Advances in the Physico-Chemical, Antimicrobial and Angiogenic Properties of Graphene-Oxide/Cellulose Nanocomposites for Wound Healing
Abstract
:1. Introduction
2. Cellulose and Cellulose Derivatives
2.1. Cellulose Physical Modifications
2.2. Cellulose Chemical Modifications
2.3. Cellulose from Agricultural Waste: Extraction Methods and Properties
3. The Graphene Oxide and Reduced Graphene Oxide
3.1. Conventional Synthesis of Graphene Oxide
3.2. Synthesis of Graphene Oxide by Waste
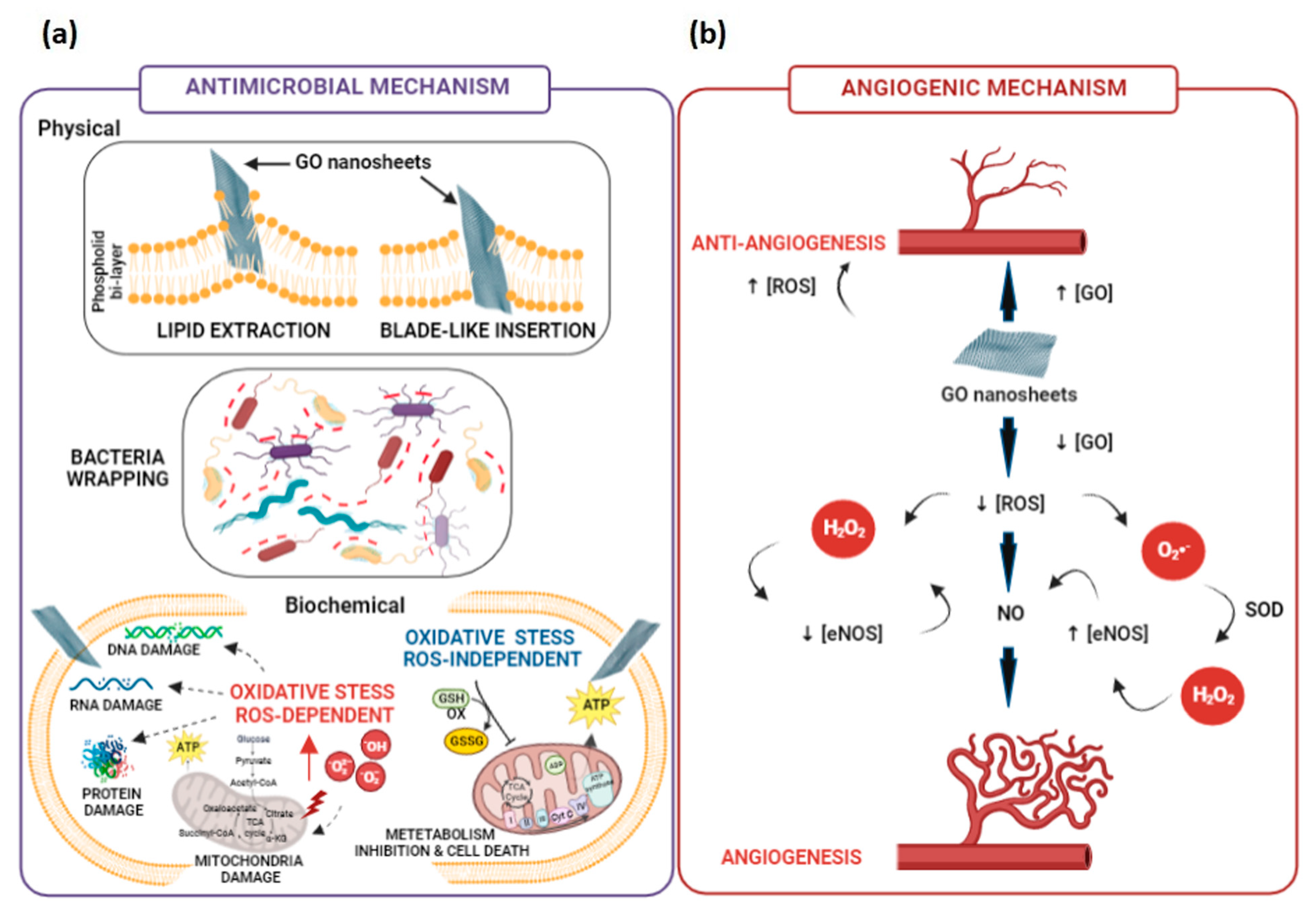
4. Short Overview of GO-Derivatives’ Role in Antimicrobial Activities and Angiogenic Mechanisms
4.1. Antimicrobial Mechanisms Involving GO-Derivatives
4.2. Angiogenic Mechanisms Involving GO-Derivatives
5. GO-Functionalised Cellulose-Based Nanocomposites for Wound Healing
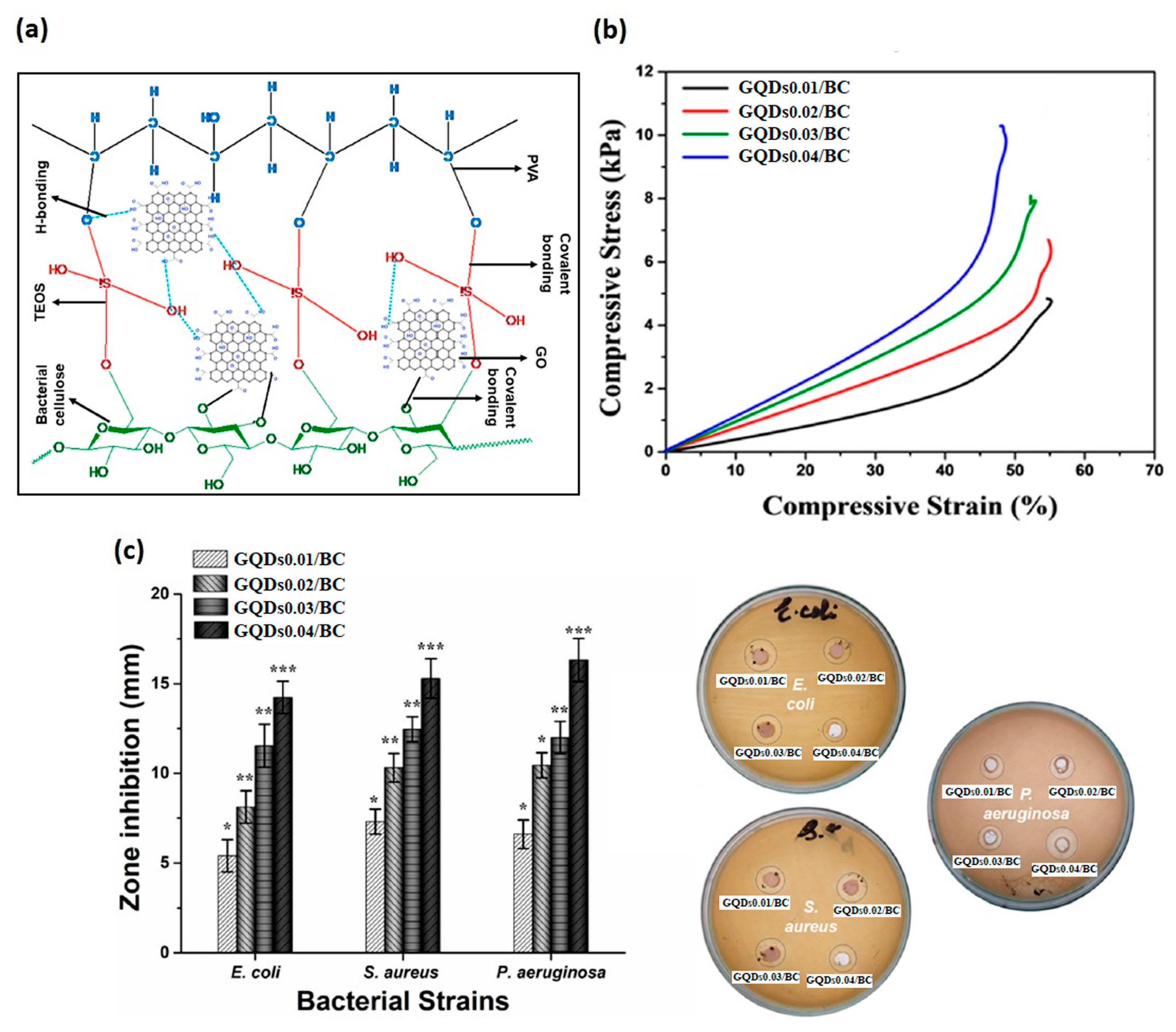
5.1. GO or rGO/Cellulose Nanocomposites
| Year | Material | Cellulose Biosource | GO Processes | Crosslinker/ Gelling Agents | Inorganic/Organic Compounds Embedded | Physico-Chemical and Mechanical Properties | In Vitro Outcomes | In Vivo Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Pure cellulose | |||||||||
| 2022 | GO/DCC | Cotton linter pulp | Hummers method | ECH:DCC | - | σcomp = 0.05–13.6 Mpa; wcomp = 0.05–1.47 MJ/m3); σtens = 0.02–2.8 Mpa; wtens = 0.01–1.49 MJ/m3 G′ = 0.4–1.5 Pa (GO effect) G″ = 5.8–12.8 Pa (GO effect) G′ = 2037 Pa at 1 rad/s (ECH effect) | E. coli death 96.6% S. aureus death 100% (8% wt GO, NIR irradiation 2 W/cm, 808 nm, 240 s) | - | [127] |
| Cellulose-derivatives without bioactive compounds | |||||||||
| 2019 | rGO/CMC | chemically modified | rGO oxidation by NaOH | HDF cells viability (>90%) S. aureus biofilm reduction 81–84%. P. aeruginosa biofilm reduction 51–62% S. aureus biofilm thickness reduction 47%, P. aeruginosa biofilm thickness reduction 40% | Higher biofilm inhibition in C. elegants infected with S. aureus than P. aeruginosa. | [128] | |||
| 2021 | GO/CMC | chemically modified | Hummers method | EA.hy926 migration promotion and increasing of the wound closure rate | Increase of newly formed blood vessels in number and in size. Highly densely packed collagen deposition. | [129] | |||
| Cellulose-derivatives with bioactive compounds | |||||||||
| 2019 | AGO/HPC | chemically modified | Hummers method | Ag/ZnO | σtens = 19.4–28.8 Mpa | E. coli inhibition zone 11.25–12.32 mm; S. aureus inhibition zone 12.18–13.76 mm | Reduction of wound size after 8 days. Newborn blood vessels filled with red blood cells after 12 days | [130] | |
| 2021 | GO/TiO2/Cur/CA | chemically modified | GO/TiO2 nanocomposite by hydrothermal method | TiO2 Cur | σtens = 35.45 Mpa; swelling ≈ 50–55% | E. coli inhibition zone 19 ± 0.2 mm; S. aureus inhibition zone 17 ± 0.1 mm; P. aeruginosa inhibition zone 16.4 ± 0.2; E. faecalis inhibition zone 14 ± 0.2 mm. Wound closure 77 ± 4.18% (12 h)–96 ± 3.26% (24 h) | [131] | ||
| 2022 | O/NA/CA | chemically modified | GO pyrolyzation of cellulose | NA (98%) | σ = 2.46 ± 0.022–4.94 ± 0.027 (·10−5 Mpa); ε = 53.95 ± 0.52–19.05 ± 0.24 %; E = 4.56–25.93 (10−7 Mpa). | HeLa cells viability (>80%) S. aureus inhibitory zone 9.15 mm | [132] | ||
| 2022 | GO/Cys/DAC GO/Meth/DAC GO/Cys/Meth/DAC | chemically modified | Cys and Meth | B. subtilis inhibition zones (23 ± 0.53 mm and 17 ± 1.27 mm), S. aureus inhibition zones (21 ± 0.58 mm and 11 ± 0.69 mm), E. coli inhibition zones (12 ± 0.51 mm and 19 ± 1.01 mm), P. aeruginosa inhibition zones (24 ± 0.50 mm and 27 ± 0.95 mm), C. albicans inhibition zones (12 ± 0.53 mm and 23 ± 0.87 mm) and C. neoformans inhibition zones (22 ± 0.52 mm and 32 ± 0.93 mm) for DAC/GO/Cys and DAC/GO/Meth, respectively. | [133] | ||||
| Cellulose with polymeric blend | |||||||||
| 2018 | rGO/PVA/CMC | chemically modified | rGO reduction under sunlight via convex lens | PVA | EA.hy926 proliferation improved up to 72 h | Number of blood vessels increase 25–62% Blood vessels thickness increase 18.4–44.5% | [134] | ||
| 2020 | rGO/Ag/PU/Cur/CA | chemically modified | Hummers method | PU | rGO/Ag Cur | σyield = 0.7 Mpa, σmax = 3.75 Mpa, and E = 0.41 (rGO effect) σyield = 0.4 Mpa, σmax = 3.4 Mpa, and E = 0.29 (curcumin effect) | P. aeruginosa death 100%, and S. aureus death 95%. | Wound healing ratio 100%. Collagen fibers deposition after 15 days | [135] |
| 2020 | Ag-ZnO@GO/k-car/KG/CMC | chemically modified | Hummers method | k-car | Ag-ZnO KG | σcomp ≈ 200 kPa; E ≈ 150 Mpa | L929 viability 100 % after 7 days. S. aureus death 96% and E. coli death 98%. | Faster ri-epithelialization process | [136] |

5.2. GO or rGO/Bacterial Cellulose Nanocomposites
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falanga, V. Wound Healing and Its Impairment in the Diabetic Foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Niu, J.; Cheng, B. Prevalence of Chronic Skin Wounds and Their Risk Factors in an Inpatient Hospital Setting in Northern China. Adv. Ski. Wound Care 2020, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Al-Arjan, W.S.; Khan, M.U.A.; Almutairi, H.H.; Alharbi, S.M.; Razak, S.I.A. PH-Responsive PVA/BC-f-GO Dressing Materials for Burn and Chronic Wound Healing with Curcumin Release Kinetics. Polymers 2022, 14, 1949. [Google Scholar] [CrossRef]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel Dressings for Advanced Wound Management. Curr. Med. Chem. 2018, 25, 5782–5797. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, A.; Landi, M.R.; Massa, A.; D’Amora, U.; Guarino, V. Hyaluronic Acid in Biomedical Fields: New Trends from Chemistry to Biomaterial Applications. Int. J. Mol. Sci. 2022, 23, 14372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; D’Amora, U.; Ronca, A.; Li, Y.; Mo, X.; Zhou, F.; Yuan, M.; Ambrosio, L.; Wu, J.; Raucci, M.G. In Vitro and in Vivo Biocompatibility and Inflammation Response of Methacrylated and Maleated Hyaluronic Acid for Wound Healing. RSC Adv. 2020, 10, 32183–32192. [Google Scholar] [CrossRef] [PubMed]
- Prelipcean, A.-M.; Iosageanu, A.; Gaspar-Pintiliescu, A.; Moldovan, L.; Craciunescu, O.; Negreanu-Pirjol, T.; Negreanu-Pirjol, B.; Mitran, R.-A.; Marin, M.; D’Amora, U. Marine and Agro-Industrial By-Products Valorization Intended for Topical Formulations in Wound Healing Applications. Materials 2022, 15, 3507. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Z.; Zou, Y.; Lu, G.; Ronca, A.; D’Amora, U.; Liang, J.; Fan, Y.; Zhang, X.; Sun, Y. Advanced Application of Collagen-Based Biomaterials in Tissue Repair and Restoration. J. Leather Sci. Eng. 2022, 4, 1–19. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A Review of Current Advancements for Wound Healing: Biomaterial Applications and Medical Devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Gardin, C.; D’Amora, U.; Calzà, L.; Ronca, A.; Tremoli, E.; Ambrosio, L.; Zavan, B. Exosomes of Mesenchymal Stem Cells Delivered from Methacrylated Hyaluronic Acid Patch Improve the Regenerative Properties of Endothelial and Dermal Cells. Biomater. Adv. 2022, 139, 213000. [Google Scholar] [CrossRef]
- D’Amora, U.; Ronca, A.; Raucci, M.G.; Dozio, S.M.; Lin, H.; Fan, Y.; Zhang, X.; Ambrosio, L. In Situ Sol-Gel Synthesis of Hyaluronan Derivatives Bio-Nanocomposite Hydrogels. Regen. Biomater. 2019, 6, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Ghosh, A.K.; Sarkar, P.; Kundu, P.P. A Mussel Mimetic, Bioadhesive, Antimicrobial Patch Based on Dopamine-Modified Bacterial Cellulose/RGO/Ag NPs: A Green Approach toward Wound-Healing Applications. ACS Sustain. Chem. Eng. 2019, 7, 12083–12097. [Google Scholar] [CrossRef]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric Hydrogels for Burn Wound Care: Advanced Skin Wound Dressings and Regenerative Templates. Burn. Trauma 2014, 2, 2321–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demitri, C.; Raucci, M.G.; Giuri, A.; De Benedictis, V.M.; Giugliano, D.; Calcagnile, P.; Sannino, A.; Ambrosio, L. Cellulose-Based Porous Scaffold for Bone Tissue Engineering Applications: Assessment of h MSC Proliferation and Differentiation. J. Biomed. Mater. Res. Part A 2016, 104, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Voicu, S.I. Recent Advances in Composites Based on Cellulose Derivatives for Biomedical Applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal Effect of Graphene Oxide and Reduced Graphene Oxide: Influence of Shape of Bacteria. Colloid Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Hasanin, M.S. Cellulose-Based Biomaterials: Chemistry and Biomedical Applications. Starch Stärke 2022, 74, 2200060. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant-vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Clemons, C.; Reiner, R.; Sabo, R.; Agarwal, U.P.; Bissessur, R.; Sojoudiasli, H.; Carreau, P.J.; Acharya, B. Towards the Scalable Isolation of Cellulose Nanocrystals from Tunicates. Sci. Rep. 2020, 10, 19090. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Bucchieri, F.; Fucarino, A.; Ronca, A.; D’Amora, U. Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources. J. Funct. Biomater. 2022, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yao, J.; Cui, Y.-N.; Zhao, T.; Che, J.; Yang, M.; Li, Z.; Gao, C. Advances in Cellulose-Based Hydrogels for Biomedical Engineering: A Review Summary. Gels 2022, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Malladi, R.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of Agriculture and Industrial Waste in the Field of Nano Cellulose and Its Recent Industrial Developments: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial Cellulose Production, Properties and Applications with Different Culture Methods—A Review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Castro, C.; Zuluaga, R.; Álvarez, C.; Putaux, J.-L.; Caro, G.; Rojas, O.J.; Mondragon, I.; Gañán, P. Bacterial Cellulose Produced by a New Acid-Resistant Strain of Gluconacetobacter Genus. Carbohydr. Polym. 2012, 89, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of Bacterial Cellulose from Industrial Wastes: A Review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly Green Conversion of Potato Peel Wastes to High Productivity Bacterial Cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution State of Cellulose in Aqueous Systems. 1. Alkaline Solvents. Cellulose 2016, 23, 247–258. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; Dacrory, S.; Hasanin, M.; Saber, E.; Kamel, S. Biocompatible Hydrogel Based on Aldehyde-Functionalized Cellulose and Chitosan for Potential Control Drug Release. Sustain. Chem. Pharm. 2021, 21, 100419. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Xing, L.; Hu, C.; Zhang, W.; Guan, L.; Gu, J. Biodegradable Cellulose I (II) Nanofibrils/Poly (Vinyl Alcohol) Composite Films with High Mechanical Properties, Improved Thermal Stability and Excellent Transparency. Int. J. Biol. Macromol. 2020, 164, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.; Huang, F.; Jiang, M.; Wei, W.; Zhou, Z. Preparation, Properties, and Applications of Natural Cellulosic Aerogels: A Review. Energy Built Environ. 2020, 1, 60–76. [Google Scholar] [CrossRef]
- Wei, Z.; Cai, C.; Huang, Y.; Wang, P.; Song, J.; Deng, L.; Fu, Y. Strong Biodegradable Cellulose Materials with Improved Crystallinity via Hydrogen Bonding Tailoring Strategy for UV Blocking and Antioxidant Activity. Int. J. Biol. Macromol. 2020, 164, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dacrory, S.; Hashem, A.H.; Kamel, S. Antimicrobial and Antiviral Activities with Molecular Docking Study of Chitosan/Carrageenan@ Clove Oil Beads. Biotechnol. J. 2022, 17, 2100298. [Google Scholar] [CrossRef]
- Dacrory, S.; Kamel, S.; Turky, G. Development of Dielectric Film Based on Cellulose Loaded Nano-Silver and Carbon for Potential Energy Storage. ECS J. Solid State Sci. Technol. 2021, 10, 123004. [Google Scholar] [CrossRef]
- Dacrory, S.; Abou Hammad, A.B.; El Nahrawy, A.M.; Abou-Yousef, H.; Kamel, S. Cyanoethyl Cellulose/BaTiO3/GO Flexible Films with Electroconductive Properties. ECS J. Solid State Sci. Technol. 2021, 10, 83004. [Google Scholar] [CrossRef]
- Al-Shemy, M.T.; Al-Sayed, A.; Dacrory, S. Fabrication of Sodium Alginate/Graphene Oxide/Nanocrystalline Cellulose Scaffold for Methylene Blue Adsorption: Kinetics and Thermodynamics Study. Sep. Purif. Technol. 2022, 290, 120825. [Google Scholar] [CrossRef]
- Dacrory, S. Development of Mesoporous Foam Based on Dicarboxylic Cellulose and Graphene Oxide for Potential Oil/Water Separation. Polym. Bull. 2022, 79, 9563–9574. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Ghica, M.-V.; Albu-Kaya, G.M.; Dinu-Pirvu, C.-E.; Popa, L.; Anuta, V.; Velescu, B.S.; Kaya, D.A.; Marin, M.M.; Prisada, R.M. Rheological Characterization of Some Cellulose Derivatives-Based Hydrogels. In Proceedings of the 9th International Conference on Advanced Materials and Systems, Online, 26–28 October 2022. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Manna, S.; Dhanalakshmi, D.; Bhowmik, M.; Jana, S.; Jana, S. Cellulose Derivative-Based Bioadhesive Blend Patch for Transdermal Drug Delivery. Front. Mater. 2022, 9, 835507. [Google Scholar] [CrossRef]
- Douglass, E.F.; Avci, H.; Boy, R.; Rojas, O.J.; Kotek, R. A Review of Cellulose and Cellulose Blends for Preparation of Bio-Derived and Conventional Membranes, Nanostructured Thin Films, and Composites. Polym. Rev. 2018, 58, 102–163. [Google Scholar] [CrossRef]
- Imeson, A. Food Stabilisers, Thickeners and Gelling Agents; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Oprea, M.; Panaitescu, D.M. Nanocellulose Hybrids with Metal Oxides Nanoparticles for Biomedical Applications. Molecules 2020, 25, 4045. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Capezzuto, F.; Lavorgna, M.; Buonocore, G.G.; Tescione, F.; Xia, H.; Ambrosio, L. Borate Cross-Linked Graphene Oxide--Chitosan as Robust and High Gas Barrier Films. Nanoscale 2016, 8, 10783–10791. [Google Scholar] [CrossRef] [Green Version]
- Raucci, M.G.; Giugliano, D.; Longo, A.; Zeppetelli, S.; Carotenuto, G.; Ambrosio, L. Comparative Facile Methods for Preparing Graphene Oxide--Hydroxyapatite for Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2204–2216. [Google Scholar] [CrossRef]
- Seo, Y.-R.; Kim, J.-W.; Hoon, S.; Kim, J.; Chung, J.H.; Lim, K.-T. Cellulose-Based Nanocrystals: Sources and Applications via Agricultural Byproducts. J. Biosyst. Eng. 2018, 43, 59–71. [Google Scholar]
- Devi, A.; Singh, A.; Bajar, S.; Pant, D.; Din, Z.U. Ethanol from Lignocellulosic Biomass: An in-Depth Analysis of Pre-Treatment Methods, Fermentation Approaches and Detoxification Processes. J. Environ. Chem. Eng. 2021, 9, 105798. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Kassem, N.; Hassan, M.L. Preparation and Characterization of Microcrystalline Cellulose from Olive Stones. Biomass Convers. Biorefinery 2021, 1–8. [Google Scholar] [CrossRef]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of Lignocellulose Using Ligninolytic Enzymes from White-Rot Fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Darwesh, O.M.; Matter, I.A.; El-Saied, H. Isolation and Characterization of Non-Cellulolytic Aspergillus Flavus EGYPTA5 Exhibiting Selective Ligninolytic Potential. Biocatal. Agric. Biotechnol. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, W.; Zhang, M.; Liu, H.; Zhang, X.; Si, C. Lignin-Containing Cellulose Nanomaterials: Preparation and Applications. Green Chem. 2021, 23, 9723–9746. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Madhu, P.; Raghav, G.R. A Comprehensive Review on Cellulose Nanocrystals and Cellulose Nanofibers: Pretreatment, Preparation, and Characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, V.; Singh, S.; Saini, S.; Gaikwad, K.K. Pine Needles Lignocellulosic Ethylene Scavenging Paper Impregnated with Nanozeolite for Active Packaging Applications. Ind. Crops Prod. 2021, 170, 113752. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the Graphene Family--A Recommended Nomenclature for Two-Dimensional Carbon Materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Yan, Y.; Nashath, F.Z.; Chen, S.; Manickam, S.; Lim, S.S.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of Graphene: Potential Carbon Precursors and Approaches. Nanotechnol. Rev. 2020, 9, 1284–1314. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and Their Nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Randviir, E.P.; Dena, A.S.A.; Banks, C.E. An Overview of Recent Applications of Reduced Graphene Oxide as a Basis of Electroanalytical Sensing Platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Ray, S. Applications of Graphene and Graphene-Oxide Based Nanomaterials; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Wan, X.; Huang, Y.; Chen, Y. Focusing on Energy and Optoelectronic Applications: A Journey for Graphene and Graphene Oxide at Large Scale. Acc. Chem. Res. 2012, 45, 598–607. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Rollo, G.; Ronca, A.; Cerruti, P.; Gan, X.P.; Fei, G.; Xia, H.; Gorokhov, G.; Bychanok, D.; Kuzhir, P.; Lavorgna, M.; et al. On the Synergistic Effect of Multi-Walled Carbon Nanotubes and Graphene Nanoplatelets to Enhance the Functional Properties of SLS 3D-Printed Elastomeric Structures. Polymers 2020, 12, 1841. [Google Scholar] [CrossRef] [PubMed]
- Ronca, A.; Rollo, G.; Cerruti, P.; Fei, G.; Gan, X.; Buonocore, G.G.; Lavorgna, M.; Xia, H.; Silvestre, C.; Ambrosio, L. Selective Laser Sintering Fabricated Thermoplastic Polyurethane/Graphene Cellular Structures with Tailorable Properties and High Strain Sensitivity. Appl. Sci. 2019, 9, 864. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, H.N.; Hussein, K.H. Graphene Oxide as a Carrier for Drug Delivery of Methotrexate. Biointerface Res. Appl. Chem. 2021, 11, 14726–14735. [Google Scholar]
- Muazim, K.; Hussain, Z. Graphene Oxide—A Platform towards Theranostics. Mater. Sci. Eng. C 2017, 76, 1274–1288. [Google Scholar] [CrossRef]
- Hussein, K.H.; Abdelhamid, H.N.; Zou, X.; Woo, H.-M. Ultrasonicated Graphene Oxide Enhances Bone and Skin Wound Regeneration. Mater. Sci. Eng. C 2019, 94, 484–492. [Google Scholar] [CrossRef]
- Dowaidar, M.; Abdelhamid, H.N.; Hällbrink, M.; Zou, X.; Langel, Ü. Graphene Oxide Nanosheets in Complex with Cell Penetrating Peptides for Oligonucleotides Delivery. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 2334–2341. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Khan, M.S.; Wu, H.-F. Graphene Oxide as a Nanocarrier for Gramicidin (GOGD) for High Antibacterial Performance. RSC Adv. 2014, 4, 50035–50046. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.-F. Synthesis of a Highly Dispersive Sinapinic Acid@ Graphene Oxide (SA@ GO) and Its Applications as a Novel Surface Assisted Laser Desorption/Ionization Mass Spectrometry for Proteomics and Pathogenic Bacteria Biosensing. Analyst 2015, 140, 1555–1565. [Google Scholar] [CrossRef]
- Ashour, R.M.; Abdelhamid, H.N.; Abdel-Magied, A.F.; Abdel-Khalek, A.A.; Ali, M.M.; Uheida, A.; Muhammed, M.; Zou, X.; Dutta, J. Rare Earth Ions Adsorption onto Graphene Oxide Nanosheets. Solvent Extr. Ion Exch. 2017, 35, 91–103. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Riedl, T.; Forster, M.; Klinowski, J. 13C and 1H MAS NMR Studies of Graphite Oxide and Its Chemically Modified Derivatives. Solid State Ionics 1997, 101, 857–862. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The Reduction of Graphene Oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A New Structural Model for Graphite Oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized Single Graphene Sheets Derived from Splitting Graphite Oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [Green Version]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and Electronic Structure of Graphene-Oxide. Nano Lett. 2009, 9, 1058–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, D.; Reifenberger, R.; Piner, R. Scanning Probe Microscopy Study of Exfoliated Oxidized Graphene Sheets. Surf. Sci. 2008, 602, 1607–1613. [Google Scholar] [CrossRef]
- Tang, H.; Liu, D.; Zhao, Y.; Yang, X.; Lu, J.; Cui, F. Molecular Dynamics Study of the Aggregation Process of Graphene Oxide in Water. J. Phys. Chem. C 2015, 119, 26712–26718. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L.; Wang, Y.; Chen, S. Molecular Dynamics Simulations of the Adsorption of DNA Segments onto Graphene Oxide. J. Phys. D Appl. Phys. 2014, 47, 505401. [Google Scholar] [CrossRef]
- Baweja, L.; Balamurugan, K.; Subramanian, V.; Dhawan, A. Hydration Patterns of Graphene-Based Nanomaterials (GBNMs) Play a Major Role in the Stability of a Helical Protein: A Molecular Dynamics Simulation Study. Langmuir 2013, 29, 14230–14238. [Google Scholar] [CrossRef]
- Sun, X.; Feng, Z.; Hou, T.; Li, Y. Mechanism of Graphene Oxide as an Enzyme Inhibitor from Molecular Dynamics Simulations. ACS Appl. Mater. Interfaces 2014, 6, 7153–7163. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, G.; Chen, L.; Wang, Y.; Wang, X.; Zeng, S. Interaction of Graphene and Its Oxide with Lipid Membrane: A Molecular Dynamics Simulation Study. J. Phys. Chem. C 2016, 120, 6225–6231. [Google Scholar] [CrossRef]
- Shih, C.-J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the PH-Dependent Behavior of Graphene Oxide Aqueous Solutions: A Comparative Experimental and Molecular Dynamics Simulation Study. Langmuir 2012, 28, 235–241. [Google Scholar] [CrossRef]
- Borthakur, P.; Boruah, P.K.; Hussain, N.; Sharma, B.; Das, M.R.; Matić, S.; Reha, D.; Minofar, B. Experimental and Molecular Dynamics Simulation Study of Specific Ion Effect on the Graphene Oxide Surface and Investigation of the Influence on Reactive Extraction of Model Dye Molecule at Water--Organic Interface. J. Phys. Chem. C 2016, 120, 14088–14100. [Google Scholar] [CrossRef]
- Brodie, B.C. XIII. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. London 1859, 149, 249–259. [Google Scholar]
- Staudenmaier, L. Verfahren Zur Darstellung Der Graphitsäure. Berichte der Dtsch. Chem. Gesellschaft 1898, 31, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Hummers Jr, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Somanathan, T.; Prasad, K.; Ostrikov, K.; Saravanan, A.; Mohana Krishna, V. Graphene Oxide Synthesis from Agro Waste. Nanomaterials 2015, 5, 826–834. [Google Scholar] [CrossRef] [Green Version]
- Faiz, M.S.A.; Azurahanim, C.A.C.; Yazid, Y.; Suriani, A.B.; Ain, M.J.S.N. Preparation and Characterization of Graphene Oxide from Tea Waste and It’s Photocatalytic Application of TiO2/Graphene Nanocomposite. Mater. Res. Express 2020, 7, 15613. [Google Scholar] [CrossRef]
- Sujiono, E.H.; Zabrian, D.; Dahlan, M.Y.; Amin, B.D.; Agus, J. Graphene Oxide Based Coconut Shell Waste: Synthesis by Modified Hummers Method and Characterization. Heliyon 2020, 6, e04568. [Google Scholar] [CrossRef]
- Bonnia, N.N.; Zanuri, A.Z.; Asli, N.A.; Masdar, N.A.; Ratim, S.; Yahaya, S.M.; Mahat, M.M.; Ramli, R. Synthesis of Graphene Oxide from Waste Carbon Tyre Using Modified Hummer’s Method. Int. J. Eng. Technol 2018, 7, 352–355. [Google Scholar] [CrossRef]
- Tian, Z.; Cao, K.; Bai, S.; He, G.; Li, J. One-Pot Transformation of Waste Toner Powder into 3D Graphene Oxide Hydrogel. ACS Sustain. Chem. Eng. 2018, 7, 496–501. [Google Scholar] [CrossRef]
- Siaw, W.C.; Tsuji, T.; Manaf, N.A.; Patah, M.F.A.; Jan, B.M. Synthesis of Graphene Oxide from Industrial Waste. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 12050. [Google Scholar] [CrossRef]
- Ghosal, K.; Sarkar, K. Biomedical Applications of Graphene Nanomaterials and Beyond. ACS Biomater. Sci. Eng. 2018, 4, 2653–2703. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Yaragalla, S.; Bhavitha, K.B.; Athanassiou, A. A Review on Graphene Based Materials and Their Antimicrobial Properties. Coatings 2021, 11, 1197. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Ali, M.E.; Appaturi, J.N.; Dinshaw, I.J.; Ong, Z.Y.; Leo, B.F. Graphene Oxide Exhibits Differential Mechanistic Action towards Gram-Positive and Gram-Negative Bacteria. Colloids Surf. B Biointerfaces 2019, 181, 6–15. [Google Scholar] [CrossRef]
- Deokar, A.R.; Lin, L.Y.; Chang, C.C.; Ling, Y.C. Single-Walled Carbon Nanotube Coated Antibacterial Paper: Preparation and Mechanistic Study. J. Mater. Chem. B 2013, 1, 2639–2646. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Romero-Vargas Castrillón, S.; Perreault, F.; De Faria, A.F.; Elimelech, M. Interaction of Graphene Oxide with Bacterial Cell Membranes: Insights from Force Spectroscopy. Environ. Sci. Technol. Lett. 2015, 2, 112–117. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pandit, S.; Rahimi, S.; Mijakovic, I. Interactions Between Graphene-Based Materials and Biological Surfaces: A Review of Underlying Molecular Mechanisms. Adv. Mater. Interfaces 2021, 8, 2101132. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Thakur, V.; Kutty, R.V. Recent Advances in Nanotheranostics for Triple Negative Breast Cancer Treatment. J. Exp. Clin. Cancer Res. 2019, 38, 430. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Gao, H. Cell Interaction with Graphene Microsheets Near-Orthogonal Cutting versus Parallel Attachment. Nanoscale 2015, 7, 5457–5467. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Shi, X.; Gao, H. Cellular Entry of Graphene Nanosheets: The Role of Thickness, Oxidation and Surface Adsorption. RSC Adv. 2013, 3, 15776–15782. [Google Scholar] [CrossRef] [Green Version]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.M.; Guo, Y.; Baulin, V.A.; Al Kobaisi, M.; Crawford, R.J.; Ivanova, E.P. Graphene Induces Formation of Pores That Kill Spherical and Rod-Shaped Bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral Dimension-Dependent Antibacterial Activity of Graphene Oxide Sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene Oxide Exhibits Broad-Spectrum Antimicrobial Activity against Bacterial Phytopathogens and Fungal Conidia by Intertwining and Membrane Perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Han, H. A New Function of Graphene Oxide Emerges: Inactivating Phytopathogenic Bacterium Xanthomonas Oryzae Pv. Oryzae. J. Nanoparticle Res. 2013, 15, 1658. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Abdal Dayem, A.; Eppakayala, V.; Kim, J.H. Oxidative Stress-Mediated Antibacterial Activity of Graphene Oxide and Reduced Graphene Oxide in Pseudomonas Aeruginosa. Int. J. Nanomed. 2012, 7, 5901. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Park, M.R.; Kwon, D.N.; Kim, J.H. Antibacterial Activity of Dithiothreitol Reduced Graphene Oxide. J. Ind. Eng. Chem. 2013, 19, 1280–1288. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Zhang, L.H.; Yun, K.; Kim, S.J. Antibacterial Efficiency of Graphene Nanosheets against Pathogenic Bacteria via Lipid Peroxidation. J. Phys. Chem. C 2012, 116, 17280–17287. [Google Scholar] [CrossRef]
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial Activity of Large-Area Monolayer Graphene Film Manipulated by Charge Transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Vimalraj, S.; Pichu, S.; Pankajam, T.; Dharanibalan, K.; Djonov, V.; Chatterjee, S. Nitric Oxide Regulates Intussusceptive-like Angiogenesis in Wound Repair in Chicken Embryo and Transgenic Zebrafish Models. Nitric Oxide 2019, 82, 48–58. [Google Scholar] [CrossRef]
- Honnegowda, T.M.; Kumar, P.; Udupa, E.G.P.; Kumar, S.; Kumar, U.; Rao, P. Role of Angiogenesis and Angiogenic Factors in Acute and Chronic Wound Healing. Plast. Aesthetic Res. 2015, 2, 243–249. [Google Scholar]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Yong, J.M.; Fu, L.; Tang, F.; Yu, P.; Kuchel, R.P.; Whitelock, J.M.; Lord, M.S. ROS-Mediated Anti-Angiogenic Activity of Cerium Oxide Nanoparticles in Melanoma Cells. ACS Biomater. Sci. Eng. 2022, 8, 512–525. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive Oxygen Species and Cancer Paradox: To Promote or to Suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Breton-Romero, R.; Lamas, S. Hydrogen Peroxide Signaling in Vascular Endothelial Cells. Redox Biol. 2014, 2, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Choe, G.; Park, J.; Lee, J.Y. Graphene Oxide-Incorporated Hydrogels for Biomedical Applications. Polym. J. 2020, 52, 823–837. [Google Scholar] [CrossRef]
- Wei, P.; Wang, L.; Xie, F.; Cai, J. Strong and Tough Cellulose—Graphene Oxide Composite Hydrogels by Multi-Modulus Components Strategy as Photothermal Antibacterial Platform. Chem. Eng. J. 2022, 431, 133964. [Google Scholar] [CrossRef]
- Ali, N.H.; Amin, M.C.I.M.; Ng, S.-F. Sodium Carboxymethyl Cellulose Hydrogels Containing Reduced Graphene Oxide (RGO) as a Functional Antibiofilm Wound Dressing. J. Biomater. Sci. Polym. Ed. 2019, 30, 629–645. [Google Scholar] [CrossRef]
- Soliman, M.; Sadek, A.A.; Abdelhamid, H.N.; Hussein, K. Graphene Oxide-Cellulose Nanocomposite Accelerates Skin Wound Healing. Res. Vet. Sci. 2021, 137, 262–273. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Wu, H.; Li, Q.; Hu, W.; Zhang, Z.; Huang, L.; Zhang, J.; Chen, D.; Deng, S.; et al. Graphene Oxide—IPDI—Ag/ZnO@ Hydroxypropyl Cellulose Nanocomposite Films for Biological Wound-Dressing Applications. ACS Omega 2019, 4, 15373–15381. [Google Scholar] [CrossRef] [Green Version]
- Prakash, J.; Venkataprasanna, K.S.; Bharath, G.; Banat, F.; Niranjan, R.; Venkatasubbu, G.D. In-Vitro Evaluation of Electrospun Cellulose Acetate Nanofiber Containing Graphene Oxide/TiO2/Curcumin for Wound Healing Application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127166. [Google Scholar] [CrossRef]
- Purnamasari, W.; Budiastanti, T.A.; Aminatun, A.; Rahmah, U.; Sumarsih, S.; Chang, J.-Y.; Fahmi, M.Z. Naproxen Release Behaviour from Graphene Oxide/Cellulose Acetate Composite Nanofibers. RSC Adv. 2022, 12, 8019–8029. [Google Scholar] [CrossRef]
- Hashem, A.H.; Hasanin, M.; Kamel, S.; Dacrory, S. A New Approach for Antimicrobial and Antiviral Activities of Biocompatible Nanocomposite Based on Cellulose, Amino Acid and Graphene Oxide. Colloids Surf. B Biointerfaces 2022, 209, 112172. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ponrasu, T.; Chandel, S.; Dixit, M.; Muthuvijayan, V. Reduced Graphene Oxide-Loaded Nanocomposite Scaffolds for Enhancing Angiogenesis in Tissue Engineering Applications. R. Soc. Open Sci. 2018, 5, 172017. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The Biomedical Potential of Cellulose Acetate/Polyurethane Nanofibrous Mats Containing Reduced Graphene Oxide/Silver Nanocomposites and Curcumin: Antimicrobial Performance and Cutaneous Wound Healing. Int. J. Biol. Macromol. 2020, 152, 418–427. [Google Scholar] [CrossRef]
- Li, X.-X.; Dong, J.-Y.; Li, Y.-H.; Zhong, J.; Yu, H.; Yu, Q.-Q.; Lei, M. Fabrication of Ag—ZnO@ Carboxymethyl Cellulose/K-Carrageenan/Graphene Oxide/Konjac Glucomannan Hydrogel for Effective Wound Dressing in Nursing Care for Diabetic Foot Ulcers. Appl. Nanosci. 2020, 10, 729–738. [Google Scholar] [CrossRef]
- Liu, Y.; Morishima, T.; Yatsui, T.; Kawazoe, T.; Ohtsu, M. Size Control of Sol--Gel-Synthesized ZnO Quantum Dots Using Photo-Induced Desorption. Nanotechnology 2011, 22, 215605. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, H.-I. Characterization and Antibacterial Properties of Genipin-Crosslinked Chitosan/Poly (Ethylene Glycol)/ZnO/Ag Nanocomposites. Carbohydr. Polym. 2012, 89, 111–116. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, F.; Meng, A.; Xie, C.; Xing, J. ZnO/Ag Micro/Nanospheres with Enhanced Photocatalytic and Antibacterial Properties Synthesized by a Novel Continuous Synthesis Method. RSC Adv. 2015, 5, 612–620. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, M.S.; Nayak, A.K. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Matharu, R.K.; Tabish, T.A.; Trakoolwilaiwan, T.; Mansfield, J.; Moger, J.; Wu, T.; Lourenço, C.; Chen, B.; Ciric, L.; Parkin, I.P.; et al. Microstructure and Antibacterial Efficacy of Graphene Oxide Nanocomposite Fibres. J. Colloid Interface Sci. 2020, 571, 239–252. [Google Scholar] [CrossRef]
- Shome, S.; Talukdar, A.D.; Upadhyaya, H. Antibacterial Activity of Curcumin and Its Essential Nanoformulations against Some Clinically Important Bacterial Pathogens: A Comprehensive Review. Biotechnol. Appl. Biochem. 2021, 69, 2357–2386. [Google Scholar] [CrossRef]
- Rai, D.; Singh, J.K.; Roy, N.; Panda, D. Curcumin Inhibits FtsZ Assembly: An Attractive Mechanism for Its Antibacterial Activity. Biochem. J. 2008, 410, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Heil, M.; Eitenmüller, I.; Schmitz-Rixen, T.; Schaper, W. Arteriogenesis versus Angiogenesis: Similarities and Differences. J. Cell. Mol. Med. 2006, 10, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Sriram, P.; Barui, A.K.; Nethi, S.K.; Veeriah, V.; Chatterjee, S.; Suresh, K.I.; Patra, C.R. Graphene Oxides Show Angiogenic Properties. Adv. Healthc. Mater. 2015, 4, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-Y.; Hu, X.-H.; Zhang, Y.-W.; Wahid, F.; Chu, L.-Q.; Jia, S.-R.; Zhong, C. Development and Antibacterial Activities of Bacterial Cellulose/Graphene Oxide-CuO Nanocomposite Films. Carbohydr. Polym. 2020, 229, 115456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, Y.; Zheng, S.; Zhong, L.; Xue, J. Preparation and Properties of Conductive Bacterial Cellulose-Based Graphene Oxide-Silver Nanoparticles Antibacterial Dressing. Carbohydr. Polym. 2021, 257, 117671. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.Z.; Marković, Z.M.; Mitić, D.D.; Zdravković, N.M.; Kozyrovska, N.O.; Bugárová, N.; Todorović Marković, B.M. Antibacterial Composite Hydrogels of Graphene Quantum Dots and Bacterial Cellulose Accelerate Wound Healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
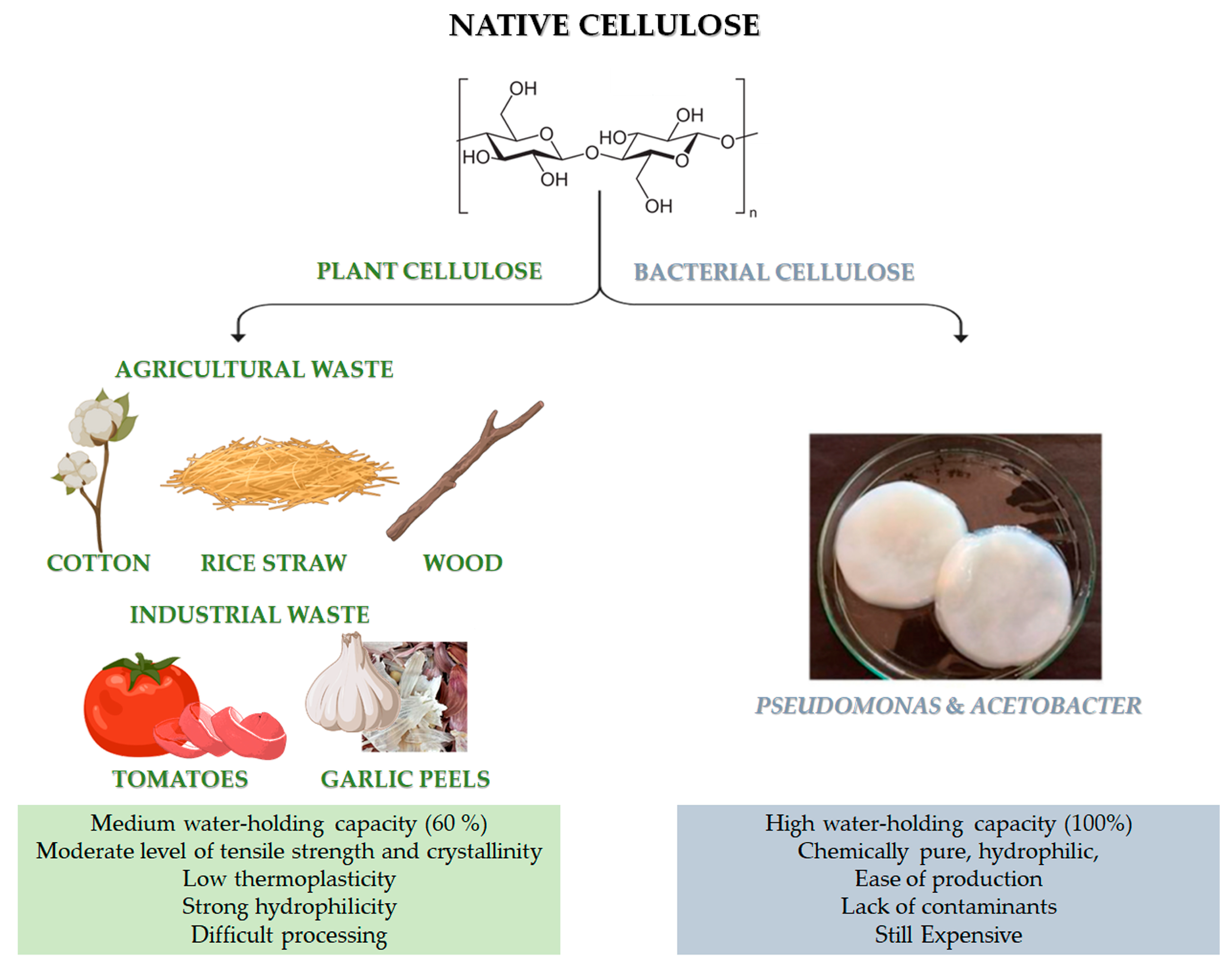





| Agriculture | Cellulose (%) |
|---|---|
| Wood | 35–50 |
| Wheat straw | 33–40 |
| Switchgrass | 30–50 |
| Bagasse | 44 |
| Olive husk | 24 |
| Sunflowers | 26 |
| Rice straw | 33 |
| Rice husk | 49 |
| Cotton | 80–95 |
| Nutshells | 25–30 |
| Banana fibers | 60–65 |
| Corn cob | 42–45 |
| Oat straw | 33–35 |
| Hazelnut shell | 29 |
| Derivative | R1 | R2 | R3 |
|---|---|---|---|
| Carboxymethyl cellulose (CMC) | COONa | COONa | COONa |
| Methylcellulose (MC) | CH3 | CH3 | CH3 |
| Dialdehyde cellulose (DAC) | H | C=O | C=O |
| Hydroxyethyl cellulose (HEC) | CH2CH2OH | CH2CH2OH | CH2CH2OH |
| Year | Waste | Physico-Chemical Process and Reagents | References |
|---|---|---|---|
| Agricultural Wastes | |||
| 2015 | Sugarcane bagasse | Muffle furnace (T = 300 °C, t = 10 min) Ferrocene | [91] |
| 2020 | Coconut shell | Carbonization (T = 600 °C, t = 3 h) + Modified Hummers method (NaNO3, H2SO4, KMnO4) | [93] |
| 2020 | Tea | Carbonization (T = 750 °C, t = 3 h, argon) + Modified Hummers method (NaNO3, H2SO4, KMnO4) | [92] |
| Industrial Wastes | |||
| 2018 | Carbon Tyre | Crushing + Modified Hummers method (NaNO3, H2SO4, KMnO4) | [94] |
| 2019 | Toner Powder | Modified Hummers method (NaNO3, H2SO4, KMnO4) | [95] |
| 2020 | Generic | Leaching (6 M HCl, T = 70 °C, t = 210 min) + Modified Hummers method (NaNO3, H2SO4, KMnO4) | [96] |
| Year | Material | Cellulose Biosource | GO Processes | Crosslinker/ Gelling Agents | Inorganic/Organic Compounds Embedded | Physico- Chemical and Mechanical Properties | In Vitro Outcomes | In Vivo Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 2019 | rGO/Ag/DOPA/BC | Gluconacetobacter xylus (MTCC7795) | Hummers method | -- | AgNO3 | σtens = 1–5.52 ± 0.07 MPa σtens = 5.21 ± 0.03 MPa (Ag NPs effect) Average resistance of 84 kΩ | E. coli inhibition zone diameter 15 ± 1 mm; P. aeruginosa inhibition zone diameter 11 ± 0.5 mm, S. aureus inhibition zone diameter 13 ± 0.5 mm, L. fusiformis inhibition zone diameter 14 ± 0.75 mm. High stimulation of NIH3T3 proliferation favouring the wound closure (18 h). | - | [12] |
| 2019 | GO-CuO/BC | Gluconacetobacter xylinus (traditional Chinese drink) | CuO | S. aureus inhibition zone diameter 16.3–18.3 mm; E. coli inhibition zone diameter 12.7–15.2 mm, B. subtilis inhibition zone diameter 27.8–28.5 mm, P. aeruginosa inhibition zone diameter 0–15.2 mm. NIH3T3 viability > 100% | - | [146] | |||
| 2021 | rGO/Ag-pDA/BC | Gluconacetobacter xylinus | Hummers method | AgNO3 | E. coli inhibition zone 6.3 mm (AgNO3 effect) | - | [147] | ||
| 2022 | GQDs/BC | Komagataeibacter oboediens (IMBG180) | - | Bacterial inhibition against S. aureus, MRSA, E. coli, P. aeruginosa and S. agalactiae. Bactericidal effect against MRSA, E. coli and P. aeruginosa. Angiogenesis stimulation validated by up-regulation of eNOS, VEGFA, MMP-9 and vimentin | [148] | ||||
| 2022 | GO/PVA/BC | - | TEOS PVA | Cur | Increase of the compressive stress and hydrophilicity by increasing the GO content | E. coli inhibition zone 5–15 mm; S. aureus inhibition zone 7.5–16 mm; P. aeruginosa inhibition zone 7–17 mm | [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amora, U.; Dacrory, S.; Hasanin, M.S.; Longo, A.; Soriente, A.; Kamel, S.; Raucci, M.G.; Ambrosio, L.; Scialla, S. Advances in the Physico-Chemical, Antimicrobial and Angiogenic Properties of Graphene-Oxide/Cellulose Nanocomposites for Wound Healing. Pharmaceutics 2023, 15, 338. https://doi.org/10.3390/pharmaceutics15020338
D’Amora U, Dacrory S, Hasanin MS, Longo A, Soriente A, Kamel S, Raucci MG, Ambrosio L, Scialla S. Advances in the Physico-Chemical, Antimicrobial and Angiogenic Properties of Graphene-Oxide/Cellulose Nanocomposites for Wound Healing. Pharmaceutics. 2023; 15(2):338. https://doi.org/10.3390/pharmaceutics15020338
Chicago/Turabian StyleD’Amora, Ugo, Sawsan Dacrory, Mohamed Sayed Hasanin, Angela Longo, Alessandra Soriente, Samir Kamel, Maria Grazia Raucci, Luigi Ambrosio, and Stefania Scialla. 2023. "Advances in the Physico-Chemical, Antimicrobial and Angiogenic Properties of Graphene-Oxide/Cellulose Nanocomposites for Wound Healing" Pharmaceutics 15, no. 2: 338. https://doi.org/10.3390/pharmaceutics15020338
APA StyleD’Amora, U., Dacrory, S., Hasanin, M. S., Longo, A., Soriente, A., Kamel, S., Raucci, M. G., Ambrosio, L., & Scialla, S. (2023). Advances in the Physico-Chemical, Antimicrobial and Angiogenic Properties of Graphene-Oxide/Cellulose Nanocomposites for Wound Healing. Pharmaceutics, 15(2), 338. https://doi.org/10.3390/pharmaceutics15020338










