Inhaled Medicines for Targeting Non-Small Cell Lung Cancer
Abstract
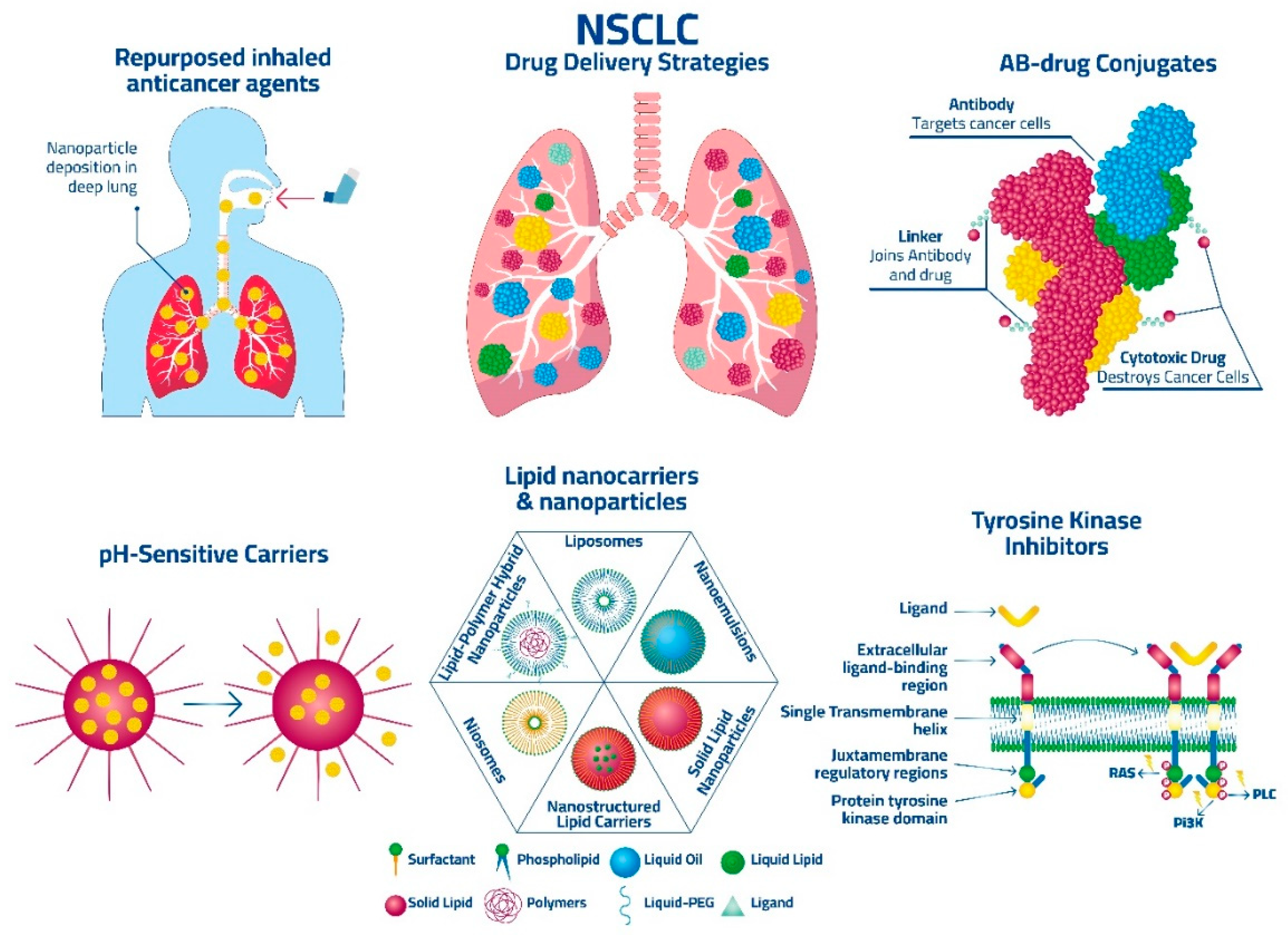
:1. Introduction
2. Lung Cancer, Etiology, and Current Practice
3. Advantages of Direct Delivery to the Lungs to Target Lung Cancer
4. Lung Surfactants and Impact on Drug Deposition
- Diffusion limitation: The surfactant layer might act as a barrier, hindering drugs from reaching deeper tissues. This can lead to reduced drug concentrations in the lungs and diminished treatment effectiveness.
- Extended residence time: The surfactant layer can prolong the duration of time that the drugs linger in the lungs. This is beneficial for drugs that are rapidly expelled, as it allows for extended exposure to lung tissue, which enhances their potency.
- Drug particle dimensions: The size of the drug particles affects their deposition depth in the lungs. Larger particles might be trapped atop the surfactant layer, which limits their penetration, while smaller particles might navigate past the layer to access the deeper lung regions.
5. Mucociliary Clearance and Mucoadhesion, Getting the Balance Right
6. Methods and Devices for Inhalation
7. Assessment of Drug Deposition, Current Methods, and Accuracy for Assessment of Deposition of Anticancer Agents
8. Effect of Particle Size and Shape on Their Lung Deposition
9. Challenges for the Delivery of Inhaled Chemotherapy
9.1. Uniform Drug Deposition
9.2. Patient Variability
9.3. Device Design and Performance
9.4. Disease-Specific Challenges
9.5. Toxicity and Side Effects
9.6. Drug Stability
10. Formulation of Anticancer Agents Using Carrier Free Technology
- Self-assembly of a singular anti-cancer drug.
- Self-assembly of multiple anti-cancer drugs.
11. Strategies for Drug Targeting of NSCLC
11.1. Active Targeting for NSCLC in Inhaled Therapies
11.1.1. Immune Checkpoint Inhibitors (ICIs)
11.1.2. Tyrosine Kinase Inhibitors (TKIs)
11.1.3. Antibody-Drug Conjugates (ADCs)
11.1.4. Personalized Inhaled Medicine Approaches
11.2. Passive Targeting for NSCLC in Inhaled Therapies
11.2.1. Use of Amorphous Solid Dispersions
11.2.2. Use of Nanoparticle Delivery Systems
11.2.3. Lipid-Based Nanoparticle Delivery Systems (LNPs)
11.2.4. Liposomes
11.2.5. Solid Lipid Nanoparticles (SLNs)
11.2.6. Nanostructured Lipid Carriers (NLCs)
11.2.7. Lipid-Polymer Hybrid Nanoparticles (LPHN)
11.2.8. Polymeric Nanoparticle Delivery Systems
11.2.9. Gold Nanoparticle Delivery Systems
11.2.10. Use of pH-Sensitive Drug Delivery Systems
| API Type | Formulation Type | Delivery Method | Reference |
|---|---|---|---|
| Azacitidine | Solution | Aerosol, Nebulizer | [134] |
| Azacitidine | Solution/Dry powder | Aerosol, Nebulizer/Dry powder, nose-only inhalation | [135] |
| Bevacizumab | Dry powder | Aerosol, nasal inhalation | [136] |
| 2-ME 2-methoxyestradiol | Nanocomposites and nanoaggregates | Intratrchial insufflation | [137] |
| 5-Fluorouracil | Lipid coated nanoparticles | Inhalation Aerosol, Nebulizer | [138,139] |
| Epirubicin | Solid Lipid Nanoparticles (SLNs) | Inhalation Aerosol, Nebulizer | [123] |
| 9-Nitrocampthotecin | Liposome | Inhalation Aerosol, Nebulizer | [140,141,142] |
| Afatinib & paclitaxel | Lipid-based nanocarriers | In vitro: Turbospin (single dose powder inhaler device). In vivo: Dry powder insufflator—Lipid-based nanocarriers | [129,143] |
| Camptothecin | Aerosolized liposomal Camptothecin | Inhalation Aerosol, Nebulizer | [144] |
| Carboplatin | Solution | Inhalation Aerosol, Nebulizer | [145] |
| Doxorubicin Celecoxib | Liposomes, EGF-modified gelatine nanoparticles, PLGA microparticles, drug conjugates | Inhalation, Aerosol, pMDI | [114,146,147,148,149,150,151] |
| Lipid-based nanocarriers | Inexpose™ nebulizer Nanolipidcarriers | [152] | |
| Celecoxib & Docetaxel | Solution | Inhalation Aerosol, MDI | [153,154] |
| Cisplatin | SLIT: Lipid vesicles | Inhalation Aerosol, nebulizer | [155] |
| Cisplatin loaded EGF-modified GP | Gelatin nanoparticle | Endotrachial installation | [156] |
| CpG & Poly I:C | Liposomal formulations | Intratracheal instillation | [157] |
| Curcumin | Nanocomposites and nanoaggregates | In vitro: Aerosol using cascade impactors | [158] |
| Liposomal formulations | Intratracheal instillation | [159] | |
| Liposomal formulations | IntratrachialInsufflator | [117] | |
| Curcuminoids | Lipid-based nanocarriers | Aerosol, Side stream jet nebulizer | [160] |
| Methotroxate | HFA-based Microparticles | Aerosol, Metered Dose Inhaler | [161] |
| Docetaxel | Liposomal formulations | Intratracheal administration | [116] |
| Docetaxel | Lipid-based nanoemulsion | OMRON MicroAIR nebulize | [162] |
| Docetaxel | Nanoparticles | DPI | [125] |
| Docetaxel and Curcumin | Nanoemulsion | In vitro: Aerosol, Anderson cascade impactor | [163] |
| Doxorubicin | Poly(butyl cyanoacrylate) nanoparticles | Aerosol, DPI | [164] |
| Effervescent nanoparticles | Intratrachial insufflator | [165] | |
| Highly porus large PLGA microparticles | Inhalation | [28,133,144,166,167,168,169,170] | |
| Liposomal formulations | Intratracheal administration using microsprayer | [171] | |
| Solution (0.4 to 9.4 mg/m2) | Aerosol, Nebulizer | [172] | |
| Liposomal formulations | Aerosol, One-jet Collison nebulizer | [173] | |
| Doxorubicin & ASO, or siRNA | LHRH receptor-targeted mesoporous silica nanoparticles | Inhalation | [114,132] |
| Doxorubicin and paclitaxel | Lipid-based nanocarriers | Collision nebulizer connected to nose-only exposure chamber | [115,126] |
| Epirubicin | Solid lipid nanoparticles | Aerosol, nebulizer | [123] |
| Erlotinib | Microparticles | Aerosol, DPI | [120] |
| Gefitinib | Lipid-based nanocarriers | Intratracheal installation | [174] |
| Cisplatin | EGF-modified Gelatin Nanoparticles (LNPs) | Aerosol, nebulizer | [27] |
| Gemcitabine & cisplatin | Niosomes | Aerosol, nebulizer | [175] |
| Gemcitabine | Solution | Aerosol, nebulizer | [176,177,178] |
| Retinoic acid and and genistein | Nanoparticles | DPI | [127] |
| Gemcitabine-HCl | Liposomal formulations | Intratracheal/Insufflator (Aerosol) | [179] |
| HC & 5-Amino levulinic acid | Cationic Liposomal nanoparticles | Endotrachial installation | [180] |
| Hyaluronan (HA)-cisplatin conjugates | Drug conjugates | Endotrachial installation | [181] |
| IL-2 | Liposomes | Aerosol, nebulizer | [181] |
| Doxorubicin | Solution | Aerosol, nebulizer | [113] |
| Cyclosporin A and paclitaxel | Liposomes | Aerosol nebulizer | [182] |
| Doxorubicin | Self-assembled albumin nanoparticles | Aerosol, nebulizer | [113] |
| Anti-carbonic anhydrase IX (CA IX) antibody, conjugated to the surface of triptolide (TPL) | Liposomes | Aerosol | [118] |
| 9-Bromo-noscapine | Lipid-based nanocarriers | Inhalation by indigenously developed apparatus | [183] |
| Myricetin | Nanoencapsulated Phospholipid Complex | In vitro: Aerosol using Aerolizer connected to Anderson Cascade Impigner | [184] |
| Silibinin | Nanoparticles | Inhalation, DPI | [128] |
| Nitro-camptothecin | Liposomes | Aerosol, nebulizer | [185,186,187] |
| Oridonin | DLPC liposome | Aerosol jet nebulizer | [188] |
| Paclitaxel | Liposomes | Aerosol, nebulizer | [168] |
| Lung surfactant mimetic and pH-responsive lipid nanovesicles | Inhalation | [139] | |
| Chitosan-coated folate-PEG nanoparticles | Endotracheal administration, Micro Sprayer Aerosolizer IA-1C | [119] | |
| Lipid-based nanocarriers | DP insufflator | [189] | |
| Lipid-based nanocarriers | Aerosol, Collison nebulizer | [190] | |
| SLN- solid lipid nanoparticle | DPI- dry powder inhaler | [122] | |
| Phospho-sulindac | Liposome | Aerosol, nebulizer | [191] |
| Quercetin | Lipid-based nanocarriers | OMRON MicroAIR nebulizer | [192,193,194] |
| Naringenin | SLN | Intratracheal instillation | [124] |
| Resveratrol | Nanocomposites and nanoaggregates | Inhalation | [195] |
| siRNA | DOTAP-modified PLGA nanoparticles | In vitro: aerosol generated by small scale powder disperser | [196] |
| Sorafenib Tosylate | Liposomal formulations | In vitro: DPI, Revolizer device | [197] |
| TAS-103 | PLGA Nanocomposites and nanoaggregates | DPI, insufflator | [198] |
| Temozolomide | Liposomal formulations | Intratracheal administration using Microsprayer IA-1C system | [199] |
| Nanocomposites and nanoaggregates | In vitro: DPI, AxahalerTM | [200] | |
| Liposomal formulations | Micro sprayer Aerosolizer Pulmonary Aerosol Kit for Mouse Model PAK-MSA | [201] | |
| Amodiaquine | Nanoparticles | Inhalation, aerosol | [202] |
| Pioglitazone | Powder | Inhalation, aerosol | [203] |
| Telmisartan | Nanoparticles | Intratumoral distribution | [204] |
| Amodiaquine | Inhalable nanoparticulate system | Inhalation, nebulizer | [202] |
| Bexarotene (Targretin) & budesonide | Powders | Inhalation, aerosol, turbuhaler | [205,206,207] |
| Itraconazole | Dry powder for inhalation | In vitro: Cyclohaler™ Dry Powder Inhaler, twin stage impigner apparatus. | [208] |
| Dry powder for inhalation | Inhalation Aerosol, DPI | [209] | |
| Solid dispersion | Inhalation Aerosol, DPI | [210] | |
| Fisetin | Dry powder | Aerosol, DPI | [211] |
| Isotretinoin | Powder | Aerosol | [212] |
| Metformin | Sterosomes | Aerosol, nebulizer | [213] |
| Pirfenidone | Liposome | Microsprayer® Aerosolizer Pulmonary Aerosol-Kit for Mouse | [214] |
12. Use of Repurposed Inhaled Anticancer Agents for Targeting NSCLC
| Drug | Phase | Device, Formulation | Patient Status (n) | Deposition in the Lung | Local Dose Limiting Toxicity | Severe Systemic Toxicity | Disease Response (n) | Reference |
|---|---|---|---|---|---|---|---|---|
| Azacitidine | Phase 1/2 | Aerosol, Nebulizer/Dry powder, nose-only inhalation | Local and metastatic lung cancer | Direct deposition into the bronchi and lung | No pulmonary toxicity | Pale skin, shortness of breath, fast heartbeat, chest pain, cough, unusual bruising or bleeding | (29%) of patients had stable disease with one partial, and one complete response | [134,135] |
| 5-FU | Pilot | Wave nebulizer, iv solution | Lung cancer [222], lung metastasis [223] | 5–15 times more concentrated in tumor compared to lung tissues | None | None | Complete response [142], Partial response [219], progressive disease [219] | [172] |
| 9-nitro-camptothecin | I | Jet nebulizer, liposome dispersion | Lung cancer and lung metastases [70] | 4–10 time more concentrated in the bronchoalveolar lavage compared to serum | Grade 2: cough, bronchial irritation Grade 3: Chemical pharyngitis | Grade 2: nausea, vomiting, anaemia, neutropenia | Partial response [155], stable disease [155] | [142] |
| Cisplatin | I | Jet nebulizer, liposome dispersion | Advanced NSCLC [61], SCLC [172] | 10–15% (radiolabelled solution) | Grade 3: Bronchitis, dyspnea, decreased lung function | Grade 3: Fatigue Grade 4: thrombosis | Stable disease [225], progressive disease [219] | [155] |
| Ib/IIa | Osteosarcoma with lung metastases [64] | N/A | Grade 2: Hoarseness | Grade 3: Nausea, vomiting | Partial response [172], stable disease [178], progressive disease [145] | [219] | ||
| Doxorubicin | I | Jet nebulizer, solution | Lung metastases: [221] sarcoma [64], Osteosarcoma [219], NSCLC [61], colorectal [221], thyroid [155], Miscellaneous [221] | Correct deposition in the lung (radiolabelled solution) | Grade 2: Cough, wheezing, dyspnea Grade 3: Hypoxia Grade 4: Respiratory distress, dyspnea | No Grade 3/4 toxicity observed | Partial response [172], stable disease [145], progressive disease [142] | [221] |
| Doxorubicin (inhaled) + cisplatin (iv) and docetaxel (iv) | I/II | Jet nebulizer, solution | Advanced NSCLC [227] | N/A | Grade 3–4: Cough, decrease in pulmonary function test | Grade 3–4: Constipation, hyponatremia, neutropenia | No significant improve in survival, most patients had stable disease | [221] |
| Gemcitabine | I | Mesh nebulizer, iv solution | NSCLC [224] | 42 ± 16% (homogenous deposition) | Grade 2–3: Cough Grade 4: bronchospasm | Grade 3: Fatigue, vomiting | Minor response [172], stable disease [219], progressive disease [219] | [178] |
| Carboplatin | I/II | Jet nebulizer, solution | NSCLC [145] | Deposition in lung parenchyma (radiolabelled solution) | Grade 2–3 Cough Grade 3: Dyspnea, hoarseness | Grade 3: Fatigue, alopecia, rash, anorexia, anemia, neutropenia, pharyngitis, mucositis Grade 4: anorexia, neutropenia | No significant improve in survival, most patients had progressive disease | [145] |
13. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Person, A.; Mintz, M.L. Anatomy and Physiology of the Respiratory Tract. In Disorders of the Respiratory Tract: Common Challenges in Primary Care; Mintz, M.L., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 11–15. [Google Scholar]
- Kenney, W.L.; Wilmore, J.H.; Costill, D.L. Physiology of Sport and Exercise; Human Kinetics: Champaign, IL, USA, 2021. [Google Scholar]
- Rocco, D.; Della Gravara, L.; Ragone, A.; Sapio, L.; Naviglio, S.; Gridelli, C. Prognostic Factors in Advanced Non-Small Cell Lung Cancer Patients Treated with Immunotherapy. Cancers 2023, 15, 4684. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.; Sapio, L.; Della Gravara, L.; Naviglio, S.; Gridelli, C. Treatment of Advanced Non-Small Cell Lung Cancer with RET Fusions: Reality and Hopes. Int. J. Mol. Sci. 2023, 24, 2433. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Olivo, M.A.; Minnix, J.A.; Fox, J.G.; Nishi, S.P.E.; Lowenstein, L.M.; Maki, K.G.; Leal, V.B.; Tina Shih, Y.C.; Cinciripini, P.M.; Volk, R.J. Smoking cessation and shared decision-making practices about lung cancer screening among primary care providers. Cancer Med. 2021, 10, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Heijnen, M.L.; Houterman, S.; Lemmens, V.E.; Louwman, M.W.; Maas, H.A.; Coebergh, J.W. Prognostic impact of increasing age and co-morbidity in cancer patients: A population-based approach. Crit. Rev. Oncol. Hematol. 2005, 55, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.L.; Christiani, D.C. Genetic susceptibility to lung cancer--light at the end of the tunnel? Carcinogenesis 2013, 34, 487–502. [Google Scholar] [CrossRef]
- Jones, R.N.; Hughes, J.M.; Weill, H. Asbestos exposure, asbestosis, and asbestos-attributable lung cancer. Thorax 1996, 51 (Suppl. S2), S9–S15. [Google Scholar] [CrossRef]
- Alberg, A.J.; Samet, J.M. Epidemiology of lung cancer. Chest 2003, 123, 21S–49S. [Google Scholar] [CrossRef]
- Kirk, G.D.; Merlo, C.; O’Driscoll, P.; Mehta, S.H.; Galai, N.; Vlahov, D.; Samet, J.; Engels, E.A. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin. Infect. Dis. 2007, 45, 103–110. [Google Scholar] [CrossRef]
- Hubbard, R.; Venn, A.; Lewis, S.; Britton, J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am. J. Respir. Crit. Care Med. 2000, 161, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288. [Google Scholar] [CrossRef] [PubMed]
- Lavacchi, D.; Mazzoni, F.; Giaccone, G. Clinical evaluation of dacomitinib for the treatment of metastatic non-small cell lung cancer (NSCLC): Current perspectives. Drug Des. Dev. Ther. 2019, 13, 3187–3198. [Google Scholar] [CrossRef]
- Robelin, P.; Hadoux, J.; Forestier, J.; Planchard, D.; Hervieu, V.; Berdelou, A.; Scoazec, J.-Y.; Valette, P.-J.; Leboulleux, S.; Ducreux, M. Characterization, prognosis, and treatment of patients with metastatic lung carcinoid tumors. J. Thorac. Oncol. 2019, 14, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Forskolin affects proliferation, migration and Paclitaxel-mediated cytotoxicity in non-small-cell lung cancer cell lines via adenylyl cyclase/cAMP axis. Eur. J. Cell Biol. 2023, 102, 151292. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Wang, R.; Chen, E.; Yang, Y.; Fan, B.; Li, Y.; Han, B.; Li, Q.; Liu, Z.; Han, Y.; et al. A forskolin-loaded nanodelivery system prevents noise-induced hearing loss. J. Control. Release 2022, 348, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Discher, D.E. Lung vascular targeting through inhalation delivery: Insight from filamentous viruses and other shapes. IUBMB Life 2011, 63, 607–612. [Google Scholar] [CrossRef]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef]
- Kaialy, W.; Nokhodchi, A. Particle engineering for improved pulmonary drug delivery through dry powder inhalers. In Pulmonary Drug Delivery: Advances and Challenges; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 171–198. [Google Scholar]
- Liu, W.K.; Liu, Q.; Chen, D.H.; Liang, H.X.; Chen, X.K.; Chen, M.X.; Qiu, S.Y.; Yang, Z.Y.; Zhou, R. Epidemiology of acute respiratory infections in children in Guangzhou: A three-year study. PLoS ONE 2014, 9, e96674. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Darwiche, K.; Krauss, L.; Huang, H.; Zachariadis, G.A.; Katsavou, A.; Hohenforst-Schmidt, W.; Papaiwannou, A.; Vogl, T.J.; Freitag, L.; et al. Inhaled cisplatin deposition and distribution in lymph nodes in stage II lung cancer patients. Future Oncol. 2013, 9, 1307–1313. [Google Scholar] [CrossRef]
- Jinturkar, K.A.; Anish, C.; Kumar, M.K.; Bagchi, T.; Panda, A.K.; Misra, A.R. Liposomal formulations of Etoposide and Docetaxel for p53 mediated enhanced cytotoxicity in lung cancer cell lines. Biomaterials 2012, 33, 2492–2507. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-L.; Su, W.-Y.; Yen, K.-C.; Yang, K.-C.; Lin, F.-H. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 2009, 30, 3476–3485. [Google Scholar] [CrossRef] [PubMed]
- Videira, M.; Almeida, A.J.; Fabra, À. Preclinical evaluation of a pulmonary delivered paclitaxel-loaded lipid nanocarrier antitumor effect. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Kandala, B.; Hochhaus, G. Pharmacometrics in pulmonary diseases. In Applied Pharmacometrics; Springer: New York, NY, USA, 2014; pp. 349–382. [Google Scholar]
- Zasadzinski, J.A.; Ding, J.; Warriner, H.E.; Bringezu, F.; Waring, A.J. The physics and physiology of lung surfactants. Curr. Opin. Colloid Interface Sci. 2001, 6, 506–513. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takei, T.; Aiba, T.; Masuda, K.; Kiuchi, A.; Fujiwara, T. Development of synthetic lung surfactants. J. Lipid Res. 1988, 27, 475–485. [Google Scholar] [CrossRef]
- Hickey, A.J. Lung deposition and clearance of pharmaceutical aerosols: What can be learned from inhalation toxicology and industrial hygiene? Aerosol Sci. Technol. 1993, 18, 290–304. [Google Scholar] [CrossRef]
- Wnek, G.; Bowlin, G. Lung Surfactants. In Encyclopedia of Biomaterials and Biomedical Engineering; Notter, R.H., Wang, Z., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 1715–1726. [Google Scholar]
- Pardeshi, C.V.; Kulkarni, A.D.; Sonawane, R.O.; Belgamwar, V.S.; Chaudhari, P.J.; Surana, S.J. Mucoadhesive nanoparticles: A roadmap to encounter the challenge of rapid nasal mucociliary clearance. Indian J. Pharm. Educ. Res. 2019, 53, S17–S27. [Google Scholar] [CrossRef]
- Sakagami, M.; Sakon, K.; Kinoshita, W.; Makino, Y. Enhanced pulmonary absorption following aerosol administration of mucoadhesive powder microspheres. J. Control. Release 2001, 77, 117–129. [Google Scholar] [CrossRef]
- Henning, A.; Schneider, M.; Nafee, N.; Muijs, L.; Rytting, E.; Wang, X.; Kissel, T.; Grafahrend, D.; Klee, D.; Lehr, C.-M. Influence of particle size and material properties on mucociliary clearance from the airways. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Ugwoke, M.I.; Verbeke, N.; Kinget, R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J. Pharm. Pharmacol. 2001, 53, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Jha, N.K.; Gupta, P.K.; Gupta, G.; Chellappan, D.K.; Zacconi, F.; Pinto, T.d.J.A. Targeting mucus barrier in respiratory diseases by chemically modified advanced delivery systems. Chem. Biol. Interact. 2022, 365, 110048. [Google Scholar] [CrossRef] [PubMed]
- Gatti, T.H.H.; Eloy, J.O.; Ferreira, L.M.B.; Silva, I.C.D.; Pavan, F.R.; Gremião, M.P.D.; Chorilli, M. Insulin-loaded polymeric mucoadhesive nanoparticles: Development, characterization and cytotoxicity evaluation. Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- Zaki, N.M.; Awad, G.A.; Mortada, N.D.; Abd ElHady, S.S. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Kaur, V.P.; Singh, A.; Singh, C. Recent advances on drug delivery applications of mucopenetrative/mucoadhesive particles: A review. J. Drug Deliv. Sci. Technol. 2022, 75, 103712. [Google Scholar] [CrossRef]
- Marttin, E.; Schipper, N.G.; Verhoef, J.C.; Merkus, F.W. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef] [PubMed]
- Bhise, S.B.; Yadav, A.V.; Avachat, A.M.; Malayandi, R. Bioavailability of intranasal drug delivery system. Asian J. Pharm. (AJP) 2008, 2, 201. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Barbi, M.S.; Sarmento, V.H.V.; Chiavacci, L.A.; Netto, F.M.; Gremião, M.P. Surfactant systems for nasal zidovudine delivery: Structural, rheological and mucoadhesive properties. J. Pharm. Pharmacol. 2010, 62, 430–439. [Google Scholar] [CrossRef]
- Nelson, H.S. Inhalation devices, delivery systems, and patient technique. Ann. Allergy Asthma Immunol. 2016, 117, 606–612. [Google Scholar] [CrossRef]
- Scichilone, N.; Benfante, A.; Bocchino, M.; Braido, F.; Paggiaro, P.; Papi, A.; Santus, P.; Sanduzzi, A. Which factors affect the choice of the inhaler in chronic obstructive respiratory diseases? Pulm. Pharmacol. Ther. 2015, 31, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F. The challenge of delivering therapeutic aerosols to asthma patients. Int. Sch. Res. Not. 2013, 2013, 102418. [Google Scholar] [CrossRef] [PubMed]
- Scheuch, G.; Bennett, W.; Borgström, L.; Clark, A.; Dalby, R.; Dolovich, M.; Fleming, J.; Gehr, P.; Gonda, I.; O’Callaghan, C. Deposition, imaging, and clearance: What remains to be done? J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, S-39–S-57. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.C.; Carvalho, S.R.; McConville, J.T. Formulations for pulmonary administration of anticancer agents to treat lung malignancies. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Heyder, J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc. Am. Thorac. Soc. 2004, 1, 315–320. [Google Scholar] [CrossRef]
- Gonda, I. Systemic delivery of drugs to humans via inhalation. J. Aerosol Med. 2006, 19, 47–53. [Google Scholar] [CrossRef]
- Snell, N.J.C.; Ganderton, D. Assessing lung deposition of inhaled medications. Consensus statement from a workshop of the British Association for Lung Research, held at the Institute of Biology, London, UK on 17 April 1998. eds. Respir. Med. 1999, 93, 123–133, Erratum in Respir. Med. 2000, 94, 918–919. [Google Scholar] [CrossRef]
- Sakagami, M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv. Drug Deliv. Rev. 2006, 58, 1030–1060. [Google Scholar] [CrossRef]
- Kabil, M.F.; Nasr, M.; Ibrahim, I.T.; Hassan, Y.A.; El-Sherbiny, I.M. New repurposed rolapitant in nanovesicular systems for lung cancer treatment: Development, in-vitro assessment and in-vivo biodistribution study. Eur. J. Pharm. Sci. 2022, 171, 106119. [Google Scholar] [CrossRef]
- Koullapis, P.; Kassinos, S.C.; Muela, J.; Perez-Segarra, C.; Rigola, J.; Lehmkuhl, O.; Cui, Y.; Sommerfeld, M.; Elcner, J.; Jicha, M. Regional aerosol deposition in the human airways: The SimInhale benchmark case and a critical assessment of in silico methods. Eur. J. Pharm. Sci. 2018, 113, 77–94. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Zhang, Z. Airflow and Particle Transport in the Human Respiratory System. Annu. Rev. Fluid Mech. 2010, 42, 301–334. [Google Scholar] [CrossRef]
- Pitcairn, G.; Newman, S. Radiolabelling of dry powder formulations. In Respiratory Drug Delivery VI; Interpharm Press: Buffalo Grove, IL, USA, 1998; Volume 397399. [Google Scholar]
- Newman, S.P.; Chan, H.-K. In vitro-in vivo correlations (IVIVCs) of deposition for drugs given by oral inhalation. Adv. Drug Deliv. Rev. 2020, 167, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Dolovich, M.; Nahmias, C.; Coates, G. Unleashing the PET: 3D imaging of the lung. In Respiratory Drug Delivery VII Niological, Pharmaceutical, Clinical and Regulatory Issues Relating to Optimized Drug Delivery by Aerosol; Serentec Press, Inc.: Raleigh, NC, USA, 2000; pp. 215–230. [Google Scholar]
- Yeh, H.; Phalen, R.; Raabe, O. Factors influencing the deposition of inhaled particles. Environ. Health Perspect. 1976, 15, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Wong, J.; Qu, L.; Chan, H.-K.; Zhou, Q.T. Powder production and particle engineering for dry powder inhaler formulations. Curr. Pharm. Des. 2015, 21, 3902–3916. [Google Scholar] [CrossRef] [PubMed]
- Telko, M.J.; Hickey, A.J. Dry powder inhaler formulation. Respir. Care 2005, 50, 1209–1227. [Google Scholar] [PubMed]
- Davies, C.; Muir, D. Deposition of inhaled particles in human lungs. Nature 1966, 211, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Martonen, T.B.; Katz, I.M. Deposition patterns of aerosolized drugs within human lungs: Effects of ventilatory parameters. Pharm. Res. 1993, 10, 871–878. [Google Scholar] [CrossRef]
- Sturm, R.; Hofmann, W. A theoretical approach to the deposition and clearance of fibers with variable size in the human respiratory tract. J. Hazard. Mater. 2009, 170, 210–218. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Schum, G. Models of human lung airways and their application to inhaled particle deposition. Bull. Math. Biol. 1980, 42, 461–480. [Google Scholar] [CrossRef]
- Stahlhofen, W.; Gebhart, J.; Heyder, J. Biological variability of regional deposition of aerosol particles in the human respiratory tract. Am. Ind. Hyg. Assoc. J. 1981, 42, 348–352. [Google Scholar] [CrossRef]
- Heyder, J.; Gebhart, J.; Rudolf, G.; Schiller, C.F.; Stahlhofen, W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986, 17, 811–825. [Google Scholar] [CrossRef]
- Heyder, J.; Rudolf, G. Mathematical models of particle deposition in the human respiratory tract. J. Aerosol Sci. 1984, 15, 697–707. [Google Scholar] [CrossRef]
- Chalupa, D.C.; Morrow, P.E.; Oberdörster, G.; Utell, M.J.; Frampton, M.W. Ultrafine particle deposition in subjects with asthma. Environ. Health Perspect. 2004, 112, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Jaques, P.A.; Kim, C.S. Measurement of total lung deposition of inhaled ultrafine particles in healthy men and women. Inhal. Toxicol. 2000, 12, 715–731. [Google Scholar] [PubMed]
- Byron, P.R. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J. Pharm. Sci. 1986, 75, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Sardeli, C.; Zarogoulidis, P.; Kosmidis, C.; Amaniti, A.; Katsaounis, A.; Giannakidis, D.; Koulouris, C.; Hohenforst-Schmidt, W.; Huang, H.; Bai, C. Inhaled chemotherapy adverse effects: Mechanisms and protection methods. Lung Cancer Manag. 2019, 8, LMT19. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.; Minguillón, M.C.; Moreno, T.; Querol, X.; de Miguel, E.; Capdevila, M.; Centelles, S.; Lazaridis, M. Deposition of aerosol particles from a subway microenvironment in the human respiratory tract. J. Aerosol Sci. 2015, 90, 103–113. [Google Scholar] [CrossRef]
- Moller, W.; Haussinger, K.; Winkler-Heil, R.; Stahlhofen, W.; Meyer, T.; Hofmann, W.; Heyder, J. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J. Appl. Physiol. 2004, 97, 2200–2206. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler-Behnke, M.; Möller, W. Ultrafine particle–lung interactions: Does size matter? J. Aerosol Med. 2006, 19, 74–83. [Google Scholar] [CrossRef]
- Moller, W.; Felten, K.; Sommerer, K.; Scheuch, G.; Meyer, G.; Meyer, P.; Haussinger, K.; Kreyling, W.G. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am. J. Respir. Crit. Care Med. 2008, 177, 426–432. [Google Scholar] [CrossRef]
- Schmid, O.; Möller, W.; Semmler-Behnke, M.; Ferron, G.A.; Karg, E.; Lipka, J.; Schulz, H.; Kreyling, W.; Stöger, T. Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers 2009, 14, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bur, M.; Henning, A.; Hein, S.; Schneider, M.; Lehr, C.-M. Inhalative nanomedicine—Opportunities and challenges. Inhal. Toxicol. 2009, 21, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Geiser, M.; Schurch, S.; Gehr, P. Influence of surface chemistry and topography of particles on their immersion into the lung’s surface-lining layer. J. Appl. Physiol. 2003, 94, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Arredouani, M.; Yang, Z.; Ning, Y.; Qin, G.; Soininen, R.; Tryggvason, K.; Kobzik, L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J. Exp. Med. 2004, 200, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Gumbleton, M. Caveolae as potential macromolecule trafficking compartments within alveolar epithelium. Adv. Drug Deliv. Rev. 2001, 49, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Palecanda, A.; Kobzik, L. Receptors for unopsonized particles: The role of alveolar macrophage scavenger receptors. Curr. Mol. Med. 2001, 1, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Madl, A.K.; Pinkerton, K.E. Health effects of inhaled engineered and incidental nanoparticles. Crit. Rev. Toxicol. 2009, 39, 629–658. [Google Scholar] [CrossRef]
- Wang, X.; Wan, W.; Lu, J.; Quan, G.; Pan, X.; Liu, P. Effects of L-leucine on the properties of spray-dried swellable microparticles with wrinkled surfaces for inhalation therapy of pulmonary fibrosis. Int. J. Pharm. 2021, 610, 121223. [Google Scholar] [CrossRef]
- Hassan, M.S.; Lau, R.W.M. Effect of particle shape on dry particle inhalation: Study of flowability, aerosolization, and deposition properties. Aaps Pharmscitech 2009, 10, 1252–1262. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Xu, Z.; Chen, L.; Luo, R.; Zhang, C.; Gao, F.; Zhang, J.; Fu, C. Celastrol: A review of useful strategies overcoming its limitation in anticancer application. Front. Pharmacol. 2020, 11, 558741. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, C.; Fan, W.; Jiang, H.; Xiang, J.; Qiu, N.; Piao, Y.; Xie, T.; Luo, Y.; Li, Z. Tumor extravasation and infiltration as barriers of nanomedicine for high efficacy: The current status and transcytosis strategy. Biomaterials 2020, 240, 119902. [Google Scholar] [CrossRef]
- Zhou, J.; Kroll, A.V.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic nanotechnology toward personalized vaccines. Adv. Mater. 2020, 32, 1901255. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Peng, P.; Dharap, S.S.; Wang, Y.; Mehlig, M.; Chandna, P.; Zhao, H.; Filpula, D.; Yang, K.; Borowski, V. Antitumor activity of poly (ethylene glycol)–camptothecin conjugate: The inhibition of tumor growth in vivo. J. Control. Release 2005, 110, 90–102. [Google Scholar] [CrossRef]
- Mei, H.; Cai, S.; Huang, D.; Gao, H.; Cao, J.; He, B. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: From intrinsic physicochemical properties to external modification. Bioact. Mater. 2022, 8, 220–240. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, B.; Xu, A.; Shen, Z.; Guo, Y.; Zhao, R.; Yao, H.; Shao, J.-W. Carrier-free, pure nanodrug formed by the self-assembly of an anticancer drug for cancer immune therapy. Mol. Pharm. 2018, 15, 2466–2478. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.-Y.; Zhang, A.-Q.; Cheng, S.-X.; Rong, L.; Zhang, X.-Z. Drug self-delivery systems for cancer therapy. Biomaterials 2017, 112, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, S.; Zhou, M.; Shi, B.; Zhang, X.; Williams, G.R.; Chen, X. Carrier-free nanodrugs for safe and effective cancer treatment. J. Control. Release 2021, 329, 805–832. [Google Scholar] [CrossRef]
- Zhang, R.; Xing, R.; Jiao, T.; Ma, K.; Chen, C.; Ma, G.; Yan, X. Carrier-free, chemophotodynamic dual nanodrugs via self-assembly for synergistic antitumor therapy. ACS Appl. Mater. Interfaces 2016, 8, 13262–13269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, X.; Yang, Y.; Liu, Z.; Tian, B.; Jie, J.; Zhang, X. Carrier-free functionalized multidrug nanorods for synergistic cancer therapy. Biomaterials 2013, 34, 8960–8967. [Google Scholar] [CrossRef]
- Shim, M.K.; Park, J.; Yoon, H.Y.; Lee, S.; Um, W.; Kim, J.-H.; Kang, S.-W.; Seo, J.-W.; Hyun, S.-W.; Park, J.H. Carrier-free nanoparticles of cathepsin B-cleavable peptide-conjugated doxorubicin prodrug for cancer targeting therapy. J. Control. Release 2019, 294, 376–389. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Zhao, R.-R.; Fang, Y.-F.; Jiang, J.-L.; Yuan, X.-T.; Shao, J.-W. Carrier-free nanodrug: A novel strategy of cancer diagnosis and synergistic therapy. Int. J. Pharm. 2019, 570, 118663. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; do Carmo Pereira, M. Nanotechnology to improve the Alzheimer’s disease therapy with natural compounds. Drug Deliv. Transl. Res. 2020, 10, 380–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; An, F.-F.; Liu, J.; Jin, S.; Zhang, J.-C.; Wang, P.C.; Zhang, X.; Lee, C.-S.; Liang, X.-J. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale 2015, 7, 13503–13510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Zhou, M.; Nan, X.; Chen, X.; Zhang, X. Mitochondrial-targeting lonidamine-doxorubicin nanoparticles for synergistic chemotherapy to conquer drug resistance. ACS Appl. Mater. Interfaces 2017, 9, 43498–43507. [Google Scholar] [CrossRef]
- Muralidharan, P.; Mallory, E.K.; Malapit, M.; Phan, H.; Ledford, J.G.; Hayes, D.; Mansour, H.M. Advanced design and development of nanoparticle/microparticle dual-drug combination lactose carrier-free dry powder inhalation aerosols. RSC Adv. 2020, 10, 41846–41856. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.-C.; Lin, X.; Zhu, X.; Yan, L.; Li, S.; Yang, X.; Zhu, G.; Rogach, A.L.; Yu, P.K. Self-monitoring and self-delivery of photosensitizer-doped nanoparticles for highly effective combination cancer therapy in vitro and in vivo. ACS Nano 2015, 9, 9741–9756. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Chen, X.; Zhou, M.; Xu, R.; Zhang, X. Smart surface coating of drug nanoparticles with cross-linkable polyethylene glycol for bio-responsive and highly efficient drug delivery. Nanoscale 2016, 8, 8118–8125. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Yang, J.C.; Lee, C.K.; Kurata, T.; Kim, D.W.; John, T.; Nogami, N.; Ohe, Y.; Mann, H.; Rukazenkov, Y.; et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 841–849. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J.; et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Pietanza, M.C.; Bauer, T.M.; Ready, N.; Morgensztern, D.; Glisson, B.S.; Byers, L.A.; Johnson, M.L.; Burris, H.A., 3rd; Robert, F.; et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017, 18, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, H.; Granger, A.; Hibbard, T.; Opesanwo, S. Pulmonary Drug Delivery of Antimicrobials and Anticancer Drugs Using Solid Dispersions. Pharmaceutics 2021, 13, 1056. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Byeon, H.J.; Choi, J.S.; Thao, L.; Kim, I.; Lee, E.S.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Inhalable self-assembled albumin nanoparticles for treating drug-resistant lung cancer. J. Control. Release 2015, 197, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Garbuzenko, O.B.; Chen, A.M.; Minko, T. Innovative strategy for treatment of lung cancer: Targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J. Drug Target. 2011, 19, 900–914. [Google Scholar] [CrossRef]
- Taratula, O.; Kuzmov, A.; Shah, M.; Garbuzenko, O.B.; Minko, T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J. Control. Release 2013, 171, 349–357. [Google Scholar] [CrossRef]
- Zhu, X.; Kong, Y.; Liu, Q.; Lu, Y.; Xing, H.; Lu, X.; Yang, Y.; Xu, J.; Li, N.; Zhao, D.; et al. Inhalable dry powder prepared from folic acid-conjugated docetaxel liposomes alters pharmacodynamic and pharmacokinetic properties relevant to lung cancer chemotherapy. Pulm. Pharmacol. Ther. 2019, 55, 50–61. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Ge, Y.; Hu, Y.; Li, M.; Jin, Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm. Sin. B 2018, 8, 440–448. [Google Scholar] [CrossRef]
- Lin, C.; Wong, B.C.K.; Chen, H.; Bian, Z.; Zhang, G.; Zhang, X.; Kashif Riaz, M.; Tyagi, D.; Lin, G.; Zhang, Y.; et al. Pulmonary delivery of triptolide-loaded liposomes decorated with anti-carbonic anhydrase IX antibody for lung cancer therapy. Sci. Rep. 2017, 7, 1097. [Google Scholar] [CrossRef]
- Rosière, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New Folate-Grafted Chitosan Derivative To Improve Delivery of Paclitaxel-Loaded Solid Lipid Nanoparticles for Lung Tumor Therapy by Inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, Z.; Barar, J.; Aghanejad, A.; Saei, A.A.; Nemati, E.; Ezzati Nazhad Dolatabadi, J.; Omidi, Y. Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Dev. Ind. Pharm. 2017, 43, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Nassimi, M.; Schleh, C.; Lauenstein, H.D.; Hussein, R.; Hoymann, H.-G.; Koch, W.; Pohlmann, G.; Krug, N.; Sewald, K.; Rittinghausen, S. A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur. J. Pharm. Biopharm. 2010, 75, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Rosière, R.; Amighi, K.; Wauthoz, N.; Dalby, R.; Byron, P.R.; Peart, J. New dry powders for inhalation containing chitosan derivative-coated solid lipid nanoparticles for targeted delivery to lung cancer cells. RDD Eur. 2015, 2015, 447–452. [Google Scholar]
- Hu, L.; Jia, Y. Preparation and characterization of solid lipid nanoparticles loaded with epirubicin for pulmonary delivery. Die Pharmazie Int. J. Pharm. Sci. 2010, 65, 585–587. [Google Scholar]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W.; Wu, C. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911–925. [Google Scholar] [CrossRef]
- Chishti, N.; Jagwani, S.; Dhamecha, D.; Jalalpure, S.; Dehghan, M.H. Preparation, optimization, and in vivo evaluation of nanoparticle-based formulation for pulmonary delivery of anticancer drug. Medicina 2019, 55, 294. [Google Scholar] [CrossRef]
- Kaur, P.; Mishra, V.; Shunmugaperumal, T.; Goyal, A.K.; Ghosh, G.; Rath, G. Inhalable spray dried lipidnanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101502. [Google Scholar] [CrossRef]
- Kamel, N.M.; Helmy, M.W.; Abdelfattah, E.Z.; Khattab, S.N.; Ragab, D.; Samaha, M.W.; Fang, J.Y.; Elzoghby, A.O. Inhalable Dual-Targeted Hybrid Lipid Nanocore-Protein Shell Composites for Combined Delivery of Genistein and All-Trans Retinoic Acid to Lung Cancer Cells. ACS Biomater. Sci. Eng. 2020, 6, 71–87. [Google Scholar] [CrossRef]
- Raval, M.; Patel, P.; Airao, V.; Bhatt, V.; Sheth, N. Novel Silibinin Loaded Chitosan-Coated PLGA/PCL Nanoparticles Based Inhalation Formulations with Improved Cytotoxicity and Bioavailability for Lung Cancer. BioNanoScience 2021, 11, 67–83. [Google Scholar] [CrossRef]
- Vanza, J.D.; Lalani, J.R.; Patel, R.B.; Patel, M.R. DOE supported optimization of biodegradable polymeric nanoparticles based dry powder inhaler for targeted delivery of afatinib in non-small cell lung cancer. J. Drug Deliv. Sci. Technol. 2023, 84, 104554. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Mukherjee, A.; Paul Manash, K. Chapter 9—Bioinspired nanoparticles-based drug delivery systems for cancer theranostics. In Biogenic Nanoparticles for Cancer Theranostics; Patra, C., Ahmad, I., Ayaz, M., Khalil, A.T., Mukherjee, S., Ovais, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 189–228. [Google Scholar]
- Guinart, A.; Perry, H.L.; Wilton-Ely, J.; Tetley, T.D. Gold nanomaterials in the management of lung cancer. Emerg. Top. Life Sci. 2020, 4, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, P.; Zhang, J.; Tian, H.; Park, K.; Chen, X. Pulmonary codelivery of doxorubicin and siRNA by pH-sensitive nanoparticles for therapy of metastatic lung cancer. Small 2015, 11, 4321–4333. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.K.; Zhong, Q.; Bielski, E.R.; da Rocha, S.R. Nanoparticles of pH-responsive, PEG–doxorubicin conjugates: Interaction with an in vitro model of lung adenocarcinoma and their direct formulation in propellant-based portable inhalers. Mol. Pharm. 2017, 14, 3866–3878. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zou, Y.; Shah, C.D.; Fan, N.; Bhagat, T.D.; Gucalp, R.; Kim, M.; Verma, A.; Piperdi, B.; Spivack, S.D. First-in-human study of inhaled Azacitidine in patients with advanced non-small cell lung cancer. Lung Cancer 2021, 154, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, P.J.; Tellez, C.S.; Grimes, M.J.; March, T.H.; Tessema, M.; Revelli, D.A.; Mallis, L.M.; Dye, W.W.; Sniegowski, T.; Badenoch, A. 5-Azacytidine inhaled dry powder formulation profoundly improves pharmacokinetics and efficacy for lung cancer therapy through genome reprogramming. Br. J. Cancer 2020, 122, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Shepard, K.B.; Vodak, D.T.; Kuehl, P.J.; Revelli, D.; Zhou, Y.; Pluntze, A.M.; Adam, M.S.; Oddo, J.C.; Switala, L.; Cape, J.L. Local treatment of non-small cell lung cancer with a spray-dried bevacizumab formulation. AAPS PharmSciTech 2021, 22, 230. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, X.; Ye, L.; Zhang, Y.; Ding, R.; Hao, Y.; Zhao, Y.; Zhang, Z.; Zhang, Y. Inhalable microspheres embedding chitosan-coated PLGA nanoparticles for 2-methoxyestradiol. J. Drug Target. 2014, 22, 421–427. [Google Scholar] [CrossRef]
- Hitzman, C.J.; Elmquist, W.F.; Wattenberg, L.W.; Wiedmann, T.S. Development of a Respirable, Sustained Release Microcarrier for 5-Fluorouracil I: In Vitro Assessment of Liposomes, Microspheres, and Lipid Coated Nanoparticles. J. Pharm. Sci. 2006, 95, 1114–1126. [Google Scholar] [CrossRef]
- Hitzman, C.J.; Wattenberg, L.W.; Wiedmann, T.S. Pharmacokinetics of 5-fluorouracil in the hamster following inhalation delivery of lipid-coated nanoparticles. J. Pharm. Sci. 2006, 95, 1196–1211. [Google Scholar] [CrossRef] [PubMed]
- Knight, V.; Kleinerman, E.S.; Waldrep, J.C.; Giovanella, B.C.; Gilbert, B.E.; Koshkina, N.V. 9-Nitrocamptothecin liposome aerosol treatment of human cancer subcutaneous xenografts and pulmonary cancer metastases in mice. Ann. N. Y. Acad. Sci. 2000, 922, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Koshkina, N.V.; Kleinerman, E.S.; Waldrep, C.; Jia, S.-F.; Worth, L.L.; Gilbert, B.E.; Knight, V. 9-Nitrocamptothecin liposome aerosol treatment of melanoma and osteosarcoma lung metastases in mice. Clin. Cancer Res. 2000, 6, 2876–2880. [Google Scholar] [PubMed]
- Verschraegen, C.F.; Gilbert, B.E.; Loyer, E.; Huaringa, A.; Walsh, G.; Newman, R.A.; Knight, V. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(s)-camptothecin in patients with advanced pulmonary malignancies. Clin. Cancer Res. 2004, 10, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Z.; Li, J.; Mo, Z.; Huang, Y.; Ma, C.; Wang, W.; Pan, X.; Wu, C. PLGA Porous Microspheres Dry Powders for Codelivery of Afatinib-Loaded Solid Lipid Nanoparticles and Paclitaxel: Novel Therapy for EGFR Tyrosine Kinase Inhibitors Resistant Nonsmall Cell Lung Cancer. Adv. Healthc. Mater. 2019, 8, e1900965. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Knight, V.; Gilbert, B.E.; Golunski, E.; Roberts, L.; Waldrep, J.C. Improved respiratory delivery of the anticancer drugs, camptothecin and paclitaxel, with 5% CO2-enriched air: Pharmacokinetic studies. Cancer Chemother. Pharmacol. 2001, 47, 451–456. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Eleftheriadou, E.; Sapardanis, I.; Zarogoulidou, V.; Lithoxopoulou, H.; Kontakiotis, T.; Karamanos, N.; Zachariadis, G.; Mabroudi, M.; Zisimopoulos, A.; et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Investig. New Drugs 2012, 30, 1628–1640. [Google Scholar] [CrossRef]
- Anabousi, S.; Bakowsky, U.; Schneider, M.; Huwer, H.; Lehr, C.-M.; Ehrhardt, C. In vitro assessment of transferrin-conjugated liposomes as drug delivery systems for inhalation therapy of lung cancer. Eur. J. Pharm. Sci. 2006, 29, 367–374. [Google Scholar] [CrossRef]
- Kim, I.; Byeon, H.J.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Doxorubicin-loaded highly porous large PLGA microparticles as a sustained-release inhalation system for the treatment of metastatic lung cancer. Biomaterials 2012, 33, 5574–5583. [Google Scholar] [CrossRef]
- Long, J.-T.; Cheang, T.-y.; Zhuo, S.-Y.; Zeng, R.-F.; Dai, Q.-S.; Li, H.-P.; Fang, S. Anticancer drug-loaded multifunctional nanoparticles to enhance the chemotherapeutic efficacy in lung cancer metastasis. J. Nanobiotechnol. 2014, 12, 1–11. [Google Scholar] [CrossRef]
- Tagami, T.; Ando, Y.; Ozeki, T. Fabrication of liposomal doxorubicin exhibiting ultrasensitivity against phospholipase A2 for efficient pulmonary drug delivery to lung cancers. Int. J. Pharm. 2017, 517, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; da Rocha, S.R. Poly (amidoamine) dendrimer–doxorubicin conjugates: In vitro characteristics and pseudosolution formulation in pressurized metered-dose inhalers. Mol. Pharm. 2016, 13, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, O.B.; Mainelis, G.; Taratula, O.; Minko, T. Inhalation treatment of lung cancer: The influence of composition, size and shape of nanocarriers on their lung accumulation and retention. Cancer Biol. Med. 2014, 11, 44. [Google Scholar]
- Patel, A.R.; Chougule, M.B.; Townley, I.; Patlolla, R.; Wang, G.; Singh, M. Efficacy of aerosolized celecoxib encapsulated nanostructured lipid carrier in non-small cell lung cancer in combination with docetaxel. Pharm. Res. 2013, 30, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Fulzele, S.V.; Shaik, M.S.; Chatterjee, A.; Singh, M. Anti-cancer effect of celecoxib and aerosolized docetaxel against human non-small cell lung cancer cell line, A549. J. Pharm. Pharmacol. 2006, 58, 327–336. [Google Scholar] [CrossRef]
- Fulzele, S.V.; Chatterjee, A.; Shaik, M.S.; Jackson, T.; Singh, M. Inhalation delivery and anti-tumor activity of celecoxib in human orthotopic non-small cell lung cancer xenograft model. Pharm. Res. 2006, 23, 2094–2106. [Google Scholar] [CrossRef]
- Wittgen, B.P.; Kunst, P.W.; Van Der Born, K.; Van Wijk, A.W.; Perkins, W.; Pilkiewicz, F.G.; Perez-Soler, R.; Nicholson, S.; Peters, G.J.; Postmus, P.E. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin. Cancer Res. 2007, 13, 2414–2421. [Google Scholar] [CrossRef]
- Xie, Y.; Aillon, K.L.; Cai, S.; Christian, J.M.; Davies, N.M.; Berkland, C.J.; Forrest, M.L. Pulmonary delivery of cisplatin–hyaluronan conjugates via endotracheal instillation for the treatment of lung cancer. Int. J. Pharm. 2010, 392, 156–163. [Google Scholar] [CrossRef]
- Loira-Pastoriza, C.; Vanvarenberg, K.; Ucakar, B.; Machado Franco, M.; Staub, A.; Lemaire, M.; Renauld, J.-C.; Vanbever, R. Encapsulation of a CpG oligonucleotide in cationic liposomes enhances its local antitumor activity following pulmonary delivery in a murine model of metastatic lung cancer. Int. J. Pharm. 2021, 600, 120504. [Google Scholar] [CrossRef]
- Taki, M.; Tagami, T.; Fukushige, K.; Ozeki, T. Fabrication of nanocomposite particles using a two-solution mixing-type spray nozzle for use in an inhaled curcumin formulation. Int. J. Pharm. 2016, 511, 104–110. [Google Scholar] [CrossRef]
- Adel, I.M.; ElMeligy, M.F.; Abdelrahim, M.E.A.; Maged, A.; Abdelkhalek, A.A.; Abdelmoteleb, A.M.M.; Elkasabgy, N.A. Design and Characterization of Spray-Dried Proliposomes for the Pulmonary Delivery of Curcumin. Int. J. Nanomed. 2021, 16, 2667–2687. [Google Scholar] [CrossRef] [PubMed]
- Al Ayoub, Y.; Gopalan, R.C.; Najafzadeh, M.; Mohammad, M.A.; Anderson, D.; Paradkar, A.; Assi, K.H. Development and evaluation of nanoemulsion and microsuspension formulations of curcuminoids for lung delivery with a novel approach to understanding the aerosol performance of nanoparticles. Int. J. Pharm. 2019, 557, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.S.; Haynes, A.; McSween, J.; Ikediobi, O.; Kanikkannan, N.; Singh, M. Inhalation delivery of anticancer agents via HFA-based metered dose inhaler using methotrexate as a model drug. J. Aerosol Med. 2002, 15, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Asmawi, A.A.; Salim, N.; Ngan, C.L.; Ahmad, H.; Abdulmalek, E.; Masarudin, M.J.; Abdul Rahman, M.B. Excipient selection and aerodynamic characterization of nebulized lipid-based nanoemulsion loaded with docetaxel for lung cancer treatment. Drug Deliv. Transl. Res. 2019, 9, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Asmawi, A.A.; Salim, N.; Abdulmalek, E.; Abdul Rahman, M.B. Modeling the Effect of Composition on Formation of Aerosolized Nanoemulsion System Encapsulating Docetaxel and Curcumin Using D-Optimal Mixture Experimental Design. Int. J. Mol. Sci. 2020, 21, 4357. [Google Scholar] [CrossRef]
- Azarmi, S.; Tao, X.; Chen, H.; Wang, Z.; Finlay, W.H.; Löbenberg, R.; Roa, W.H. Formulation and cytotoxicity of doxorubicin nanoparticles carried by dry powder aerosol particles. Int. J. Pharm. 2006, 319, 155–161. [Google Scholar] [CrossRef]
- Al-Hallak, M.K.; Sarfraz, M.K.; Azarmi, S.; Roa, W.H.; Finlay, W.H.; Rouleau, C.; Löbenberg, R. Distribution of effervescent inhalable nanoparticles after pulmonary delivery: An in vivo study. Ther. Deliv. 2012, 3, 725–734. [Google Scholar] [CrossRef]
- Hershey, A.E.; Kurzman, I.D.; Forrest, L.J.; Bohling, C.A.; Stonerook, M.; Placke, M.E.; Imondi, A.R.; Vail, D.M. Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: Proof of principle using dogs with spontaneously occurring tumors as a model. Clin. Cancer Res. 1999, 5, 2653–2659. [Google Scholar]
- Alipour, S.; Montaseri, H.; Tafaghodi, M. Inhalable, large porous PLGA microparticles loaded with paclitaxel: Preparation, in vitro and in vivo characterization. J. Microencapsul. 2015, 32, 661–668. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Waldrep, J.C.; Roberts, L.E.; Golunski, E.; Melton, S.; Knight, V. Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin. Cancer Res. 2001, 7, 3258–3262. [Google Scholar]
- Gill, K.K.; Nazzal, S.; Kaddoumi, A. Paclitaxel loaded PEG5000–DSPE micelles as pulmonary delivery platform: Formulation characterization, tissue distribution, plasma pharmacokinetics, and toxicological evaluation. Eur. J. Pharm. Biopharm. 2011, 79, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Meenach, S.A.; Anderson, K.W.; Hilt, J.Z.; McGarry, R.C.; Mansour, H.M. Characterization and aerosol dispersion performance of advanced spray-dried chemotherapeutic PEGylated phospholipid particles for dry powder inhalation delivery in lung cancer. Eur. J. Pharm. Sci. 2013, 49, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Kubota, M.; Ozeki, T. Effective Remote Loading of Doxorubicin into DPPC/Poloxamer 188 Hybrid Liposome to Retain Thermosensitive Property and the Assessment of Carrier-Based Acute Cytotoxicity for Pulmonary Administration. J. Pharm. Sci. 2015, 104, 3824–3832. [Google Scholar] [CrossRef] [PubMed]
- Tatsumura, T.; Koyama, S.; Tsujimoto, M.; Kitagawa, M.; Kagamimori, S. Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: Fundamental and clinical. Br. J. Cancer 1993, 68, 1146–1149. [Google Scholar] [CrossRef]
- Mainelis, G.; Seshadri, S.; Garbuzenko, O.B.; Han, T.; Wang, Z.; Minko, T. Characterization and application of a nose-only exposure chamber for inhalation delivery of liposomal drugs and nucleic acids to mice. J. Aerosol. Med. Pulm. Drug Deliv. 2013, 26, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, R.; Li, M.; Bao, J.; Chen, Y.; Ge, Y.; Jin, Y. Comparative study of intratracheal and oral gefitinib for the treatment of primary lung cancer. Eur. J. Pharm. Sci. 2020, 149, 105352. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Saimi, N.I.; Salim, N.; Ahmad, N.; Abdulmalek, E.; Abdul Rahman, M.B. Aerosolized Niosome Formulation Containing Gemcitabine and Cisplatin for Lung Cancer Treatment: Optimization, Characterization and In Vitro Evaluation. Pharmaceutics 2021, 13, 59. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Kleinerman, E.S. Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int. J. Cancer 2005, 116, 458–463. [Google Scholar] [CrossRef]
- Gordon, N.; Koshkina, N.V.; Jia, S.-F.; Khanna, C.; Mendoza, A.; Worth, L.L.; Kleinerman, E.S. Corruption of the Fas pathway delays the pulmonary clearance of murine osteosarcoma cells, enhances their metastatic potential, and reduces the effect of aerosol gemcitabine. Clin. Cancer Res. 2007, 13, 4503–4510. [Google Scholar] [CrossRef]
- Lemarie, E.; Vecellio, L.; Hureaux, J.; Prunier, C.; Valat, C.; Grimbert, D.; Boidron-Celle, M.; Giraudeau, B.; le Pape, A.; Pichon, E. Aerosolized gemcitabine in patients with carcinoma of the lung: Feasibility and safety study. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 261–270. [Google Scholar] [CrossRef]
- Gandhi, M.; Pandya, T.; Gandhi, R.; Patel, S.; Mashru, R.; Misra, A.; Tandel, H. Inhalable liposomal dry powder of gemcitabine-HCl: Formulation, in vitro characterization and in vivo studies. Int. J. Pharm. 2015, 496, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhuang, B.; Zhang, G.; Li, M.; Jin, Y. Pulmonary delivery of cationic liposomal hydroxycamptothecin and 5-aminolevulinic acid for chemo-sonodynamic therapy of metastatic lung cancer. Int. J. Pharm. 2021, 601, 120572. [Google Scholar] [CrossRef] [PubMed]
- Skubitz, K.M.; Anderson, P.M. Inhalational interleukin-2 liposomes for pulmonary metastases: A phase I clinical trial. Anti-Cancer Drugs 2000, 11, 555–563. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Golunski, E.; Roberts, L.E.; Gilbert, B.E.; Knight, V. Cyclosporin A aerosol improves the anticancer effect of paclitaxel aerosol in mice. J. Aerosol Med. 2004, 17, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Kaur, K.; Pandey, R.S.; Jain, U.K.; Chandra, R.; Madan, J. Inhalable nanostructured lipid particles of 9-bromo-noscapine, a tubulin-binding cytotoxic agent: In vitro and in vivo studies. J. Colloid Interface Sci. 2015, 445, 219–230. [Google Scholar] [CrossRef]
- Nafee, N.; Gaber, D.M.; Elzoghby, A.O.; Helmy, M.W.; Abdallah, O.Y. Promoted Antitumor Activity of Myricetin against Lung Carcinoma Via Nanoencapsulated Phospholipid Complex in Respirable Microparticles. Pharm. Res. 2020, 37, 82. [Google Scholar] [CrossRef]
- Boehle, A.; Kurdow, R.; Boenicke, L.; Schniewind, B.; Faendrich, F.; Dohrmann, P.; Kalthoff, H. Wortmannin inhibits growth of human non-small-cell lung cancer in vitro and in vivo. Langenbecks Arch. Surg. 2002, 387, 234–239. [Google Scholar] [CrossRef]
- Hemström, T.H.; Sandström, M.; Zhivotovsky, B. Inhibitors of the PI3-kinase/Akt pathway induce mitotic catastrophe in non-small cell lung cancer cells. Int. J. Cancer 2006, 119, 1028–1038. [Google Scholar] [CrossRef]
- Zhang, T.; Cui, G.-B.; Zhang, J.; Zhang, F.; Zhou, Y.-A.; Jiang, T.; Li, X.-F. Inhibition of PI3 kinases enhances the sensitivity of non-small cell lung cancer cells to ionizing radiation. Oncol. Rep. 2010, 24, 1683–1689. [Google Scholar]
- Zhu, L.; Li, M.; Liu, X.; Du, L.; Jin, Y. Inhalable oridonin-loaded poly(lactic-co-glycolic)acid large porous microparticles for in situ treatment of primary non-small cell lung cancer. Acta Pharm. Sin. B 2017, 7, 80–90. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.; Goyal, A.K. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 2016, 23, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, O.B.; Kuzmov, A.; Taratula, O.; Pine, S.R.; Minko, T. Strategy to enhance lung cancer treatment by five essential elements: Inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics 2019, 9, 8362–8376. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.W.; Wong, C.C.; Alston, N.; Mackenzie, G.G.; Huang, L.; Ouyang, N.; Xie, G.; Wiedmann, T.; Rigas, B. Aerosol administration of phospho-sulindac inhibits lung tumorigenesis. Mol. Cancer Ther. 2013, 12, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.K.; Zhang, X.; Wong, K.H.; Chen, H.; Liu, Q.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy. Int. J. Nanomed. 2019, 14, 2879–2902. [Google Scholar] [CrossRef] [PubMed]
- Arbain, N.H.; Basri, M.; Salim, N.; Wui, W.T.; Abdul Rahman, M.B. Development and Characterization of Aerosol Nanoemulsion System Encapsulating Low Water Soluble Quercetin for Lung Cancer Treatment. Mater. Today Proc. 2018, 5, S137–S142. [Google Scholar] [CrossRef]
- Arbain, N.H.; Salim, N.; Masoumi, H.R.F.; Wong, T.W.; Basri, M.; Abdul Rahman, M.B. In vitro evaluation of the inhalable quercetin loaded nanoemulsion for pulmonary delivery. Drug Deliv. Transl. Res. 2019, 9, 497–507. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Prasad, N.R.; Ganamani, A.; Balamurugan, E. Anticancer activity of resveratrol-loaded gelatin nanoparticles on NCI-H460 non-small cell lung cancer cells. Biomed. Prev. Nutr. 2013, 3, 64–73. [Google Scholar] [CrossRef]
- Jensen, D.K.; Jensen, L.B.; Koocheki, S.; Bengtson, L.; Cun, D.; Nielsen, H.M.; Foged, C. Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA. J. Control. Release 2012, 157, 141–148. [Google Scholar] [CrossRef]
- Patel, K.; Bothiraja, C.; Mali, A.; Kamble, R. Investigation of sorafenib tosylate loaded liposomal dry powder inhaler for the treatment of non-small cell lung cancer. Part. Sci. Technol. 2021, 39, 990–999. [Google Scholar] [CrossRef]
- Tomoda, K.; Ohkoshi, T.; Hirota, K.; Sonavane, G.S.; Nakajima, T.; Terada, H.; Komuro, M.; Kitazato, K.; Makino, K. Preparation and properties of inhalable nanocomposite particles for treatment of lung cancer. Colloids Surf. B Biointerfaces 2009, 71, 177–182. [Google Scholar] [CrossRef]
- Hamzawy, M.A.; Abo-Youssef, A.M.; Salem, H.F.; Mohammed, S.A. Antitumor activity of intratracheal inhalation of temozolomide (TMZ) loaded into gold nanoparticles and/or liposomes against urethane-induced lung cancer in BALB/c mice. Drug Deliv. 2017, 24, 599–607. [Google Scholar] [CrossRef]
- Wauthoz, N.; Deleuze, P.; Saumet, A.; Duret, C.; Kiss, R.; Amighi, K. Temozolomide-based dry powder formulations for lung tumor-related inhalation treatment. Pharm Res 2011, 28, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, X.; Chen, H.; Bian, Z.; Zhang, G.; Riaz, M.K.; Tyagi, D.; Lin, G.; Zhang, Y.; Wang, J.; et al. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv. 2018, 25, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Kulkarni, N.S.; Chauhan, G.; Shukla, S.K.; Elbatanony, R.; Patel, B.; Kunda, N.K.; Muth, A.; Gupta, V. Development of pharmaceutically scalable inhaled anti-cancer nanotherapy—Repurposing amodiaquine for non-small cell lung cancer (NSCLC). Mater. Sci. Eng. C 2020, 115, 111139. [Google Scholar] [CrossRef] [PubMed]
- Seabloom, D.E.; Galbraith, A.R.; Haynes, A.M.; Antonides, J.D.; Wuertz, B.R.; Miller, W.A.; Miller, K.A.; Steele, V.E.; Suen, C.S.; O’Sullivan, M.G.; et al. Safety and Preclinical Efficacy of Aerosol Pioglitazone on Lung Adenoma Prevention in A/J Mice. Cancer Prev. Res. 2017, 10, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Godugu, C.; Patel, A.R.; Doddapaneni, R.; Marepally, S.; Jackson, T.; Singh, M. Inhalation delivery of Telmisartan enhances intratumoral distribution of nanoparticles in lung cancer models. J. Control. Release 2013, 172, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, W.; Yi, Y.; Zhang, Z.; Lubet, R.A.; You, M. Preventive Effects of Bexarotene and Budesonide in a Genetically Engineered Mouse Model of Small Cell Lung CancerBexarotene Inhibits Small Cell Lung Carcinoma in Mice. Cancer Prev. Res. 2009, 2, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Balansky, R.; Ganchev, G.; Iltcheva, M.; Steele, V.E.; De Flora, S. Prevention of cigarette smoke–induced lung tumors in mice by budesonide, phenethyl isothiocyanate, and N-acetylcysteine. Int. J. Cancer 2010, 126, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; LeRiche, J.C.; McWilliams, A.; MacAulay, C.; Dyachkova, Y.; Szabo, E.; Mayo, J.; Schellenberg, R.; Coldman, A.; Hawk, E. A randomized phase IIb trial of pulmicort turbuhaler (budesonide) in people with dysplasia of the bronchial epithelium. Clin. Cancer Res. 2004, 10, 6502–6511. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Gilani, K.; Moazeni, E.; Ghazi-Khansari, M.; Najafabadi, A.R.; Mohajel, N. Development of chitosan-based nanoparticles for pulmonary delivery of itraconazole as dry powder formulation. Powder Technol. 2012, 222, 65–70. [Google Scholar] [CrossRef]
- Lin, L.; Quan, G.; Peng, T.; Huang, Z.; Singh, V.; Lu, M.; Wu, C. Development of fine solid-crystal suspension with enhanced solubility, stability, and aerosolization performance for dry powder inhalation. Int. J. Pharm. 2017, 533, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Duret, C.; Wauthoz, N.; Sebti, T.; Vanderbist, F.; Amighi, K. Solid dispersions of itraconazole for inhalation with enhanced dissolution, solubility and dispersion properties. Int. J. Pharm. 2012, 428, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Mohtar, N.; Taylor, K.M.; Sheikh, K.; Somavarapu, S. Design and development of dry powder sulfobutylether-β-cyclodextrin complex for pulmonary delivery of fisetin. Eur. J. Pharm. Biopharm. 2017, 113, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dahl, A.R.; Grossi, I.M.; Houchens, D.P.; Scovell, L.J.; Placke, M.E.; Imondi, A.R.; Stoner, G.D.; De Luca, L.M.; Wang, D.; Mulshine, J.L. Inhaled isotretinoin (13-cis retinoic acid) is an effective lung cancer chemopreventive agent in A/J mice at low doses: A pilot study. Clin. Cancer Res. 2000, 6, 3015–3024. [Google Scholar]
- Osama, H.; Sayed, O.M.; Hussein, R.R.S.; Abdelrahim, M.; Elberry, A.A. Design, optimization, characterization, and in vivo evaluation of sterosomes as a carrier of metformin for treatment of lung cancer. J. Liposome Res. 2020, 30, 150–162. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Farrales, P.T.; Kunda, N.K.; Muth, A.; Gupta, V. Systematic Development and Optimization of Inhalable Pirfenidone Liposomes for Non-Small Cell Lung Cancer Treatment. Pharmaceutics 2020, 12, 206. [Google Scholar] [CrossRef]
- Verco, J.; Johnston, W.; Frost, M.; Baltezor, M.; Kuehl, P.J.; Lopez, A.; Gigliotti, A.; Belinsky, S.A.; Wolff, R.; diZerega, G. Inhaled submicron particle paclitaxel (NanoPac) induces tumor regression and immune cell infiltration in an orthotopic athymic nude rat model of non-small cell lung cancer. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 266–277. [Google Scholar] [CrossRef]
- Borghaei, H.; Langer, C.J.; Millenson, M.; Ruth, K.J.; Litwin, S.; Tuttle, H.; Seldomridge, J.S.; Rovito, M.; Mintzer, D.; Cohen, R. Phase II study of paclitaxel, carboplatin, and cetuximab as first line treatment, for patients with advanced non-small cell lung cancer (NSCLC): Results of OPN-017. J. Thorac. Oncol. 2008, 3, 1286–1292. [Google Scholar] [CrossRef]
- Rosière, R.; Berghmans, T.; De Vuyst, P.; Amighi, K.; Wauthoz, N. The position of inhaled chemotherapy in the care of patients with lung tumors: Clinical feasibility and indications according to recent pharmaceutical progresses. Cancers 2019, 11, 329. [Google Scholar] [CrossRef]
- Wauthoz, N.; Rosière, R.; Amighi, K. Inhaled cytotoxic chemotherapy: Clinical challenges, recent developments, and future prospects. Expert Opin. Drug Deliv. 2021, 18, 333–354. [Google Scholar] [CrossRef]
- Chou, A.J.; Gupta, R.; Bell, M.D.; Riewe, K.O.D.; Meyers, P.A.; Gorlick, R. Inhaled lipid cisplatin (ILC) in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. Pediatr. Blood Cancer 2013, 60, 580–586. [Google Scholar] [CrossRef]
- Otterson, G.A.; Villalona-Calero, M.A.; Sharma, S.; Kris, M.G.; Imondi, A.; Gerber, M.; White, D.A.; Ratain, M.J.; Schiller, J.H.; Sandler, A. Phase I study of inhaled Doxorubicin for patients with metastatic tumors to the lungs. Clin. Cancer Res. 2007, 13, 1246–1252. [Google Scholar] [CrossRef]
- Otterson, G.A.; Villalona-Calero, M.A.; Hicks, W.; Pan, X.; Ellerton, J.A.; Gettinger, S.N.; Murren, J.R. Phase I/II Study of inhaled doxorubicin combined with platinum-based therapy for advanced non–small cell lung cancer. Clin. Cancer Res. 2010, 16, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F.; Buttini, F.; Usmani, O.S. 100 years of drug delivery to the lungs. In Concepts and Principles of Pharmacology: 100 Years of the Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2019; pp. 143–159. [Google Scholar]
- Gagnadoux, F.; Hureaux, J.; Vecellio, L.; Urban, T.; Le Pape, A.; Valo, I.; Montharu, J.; Leblond, V.; Boisdron-Celle, M.; Lerondel, S.J. Aerosolized chemotherapy. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, K.; Zarogoulidis, P.; Karamanos, N.K.; Domvri, K.; Chatzaki, E.; Constantinidis, T.C.; Kakolyris, S.; Zarogoulidis, K. Efficacy versus safety concerns for aerosol chemotherapy in non-small-cell lung cancer: A future dilemma for micro-oncology. Future Oncol. 2013, 9, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Martínez, M.A.; Ramos Merino, M.; Santos Gago, J.M.; Álvarez Sabucedo, L.M.; Wanden-Berghe, C.; Sanz-Valero, J. Guidelines for safe handling of hazardous drugs: A systematic review. PLoS ONE 2018, 13, e0197172. [Google Scholar] [CrossRef]
- Charpidou, A.G.; Gkiozos, I.; Tsimpoukis, S.; Apostolaki, D.; Dilana, K.D.; Karapanagiotou, E.M.; Syrigos, K.N. Therapy-induced toxicity of the lungs: An overview. Anticancer Res. 2009, 29, 631–639. [Google Scholar] [PubMed]
- Lippmann, M.; Yeates, D.; Albert, R. Deposition, retention, and clearance of inhaled particles. Occup. Environ. Med. 1980, 37, 337–362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Khatib, A.O.; El-Tanani, M.; Al-Obaidi, H. Inhaled Medicines for Targeting Non-Small Cell Lung Cancer. Pharmaceutics 2023, 15, 2777. https://doi.org/10.3390/pharmaceutics15122777
Al Khatib AO, El-Tanani M, Al-Obaidi H. Inhaled Medicines for Targeting Non-Small Cell Lung Cancer. Pharmaceutics. 2023; 15(12):2777. https://doi.org/10.3390/pharmaceutics15122777
Chicago/Turabian StyleAl Khatib, Arwa Omar, Mohamed El-Tanani, and Hisham Al-Obaidi. 2023. "Inhaled Medicines for Targeting Non-Small Cell Lung Cancer" Pharmaceutics 15, no. 12: 2777. https://doi.org/10.3390/pharmaceutics15122777
APA StyleAl Khatib, A. O., El-Tanani, M., & Al-Obaidi, H. (2023). Inhaled Medicines for Targeting Non-Small Cell Lung Cancer. Pharmaceutics, 15(12), 2777. https://doi.org/10.3390/pharmaceutics15122777






