin OD1 | [91] |
| ProTx-II (β/ω-terafotoxina-Tp2a) | Thrixopelma pruriens | - | Intracerebroventricular (i.cv) injection | Nav1.7 channel inhibitor | Acute thermal pain model induced by hot plate and mechanical allodynia | [96] |
4.4. Frogs Analgesic Peptides
| Toxin/ Molecule | Species | Production | Administration | Mechanism of Analgesia | Model | Ref. |
|---|---|---|---|---|---|---|
| Bufalin | Bufo gargarizans | Purified from venom | Intraperitoneal (i.p.) injection | VGSC activity inhibitor | Pain formalin-induced paw licking, pain carrageenan-induced thermal and mechanical hyperalgesia | [114] |
4.5. Bee Analgesic Peptides
| Toxin/ Molecule | Species | Production | Administration | Mechanism of Analgesia | Model | Ref. |
|---|---|---|---|---|---|---|
| Bee venom | Apis mellifera | Sigma-Aldrich® | Subcutaneous (i.s.) injection | Suppression of glial cell activation ipsilateral, dorsal spinal cord | Spinal cord injury (SCI)-induced allodynia and thermal hyperalgesia | [116] |
| Bee venom | Apis mellifera | Sigma-Aldrich® | Subcutaneous (i.s.) injection | α-2 adrenergic receptors activation | Oxaliplatin-induced mechanical allodynia | [117] |
| Bee venom | Apis mellifera | - | Subcutaneous (i.s.) injection | Decrease of NK-1 receptor expression in dorsal root ganglia (DRG) | Complex regional pain syndrome type-1 (CRPS-I) induced mechanical allodynia | [118] |
| Bee venom | Apis mellifera | Jayeonsaeng TJ® | Subcutaneous (i.s.) injection | Increase of the action potential threshold in A-fiber DRG neurons | Oxaliplatin-induced neuropathic pain, cold and mechanical allodynia | [119] |
| Bee venom | Apis mellifera | Jayeonsaeng TJ® | Subcutaneous (i.s.) injection | α-2 adrenergic receptors activation with the involvement of noradrenergic nuclei of the locus coeruleus (LC) | Vincristine-induced cold and mechanical hypersensitivity | [120] |
| Bee venom | Apis mellifera | Apis Injeel® | Intraperitoneal (i.p.) Injection | Inhibitory activity of the COX pathway | Complete Freund’s adjuvant-induced arthritic rats | [124] |
| Bee venom | Apis mellifera | Sigma-Aldrich® | Subcutaneous (i.s.) injection | Inhibitory effect on the expression of substance P in the peripheral and central nervous systems | Scalding-burn-model-induced mechanical allodynia | [121] |
| Bee venom + Morphine | Bee venom from Apis mellifera | Sigma-Aldrich® | Intrathecal (i.t.) injection | Spinal opioidergic and 5-HT3 receptors modulate the analgesia | Oxaliplatin-induced neuropathic pain, cold and mechanical allodynia | [125] |
| Bee venom + Venlafaxine | Bee venom from Apis mellifera | Jayeonsaeng TJ® | Intrathecal (i.t.) injection | α-2 adrenergic receptors activation and serotonergic receptors (5-HT1/5-HT2 and 5-HT3) | Paclitaxel-induced cold and mechanical allodynia | [126] |
| Bee venom and melittin | Apis mellifera | Purified from bee venom | Subcutaneous (i.s.) injection | α-2 adrenergic receptor activation | Paclitaxel-induced mechanical hyperalgesia | [122] |
| Melittin | Apis mellifera | Sigma Aldrich® | Intraprostatic injection | Suppression of COX-2 expression | Complete Freund’s adjuvant-induced prostatitis | [127] |
| Melittin | Apis mellifera | Purified from bee venom—obtained from Sigma-Aldrich® | Subcutaneous (i.s.) injection | Spinal α-1 and α-2 adrenergic receptor activation | Oxaliplatin-induced mechanical and cold allodynia | [128] |
| Phospholipase A2 (bvPLA2) | Apis mellifera | Sigma-Aldrich® | Intraperitoneal (i.p.) injection | Activation of the noradrenergic system, via α2-adrenergic receptors | Oxaliplatin-induced neuropathic pain—cold and mechanical allodynia | [123] |
| bvPLA2 | Apis mellifera | Sigma-Aldrich® | Intraperitoneal (i.p.) injection | Suppressing immune responses in the DRG by regulatory T cells (Tregs) | Oxaliplatin-induced neuropathic pain in Treg-depleted mice | [128] |
4.6. Mollusk Analgesic Peptides
5. Challenges in Integrating Venom-Derived Toxins for Pharmaceutical Pain Relief Solutions
6. Future Perspectives of Using Toxins as Novel Analgesics
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Maatuf, Y.; Geron, M.; Priel, A. The Role of Toxins in the Pursuit for Novel Analgesics. Toxins 2019, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.; Siniavin, A.; Kasheverov, I.; Tsetlin, V. Antiviral Effects of Animal Toxins: Is There a Way to Drugs? Int. J. Mol. Sci. 2022, 23, 3634. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.-M.; Fajloun, Z. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- Sartim, M.A.; Menaldo, D.L.; Sampaio, S.V. Immunotherapeutic Potential of Crotoxin: Anti-Inflammatory and Immunosuppressive Properties. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 39. [Google Scholar] [CrossRef]
- dos Santos Cavalcante, J.; Júnior, F.A.; Jorge, R.J.; Almeida, C. Pain Modulated by Bothrops Snake Venoms: Mechanisms of Nociceptive Signaling and Therapeutic Perspectives. Toxicon 2021, 201, 105–114. [Google Scholar] [CrossRef]
- Garland, E.L. Pain Processing in the Human Nervous System. Prim. Care: Clin. Off. Pract. 2012, 39, 561–571. [Google Scholar] [CrossRef]
- Peirs, C.; Seal, R.P. Neural Circuits for Pain: Recent Advances and Current Views. Science (1979) 2016, 354, 578–584. [Google Scholar] [CrossRef]
- Yam, M.; Loh, Y.; Tan, C.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef]
- Roeckel, L.-A.; Le Coz, G.-M.; Gavériaux-Ruff, C.; Simonin, F. Opioid-Induced Hyperalgesia: Cellular and Molecular Mechanisms. Neuroscience 2016, 338, 160–182. [Google Scholar] [CrossRef]
- Jensen, T.S.; Finnerup, N.B. Allodynia and Hyperalgesia in Neuropathic Pain: Clinical Manifestations and Mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef]
- Viana, F. Nociceptors: Thermal Allodynia and Thermal Pain. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–119. [Google Scholar]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic Pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef]
- Aurilio, C.; Pota, V.; Pace, M.C.; Passavanti, M.B.; Barbarisi, M. Ionic Channels and Neuropathic Pain: Phisiopatology and Applications. J. Cell Physiol. 2008, 215, 8–14. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Kaur, S.; Pandhi, P.; Dutta, P. Painful Diabetic Neuropathy: An Update. Ann. Neurosci. 2011, 18, 168–175. [Google Scholar] [CrossRef]
- Mallick-Searle, T.; Snodgrass, B.; Brant, J. Postherpetic Neuralgia: Epidemiology, Pathophysiology, and Pain Management Pharmacology. J. Multidiscip. Healthc. 2016, 9, 447–454. [Google Scholar] [CrossRef]
- Gambeta, E.; Chichorro, J.G.; Zamponi, G.W. Trigeminal Neuralgia: An Overview from Pathophysiology to Pharmacological Treatments. Mol. Pain 2020, 16, 174480692090189. [Google Scholar] [CrossRef]
- Lu, H.-J.; Fu, Y.-Y.; Wei, Q.-Q.; Zhang, Z.-J. Neuroinflammation in HIV-Related Neuropathic Pain. Front. Pharmacol. 2021, 12, 653852. [Google Scholar] [CrossRef]
- Edwards, H.; Mulvey, M.; Bennett, M. Cancer-Related Neuropathic Pain. Cancers 2019, 11, 373. [Google Scholar] [CrossRef]
- Schmid, A.B.; Fundaun, J.; Tampin, B. Entrapment Neuropathies: A Contemporary Approach to Pathophysiology, Clinical Assessment, and Management. Pain Rep. 2020, 5, e829. [Google Scholar] [CrossRef]
- Shipton, E. Post-surgical neuropathic pain. ANZ J. Surg. 2008, 78, 548–555. [Google Scholar] [CrossRef]
- Niccolaï, P.; Ouchchane, L.; Libier, M.; Beouche, F.; Belon, M.; Vedrinne, J.-M.; El Drayi, B.; Vallet, L.; Ruiz, F.; Biermann, C.; et al. Persistent Neuropathic Pain after Inguinal Herniorrhaphy Depending on the Procedure (Open Mesh v. Laparoscopy): A Propensity-Matched Analysis. Can. J. Surg. 2015, 58, 114–120. [Google Scholar] [CrossRef]
- Subedi, B.; Grossberg, G.T. Phantom Limb Pain: Mechanisms and Treatment Approaches. Pain Res. Treat. 2011, 2011, 864605. [Google Scholar] [CrossRef]
- Valentine, W.M. Toxic Peripheral Neuropathies: Agents and Mechanisms. Toxicol. Pathol. 2020, 48, 152–173. [Google Scholar] [CrossRef]
- Cook, A.D.; Christensen, A.D.; Tewari, D.; McMahon, S.B.; Hamilton, J.A. Immune Cytokines and Their Receptors in Inflammatory Pain. Trends Immunol. 2018, 39, 240–255. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Gonçalves dos Santos, G.; Delay, L.; Yaksh, T.L.; Corr, M. Neuraxial Cytokines in Pain States. Front. Immunol. 2020, 10, 3061. [Google Scholar] [CrossRef]
- Price, T.J.; Ray, P.R. Recent Advances toward Understanding the Mysteries of the Acute to Chronic Pain Transition. Curr. Opin. Physiol. 2019, 11, 42–50. [Google Scholar] [CrossRef]
- Richard, A.M.; Srinivasa, N.R.; James, N.C. Neural Mechanisms of Primary Hyperalgesia. In Neurobiology of Nociceptors; Oxford University Press: Oxford, UK, 1996; pp. 370–389. [Google Scholar]
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain Regulation by Non-Neuronal Cells and Inflammation. Science (1979) 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Ashmawi, H.A.; Freire, G.M.G. Peripheral and Central Sensitization. Rev. Dor 2016, 17, 31–34. [Google Scholar] [CrossRef]
- Costigan, M.; Woolf, C.J. Pain: Molecular Mechanisms. J. Pain 2000, 1, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Cid-Uribe, J.I.; Veytia-Bucheli, J.I.; Romero-Gutierrez, T.; Ortiz, E.; Possani, L.D. Scorpion Venomics: A 2019 Overview. Expert Rev. Proteom. 2020, 17, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Langenegger, N.; Nentwig, W.; Kuhn-Nentwig, L. Spider Venom: Components, Modes of Action, and Novel Strategies in Transcriptomic and Proteomic Analyses. Toxins 2019, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Giralt, E. Three Valuable Peptides from Bee and Wasp Venoms for Therapeutic and Biotechnological Use: Melittin, Apamin and Mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef]
- Clarke, B.T. The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol. Rev. 2007, 72, 365–379. [Google Scholar] [CrossRef]
- Zhao, Y.; Antunes, A. Biomedical Potential of the Neglected Molluscivorous and Vermivorous Conus Species. Mar. Drugs 2022, 20, 105. [Google Scholar] [CrossRef]
- Bjørn-Yoshimoto, W.E.; Ramiro, I.B.L.; Yandell, M.; McIntosh, J.M.; Olivera, B.M.; Ellgaard, L.; Safavi-Hemami, H. Curses or Cures: A Review of the Numerous Benefits Versus the Biosecurity Concerns of Conotoxin Research. Biomedicines 2020, 8, 235. [Google Scholar] [CrossRef]
- Tosti, E.; Boni, R.; Gallo, A. Pathophysiological Responses to Conotoxin Modulation of Voltage-Gated Ion Currents. Mar. Drugs 2022, 20, 282. [Google Scholar] [CrossRef]
- Hatakeyama, D.M.; Tasima, L.J.; Bravo-Tobar, C.A.; Serino-Silva, C.; Tashima, A.K.; Rodrigues, C.F.; Aguiar, W.D.; Galizio, N.D.; Lima, E.O.; Kavazoi, V.K.; et al. Venom Complexity of Bothrops Atrox (Common Lancehead) Siblings. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200018. [Google Scholar] [CrossRef]
- Valente, R.H.; Guimarães, P.R.; Junqueira, M.; Neves-Ferreira, A.G.C.; Soares, M.R.; Chapeaurouge, A.; Trugilho, M.R.O.; León, I.R.; Rocha, S.L.G.; Oliveira-Carvalho, A.L.; et al. Bothrops Insularis Venomics: A Proteomic Analysis Supported by Transcriptomic-Generated Sequence Data. J. Proteom. 2009, 72, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Mora-Obando, D.; Guerrero-Vargas, J.A.; Prieto-Sánchez, R.; Beltrán, J.; Rucavado, A.; Sasa, M.; Gutiérrez, J.M.; Ayerbe, S.; Lomonte, B. Proteomic and Functional Profiling of the Venom of Bothrops Ayerbei from Cauca, Colombia, Reveals Striking Interspecific Variation with Bothrops Asper Venom. J. Proteom. 2014, 96, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.F.; Portes-Junior, J.A.; Nicolau, C.A.; Bernardoni, J.L.; Nishiyama, M.Y., Jr.; Amazonas, D.R.; Freitas-de-Sousa, L.A.; Mourão, R.H.; Chalkidis, H.M.; Valente, R.H.; et al. Functional Proteomic Analyses of Bothrops Atrox Venom Reveals Phenotypes Associated with Habitat Variation in the Amazon. J. Proteom. 2017, 159, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Simizo, A.; Kitano, E.S.; Sant’Anna, S.S.; Grego, K.F.; Tanaka-Azevedo, A.M.; Tashima, A.K. Comparative Gender Peptidomics of Bothrops Atrox Venoms: Are There Differences between Them? J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200055. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.S.; Brito, I.M.; De Oliveira, L.A.; De Barros, L.C.; Almeida, C.; Rossini, B.C.; Sousa, D.L.; Alves, R.S.; Jorge, R.J.; Santos, L.D. Experimental Bothrops Atrox Envenomation: Blood Plasma Proteome Effects after Local Tissue Damage and Perspectives on Thromboinflammation. Toxins 2022, 14, 613. [Google Scholar] [CrossRef]
- Cavalcante, J.S.; Borges da Silva, W.R.G.; de Oliveira, L.A.; Brito, I.M.C.; Muller, K.S.; Vidal, I.S.J.; dos Santos, L.D.; Jorge, R.J.B.; Almeida, C.; de Lima Bicho, C. Blood Plasma Proteome Alteration after Local Tissue Damage Induced by Bothrops Erythromelas Snake Venom in Mice. J. Proteom. 2022, 269, 104742. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Cavalcante, J.; de Almeida, C.A.; Clasen, M.A.; da Silva, E.L.; de Barros, L.C.; Marinho, A.D.; Rossini, B.C.; Marino, C.L.; Carvalho, P.C.; Jorge, R.J.; et al. A Fingerprint of Plasma Proteome Alteration after Local Tissue Damage Induced by Bothrops Leucurus Snake Venom in Mice. J. Proteom. 2022, 253, 104464. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, K.; Hemshekhar, M.; Thushara, R.M.; Santhosh, M.S.; Sundaram, M.S.; Kemparaju, K.; Girish, K.S. Inflammation and Oxidative Stress in Viper Bite: An Insight within and Beyond. Toxicon 2015, 98, 89–97. [Google Scholar] [CrossRef]
- Cavalcante, J.D.; de Almeida, D.E.; Moraes, M.S.; Santos, S.R.; Pincinato, P.M.; Riciopo, P.M.; de Oliveira, L.L.; Monteiro, W.M.; Ferreira-Junior, R.S. Challenges and Opportunities in Clinical Diagnostic Routine of Envenomation Using Blood Plasma Proteomics. Toxins 2023, 15, 180. [Google Scholar] [CrossRef]
- Cavalcante, J.S.; de Almeida, D.E.G.; Santos-Filho, N.A.; Sartim, M.A.; de Almeida Baldo, A.; Brasileiro, L.; Albuquerque, P.L.; Oliveira, S.S.; Sachett, J.A.G.; Monteiro, W.M.; et al. Crosstalk of Inflammation and Coagulation in Bothrops Snakebite Envenoming: Endogenous Signaling Pathways and Pathophysiology. Int. J. Mol. Sci. 2023, 24, 11508. [Google Scholar] [CrossRef]
- Albuquerque, P.L.; Paiva, J.H.; Martins, A.M.; Meneses, G.C.; Silva Júnior, G.B.; Buckley, N.; Daher, E.D. Clinical Assessment and Pathophysiology of Bothrops Venom-Related Acute Kidney Injury: A Scoping Review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Bordon, K.C.F.; Wiezel, G.A.; Amorim, F.G.; Arantes, E.C. Arthropod Venom Hyaluronidases: Biochemical Properties and Potential Applications in Medicine and Biotechnology. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 43. [Google Scholar] [CrossRef]
- Nait Mohamed, F.A.; Laraba-Djebari, F. Scorpion Envenomation: A Deadly Illness Requiring an Effective Therapy. Toxin Rev. 2021, 40, 592–605. [Google Scholar] [CrossRef]
- Schmidt, J.O. Clinical Consequences of Toxic Envenomations by Hymenoptera. Toxicon 2018, 150, 96–104. [Google Scholar] [CrossRef]
- RA Mans, D.; Pawirodihardjo, J.; Djotaroeno, M.; Friperson, P. Exploring the Global Animal Biodiversity in the Search for New Drugs -Amphibians. J. Transl. Sci. 2021, 7, 2–17. [Google Scholar] [CrossRef]
- Bordon, K.D.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Trim, S.A.; Trim, C.M. Venom: The Sharp End of Pain Therapeutics. Br. J. Pain 2013, 7, 179–188. [Google Scholar] [CrossRef]
- Hayashi, M.A.F.; Oliveira, E.B.; Kerkis, I.; Karpel, R.L. Crotamine: A Novel Cell-Penetrating Polypeptide Nanocarrier with Potential Anti-Cancer and Biotechnological Applications. Methods Mol. Biol. 2012, 906, 337–352. [Google Scholar] [CrossRef]
- Stábeli, R.G.; Marcussi, S.; Carlos, G.B.; Pietro, R.C.L.R.; Selistre-De-Araújo, H.S.; Giglio, J.R.; Oliveira, E.B.; Soares, A.M. Platelet Aggregation and Antibacterial Effects of an L-Amino Acid Oxidase Purified from Bothrops Alternatus Snake Venom. Bioorg Med. Chem. 2004, 12, 2881–2886. [Google Scholar] [CrossRef]
- Abbade, L.P.; Barraviera, S.R.; Silvares, M.R.; Lima, A.B.; Haddad, G.R.; Gatti, M.A.; Medolago, N.B.; Rigotto Carneiro, M.T.; Dos Santos, L.D.; Ferreira, R.S., Jr.; et al. Treatment of Chronic Venous Ulcers With Heterologous Fibrin Sealant: A Phase I/II Clinical Trial. Front. Immunol. 2021, 12, 83. [Google Scholar] [CrossRef]
- FERREIRA, S.H. A Bradykinin-Potentiating Factor (Bpf) Present in the Venom of Bothrops Jararaca. Br. J. Pharmacol. Chemother. 1965, 24, 163. [Google Scholar] [CrossRef]
- Diochot, S.; Alloui, A.; Rodrigues, P.; Dauvois, M.; Friend, V.; Aissouni, Y.; Eschalier, A.; Lingueglia, E.; Baron, A. Analgesic Effects of Mambalgin Peptide Inhibitors of Acid-Sensing Ion Channels in Inflammatory and Neuropathic Pain. Pain 2016, 157, 552–559. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Xu, X.; Zhang, Y.; Gong, X.; Yang, Z.; Zhang, H.; Tang, D.; Liang, S.; Liu, Z. Naja Atra Venom Peptide Reduces Pain by Selectively Blocking the Voltage-Gated Sodium Channel Nav1.8. J. Biol. Chem. 2019, 294, 7324–7334. [Google Scholar] [CrossRef]
- Sant’Anna, M.B.; Lopes, F.S.R.; Kimura, L.F.; Giardini, A.C.; Sant’Anna, O.A.; Picolo, G. Crotoxin Conjugated to SBA-15 Nanostructured Mesoporous Silica Induces Long-Last Analgesic Effect in the Neuropathic Pain Model in Mice. Toxins 2019, 11, 679. [Google Scholar] [CrossRef]
- Teixeira, N.B.; Sant’Anna, M.B.; Giardini, A.C.; Araujo, L.P.; Fonseca, L.A.; Basso, A.S.; Cury, Y.; Picolo, G. Crotoxin Down-Modulates pro-Inflammatory Cells and Alleviates Pain on the MOG35-55-Induced Experimental Autoimmune Encephalomyelitis, an Animal Model of Multiple Sclerosis. Brain Behav. Immun. 2020, 84, 253–268. [Google Scholar] [CrossRef]
- Konno, K.; Picolo, G.; Gutierrez, V.P.; Brigatte, P.; Zambelli, V.O.; Camargo, A.C.M.; Cury, Y. Crotalphine, a Novel Potent Analgesic Peptide from the Venom of the South American Rattlesnake Crotalus Durissus Terrificus. Peptides 2008, 29, 1293–1304. [Google Scholar] [CrossRef]
- Bressan, E.; Touska, F.; Vetter, I.; Kistner, K.; Kichko, T.I.; Teixeira, N.B.; Picolo, G.; Cury, Y.; Lewis, R.J.; Fischer, M.J.M.; et al. Crotalphine Desensitizes TRPA1 Ion Channels to Alleviate Inflammatory Hyperalgesia. Pain 2016, 157, 2504–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhao, J.; Yang, Q.; Ye, Y. Cobra Neurotoxin Produces Central Analgesic and Hyperalgesic Actions via Adenosine A 1 and A 2A Receptors. Mol. Pain 2017, 13, 174480691772033. [Google Scholar] [CrossRef]
- Liang, Y.-X.; Zhang, Z.-Y.; Zhang, R. Antinociceptive Effect of Najanalgesin from Naja Naja Atra in a Neuropathic Pain Model via Inhibition of C-Jun NH2-Terminal Kinase. Chin. Med. J. 2015, 128, 2340–2345. [Google Scholar] [CrossRef]
- Gong, S.; Liang, Q.; Zhu, Q.; Ding, D.; Yin, Q.; Tao, J.; Jiang, X. Nicotinic Acetylcholine Receptor A7 Subunit Is Involved in the Cobratoxin-Induced Antinociception in an Animal Model of Neuropathic Pain. Toxicon 2015, 93, 31–36. [Google Scholar] [CrossRef]
- Kampo, S.; Ahmmed, B.; Zhou, T.; Owusu, L.; Anabah, T.W.; Doudou, N.R.; Kuugbee, E.D.; Cui, Y.; Lu, Z.; Yan, Q.; et al. Scorpion Venom Analgesic Peptide, BmK AGAP Inhibits Stemness, and Epithelial-Mesenchymal Transition by Down-Regulating PTX3 in Breast Cancer. Front. Oncol. 2019, 9, 21. [Google Scholar] [CrossRef]
- Cao, Q.; Lu, W.; Cai, X.; Hu, C.; Wang, C.; Ye, J.; Yan, H.; Yang, Y.; Wang, Z.; Huo, J.; et al. In Vitro Refolding and Functional Analysis of Polyhistidine-Tagged Buthus Martensii Karsch Antitumor-Analgesic Peptide Produced in Escherichia Coli. Biotechnol. Lett. 2015, 37, 2461–2466. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, X.; Hou, X.; Sun, J.; Kong, X.; Sun, Y.; Liu, Z.; Ma, Y.; Niu, Y.; Song, Y.; et al. A Mutant of the Buthus Martensii Karsch Antitumor-Analgesic Peptide Exhibits Reduced Inhibition to HNav1.4 and HNav1.5 Channels While Retaining Analgesic Activity. J. Biol. Chem. 2017, 292, 18270–18280. [Google Scholar] [CrossRef]
- Ruan, J.-P.; Mao, Q.-H.; Lu, W.-G.; Cai, X.-T.; Chen, J.; Li, Q.-; Fu, Q.-; Yan, H.-J.; Cao, J.-L.; Cao, P. Inhibition of Spinal MAPKs by Scorpion Venom Peptide BmK AGAP Produces a Sensory-Specific Analgesic Effect. Mol. Pain 2018, 14, 174480691876123. [Google Scholar] [CrossRef]
- Maatoug, R.; Jebali, J.; Guieu, R.; De Waard, M.; Kharrat, R. BotAF, a New Buthus Occitanus Tunetanus Scorpion Toxin, Produces Potent Analgesia in Rodents. Toxicon 2018, 149, 72–85. [Google Scholar] [CrossRef]
- Li, Z.; Hu, P.; Wu, W.; Wang, Y. Peptides with Therapeutic Potential in the Venom of the Scorpion Buthus Martensii Karsch. Peptides 2019, 115, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.K.; Bochi, G.V.; Pereira, A.L.; Adamante, G.; Ferro, P.R.; Dal-Toé De Prá, S.; Milioli, A.M.; Damiani, A.P.; da Silveira Prestes, G.; Dalenogare, D.P.; et al. TsNTxP, a Non-Toxic Protein from Tityus Serrulatus Scorpion Venom, Induces Antinociceptive Effects by Suppressing Glutamate Release in Mice. Eur. J. Pharmacol. 2019, 855, 65–74. [Google Scholar] [CrossRef]
- Bagheri-Ziari, S.; Shahbazzadeh, D.; Sardari, S.; Sabatier, J.-M.; Pooshang Bagheri, K. Discovery of a New Analgesic Peptide, Leptucin, from the Iranian Scorpion, Hemiscorpius Lepturus. Molecules 2021, 26, 2580. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Y.; Wang, X.; Zhang, Z.; Zhang, J.; Zhao, Y. The Role of the Arginine Residue in Site RC for the Analgesic Activity of the Recombinant Chinese Scorpion Buthus Martensii Karsch, BmK AGP-SYPU1. Comput. Biol. Chem. 2018, 74, 247–252. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, E.; Sang, M.; Wang, Z.; Zhang, Y.; Ye, J.; Zhou, Q.; Zhao, C.; Hu, C.; Lu, W.; et al. Makatoxin-3, a Thermostable Nav1.7 Agonist from Buthus Martensii Karsch (BmK) Scorpion Elicits Non-Narcotic Analgesia in Inflammatory Pain Models. J. Ethnopharmacol. 2022, 288, 114998. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.R.; Wingerd, J.S.; Brust, A.; Bladen, C.; Ragnarsson, L.; Herzig, V.; Deuis, J.R.; Dutertre, S.; Vetter, I.; Zamponi, G.W.; et al. Discovery and Mode of Action of a Novel Analgesic β-Toxin from the African Spider Ceratogyrus Darlingi. PLoS ONE 2017, 12, e0182848. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Dekan, Z.; Smith, J.J.; Deuis, J.R.; Vetter, I.; Herzig, V.; Alewood, P.F.; King, G.F.; Lewis, R.J. Modulatory Features of the Novel Spider Toxin Μ-TRTX-Df1a Isolated from the Venom of the Spider Davus Fasciatus. Br. J. Pharmacol. 2017, 174, 2528–2544. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Huang, H.; Wang, G.; Yang, S.; Lu, Q.; Liu, J.; Lai, R.; Rong, M. A Novel Toxin from Haplopelma Lividum Selectively Inhibits the NaV1.8 Channel and Possesses Potent Analgesic Efficacy. Toxins 2016, 9, 7. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Chen, Y.; Xu, D.; Zhang, P.; Wang, X. Newly Discovered Action of HpTx3 from Venom of Heteropoda Venatoria on Nav1.7 and Its Pharmacological Implications in Analgesia. Toxins 2019, 11, 680. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Dekan, Z.; Rosengren, K.J.; Erickson, A.; Vetter, I.; Deuis, J.R.; Herzig, V.; Alewood, P.F.; King, G.F.; Lewis, R.J. Identification and Characterization of ProTx-III [μ -TRTX-Tp1a], a New Voltage-Gated Sodium Channel Inhibitor from Venom of the Tarantula Thrixopelma Pruriens. Mol. Pharmacol. 2015, 88, 291–303. [Google Scholar] [CrossRef]
- da Silva, J.F.; Castro-Junior, C.J.; Oliveira, S.M.; Dalmolin, G.D.; Silva, C.R.; Vieira, L.B.; Diniz, D.M.; do Nascimento Cordeiro, M.; Ferreira, J.; Souza, A.H.; et al. Characterization of the Antinociceptive Effect of PhTx3-4, a Toxin from Phoneutria Nigriventer, in Models of Thermal, Chemical and Incisional Pain in Mice. Toxicon 2015, 108, 53–61. [Google Scholar] [CrossRef]
- Emerich, B.; Ferreira, R.; Cordeiro, M.; Borges, M.; Pimenta, A.; Figueiredo, S.; Duarte, I.; de Lima, M. δ-Ctenitoxin-Pn1a, a Peptide from Phoneutria Nigriventer Spider Venom, Shows Antinociceptive Effect Involving Opioid and Cannabinoid Systems, in Rats. Toxins 2016, 8, 106. [Google Scholar] [CrossRef]
- Freitas, A.C.N.; Pacheco, D.F.; Machado, M.F.M.; Carmona, A.K.; Duarte, I.D.G.; de Lima, M.E. PnPP-19, a Spider Toxin Peptide, Induces Peripheral Antinociception through Opioid and Cannabinoid Receptors and Inhibition of Neutral Endopeptidase. Br. J. Pharmacol. 2016, 173, 1491–1501. [Google Scholar] [CrossRef]
- Freitas, A.C.N.; Silva, G.C.; Pacheco, D.F.; Pimenta, A.M.C.; Lemos, V.S.; Duarte, I.D.G.; de Lima, M.E. The Synthetic Peptide PnPP-19 Induces Peripheral Antinociception via Activation of NO/CGMP/KATP Pathway: Role of ENOS and NNOS. Nitric Oxide 2017, 64, 31–38. [Google Scholar] [CrossRef]
- Stanciu, G.-D.; Luca, A.; Marza, A.; Alexa-Stratulat, T.; Tudorancea, I.; Bild, W.; Rezus, E.; Rezus, C.; Tamba, B.I. Intracerebroventricular Coadministration of Protoxin-II and Trace Elements in Rats Enhances the Analgesic Effect of the 1.7 Voltage-Gate Sodium Channel Blocker. Biomed. Res. Int. 2019, 2019, 8057803. [Google Scholar] [CrossRef]
- Rigo, F.K.; Trevisan, G.; De Prá, S.D.-T.; Cordeiro, M.N.; Borges, M.H.; Silva, J.F.; Santa Cecilia, F.V.; de Souza, A.H.; de Oliveira Adamante, G.; Milioli, A.M.; et al. The Spider Toxin Phα1β Recombinant Possesses Strong Analgesic Activity. Toxicon 2017, 133, 145–152. [Google Scholar] [CrossRef]
- da Silva Junior, C.A.; de Castro Junior, C.J.; Pereira, E.M.R.; Binda, N.S.; da Silva, J.F.; do Nascimento Cordeiro, M.; Diniz, D.M.; Cecilia, F.S.; Ferreira, J.; Gomez, M.V. The Inhibitory Effect of Phα1β Toxin on Diabetic Neuropathic Pain Involves the CXCR4 Chemokine Receptor. Pharmacol. Rep. 2020, 72, 47–54. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dekan, Z.; Wingerd, J.S.; Smith, J.J.; Munasinghe, N.R.; Bhola, R.F.; Imlach, W.L.; Herzig, V.; Armstrong, D.A.; Rosengren, K.J.; et al. Pharmacological Characterisation of the Highly NaV1.7 Selective Spider Venom Peptide Pn3a. Sci. Rep. 2017, 7, 40883. [Google Scholar] [CrossRef]
- Deng, M.; Jiang, L.; Luo, X.; Tao, H.; Liang, S. Jingzhaotoxin-X, a Gating Modifier of Kv4.2 and Kv4.3 Potassium Channels Purified from the Venom of the Chinese Tarantula Chilobrachys Jingzhao. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, D.; Huang, B.; Yang, Q.; Zhang, Q.; Chen, M.; Rong, M.; Liu, Z. Discovery of a Novel Nav1.7 Inhibitor From Cyriopagopus Albostriatus Venom With Potent Analgesic Efficacy. Front. Pharmacol. 2018, 9, 1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, D.; Zhang, Q.; Huang, B.; Yang, Q.; Tang, D.; Chen, M.; Rong, M.; Liu, Z. Μ-TRTX-Ca1a: A Novel Neurotoxin from Cyriopagopus Albostriatus with Analgesic Effects. Acta Pharmacol. Sin. 2019, 40, 859–866. [Google Scholar] [CrossRef]
- Gonçalves, T.C.; Benoit, E.; Kurz, M.; Lucarain, L.; Fouconnier, S.; Combemale, S.; Jaquillard, L.; Schombert, B.; Chambard, J.; Boukaiba, R.; et al. From Identification to Functional Characterization of Cyriotoxin-1a, an Antinociceptive Toxin from the Spider Cyriopagopus schioedtei. Br. J. Pharmacol. 2019, 176, 1298–1314. [Google Scholar] [CrossRef]
- Chen, C.; Xu, B.; Shi, X.; Zhang, M.; Zhang, Q.; Zhang, T.; Zhao, W.; Zhang, R.; Wang, Z.; Li, N.; et al. GpTx-1 and [Ala5, Phe6, Leu26, Arg28]GpTx-1, Two Peptide Na V 1.7 Inhibitors: Analgesic and Tolerance Properties at the Spinal Level. Br. J. Pharmacol. 2018, 175, 3911–3927. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Silva, C.R.; Trevisan, G.; Villarinho, J.G.; Cordeiro, M.N.; Richardson, M.; Borges, M.H.; Castro, C.J.; Gomez, M.V.; Ferreira, J. Antinociceptive Effect of a Novel Armed Spider Peptide Tx3-5 in Pathological Pain Models in Mice. Pflug. Arch. 2016, 468, 881–894. [Google Scholar] [CrossRef]
- Tonello, R.; Fusi, C.; Materazzi, S.; Marone, I.M.; De Logu, F.; Benemei, S.; Gonçalves, M.C.; Coppi, E.; Castro-Junior, C.J.; Gomez, M.V.; et al. The Peptide Phα1β, from Spider Venom, Acts as a TRPA1 Channel Antagonist with Antinociceptive Effects in Mice. Br. J. Pharmacol. 2017, 174, 57–69. [Google Scholar] [CrossRef]
- Oliveira, C.F.; Alves, D.P.; Emerich, B.L.; Figueiredo, S.G.; Cordeiro, M.D.; Borges, M.H.; Richardson, M.; Pimenta, A.M.; Duarte, I.D.; Lima, M.E. Antinociceptive Effect of PnTx4(5-5), a Peptide from Phoneutria Nigriventer Spider Venom, in Rat Models and the Involvement of Glutamatergic System. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190022. [Google Scholar] [CrossRef]
- Rigo, F.K.; Rossato, M.F.; Borges, V.; Silva, J.F.; Pereira, E.M.; Ávila, R.A.; Trevisan, G.; Santos, D.C.; Diniz, D.M.; Silva, M.A.; et al. Analgesic and Side Effects of Intravenous Recombinant Phα1β. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190070. [Google Scholar] [CrossRef]
- Antunes, F.T.T.; Angelo, S.G.; Dallegrave, E.; Picada, J.N.; Marroni, N.P.; Schemitt, E.; Ferraz, A.G.; Gomez, M.V.; de Souza, A.H. Recombinant Peptide Derived from the Venom the Phoneutria Nigriventer Spider Relieves Nociception by Nerve Deafferentation. Neuropeptides 2020, 79, 101980. [Google Scholar] [CrossRef]
- Emerich, B.L.; Ferreira, R.C.M.; Machado-de-Avila, R.A.; Resende, J.M.; Duarte, I.D.G.; de Lima, M.E. PnAn13, an Antinociceptive Synthetic Peptide Inspired in the Phoneutria Nigriventer Toxin PnTx4(6–1) (δ-Ctenitoxin-Pn1a). Toxicon X 2020, 7, 100045. [Google Scholar] [CrossRef]
- Tonello, R.; Trevisan, G.; Luckemeyer, D.; Castro-Junior, C.J.; Gomez, M.V.; Ferreira, J. Phα1β, a Dual Blocker of TRPA1 and Cav2.2, as an Adjuvant Drug in Opioid Therapy for Postoperative Pain. Toxicon 2020, 188, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Garcia Mendes, M.P.; Carvalho dos Santos, D.; Rezende, M.J.S.; Assis Ferreira, L.C.; Rigo, F.K.; José de Castro Junior, C.; Gomez, M.V. Effects of Intravenous Administration of Recombinant Phα1β Toxin in a Mouse Model of Fibromyalgia. Toxicon 2021, 195, 104–110. [Google Scholar] [CrossRef]
- Dornelles, M.F.; Marques, M.G.B.; Renner, M.F. Revisão Sobre Toxinas de Anura (Tetrapoda, Lissamphibia) e Suas Aplicações Biotecnológicas. Ciência Em Mov. 2010, 12, 103–114. [Google Scholar] [CrossRef]
- Tao, J.; Jiang, F.; Liu, C.; Liu, Z.; Zhu, Y.; Xu, J.; Ge, Y.; Xu, K.; Yin, P. Modulatory Effects of Bufalin, an Active Ingredient from Toad Venom on Voltage-Gated Sodium Channels. Mol. Biol. Rep. 2018, 45, 721–740. [Google Scholar] [CrossRef]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 2019, 10, 2090. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Roh, D.-H.; Choi, J.-W.; Ryu, Y.; Lee, J.-H. Repetitive Treatment with Diluted Bee Venom Attenuates the Induction of Below-Level Neuropathic Pain Behaviors in a Rat Spinal Cord Injury Model. Toxins 2015, 7, 2571–2585. [Google Scholar] [CrossRef]
- Yeo, J.-H.; Yoon, S.-Y.; Kwon, S.-K.; Kim, S.-J.; Lee, J.-H.; Beitz, A.J.; Roh, D.-H. Repetitive Acupuncture Point Treatment with Diluted Bee Venom Relieves Mechanical Allodynia and Restores Intraepidermal Nerve Fiber Loss in Oxaliplatin-Induced Neuropathic Mice. J. Pain 2016, 17, 298–309. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Kim, Y.; Choi, J.; Jeon, S.; Kim, D.; Jeong, H.; Lee, Y.; Park, H. Antiallodynic Effects of Bee Venom in an Animal Model of Complex Regional Pain Syndrome Type 1 (CRPS-I). Toxins 2017, 9, 285. [Google Scholar] [CrossRef]
- Lee, J.H.; Gang, J.; Yang, E.; Kim, W.; Jin, Y.-H. Bee Venom Acupuncture Attenuates Oxaliplatin-Induced Neuropathic Pain by Modulating Action Potential Threshold in A-Fiber Dorsal Root Ganglia Neurons. Toxins 2020, 12, 737. [Google Scholar] [CrossRef]
- Li, D.; Chung, G.; Kim, S.K. The Involvement of Central Noradrenergic Pathway in the Analgesic Effect of Bee Venom Acupuncture on Vincristine-Induced Peripheral Neuropathy in Rats. Toxins 2020, 12, 775. [Google Scholar] [CrossRef]
- Kang, D.-W.; Choi, J.-G.; Kim, J.; Park, J.B.; Lee, J.-H.; Kim, H.-W. Bee Venom Reduces Burn-Induced Pain via the Suppression of Peripheral and Central Substance P Expression in Mice. J. Vet. Sci. 2021, 22, e9. [Google Scholar] [CrossRef]
- Choi, J.; Jeon, C.; Lee, J.; Jang, J.; Quan, F.; Lee, K.; Kim, W.; Kim, S. Suppressive Effects of Bee Venom Acupuncture on Paclitaxel-Induced Neuropathic Pain in Rats: Mediation by Spinal A2-Adrenergic Receptor. Toxins 2017, 9, 351. [Google Scholar] [CrossRef]
- Li, D.; Lee, Y.; Kim, W.; Lee, K.; Bae, H.; Kim, S. Analgesic Effects of Bee Venom Derived Phospholipase A2 in a Mouse Model of Oxaliplatin-Induced Neuropathic Pain. Toxins 2015, 7, 2422–2434. [Google Scholar] [CrossRef]
- El-tedawy, D.; Abd-alhaseeb, M.; Helmy, M.; Ghoneim, A. Systemic Bee Venom Exerts Anti-arthritic and Anti-inflammatory Properties in a Rat Model of Arthritis. Biomed. Rep. 2020, 13, 20. [Google Scholar] [CrossRef]
- Kim, W.; Kim, M.; Go, D.; Min, B.-I.; Na, H.; Kim, S. Combined Effects of Bee Venom Acupuncture and Morphine on Oxaliplatin-Induced Neuropathic Pain in Mice. Toxins 2016, 8, 33. [Google Scholar] [CrossRef]
- Li, D.; Yoo, J.H.; Kim, S.K. Long-Lasting and Additive Analgesic Effects of Combined Treatment of Bee Venom Acupuncture and Venlafaxine on Paclitaxel-Induced Allodynia in Mice. Toxins 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, B.-P.; Cai, L. Therapeutic Effect of Melittin on a Rat Model of Chronic Prostatitis Induced by Complete Freund’s Adjuvant. Biomed. Pharmacother. 2017, 90, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kim, W.; Shin, D.; Jung, Y.; Bae, H.; Kim, S. Preventive Effects of Bee Venom Derived Phospholipase A2 on Oxaliplatin-Induced Neuropathic Pain in Mice. Toxins 2016, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Hemami, H.; Brogan, S.E.; Olivera, B.M. Pain Therapeutics from Cone Snail Venoms: From Ziconotide to Novel Non-Opioid Pathways. J. Proteom. 2019, 190, 12–20. [Google Scholar] [CrossRef]
- Gorson, J.; Holford, M. Small Packages, Big Returns: Uncovering the Venom Diversity of Small Invertebrate Conoidean Snails. Integr. Comp. Biol. 2016, 56, 962–972. [Google Scholar] [CrossRef]
- Yu, S.; Du, T.; Liu, Z.; Wu, Q.; Feng, G.; Dong, M.; Zhou, X.; Jiang, L.; Dai, Q. Im10A, a Short Conopeptide Isolated from Conus Imperialis and Possesses Two Highly Concentrated Disulfide Bridges and Analgesic Activity. Peptides 2016, 81, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, S.; Min, X.; Shao, M.; Chen, Y.; Chen, Z.; Zhou, M. A Novel μ-Conotoxin from Worm-Hunting Conus Tessulatus That Selectively Inhibit Rat TTX-Resistant Sodium Currents. Toxicon 2017, 130, 11–18. [Google Scholar] [CrossRef]
- Liu, Z.; Bartels, P.; Sadeghi, M.; Du, T.; Dai, Q.; Zhu, C.; Yu, S.; Wang, S.; Dong, M.; Sun, T.; et al. A Novel α-Conopeptide Eu1.6 Inhibits N-Type (CaV2.2) Calcium Channels and Exhibits Potent Analgesic Activity. Sci. Rep. 2018, 8, 1004. [Google Scholar] [CrossRef]
- Sousa, S.R.; McArthur, J.R.; Brust, A.; Bhola, R.F.; Rosengren, K.J.; Ragnarsson, L.; Dutertre, S.; Alewood, P.F.; Christie, M.J.; Adams, D.J.; et al. Novel Analgesic ω-Conotoxins from the Vermivorous Cone Snail Conus Moncuri Provide New Insights into the Evolution of Conopeptides. Sci. Rep. 2018, 8, 13397. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Xu, N.; Liu, Z.; Ding, R.; Yu, S.; Dong, M.; Wang, S.; Shen, J.; Tae, H.-S.; Adams, D.J.; et al. Targeting of N-Type Calcium Channels via GABA B -Receptor Activation by α-Conotoxin Vc1.1 Variants Displaying Improved Analgesic Activity. J. Med. Chem. 2018, 61, 10198–10205. [Google Scholar] [CrossRef] [PubMed]
- Belgi, A.; Burnley, J.V.; MacRaild, C.A.; Chhabra, S.; Elnahriry, K.A.; Robinson, S.D.; Gooding, S.G.; Tae, H.-S.; Bartels, P.; Sadeghi, M.; et al. Alkyne-Bridged α-Conotoxin Vc1.1 Potently Reverses Mechanical Allodynia in Neuropathic Pain Models. J. Med. Chem. 2021, 64, 3222–3233. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Grundy, L.; Deiteren, A.; Harrington, A.M.; O’Donnell, T.; Maddern, J.; Moore, J.; Garcia-Caraballo, S.; Rychkov, G.Y.; Yu, R.; et al. Cyclic Analogues of A-conotoxin Vc1.1 Inhibit Colonic Nociceptors and Provide Analgesia in a Mouse Model of Chronic Abdominal Pain. Br. J. Pharmacol. 2018, 175, 2384–2398. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Zhangsun, D.; Yu, G.; Su, R.; Luo, S. The α9α10 Nicotinic Acetylcholine Receptor Antagonist AO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain. Mar. Drugs 2019, 17, 265. [Google Scholar] [CrossRef]
- Liu, C.; Wu, P.; Zhu, H.; Grieco, P.; Yu, R.; Gao, X.; Wu, G.; Wang, D.; Xu, H.; Qi, W. Rationally Designed α-Conotoxin Analogues Maintained Analgesia Activity and Weakened Side Effects. Molecules 2019, 24, 337. [Google Scholar] [CrossRef]
- Gajewiak, J.; Christensen, S.B.; Dowell, C.; Hararah, F.; Fisher, F.; Huynh, P.N.; Olivera, B.M.; McIntosh, J.M. Selective Penicillamine Substitution Enables Development of a Potent Analgesic Peptide That Acts through a Non-Opioid-Based Mechanism. J. Med. Chem. 2021, 64, 9271–9278. [Google Scholar] [CrossRef]
- Hasan, M.d.M.; Starobova, H.; Mueller, A.; Vetter, I.; Lewis, R.J. Subcutaneous ω-Conotoxins Alleviate Mechanical Pain in Rodent Models of Acute Peripheral Neuropathy. Mar. Drugs 2021, 19, 106. [Google Scholar] [CrossRef]
- Liu, X.; Yao, G.; Wang, K.; Liu, Y.; Wan, X.; Jiang, H. Structural and Functional Characterization of Conotoxins from Conus Achatinus Targeting NMDAR. Mar. Drugs 2020, 18, 135. [Google Scholar] [CrossRef]
- Zheng, N.; Christensen, S.B.; Dowell, C.; Purushottam, L.; Skalicky, J.J.; McIntosh, J.M.; Chou, D.H.-C. Discovery of Methylene Thioacetal-Incorporated α-RgIA Analogues as Potent and Stable Antagonists of the Human A9α10 Nicotinic Acetylcholine Receptor for the Treatment of Neuropathic Pain. J. Med. Chem. 2021, 64, 9513–9524. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, Y.; Chen, J.; Zhang, Y.; Tao, X.; Dai, Q.; Wang, Y.; Li, S.; Dong, M. TAT-Modified ω-Conotoxin MVIIA for Crossing the Blood-Brain Barrier. Mar. Drugs 2019, 17, 286. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.; Zolgharnein, H.; Ronagh, M.T.; Moghaddam, J.A.; Crüsemann, M. Conus Coronatus and Conus Frigidus Venom: A New Source of Conopeptides with Analgesic Activity. Avicenna J. Med. Biotechnol. 2020, 12, 179–185. [Google Scholar] [PubMed]
- Chen, J.; Liu, X.; Yu, S.; Liu, J.; Chen, R.; Zhang, Y.; Jiang, L.; Dai, Q. A Novel ω-Conotoxin Bu8 Inhibiting N-Type Voltage-Gated Calcium Channels Displays Potent Analgesic Activity. Acta Pharm. Sin. B 2021, 11, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Wu, Y.; Zhao, D.; Zhao, B.; Wang, F.; Ren, S.; Wen, Y.; Gu, J.; Zhang, L.; Liu, K.; et al. Discovery and Characterization of the Novel Conotoxin Lv1d from Conus Lividus That Presents Analgesic Activity. Toxicon 2021, 194, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Niu, J.; Wu, Y.; Di, Z.; Wang, F.; Zhang, L.; Liu, K.; Zhao, B.; Wang, L. Discovery of a Novel Cysteine Framework XXIV Conotoxin from Conus Striatus, S24a, with Potential Analgesic Activity. Int. J. Pept. Res. Ther. 2021, 27, 615–625. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Nabil, Z.I.; El-Naggar, M.S. Prospecting for Candidate Molecules from Conus Virgo Toxins to Develop New Biopharmaceuticals. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28. [Google Scholar] [CrossRef]
- Williams, J.A.; Day, M.; Heavner, J.E. Ziconotide: An Update and Review. Expert. Opin. Pharmacother. 2008, 9, 1575–1583. [Google Scholar] [CrossRef]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Administration Strategies for Proteins and Peptides. Int. J. Pharm. 2014, 477, 578–589. [Google Scholar] [CrossRef]
- Abram, S.E.; Mampilly, G.A.; Milosavljevic, D. Assessment of the Potency and Intrinsic Activity of Systemic versus Intrathecal Opioids in Rats. Anesthesiology 1997, 87, 127–134. [Google Scholar] [CrossRef]
- Mercadante, S. Problems of Long-Term Spinal Opioid Treatment in Advanced Cancer Patients. Pain 1999, 79, 1–13. [Google Scholar] [CrossRef]
- Morishita, M.; Peppas, N.A. Is the Oral Route Possible for Peptide and Protein Drug Delivery? Drug Discov. Today 2006, 11, 905–910. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The Future of Peptide-Based Drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.X.L.; Danquah, M.K. Drug and Protein Encapsulation by Emulsification: Technology Enhancement Using Foam Formulations. Chem. Eng. Technol. 2012, 35, 618–626. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Drechsler, M.; Garamus, V.M.; Lesieur, S. Protein Entrapment in PEGylated Lipid Nanoparticles. Int. J. Pharm. 2013, 454, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W. Solid Lipid Nanoparticles Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Swaminathan, J.; Ehrhardt, C. Liposomal Delivery of Proteins and Peptides. Expert. Opin. Drug Deliv. 2012, 9, 1489–1503. [Google Scholar] [CrossRef]
- Wang, Y.; Selomulya, C. Spray Drying Strategy for Encapsulation of Bioactive Peptide Powders for Food Applications. Adv. Powder Technol. 2020, 31, 409–415. [Google Scholar] [CrossRef]
- Ganesh, A.N.; Heusser, C.; Garad, S.; Sánchez-Félix, M.V. Patient-Centric Design for Peptide Delivery: Trends in Routes of Administration and Advancement in Drug Delivery Technologies. Med. Drug Discov. 2021, 9, 100079. [Google Scholar] [CrossRef]
- Zhao, Q.; Xu, W.; Xing, L.; Lin, Z. Recombinant Production of Medium- to Large-Sized Peptides in Escherichia Coli Using a Cleavable Self-Aggregating Tag. Microb. Cell Fact. 2016, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Zhang, X.; Liu, C.-F. Progress in Chemical Synthesis of Peptides and Proteins. Trans. Tianjin Univ. 2017, 23, 401–419. [Google Scholar] [CrossRef]
- Santos, L.; Oliveira, C.; Vasconcelos, B.M.; Vilela, D.; Melo, L.; Ambrósio, L.; da Silva, A.; Murback, L.; Kurissio, J.; Cavalcante, J.; et al. Good Management Practices of Venomous Snakes in Captivity to Produce Biological Venom-Based Medicines: Achieving Replicability and Contributing to Pharmaceutical Industry. J. Toxicol. Environ. Health Part B 2021, 24, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]

| Toxin/ Molecule | Species | Production | Administration | Mechanism of Analgesia | Model | Ref. |
|---|---|---|---|---|---|---|
| µ-EPTX-Na1 | Naja atra | Purified from venom | Intraplantar (i.pl.) injection | Nav1.8 channel inhibitor | Acute inflammation models induced by formalin and acetic acid, chronic inflammation pain model induced by complete Freund’s adjuvant and partial nerve ligation-induced allodynia. | [69] |
| Mambalgin-1 | Dendroaspis polylepis polylepis | Purified from venom | Intravenous (i.v.) and intratechal (i.t.) injection | Acid-sensing ion channel (ASIC) inhibitors | Motor behavior tests such as accelerated rotarod test and grip strength test. Pain and inflammation models induced by carrageenan, thermal and mechanical pain test by von Frey | [68] |
| Mambalgin-3 | Dendroaspis polylepis polylepis | Purified from venom | Intravenous (i.v.), intrathecal (i.t.), and intraplantar (i.pl.) injection | Acid-sensing ion channel (ASIC) inhibitors | Motor behavior tests such as accelerated rotarod test and grip strength test. Pain and inflammation models induced by carrageenan, thermal and mechanical pain test by von Frey | [68] |
| Cobra neurotoxin | Naja naja atra | Purified from venom | Intraperitoneal (i.p.) injection | Adenosine receptor (A1 and A2A) pathway activation | Acute pain model induced by hot plate and spinal cord injury | [74] |
| Najanalgesin | Naja naja atra | Purified from venom | Intrathecal (i.t.) injection | c-Jun N-terminal kinase (JNK) inhibitor | Neuropathic pain induced by spinal nerve ligation | [75] |
| Crotoxin (CTX) | Crotalus durissus terrificus | Purified from venom | Subcutaneous injection (s.c.) | Formyl Peptide, α2-Adrenergic and Muscarinic Receptors | Acute and chronic phases of hypernociception induced by partial sciatic nerve ligation | [70] |
| Crotoxin (CTX) | Crotalus durissus terrificus | Purified from venom | Subcutaneous injection (s.c.) | Analgesia dependent on formyl peptide, lipoxygenase and muscarinic receptors | Pain on the MOG 35-55-induced experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis | [71] |
| Crotalphine | Crotalus durissus terrificus | Chemical synthesis | Oral administration (p.o.) | TRPA1 | The ciguatoxin-induced cold hypersensitivity, and the bradykinin-induced and zymosan-induced mechanical hypersensitivity | [73] |
| Cobratoxin (CbTX) | Naja naja kaouthia | Purified from venom | Intrathecal (i.t.) injection | α7 nicotinic acetylcholine receptor (nAChRs) | Acute pain model induced by hot plate and tail-flick | [76] |
| Toxin/ Molecule | Species | Production | Administration | Mechanism of Analgesia | Model | Ref. |
|---|---|---|---|---|---|---|
| rAGAP | Buthus martensii | Recombinant | Injection application location not specified | CHis6-rAGAP and NHis6-rAGAP | Xenograft tumor mouse model | [78] |
| AGAP W38G | Buthus martensii | Purified from venom | Intraplantar (i.pl.) injection | Nav1.7 and Nav1.8 channel inhibitor | An acute thermal pain model induced by a hot plate and an acute inflammation model induced by formalin | [79] |
| BmK AGP-SYPU1 | Buthus martensii | Recombinant | Intraperitoneal (i.p.) injection | Arginine residue at position 58 | Mouse-twisting pain model | [85] |
| BmK AGAP | Buthus martensii | Recombinant | Intrathecal (i.t.) injection | MAPK inhibitor | An acute inflammation model induced by formalin, a thermal pain model induced by hot plate, and mechanical allodynia | [80] |
| BotAF | Buthus occitanus tunetanus | Purified from venom | Intraperitoneal (i.p.), intrathecal (i.t.) and intraplantar (i.pl.) injections | Ion channel inhibitor | An acute thermal pain model induced by a hot plate, a nociception model induced by a shutter-controlled lamp, and an inflammation model induced by formalin and acetic acid writhing | [81] |
| BmK AGAP | Buthus martensii | Purified from venom | Intraplantar (i.pl.) injection | Kv1.3 channel and MAPK inhibitor | An acute inflammation model induced by formalin | [82] |
| BmK AGAP | Buthus martensii | Recombinant | Intraperitoneal (i.p.) injection | MCF-7 and MDA-MB-231 migration inhibitor | Xenograft tumor mouse model | [77] |
| Makatoxin-3 (MkTxs) | Buthus martensii | Purified from venom | Intraperitoneal (i.p.) injection | Nav1.7 inhibitor | Acute nociception induced by formalin test and Freund’s adjuvant (CFA) induced mechanical pain model | [86] |
| TsNTxP | Tityus serrulatus | - | Intraperitoneal (i.p.) injection | Nav channel inhibitor | Acute nociception induced by a water bath and neuropathic pain model induced by CCI model | [83] |
| Leptucin | Hemiscorpius lepturus | Chemical synthesis | Intraperitoneal (i.p.) injection | Ion channel inhibitor | Acute thermal pain model induced by hot plate and nociception model using tail flick test | [84] |
| Toxin/ Molecule | Species | Production | Administration | Mechanism of Analgesia | Model | Ref. |
|---|---|---|---|---|---|---|
| Im10A (conotoxin) | Conus imperialis | Synthesized | Intramuscular injection (i.m.) | - | Rat PNL model | [131] |
| μ-Conotoxin TsIIIA | Conus tessulatus | Synthesized | Intrathecal (i.t.) injection | Inhibition of TTX (tetrodotoxin)-resistant sodium currents in DRG neurons | Hot-plate model | [132] |
| α-Conopeptide Eu1.6 | Conus eburneus | Synthesized | Intramuscular injection (i.m.) and intravenous (i.v.) injection | Inhibition of high voltage-activated N-type calcium channel currents (Cav2.2) in isolated mouse dorsal root ganglia (DRG) neurons | Rat partial sciatic nerve injury (PNL model) and chronic constriction injury pain (CCI model) models | [102] |
| ω-Conotoxins: MoVIA and MoVIB | Conus Moncuri | Purified from venom and synthesized | Intrathecal (i.t.) injection | Inhibition of rat Cav2.2 channels | PNL-induced neuropathic pain | [134] |
| α-Conotoxin Vc1.1 Variants | - | Synthesized | Intramuscular (im.i), injection | Inhibition of G protein-coupled γ-aminobutyric acid type B receptors (GABABR) coupled Cav2.2 channels in rat DRG neurons | PNL and CCI models | [135] |
| cVc1.1 and cVc1.1 analogues: [C2H, C8F]cVc1.1 and [N9W]cVc1.1 | - | Synthesized | Intra-colonic administration | Reduction in the excitability of DRG neurons | Chronic visceral hypersensitivity (CVH model) | [142] |
| ω -Conotoxin MVIIA modified | - | Synthesized | Intravenously (i.v.) injection and intranasally injection | - | Hot-Plate model | [144] |
| αO-Conotoxin GeXIVA | Conus generalis | Synthesized | Intramuscular (im.i), injection | - | Oxaliplatin-induced neuropathic pain, cold and mechanical allodynia | [137] |
| Partially purified conotoxins (C1-C7) of C. coronatus and C. frigidus (F1-F6). | Conus coronatus and Conus frigidus | Purified from Venom | Intraperitoneal (i.p.) injection | Only C2 had analgesic effects in both tested models; mechanism of analgesia: not studied | Hot-plate model and formalin-induced pain | [145] |
| BuIA conotoxin analogues | Conus bullatus | Synthesized | Intraperitoneal (i.p.) injection | All the analogs showed the same analgesic activity of BuIA Mechanism of analgesia: not studied | Hot-plate model and paclitaxel-induced neuropathic pain | [138] |
| ω-conotoxin Bu8 | Conus bullatus | Synthesized | Intraperitoneal (i.p.) injection | Inhibition of Cav2.2 | Hot-plate model and analgesic activity to acute pain and inflammatory pain | [146] |
| Conotoxin-Ac1// Conotoxin-Ac1-O6P (variant) | Conus achatinus | Synthesized | Intrathecal (i.t.) injection | Inhibition of NR2B ion channels | Hot-plate and tail-flick models | [141] |
| α-Conotoxin Vc1.1 modified | Conus victoriae | Purified from venom and modified | Intramuscular (im.i), injection | Activation of GABABRs expressed in DRG | Spinal nerve ligation-induced neuropathic pain (SNL model), mechanical allodynia | [136] |
| α4/7-Conotoxin (Lv1d) | Conus lividus | Synthesized | Intrathecal (i.t.) injection (mouse hot plate model) Intramedullary injection (formalin-induced pain model) | - | Mouse hot-plate and formalin-induced pain models | [147] |
| µ-Conotoxin S24a | Conus striatus | Synthesized | Intrathecal (i.t.) injection | - | Mouse hot-plate and formalin-induced pain models | [148] |
| ω-Conotoxin | Conus virgo | Purified from venom and modified | Intraperitoneal (i.p.) injection | - | Central analgesic assay (tail immersion test) and Peripheral analgesic assay (acetic acid-induced writhing test) | [149] |
| α-CTx RgIA analogues (RgIA-5524) | - | Synthesized | Subcutaneous (i.s.) injection | Inhibition of α9-containing nicotinic acetylcholine receptors (nAChRs) | Oxaliplatin-induced neuropathic pain | [143] |
| α-Conotoxin RgIA analogues (RgIA-5474) | - | Synthesized | Subcutaneous (i.s.) injection | - | Oxaliplatin-induced neuropathic pain, cold allodynia | [139] |
| ω–Conotoxins: MVIIA, GVIA and CVIF | - | Obtained from Alomone Labs (Jerusalem, Israel). | Intraplantar (i.pl.). injection | - | Post-surgical pain (PSP model) and cisplatin-induced pain neuropathy and oxaliplatin-induced neuropathic pain | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.F.M.; Cavalcante, J.S.; Angstmam, D.G.; Almeida, C.; Soares, G.S.; Pucca, M.B.; Ferreira Junior, R.S. Unveiling the Pain Relief Potential: Harnessing Analgesic Peptides from Animal Venoms. Pharmaceutics 2023, 15, 2766. https://doi.org/10.3390/pharmaceutics15122766
Pereira AFM, Cavalcante JS, Angstmam DG, Almeida C, Soares GS, Pucca MB, Ferreira Junior RS. Unveiling the Pain Relief Potential: Harnessing Analgesic Peptides from Animal Venoms. Pharmaceutics. 2023; 15(12):2766. https://doi.org/10.3390/pharmaceutics15122766
Chicago/Turabian StylePereira, Ana Flávia Marques, Joeliton S. Cavalcante, Davi Gomes Angstmam, Cayo Almeida, Gean S. Soares, Manuela B. Pucca, and Rui Seabra Ferreira Junior. 2023. "Unveiling the Pain Relief Potential: Harnessing Analgesic Peptides from Animal Venoms" Pharmaceutics 15, no. 12: 2766. https://doi.org/10.3390/pharmaceutics15122766
APA StylePereira, A. F. M., Cavalcante, J. S., Angstmam, D. G., Almeida, C., Soares, G. S., Pucca, M. B., & Ferreira Junior, R. S. (2023). Unveiling the Pain Relief Potential: Harnessing Analgesic Peptides from Animal Venoms. Pharmaceutics, 15(12), 2766. https://doi.org/10.3390/pharmaceutics15122766









