Lipid Liquid Crystal Nanoparticles: Promising Photosensitizer Carriers for the Treatment of Infected Cutaneous Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GaPP-LCNP
2.3. Characterization of Nanoparticles Diameter
2.4. Determination of GaPP Concentration
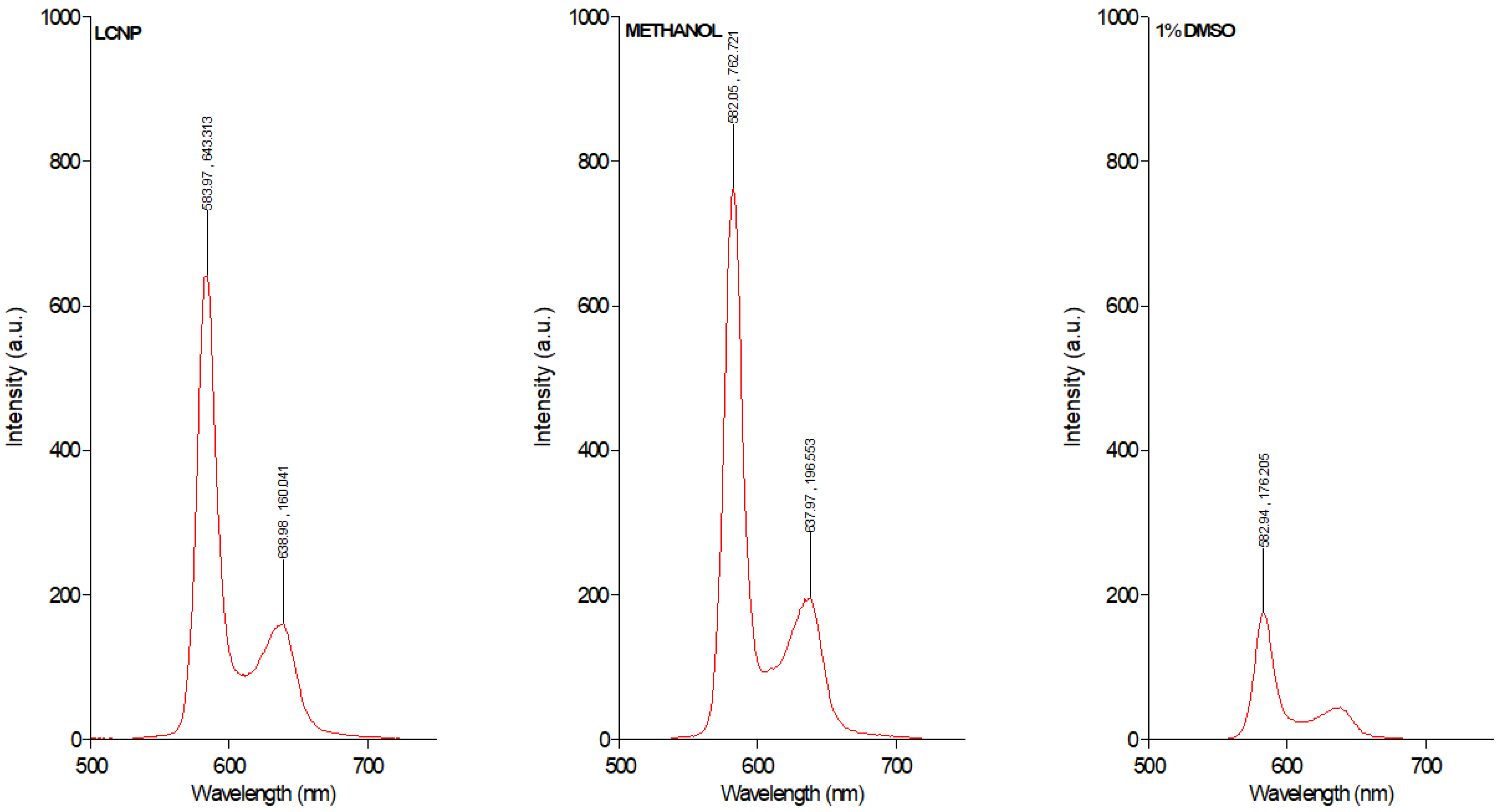
2.5. Spectrosocpic Studies
2.6. Illumination Set Up
2.7. Antibiofilm Activity of GaPP-LCNP In Vitro
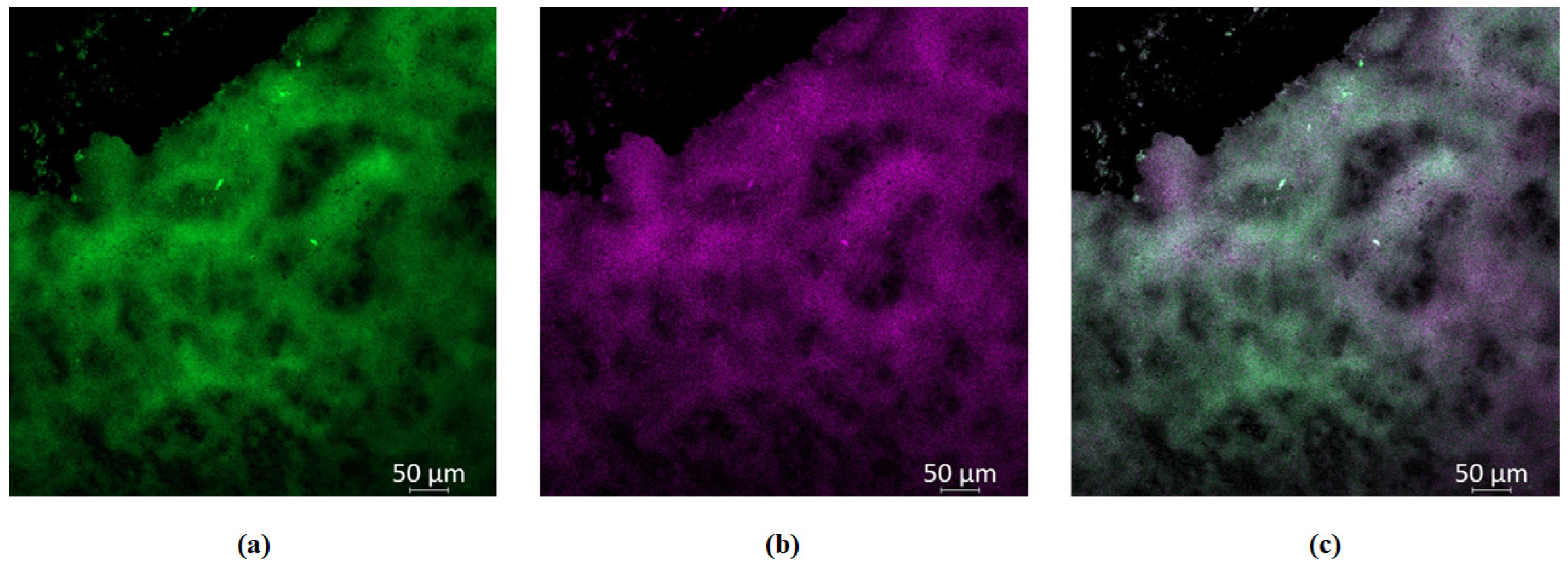
2.8. Study of GaPP-LCNP Distribution in Biofilms In Vitro
2.9. Antibiofilm Activity in an Ex Vivo Infection Model
2.10. Efficacy Study of GaPP-LCNP in a Chronic Infected Wound in Mice
2.11. Wound Healing Assessment
3. Results and Discussion
3.1. Preparation and Characterization of Dispersible GaPP-LCNP
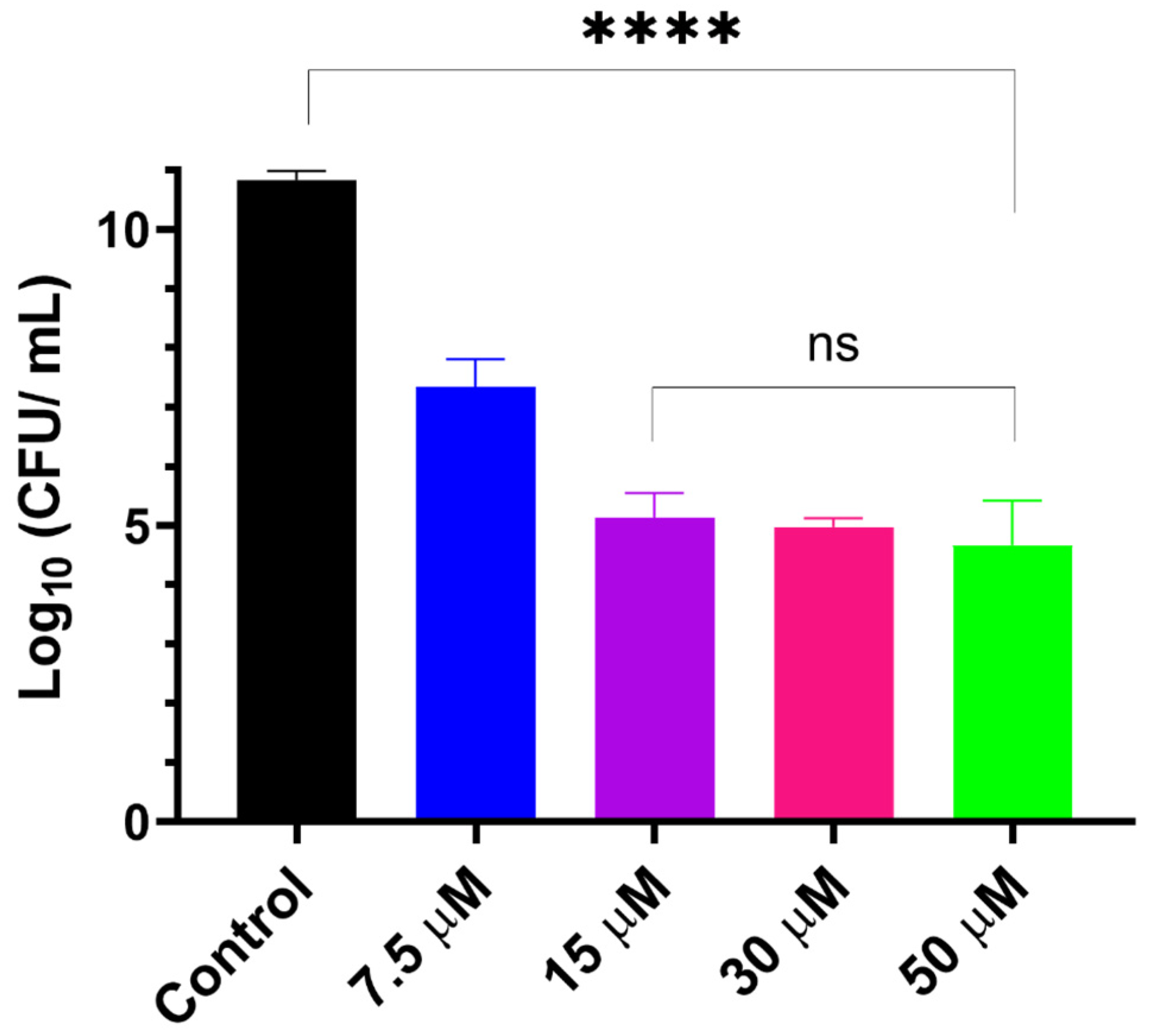
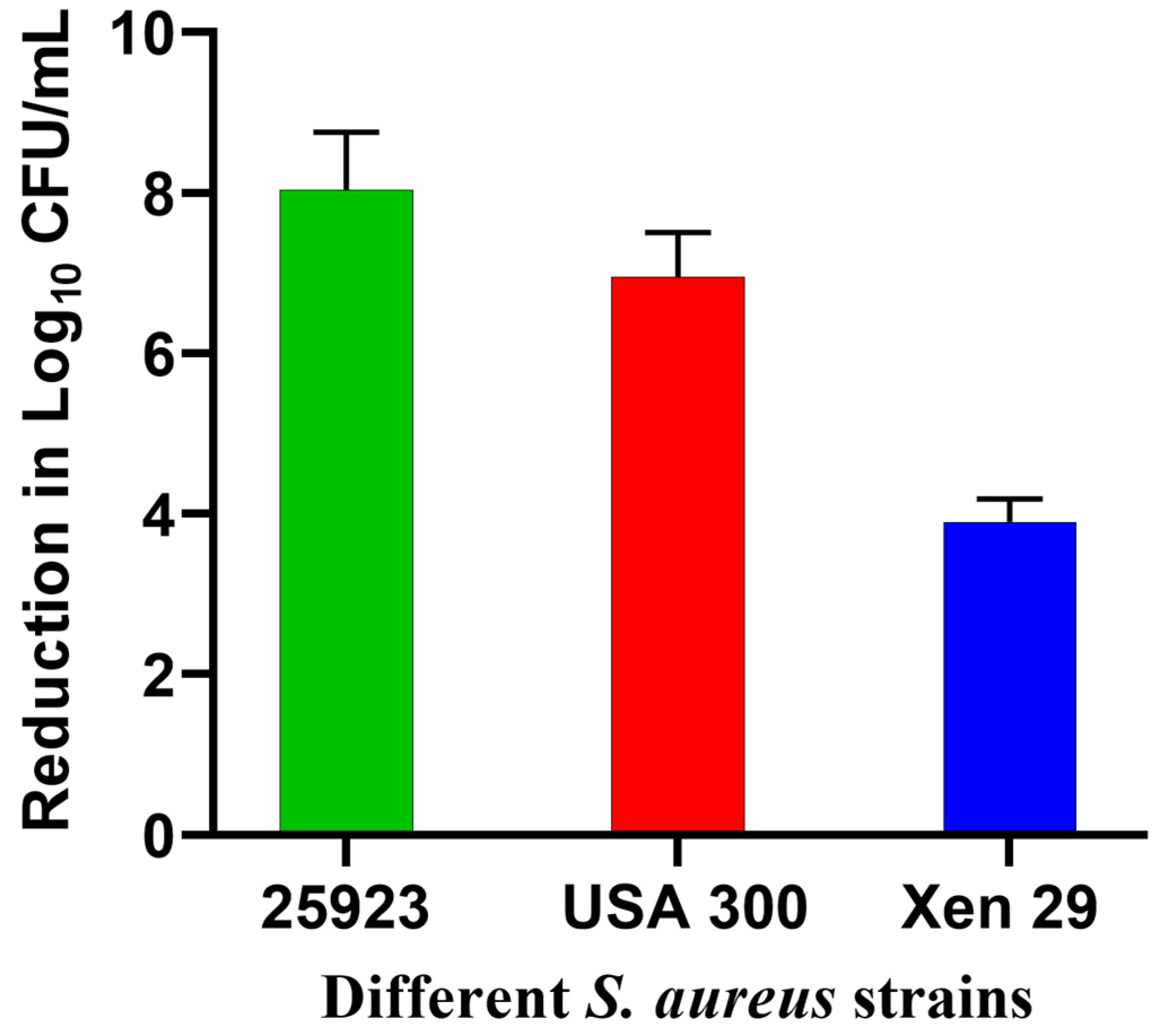
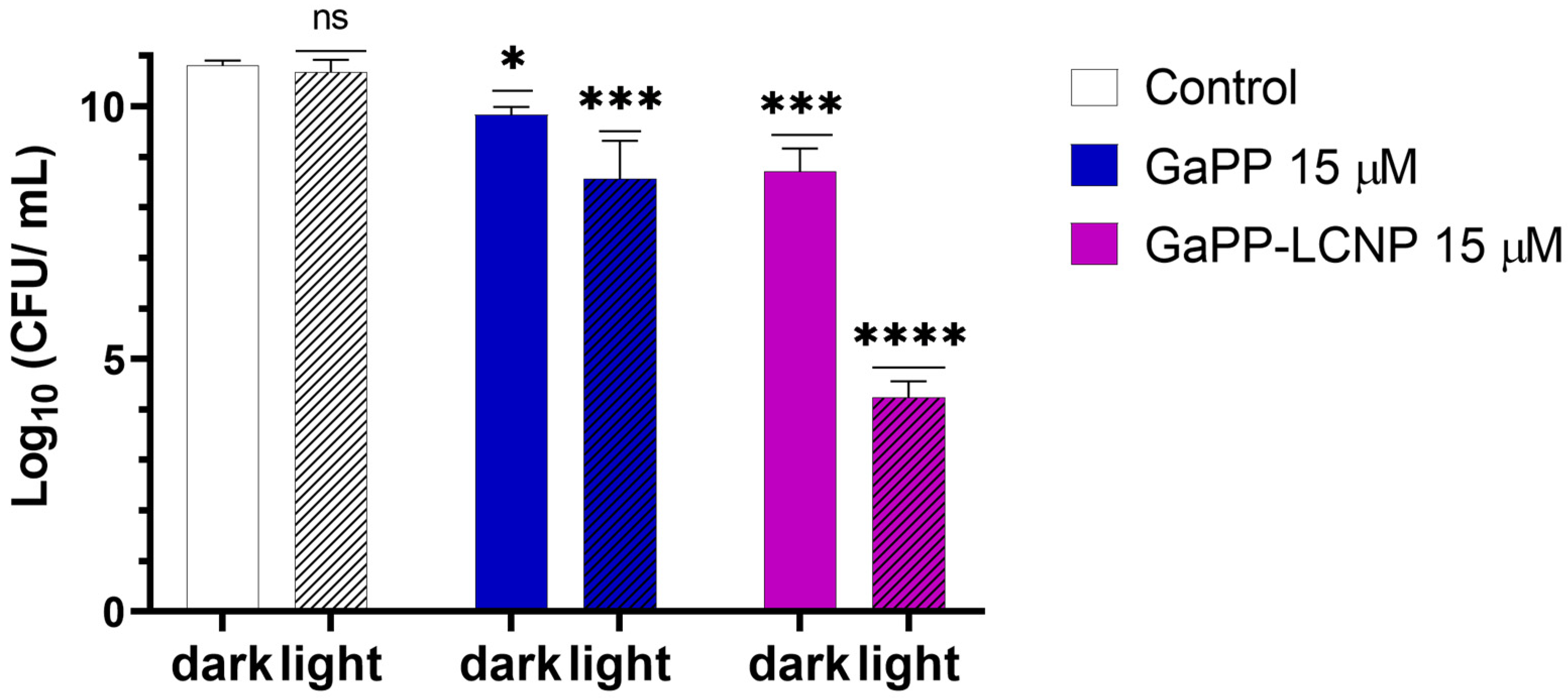
3.2. Evaluation of Antibiofilm Activity In Vitro
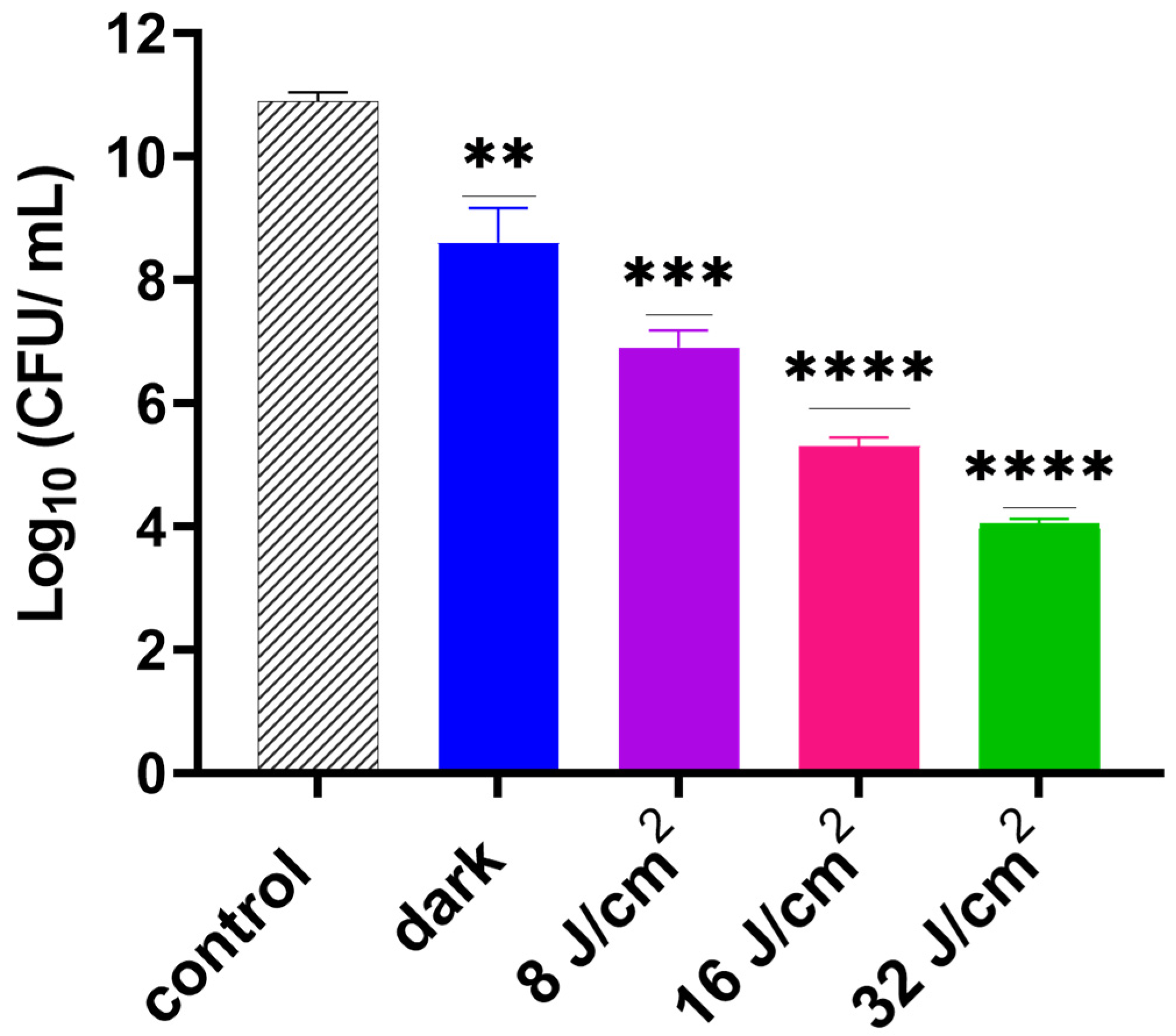
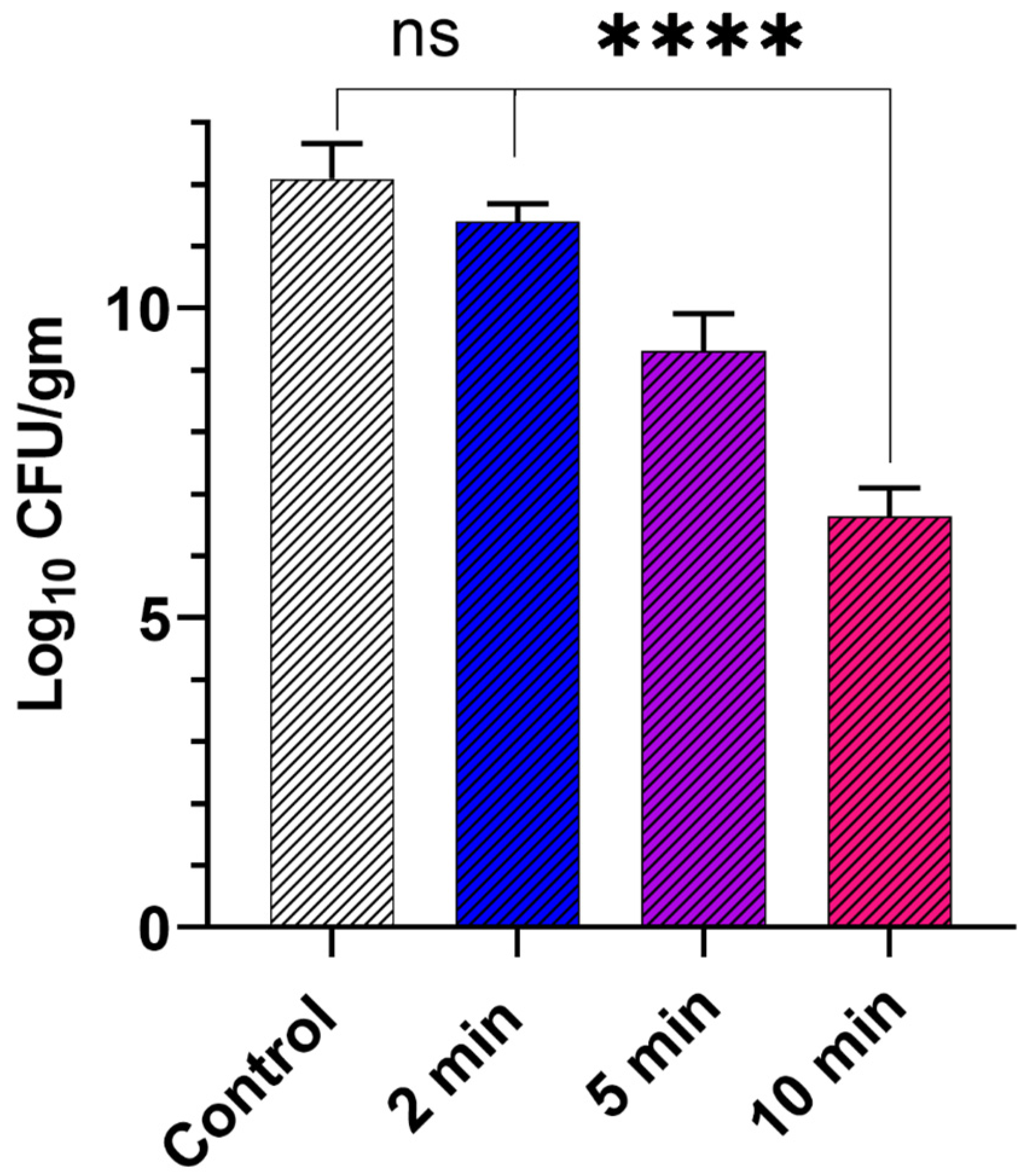
3.3. Efficacy Study on Infected Skin Ex Vivo Model
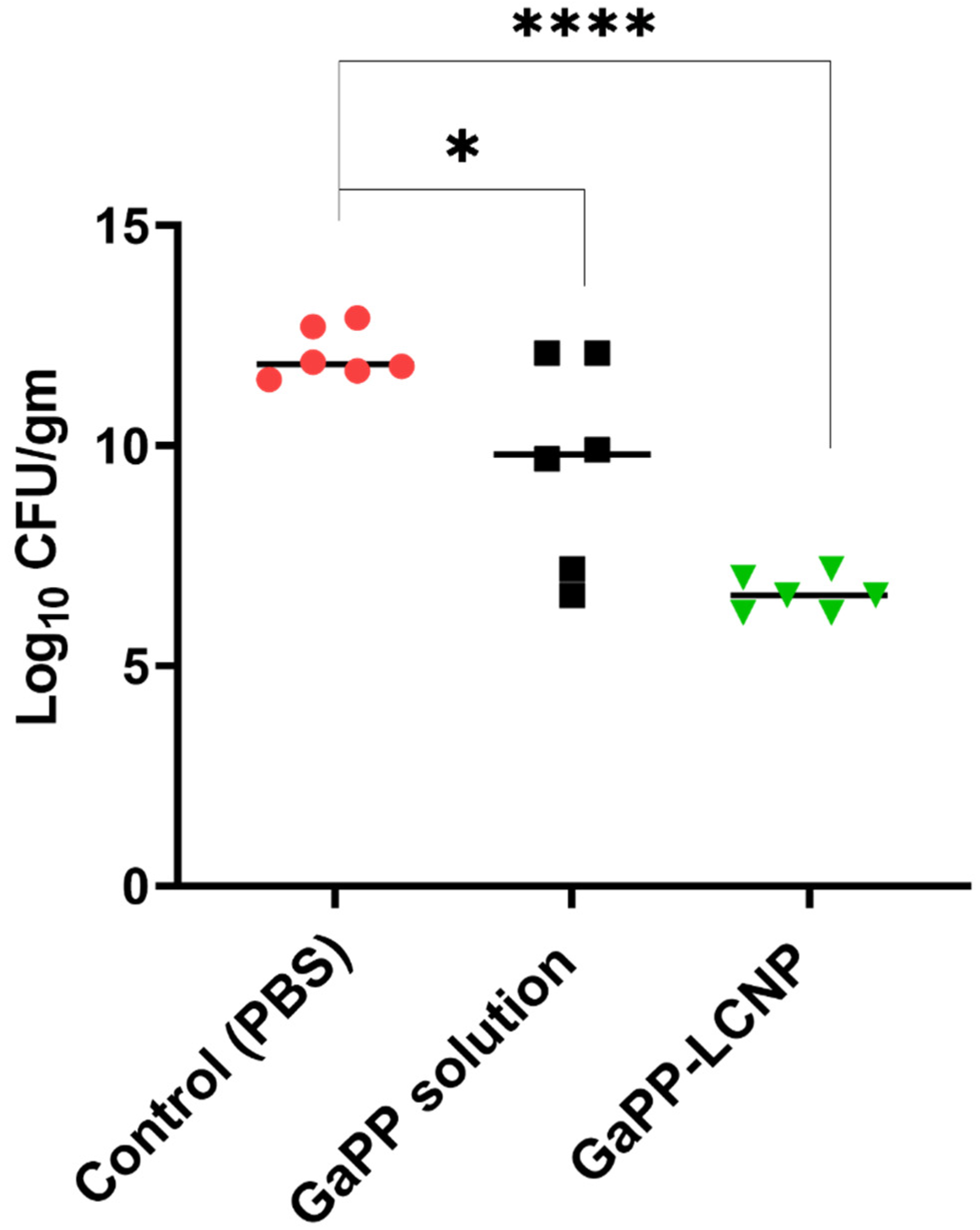
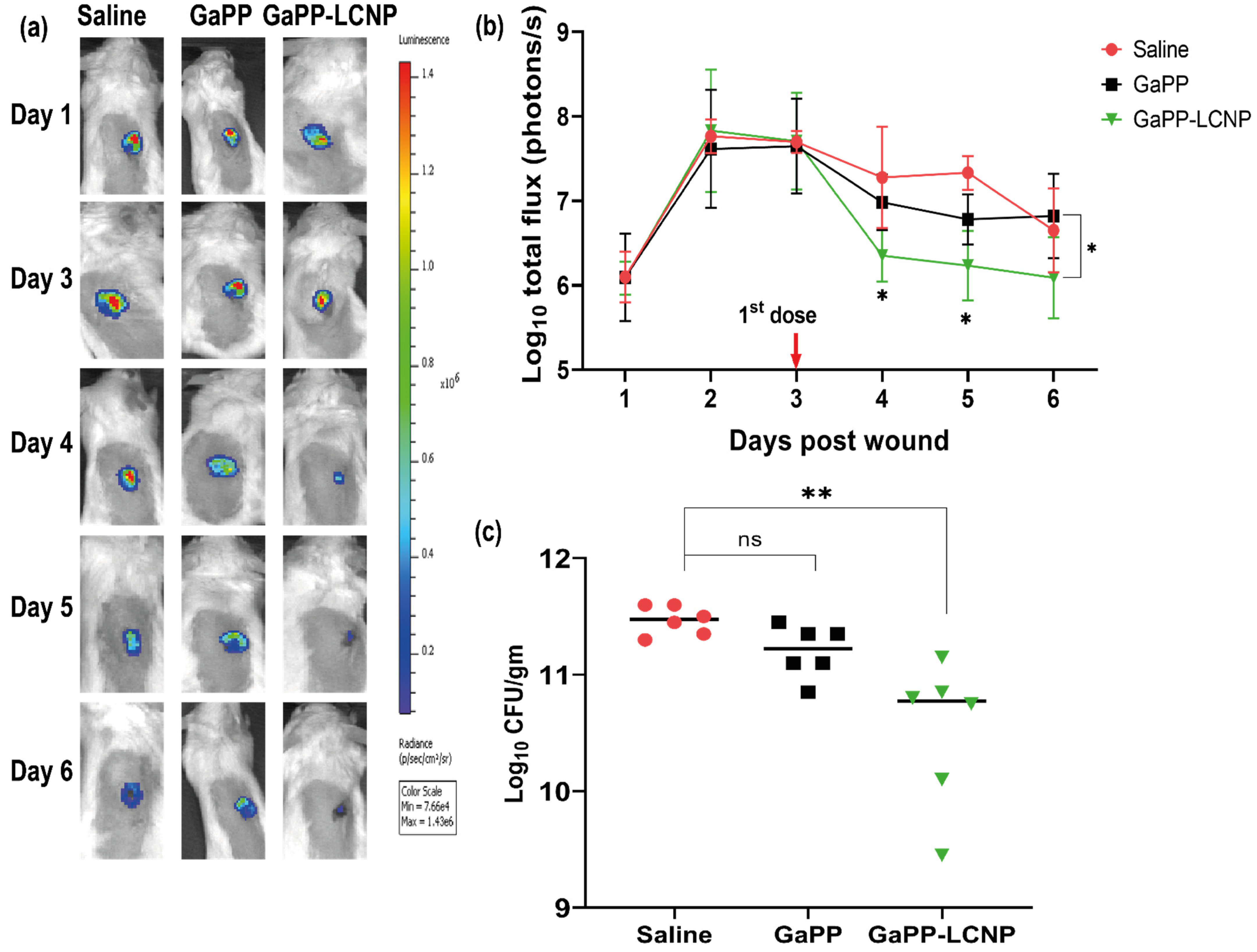
3.4. Efficacy Study in Infected Wounds In Vivo
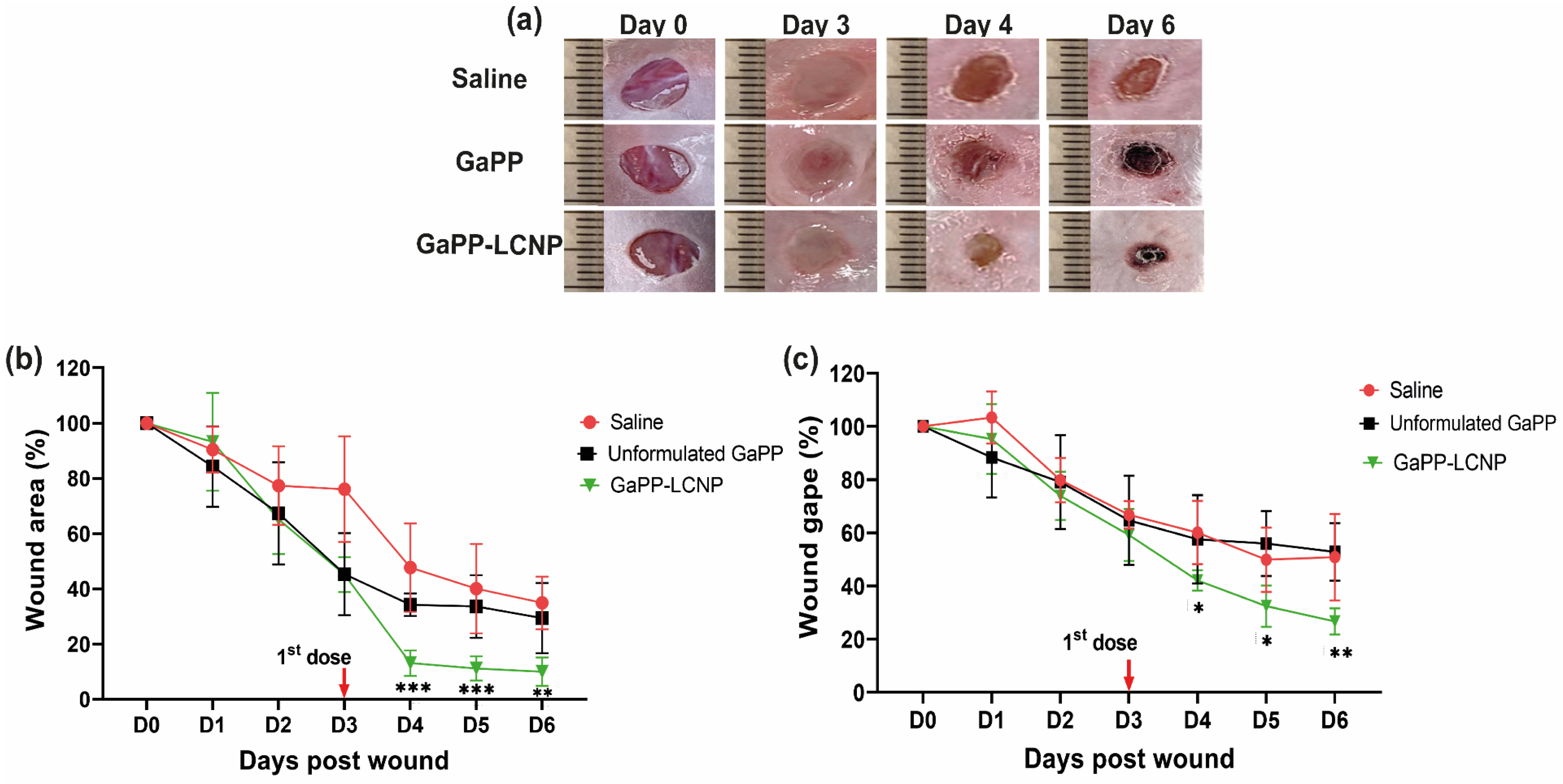
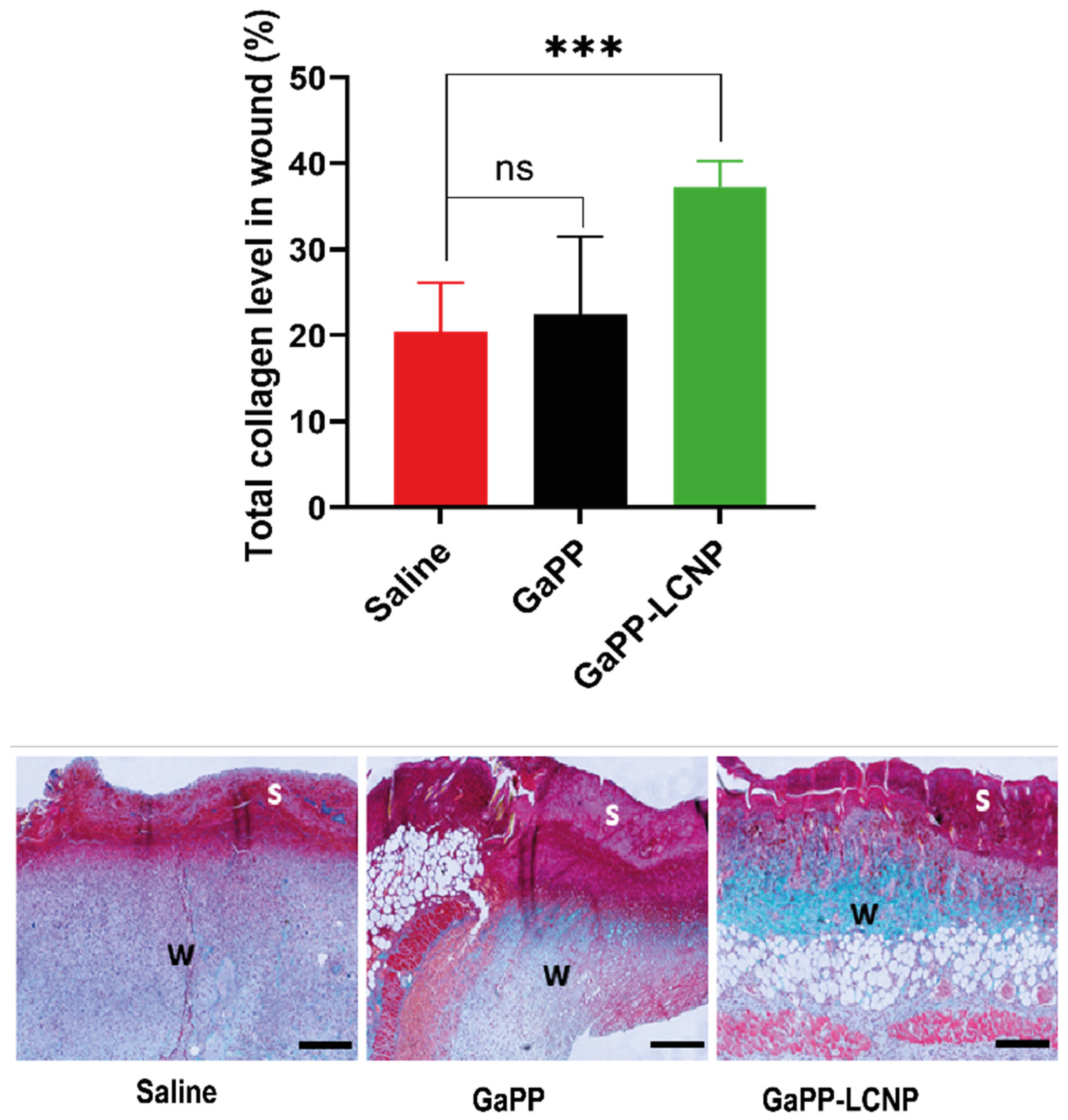
3.5. Effect of GaPP-LCNP on Wound Healing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, B.M.; Järbrink, K.; Martinengo, L.; Car, J.; Harding, K.; Schmidtchen, A. Need for Improved Definition of "Chronic Wounds" in Clinical Studies. Acta Derm. Venereol. 2018, 98, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Haidari, H.; Bright, R.; Garg, S.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Eradication of Mature Bacterial Biofilms with Concurrent Improvement in Chronic Wound Healing Using Silver Nanoparticle Hydrogel Treatment. Biomedicines 2021, 9, 1182. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Maheswary, T.; Nurul, A.A.; Fauzi, M.B. The Insights of Microbes’ Roles in Wound Healing: A Comprehensive Review. Pharmaceutics 2021, 13, 981. [Google Scholar] [CrossRef]
- Shami, A.; Al-Mijalli, S.; Pongchaikul, P.; Al-Barrag, A.; AbduRahim, S. The prevalence of the culturable human skin aerobic bacteria in Riyadh, Saudi Arabia. BMC Microbiol. 2019, 19, 189. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial biofilms. J. Cell. Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.; Thomas, N.; Barnes, T.J.; Prestidge, C.A. Nanomaterials enabling clinical translation of antimicrobial photodynamic therapy. J. Control. Release 2022, 346, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-N.; Hsu, R.; Chen, H.; Wong, T.-W. Daylight Photodynamic Therapy: An Update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.B.; Ferrisse, T.M.; Fontana, C.R.; Basso, F.G.; Brighenti, F.L. Photodynamic therapy for treating infected skin wounds: A systematic review and meta-analysis from randomized clinical trials. Photodiagnosis Photodyn. Ther. 2022, 40, 103118. [Google Scholar] [CrossRef] [PubMed]
- Oyama, J.; Fernandes Herculano Ramos-Milaré, Á.C.; Lopes Lera-Nonose, D.S.S.; Nesi-Reis, V.; Galhardo Demarchi, I.; Alessi Aristides, S.M.; Juarez Vieira Teixeira, J.; Gomes Verzignassi Silveira, T.; Campana Lonardoni, M.V. Photodynamic therapy in wound healing in vivo: A systematic review. Photodiagnosis Photodyn. Ther. 2020, 30, 101682. [Google Scholar] [CrossRef]
- Awad, M.; Barnes, T.J.; Joyce, P.; Thomas, N.; Prestidge, C.A. Liquid crystalline lipid nanoparticle promotes the photodynamic activity of gallium protoporphyrin against S. aureus biofilms. J. Photochem. Photobiol. B Biol. 2022, 232, 112474. [Google Scholar] [CrossRef]
- Hu, W.-P.; Wang, J.-J.; Yu, C.-L.; Lan, C.-C.E.; Chen, G.-S.; Yu, H.-S. Helium-Neon Laser Irradiation Stimulates Cell Proliferation through Photostimulatory Effects in Mitochondria. J. Investig. Dermatol. 2007, 127, 2048–2057. [Google Scholar] [CrossRef]
- Rudenko, T.G.; Shekhter, A.B.; Guller, A.E.; Aksenova, N.A.; Glagolev, N.N.; Ivanov, A.V.; Aboyants, R.K.; Kotova, S.L.; Solovieva, A.B. Specific Features of Early Stage of the Wound Healing Process Occurring Against the Background of Photodynamic Therapy Using Fotoditazin Photosensitizer–Amphiphilic Polymer Complexes. Photochem. Photobiol. 2014, 90, 1413–1422. [Google Scholar] [CrossRef]
- Sun, Y.; Ogawa, R.; Xiao, B.H.; Feng, Y.X.; Wu, Y.; Chen, L.H.; Gao, X.H.; Chen, H.D. Antimicrobial photodynamic therapy in skin wound healing: A systematic review of animal studies. Int. Wound J. 2020, 17, 285–299. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Chien, H.-F.; Chang, P.-H.; Chen, Y.-C.; Jay, M.; Tsai, T.; Chen, C.-T. Photodynamic inactivation of chlorin e6-loaded CTAB-liposomes against Candida albicans. Lasers Surg. Med. 2013, 45, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.; Barnes, T.J.; Thomas, N.; Joyce, P.; Prestidge, C.A. Gallium Protoporphyrin Liquid Crystalline Lipid Nanoparticles: A Third-Generation Photosensitizer against Pseudomonas aeruginosa Biofilms. Pharmaceutics 2022, 14, 2124. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.R.; Carvalho-Wodarz, C.d.S.; Horstmann, J.C.; Lehr, C.-M.; Prestidge, C.A.; Thomas, N. Tobramycin Liquid Crystal Nanoparticles Eradicate Cystic Fibrosis-Related Pseudomonas aeruginosa Biofilms. Small 2021, 17, 2100531. [Google Scholar] [CrossRef]
- Thorn, C.R.; Wignall, A.; Kopecki, Z.; Kral, A.; Prestidge, C.A.; Thomas, N. Liquid Crystal Nanoparticles Enhance Tobramycin Efficacy in a Murine Model of Pseudomonas aeruginosa Biofilm Wound Infection. ACS Infect. Dis. 2022, 8, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Haidari, H.; Bright, R.; Strudwick, X.L.; Garg, S.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Multifunctional ultrasmall AgNP hydrogel accelerates healing of S. aureus infected wounds. Acta Biomater. 2021, 128, 420–434. [Google Scholar] [CrossRef]
- Kopecki, Z.; Arkell, R.; Powell, B.C.; Cowin, A.J. Flightless I Regulates Hemidesmosome Formation and Integrin-Mediated Cellular Adhesion and Migration during Wound Repair. J. Investig. Dermatol. 2009, 129, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Kopecki, Z.; Stevens, N.E.; Yang, G.N.; Melville, E.; Cowin, A.J. Recombinant Leucine-Rich Repeat Flightless-Interacting Protein-1 Improves Healing of Acute Wounds through Its Effects on Proliferation Inflammation and Collagen Deposition. Int. J. Mol. Sci. 2018, 19, 2014. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Ansari, M.J.; Singh, A.; Hassan, A.; Abdelgawad, M.A.; Shrivastav, P.; Abualsoud, B.M.; Amaral, L.S.; Pramanik, S. Cubosomes as an emerging platform for drug delivery: A review of the state of the art. J. Mater. Chem. B 2022, 10, 2781–2819. [Google Scholar] [CrossRef]
- Spicer, P.T.; Hayden, K.L.; Lynch, M.L.; Ofori-Boateng, A.; Burns, J.L. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir 2001, 17, 5748–5756. [Google Scholar] [CrossRef]
- Pinter, T.B.J.; Dodd, E.L.; Bohle, D.S.; Stillman, M.J. Spectroscopic and Theoretical Studies of Ga(III)protoporphyrin-IX and Its Reactions with Myoglobin. Inorg. Chem. 2012, 51, 3743–3753. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical Properties of Protoporphyrin IX, Pyropheophorbide-a, and Photofrin® in Different Conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Scolaro, L.M.; Castriciano, M.; Romeo, A.; Patanè, S.; Cefalì, E.; Allegrini, M. Aggregation Behavior of Protoporphyrin IX in Aqueous Solutions: Clear Evidence of Vesicle Formation. J. Phys. Chem. B 2002, 106, 2453–2459. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, P.; Jiang, D.; Yang, G.; Xue, Y.; Tang, Z.; Zhang, M.; Wang, H.; Jiang, X.; Wu, Y.; et al. In Situ Catalytic Reaction for Solving the Aggregation of Hydrophobic Photosensitizers in Tumor. ACS Appl. Mater. Interfaces 2020, 12, 5624–5632. [Google Scholar] [CrossRef]
- Plenagl, N.; Seitz, B.S.; Reddy Pinnapireddy, S.; Jedelská, J.; Brüßler, J.; Bakowsky, U. Hypericin Loaded Liposomes for Anti-Microbial Photodynamic Therapy of Gram-Positive Bacteria. Int. J. Pharm. 2018, 215, 1700837. [Google Scholar] [CrossRef]
- Charron, D.M.; Yousefalizadeh, G.; Buzzá, H.H.; Rajora, M.A.; Chen, J.; Stamplecoskie, K.G.; Zheng, G. Photophysics of J-Aggregating Porphyrin-Lipid Photosensitizers in Liposomes: Impact of Lipid Saturation. Langmuir 2020, 36, 5385–5393. [Google Scholar] [CrossRef]
- Azmi, I.D.; Moghimi, S.M.; Yaghmur, A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef]
- Albayaty, Y.N.; Thomas, N.; Jambhrunkar, M.; Al-Hawwas, M.; Kral, A.; Thorn, C.R.; Prestidge, C.A. Enzyme responsive copolymer micelles enhance the anti-biofilm efficacy of the antiseptic chlorhexidine. Int. J. Pharm. 2019, 566, 329–341. [Google Scholar] [CrossRef]
- Zhang, J.; Suo, Y.; Zhang, D.; Jin, F.; Zhao, H.; Shi, C. Genetic and Virulent Difference Between Pigmented and Non-pigmented Staphylococcus aureus. Front. Microbiol. 2018, 9, 598. [Google Scholar] [CrossRef]
- Yarwood, J.M.; Paquette, K.M.; Tikh, I.B.; Volper, E.M.; Greenberg, E.P. Generation of Virulence Factor Variants in Staphylococcus aureus Biofilms. J. Bacteriol. 2007, 189, 7961–7967. [Google Scholar] [CrossRef]
- Ooi, M.L.; Richter, K.; Drilling, A.J.; Thomas, N.; Prestidge, C.A.; James, C.; Moratti, S.; Vreugde, S.; Psaltis, A.J.; Wormald, P.-J. Safety and Efficacy of Topical Chitogel-Deferiprone-Gallium Protoporphyrin in Sheep Model. Front. Microbiol. 2018, 9, 917. [Google Scholar] [CrossRef]
- Graf, A.C.; Leonard, A.; Schäuble, M.; Rieckmann, L.M.; Hoyer, J.; Maass, S.; Lalk, M.; Becher, D.; Pané-Farré, J.; Riedel, K. Virulence Factors Produced by Staphylococcus aureus Biofilms Have a Moonlighting Function Contributing to Biofilm Integrity. Mol. Cell. Proteom. 2019, 18, 1036–1053. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.; Tulsiyan, K.D.; Kumari, A.; Das, R.; Biswal, H.S. Thiolumazines as Heavy-Atom-Free Photosensitizers for Applications in Daylight Photodynamic Therapy: Insights from Ultrafast Excited-State Dynamics. J. Phys. Chem. B 2022, 126, 6083–6094. [Google Scholar] [CrossRef]
- Atif, M. A study on the effects of photosensitizer concentration on singlet oxygen mediated photobleaching. Laser Phys. 2013, 23, 055603. [Google Scholar] [CrossRef]
- Hazan, R.; Que, Y.-A.; Maura, D.; Rahme, L.G. A method for high throughput determination of viable bacteria cell counts in 96-well plates. BMC Microbiol. 2012, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Dyett, B.P.; Yu, H.; Sarkar, S.; Strachan, J.B.; Drummond, C.J.; Conn, C.E. Uptake Dynamics of Cubosome Nanocarriers at Bacterial Surfaces and the Routes for Cargo Internalization. ACS Appl. Mater. Interfaces 2021, 13, 53530–53540. [Google Scholar] [CrossRef]
- Bazylińska, U.; Kulbacka, J.; Schmidt, J.; Talmon, Y.; Murgia, S. Polymer-free cubosomes for simultaneous bioimaging and photodynamic action of photosensitizers in melanoma skin cancer cells. J. Colloid Interface Sci. 2018, 522, 163–173. [Google Scholar] [CrossRef]
- Nafee, N.; Youssef, A.; El-Gowelli, H.; Asem, H.; Kandil, S. Antibiotic-free nanotherapeutics: Hypericin nanoparticles thereof for improved in vitro and in vivo antimicrobial photodynamic therapy and wound healing. Int. J. Pharm. 2013, 454, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Esfahani, H.; Zandsalimi, K.; Shahidi, F.K.; Abrahamse, H. Accelerating skin regeneration and wound healing by controlled ROS from photodynamic treatment. Inflamm. Regen. 2022, 42, 40. [Google Scholar] [CrossRef]
- Hoffmann, M.H.; Griffiths, H.R. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: Evidence from preclinical models. Free. Radic. Biol. Med. 2018, 125, 62–71. [Google Scholar] [CrossRef]
- Chan, E.C.; Peshavariya, H.M.; Liu, G.-S.; Jiang, F.; Lim, S.-Y.; Dusting, G.J. Nox4 modulates collagen production stimulated by transforming growth factor β1 in vivo and in vitro. Biochem. Biophys. Res. Commun. 2013, 430, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Li, R.; Zhu, L.; Yu, X.; Zhu, S.; Pang, L.; Ma, J.; Du, L.; Jin, Y. Wound healing of laser injured skin with glycerol monooleicate cubic liquid crystal. Burns 2020, 46, 1381–1388. [Google Scholar] [CrossRef] [PubMed]

| Sample | z-Average Diameter (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) | EE% | DL W/W% |
|---|---|---|---|---|---|
| Blank LCNP | 182 ± 2.3 | 0.16 ± 0.03 | −23.6 ± 1.2 | ||
| GaPP-LCNP | 178 ± 3.1 | 0.19 ± 0.04 | −30.5 ± 2.2 | 98.3 % ± 4.3 | 3.3 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, M.; Kopecki, Z.; Barnes, T.J.; Wignall, A.; Joyce, P.; Thomas, N.; Prestidge, C.A. Lipid Liquid Crystal Nanoparticles: Promising Photosensitizer Carriers for the Treatment of Infected Cutaneous Wounds. Pharmaceutics 2023, 15, 305. https://doi.org/10.3390/pharmaceutics15020305
Awad M, Kopecki Z, Barnes TJ, Wignall A, Joyce P, Thomas N, Prestidge CA. Lipid Liquid Crystal Nanoparticles: Promising Photosensitizer Carriers for the Treatment of Infected Cutaneous Wounds. Pharmaceutics. 2023; 15(2):305. https://doi.org/10.3390/pharmaceutics15020305
Chicago/Turabian StyleAwad, Muhammed, Zlatko Kopecki, Timothy J. Barnes, Anthony Wignall, Paul Joyce, Nicky Thomas, and Clive A. Prestidge. 2023. "Lipid Liquid Crystal Nanoparticles: Promising Photosensitizer Carriers for the Treatment of Infected Cutaneous Wounds" Pharmaceutics 15, no. 2: 305. https://doi.org/10.3390/pharmaceutics15020305
APA StyleAwad, M., Kopecki, Z., Barnes, T. J., Wignall, A., Joyce, P., Thomas, N., & Prestidge, C. A. (2023). Lipid Liquid Crystal Nanoparticles: Promising Photosensitizer Carriers for the Treatment of Infected Cutaneous Wounds. Pharmaceutics, 15(2), 305. https://doi.org/10.3390/pharmaceutics15020305










