Surface Modification of Titanate Nanotubes with a Carboxylic Arm for Further Functionalization Intended to Pharmaceutical Applications
Abstract
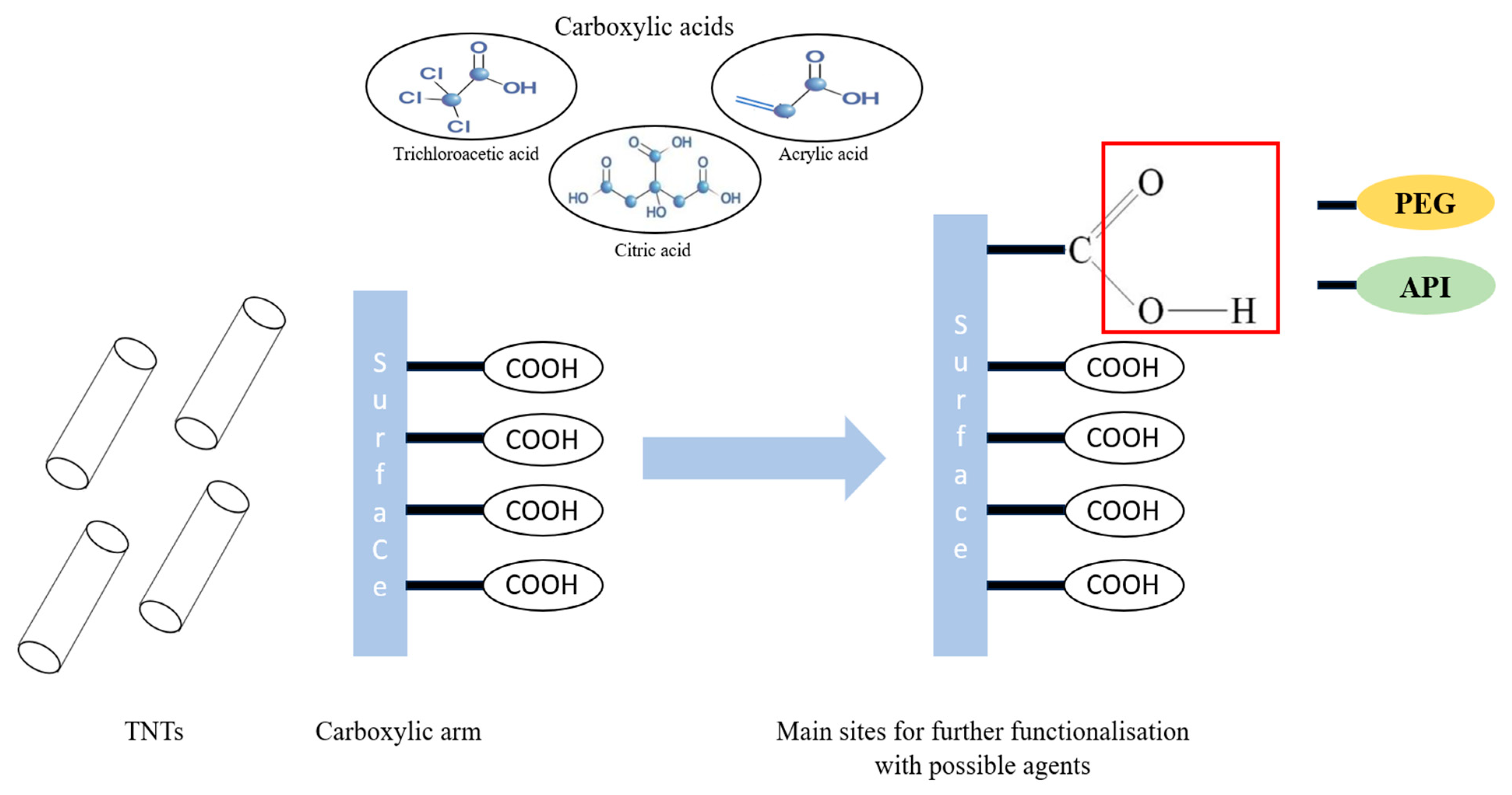
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of TNTs
2.2.2. Preparation of Carboxylic Acid Functionalized TNTs
2.2.3. Functionalization with PEG
2.2.4. Structural Investigations
2.2.5. Morphology
2.2.6. Cell Viability Studies
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Shi, L.; He, W.X.; Han, D.; Yan, Y.; Niu, Z.Y.; Shi, S.G. Bioinspired micro/nano fabrication on dental implant-bone interface. Appl. Surf. Sci. 2013, 265, 480–488. [Google Scholar] [CrossRef]
- Popat, K.C.; Leoni, L.; Grimes, C.A.; Desai, T.A. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 2007, 28, 3188–3197. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Teng, H. Regulation of the physical characteristics of titania nanotube aggregates synthesized from hydrothermal treatment. Chem. Mater. 2004, 16, 4352–4358. [Google Scholar] [CrossRef]
- Adeleye, A.T.; John, K.I.; Adeleye, P.G.; Akande, A.A.; Banjoko, O.O. One-dimensional titanate nanotube materials: Heterogeneous solid catalysts for sustainable synthesis of biofuel precursors/value-added chemicals—A review. J. Mater. Sci. 2021, 56, 18391–18416. [Google Scholar] [CrossRef]
- Wei, M.D.; Konishi, Y.; Zhou, H.S.; Sugihara, H.; Arakawa, H. Utilization of titanate nanotubes as an electrode material in dye-sensitized solar cells. J. Electrochem. Soc. 2006, 153, A1232–A1236. [Google Scholar] [CrossRef]
- Hinojosa-Reyes, M.; Camposeco-Solis, R.; Ruiz, F. H2Ti3O7 titanate nanotubes for highly effective adsorption of basic fuchsin dye for water purification. Microporous Mesoporous Mater. 2019, 276, 183–191. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Mejia-Centeno, I.; Navarrete, J.; Gomez, R. Effect of the Ti/Na molar ratio on the acidity and the structure of TiO2 nanostructures: Nanotubes, nanofibers and nanowires. Mater. Charact. 2014, 90, 113–120. [Google Scholar] [CrossRef]
- Ranjous, Y.; Regdon, G.; Pintye-Hodi, K.; Sovany, T. Standpoint on the priority of TNTs and CNTs as targeted drug delivery systems. Drug Discov. Today 2019, 24, 1704–1709. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, L.; Yao, C.; Fang, J.; Wu, M. Cytotoxicity Evaluation of pH-Controlled Antitumor Drug Release System of Titanium Dioxide Nanotubes. J. Nanosci. Nanotechnol. 2015, 15, 4143–4148. [Google Scholar] [CrossRef]
- Loiseau, A.; Boudon, J.; Oudot, A.; Moreau, M.; Boidot, R.; Chassagnon, R.; Said, N.M.; Roux, S.; Mirjolet, C.; Millot, N. Titanate Nanotubes Engineered with Gold Nanoparticles and Docetaxel to Enhance Radiotherapy on Xenografted Prostate Tumors. Cancers 2019, 11, 1962. [Google Scholar] [CrossRef]
- Kalbacova, M.; Macak, J.; Schmidt-Stein, F.; Mierke, C.; Schmuki, P. TiO2 nanotubes: Photocatalyst for cancer cell killing. Phys. Status Solidi–Rapid Res. Lett. 2008, 2, 194–196. [Google Scholar] [CrossRef]
- Mandal, S.S.; Jose, D.; Bhattacharyya, A. Role of surface chemistry in modulating drug release kinetics in titania nanotubes. Mater. Chem. Phys. 2014, 147, 247–253. [Google Scholar] [CrossRef]
- Khoshnood, N.; Zamanian, A.; Massoudi, A. Mussel-inspired surface modification of titania nanotubes as a novel drug delivery system. Mater. Sci. Eng. C 2017, 77, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.C.; Campos, C.H.; Diáz, C.; Jiménez, V.A.; Vidal, F.; Guzmán, L.; Alderete, J.B. PAMAM-grafted TiO2 nanotubes as novel versatile materials for drug delivery applications. Mater. Sci. Eng. C 2016, 65, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, C.; Luo, F.; Li, P.; Xiao, X. P25 nanoparticles decorated on titania nanotubes arrays as effective drug delivery system for ibuprofen. Appl. Surf. Sci. 2015, 324, 621–626. [Google Scholar] [CrossRef]
- Sipos, B.; Pintye-Hodi, K.; Regdon, G., Jr.; Konya, Z.; Viana, M.; Sovany, T. Investigation of the Compressibility and Compactibility of Titanate Nanotube-API Composites. Materials 2018, 11, 2582. [Google Scholar] [CrossRef]
- Sipos, B.; Regdon, G.; Konya, Z.; Pintye-Hodi, K.; Sovany, T. Comparative study on the rheological properties and tablettability of various APIs and their composites with titanate nanotubes. Powder Technol. 2017, 321, 419–427. [Google Scholar] [CrossRef]
- Sipos, B.; Pintye-Hodi, K.; Konya, Z.; Kelemen, A.; Regdon, G.; Sovany, T. Physicochemical characterisation and investigation of the bonding mechanisms of API-titanate nanotube composites as new drug carrier systems. Int. J. Pharm. 2017, 518, 119–129. [Google Scholar] [CrossRef]
- Song, Y.Y.; Schmidt-Stein, F.; Bauer, S.; Schmuki, P. Amphiphilic TiO2 nanotube arrays: An actively controllable drug delivery system. J. Am. Chem. Soc. 2009, 131, 4230–4232. [Google Scholar] [CrossRef]
- Ranjous, Y.; Kósa, D.; Ujhelyi, Z.; Regdon, G., Jr.; Nagy, K.A.; Szenti, I.; Kónya, Z.; Bácskay, I.; Sovány, T. Evaluation of the permeability and in vitro cytotoxicity of functionalized titanate nanotubes on Caco-2 cell line. Acta Pharm. Hung. 2021, 91, 31–39. [Google Scholar] [CrossRef]
- Chen, X.; Cai, K.; Fang, J.; Lai, M.; Hou, Y.; Li, J.; Luo, Z.; Hu, Y.; Tang, L. Fabrication of selenium-deposited and chitosan-coated titania nanotubes with anticancer and antibacterial properties. Colloids Surf. B Biointerfaces 2013, 103, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Mohan, L.; Anandan, C.; Rajendran, N. Drug release characteristics of quercetin-loaded TiO2 nanotubes coated with chitosan. Int. J. Biol. Macromol. 2016, 93, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Gombotz, W.R.; Wang, G.H.; Horbett, T.A.; Hoffman, A.S. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 1991, 25, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Popat, K.C.; Desai, T.A. Controlling nonspecific protein interactions in silicon biomicrosystems with nanostructured poly(ethylene glycol) films. Langmuir 2002, 18, 8728–8731. [Google Scholar] [CrossRef]
- Kingshott, P.; McArthur, S.; Thissen, H.; Castner, D.G.; Griesser, H.J. Ultrasensitive probing of the protein resistance of PEG surfaces by secondary ion mass spectrometry. Biomaterials 2002, 23, 4775–4785. [Google Scholar] [CrossRef] [PubMed]
- Lipka, J.; Semmler-Behnke, M.; Sperling, R.A.; Wenk, A.; Takenaka, S.; Schleh, C.; Kissel, T.; Parak, W.J.; Kreyling, W.G. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials 2010, 31, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Mano, S.S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Effect of Polyethylene Glycol Modification of TiO2 Nanoparticles on Cytotoxicity and Gene Expressions in Human Cell Lines. Int. J. Mol. Sci. 2012, 13, 3703–3717. [Google Scholar] [CrossRef]
- Marques, T.M.F.; Sales, D.A.; Silva, L.S.; Bezerra, R.D.S.; Silva, M.S.; Osajima, J.A.; Ferreira, O.P.; Ghosh, A.; Silva, E.C.; Viana, B.C.; et al. Amino-functionalized titanate nanotubes for highly efficient removal of anionic dye from aqueous solution. Appl. Surf. Sci. 2020, 512, 145659. [Google Scholar] [CrossRef]
- Nagasawa, S.; Yudasaka, M.; Hirahara, K.; Ichihashi, T.; Iijima, S. Effect of oxidation on single-wall carbon nanotubes. Chem. Phys. Lett. 2000, 328, 374–380. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef]
- Abo-Shosha, M.; El-Zairy, M.; Ibrahim, N. Preparation and rheology of new synthetic thickeners based on polyacrylic acid. Dye. Pigment. 1994, 24, 249–257. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Amr, A.; Eid, B.M.; Mohamed, Z.E.; Fahmy, H.M. Poly(acrylic acid)/poly(ethylene glycol) adduct for attaining multifunctional cellulosic fabrics. Carbohydr. Polym. 2012, 89, 648–660. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Abdullah, F.Z.; Ma’amor, A.; Daud, N.A.; Abd Hamid, S.B. Selective Synthesis of Peg-Monoester Using Cesium Heteropoly Acid as Heterogeneous Catalyst. Quim. Nova 2017, 40, 506–512. [Google Scholar] [CrossRef]
- Borges, M.E.; Diaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sust. Energ. Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Ranjous, Y.; Regdon Jr, G.; Pintye-Hódi, K.; Varga, T.; Szenti, I.; Kónya, Z.; Sovány, T. Optimization of the Production Process and Product Quality of Titanate Nanotube–Drug Composites. Nanomaterials 2019, 9, 1406. [Google Scholar] [CrossRef] [PubMed]
- Pouran, H.; Colodrero, R.P.; Wu, S.; Hix, G.; Zakharova, J.; Zhang, H. Assessment of ATR-FTIR spectroscopy with multivariate analysis to investigate the binding mechanisms of Ag and TiO2 nanoparticles to Chelex®-100 or Metsorb™ for the DGT technique. Anal. Methods 2020, 12, 959. [Google Scholar] [CrossRef]
- Liew, C.-M.; Ng, H.M.; Numan, A.; Ramesh, S. Poly(Acrylic acid)–Based Hybrid Inorganic–Organic Electrolytes Membrane for Electrical Double Layer Capacitors Application. Polymers 2016, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Bavykin, D.V.; Friedrich, J.M.; Lapkin, A.A.; Walsh, F.C. Stability of aqueous suspensions of titanate nanotubes. Chem. Mater. 2006, 18, 1124–1129. [Google Scholar] [CrossRef]
- Papa, A.-L.; Maurizi, L.; Vandroux, D.; Walker, P.; Millot, N. Synthesis of titanate nanotubes directly coated with USPIO in hydrothermal conditions: A new detectable nanocarrier. J. Phys. Chem. C 2011, 115, 19012–19017. [Google Scholar] [CrossRef]
- Papa, A.L.; Dumont, L.; Vandroux, D.; Millot, N. Titanate nanotubes: Towards a novel and safer nanovector for cardiomyocytes. Nanotoxicology 2013, 7, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Cruje, C.; Chithrani, D.J.J.N.R. Polyethylene glycol density and length affects nanoparticle uptake by cancer cells. J. Nanomed. Res. 2014, 1, 8. [Google Scholar] [CrossRef]
- Kamal, N.; Zaki, A.H.; El-Shahawy, A.A.; Sayed, O.M.; El-Dek, S.I. Changing the morphology of one-dimensional titanate nanostructures affects its tissue distribution and toxicity. Toxicol. Ind. Health 2020, 36, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, F.; Konya, Z.; Razga, Z.; Vecsernyes, M.; Kasa, P., Jr.; Pintye-Hodi, K.; Bacskay, I. Investigation of the cytotoxic effects of titanate nanotubes on Caco-2 cells. AAPS PharmSciTech 2014, 15, 858–861. [Google Scholar] [CrossRef]
- Wadhwa, S.; Rea, C.; O’Hare, P.; Mathur, A.; Roy, S.S.; Dunlop, P.S.; Byrne, J.A.; Burke, G.; Meenan, B.; McLaughlin, J.A. Comparative in vitro cytotoxicity study of carbon nanotubes and titania nanostructures on human lung epithelial cells. J. Hazard. Mater. 2011, 191, 56–61. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saker, R.; Jójárt-Laczkovich, O.; Regdon, G., Jr.; Takács, T.; Szenti, I.; Bózsity-Faragó, N.; Zupkó, I.; Sovány, T. Surface Modification of Titanate Nanotubes with a Carboxylic Arm for Further Functionalization Intended to Pharmaceutical Applications. Pharmaceutics 2023, 15, 2780. https://doi.org/10.3390/pharmaceutics15122780
Saker R, Jójárt-Laczkovich O, Regdon G Jr., Takács T, Szenti I, Bózsity-Faragó N, Zupkó I, Sovány T. Surface Modification of Titanate Nanotubes with a Carboxylic Arm for Further Functionalization Intended to Pharmaceutical Applications. Pharmaceutics. 2023; 15(12):2780. https://doi.org/10.3390/pharmaceutics15122780
Chicago/Turabian StyleSaker, Ranim, Orsolya Jójárt-Laczkovich, Géza Regdon, Jr., Tamás Takács, Imre Szenti, Noémi Bózsity-Faragó, István Zupkó, and Tamás Sovány. 2023. "Surface Modification of Titanate Nanotubes with a Carboxylic Arm for Further Functionalization Intended to Pharmaceutical Applications" Pharmaceutics 15, no. 12: 2780. https://doi.org/10.3390/pharmaceutics15122780
APA StyleSaker, R., Jójárt-Laczkovich, O., Regdon, G., Jr., Takács, T., Szenti, I., Bózsity-Faragó, N., Zupkó, I., & Sovány, T. (2023). Surface Modification of Titanate Nanotubes with a Carboxylic Arm for Further Functionalization Intended to Pharmaceutical Applications. Pharmaceutics, 15(12), 2780. https://doi.org/10.3390/pharmaceutics15122780




