3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals
Abstract
:1. Personalised Medicine
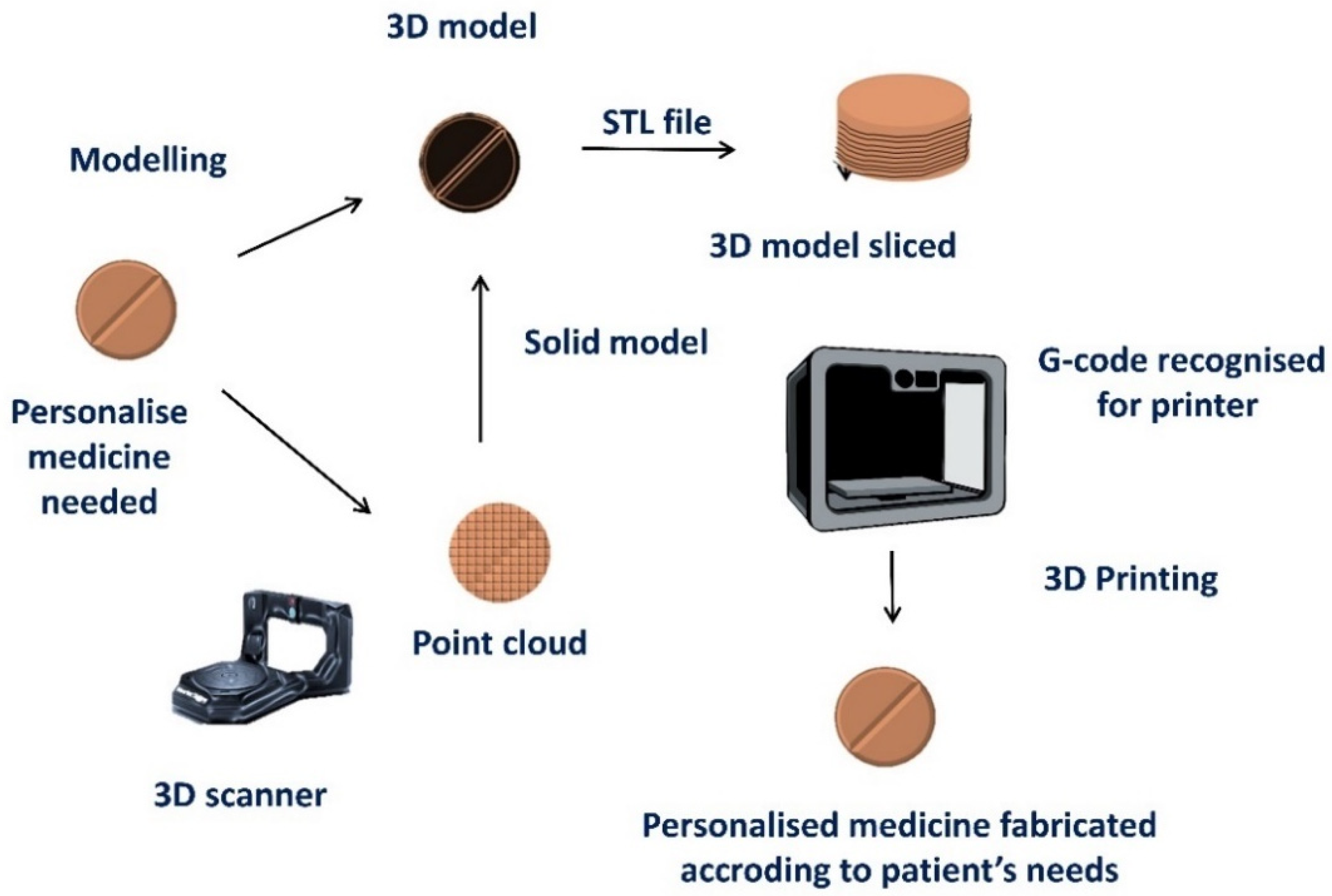
2. 3D Printing of Medicines
2.1. What Applications Can 3D Printing Have for Healthcare Professionals?
2.2. Which Technical Considerations Should Be Born in Mind before 3D-Printing Personalized Medicines?
2.3. What Are the Great Challenges to Bringing This Technology to Clinical Practice?
2.4. What Are the Differences between Conventional Drug Manufacturing and 3D Printing?
2.5. How the Pharmaceutical Ink Can Be Manufactured for 3D Printing?
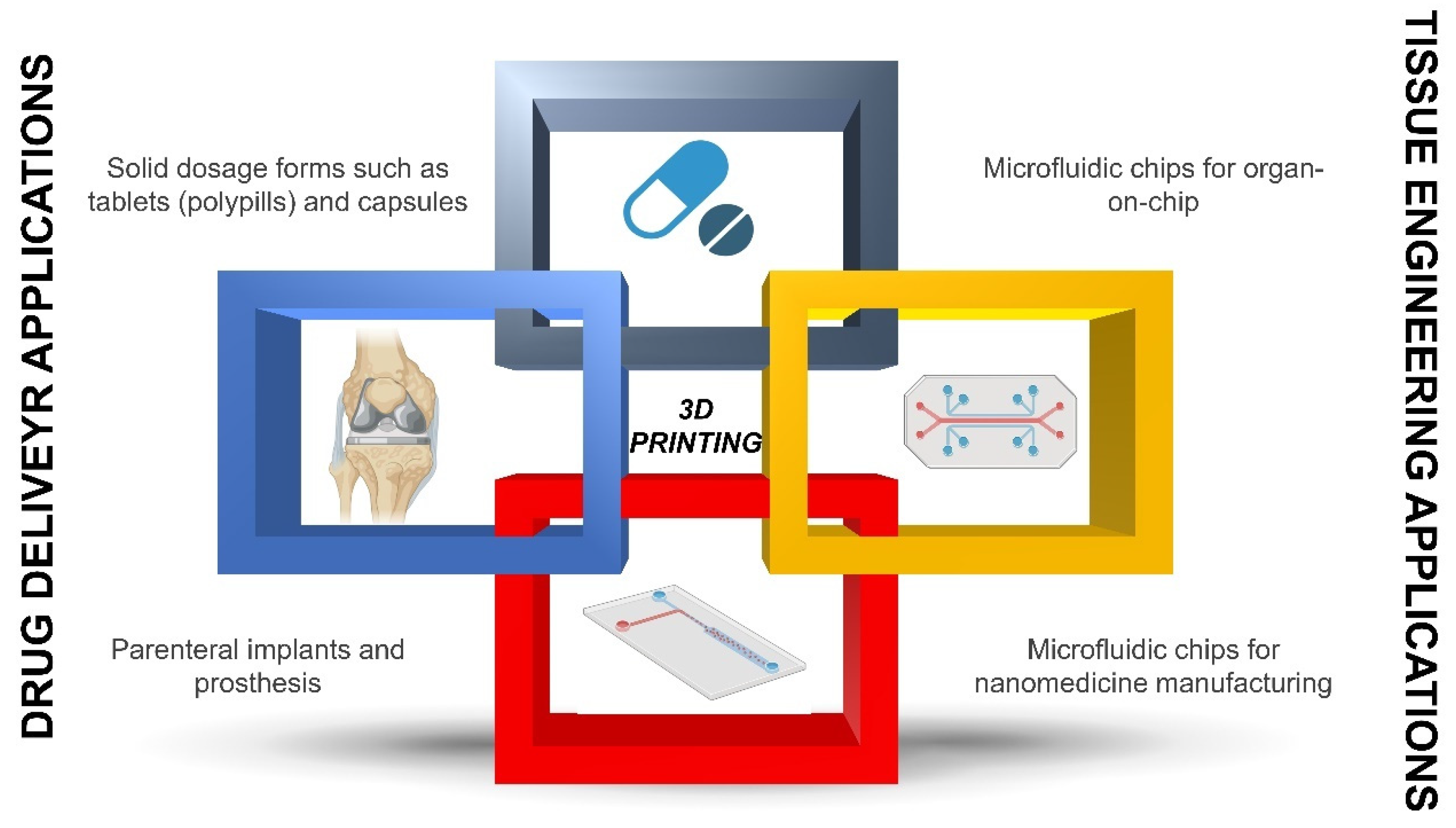
3. Implementation of 3D Printing in Personalized Solid, Topical, Parenteral Dosage Forms and Medical Devices
3.1. Solid Dosage Forms
3.2. 3D-Printed Medical Devices
3.3. 3D-Printed Implants
3.4. Semisolid and Locally Applied Drugs
4. Implementation of 3D Printing in Personalised Biopharmaceuticals
5. Implementation of 3D Printing in Personalised Nanomedicines
5.1. 3D Printed Nanomedicines
5.2. Conventional Batch-to-Batch Approach versus Continuous Manufacturing Using Microfluidics Chips
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission Personalised Medicines. Available online: https://research-and-innovation.ec.europa.eu/research-area/health/personalised-medicine_en (accessed on 9 November 2022).
- Nimmesgern, E.; Norstedt, I.; Draghia-Akli, R. Enabling personalized medicine in Europe by the European Commission’s funding activities. Pers. Med. 2017, 14, 355–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimmesgern, E.; Benediktsson, I.; Norstedt, I. Personalized Medicine in Europe. Clin. Transl. Sci. 2017, 10, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Giacomo, G.D.A.D.; Cury, P.R.; da Silva, A.M.; da Silva, J.V.; Ajzen, S.A. Surgical guides for flapless dental implant placement and immediate definitive prosthesis installation by using selective laser melting and sintering for 3D metal and polymer printing: A clinical report. J. Prosthet. Dent. 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Wu, C.-H.; Lin, C.-L. Design of a patient-specific mandible reconstruction implant with dental prosthesis for metal 3D printing using integrated weighted topology optimization and finite element analysis. J. Mech. Behav. Biomed. Mater. 2020, 105, 103700. [Google Scholar] [CrossRef]
- Pan, S.; Liu, B. Research progress on 3D printing metal powders used in cranio-maxillofacial prosthesis. West China J. Stomatol. 2019, 37, 438–442. (In Chinese) [Google Scholar] [CrossRef]
- Klemm, I.M.; García-Arranz, J.; Özcan, M. 3D Metal Printing—Additive Manufacturing Technologies for Frameworks of ImplantBorne Fixed Dental Prosthesis. 2017, 143–147. Eur. J. Prosthodont. Restor. Dent. 2017, 25, 143–147. [Google Scholar] [CrossRef]
- Moroni, S.; Casettari, L.; Lamprou, D.A. 3D and 4D Printing in the Fight against Breast Cancer. Biosensors 2022, 12, 568. [Google Scholar] [CrossRef]
- Serrano, D.R.; Terres, M.C.; Lalatsa, A. Applications of 3D printing in cancer. J. 3D Print. Med. 2018, 2, 115–127. [Google Scholar] [CrossRef]
- Baert, Y.; Ruetschle, I.; Cools, W.; Oehme, A.; Lorenz, A.; Marx, U.; Goossens, E.; Maschmeyer, I. A multi-organ-chip co-culture of liver and testis equivalents: A first step toward a systemic male reprotoxicity model. Hum. Reprod. 2020, 35, 1029–1044. [Google Scholar] [CrossRef]
- Yuste, I.; Luciano, F.; González-Burgos, E.; Lalatsa, A.; Serrano, D. Mimicking bone microenvironment: 2D and 3D in vitro models of human osteoblasts. Pharmacol. Res. 2021, 169, 105626. [Google Scholar] [CrossRef]
- Nguyen, V.V.T.; Ye, S.; Gkouzioti, V.; van Wolferen, M.E.; Yengej, F.Y.; Melkert, D.; Siti, S.; de Jong, B.; Besseling, P.J.; Spee, B.; et al. A human kidney and liver organoid-based multi-organ-on-a-chip model to study the therapeutic effects and biodistribution of mesenchymal stromal cell-derived extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12280. [Google Scholar] [CrossRef] [PubMed]
- Marzagalli, M.; Pelizzoni, G.; Fedi, A.; Vitale, C.; Fontana, F.; Bruno, S.; Poggi, A.; Dondero, A.; Aiello, M.; Castriconi, R.; et al. A multi-organ-on-chip to recapitulate the infiltration and the cytotoxic activity of circulating NK cells in 3D matrix-based tumor model. Front. Bioeng. Biotechnol. 2022, 10, 945149. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N. Modeling multi-organ systems on a chip. Nat. Methods 2022, 19, 641. [Google Scholar] [CrossRef]
- Chiado, A.; Palmara, G.; Chiappone, A.; Tanzanu, C.; Pirri, C.F.; Roppolo, I.; Frascell, F. A modular 3D printed lab-on-a-chip for early cancer detection. Lab Chip 2020, 20, 665–674. [Google Scholar] [CrossRef]
- Knowlton, S.; Yu, C.H.; Ersoy, F.; Emadi, S.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic chips with patterned, cell-laden hydrogel constructs. Biofabrication 2016, 8, 025019. [Google Scholar] [CrossRef] [Green Version]
- Lepowsky, E.; Amin, R.; Tasoglu, S. Assessing the Reusability of 3D-Printed Photopolymer Microfluidic Chips for Urine Processing. Micromachines 2018, 9, 520. [Google Scholar] [CrossRef] [Green Version]
- Shan, H.; Lin, Q.; Wang, D.; Sun, X.; Quan, B.; Chen, X.; Chen, Z. 3D Printed Integrated Multi-Layer Microfluidic Chips for Ultra-High Volumetric Throughput Nanoliposome Preparation. Front. Bioeng. Biotechnol. 2021, 9, 773705. [Google Scholar] [CrossRef]
- Serrano, D.R.; Cerda, J.R.; Fernandez-Garcia, R.; Pérez-Ballesteros, L.F.; Ballesteros, M.P.; Lalatsa, A. Market Demands in 3D Printing Pharmaceuticals Products. In 3D Printing Technology in Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–183. [Google Scholar] [CrossRef]
- Ayyoubi, S.; Cerda, J.R.; Fernández-García, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Medicines 3D-Printed in Seven Seconds. Available online: https://phys.org/news/2022-03-medicines-3d-printed-seconds.html (accessed on 15 November 2022).
- Fernández-García, R.; Prada, M.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Oral Fixed-Dose Combination Pharmaceutical Products: Industrial Manufacturing Versus Personalized 3D Printing. Pharm. Res. 2020, 37, 132. [Google Scholar] [CrossRef] [PubMed]
- Beer, N.; Hegger, I.; Kaae, S.; De Bruin, M.L.; Genina, N.; Alves, T.L.; Hoebert, J.; Sporrong, S.K. Scenarios for 3D printing of personalized medicines—A case study. Explor. Res. Clin. Soc. Pharm. 2021, 4, 100073. [Google Scholar] [CrossRef]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef] [Green Version]
- Pinho, L.A.G.; Lima, A.L.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Marreto, R.N.; Cunha-Filho, M. Preformulation Studies to Guide the Production of Medicines by Fused Deposition Modeling 3D Printing. AAPS Pharm. Sci. Tech. 2021, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Quodbach, J.; Bogdahn, M.; Breitkreutz, J.; Chamberlain, R.; Eggenreich, K.; Elia, A.G.; Gottschalk, N.; Gunkel-Grabole, G.; Hoffmann, L.; Kapote, D.; et al. Quality of FDM 3D Printed Medicines for Pediatrics: Considerations for Formulation Development, Filament Extrusion, Printing Process and Printer Design. Ther. Innov. Regul. Sci. 2022, 56, 910–928. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.A.; Lima, A.L.; Gratieri, T.; Gelfuso, G.M.; Sa-Barreto, L.L.; Cunha-Filho, M. Compatibility and stability studies involving polymers used in fused deposition modeling 3D printing of medicines. J. Pharm. Anal. 2021, 12, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Korte, C.; Quodbach, J. Formulation development and process analysis of drug-loaded filaments manufactured via hot-melt extrusion for 3D-printing of medicines. Pharm. Dev. Technol. 2018, 23, 1117–1127. [Google Scholar] [CrossRef]
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Knief, P.; Ballesteros, M.P.; Keeble, W.; Barbu, E.; Healy, A.M.; Lalatsa, A.; Serrano, D.R. Personalised 3D Printed Medicines: Optimising Material Properties for Successful Passive Diffusion Loading of Filaments for Fused Deposition Modelling of Solid Dosage Forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Eutionnat-Diffo, P.A.; Chen, Y.; Guan, J.; Cayla, A.; Campagne, C.; Zeng, X.; Nierstrasz, V. Stress, strain and deformation of poly-lactic acid filament deposited onto polyethylene terephthalate woven fabric through 3D printing process. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Godec, D.; Cano, S.; Holzer, C.; Gonzalez-Gutierrez, J. Optimization of the 3D Printing Parameters for Tensile Properties of Specimens Produced by Fused Filament Fabrication of 17-4PH Stainless Steel. Materials 2020, 13, 774. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, G.; Kotsilkova, R.; Ivanov, E.; Petrova-Doycheva, I.; Menseidov, D.; Georgiev, V.; Di Maio, R.; Silvestre, C. Effects of Filament Extrusion, 3D Printing and Hot-Pressing on Electrical and Tensile Properties of Poly(Lactic) Acid Composites Filled with Carbon Nanotubes and Graphene. Nanomaterials 2019, 10, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhou, Y.; Lin, X.; Yang, Q.; Yang, G. Printability of External and Internal Structures Based on Digital Light Processing 3D Printing Technique. Pharmaceutics 2020, 12, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, S.; De Wever, L.; Vanhoorne, V.; De Beer, T.; Vervaet, C. Influence of Print Settings on the Critical Quality Attributes of Extrusion-Based 3D-Printed Caplets: A Quality-by-Design Approach. Pharmaceutics 2021, 13, 2068. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.; Hossain, S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nambiar, A.G.; Singh, M.; Mali, A.R.; Serrano, D.R.; Kumar, R.; Healy, A.M.; Agrawal, A.K.; Kumar, D. Continuous Manufacturing and Molecular Modeling of Pharmaceutical Amorphous Solid Dispersions. AAPS Pharm. Sci. Technol. 2022, 23, 1–26. [Google Scholar] [CrossRef]
- Sánchez-Guirales, S.A.; Jurado, N.; Kara, A.; Lalatsa, A.; Serrano, D.R. Understanding Direct Powder Extrusion for Fabrication of 3D Printed Personalised Medicines: A Case Study for Nifedipine Minitablets. Pharmaceutics 2021, 13, 1583. [Google Scholar] [CrossRef]
- Malebari, A.M.; Kara, A.; Khayyat, A.N.; Mohammad, K.A.; Serrano, D.R. Development of Advanced 3D-Printed Solid Dosage Pediatric Formulations for HIV Treatment. Pharmaceuticals 2022, 15, 435. [Google Scholar] [CrossRef]
- Boniatti, J.; Januskaite, P.; Fonseca, L.; Viçosa, A.; Amendoeira, F.; Tuleu, C.; Basit, A.; Goyanes, A.; Ré, M.-I. Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations. Pharmaceutics 2021, 13, 1114. [Google Scholar] [CrossRef]
- Mendibil, X.; Tena, G.; Duque, A.; Uranga, N.; Campanero, M.; Alonso, J. Direct Powder Extrusion of Paracetamol Loaded Mixtures for 3D Printed Pharmaceutics for Personalized Medicine via Low Temperature Thermal Processing. Pharmaceutics 2021, 13, 907. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control Release 2021, 332, 367–389. [Google Scholar] [CrossRef]
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A. Pressure-assisted microsyringe 3D printing of oral films based on pullulan and hydroxypropyl methylcellulose. Int. J. Pharm. 2021, 595, 120197. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Yoshimura, N.; Goto, E.; Noda, T.; Ozeki, T. Fabrication of Muco-Adhesive Oral Films by the 3D Printing of Hydroxypropyl Methylcellulose-Based Catechin-Loaded Formulations. Biol. Pharm. Bull. 2019, 42, 1898–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; You, Y.; Jiang, W.; Wang, B.; Wu, Q.; Dai, K. 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci. Adv. 2020, 6, eaay1422. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2019, 11, 013001. [Google Scholar] [CrossRef]
- Kim, D.; Kim, M.; Lee, J.; Jang, J. Review on Multicomponent Hydrogel Bioinks Based on Natural Biomaterials for Bioprinting 3D Liver Tissues. Front. Bioeng. Biotechnol. 2022, 10, 764682. [Google Scholar] [CrossRef]
- de Souza, T.V.; Pastena Giorno, L.; Malmonge, S.M.; Santos, A.R., Jr. Bioprinting: From Technique to Application in Tissue Engineering and Regenerative Medicine. Curr. Mol. Med. 2022. [Google Scholar] [CrossRef]
- Ramadan, Q.; Zourob, M. 3D Bioprinting at the Frontier of Regenerative Medicine, Pharmaceutical, and Food Industries. Front. Med. Technol. 2020, 2, 607648. [Google Scholar] [CrossRef]
- Arrigoni, C.; Gilardi, M.; Bersini, S.; Candrian, C.; Moretti, M. Bioprinting and Organ-on-Chip Applications Towards Personalized Medicine for Bone Diseases. Stem Cell Rev. Rep. 2017, 13, 407–417. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: A controlled release study. Int. J. Pharm. 2020, 584, 119428. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Saxena, P.; Pant, V.A.; Pant, A.B. Release and toxicity of dental resin composite. Toxicol. Int. 2012, 19, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin. Oral Investig. 2008, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Darmani, H.; Al-Hiyasat, A.S. The resin monomer triethylene glycol dimethacrylate exhibits reproductive toxicity in male mice. Reprod. Fertil. Dev. 2005, 17, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.; Parma-Benfenati, S.; Quinti, F.; Galletti, P.; Sava, C.; Sava, C.; Kim, D. Clinical and Histologic Evaluations of SLA Dental Implants. Int. J. Periodontics Restor. Dent. 2017, 37, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueche, Y.; Sanchez-Ballester, N.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective Laser Sintering (SLS), a New Chapter in the Production of Solid Oral Forms (SOFs) by 3D Printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef] [PubMed]
- Aprecia Pahrmaceuticals. Spritam. Available online: https://www.aprecia.com/ (accessed on 13 November 2022).
- Windolf, H.; Chamberlain, R.; Breitkreutz, J.; Quodbach, J. 3D Printed Mini-Floating-Polypill for Parkinson’s Disease: Combination of Levodopa, Benserazide, and Pramipexole in Various Dosing for Personalized Therapy. Pharmaceutics 2022, 14, 931. [Google Scholar] [CrossRef]
- Pereira, B.C.; Isreb, A.; Forbes, R.T.; Dores, F.; Habashy, R.; Petit, J.-B.; Alhnan, M.A.; Oga, E.F. ‘Temporary Plasticiser’: A novel solution to fabricate 3D printed patient-centred cardiovascular ‘Polypill’ architectures. Eur. J. Pharm. Biopharm. 2019, 135, 94–103. [Google Scholar] [CrossRef]
- Chen, P.; Luo, H.; Huang, S.; Liu, J.; Lin, M.; Yang, F.; Ban, J.; Huang, Z.; Lu, Z.; Xie, Q.; et al. Preparation of High-Drug-Loaded Clarithromycin Gastric-Floating Sustained-Release Tablets Using 3D Printing. AAPS Pharm. Sci. Tech. 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaurkhede, S.G.; Osipitan, O.O.; Dromgoole, G.; Spencer, S.A.; Di Pasqua, A.J.; Deng, J. 3D Printing and Dissolution Testing of Novel Capsule Shells for Use in Delivering Acetaminophen. J. Pharm. Sci. 2021, 110, 3829–3837. [Google Scholar] [CrossRef]
- Russi, L.; Gaudio, C. 3D printed multicompartmental capsules for a progressive drug release. Ann. 3d Print. Med. 2021, 3, 100026. [Google Scholar] [CrossRef]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [Green Version]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Tan, H.X.; Awad, A.; Buanz, A.; Gaisford, S.; Basit, A.W.; Goyanes, A. Track-and-trace: Novel anti-counterfeit measures for 3D printed personalized drug products using smart material inks. Int. J. Pharm. 2019, 567, 118443. [Google Scholar] [CrossRef]
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172. [Google Scholar] [CrossRef]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef]
- Goh, O.; Goh, W.J.; Lim, S.H.; Hoo, G.S.; Liew, R.; Ng, T.M. Preferences of Healthcare Professionals on 3D-Printed Tablets: A Pilot Study. Pharmaceutics 2022, 14, 1521. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Piñeiro, G.D.; Montero, J.M.G.; Diaz, M.J.L.; Barcia, M.G.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef] [PubMed]
- Triastek, Novel 3D Printed Drug in Clinicla Trials. Available online: https://www.europeanpharmaceuticalreview.com/news/176673/clinical-trials-authorised-ulcerative-colitis-3d-printed-drug/ (accessed on 29 December 2022).
- Clinical Trials on 3D Printed Medical Devices and Prostheses. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=3D+printed+medicine&cntry=&state=&city=&dist= (accessed on 29 December 2022).
- Chua, K.; Khan, I.; Malhotra, R.; Zhu, D. Additive manufacturing and 3D printing of metallic biomaterials. Eng. Regen. 2021, 2, 288–299. [Google Scholar] [CrossRef]
- Francis, A.; Yang, Y.; Virtanen, S.; Boccaccini, A.R. Iron and iron-based alloys for temporary cardiovascular applications. J. Mater. Sci. Mater. Med. 2015, 26, 138. [Google Scholar] [CrossRef]
- Yang, K.; Ren, Y. Nickel-free austenitic stainless steels for medical applications. Sci. Technol. Adv. Mater. 2010, 11, 014105. [Google Scholar] [CrossRef]
- Jakubowicz, J. Special Issue: Ti-Based Biomaterials: Synthesis, Properties and Applications. Materials 2020, 13, 1696. [Google Scholar] [CrossRef] [Green Version]
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: A review. Biometals 2019, 32, 185–193. [Google Scholar] [CrossRef]
- Liu, Y.; Du, T.; Qiao, A.; Mu, Y.; Yang, H. Zinc-Based Biodegradable Materials for Orthopaedic Internal Fixation. J. Funct. Biomater. 2022, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, K.R.; Hanenbaum, C.L.; Carbone, E.J.; Lo, K.W.; Laurencin, C.T.; Walker, J.; Nair, L.S. Pain management via local anesthetics and responsive hydrogels. Ther. Deliv. 2015, 6, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Cudworth, G. Therapeutic applications of implantable drug delivery systems. J. Pharmacol. Toxicol. Methods 1998, 40, 1–12. [Google Scholar] [CrossRef]
- Gimeno, M.; Pinczowski, P.; Pérez, M.; Giorello, A.; Martínez, M.; Santamaría, J.; Arruebo, M.; Luján, L. A controlled antibiotic release system to prevent orthopedic-implant associated infections: An in vitro study. Eur. J. Pharm. Biopharm. 2015, 96, 264–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, S.; Domínguez-Robles, J.; Donnelly, R.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Yang, Y.; Huang, R.; Shi, X.; Chen, H.; Wang, J.; Chen, Y.; Tan, Y.; Tan, Z. E-Jet 3D-Printed Scaffolds as Sustained Multi-Drug Delivery Vehicles in Breast Cancer Therapy. Pharm. Res. 2019, 36, 182. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.J.; Ciurana, J. 3D-printed bioabsordable polycaprolactone stent: The effect of process parameters on its physical features. Mater. Des. 2018, 137, 430–437. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.-H.; Ahn, C.B.; Hong, J.H.; Son, K.H.; Lee, J.W. Development of a 3D-Printed Drug-Eluting Stent for Treating Obstructive Salivary Gland Disease. ACS Biomater. Sci. Eng. 2019, 5, 3572–3581. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D printing of a bladder device for intravesical drug delivery. Mater. Sci. Eng. C 2021, 120, 111773. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Van Kogelenberg, S.; Yue, Z.; Dinoro, J.; Baker, C.S.; Wallace, G.G. Three-Dimensional Printing and Cell Therapy for Wound Repair. Adv. Wound Care 2018, 7, 145–156. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, X.; Liu, Y. Multifunctional 3D printed porous GelMA/xanthan gum based dressing with biofilm control and wound healing activity. Mater. Sci. Eng. C 2021, 131, 112493. [Google Scholar] [CrossRef]
- Lazaridou, M.; Bikiaris, D.N.; Lamprou, D.A. 3D Bioprinted Chitosan-Based Hydrogel Scaffolds in Tissue Engineering and Localised Drug Delivery. Pharmaceutics 2022, 14, 1978. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef] [PubMed]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Elahpour, N.; Pahlevanzadeh, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. Int. J. Pharm. 2021, 597, 120301. [Google Scholar] [CrossRef]
- Uddin, J.; Scoutaris, N.; Economidou, S.N.; Giraud, C.; Chowdhry, B.Z.; Donnelly, R.F.; Douroumis, D. 3D printed microneedles for anticancer therapy of skin tumours. Mater. Sci. Eng. C 2020, 107, 110248. [Google Scholar] [CrossRef]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.; Sharma, P.K.; Murty, U.S.; Mohan, N.H.; Thomas, R.; Dwivedy, S.K.; Banerjee, S. 3D printed hollow microneedles array using stereolithography for efficient transdermal delivery of rifampicin. Int. J. Pharm. 2021, 605, 120815. [Google Scholar] [CrossRef] [PubMed]
- Farias, C.; Lyman, R.; Hemingway, C.; Chau, H.; Mahacek, A.; Bouzos, E.; Mobed-Miremadi, M. Three-Dimensional (3D) Printed Microneedles for Microencapsulated Cell Extrusion. Bioengineering 2018, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D printed drug-eluting contact lenses. J. Pharm. Pharmacol. 2022, 74, 1467–1476. [Google Scholar] [CrossRef]
- Alam, F.; Elsherif, M.; AlQattan, B.; Salih, A.; Lee, S.M.; Yetisen, A.K.; Park, S.; Butt, H. 3D Printed Contact Lenses. ACS Biomater. Sci. Eng. 2021, 7, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Z.; Shi, Q.; Zhao, H.; Pan, G.Q.; Shao, L.H.; Wang, J.F.; Liu, H.W. In vivo study of dual functionalized mussel-derived bioactive peptides promoting 3D-printed porous Ti6Al4V scaffolds for repair of rabbit femoral defects. J. Biomater. Appl. 2022, 37, 942–958. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Gao, J.; Yu, X.; Shi, J.; Chen, J.; Yu, L.; Chen, S.; Ding, J. 3D-Printed Porous Scaffolds of Hydrogels Modified with TGF-β1 Binding Peptides to Promote In Vivo Cartilage Regeneration and Animal Gait Restoration. ACS Appl. Mater. Interfaces 2022, 14, 15982–15995. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, C.; Liu, C.; Li, J.; Peng, Z.; Guo, J.; Zhu, L. Stem Cell-Seeded 3D-Printed Scaffolds Combined with Self-Assembling Peptides for Bone Defect Repair. Tissue Eng. Part A 2022, 28, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lou, Q.; Wang, L.; Min, S.; Zhao, M.; Quan, C. Immobilization of BMP-2-derived peptides on 3D-printed porous scaffolds for enhanced osteogenesis. Biomed. Mater. 2019, 15, 015002. [Google Scholar] [CrossRef]
- Alexa, R.L.; Cucuruz, A.; Ghițulică, C.-D.; Voicu, G.; Balahura, L.-R.S.; Dinescu, S.; Vlasceanu, G.M.; Iovu, H.; Serafim, A.; Ianchis, R.; et al. 3D Printed Composite Scaffolds of GelMA and Hydroxyapatite Nanopowders Doped with Mg/Zn Ions to Evaluate the Expression of Genes and Proteins of Osteogenic Markers. Nanomaterials 2022, 12, 3420. [Google Scholar] [CrossRef]
- Song, X.; Li, X.; Wang, F.; Wang, L.; Lv, L.; Xie, Q.; Zhang, X.; Shao, X. Bioinspired Protein/Peptide Loaded 3D Printed PLGA Scaffold Promotes Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 832727. [Google Scholar] [CrossRef]
- Murphy, R.D.; Garcia, R.V.; Heisede, A.; Hawkera, C.J. Peptides as 3D printable feedstocks: Design strategies and emerging applications. Prog. Polym. Sci. 2022, 124, 101487. [Google Scholar] [CrossRef]
- Serrano, D.R.; Lalatsa, A. Peptide pills for brain diseases? Reality and future perspectives. Ther. Deliv. 2013, 4, 479–501. [Google Scholar] [CrossRef]
- Nguyen, K.T.T.; Heijningen, F.F.M.; Zillen, D.; van Bommel, K.J.C.; van Ee, R.J.; Frijlink, H.W.; Hinrichs, W.L.J. Formulation of a 3D Printed Biopharmaceutical: The Development of an Alkaline Phosphatase Containing Tablet with Ileo-Colonic Release Profile to Treat Ulcerative Colitis. Pharmaceutics 2022, 14, 2179. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Wu, Y.; Oh, Y.-K. On-demand delivery of protein drug from 3D-printed implants. J. Control. Release 2022, 349, 133–142. [Google Scholar] [CrossRef]
- European Commission Recommendation (2011/696/EU). Available online: https://ec.europa.eu/environment/chemicals/nanotech/faq/definition_en.htm (accessed on 5 October 2022).
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, R.; de Pablo, E.; Ballesteros, M.P.; Serrano, D.R. Unmet clinical needs in the treatment of systemic fungal infections: The role of amphotericin B and drug targeting. Int. J. Pharm. 2017, 525, 139–148. [Google Scholar] [CrossRef]
- Ruiz, H.K.; Serrano, D.R.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Bolás-Fernández, F.; Torrado, J.J.; Molero, G. New amphotericin B-gamma cyclodextrin formulation for topical use with synergistic activity against diverse fungal species and Leishmania spp. Int. J. Pharm. 2014, 473, 148–157. [Google Scholar] [CrossRef]
- Serrano, D.; Ruiz-Saldaña, H.; Molero, G.; Ballesteros, M.; Torrado, J. A novel formulation of solubilised amphotericin B designed for ophthalmic use. Int. J. Pharm. 2012, 437, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Lalatsa, A.; Emeriewen, K.; Protopsalti, V.; Skelton, G.; Saleh, G.M. Developing transcutaneous nanoenabled anaesthetics for eyelid surgery. Br. J. Ophthalmol. 2016, 100, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalatsa, A.; Schätzlein, A.; Garrett, N.; Moger, J.; Briggs, M.; Godfrey, L.; Iannitelli, A.; Freeman, J.; Uchegbu, I. Chitosan amphiphile coating of peptide nanofibres reduces liver uptake and delivers the peptide to the brain on intravenous administration. J. Control. Release 2015, 197, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Ge, J.; Gao, Y.; Chen, L.; Cui, J.; Zeng, J.; Gao, M. Ultrasmall superparamagnetic iron oxide nanoparticles: A next generation contrast agent for magnetic resonance imaging. WIREs Nanomed. Nanobiotechnology 2022, 14, e1740. [Google Scholar] [CrossRef]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int. J. Pharm. 2020, 573, 118817. [Google Scholar] [CrossRef]
- Fernández-García, R.; Statts, L.; De Jesus, J.A.; Ayuela, M.A.D.; Bautista, L.; Simão, R.; Bolás-Fernández, F.; Ballesteros, M.P.; Laurenti, M.D.; Passero, L.F.D.; et al. Ultradeformable Lipid Vesicles Localize Amphotericin B in the Dermis for the Treatment of Infectious Skin Diseases. ACS Infect. Dis. 2020, 6, 2647–2660. [Google Scholar] [CrossRef]
- Lalatsa, A.; Statts, L.; de Jesus, J.A.; Adewusi, O.; Dea-Ayuela, M.A.; Bolas-Fernandez, F.; Laurenti, M.D.; Passero, L.F.D.; Serrano, D.R. Topical buparvaquone nano-enabled hydrogels for cutaneous leishmaniasis. Int. J. Pharm. 2020, 588, 119734. [Google Scholar] [CrossRef]
- Bezerra-Souza, A.; Jesus, J.A.; Laurenti, M.D.; Lalatsa, A.; Serrano, D.R.; Passero, L.F.D. Nanoemulsified Butenafine for Enhanced Performance against Experimental Cutaneous Leishmaniasis. J. Immunol. Res. 2021, 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pineros, I.; Slowing, K.; Serrano, D.R.; de Pablo, E.; Ballesteros, M.P. Analgesic and anti-inflammatory controlled-released injectable microemulsion: Pseudo-ternary phase diagrams, in vitro, ex vivo and in vivo evaluation. Eur. J. Pharm. Sci. 2017, 101, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Shahba, A.; Mohsin, K.; Alanazi, F.K. Novel Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Oral Delivery of Cinnarizine: Design, Optimization, and In-Vitro Assessment. AAPS Pharm. Sci. Technol. 2012, 13, 967–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezerra-Souza, A.; Fernandez-Garcia, R.; Rodrigues, G.F.; Bolas-Fernandez, F.; Laurenti, M.D.; Passero, L.F.; Lalatsa, A.; Serrano, D.R. Repurposing Butenafine as An Oral Nanomedicine for Visceral Leishmaniasis. Pharmaceutics 2019, 11, 353. [Google Scholar] [CrossRef] [Green Version]
- Rolon, M.; Hanna, E.; Vega, C.; Coronel, C.; Dea-Ayuela, M.A.; Serrano, D.R.; Lalatsa, A. Solid Nanomedicines of Nifurtimox and Benznidazole for the Oral Treatment of Chagas Disease. Pharmaceutics 2022, 14, 1822. [Google Scholar] [CrossRef]
- Smith, L.A.; Serrano, D.R.; Mauger, M.; Bolás-Fernández, F.; Dea-Ayuela, M.A.; Lalatsa, A. Orally Bioavailable and Effective Buparvaquone Lipid-Based Nanomedicines for Visceral Leishmaniasis. Mol. Pharm. 2018, 15, 2570–2583. [Google Scholar] [CrossRef]
- Serrano, D.R.; Lalatsa, A. Oral amphotericin B: The journey from bench to market. J. Drug Deliv. Sci. Technol. 2017, 42, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Serrano, D.R.; Lalatsa, A.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Garrett, N.L.; Moger, J.; Guarro, J.; Capilla, J.; Ballesteros, M.P.; Schätzlein, A.G.; et al. Oral Particle Uptake and Organ Targeting Drives the Activity of Amphotericin B Nanoparticles. Mol. Pharm. 2015, 12, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Serrano, D.R.; Hernández, L.; Fleire, L.; González-Alvarez, I.; Montoya, A.; Ballesteros, M.P.; Dea-Ayuela, M.A.; Miró, G.; Bolás-Fernández, F.; Torrado, J.J. Hemolytic and pharmacokinetic studies of liposomal and particulate amphotericin B formulations. Int. J. Pharm. 2013, 447, 38–46. [Google Scholar] [CrossRef]
- Nam, L.; Coll, C.; Erthal, L.C.S.; de la Torre, C.; Serrano, D.; Martínez-Máñez, R.; Santos-Martínez, M.J.; Ruiz-Hernández, E. Drug Delivery Nanosystems for the Localized Treatment of Glioblastoma Multiforme. Materials 2018, 11, 779. [Google Scholar] [CrossRef] [Green Version]
- Fisusi, F.A.; Siew, A.; Chooi, K.W.; Okubanjo, O.; Garrett, N.; Lalatsa, K.; Serrano, D.; Summers, I.; Moger, J.; Stapleton, P.; et al. Lomustine Nanoparticles Enable Both Bone Marrow Sparing and High Brain Drug Levels—A Strategy for Brain Cancer Treatments. Pharm. Res. 2016, 33, 1289–1303. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Jambhulkar, S.; Zhu, Y.; Ravichandran, D.; Kakarla, M.; Vernon, B.; Lott, D.G.; Cornella, J.L.; Shefi, O.; Miquelard-Garnier, G.; et al. 3D printing for polymer/particle-based processing: A review. Compos. Part B Eng. 2021, 223, 109102. [Google Scholar] [CrossRef]
- de Oliveira, T.V.; de Oliveira, R.S.; dos Santos, J.; Funk, N.L.; Petzhold, C.L.; Beck, R.C.R. Redispersible 3D printed nanomedicines: An original application of the semisolid extrusion technique. Int. J. Pharm. 2022, 624, 122029. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef] [PubMed]
- Barber, B.W.; Dumont, C.; Caisse, P.; Simon, G.P.; Boyd, B.J. A 3D-Printed Polymer–Lipid-Hybrid Tablet towards the Development of Bespoke SMEDDS Formulations. Pharmaceutics 2021, 13, 2107. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.; Mohammed, A.; Ahmad, J.; Abdullah, M.; Saleh, E. 3D Printing of Dapagliflozin Containing Self-Nanoemulsifying Tablets: Formulation Design and In Vitro Characterization. Pharmaceutics 2021, 13, 993. [Google Scholar] [CrossRef]
- Chatzitakia, A.T.; Tsongas, K.; Tzimtzimis, E.K.; Tzetsis, D.; Bouropoulos, N.; Barpalexisa, P.; Eleftheriadisa, G.K.; Fatourus, D.G. 3D printing of patient-tailored SNEDDS-based suppositories of lidocaine. J. Drug Deliv. Sci. Technol. 2021, 61, 102292. [Google Scholar] [CrossRef]
- Serrano, D.R.; Gallagher, K.H.; Healy, A.M. Emerging Nanonisation Technologies: Tailoring Crystalline Versus Amorphous Nanomaterials. Curr. Top. Med. Chem. 2015, 15, 2327–2340. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- Serrano, D.; Persoons, T.; D’Arcy, D.M.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Modelling and shadowgraph imaging of cocrystal dissolution and assessment of in vitro antimicrobial activity for sulfadimidine/4-aminosalicylic acid cocrystals. Eur. J. Pharm. Sci. 2016, 89, 125–136. [Google Scholar] [CrossRef]
- Colombo, A.P.; Briancon, S.; Lieto, J.; Fessi, H. Project, Design, and Use of a Pilot Plant for Nanocapsule Production. Drug Dev. Ind. Pharm. 2001, 27, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Grossjohann, C.; Serrano, D.R.; Paluch, K.J.; O’Connell, P.; Vella-Zarb, L.; Manesiotis, P.; McCabe, T.; Tajber, L.; Corrigan, O.I.; Healy, A.M. Polymorphism in Sulfadimidine/4-Aminosalicylic Acid Cocrystals: Solid-State Characterization and Physicochemical Properties. J. Pharm. Sci. 2015, 104, 1385–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, D.R.; Walsh, D.; O’Connell, P.; Mugheirbi, N.A.; Worku, Z.A.; Bolas-Fernandez, F.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating. Eur. J. Pharm. Biopharm. 2018, 124, 13–27. [Google Scholar] [CrossRef]
- Rolón, M.; Serrano, D.R.; Lalatsa, A.; de Pablo, E.; Torrado, J.J.; Ballesteros, M.P.; Healy, A.M.; Vega, C.; Coronel, C.; Bolás-Fernández, F.; et al. Engineering Oral and Parenteral Amorphous Amphotericin B Formulations against Experimental Trypanosoma cruzi Infections. Mol. Pharm. 2017, 14, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, D.R.; O’Connell, P.; Paluch, K.J.; Walsh, D.; Healy, A.M. Cocrystal habit engineering to improve drug dissolution and alter derived powder properties. J. Pharm. Pharmacol. 2016, 68, 665–677. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.; Serrano, D.R.; Worku, Z.A.; Madi, A.M.; O’Connell, P.; Twamley, B.; Healy, A.M. Engineering of pharmaceutical cocrystals in an excipient matrix: Spray drying versus hot melt extrusion. Int. J. Pharm. 2018, 551, 241–256. [Google Scholar] [CrossRef]
- Walsh, D.; Serrano, D.R.; Worku, Z.A.; Norris, B.A.; Healy, A.M. Production of cocrystals in an excipient matrix by spray drying. Int. J. Pharm. 2018, 536, 467–477. [Google Scholar] [CrossRef]
- Inada, Y. Continuous Manufacturing Development in Pharmaceutical and Fine Chemicals Industries. Available online: https://www.mitsui.com/mgssi/en/report/detail/__icsFiles/afieldfile/2020/04/08/1912m_inada_e_1.pdf (accessed on 15 November 2022).
- Matji, A.; Donato, N.; Gagol, A.; Morales, E.; Carvajal, L.; Serrano, D.R.; Worku, Z.A.; Healy, A.M.; Torrado, J.J. Predicting the critical quality attributes of ibuprofen tablets via modelling of process parameters for roller compaction and tabletting. Int. J. Pharm. 2019, 565, 209–218. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- González-González, O.; Ramirez, I.O.; Ramirez, B.I.; O’Connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef]
- Bosetti, R. Cost–effectiveness of nanomedicine: The path to a future successful and dominant market? Nanomedicine 2015, 10, 1851–1853. [Google Scholar] [CrossRef]
- Garg, S.; Heuck, G.; Ip, S.; Ramsay, E. Microfluidics: A transformational tool for nanomedicine development and production. J. Drug Target. 2016, 24, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Beck-Broichsitter, M.; Bøtker, J.P.; Malmsten, M.; Rantanen, J.; Bohr, A. Transforming nanomedicine manufacturing toward Quality by Design and microfluidics. Adv. Drug Deliv. Rev. 2018, 128, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Osouli-Bostanabad, K.; Puliga, S.; Serrano, D.R.; Bucchi, A.; Halbert, G.; Lalatsa, A. Microfluidic Manufacture of Lipid-Based Nanomedicines. Pharmaceutics 2022, 14, 1940. [Google Scholar] [CrossRef]
- Khadke, S.; Roces, C.B.; Cameron, A.; Devitt, A.; Perrie, Y. Formulation and manufacturing of lymphatic targeting liposomes using microfluidics. J. Control. Release 2019, 307, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.; Takami, A.; Kim, J.H.; Dong, A.; Chen, A.; Valerio, R.; Gunn, S.; Erogbogbo, F. Herringbone-Patterned 3D-Printed Devices as Alternatives to Microfluidics for Reproducible Production of Lipid Polymer Hybrid Nanoparticles. ACS Omega 2019, 4, 4650–4657. [Google Scholar] [CrossRef] [Green Version]
- Streck, S.; Neumann, H.; Nielsen, H.M.; Rades, T.; McDowell, A. Comparison of bulk and microfluidics methods for the formulation of poly-lactic-co-glycolic acid (PLGA) nanoparticles modified with cell-penetrating peptides of different architectures. Int. J. Pharm. X 2019, 1, 100030. [Google Scholar] [CrossRef]
- Martins, J.P.; Torrieri, G.; Santos, H.A. The importance of microfluidics for the preparation of nanoparticles as advanced drug delivery systems. Expert Opin. Drug Deliv. 2018, 15, 469–479. [Google Scholar] [CrossRef]
- Tiboni, M.; Tiboni, M.; Pierro, A.; Del Papa, M.; Sparaventi, S.; Cespi, M.; Casettari, L. Microfluidics for nanomedicines manufacturing: An affordable and low-cost 3D printing approach. Int. J. Pharm. 2021, 599, 120464. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, J.Y.; Shumate, L.; Fedak, R.; DeVoe, D.L. High Throughput Nanoliposome Formation Using 3D Printed Microfluidic Flow Focusing Chips. Adv. Mater. Technol. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Bressan, L.P.; Adamo, C.B.; Quero, R.F.; de Jesus, D.P.; da Silva, J.A.F. A simple procedure to produce FDM-based 3D-printed microfluidic devices with an integrated PMMA optical window. Anal. Methods 2019, 11, 1014–1020. [Google Scholar] [CrossRef]
- Kara, A.; Vassiliadou, A.; Ongoren, B.; Keeble, W.; Hing, R.; Lalatsa, A.; Serrano, D.R. Engineering 3D Printed Microfluidic Chips for the Fabrication of Nanomedicines. Pharmaceutics 2021, 13, 2134. [Google Scholar] [CrossRef]
- Rolley, N.; Bonnin, M.; Lefebvre, G.; Verron, S.; Bargiel, S.; Robert, L.; Riou, J.; Simonsson, C.; Bizien, T.; Gimel, J.-C.; et al. Galenic Lab-on-a-Chip concept for lipid nanocapsules production. Nanoscale 2021, 13, 11899–11912. [Google Scholar] [CrossRef]
- Tiboni, M.; Benedetti, S.; Skouras, A.; Curzi, G.; Perinelli, D.R.; Palmieri, G.F.; Casettari, L. 3D-printed microfluidic chip for the preparation of glycyrrhetinic acid-loaded ethanolic liposomes. Int. J. Pharm. 2020, 584, 119436. [Google Scholar] [CrossRef]
- Ballacchino, G.; Weaver, E.; Mathew, E.; Dorati, R.; Genta, I.; Conti, B.; Lamprou, D.A. Manufacturing of 3D-Printed Microfluidic Devices for the Synthesis of Drug-Loaded Liposomal Formulations. Int. J. Mol. Sci. 2021, 22, 8064. [Google Scholar] [CrossRef]
- Khorshid, S.; Montanari, M.; Benedetti, S.; Moroni, S.; Aluigi, A.; Canonico, B.; Papa, S.; Tiboni, M.; Casettari, L. A microfluidic approach to fabricate sucrose decorated liposomes with increased uptake in breast cancer cells. Eur. J. Pharm. Biopharm. 2022, 178, 53–64. [Google Scholar] [CrossRef]
- dos Santos, J.; Oliveira, R.S.; Oliveira, T.V.; Velho, M.C.; Konrad, M.V.; da Silva, G.S.; Deon, M.; Beck, R.C.R. 3D Printing and Nanotechnology: A Multiscale Alliance in Personalized Medicine. Adv. Funct. Mater. 2021, 31, 2009691. [Google Scholar] [CrossRef]
- Jain, K.; Shukla, R.; Yadav, A.; Ujjwal, R.; Flora, S. 3D Printing in Development of Nanomedicines. Nanomaterials 2021, 11, 420. [Google Scholar] [CrossRef]






| Printing Technique | Type | Key Parameters | Advantages | Challenges | Type of Medicines |
|---|---|---|---|---|---|
| Nozzle-based deposition | FDM | Temperature of extrusion Layer height Speed of printing Filament composition and diameter Tg composite | High mechanical strength Availability of pharmaceutical-grade excipients | A suitable filament is required for printingHigh temperatures are usually necessary Thermolabile drugs | Solid dosage forms (easier to obtain sustained-release tablets rather than immediate-release ones) Parenteral implants |
| DPE | Temperature of extrusion Layer height Speed of printing Powder mixture | High mechanical strength Availability of pharmaceutical-grade excipients No need for filament prefabrication | High temperature of extrusion Lack of homogeneity during the process Thermolabile drugs | Solid dosage forms (easier to obtain sustained-release tablets rather than immediate-release ones) Parenteral implants | |
| PAM | Viscosity of the material Speed of printing Layer height Composition of the ink | No need for high temperature High cell biocompatibility | Solvent removal in the postprinting step Poor mechanical strength | Tissue engineering Solid dosage forms | |
| Laser-based writing | SLA | Laser power intensity Time of exposure Type of resin UV wavelength | High resolution No need for high temperature | Toxicity of the resin Postprinting step necessary to remove unsolidified resin UV-sensitive drugs | Dentistry Microfluidic chip fabrication |
| SLS | Laser power intensity Time of exposure Type of powder mixture | High resolution No need for solvent | Risk of degradation by laser exposure Excessive waste of powder mixture | Solid dosage forms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. https://doi.org/10.3390/pharmaceutics15020313
Serrano DR, Kara A, Yuste I, Luciano FC, Ongoren B, Anaya BJ, Molina G, Diez L, Ramirez BI, Ramirez IO, et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics. 2023; 15(2):313. https://doi.org/10.3390/pharmaceutics15020313
Chicago/Turabian StyleSerrano, Dolores R., Aytug Kara, Iván Yuste, Francis C. Luciano, Baris Ongoren, Brayan J. Anaya, Gracia Molina, Laura Diez, Bianca I. Ramirez, Irving O. Ramirez, and et al. 2023. "3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals" Pharmaceutics 15, no. 2: 313. https://doi.org/10.3390/pharmaceutics15020313
APA StyleSerrano, D. R., Kara, A., Yuste, I., Luciano, F. C., Ongoren, B., Anaya, B. J., Molina, G., Diez, L., Ramirez, B. I., Ramirez, I. O., Sánchez-Guirales, S. A., Fernández-García, R., Bautista, L., Ruiz, H. K., & Lalatsa, A. (2023). 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics, 15(2), 313. https://doi.org/10.3390/pharmaceutics15020313








