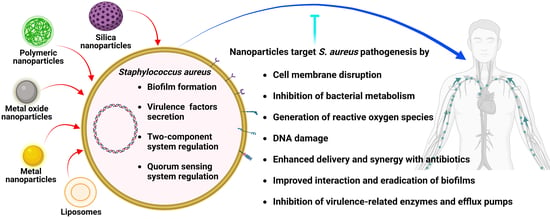
Regulation of Staphylococcus aureus Virulence and Application of Nanotherapeutics to Eradicate S. aureus Infection
Abstract
:1. Introduction
2. Host Defense and Pathogenesis in Staphylococcus aureus
2.1. Staphylococcus aureus and Its Impact on Human Health
2.2. The Host Immune Response to Staphylococcus aureus
2.3. Immune Evasion by Staphylococcus aureus
2.4. Challenging Issues of Staphylococcus aureus in Healthcare
2.5. Biofilm Formation in Staphylococcus aureus
2.5.1. Overview of Biofilm Formation
2.5.2. Biofilm Matrixome and Dynamics
2.6. Virulence Regulation in Staphylococcus aureus
2.6.1. Regulation of the Two-Component System AgrAC in Staphylococcus aureus
| Regulators | Functions |
|---|---|
| AgrCA | Cell-to-cell communication system, where the bacteria communicate with self-produced autoinducing peptide Essential for biofilm disassembly and initial attachment Agr regulation represses adhesins and stimulates phenol-soluble modulins and proteases |
| AirSR/YhcSR | Involved in cellular homeostasis and energy production Important for aerobic and anaerobic growth Positively regulates the expression of the sspABC operon |
| ArlRS | Positive regulator of MgrA and Spx Regulates many cellular processes, including cell wall-anchored adhesins, virulence factors, polysaccharides, and capsular synthesis genes |
| BceRS | Positive regulator of bceAB and vraDE genes Confers resistance towards bacteriocins by transporting bacteriocins outside the cytoplasm through BceAB and VraDE proteins |
| BraSR | Confers resistance towards nisin A and nukacin ISK-1 Exhibits significant regulatory effects on the symbiosis of S. aureus and the type I bacteriocin strain |
| CodY | Strain-dependent regulation of PIA Positive regulator of biofilm formation through the induction of ica operon Cytoplasmic regulator for metabolic response Positive regulator of virulence factor protease |
| GraRS/ApsRS | Belongs to the intramembrane-sensing histidine kinase (IM-HK) familyPositively regulates expression of the dlt operon Essential in evading host defense mechanisms such as neutrophil killing and cationic AMPs |
| HptRS | Hexose phosphate transporter Primarily involved with hptA (initiates autophosphorylation of hptS based on the phosphate concentration), uhpT (downstream regulatory protein transports phosphate/fosfomycin into the bacterial cell to maintain physiological metabolism) Mutation reduces the uptake of fosfomycin (structure similar to phosphoenolpyruvate) and increases the bacterial resistance |
| HssRS | Heme sensor system responding to heme exposure Positive regulator of the efflux pump HrtAB (heme-regulated transporter), which plays a role in maintaining intracellular heme homeostasis Found to have a role in regulating virulence and modulating host immune response |
| KdpDE | Involved in sensing potassium (K+) limitation or salt stress It plays a role in the expression of genes involved in capsule biosynthesis, amino acid and central metabolism |
| LytRS | Regulates cell lysis and induces the expression of irgAB Plays a predominant role in eDNA-mediated biofilm formation |
| MgrA | SarA family cytoplasmic regulator and prime effector of the ArlRS system Repression of adhesins and negative regulator of biofilm formation |
| NreCB | Involved in oxygen sensing; converts nitrate and nitrite as final oxygen acceptors Regulates the gene clusters involved in nitrate (narGHJI) and nitrite reduction (nirRBD) Expression is controlled by NreA, which inhibits NreB autophosphorylation |
| NsaRS | Belongs to the IM-HK family Important in conferring resistance towards nisin |
| PhoRP | Involved in response to inorganic phosphate starvation Positive regulator of pstSCAB and nptA, and also modulates the expression of pitA |
| Rot | SarA family cytoplasmic regulator and prime effector of the Agr QS system Positive regulator of biofilm formation through protease repression and adhesin induction |
| SaeRS | Stimulates adhesin and nuclease production and is very much crucial in the biofilm maturation process |
| SarA | Positive regulator of biofilm formation through the induction of ica operon Induces biofilm formation through the repression of protease production |
| SigB | Strain-dependent regulation of PIA Positive regulator of virulence factors protease and nucleaseEssential for initial attachment and biofilm disassembly Cytoplasmic regulator for stress response |
| SrrAB | Global regulator for S. aureus virulence Critical for survival under environmental stress Regulation of genes involved in anaerobic metabolism, nitrous oxide detoxification, cytochrome biosynthesis and assembly, biofilm formation, hydrogen peroxide metabolism, and programmed cell death |
| VraRS | Associated with vancomycin resistance Essential for cell wall synthesis Positive regulator of PBP2, SgtB, and MurZ |
| WalKR/YycGF | Essential for cell wall metabolism and cell viability Positive regulator of AtlA, SsaA, IsaA, and LytM Regulates the expression of host matrix interaction proteins, cytolytic toxins, and proteins involved in host immune evasion |
2.6.2. Intercellular Adhesin-Mediated Biofilm Formation and Beyond
2.7. Role of Mobile Genetic Elements in Antibiotic Resistance and Pathogenesis in Staphylococcus aureus
3. Nanotechnology-Based Approaches to Target Staphylococcus aureus Pathogenesis
3.1. Synthesis of NPs
3.2. Inorganic NPs
3.2.1. Gold NPs
3.2.2. Silver NPs
3.2.3. Copper NPs
3.2.4. Metal Oxide NPs
3.2.5. Action Mechanism of Metallic NPs
3.2.6. Silica-Based NPs
3.2.7. Quantum Dots and Carbon Nanodots
| Reducing or Capping Agent/Encapsulated Drug | Properties of the NPs | Biological Activities | Reference(s) |
|---|---|---|---|
| Gold NPs (GNPs) | |||
| Padina tetrastromatica-mediated synthesis of GNPs | M: Green synthesis AS: 1–20 nm S: Spherical PDI: ~23 nm | GNPs showed an MIC of 25 µg/mL Higher concentrations of GNPs also exhibited biofilm-eradicating ability | [173] |
| Polypeptide polymer-conjugated GNPs | M: Chemical reduction method S: Spherical AS: 23 nm AZP: 24 mV | Polypeptide-conjugated GNPs exhibited potent antibacterial activities against clinical isolates of MDR Gram-positive bacteria, such as MRSA Excellent in vitro and in vivo biocompatibility Studies with the antioxidant N-acetyl-L-cysteine suggested that oxidative stress is responsible for the antibacterial activity of these GNPs | [174] |
| Caffeine-loaded GNPs | S: Spherical AS: 77.9 nm | MIC was 512 µg/mL Biofilm inhibitory and biofilm eradication concentrations of 256 and 512 µg/mL, respectively GNPs eradicated persister cells of S. aureus | [124] |
| Silver NPs (SNPs) | |||
| Desertifilum sp.-mediated synthesis of SNPs | M: Green synthesis S: Spherical AS: 4.5–26 nm | Comparing the growth inhibitory activity against different pathogens, MRSA was more susceptible to the SNPs (MIC 1.2 mg/mL) Anti-staphylococcal activity of SNPs was related to ROS-induced oxidative stress | [175] |
| SNPs | M: Microwave technique S: Spherical AS: 1-3 nm AZP: Positively charged | Interaction between SNPs and bacterial cell wall caused leakage of cytoplasmic material MIC of SNPs was 12.5 ppm against S. aureus Eradication of mixed species biofilms (Candida albicans and S. aureus) in a dose-dependent manner, with 0.53 ppm as the IC50 value SNP-functionalized catheter material was less prone to mixed species biofilm formation | [176,177] |
| Commercial SNPs | AS: 10 nm | Photolysis of staphyloxanthin via blue light increased the anti-staphylococcal activity of SNPs Blue light reduced the MIC of SNPs from 10 µg/mL to 1 µg/mL, which is safer for mammalian cells Photolysis of staphyloxanthin increased the uptake of SNPs into the bacterial cells | [178] |
| Piper longum mediated-synthesis of SNPs | M: Green synthesis S: Spherical AS: 10–40 nm | SNPs were active against Bacillus cereus, S. aureus, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, P. aeruginosa, and Salmonella typhi SNPs were active after three months of storage | [179] |
| Gardenia thailandica leaf extract-mediated synthesis of SNPs | M: Green synthesis S: Spherical AS: 11.02–17.92 nm AZP: −6.54 ± 0.6mV | MIC of the SNPs against S. aureus ranged from 4 to 64 µg/mL SNP at 4 × MIC and 8 × MIC eradicated the S. aureus cells at 2 h and 1 h, respectively SNPs decreased the expression of efflux pump genes norA, norB, and norC | [180] |
| Copper (CuNPs) and copper oxide NPs (CuONPs) | |||
| Curcuma longa or Zingiber officinale extract-mediated synthesis of CuNPs | M: Green synthesis S: Spherical AS: 20–100 nm | Agar well diffusion assay showed the antibacterial effect of CuNPs (1 and 5 mM) produced with C. longa was higher than those produced with Z. officinale | [135] |
| TH4+/CuNPs Virkon S/CuNPs Tek-Trol/CuNPs Peracetic/CuNPs DC&R/CuNPs | AS: The ranges of particle size were 79.88–100.62 nm (TH4+), 77.74–116.49 nm (Virkon S), 82.32–115.91nm (Tek-Trol), 90.25–105.07 nm (Peracetic), and 115.15–144.86 nm (DC&R) AZP: 2.92 and 3.43 mV | The ability of disinfectant-loaded CuNPs to eliminate the viable bacterial colonies in biofilm surfaces was studied with different concentrations and time points At a contact time of 5 min TH4+/CuNPs (1%), Tek-Trol/CuNPs (1%), DC&R/CuNPs (16%), 10 min Peracetic/CuNPs (0.5%), or 20 min Virkon S/CuNPs (2%), significantly reduced the total viable count of S. aureus | [136] |
| CuNPs and CuONPs | M: Plasma arc discharge method AS: 78 nm (CuNPs) and 67 nm (CuONPs) | Tested against different bacteria, CuNPs and CuONPs showed the highest zone of inhibition against S. aureus NPs induced ROS production, protein denaturation, DNA damage, and cell death | [112] |
| CuNPs | AS: 25 nm | CuNPs showed significant anti-staphylococcal activity with reduced toxicity against fibroblasts (at 6.25 µg/mL concentration) In vivo studies using S. aureus-induced mastitis rat model indicated that CuNPs improved clinical signs faster (three days) than gentamycin (four days) CuNPs reversed the S. aureus-induced histopathological changes in the mammary gland and, on the 5th day after treatment, bacterial load, mammary gland weight, and oxidative stress parameters were lower compared to the disease control and antibiotic-treated animals | [181] |
| Other metallic NPs | |||
| Lactobacillus plantarum TA4-mediated synthesis of ZnONPs | S: Oval AS: 29.7 nm | ZnONPs were effective against S. aureus from poultry samples (disc diffusion assay) MIC and MBC values were 30 and 100 µg/mL, respectively ZnONPs inhibited biofilm formation in a dose-dependent manner The results suggested that ROS generation was the underlying antibacterial mechanism | [182] |
| Pancreatin-doped ZnONPs | M: Precipitation method S: Hexagonal AS: 85 nm | Antibacterial and virulence inhibitory activity against MRSA Protein leakage and generation of ROS were possible antibacterial mechanisms Pancreatin-doped ZnONPs sensitized the cells to vancomycin | [183] |
| Rhamnolipid-coated ZnONPs | AS: From 40 to 55 nm S: Spherical | NPs at 0.5 mg/mL had low toxicity to fibroblast cells and low hemolytic activity NPs treatment reduced the bacterial burden in infected wound in rats, revealing a rapid wound healing within five days compared to the rhamnolipid- and clindamycin-treated wounds In histopathological analysis, the NP-treated animals showed rapid remodeling of the epidermis and the presence of ample amounts of dermal cells on the 5th day of treatment | [184,185] |
| Aspergillus terreus S1 mediated-synthesis of MgONPs | M: Green synthesis S: Spherical AS: 8–38 nm PDI: 0.2 | Growth inhibitory activity (MIC 200 μg/mL) against B. subtilis (13.3 ± 1.9 mm, inhibition zone), E. coli (11.3 ± 0.6 mm), C. albicans (12.8 ± 0.3 mm), P. aeruginosa (14.7 ± 1.9 mm), and S. aureus (11.3 ± 0.6 mm) | [186] |
| Carum copticum extract-mediated synthesis of TiONPs | M: Green synthesis S: Spherical or spheroid shaped AS: ~12 nm | Inhibition of EPS secretion and rupture of preformed biofilms of S. aureus | [187] |
| Ochradenus arabicus leaf extract-mediated synthesis of TiONPs | M: Green synthesis AS: 26.48 nm | MIC of the TiONPs was 32 µg/mL TiONPs at 0.5 × MIC inhibited biofilm formation and EPS production by MRSA to approx. 50% MRSA strains increased production of ROS upon treatment with the TiONPs | [188] |
| Mesoporous Silica NPs (MSNs) | |||
| Enzyme-functionalized MSN | M: Stober method S: Spherical AS: Lys@MSN (38 ± 5 nm), Ser@MSN (31 ± 7 nm), and DN@MSN (35 ± 4 nm) AZP: Lys@MSN (+12 ± 5 mV), Ser@MSN (−22 ± 5 mV), and DN@MSN (+27 ± 5 mV) | Enzymes lysostaphin (Lys@MSN), serrapeptase (Ser@MSN), and DNase I (DN@MSN) were immobilized in MSNs Lys@MSNs targeted MRSA and MSSA growth by inducing cell lysis The other two enzymes immobilized in MSNs targeted the biofilm formation of S. aureus by hampering the production of proteins and eDNA Lys@MSNs showed a 7.5- and 5-fold decrease in MIC and MBIC values compared to free lysostaphin | [189] |
| Rifampicin-loaded MSN | M: Solvent extraction (e) and calcination (c) methods AS: 40 nm (c & e), 80 nm (c) AZP: 15 (40e), 13 (40c), and 14 mV (80c) DL: 29 (40e), 33 (40c), and 38% (80c) | Hydrophilic e-MSN particles (prepared using solvent extraction) demonstrated a > 2-fold increase in Caco-2 cell uptake MSNs were efficacious against small colony variant S. aureus hosted within Caco-2 cells Compared to free rifampicin, the MSNs loaded with rifampicin reduced the level of S. aureus in Caco-2 cells 2.5-fold | [158] |
| Moxifloxacin/rifampicin-loaded MSN (gelatine/colistin coated) | M: Stober method AS: 396 nm AZP: -29.2 ± 0.65 mV | Antibiotic-loaded MSNs were studied against MRSA osteomyelitis both in vitro and in vivo MIC of the moxifloxacin and rifampicin MSNs were 3.906 and 0.977 µg/mL, respectively Intraosseous injection of MSNs decorated with aspartic acid hexapeptide (D6, affinity towards bone tissue) reduced S. aureus load to 92% in infected rabbit femurs within 24 h MSNs showed no toxicity towards osteoblasts and macrophages in vitro, but some effects on osteoclasts over time (72 h) NPs reduced biofilm formation and the expression of the proteases staphopain, SplF, and V8 protease, whereas they increased the expression of aureolysin and the transcriptional regulator protein Rot | [190] |
| Vancomycin-loaded MSN | M: Impregnation approach S: Spherical AS: 100 nm AZP: +26.5 mV | Antibiotic-loaded MSNs targeting bone and MRSA presented an MIC of 16 µg/mL Compared to treatment with free vancomycin, the targeted MSNs improved the recovery from orthopedic implant-related infections with MRSA in rats Hemolytic and studies with bone marrow mesenchymal stem cells indicated the biocompatibility of the MSNs, and no abnormalities were observed in the heart, spleen, liver, lung, or kidneys of treated rats | [191] |
| Quantum dots (QDs) and Carbon nanodots (CND) | |||
| p-Coumaric acid QDs | M: Wet milling approach AS: 8.9 ± 3.7 nm AZP: −3.73 mV | Antimicrobial activity against a wide spectrum of foodborne microorganisms At minimal lethal concentration (250 µg/mL), 99.9% killing of bacterial cells was observed throughout the experiment time | [192] |
| Carbon QDs from gentamycin sulfate | M: Calcination method (180 °C optimal temperature) S: Spherical AS: 2–8 nm AZP: 10.9 mV | QDs effectively cleared bacterial pathogens like E. coli and S. aureus (MIC was 1.59 and 50.8 ng/mL at pH 5.5 and 7.4, respectively) QDs at 80 µg/mL eradicated (90%) preformed biofilms, whereas the gentamycin sulfate at the same concentration reduced only 10% of the biofilms QDs showed a low toxic profile against mammalian 3T3 cells, even at 2 mg/mL concentration | [193] |
| Carbon dots from m-aminophenol and tartaric acid | M: Hydrothermal method S: Spherical AS: 5–9 nm AZP: +33.2 ± 0.99 mV | The positively charged carbon dots showed anti-staphylococcal activity and low toxicity toward HeLa cells The carbon dots were selectively absorbed on the cell surface through electrostatic interactions | [194] |
| Carbon dots from levofloxacin hydrochloride | S: Spherical AS: 1.25 nm | MIC of the carbon dots against S. aureus was 128 µg/mL Mechanisms of electrostatic interaction for surface adherence and bacterial cell wall disruption were implicated in the antibacterial action No cytotoxicity was observed towards 293T cells (viability greater than 80% at a concentration of 100 μg/mL) | [195] |
| Negatively charged CNDs | M: Microwave-assisted synthesis AS: 2.5 nm AZP: −11.06 mV | Inhibitory activity against MRSA and vancomycin-intermediate S. aureus (MIC of 630 μg/mL) | [196] |
| CNDs from curcumin and citric acid | M: Hydrothermal method AZD: −15.1 mV | CNDs showed a broad range of antimicrobial and antibiofilm activity Bactericidal efficiency was maximal at 375 μg/mL against S. aureus, E. coli, P. aeruginosa, and B. subtilis | [172] |
3.3. Organic NPs
3.3.1. Lipid-Based NPs
Liposomes
Niosomes
Quatsomes
Micelles
Stimulated Phase-Shift Acoustic Nanodroplets/Nanobubbles
Solid-Lipid NPs
3.3.2. Polymer Based NPs
Polymeric NPs
Dendrimers
Cyclodextrins
| Reducing or Capping Agent/ Encapsulated Drug | Properties of the NPs | Biological Activities | Reference |
|---|---|---|---|
| Liposomes | |||
| Lecithin and Tween-80 liposomes with Laurus nobilis leaf extract | M: Ultrasound AS: 99.05 ± 2.98 nm EE: 73.76 ± 1.10% | MIC and MBC of plant extracts were between 100 and 500 ppm At 1500 ppm, the loaded liposomes inhibited oxidation, bacterial growth, and spoilage of minced beef inoculated with E. coli and S. aureus | [261] |
| Lecithin liposomes with co-encapsulated berberine and curcumin | M: Film hydration AS: 253 ± 22 nm AZP: −57 ± 4 mV EE: 57 ± 3% | MIC of free berberine and curcumin were 62 and 250 µg/mL, respectively Encapsulation reduced the MIC of the drugs by approximately half and more efficiently prevented MRSA biofilm formation Free berberine and curcumin combinations showed an MIC of 31/16 µg/mL with an FIC index of 0.56 (no interaction), while the dual drug-loaded liposomes showed an MIC of 8/10 µg/mL with an FIC index of 0.13 (synergy) The liposomes were more efficient than clindamycin in reducing intracellular infection | [262] |
| Niosomes | |||
| Ciprofloxacin-loaded niosomes | M: Remote-loading technique S: Spherical AS: 123 nm PDI: 0.198 EE: 79.25% | Stable ciprofloxacin-loaded niosomes showed MIC in the range of 2–4 µg/mL against the S. aureus strains, a 4- to 5-fold increase in antibacterial potency compared to the free drug Sub-MIC inhibited the biofilm formation of ciprofloxacin-resistant S. aureus and down-regulated the icaB gene | [217] |
| Cefazolin-loaded niosomes | M: Film hydration S: Spherical AS: 100 nm AZP: −63 mV | Cefazolin-containing niosomes removed one- to five-day-old biofilms in a concentration-dependent manner (MRSA isolates from patients with pressure sores and diabetic ulcers) Histopathological results indicated that mice treated with cefazolin-loaded niosomes recovered faster than those treated with the free drug or the untreated group | [263] |
| Quatsomes | |||
| Vancomycin-loaded quatsomes from quaternary bicephalic surfactants and cholesterol | M: Sonication/dispersion method AS: 123 nm AZP: 0.169 mV EE: 52.2% | The pH-responsive quatsomes showed 32- and 8-fold lower MICs against MRSA at pH 6 and 7.4, respectively, compared to the free vancomycin The drug-loaded quatsomes caused more significant membrane damage, had a bactericidal effect, and counteracted MRSA biofilms in vitro In a mouse skin infection model, the quatsome formulation performed better than the free antibiotic | [220] |
| Cetylpyridinium chloride (CPC)-quatsomes | ND | No toxicity towards human airway epithelial (NuLi-1) cells Low concentration inhibited the planktonic and biofilm cells of S. aureus and P. aeruginosa | [221] |
| Micelles | |||
| Platensimycin-loaded micelles constructed using [poly(lactic-co-glycolic acid)-poly(2-ethyl-2-oxazoline) (PLGA−PEOz)] and PLGA-poly(ethylene glycol) (PLGA-PEG) | AS: 183 nm (PLGA−PEOz), 195 nm (PLGA-PEG) AZP: -5.37 (PLGA−PEOz), −5.42 (PLGA-PEG) EE: 41.7% (PLGA−PEOz), 40.4% (PLGA-PEG) | Improved results against intracellular MRSA in a macrophage infection model Compared to the free drug, drug-loaded micelles showed higher potential against MRSA-induced peritonitis in mice (dose 20 mg/kg, increased survival and reduced colonization) The drug-loaded micelles were not toxic to the cells nor the animals Cmax after i.p. injection of the free drug was 28 ± 9 μg/mL, but concentrations greater than 50 μg/mL were measured after administering the encapsulated drug | [225] |
| Solid Lipid NPs (SLNs) | |||
| Curcumin-loaded SLNs | M: Microemulsion method S: Spherical AS: 126.87 ± 0.94 nm PDI: 0.21 ZP: 30 ± 0.3 mV EE: 99.96% DL: 1.8% | Curcumin SLNs were effective against pathogens such as S. aureus and E. coli Lower MIC value (142 μg/mL) than free curcumin (1000 μg/mL) The curcumin SLNs reduced the pathogens’ cell counts in contaminated food for eight days | [264] |
| Anacardic acid encapsulated in SLNs | M: Hot homogenization S: Spherical AS: 203.6 ± 3.05 nm PDI: 0.277 ± 0.02 ZP: −21.4 ± 2.81 mV DL: 76.4 ± 1.9% | Stable for 90 days and non-toxic to the human keratinocyte cell line HaCat High anti-staphylococcal and biofilm inhibitory activities | [229] |
| Polymeric NPs | |||
| Rifampicin-loaded poly-lactic acid NPs | M: Nanoprecipitation S: Spherical AS: 144 nm PDI: 0.08 AZP: −56 ± 5 mV DL: 2.2% EE: 90.5% | NPs coated with poly-lysine were more active against the growth and biofilms of S. aureus, presumably due to enhanced interaction and slow penetration into S. aureus biofilms | [265] |
| Citrus reticulata essential oil loaded in chitosan NPs | AS: 131–162 nm EE: 67.32%–82.35% AZP: 30 mV | The loaded NPs disturbed bacterial cell membranes and displayed high anti-staphylococcal activity, as well as inhibition of biofilm formation and premature biofilms of S. aureus | [266] |
| Chitosan functionalized SNP by Sygyzium aromaticum | M: Biogenic synthesis S: Spherical AS: 30–40 nm | Effective against MRSA and VRSA Lethal toxicity towards HeLa cells and brine shrimp was observed at 325 μg/mL, which is three times higher than the effective concentration showing anticoagulation, antiplatelet, and thrombolytic activities | [267] |
| Dendrimers | |||
| Platensimycin-loaded PLGA and PAMAM dendrimer NPs | M: Emulsification-evaporation AS: 175.6 nm (PLGA) and 218.1 nm (PAMAM) PDI: 0.10 (PLGA) and 0.17 (PAMAM) AZP: −17.7 mV (PLGA) and 17.2 mV (PAMAM) DL: 7.81% (PLGA) and 8.42% (PAMAM) EE: 62.1% (PLGA) and 63.2% (PAMAM) | Inhibited MRSA growth and biofilms and killed the bacteria in a macrophage cell model more efficiently than the free drug Treatment with both types of drug-loaded NPs was effective against MRSA peritoneal infection in the mice models, with reduction of MRSA in the blood and kidneys, and full survival for 7 days, while the animals treated with the same dose of free drug (10 mg/kg, i.p.) died in 24 h In pharmacokinetic study in rats, the NPs formulations provided a 2- to 4-fold higher AUC and extended the mean residence time of the drug (Cmax approx. 80 μg/mL) Loaded PLGA and PAMAM NPs showed no appreciable effect on RAW 264.7 cell viability at concentrations well above those providing antibacterial activity (below 100 μg/mL) | [247] |
| PAMAM dendrimers with amide-conjugated vancomycin and incorporated SNP | M: Drug-PAMAM with amide conjugation AS: Dual drug-conjugated dendrimers with 68 nm AZP: 27.5 mV | 5–7-log reduction in colony-forming units of VRSA Antimicrobial resistance induction was not detected in a susceptible strain, in contrast to using the free antibiotic Good biocompatibility with IH 3T3 fibroblasts and HUVEC cells (up to 8 µg SNP/mL) and low hemolytic effects Irrigation of infected wounds in mice with the dual-drug dendrimers cleared VRSA and reduced the accumulation of granulocytes at the wound site more efficiently than the free antibiotic or the SNP-only PAMAM dendrimers | [249] |
| Polyurea (PURE) oligoethyleneimine (OEI) dendrimers | M: Grafting oligo-(2-ethyl-oxazoline) in polyurea dendrimer, followed by acid hydrolysis AZP: cationic Mw 82,871 g/mol (PURE-G4-OEI-48) and 160,788 g/mol (PURE-G3-OEI-24) | MIC and MBC against MRSA, MSSA, Streptococcus pneumonia, Gram-negative bacteria and Candida strains below 10 μM (lower than 1 μM in the case of MRSA) PURE-G4-OEI-48 effective against Pseudomonas aeruginosa and MRSA infections in a Galleria mellonella insect model Up to 6 μM, no toxicity was observed against human bronchial epithelial 16HBE14o- and vaginal VK2 (E6/E7) cell lines, nor an effect on the health index scores of G. mellonella Live/dead assays, SEM, and molecular dynamic simulations supported a fast-killing mechanism via membrane disruption | [254] |
4. Application of NPs in Antimicrobial and Antibiofilm Coatings
5. Challenges and Prospects of Research in the Field
5.1. Biological Behavior of NPs
5.2. Limitation in NPs Production
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wright, G.D. Mechanisms of resistance to antibiotics. Curr. Opin. Chem. Biol. 2003, 7, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Kjelleberg, S.; Normark, S.; Nyman, L.; Uhlin, B.E.; Åkerlund, B. Microbial biofilm formation: A need to act. J. Intern. Med. 2014, 276, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Alonso, B.; Pérez-Granda, M.J.; Latorre, M.C.; Sánchez-Carrillo, C.; Bouza, E.; Muñoz, P.; Guembe, M. Production of biofilm by Staphylococcus aureus: Association with infective endocarditis? Enferm. Infecc. Y Microbiol. Clín. 2021, 40, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Gittins, D.I.; Bethell, D.; Schiffrin, D.J.; Nichols, R.J. A nanometre-scale electronic switch consisting of a metal cluster and redox-addressable groups. Nature 2000, 408, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, M.; Sneha, K.; Yun, Y.-S. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour. Technol. 2010, 101, 7958–7965. [Google Scholar] [CrossRef]
- Elegbede, J.A.; Lateef, A. Green synthesis of silver (Ag), gold (Au), and silver–gold (Ag–Au) alloy nanoparticles: A review on recent advances, trends, and biomedical applications. In Nanotechnology and Nanomaterial Applications in Food, Health, and Biomedical Sciences; Apple Academic Press: Palm Bay, FI, USA, 2019; pp. 3–89. [Google Scholar]
- Lateef, A.; Ojo, S.A.; Elegbede, J.A.; Akinola, P.O.; Akanni, E.O. Nanomedical applications of nanoparticles for blood coagulation disorders. Environ. Nanotechnol. 2018, 14, 243–277. [Google Scholar]
- Pinto, R.M.; Lopes-de-Campos, D.; Martins, M.C.L.; Van Dijck, P.; Nunes, C.; Reis, S. Impact of nanosystems in Staphylococcus aureus biofilms treatment. FEMS Microbiol. Rev. 2019, 43, 622–641. [Google Scholar] [CrossRef] [Green Version]
- Alavi, M.; Rai, M. Recent advances in antibacterial applications of metal nanoparticles (MNPs) and metal nanocomposites (MNCs) against multidrug-resistant (MDR) bacteria. Expert Rev. Anti-Infect. Ther. 2019, 17, 419–428. [Google Scholar] [CrossRef]
- Boswell, G.W.; Buell, D.; Bekersky, I. AmBisome (Liposomal Amphotericin B): A Comparative Review. J. Clin. Pharmacol. 1998, 38, 583–592. [Google Scholar] [CrossRef]
- Gupta, N.; Rai, D.B.; Jangid, A.K.; Kulhari, H. Chapter 7—Use of nanotechnology in antimicrobial therapy. In Methods in Microbiology; Gurtler, V., Ball, A.S., Soni, S., Eds.; Academic Press: Cambridge, UK, 2019; Volume 46, pp. 143–172. [Google Scholar]
- Bassetti, M.; Vena, A.; Russo, A.; Peghin, M. Inhaled liposomal antimicrobial delivery in lung infections. Drugs 2020, 80, 1309–1318. [Google Scholar] [CrossRef]
- Shimanovich, U.; Gedanken, A. Nanotechnology solutions to restore antibiotic activity. J. Mater. Chem. B 2016, 4, 824–833. [Google Scholar] [CrossRef]
- Kaur, K.; Reddy, S.; Barathe, P.; Shriram, V.; Anand, U.; Proćków, J.; Kumar, V. Combating Drug-Resistant Bacteria Using Photothermally Active Nanomaterials: A Perspective Review. Front. Microbiol. 2021, 12, 747019. [Google Scholar] [CrossRef]
- Alavi, M.; Jabari, E.; Jabbari, E. Functionalized carbon-based nanomaterials and quantum dots with antibacterial activity: A review. Expert Rev. Anti-Infect. Ther. 2021, 19, 35–44. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Herold, B.C.; Immergluck, L.C.; Maranan, M.C.; Lauderdale, D.S.; Gaskin, R.E.; Boyle-Vavra, S.; Leitch, C.D.; Daum, R.S. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998, 279, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Sievert, D.M.; Rudrik, J.T.; Patel, J.B.; McDonald, L.C.; Wilkins, M.J.; Hageman, J.C. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin. Infect. Dis. 2008, 46, 668–674. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014.
- Rowe, S.E.; Beam, J.E.; Conlon, B.P. Recalcitrant Staphylococcus aureus infections: Obstacles and solutions. Infect. Immun. 2021, 89, e00694-20. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.R.; van Strijp, J.A.G.; Bagnoli, F.; Manetti, A.G.O. Virulence Gene Expression of Staphylococcus aureus in Human Skin. Front. Microbiol. 2021, 12, 692023. [Google Scholar] [CrossRef]
- Kannappan, A.; Gowrishankar, S.; Srinivasan, R.; Pandian, S.K.; Ravi, A.V. Antibiofilm activity of Vetiveria zizanioides root extract against methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2017, 110, 313–324. [Google Scholar] [CrossRef]
- Algharib, S.A.; Dawood, A.; Xie, S. Nanoparticles for treatment of bovine Staphylococcus aureus mastitis. Drug Deliv. 2020, 27, 292–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyle, E.; Stroh, P.; Günther, F.; Hoppy-Tichy, T.; Wagner, C.; Hänsch, G.M. Destruction of bacterial biofilms by polymorphonuclear neutrophils: Relative contribution of phagocytosis, DNA release, and degranulation. Int. J. Artif. Organs 2010, 33, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Stroh, P.; Günther, F.; Meyle, E.; Prior, B.; Wagner, C.; Hänsch, G.M. Host defence against Staphylococcus aureus biofilms by polymorphonuclear neutrophils: Oxygen radical production but not phagocytosis depends on opsonisation with immunoglobulin G. Immunobiology 2011, 216, 351–357. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, F.R.; Diep, B.A.; Otto, M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect. Dis. Clin. N. Am. 2009, 23, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Guenther, F.; Stroh, P.; Wagner, C.; Obst, U.; Hänsch, G.M. Phagocytosis of staphylococci biofilms by polymorphonuclear neutrophils: S. aureus and S. epidermidis differ with regard to their susceptibility towards the host defense. Int. J. Artif. Organs 2009, 32, 565–573. [Google Scholar] [PubMed]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microb. Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Alegre, M.-L.; Chen, L.; David, M.Z.; Bartman, C.; Boyle-Vavra, S.; Kumar, N.; Chong, A.S.; Daum, R.S. Impact of Staphylococcus aureus USA300 colonization and skin infections on systemic immune responses in humans. J. Immunol. 2016, 197, 1118–1126. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Pastacaldi, C.; Lewis, P.; Howarth, P. Staphylococci and staphylococcal superantigens in asthma and rhinitis: A systematic review and meta-analysis. Allergy 2011, 66, 549–555. [Google Scholar] [CrossRef]
- Kim, M.R.; Hong, S.W.; Choi, E.B.; Lee, W.H.; Kim, Y.S.; Jeon, S.; Jang, M.; Gho, Y.; Kim, Y.K. Staphylococcus aureus-derived extracellular vesicles induce neutrophilic pulmonary inflammation via both T h1 and T h17 cell responses. Allergy 2012, 67, 1271–1281. [Google Scholar] [CrossRef]
- Zhang, R.; Shebes, M.A.; Kho, K.; Scaffidi, S.J.; Meredith, T.C.; Yu, W. Spatial regulation of protein A in Staphylococcus aureus. Mol. Microbiol. 2021, 116, 589–605. [Google Scholar] [CrossRef]
- Visai, L.; Yanagisawa, N.; Josefsson, E.; Tarkowski, A.; Pezzali, I.; Rooijakkers, S.H.; Foster, T.J.; Speziale, P. Immune evasion by Staphylococcus aureus conferred by iron-regulated surface determinant protein IsdH. Microbiology 2009, 155, 667–679. [Google Scholar] [CrossRef]
- Sharp, J.A.; Echague, C.G.; Hair, P.S.; Ward, M.D.; Nyalwidhe, J.O.; Geoghegan, J.A.; Foster, T.J.; Cunnion, K.M. Staphylococcus aureus surface protein SdrE binds complement regulator factor H as an immune evasion tactic. PLoS ONE 2012, 7, e38407. [Google Scholar] [CrossRef]
- Hallett, A.; Cooper, R. Complement activation in Staphylococcus aureus bacteraemia. Clin. Exp. Immunol. 1980, 40, 306. [Google Scholar] [PubMed]
- Diep, B.A.; Sensabaugh, G.F.; Somboona, N.S.; Carleton, H.A.; Perdreau-Remington, F. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J. Clin. Microbiol. 2004, 42, 2080–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jong, N.; van Kessel, K.; van Strijp, J. Immune evasion by Staphylococcus aureus. Microbiol. Spectr. 2019, 7, 7.2.20. [Google Scholar] [CrossRef] [PubMed]
- See, I.; Wesson, P.; Gualandi, N.; Dumyati, G.; Harrison, L.H.; Lesher, L.; Nadle, J.; Petit, S.; Reisenauer, C.; Schaffner, W. Socioeconomic factors explain racial disparities in invasive community-associated methicillin-resistant Staphylococcus aureus disease rates. Clin. Infect. Dis. 2017, 64, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, N.A.; Hussein, N.R. Staphylococcus aureus: An Overview of Discovery, Characteristics, Epidemiology, Virulence Factors and Antimicrobial Sensitivity. Eur. J. Mol. Clin. Med. 2021, 8, 1160–1183. [Google Scholar]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.-H.L.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef]
- Nurjadi, D.; Friedrich-Jänicke, B.; Schäfer, J.; Van Genderen, P.J.; Goorhuis, A.; Perignon, A.; Neumayr, A.; Mueller, A.; Kantele, A.; Schunk, M. Skin and soft tissue infections in intercontinental travellers and the import of multi-resistant Staphylococcus aureus to Europe. Clin. Microbiol. Infect. 2015, 21, 567.e1–567.e10. [Google Scholar] [CrossRef] [Green Version]
- Walter, J.; Haller, S.; Quinten, C.; Kärki, T.; Zacher, B.; Eckmanns, T.; Sin, M.A.; Plachouras, D.; Kinross, P.; Suetens, C. Healthcare-associated pneumonia in acute care hospitals in European Union/European Economic Area countries: An analysis of data from a point prevalence survey, 2011 to 2012. Eurosurveillance 2018, 23, 1700843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stauffer, B.S. aureus Sphingomyelinase is a state-dependent inhibitor of the cystic fibrosis transmembrane conductance regulator (CFTR). Biophys. J. 2017, 112, 254a. [Google Scholar] [CrossRef]
- Bauer, T.; Torres, A.; Ferrer, R.; Heyer, C.; Schultze-Werninghaus, C.; Rasche, K. Biofilm formation in endotracheal tubes. Association between pneumonia and the persistence of pathogens. Monaldi Arch. Chest Dis. 2002, 57, 84–87. [Google Scholar] [PubMed]
- Viola, G.M.; Darouiche, R.O. Cardiovascular implantable device infections. Curr. Infect. Dis. Rep. 2011, 13, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Brien, L.; Kerrigan, S.W.; Kaw, G.; Hogan, M.; Penadés, J.; Litt, D.; Fitzgerald, D.J.; Foster, T.J.; Cox, D. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: Roles for the clumping factors ClfA and ClfB, the serine–aspartate repeat protein SdrE and protein A. Mol. Microbiol. 2002, 44, 1033–1044. [Google Scholar]
- Yakut, N.; Soysal, A.; Kepenekli Kadayifci, E.; Dalgic, N.; Yılmaz Ciftdogan, D.; Karaaslan, A.; Akkoc, G.; Ocal Demir, S.; Cagan, E.; Celikboya, E. Ventriculoperitoneal shunt infections and re-infections in children: A multicentre retrospective study. Br. J. Neurosurg. 2018, 32, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.L.; Lilani, S.; Shirpurkar, R.; Chande, C.; Joshi, S.; Chowdhary, A. Coagulase-negative staphylococci: Emerging pathogen in central nervous system shunt infection. Indian J. Med. Microbiol. 2017, 35, 120–123. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Drake, J.M.; Lamberti-Pasculli, M. Cerebrospinal fluid shunt infection: A prospective study of risk factors. J. Neurosurg. 2001, 94, 195–201. [Google Scholar] [CrossRef]
- Chang, Y.; Tai, C.; Hsieh, P.; Ueng, S. Gentamicin in bone cement: A potentially more effective prophylactic measure of infection in joint arthroplasty. Bone Jt. Res. 2013, 2, 220–226. [Google Scholar] [CrossRef]
- Beam, E.; Osmon, D. Prosthetic joint infection update. Infect. Dis. Clin. 2018, 32, 843–859. [Google Scholar]
- Gad, G.F.M.; El-Feky, M.A.; El-Rehewy, M.S.; Hassan, M.A.; Abolella, H.; Abd El-Baky, R.M. Detection of icaA, icaD genes and biofilm production by Staphylococcus aureus and Staphylococcus epidermidis isolated from urinary tract catheterized patients. J. Infect. Dev. Ctries. 2009, 3, 342–351. [Google Scholar] [PubMed]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [Green Version]
- Kannappan, A.; Durgadevi, R.; Srinivasan, R.; Lagoa, R.J.L.; Packiavathy, I.A.S.V.; Pandian, S.K.; Veera Ravi, A. 2-Hydroxy-4-methoxybenzaldehyde from Hemidesmus indicus is antagonistic to Staphylococcus epidermidis biofilm formation. Biofouling 2020, 36, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial biofilm inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Bassler, B.L. Surviving as a community: Antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells and the riddle of biofilm survival. Biochemistry 2005, 70, 267–274. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhargava, A. Biofilms and human health. Biotechnol. Lett. 2016, 38, 1–22. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, X.; Xie, J.; Xu, Z.; Liu, G.; Zhang, G. Investigation of formation of bacterial biofilm upon dead siblings. Langmuir 2018, 35, 7405–7413. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, S.; Ganjloo, S.; Pourmand, M.R.; Mashhadi, R.; Ghazvini, K. Epidemiology of efflux pumps genes mediating resistance among Staphylococcus aureus; A systematic review. Microb. Pathog. 2020, 139, 103850. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wang, Y.; Villanueva, I.; Ho, P.L.; Davies, J.; Kao, R.Y.T. Construction of a multiplex promoter reporter platform to monitor Staphylococcus aureus virulence gene expression and the identification of usnic acid as a potent suppressor of psm gene expression. Front. Microbiol. 2016, 7, 1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronesky, D.; Wu, Z.; Marzi, S.; Walter, P.; Geissmann, T.; Moreau, K.; Vandenesch, F.; Caldelari, I.; Romby, P. Staphylococcus aureus RNAIII and its regulon link quorum sensing, stress responses, metabolic adaptation, and regulation of virulence gene expression. Annu. Rev. Microbiol. 2016, 70, 299–316. [Google Scholar] [CrossRef]
- Tiwari, S.; Jamal, S.B.; Hassan, S.S.; Carvalho, P.V.; Almeida, S.; Barh, D.; Ghosh, P.; Silva, A.; Castro, T.L.; Azevedo, V. Two-component signal transduction systems of pathogenic bacteria as targets for antimicrobial therapy: An overview. Front. Microbiol. 2017, 8, 1878. [Google Scholar] [CrossRef] [Green Version]
- Felden, B.; Vandenesch, F.; Bouloc, P.; Romby, P. The Staphylococcus aureus RNome and its commitment to virulence. PLoS Pathog. 2011, 7, e1002006. [Google Scholar] [CrossRef]
- Reyes, D.; Andrey, D.O.; Monod, A.; Kelley, W.L.; Zhang, G.; Cheung, A.L. Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J. Bacteriol. 2011, 193, 6020–6031. [Google Scholar] [CrossRef] [Green Version]
- Valle, J.; Toledo-Arana, A.; Berasain, C.; Ghigo, J.M.; Amorena, B.; Penadés, J.R.; Lasa, I. SarA and not σB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 2003, 48, 1075–1087. [Google Scholar] [CrossRef]
- Tan, L.; Li, S.R.; Jiang, B.; Hu, X.M.; Li, S. Therapeutic targeting of the Staphylococcus aureus accessory gene regulator (agr) system. Front. Microbiol. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Karthik, L.; Kumar, G.; Keswani, T.; Bhattacharyya, A.; Chandar, S.S.; Bhaskara Rao, K. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PLoS ONE 2014, 9, e90972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boles, B.R.; Horswill, A.R. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; López-Redondo, M.; Miguel-Romero, L.; Kulhankova, K.; Cahill, M.P.; Tran, P.M.; Kinney, K.J.; Kilgore, S.H.; Al-Tameemi, H.; Herfst, C.A. The SrrAB two-component system regulates Staphylococcus aureus pathogenicity through redox sensitive cysteines. Proc. Natl. Acad. Sci. USA 2020, 117, 10989–10999. [Google Scholar] [CrossRef]
- Kolar, S.L.; Nagarajan, V.; Oszmiana, A.; Rivera, F.E.; Miller, H.K.; Davenport, J.E.; Riordan, J.T.; Potempa, J.; Barber, D.S.; Koziel, J.; et al. NsaRS is a cell-envelope-stress-sensing two-component system of Staphylococcus aureus. Microbiology 2011, 157, 2206–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, D.; Herbert, S.; Kristian, S.A.; Khosravi, A.; Nizet, V.; Götz, F.; Peschel, A. The GraRS regulatory system controls Staphylococcus aureus susceptibility to antimicrobial host defenses. BMC Microbiol. 2008, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.W.; Yang, J.; Guo, H.; Ji, Y. The AirSR two-component system contributes to Staphylococcus aureus survival in human blood and transcriptionally regulates sspABC operon. Front. Microbiol. 2015, 6, 682. [Google Scholar] [CrossRef]
- Torres, V.J.; Stauff, D.L.; Pishchany, G.; Bezbradica, J.S.; Gordy, L.E.; Iturregui, J.; Anderson, K.L.; Dunman, P.M.; Joyce, S.; Skaar, E.P. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe 2007, 1, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Lin, K.; Liu, Y.; Zhang, H.; Lei, L. Two-component signaling pathways modulate drug resistance of Staphylococcus aureus (Review). Biomed. Rep. 2020, 13, 5. [Google Scholar] [CrossRef]
- Rapun-Araiz, B.; Haag, A.F.; Solano, C.; Lasa, I. The impact of two-component sensorial network in staphylococcal speciation. Curr. Opin. Microbiol. 2020, 55, 40–47. [Google Scholar] [CrossRef]
- O’Gara, J.P. ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 2007, 270, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cue, D.; Lei, M.G.; Luong, T.T.; Kuechenmeister, L.; Dunman, P.M.; O’Donnell, S.; Rowe, S.; O’Gara, J.P.; Lee, C.Y. Rbf promotes biofilm formation by Staphylococcus aureus via repression of icaR, a negative regulator of icaADBC. J. Bacteriol. 2009, 191, 6363–6373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuong, C.; Kocianova, S.; Voyich, J.M.; Yao, Y.; Fischer, E.R.; DeLeo, F.R.; Otto, M. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 2004, 279, 54881–54886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrigan, R.M.; Rigby, D.; Handley, P.; Foster, T.J. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology 2007, 153, 2435–2446. [Google Scholar] [CrossRef]
- Gruszka, D.T.; Wojdyla, J.A.; Bingham, R.J.; Turkenburg, J.P.; Manfield, I.W.; Steward, A.; Leech, A.P.; Geoghegan, J.A.; Foster, T.J.; Clarke, J.; et al. Staphylococcal biofilm-forming protein has a contiguous rod-like structure. Proc. Natl. Acad. Sci. USA 2012, 109, E1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Zhao, L.; Xue, T.; Sun, B. Staphylococcus aureus autoinducer-2 quorum sensing decreases biofilm formation in an icaR-dependent manner. BMC Microbiol. 2012, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, P.; Rowe, S.E.; Pozzi, C.; Waters, E.M.; O’Gara, J.P. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect. Immun. 2011, 79, 1153–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.P.; Bischoff, M.; Senn, M.M.; Berger-Bächi, B. The σB regulon influences internalization of Staphylococcus aureus by osteoblasts. Infect. Immun. 2003, 71, 4167–4170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chongtrakool, P.; Ito, T.; Ma, X.X.; Kondo, Y.; Trakulsomboon, S.; Tiensasitorn, C.; Jamklang, M.; Chavalit, T.; Song, J.-H.; Hiramatsu, K. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: A proposal for a new nomenclature for SCC mec elements. Antimicrob. Agents Chemother. 2006, 50, 1001–1012. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Cui, L.; Oguchi, A.; Aoki, K.-i.; Nagai, Y. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]
- Malachowa, N.; DeLeo, F.R. Mobile genetic elements of Staphylococcus aureus. Cell. Mol. Life Sci. 2010, 67, 3057–3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, M. Staphylococcus aureus toxin gene hitchhikes on a transferable antibiotic resistance element. Virulence 2010, 1, 49–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Murray, P.R.; Huskins, W.C.; Jernigan, J.A.; McDonald, L.C.; Clark, N.C.; Anderson, K.F.; McDougal, L.K.; Hageman, J.C.; Olsen-Rasmussen, M. Dissemination of an Enterococcus Inc18-Like vanA plasmid associated with vancomycin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 4314–4320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shallcross, L.J.; Fragaszy, E.; Johnson, A.M.; Hayward, A.C. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 43–54. [Google Scholar] [CrossRef] [PubMed]
- CheungGY, W. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011, 79, 1927–1935. [Google Scholar] [CrossRef] [Green Version]
- Genestier, A.-L.; Michallet, M.-C.; Prévost, G.; Bellot, G.; Chalabreysse, L.; Peyrol, S.; Thivolet, F.; Etienne, J.; Lina, G.; Vallette, F.M.; et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Investig. 2005, 115, 3117–3127. [Google Scholar] [CrossRef]
- Joo, H.-S.; Cheung, G.Y.; Otto, M. Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives. J. Biol. Chem. 2011, 286, 8933–8940. [Google Scholar] [CrossRef] [Green Version]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef]
- Salah, R.; Karmy, M.; Abdelraouf, A.; Kotb, S. Evaluation of the bactericidal effect of silver nanoparticles against methicillin resistant Staphylococcus aureus (MRSA) and methicillin sensitive Staphylococcus aureus (MSSA) strains isolated from mastitic milk of small ruminants and their surrounding environment in Aswan, Egypt. J. Vet. Med. Res. 2021, 27, 143–151. [Google Scholar]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–biofilm interactions: The role of the EPS matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Singh, B.; Vuddanda, P.R.; Vijayakumar, M.; Kumar, V.; Saxena, P.S.; Singh, S. Cefuroxime axetil loaded solid lipid nanoparticles for enhanced activity against S. Aureus Biofilm. Colloids Surf. B Biointerfaces 2014, 121, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef] [PubMed]
- Rane, A.V.; Kanny, K.; Abitha, V.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. [Google Scholar]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Xiao, X.; Zhu, Y.; Liao, J.; Wang, T.; Sun, W.; Tong, Z. High-efficient and synergetic antibacterial nanocomposite hydrogel with quaternized chitosan/Ag nanoparticles prepared by one-pot UV photochemical synthesis. Biopolymers 2020, 111, e23354. [Google Scholar] [CrossRef] [PubMed]
- Alothman, A.A.; Albaqami, M.D. Nano-sized Cu(II) and Zn(II) complexes and their use as a precursor for synthesis of CuO and ZnO nanoparticles: A study on their sonochemical synthesis, characterization, and DNA-binding/cleavage, anticancer, and antimicrobial activities. Appl. Organomet. Chem. 2020, 34, e5827. [Google Scholar] [CrossRef]
- Goswami, S.; Sahareen, T.; Singh, M.; Kumar, S. Role of biogenic silver nanoparticles in disruption of cell–cell adhesion in Staphylococcus aureus and Escherichia coli biofilm. J. Ind. Eng. Chem. 2015, 26, 73–80. [Google Scholar] [CrossRef]
- Lozhkomoev, A.; Bakina, O.; Pervikov, A.; Kazantsev, S.; Glazkova, E. Synthesis of CuO–ZnO composite nanoparticles by electrical explosion of wires and their antibacterial activities. J. Mater. Sci. Mater. Electron. 2019, 30, 13209–13216. [Google Scholar] [CrossRef]
- Tharchanaa, S.; Priyanka, K.; Preethi, K.; Shanmugavelayutham, G. Facile synthesis of Cu and CuO nanoparticles from copper scrap using plasma arc discharge method and evaluation of antibacterial activity. Mater. Technol. 2021, 36, 97–104. [Google Scholar] [CrossRef]
- Khashan, K.S.; Sulaiman, G.M.; Abdulameer, F.A.; Albukhaty, S.; Ibrahem, M.A.; Al-Muhimeed, T.; AlObaid, A.A. Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid. Appl. Sci. 2021, 11, 4623. [Google Scholar] [CrossRef]
- Zhang, S.; Li, R.; Huang, D.; Ren, X.; Huang, T.-S. Antibacterial modification of PET with quaternary ammonium salt and silver particles via electron-beam irradiation. Mater. Sci. Eng. C 2018, 85, 123–129. [Google Scholar] [CrossRef]
- Kankala, R.K.; Wang, S.B.; Chen, A.Z. Nanoarchitecting Hierarchical Mesoporous Siliceous Frameworks: A New Way Forward. Iscience 2020, 23, 101687. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.D.; Nogueira, B.R.; Rostelato, M.E.C. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Kherde, Y.; Aguilar, Z.P.; Zystein, L.; Rodgers, H.M.; Hamilton, W. Gold nanoparticles: Various methods of synthesis and antibacterial applications. Front. Biosci. 2014, 19, 1320–1344. [Google Scholar]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold nanoparticles: An efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Dasari, T.S.; Zhang, Y.; Yu, H. Antibacterial activity and cytotoxicity of gold (I) and (III) ions and gold nanoparticles. Biochem. Pharmacol. 2015, 4, 199. [Google Scholar] [CrossRef]
- Boda, S.K.; Broda, J.; Schiefer, F.; Weber-Heynemann, J.; Hoss, M.; Simon, U.; Basu, B.; Jahnen-Dechent, W. Cytotoxicity of ultrasmall gold nanoparticles on planktonic and biofilm encapsulated Gram-positive staphylococci. Small 2015, 11, 3183–3193. [Google Scholar] [CrossRef]
- Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E. Investigation of nanoparticle metallic core antibacterial activity: Gold and silver nanoparticles against Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 1905. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; El-Agamy Farh, M.; Yang, D.C. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 811–816. [Google Scholar] [CrossRef]
- Khan, F.; Park, S.-K.; Bamunuarachchi, N.I.; Oh, D.; Kim, Y.-M. Caffeine-loaded gold nanoparticles: Antibiofilm and anti-persister activities against pathogenic bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 3717–3731. [Google Scholar] [CrossRef]
- Bar, H. One pot green synthesis of gold nanoparticles using Piper betle leaf extract and their antibacterial activities. Adv. Mater. Res. 2021, 1163, 106–116. [Google Scholar] [CrossRef]
- Hameed, S.; Wang, Y.; Zhao, L.; Xie, L.; Ying, Y. Shape-dependent significant physical mutilation and antibacterial mechanisms of gold nanoparticles against foodborne bacterial pathogens (Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus) at lower concentrations. Mater. Sci. Eng. C 2020, 108, 110338. [Google Scholar] [CrossRef]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Hussein, E.A.M.; Mohammad, A.A.-H.; Harraz, F.A.; Ahsan, M.F. Biologically synthesized silver nanoparticles for enhancing tetracycline activity against Staphylococcus aureus and Klebsiella pneumoniae. Braz. Arch. Biol. Technol. 2019, 62. [Google Scholar] [CrossRef]
- Zautner, A.E.; Krause, M.; Stropahl, G.; Holtfreter, S.; Frickmann, H.; Maletzki, C.; Kreikemeyer, B.; Pau, H.W.; Podbielski, A. Intracellular persisting Staphylococcus aureus is the major pathogen in recurrent tonsillitis. PLoS ONE 2010, 5, e9452. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Dietz, M.J.; Hughes, K.; Xing, M.; Li, B. Silver nanoparticles present high intracellular and extracellular killing against Staphylococcus aureus. J. Antimicrob. Chemother. 2019, 74, 1578–1585. [Google Scholar] [CrossRef]
- Parveen, F.; Sannakki, B.; Mandke, M.V.; Pathan, H.M. Copper nanoparticles: Synthesis methods and its light harvesting performance. Sol. Energy Mater. Sol. Cells 2016, 144, 371–382. [Google Scholar] [CrossRef]
- Varghese, B.; Kurian, M.; Krishna, S.; Athira, T. Biochemical synthesis of copper nanoparticles using Zingiber officinalis and Curcuma longa: Characterization and antibacterial activity study. Mater. Today Proc. 2020, 25, 302–306. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Elgohary, F.A.; Zakaria, A.I.; Elkenany, R.M.; El-Khateeb, A.Y. Novel eradication methods for Staphylococcus aureus biofilm in poultry farms and abattoirs using disinfectants loaded onto silver and copper nanoparticles. Environ. Sci. Pollut. Res. 2020, 27, 30716–30728. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mao, S.; Xu, Z.; Ding, W. Various antibacterial mechanisms of biosynthesized copper oxide nanoparticles against soilborne Ralstonia solanacearum. RSC Adv. 2019, 9, 3788–3799. [Google Scholar] [CrossRef] [Green Version]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef] [PubMed]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Deng, L.; Zou, L.; Feng, F.; Zhang, H. Hydrophobic Ethylcellulose/Gelatin Nanofibers Containing Zinc Oxide Nanoparticles for Antimicrobial Packaging. J. Agric. Food Chem. 2018, 66, 9498–9506. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Yamayoshi, I.; Mathew, S.; Lin, H.; Nayfach, J.; Simon, S.I. Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Ann. Biomed. Eng. 2013, 41, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.-H.; Tsai, P.-I.; Huang, S.-W.; Sun, J.-S.; Chang, J.Z.-C.; Shen, H.-H.; Chen, S.-Y.; Lin, F.H.; Hsu, L.-T.; Chen, Y.-C. Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis. BMC Infect. Dis. 2017, 17, 516. [Google Scholar] [CrossRef] [Green Version]
- Aureliano, M.; Marques-da-Silva, D.; Serrano, A.; Martins, J.; Faleiro, L.; Fonseca, C.; Fraqueza, G.; Lagoa, R. Polyoxometalates with anticancer, antibacterial and antiviral activities. In Polyoxometalates: Advances, Properties, and Applications; Rubio, L., Vilela, J., Artetxe, B., Gutiérrez-Zorrilla, J., Eds.; Jenny Stanford Publishing: United Square, Singapore, 2022; pp. 309–358. [Google Scholar]
- Ekielski, A. Interactions Between Food Ingredients and Nanocomponents Used for Composite Packaging. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 669–674. [Google Scholar]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium oxide nanoparticles: Effective agricultural antibacterial agent against Ralstonia Solanacearum. Front. Microbiol. 2018, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, S.; Shahzad, K.; Mushtaq, S.; Ali, I.; Rafe, M.H.; Fazal-ul-Karim, S.M. Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater. Res. Express 2019, 6, 105409. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Thamphiwatana, S.; Angsantikul, P.; Zhang, L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 532–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidi, S.; Misba, L.; Khan, A.U. Nano-therapeutics: A revolution in infection control in post antibiotic era. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2281–2301. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Swiecicka, I.; Wilczewska, A.Z.; Misztalewska, I.; Kalska-Szostko, B.; Bienias, K.; Bucki, R.; Car, H. Gold-functionalized magnetic nanoparticles restrict growth of Pseudomonas aeruginosa. Int. J. Nanomed. 2014, 9, 2217. [Google Scholar]
- Su, Y.; Zheng, X.; Chen, Y.; Li, M.; Liu, K. Alteration of intracellular protein expressions as a key mechanism of the deterioration of bacterial denitrification caused by copper oxide nanoparticles. Sci. Rep. 2015, 5, 15824. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. [Google Scholar] [CrossRef] [Green Version]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124. [Google Scholar] [CrossRef] [Green Version]
- Gounani, Z.; Asadollahi, M.A.; Pedersen, J.N.; Lyngsø, J.; Pedersen, J.S.; Arpanaei, A.; Meyer, R.L. Mesoporous silica nanoparticles carrying multiple antibiotics provide enhanced synergistic effect and improved biocompatibility. Colloids Surf. B Biointerfaces 2019, 175, 498–508. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Lu, Y.; Broadnax, A.D.; Hunter, R.A.; Carpenter, A.W.; Schoenfisch, M.H. Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 9322–9329. [Google Scholar] [CrossRef] [PubMed]
- Joyce, P.; Ulmefors, H.; Maghrebi, S.; Subramaniam, S.; Wignall, A.; Jõemetsa, S.; Höök, F.; Prestidge, C.A. Enhancing the cellular uptake and antibacterial activity of rifampicin through encapsulation in mesoporous silica nanoparticles. Nanomaterials 2020, 10, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.-G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhu, Y.; Wu, T.; Wang, L.; Yuan, Y.; Chen, J.; Hu, Y.; Pang, J. Enhanced functional properties of biopolymer film incorporated with curcurmin-loaded mesoporous silica nanoparticles for food packaging. Food Chem. 2019, 288, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Tamanna, T.; Landersdorfer, C.B.; Ng, H.J.; Bulitta, J.B.; Wood, P.; Yu, A. Prolonged and continuous antibacterial and anti-biofilm activities of thin films embedded with gentamicin-loaded mesoporous silica nanoparticles. Appl. Nanosci. 2018, 8, 1471–1482. [Google Scholar] [CrossRef]
- Vijayan, V.; Uthaman, S.; Park, I.-K. Cell membrane coated nanoparticles: An emerging biomimetic nanoplatform for targeted bioimaging and therapy. Biomim. Med. Mater. 2018, 1064, 45–59. [Google Scholar]
- Gao, W.; Zhang, L. Coating nanoparticles with cell membranes for targeted drug delivery. J. Drug Target. 2015, 23, 619–626. [Google Scholar] [CrossRef]
- Yang, S.; Han, X.; Yang, Y.; Qiao, H.; Yu, Z.; Liu, Y.; Wang, J.; Tang, T. Bacteria-targeting nanoparticles with microenvironment-responsive antibiotic release to eliminate intracellular Staphylococcus aureus and associated infection. ACS Appl. Mater. Interfaces 2018, 10, 14299–14311. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Haselmann, G.M.; Wang, J.; Eder, D.; Schneider-Stickler, B. Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohydr. Polym. 2018, 200, 35–42. [Google Scholar] [CrossRef]
- Singh, A.K.; Prakash, P.; Singh, R.; Nandy, N.; Firdaus, Z.; Bansal, M.; Singh, R.K.; Srivastava, A.; Roy, J.K.; Mishra, B. Curcumin quantum dots mediated degradation of bacterial biofilms. Front. Microbiol. 2017, 8, 1517. [Google Scholar] [CrossRef] [Green Version]
- Meikhail, M.; Abdelghany, A.; Awad, W. Role of CdSe quantum dots in the structure and antibacterial activity of chitosan/poly ε-caprolactone thin films. Egypt. J. Basic Appl. Sci. 2018, 5, 138–144. [Google Scholar] [CrossRef]
- Wansapura, P.T.; Dassanayake, R.S.; Hamood, A.; Tran, P.; Moussa, H.; Abidi, N. Preparation of chitin-CdTe quantum dots films and antibacterial effect on Staphylococcus aureus and Pseudomonas aeruginosa. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Openda, Y.I.; Sen, P.; Managa, M.; Nyokong, T. Acetophenone substituted phthalocyanines and their graphene quantum dots conjugates as photosensitizers for photodynamic antimicrobial chemotherapy against Staphylococcus aureus. Photodiagnosis Photodyn. Ther. 2020, 29, 101607. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Luong, J.H.; Gedanken, A. Green synthesis of multifunctional carbon dots with antibacterial activities. Nanomaterials 2021, 11, 369. [Google Scholar] [CrossRef]
- Lu, F.; Ma, Y.; Wang, H.; Zhang, M.; Wang, B.; Zhang, Y.; Huang, H.; Liao, F.; Liu, Y.; Kang, Z. Water-solvable carbon dots derived from curcumin and citric acid with enhanced broad-spectrum antibacterial and antibiofilm activity. Mater. Today Commun. 2021, 26, 102000. [Google Scholar] [CrossRef]
- Salam, F.D.; Vinita, M.N.; Puja, P.; Prakash, S.; Yuvakkumar, R.; Kumar, P. Anti-bacterial and anti-biofilm efficacies of bioinspired gold nanoparticles. Mater. Lett. 2020, 261, 126998. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Liu, L.; Xiao, X.; Cong, Z.; Shao, N.; Qiao, Z.; Chen, K.; Liu, S.; Zhang, H.; et al. The membrane-targeting mechanism of host defense peptides inspiring the design of polypeptide-conjugated gold nanoparticles exhibiting effective antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Mater. Chem. B 2021, 9, 5092–5101. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel biogenic silver nanoparticle-induced reactive oxygen species inhibit the biofilm formation and virulence activities of methicillin-resistant Staphylococcus aureus (MRSA) strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Lara, H.H.; Lopez-Ribot, J.L. Inhibition of mixed biofilms of Candida albicans and methicillin-resistant Staphylococcus aureus by positively charged silver nanoparticles and functionalized silicone elastomers. Pathogens 2020, 9, 784. [Google Scholar] [CrossRef]
- Romero-Urbina, D.G.; Lara, H.H.; Velázquez-Salazar, J.J.; Arellano-Jiménez, M.J.; Larios, E.; Srinivasan, A.; Lopez-Ribot, J.L.; Yacamán, M.J. Ultrastructural changes in methicillin-resistant Staphylococcus aureus induced by positively charged silver nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2396–2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jusuf, S.; Hui, J.; Dong, P.-T.; Cheng, J.-X. Staphyloxanthin photolysis potentiates low concentration silver nanoparticles in eradication of methicillin-resistant Staphylococcus aureus. J. Phys. Chem. C 2020, 124, 5321–5330. [Google Scholar] [CrossRef]
- Huang, H.; Shan, K.; Liu, J.; Tao, X.; Periyasamy, S.; Durairaj, S.; Jiang, Z.; Jacob, J.A. Synthesis, optimization and characterization of silver nanoparticles using the catkin extract of Piper longum for bactericidal effect against food-borne pathogens via conventional and mathematical approaches. Bioorg. Chem. 2020, 103, 104230. [Google Scholar] [CrossRef]
- Attallah, N.G.M.; Elekhnawy, E.; Negm, W.A.; Hussein, I.A.; Mokhtar, F.A.; Al-Fakhrany, O.M. In vivo and in vitro antimicrobial activity of biogenic silver nanoparticles against Staphylococcus aureus clinical isolates. Pharmaceuticals 2022, 15, 194. [Google Scholar] [CrossRef]
- Taifa, S.; Muhee, A.; Bhat, R.A.; Nabi, S.U.I.; Roy, A.; Rather, G.A.; Khan, A.A.; Bashir, S.M.; Patwekar, M.; Wahab, S.; et al. Evaluation of therapeutic efficacy of copper nanoparticles in Staphylococcus aureus-induced rat mastitis model. J. Nanomater. 2022, 2022, 7124114. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.A.; Mohamad, R.; Hasanah Zaidan, U.; Samsudin, A.A. Antibacterial potential of biosynthesized zinc oxide nanoparticles against poultry-associated foodborne pathogens: An in vitro study. Animals 2021, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Vishakha, K.; Das, S.; Dutta, M.; Mukherjee, D.; Mondal, J.; Mondal, S.; Ganguli, A. Antibacterial, anti-biofilm activity and mechanism of action of pancreatin doped zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus. Colloids Surf. B Biointerfaces 2020, 190, 110921. [Google Scholar] [CrossRef]
- Malakar, C.; Kashyap, B.; Kalita, M.C.; Deka, S. Wound healing efficacy of rhamnolipid-coated zinc oxide nanoparticle along with its in vivo antibacterial efficacy against Staphylococcus aureus. Exp. Dermatol. 2022. [Google Scholar] [CrossRef]
- Malakar, C.; Patowary, K.; Deka, S.; Kalita, M.C. Synthesis, characterization, and evaluation of antibacterial efficacy of rhamnolipid-coated zinc oxide nanoparticles against Staphylococcus aureus. World J. Microbiol. Biotechnol. 2021, 37, 193. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.; Eid, A.M.; Hassan, S.E.-D.; Salem, S.S.; Radwan, A.A.; Halawa, M.; Saleh, F.M.; Saad, H.A.; Saied, E.M.; Fouda, A. The catalytic activity of biosynthesized magnesium oxide nanoparticles (MgO-NPs) for inhibiting the growth of pathogenic microbes, tanning effluent treatment, and chromium ion removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Altaf, M.; Zeyad, M.T.; Hashmi, M.A.; Manoharadas, S.; Hussain, S.A.; Abuhasil, M.S.A.; Almuzaini, M.A.M. Effective inhibition and eradication of pathogenic biofilms by titanium dioxide nanoparticles synthesized using Carum copticum extract. RSC Adv. 2021, 11, 19248–19257. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Husain, F.M.; Qais, F.A.; Alam, P.; Ahmad, I.; Albalawi, T.; Ahmad, N.; Alam, M.; Baig, M.H.; Dong, J.-J. Bio-fabrication of titanium oxide nanoparticles from Ochradenus arabicus to obliterate biofilms of drug-resistant Staphylococcus aureus and Pseudomonas aeruginosa isolated from diabetic foot infections. Appl. Nanosci. 2021, 11, 375–387. [Google Scholar] [CrossRef]
- Devlin, H.; Fulaz, S.; Hiebner, D.W.; O’Gara, J.P.; Casey, E. Enzyme-functionalized mesoporous silica nanoparticles to target Staphylococcus aureus and disperse biofilms. Int. J. Nanomed. 2021, 16, 1929–1942. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Correa, J.J.; Gisbert-Garzarán, M.; Mediero, A.; Fernández-Aceñero, M.J.; de-Pablo-Velasco, D.; Lozano, D.; Esteban, J.; Vallet-Regí, M. Antibiotic delivery from bone-targeted mesoporous silica nanoparticles for the treatment of osteomyelitis caused by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2022, 154, 608–625. [Google Scholar] [CrossRef]
- Nie, B.e.; Huo, S.; Qu, X.; Guo, J.; Liu, X.; Hong, Q.; Wang, Y.; Yang, J.; Yue, B. Bone infection site targeting nanoparticle-antibiotics delivery vehicle to enhance treatment efficacy of orthopedic implant related infection. Bioact. Mater. 2022, 16, 134–148. [Google Scholar] [CrossRef]
- Zulkarnain, M.Z.; Tong, W.Y.; Tan, W.-N.; Leong, C.R.; Md Yusof, F.A.; Wahidin, S.; Attifah, N.R.; Zubaidah, S. p-Coumaric acid quantum dots inhibit beta lactam resistant foodborne microorganisms. Mater. Today Proc. 2020, 31, 48–53. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Cao, W.; Zhang, G.; Yang, X.; Gong, X.; Xing, X. Low-toxicity carbon quantum dots derived from gentamicin sulfate to combat antibiotic resistance and eradicate mature biofilms. Chem. Commun. 2020, 56, 2316–2319. [Google Scholar] [CrossRef]
- Wang, H.; Lu, F.; Ma, C.; Ma, Y.; Zhang, M.; Wang, B.; Zhang, Y.; Liu, Y.; Huang, H.; Kang, Z. Carbon dots with positive surface charge from tartaric acid and m-aminophenol for selective killing of Gram-positive bacteria. J. Mater. Chem. B 2021, 9, 125–130. [Google Scholar] [CrossRef]
- Liang, J.; Li, W.; Chen, J.; Huang, X.; Liu, Y.; Zhang, X.; Shu, W.; Lei, B.; Zhang, H. Antibacterial activity and synergetic mechanism of carbon dots against Gram-positive and -negative bacteria. ACS Appl. Bio. Mater. 2021, 4, 6937–6945. [Google Scholar] [CrossRef]
- Kung, J.-C.; Tseng, I.T.; Chien, C.-S.; Lin, S.-H.; Wang, C.-C.; Shih, C.-J. Microwave assisted synthesis of negative-charge carbon dots with potential antibacterial activity against multi-drug resistant bacteria. RSC Adv. 2020, 10, 41202–41208. [Google Scholar] [CrossRef]
- Kumar, R. Chapter 8—Lipid-based nanoparticles for drug-delivery systems. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–284. [Google Scholar]
- Walduck, A.; Sangwan, P.; Vo, Q.A.; Ratcliffe, J.; White, J.; Muir, B.W.; Tran, N. Treatment of Staphylococcus aureus skin infection in vivo using rifampicin loaded lipid nanoparticles. RSC Adv. 2020, 10, 33608–33619. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49. [Google Scholar]
- Mitchell, S.L.; Carlson, E.E. Tiny things with enormous impact: Nanotechnology in the fight against infectious disease. ACS Infect. Dis. 2018, 4, 1432–1435. [Google Scholar] [CrossRef]
- Pinheiro, M.; Magalhães, J.; Reis, S. Antibiotic interactions using liposomes as model lipid membranes. Chem. Phys. Lipids 2019, 222, 36–46. [Google Scholar] [CrossRef]
- Dong, D.; Thomas, N.; Thierry, B.; Vreugde, S.; Prestidge, C.A.; Wormald, P.-J. Distribution and inhibition of liposomes on Staphylococcus aureus and Pseudomonas aeruginosa biofilm. PLoS ONE 2015, 10, e0131806. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.E.; Crauwels, H.M.; Basstanie, E.D. Formulation and pharmacology of long-acting rilpivirine. Curr. Opin. HIV AIDS 2015, 10, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Berti, I.R.; Dell’Arciprete, M.L.; Dittler, M.L.; Miñan, A.; de Mele, M.F.L.; Gonzalez, M. Delivery of fluorophores by calcium phosphate-coated nanoliposomes and interaction with Staphylococcus aureus biofilms. Colloids Surf. B Biointerfaces 2016, 142, 214–222. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling 2016, 32, 215–225. [Google Scholar] [CrossRef]
- Zomorodian, K.; Veisi, H.; Mousavi, S.M.; Ataabadi, M.S.; Yazdanpanah, S.; Bagheri, J.; Mehr, A.P.; Hemmati, S.; Veisi, H. Modified magnetic nanoparticles by PEG-400-immobilized Ag nanoparticles (Fe(3)O(4)@PEG-Ag) as a core/shell nanocomposite and evaluation of its antimicrobial activity. Int. J. Nanomed. 2018, 13, 3965–3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Zhong, D.; Li, W.; Lin, X.; He, J.; Sun, Y.; Wu, Y.; Shi, M.; Chen, X.; Xu, F.; et al. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today 2022, 42, 101368. [Google Scholar] [CrossRef]
- Ferreira, M.; Pinto, S.; Aires-da-Silva, F.; Bettencourt, A.; Aguiar, S.; Gaspar, M. Liposomes as a nanoplatform to improve the delivery of antibiotics into Staphylococcus aureus biofilms. Pharmaceutics 2021, 13, 321. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Sung, C.T.; Aljuffali, I.A.; Chen, C.-H.; Hu, K.-Y.; Fang, J.-Y. Intravenous anti-MRSA phosphatiosomes mediate enhanced affinity to pulmonary surfactants for effective treatment of infectious pneumonia. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 215–225. [Google Scholar] [CrossRef]
- Silva, N.C.; Silva, S.; Sarmento, B.; Pintado, M. Chitosan nanoparticles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Deliv. 2015, 22, 885–893. [Google Scholar] [CrossRef]
- Vyas, S.; Sihorkar, V.; Jain, S. Mannosylated liposomes for bio-film targeting. Int. J. Pharm. 2007, 330, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.N.I.M.; Chen, X.Y.; Al-Zubaidi, Z.M.; Azhari, H.; Khaitir, T.M.N.; Ng, P.Y.; Buang, F.; Tan, G.C.; Wong, Y.P.; Said, M.M.; et al. Surface-engineered liposomes for dual-drug delivery targeting strategy against methicillin-resistant Staphylococcus aureus (MRSA). Asian J. Pharm. Sci. 2022, 17, 102–119. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Wang, X.; Chen, Y.; Wu, F.; Men, K.; Xu, T.; Luo, Y.; Yang, L. Novel antimicrobial peptide–modified azithromycin-loaded liposomes against methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2016, 11, 6781. [Google Scholar] [CrossRef] [Green Version]
- Sahin, N.O. Niosomes as nanocarrier systems. In Nanomaterials and Nanosystems for Biomedical Applications; Mozafari, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 67–81. [Google Scholar] [CrossRef]
- Mirzaie, A.; Peirovi, N.; Akbarzadeh, I.; Moghtaderi, M.; Heidari, F.; Yeganeh, F.E.; Noorbazargan, H.; Mirzazadeh, S.; Bakhtiari, R. Preparation and optimization of ciprofloxacin encapsulated niosomes: A new approach for enhanced antibacterial activity, biofilm inhibition and reduced antibiotic resistance in ciprofloxacin-resistant methicillin-resistance Staphylococcus aureus. Bioorg. Chem. 2020, 103, 104231. [Google Scholar] [CrossRef]
- Kashef, M.T.; Saleh, N.M.; Assar, N.H.; Ramadan, M.A. The antimicrobial activity of ciprofloxacin-loaded niosomes against ciprofloxacin-resistant and biofilm-forming Staphylococcus aureus. Infect. Drug Resist. 2020, 13, 1619. [Google Scholar] [CrossRef]
- Grimaldi, N.; Andrade, F.; Segovia, N.; Ferrer-Tasies, L.; Sala, S.; Veciana, J.; Ventosa, N. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 2016, 45, 6520–6545. [Google Scholar] [CrossRef] [Green Version]
- Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Elrashedy, A.A.; Mocktar, C.; Nkambule, B.; Soliman, M.E.; Govender, T. Formulation of pH-responsive quatsomes from quaternary bicephalic surfactants and cholesterol for enhanced delivery of vancomycin against methicillin resistant Staphylococcus aureus. Pharmaceutics 2020, 12, 1093. [Google Scholar] [CrossRef]
- Dong, D.; Thomas, N.; Ramezanpour, M.; Psaltis, A.J.; Huang, S.; Zhao, Y.; Thierry, B.; Wormald, P.-J.; Prestidge, C.A.; Vreugde, S. Inhibition of Staphylococcus aureus and Pseudomonas aeruginosa biofilms by quatsomes in low concentrations. Exp. Biol. Med. 2020, 245, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Dalili, D.; Amini, M.; Faramarzi, M.A.; Fazeli, M.R.; Khoshayand, M.R.; Samadi, N. Isolation and structural characterization of Coryxin, a novel cyclic lipopeptide from Corynebacterium xerosis NS5 having emulsifying and anti-biofilm activity. Colloids Surf. B Biointerfaces 2015, 135, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Peng, Y.; Su, M.; Zhu, S.; Pan, J.; Shen, B.; Duan, Y.; Huang, Y. Platensimycin-encapsulated liposomes or micelles as biosafe nanoantibiotics exhibited strong antibacterial activities against methicillin-resistant Staphylococcus aureus infection in mice. Mol. Pharm. 2020, 17, 2451–2462. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Z.; Du, Q.; Li, P.; Wang, Z.; Wang, A. Stimulated phase-shift acoustic nanodroplets enhance vancomycin efficacy against methicillin-resistant Staphylococcus aureus biofilms. Int. J. Nanomed. 2017, 12, 4679. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Banche, G.; Luganini, A.; Finesso, N.; Allizond, V.; Gulino, G.R.; Khadjavi, A.; Spagnolo, R.; Tullio, V.; Giribaldi, G. Vancomycin-loaded nanobubbles: A new platform for controlled antibiotic delivery against methicillin-resistant Staphylococcus aureus infections. Int. J. Pharm. 2017, 523, 176–188. [Google Scholar] [CrossRef] [Green Version]
- Durham, P.G.; Sidders, A.E.; Beam, J.E.; Kedziora, K.M.; Dayton, P.A.; Conlon, B.P.; Papadopoulou, V.; Rowe, S.E. Harnessing ultrasound-stimulated phase change contrast agents to improve antibiotic efficacy against methicillin-resistant Staphylococcus aureus biofilms. Biofilm 2021, 3, 100049. [Google Scholar] [CrossRef]
- Anjum, M.M.; Patel, K.K.; Dehari, D.; Pandey, N.; Tilak, R.; Agrawal, A.K.; Singh, S. Anacardic acid encapsulated solid lipid nanoparticles for Staphylococcus aureus biofilm therapy: Chitosan and DNase coating improves antimicrobial activity. Drug Deliv. Transl. Res. 2021, 11, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Fazly Bazzaz, B.; Khameneh, B.; Namazi, N.; Iranshahi, M.; Davoodi, D.; Golmohammadzadeh, S. Solid lipid nanoparticles carrying Eugenia caryophyllata essential oil: The novel nanoparticulate systems with broad-spectrum antimicrobial activity. Lett. Appl. Microbiol. 2018, 66, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Chi, Z.; Liu, C. Chinese white wax solid lipid nanoparticles as a novel nanocarrier of curcumin for inhibiting the formation of Staphylococcus aureus biofilms. Nanomaterials 2019, 9, 763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar]
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M.; Ki, M.-H.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Bacteria-targeted clindamycin loaded polymeric nanoparticles: Effect of surface charge on nanoparticle adhesion to MRSA, antibacterial activity, and wound healing. Pharmaceutics 2019, 11, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, N.; Thorn, C.; Richter, K.; Thierry, B.; Prestidge, C. Efficacy of poly-lactic-co-glycolic acid micro-and nanoparticles of ciprofloxacin against bacterial biofilms. J. Pharm. Sci. 2016, 105, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Manukumar, H.; Chandrasekhar, B.; Rakesh, K.; Ananda, A.; Nandhini, M.; Lalitha, P.; Sumathi, S.; Qin, H.-L.; Umesha, S. Novel TC@ AgNPs mediated biocidal mechanism against biofilm associated methicillin-resistant Staphylococcus aureus (Bap-MRSA) 090, cytotoxicity and its molecular docking studies. MedChemComm 2017, 8, 2181–2194. [Google Scholar] [CrossRef]
- Zhang, X.; Manukumar, H.; Rakesh, K.; Karthik, C.; Prasad, H.N.; Swamy, S.N.; Mallu, P.; Mohammed, Y.H.E.; Qin, H.-L. Role of BP* C@ AgNPs in Bap-dependent multicellular behavior of clinically important methicillin-resistant Staphylococcus aureus (MRSA) biofilm adherence: A key virulence study. Microb. Pathog. 2018, 123, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef] [PubMed]
- Siddhardha, B.; Pandey, U.; Kaviyarasu, K.; Pala, R.; Syed, A.; Bahkali, A.H.; Elgorban, A.M. Chrysin-Loaded Chitosan Nanoparticles Potentiates Antibiofilm Activity against Staphylococcus aureus. Pathogens 2020, 9, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asli, A.; Brouillette, E.; Ster, C.; Ghinet, M.G.; Brzezinski, R.; Lacasse, P.; Jacques, M.; Malouin, F. Antibiofilm and antibacterial effects of specific chitosan molecules on Staphylococcus aureus isolates associated with bovine mastitis. PLoS ONE 2017, 12, e0176988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breser, M.L.; Felipe, V.; Bohl, L.P.; Orellano, M.S.; Isaac, P.; Conesa, A.; Rivero, V.E.; Correa, S.G.; Bianco, I.D.; Porporatto, C. Chitosan and cloxacillin combination improve antibiotic efficacy against different lifestyle of coagulase-negative Staphylococcus isolates from chronic bovine mastitis. Sci. Rep. 2018, 8, 5081. [Google Scholar] [CrossRef] [PubMed]
- Jamil, B.; Habib, H.; Abbasi, S.A.; Ihsan, A.; Nasir, H.; Imran, M. Development of cefotaxime impregnated chitosan as nano-antibiotics: De novo strategy to combat biofilm forming multi-drug resistant pathogens. Front. Microbiol. 2016, 7, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, J.; Vanat, P.; Marques-da-Silva, D.; Rodrigues, J.R.; Lagoa, R. Metal alginates for polyphenol delivery systems: Studies on crosslinking ions and easy-to-use patches for release of protective flavonoids in skin. Bioact. Mater. 2020, 5, 447–457. [Google Scholar] [CrossRef]
- Scolari, I.; Paez, P.; Musri, M.; Petiti, J.; Torres, A.; Granero, G. Rifampicin loaded in alginate/chitosan nanoparticles as a promising pulmonary carrier against Staphylococcus aureus. Drug Deliv. Transl. Res. 2020, 10, 1403–1417. [Google Scholar] [CrossRef]
- Pavelková, M.; Vysloužil, J.; Kubová, K.; Pavloková, S.; Molinková, D.; Celer, V.; Pechová, A.; Mašek, J.; Vetchý, D. Assessment of antimicrobic, antivirotic and cytotoxic potential of alginate beads cross-linked by bivalent ions for vaginal administration. Pharmaceutics 2021, 13, 165. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Malkoch, M.; García-Gallego, S. Introduction to dendrimers and other dendritic polymers. In Dendrimer Chemistry: Synthetic Approaches Towards Complex Architectures; The Royal Society of Chemistry: London, UK, 2020; pp. 1–20. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Feng, X.; Bai, E.; Xiong, Y.; Zhu, X.; Shen, B.; Duan, Y.; Huang, Y. Platensimycin-encapsulated poly(lactic-co-glycolic acid) and poly(amidoamine) dendrimers nanoparticles with enhanced anti-staphylococcal activity in vivo. Bioconjugate Chem. 2020, 31, 1425–1437. [Google Scholar] [CrossRef]
- Gholami, M.; Mohammadi, R.; Arzanlou, M.; Dourbash, F.A.; Kouhsari, E.; Majidi, G.; Mohseni, S.M.; Nazari, S. In vitro antibacterial activity of poly (amidoamine)-G7 dendrimer. BMC Infect. Dis. 2017, 17, 395. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Liu, S.; Yu, T.; Wu, R.; Ren, Y.; van der Mei, H.C.; Liu, J.; Busscher, H.J. PAMAM dendrimers with dual-conjugated vancomycin and Ag-nanoparticles do not induce bacterial resistance and kill vancomycin-resistant Staphylococci. Acta Biomater. 2021, 123, 230–243. [Google Scholar] [CrossRef]
- Svenningsen, S.W.; Frederiksen, R.F.; Counil, C.; Ficker, M.; Leisner, J.J.; Christensen, J.B. Synthesis and antimicrobial properties of a ciprofloxacin and pamam-dendrimer conjugate. Molecules 2020, 25, 1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omolo, C.A.; Kalhapure, R.S.; Agrawal, N.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. A hybrid of mPEG-b-PCL and G1-PEA dendrimer for enhancing delivery of antibiotics. J. Control. Release 2018, 290, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N.; Overy, D.P.; Lanteigne, M.; McQuillan, K.; Kerr, R.G. Antimicrobial Organometallic Dendrimers with Tunable Activity against Multidrug-Resistant Bacteria. Biomacromolecules 2015, 16, 3694–3703. [Google Scholar] [CrossRef]
- Thissa, N. Siriwardena, Michaela Stach, Runze He, Bee-Ha Gan, Sacha Javor, Marc Heitz, Lan Ma, Xiangju Cai, Peng Chen, Dengwen Wei, Hongtao Li, Jun Ma, Thilo Köhler, Christian van Delden, Tamis Darbre, and Jean-Louis Reymond. Lipidated Peptide Dendrimers Killing Multidrug-Resistant Bacteria. J. Am. Chem. Soc. 2018, 140, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Pinto, S.N.; Mil-Homens, D.; Pires, R.F.; Alves, M.M.; Serafim, G.; Martinho, N.; Melo, M.; Fialho, A.M.; Bonifácio, V.D. Core-shell polycationic polyurea pharmadendrimers: New-generation of sustainable broad-spectrum antibiotics and antifungals. Biomater. Science 2022, 10, 5197–5207. [Google Scholar] [CrossRef]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef]
- Jaiswal, S.; Duffy, B.; Jaiswal, A.K.; Stobie, N.; McHale, P. Enhancement of the antibacterial properties of silver nanoparticles using β-cyclodextrin as a capping agent. Int. J. Antimicrob. Agents 2010, 36, 280–283. [Google Scholar] [CrossRef] [Green Version]
- Rajewski, R.A.; Stella, V.J. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J. Pharm. Sci. 1996, 85, 1142–1169. [Google Scholar] [CrossRef]
- Lin, L.; Mao, X.; Sun, Y.; Cui, H. Antibacterial mechanism of artemisinin/beta-cyclodextrins against methicillin-resistant Staphylococcus aureus (MRSA). Microb. Pathog. 2018, 118, 66–73. [Google Scholar] [CrossRef]
- Pinho, E.; Soares, G.; Henriques, M. Evaluation of antibacterial activity of caffeic acid encapsulated by β-cyclodextrins. J. Microencapsul. 2015, 32, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, F.d.S.; Freitas, T.S.d.; Cruz, R.P.d.; Costa, M.d.S.; Pereira, R.L.S.; Quintans-Júnior, L.J.; Andrade, T.d.A.; Menezes, P.d.P.; Sousa, B.M.H.d.; Nunes, P.S.; et al. Evaluation of the antibacterial and modulatory potential of α-bisabolol, β-cyclodextrin and α-bisabolol/β-cyclodextrin complex. Biomed. Pharmacother. 2017, 92, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Tometri, S.S.; Ahmady, M.; Ariaii, P.; Soltani, M.S. Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome and its effect on oxidative, microbial, bacterial and sensory properties of minced beef. J. Food Meas. Charact. 2020, 14, 3333–3344. [Google Scholar] [CrossRef]
- Bhatia, E.; Sharma, S.; Jadhav, K.; Banerjee, R. Combinatorial liposomes of berberine and curcumin inhibit biofilm formation and intracellular methicillin resistant Staphylococcus aureus infections and associated inflammation. J. Mater. Chem. B 2021, 9, 864–875. [Google Scholar] [CrossRef]
- Zafari, M.; Adibi, M.; Chiani, M.; Bolourchi, N.; Barzi, S.M.; Nosrati, M.S.S.; Bahari, Z.; Shirvani, P.; Noghabi, K.A.; Ebadi, M. Effects of cefazolin-containing niosome nanoparticles against methicillin-resistant Staphylococcus aureus biofilm formed on chronic wounds. Biomed. Mater. 2021, 16, 035001. [Google Scholar] [CrossRef] [PubMed]
- Alanchari, M.; Mohammadi, M.; Yazdian, F.; Ahangari, H.; Ahmadi, N.; Emam-Djomeh, Z.; Homayouni-Rad, A.; Ehsani, A. Optimization and antimicrobial efficacy of curcumin loaded solid lipid nanoparticles against foodborne bacteria in hamburger patty. J. Food Sci. 2021, 86, 2242–2254. [Google Scholar] [CrossRef]
- Da Costa, D.; Exbrayat-Héritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnol. 2021, 19, 12. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) essential oil incorporated into chitosan nanoparticles: Characterization, anti-biofilm properties and application in pork preservation. Int. J. Biol. Macromol. 2021, 185, 620–628. [Google Scholar] [CrossRef]
- Asghar, M.A.; Yousuf, R.I.; Shoaib, M.H.; Asghar, M.A. Antibacterial, anticoagulant and cytotoxic evaluation of biocompatible nanocomposite of chitosan loaded green synthesized bioinspired silver nanoparticles. Int. J. Biol. Macromol. 2020, 160, 934–943. [Google Scholar] [CrossRef]
- Urien, J.-M.; Camus, C.; Leclercq, C.; Dejoies, L.; Mabo, P.; Martins, R.; Boukthir, S.; Bénézit, F.; Behar, N.; Revest, M. The emergence of Staphylococcus aureus as the primary cause of cardiac device-related infective endocarditis. Infection 2021, 49, 999–1006. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J. Recent nanotechnology approaches for prevention and treatment of biofilm-associated infections on medical devices. BioMed Res. Int. 2016, 2016, 1851242. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef]
- Natan, M.; Banin, E. From Nano to Micro: Using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol. Rev. 2017, 41, 302–322. [Google Scholar] [CrossRef] [Green Version]
- Phuengkham, H.; Nasongkla, N. Development of antibacterial coating on silicone surface via chlorhexidine-loaded nanospheres. J. Mater. Sci. Mater. Med. 2015, 26, 78. [Google Scholar] [CrossRef]
- Srisang, S.; Wongsuwan, N.; Boongird, A.; Ungsurungsie, M.; Wanasawas, P.; Nasongkla, N. Multilayer nanocoating of Foley urinary catheter by chlorhexidine-loaded nanoparticles for prolonged release and anti-infection of urinary tract. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1081–1089. [Google Scholar] [CrossRef]
- Sehmi, S.K.; Noimark, S.; Weiner, J.; Allan, E.; MacRobert, A.J.; Parkin, I.P. Potent antibacterial activity of copper embedded into silicone and polyurethane. ACS Appl. Mater. Interfaces 2015, 7, 22807–22813. [Google Scholar] [CrossRef] [PubMed]
- Zangirolami, A.C.; Dias, L.D.; Blanco, K.C.; Vinagreiro, C.S.; Inada, N.M.; Arnaut, L.G.; Pereira, M.M.; Bagnato, V.S. Avoiding ventilator-associated pneumonia: Curcumin-functionalized endotracheal tube and photodynamic action. Proc. Natl. Acad. Sci. USA 2020, 117, 22967–22973. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Nano-TiO2 reinforced PEEK/PEI blends as biomaterials for load-bearing implant applications. ACS Appl. Mater. Interfaces 2015, 7, 5561–5573. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, Q.; Fu, H.; Wang, D.; Gao, Y.; Zhang, M.; Liu, H. Construction and performance evaluation of a sustained release implant material polyetheretherketone with antibacterial properties. Mater. Sci. Eng. C 2021, 126, 112109. [Google Scholar] [CrossRef] [PubMed]
- Natan, M.; Edin, F.; Perkas, N.; Yacobi, G.; Perelshtein, I.; Segal, E.; Homsy, A.; Laux, E.; Keppner, H.; Rask-Andersen, H. Two are better than one: Combining ZnO and MgF2 nanoparticles reduces Streptococcus pneumoniae and Staphylococcus aureus biofilm formation on cochlear implants. Adv. Funct. Mater. 2016, 26, 2473–2481. [Google Scholar] [CrossRef]
- Andersen, B.M. Hospital Textiles. In Prevention and Control of Infections in Hospitals; Springer: Berlin/Heidelberg, Germany, 2019; pp. 907–917. [Google Scholar]
- Petkova, P.; Francesko, A.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Simultaneous sonochemical-enzymatic coating of medical textiles with antibacterial ZnO nanoparticles. Ultrason. Sonochem. 2016, 29, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Perelshtein, I.; Lipovsky, A.; Perkas, N.; Tzanov, T.; Arguirova, M.; Leseva, M.; Gedanken, A. Making the hospital a safer place by sonochemical coating of all its textiles with antibacterial nanoparticles. Ultrason. Sonochem. 2015, 25, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhiya, S.; Dkhar, S.A.; Surendiran, A. Emerging trends of nanomedicine–an overview. Fundam. Clin. Pharmacol. 2009, 23, 263–269. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V. Exposure to inorganic nanoparticles: Routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics 2017, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Bakand, S.; Hayes, A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Shah, J. Safety and toxicological considerations of nanomedicines: The future directions. Curr. Clin. Pharmacol. 2017, 12, 73–82. [Google Scholar] [CrossRef]
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Research 2018, 7, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Gupta, I.; Chaud, M.V.; Dos Santos, C.A. Broadening the spectrum of small-molecule antibacterials by metallic nanoparticles to overcome microbial resistance. Int. J. Pharm. 2017, 532, 139–148. [Google Scholar] [CrossRef]
- Sukwong, P.; Somkid, K.; Kongseng, S.; Pissuwan, D.; Yoovathaworn, K. Respiratory tract toxicity of titanium dioxide nanoparticles and multi-walled carbon nanotubes on mice after intranasal exposure. Micro Nano Lett. 2016, 11, 183–187. [Google Scholar] [CrossRef]
- Emilian Leucuta, S. Systemic and biophase bioavailability and pharmacokinetics of nanoparticulate drug delivery systems. Curr. Drug Deliv. 2013, 10, 208–240. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid.-Based Complement. Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafner, A.; Lovrić, J.; Lakoš, G.P.; Pepić, I. Nanotherapeutics in the EU: An overview on current state and future directions. Int. J. Nanomed. 2014, 9, 1005–1023. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, P.; Subramony, J.A. Nanotherapeutics in oral and parenteral drug delivery: Key learnings and future outlooks as we think small. J. Control. Release 2018, 272, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef]
- Kranjec, C.; Ovchinnikov, K.V.; Grønseth, T.; Ebineshan, K.; Srikantam, A.; Diep, D.B. A bacteriocin-based antimicrobial formulation to effectively disrupt the cell viability of methicillin-resistant Staphylococcus aureus (MRSA) biofilms. NPJ Biofilms Microbiomes 2020, 6, 58. [Google Scholar] [CrossRef]
- Ravindran, D.; Ramanathan, S.; Arunachalam, K.; Jeyaraj, G.; Shunmugiah, K.; Arumugam, V. Phytosynthesized silver nanoparticles as antiquorum sensing and antibiofilm agent against the nosocomial pathogen Serratia marcescens: An in vitro study. J. Appl. Microbiol. 2018, 124, 1425–1440. [Google Scholar] [CrossRef]
- Qin, H.; Cao, H.; Zhao, Y.; Zhu, C.; Cheng, T.; Wang, Q.; Peng, X.; Cheng, M.; Wang, J.; Jin, G.; et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials 2014, 35, 9114–9125. [Google Scholar] [CrossRef]
- Gan, B.H.; Siriwardena, T.N.; Javor, S.; Darbre, T.; Reymond, J.L. Fluorescence imaging of bacterial killing by antimicrobial peptide dendrimer G3KL. ACS Infect. Dis. 2019, 5, 2164–2173. [Google Scholar] [CrossRef]
- Duan, F.; Feng, X.; Jin, Y.; Liu, D.; Yang, X.; Zhou, G.; Liu, D.; Li, Z.; Liang, X.-J.; Zhang, J. Metal–carbenicillin framework-based nanoantibiotics with enhanced penetration and highly efficient inhibition of MRSA. Biomaterials 2017, 144, 155–165. [Google Scholar] [CrossRef]


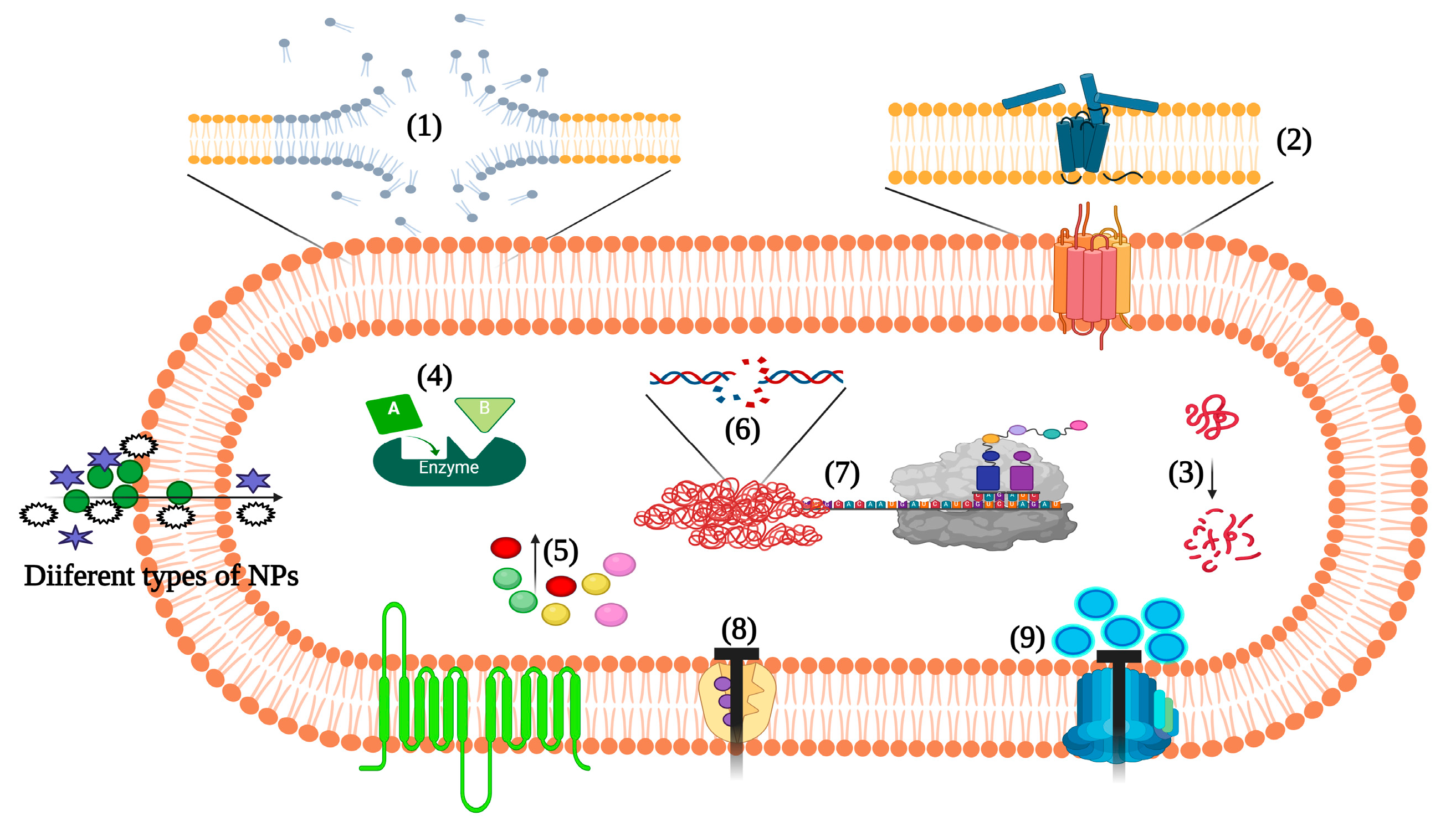
| Virulence Processes | Virulence Factors Involved | Responsible Genes |
|---|---|---|
| Attachment | Microbial surface components recognizing adhesive matrix molecules involving surface proteins such as bone sialoprotein-binding protein, fibronectin-binding proteins, clumping factors, and fibrinogen-binding protein | bbp, fnbA, fnbB, clfA, clfB, sdrD, sasG, fib, and cna |
| Invasion or tissue penetration | Enzymes able to break down lipids, phospholipids, proteins (elastin), DNA, and hyaluronic acid | hysA, nuc, gehB, plc, sepA, sspA, and V8 |
| Destroying or evading host immune system | Pore-forming toxins like leukocidins, phenol-soluble modulins, protein A, CHIPS, Eap, staphyloxanthin, staphylococcal complement inhibitor, and capsular polysaccharides | lukS-PV, hlg, lukF-PV, crtN, spa, psm-a gene cluster, chp, scn, eap, cap5, and 8 gene clusters |
| Persistence and tolerance | Factors involved in intracellular persistence and biofilm formation such as polysaccharide intracellular adhesions, and formation of small colony variants | ica operon, dnaK, and hemB mutation |
| Toxins mediated infections and sepsis | Toxic shock syndrome toxin-1, α-toxin, enterotoxins, exfoliative toxins A and B, and lipoteichoic acid | sea, hla, tstH, eta, and etb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunachalam, K.; Pandurangan, P.; Shi, C.; Lagoa, R. Regulation of Staphylococcus aureus Virulence and Application of Nanotherapeutics to Eradicate S. aureus Infection. Pharmaceutics 2023, 15, 310. https://doi.org/10.3390/pharmaceutics15020310
Arunachalam K, Pandurangan P, Shi C, Lagoa R. Regulation of Staphylococcus aureus Virulence and Application of Nanotherapeutics to Eradicate S. aureus Infection. Pharmaceutics. 2023; 15(2):310. https://doi.org/10.3390/pharmaceutics15020310
Chicago/Turabian StyleArunachalam, Kannappan, Poonguzhali Pandurangan, Chunlei Shi, and Ricardo Lagoa. 2023. "Regulation of Staphylococcus aureus Virulence and Application of Nanotherapeutics to Eradicate S. aureus Infection" Pharmaceutics 15, no. 2: 310. https://doi.org/10.3390/pharmaceutics15020310
APA StyleArunachalam, K., Pandurangan, P., Shi, C., & Lagoa, R. (2023). Regulation of Staphylococcus aureus Virulence and Application of Nanotherapeutics to Eradicate S. aureus Infection. Pharmaceutics, 15(2), 310. https://doi.org/10.3390/pharmaceutics15020310