Orodispersible Films—Current State of the Art, Limitations, Advances and Future Perspectives
Abstract
1. Introduction
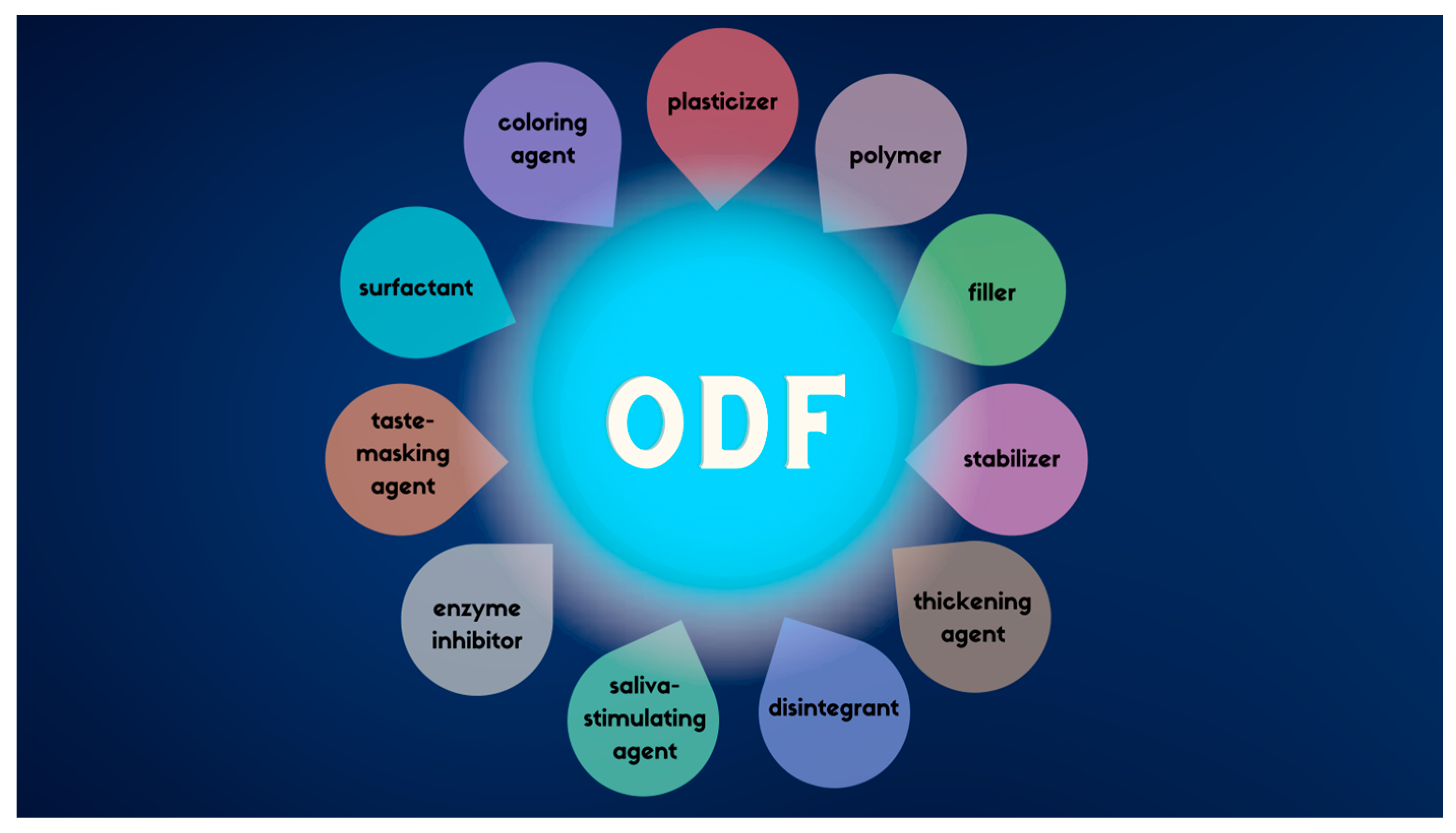
2. Technological and Formulation Overview
3. Personalized Therapy
4. Limitations of ODF’s
4.1. Drug Loading Capacity
4.2. Bitter Taste
4.3. Exposure to Humidity
4.4. Manufacturing Method Limitations
5. ODFs in Science and in Markets
| Drug Name | The Active Substance | Dose | Region | Producer | Drug Action/Application | References |
|---|---|---|---|---|---|---|
| Ashwagandha | Ashwagandha |
| Indie/worldwide | Aavishkar | Anti-inflammatory, antioxidant | [172,173] |
| Astaxanthin | Astaxanthin |
| Indie/worldwide | Aavishkar | Antioxidant | [172,173] |
| B 12 | B 12 |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| B 12 | Biotin, D3, Folic acid |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Breath Freshener | Spearmint, Peppermint | Indie/worldwide | Aavishkar | Refreshing the breath | [172,173] | |
| Caffeine | Caffeine, L-Theanine, B12 |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Clove oil | Clove oil |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| CoEnzyme Q-10 | CoEnzyme Q-10 |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Cold | AP-Bio—Andrographis Paniculata |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Curcumin | Curcumin |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Echinacea | Echinacea, D3, Manuka Honey Propolis |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Elderberry | Elderberry |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Expectorant | Hedera Helix |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| D3 | D3 |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| GC Well Being ODF | Sodium Selenite Pentahydrate |
| South Korea | C.L.Pharm Co., Ltd. | Support deficiency that cannot be supplemented through nutrition supply | [143] |
| Glycine | Glycine, B1, B6, B12 |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Iron | Iron |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| K2 + D3 | K2 + D3 |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Listerine Pocketpaks | Eucalyptol, Thymol, Menthol | USA | Johnson&Johnson | Freshening breath | [174] | |
| Lutein | Lutein, Zeaxanthin |
| Indie/worldwide | Aavishkar | sSpplementation | [172,173] |
| Lysozyme | Lysozyme, B6 |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Melatonin | Melatonin, Valerian, B6 |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Multivitamin | Vitamin: A, B5, B6, B7, B9, C, B12, D3, K2, Iodine |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Probiotics | Bacillus Coagulans |
| Indie/worldwide | Aavishkar | Supplementation | [172,173] |
| Resveratrol | Resveratrol |
| Indie/worldwide | Aavishkar | Antioxidant | [172,173] |
| Spice Mix | Cinnamon, Turmeric, Bee Propolis, Garlic, Piperine, Fennel, Fenugreek, Cardamon oil, Clove oil, Capsaicin, Tulsi, Saffron | Indie/worldwide | Aavishkar | [172,173] | ||
| Snoreeze Oral Strips | Peppermint oil, Tocopheryl acetate, Menthol | PL, UK, Europa | PASSION FOR LIFE HEALTHCARE | Snoring relief | [175] | |
| Tusheel ODF | Hedera helix L., Folium |
| EU | Heel | As an expectorant in case of a productive cough | [176] |
| Vitafol (Strips) |
|
| USA | Exeltis | Prenatal vitamin | [177] |
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lau, E.T.L.; Steadman, K.J.; Cichero, J.A.Y.; Nissen, L.M. Dosage form modification and oral drug delivery in older people. Adv. Drug Deliv. Rev. 2018, 135, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Umay, E.; Ozturk, E.; Gurcay, E.; Delibas, O.; Celikel, F. Swallowing in Parkinson’s disease: How is it affected? Clin. Neurol. Neurosurg. 2019, 177, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Lucci, N.; McConnell, C.; Biddle, C. Understanding Normal and Abnormal Swallowing: Patient Safety Considerations for the Perianesthetic Nurse. J. Perianesthesia Nurs. 2018, 33, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, D.P.; Kamath, V.D.; Stewart, J.T. Swallowing Disorders in Schizophrenia. Dysphagia 2017, 32, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Ruggiero, C.; Patriti, A.; Marano, L. Diagnostic Assessment and Management of Dysphagia in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 50, 947–955. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Guidance for Industry—Size, Shape, and other Physical Attributes of Generic Tablets and Capsules. 2015. Available online: https://www.fda.gov/files/drugs/published/Size--Shape--and-Other-Physical-Attributes-of-Generic-Tablets-and-Capsules.pdf?next=/answers/six-tips-to-avoid-getting-pill-stuck-in-your-throat/avoid-pill-getting-stuck-in-throat/ (accessed on 3 January 2023).
- Krampe, R.; Visser, J.C.; Frijlink, H.W.; Breitkreutz, J.; Woerdenbag, H.J.; Preis, M. Oromucosal film preparations: Points to consider for patient centricity and manufacturing processes. Expert Opin. Drug Deliv. 2016, 13, 493–506. [Google Scholar] [CrossRef]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef]
- Kumar Thummala, U.; Maddi, E.G.; Rani Avula, P. Optimization and development of orodispersible films for ledipasvir and sofosbuvir through solid dispersion using Box-Behnken design. Res. J. Pharm. Dos. Forms Technol. 2021, 13, 201–208. [Google Scholar] [CrossRef]
- Khan, Q.; Siddique, M.I.; Rasool, F.; Naeem, M.; Usman, M.; Zaman, M. Development and characterization of orodispersible film containing cefixime trihydrate. Drug Dev. Ind. Pharm. 2020, 46, 2070–2080. [Google Scholar] [CrossRef]
- Ahmad, A.; Butt, M.H.; Misbah, S.; Saleem, R.T. Development and Evaluation of Orodispersible Films by Solvent Casting Method Using Eletriptan Hydrobromide as a Model Drug. Lat. Am. J. Pharm. 2020, 39, 1951–1956. [Google Scholar]
- Fael, H.; Demirel, A.L. Tannic acid as a co-former in co-amorphous systems: Enhancing their physical stability, solubility and dissolution behavior. Int. J. Pharm. 2020, 581, 119284. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guan, R.; Gao, S.; Song, W.; Liu, Y.; Yang, Y.; Liu, H. Designing orodispersible films containing everolimus for enhanced compliance and bioavailability. Expert Opin. Drug Deliv. 2020, 17, 1499–1508. [Google Scholar] [CrossRef]
- Lai, K.L.; Fang, Y.; Han, H.; Li, Q.; Zhang, S.; Li, H.Y.; Chow, S.F.; Lam, T.N.; Lee, W.Y.T. Orally-dissolving film for sublingual and buccal delivery of ropinirole. Colloids Surf. B. Biointerfaces. 2018, 163, 9–18. [Google Scholar] [CrossRef]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Öblom, H.; Sjöholm, E.; Rautamo, M.; Sandler, N. Towards printed pediatric medicines in hospital pharmacies: Comparison of 2d and 3d-printed orodispersiblewarfarin films with conventional oral powders in unit dose sachets. Pharmaceutics 2019, 11, 334. [Google Scholar] [CrossRef]
- Yan, T.T.; Lv, Z.F.; Tian, P.; Lin, M.M.; Lin, W.; Huang, S.Y.; Chen, Y.Z. Semi-solid extrusion 3D printing ODFs: An individual drug delivery system for small scale pharmacy. Drug Dev. Ind. Pharm. 2020, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.S.; Gowda, D.V.; Kumar, T.P.; Rosenholm, J.M. A Comprehensive Review of Patented Technologies to Fabricate Orodispersible Films: Proof of Patent Analysis (2000–2020). Pharmaceutics 2022, 14, 820. [Google Scholar] [CrossRef]
- Tian, Y.; Bhide, Y.C.; Woerdenbag, H.J.; Huckriede, A.L.W.W.; Frijlink, H.W.; Hinrichs, W.L.J.J.; Visser, J.C. Development of an Orodispersible Film Containing Stabilized Influenza Vaccine. Pharmaceutics 2020, 12, 245. [Google Scholar] [CrossRef]
- Lordello, V.B.; Meneguin, A.B.; de Annunzio, S.R.; Taranto, M.P.; Chorilli, M.; Fontana, C.R.; Cavallini, D.C.U. Orodispersible Film Loaded with Enterococcus faecium CRL183 Presents Anti-Candida albicans Biofilm Activity In Vitro. Pharmaceutics 2021, 13, 998. [Google Scholar] [CrossRef]
- Visser, J.C.; Eugresya, G.; Hinrichs, W.L.J.; Tjandrawinata, R.R.; Avanti, C.; Frijlink, H.W.; Woerdenbag, H.J. Development of orodispersible films with selected Indonesian medicinal plant extracts. J. Herb. Med. 2017, 7, 37–46. [Google Scholar] [CrossRef]
- Cupone, I.E.; Dellera, E.; Marra, F.; Giori, A.M. Development and characterization of an orodispersible film for Vitamin D3 supplementation. Molecules 2020, 25, 5851. [Google Scholar] [CrossRef]
- Olechno, K.; Maciejewski, B.; Głowacz, K.; Lenik, J.; Ciosek-Skibińska, P.; Basa, A.; Winnicka, K. Orodispersible Films with Rupatadine Fumarate Enclosed in Ethylcellulose Microparticles as Drug Delivery Platform with Taste-Masking Effect. Materials 2022, 15, 2126. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Emmendörffer, J.F.; Bunjes, H. Orodispersible films: A delivery platform for solid lipid nanoparticles? Pharmaceutics 2021, 13, 2162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, T.; Zou, Y.; Han, P.; Liu, K. Self-microemulsifying oral fast dissolving films of vitamin D3 for infants: Preparation and characterization. Food Sci. Nutr. 2019, 7, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopeia Commission, Oromucosal Preparations 10.3/1807, European Pharmacopeia 10.3 ed. Straßbourg: European Directorate for the Quality of Medicines (EDQM) Council of Europe, France. 2021. Available online: https://pheur.edqm.eu/home (accessed on 3 December 2022).
- Triaminic Thin Strips Day Time Cold & Cough Side Effects: Common, Severe, Long Term—Drugs.com. Available online: https://www.drugs.com/sfx/triaminic-thin-strips-day-time-cold-cough-side-effects.html (accessed on 3 January 2023).
- Theraflu Thin Strips Multi Symptom Side Effects: Common, Severe, Long Term—Drugs.com. Available online: https://www.drugs.com/sfx/theraflu-thin-strips-multi-symptom-side-effects.html (accessed on 3 January 2023).
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simões, S. Outlining critical quality attributes (CQAs) as guidance for the development of orodispersible films. Pharm. Dev. Technol. 2017, 22, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.C.; Dohmen, W.M.C.; Hinrichs, W.L.J.; Breitkreutz, J.; Frijlink, H.W.; Woerdenbag, H.J. Quality by design approach for optimizing the formulation and physical properties of extemporaneously prepared orodispersible films. Int. J. Pharm. 2015, 485, 70–76. [Google Scholar] [CrossRef]
- Borges, A.F.S. Development of Novel Pharmaceutical Forms for Oral Administration of Bioactive Agents. PQDT-Glob. 2015, 335, 27981776. [Google Scholar]
- US Department of Health and Human Services; Food and Drug Administration. Orally Disintegrating Tablets. Guidance for Industry. US Government Printing Office. 2008. Available online: https://www.fda.gov/media/70877/download (accessed on 3 January 2023).
- Garsuch, V.; Breitkreutz, J. Comparative investigations on different polymers for the preparation of fast-dissolving oral films. J. Pharm. Pharmacol. 2010, 62, 539–545. [Google Scholar] [CrossRef]
- Steiner, D.; Finke, J.H.; Kwade, A. Model-based description of disintegration time and dissolution rate of nanoparticle-loaded orodispersible films. Eur. J. Pharm. Sci. 2019, 132, 18–26. [Google Scholar] [CrossRef]
- Zhang, L.; Aloia, M.; Pielecha-Safira, B.; Lin, H.; Rajai, P.M.; Kunnath, K.; Davé, R.N. Impact of Superdisintegrants and Film Thickness on Disintegration Time of Strip Films Loaded With Poorly Water-Soluble Drug Microparticles. J Pharm Sci. 2018, 107, 2107–2118. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Nishimatsu, T.; Tahara, K.; Takeuchi, H. Novel use of insoluble particles as disintegration enhancers for orally disintegrating films. J. Drug Deliv. Sci. Technol. 2019, 54, 101310. [Google Scholar] [CrossRef]
- Ṣen Karaman, D.; Patrignani, G.; Rosqvist, E.; Smått, J.H.; Orłowska, A.; Mustafa, R.; Preis, M.; Rosenholm, J.M. Mesoporous silica nanoparticles facilitating the dissolution of poorly soluble drugs in orodispersible films. Eur. J. Pharm. Sci. 2018, 122, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Tran, T.T.D. Recent Strategic Developments in the Use of Superdisintegrants for Drug Delivery. Curr. Pharm Des. 2020, 26, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Badr-Eldin, S.M.; Ahmed, T.A.; Ismail, H.R. Aripiprazole-cyclodextrin binary systems for dissolution enhancement: Effect of preparation technique, cyclodextrin type and molar ratio. Iran. J. Basic Med. Sci. 2013, 16, 1223–1231. [Google Scholar]
- Mihajlovic, T.; Kachrimanis, K.; Graovac, A.; Djuric, Z.; Ibric, S. Improvement of aripiprazole solubility by complexation with (2-hydroxy)propyl-β-cyclodextrin using spray drying technique. AAPS PharmSciTech 2012, 13, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, X.; Lian, R.; Zheng, S.; Yin, Z.; Lu, Y.; Wu, W. Enhanced dissolution and oral bioavailability of aripiprazole nanosuspensions prepared by nanoprecipitation/homogenization based on acid-base neutralization. Int. J. Pharm. 2012, 438, 287–295. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Steiner, D.; Tidau, M.; Finke, J.H. Embedding of Poorly Water-Soluble Drugs in Orodispersible Films—Comparison of Five Formulation Strategies. Pharmaceutics 2022, 15, 17. [Google Scholar] [CrossRef]
- Islam, N.; Irfan, M.; Zahoor, A.F.; Iqbal, M.S.; Syed, H.K.; Khan, I.U.; Rasul, A.; Khan, S.-U.-D.; Alqahtani, A.M.; Ikram, M.; et al. Improved Bioavailability of Ebastine through Development of Transfersomal Oral Films. Pharmaceutics 2021, 13, 1315. [Google Scholar] [CrossRef]
- Łyszczarz, E.; Hofmanová, J.; Szafraniec-Szczęsny, J.; Jachowicz, R. Orodispersible films containing ball milled aripiprazole-poloxamer® 407 solid dispersions. Int. J. Pharm. 2020, 575, 118955. [Google Scholar] [CrossRef]
- Jamróz, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Baek, S.H.; Lee, B.J.; Jin, H.E. Orodispersible polymer films with the poorly water-soluble drug, olanzapine: Hot-melt pneumatic extrusion for single-process 3D printing. Pharmaceutics 2020, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Speer, I.; Preis, M.; Breitkreutz, J. Novel Dissolution Method for Oral Film Preparations with Modified Release Properties. AAPS PharmSciTech 2019, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Speer, I.; Preis, M.; Breitkreutz, J. Prolonged drug release properties for orodispersible films by combining hot-melt extrusion and solvent casting methods. Eur. J. Pharm. Biopharm. 2018, 129, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Hussain, A.; Shah, P.A.; Raza, S.A.; Anwer, U.U.; Shamim, R.; Rasool, F.; Hafiz, M.A.; Bukhari, N.I. Development of Optimized Sumatriptan–Prochlorperazine Combined Orodispersible Films Without Disintegrant: In vitro, ex vivo and in vivo Characterization. AAPS PharmSciTech 2022, 23, 156. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Hussain, A.; Shah, P.A.; Ishtiaq, S.; Ali, E.; Abbas, N.; Bukhari, N.I. Simultaneous quantification of sumatriptan succinate and prochlorperazine maleate in orodispersible films using two validated UV-spectroscopic methods. Pak. J. Pharm. Sci. 2022, 35, 183–194. [Google Scholar] [CrossRef]
- Thabet, Y.; Lunter, D.; Breitkreutz, J. Continuous manufacturing and analytical characterization of fixed-dose, multilayer orodispersible films. Eur. J. Pharm. Sci. 2018, 117, 236–244. [Google Scholar] [CrossRef]
- Göbel, A.; Breitkreutz, J. Concept of Orodispersible or Mucoadhesive “Tandem Films” and Their Pharmaceutical Realization. Pharmaceutics 2022, 14, 264. [Google Scholar] [CrossRef]
- Patocka, J.; Wu, Q.; Nepovimova, E.; Kuca, K. Phenytoin—An anti-seizure drug: Overview of its chemistry, pharmacology and toxicology. Food Chem. Toxicol. 2020, 142, 111393. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Pattanaseri, K.; Hidalgo-Mazzei, D.; Taylor, D.; Young, A.H. Is lithium monitoring NICE? Lithium monitoring in a UK secondary care setting. J. Psychopharmacol. 2018, 32, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Than, Y.M.; Titapiwatanakun, V. Tailoring immediate release FDM 3D printed tablets using a quality by design (QbD) approach. Int. J. Pharm. 2021, 599, 120402. [Google Scholar] [CrossRef] [PubMed]
- Mwema, F.M.; Akinlabi, E.T. Basics of Fused Deposition Modelling (FDM). Fused Depos. Model. 2020, 30, 1–15. [Google Scholar] [CrossRef]
- Domsta, V.; Seidlitz, A. 3D-Printing of Drug-Eluting Implants: An Overview of the Current Developments Described in the Literature. Molecules 2021, 26, 4066. [Google Scholar] [CrossRef]
- Steiner, D.; Finke, J.H.; Kwade, A. SOFTs—Structured orodispersible film templates. Eur. J. Pharm. Biopharm. 2019, 137, 209–217. [Google Scholar] [CrossRef]
- Oh, B.C.; Jin, G.; Park, C.; Park, J.B.; Lee, B.J. Preparation and evaluation of identifiable quick response (QR)-coded orodispersible films using 3D printer with directly feeding nozzle. Int. J. Pharm. 2020, 584, 119405. [Google Scholar] [CrossRef]
- Windolf, H.; Chamberlain, R.; Breitkreutz, J.; Quodbach, J. 3D Printed Mini-Floating-Polypill for Parkinson’s Disease: Combination of Levodopa, Benserazide, and Pramipexole in Various Dosing for Personalized Therapy. Pharmaceutics 2022, 14, 931. [Google Scholar] [CrossRef]
- Morath, B.; Sauer, S.; Zaradzki, M.; Wagner, A.H. Orodispersible films—Recent developments and new applications in drug delivery and therapy. Biochem. Pharmacol. 2022, 200, 115036. [Google Scholar] [CrossRef]
- Carolina Visser, J.; Weggemans, O.A.F.; Boosman, R.J.; Loos, K.U.; Frijlink, H.W.; Woerdenbag, H.J. Increased drug load and polymer compatibility of bilayered orodispersible films. Eur. J. Pharm. Sci. 2017, 107, 183–190. [Google Scholar] [CrossRef]
- Woertz, C.; Kleinebudde, P. Development of orodispersible polymer films containing poorly water soluble active pharmaceutical ingredients with focus on different drug loadings and storage stability. Int. J. Pharm. 2015, 493, 134–145. [Google Scholar] [CrossRef]
- Centkowska, K.; Ławrecka, E.; Sznitowska, M. Technology of orodispersible polymer films with micronized loratadine—Influence of different drug loadings on film properties. Pharmaceutics 2020, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Umemura, K.; Tahara, K.; Takeuchi, H. Formulation design of hydroxypropyl cellulose films for use as orally disintegrating dosage forms. J. Drug Deliv. Sci. Technol. 2018, 46, 93–100. [Google Scholar] [CrossRef]
- Ren, T.; Lin, X.; Zhang, Q.; You, D.; Liu, X.; Tao, X.; Gou, J.; Zhang, Y.; Yin, T.; He, H.; et al. Encapsulation of Azithromycin Ion Pair in Liposome for Enhancing Ocular Delivery and Therapeutic Efficacy on Dry Eye. Mol. Pharm. 2018, 15, 4862–4871. [Google Scholar] [CrossRef] [PubMed]
- Lozoya-Agullo, I.; Planelles, M.; Merino-Sanjuán, M.; Bermejo, M.; Sarmento, B.; González-Álvarez, I.; González-Álvarez, M. Ion-pair approach coupled with nanoparticle formation to increase bioavailability of a low permeability charged drug. Int. J. Pharm. 2019, 557, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guan, J.; Wan, X.; Shang, R.; Shi, X.; Fang, L.; Liu, C. The Improved Cargo Loading and Physical Stability of Ibuprofen Orodispersible Film: Molecular Mechanism of Ion-Pair Complexes on Drug-Polymer Miscibility. J. Pharm. Sci. 2020, 109, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Khadra, I.; Obeid, M.A.; Dunn, C.; Watts, S.; Halbert, G.; Ford, S.; Mullen, A. Characterisation and optimisation of diclofenac sodium orodispersible thin film formulation. Int. J. Pharm. 2019, 561, 43–46. [Google Scholar] [CrossRef]
- Abou-taleb, H.A.; Mustafa, W.W.; Makram, T.S.; Abdelaty, L.N.; Salem, H.; Abdelkader, H. Vardenafil Oral Dispersible Films (ODFs) with Advanced Dissolution, Palatability, and Bioavailability. Pharmaceutics 2022, 14, 517. [Google Scholar] [CrossRef]
- Ryeong, H.; Su, P.; Seok, H.; Mok, K.; Ju, H.; Kim, Y.; Woong, C.; Eun, P.; Park, S.; Park, H.R.; et al. Formulation of sustained-release orodispersible film containing drug–resin complexes of donepezil hydrochloride. J. Pharm. Investig. 2022, 52, 259–272. [Google Scholar] [CrossRef]
- Liu, T.; Wan, X.; Luo, Z.; Liu, C.; Quan, P.; Cun, D.; Fang, L. A donepezil/cyclodextrin complexation orodispersible film: Effect of cyclodextrin on taste-masking based on dynamic process and in vivo drug absorption. Asian J. Pharm. Sci. 2019, 14, 183–192. [Google Scholar] [CrossRef]
- Marzouk, M.A.; Osman, D.A.; Mohamed, O.S. In vitro and in vivo evaluation of taste-masked orodispersible tablets of fluoxetine hydrochloride for the treatment of depression. Drug Dev. Ind. Pharm. 2021, 47, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Olechno, K.; Grilc, N.K.; Zupančič, Š.; Winnicka, K. Incorporation of Ethylcellulose Microparticles Containing a Model Drug with a Bitter Taste into Nanofibrous Mats by the Electrospinning Technique—Preliminary Studies. Materials 2022, 15, 5286. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Jain, A.; Laghate, G.; Prabhudesai, D. Pharmaceutical excipients. Remingt. Sci. Pract. Pharm. 2020, 3, 633–643. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose–A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, O.A. Premium ethylcellulose polymer based architectures at work in drug delivery. Int. J. Pharm. X 2019, 1, 100023. [Google Scholar] [CrossRef]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The Application of 3D Printing in the Formulation of Multilayered Fast Dissolving Oral Films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef]
- Kraan, H.; Vrieling, H.; Czerkinsky, C.; Jiskoot, W.; Kersten, G.; Amorij, J.P. Buccal and sublingual vaccine delivery. J. Control. Release 2014, 190, 580–592. [Google Scholar] [CrossRef]
- Singh, H.; Singla, Y.P.; Narang, R.S.; Pandita, D.; Singh, S.; Narang, J.K. Frovatriptan loaded hydroxy propyl methyl cellulose/treated chitosan based composite fast dissolving sublingual films for management of migraine. J. Drug Deliv. Sci. Technol. 2018, 47, 230–239. [Google Scholar] [CrossRef]
- Okonogi, S.; Kaewpinta, A.; Chaijareenont, P. Stability and influence of storage conditions on nanofibrous film containing tooth whitening agent. Pharmaceutics 2021, 13, 449. [Google Scholar] [CrossRef]
- Selmin, F.; Khalid, G.M.; Musazzi, U.M.; Demartin, F.; Minghetti, P.; Cilurzo, F. Relevance of production method on the physical stability and in vitro biopharmaceutical performances of olanzapine orodispersible film. Int. J. Pharm. 2021, 603, 120697. [Google Scholar] [CrossRef]
- Foo, W.C.; Khong, Y.M.; Gokhale, R.; Chan, S.Y. A novel unit-dose approach for the pharmaceutical compounding of an orodispersible film. Int. J. Pharm. 2018, 539, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Gronkowsky, D.; Grytzan, D.; Breitkreutz, J. Comparative study on novel test systems to determine disintegration time of orodispersible films. J. Pharm. Pharmacol. 2014, 66, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef] [PubMed]
- El-Bary, A.A.; Al Sharabi, I.; Haza’a, B.S. Effect of casting solvent, film-forming agent and solubilizer on orodispersible films of a polymorphic poorly soluble drug: An in vitro/in silico study. Drug Dev. Ind. Pharm. 2019, 45, 1751–1769. [Google Scholar] [CrossRef] [PubMed]
- Janigová, N.; Elbl, J.; Pavloková, S.; Gajdziok, J. Effects of Various Drying Times on the Properties of 3D Printed Orodispersible Films. Pharmaceutics 2022, 14, 250. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Khalid, G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2020, 576, 118963. [Google Scholar] [CrossRef]
- Turković, E.; Vasiljević, I.; Drašković, M.; Parojčić, J. Orodispersible films—Pharmaceutical development for improved performance: A review. J. Drug Deliv. Sci. Technol. 2022, 75, 103708. [Google Scholar] [CrossRef]
- He, M.; Zhu, L.; Yang, N.; Li, H.; Yang, Q. Recent advances of oral film as platform for drug delivery. Int. J. Pharm. 2021, 604, 120759. [Google Scholar] [CrossRef]
- Matić, J.; Alva, C.; Witschnigg, A.; Eder, S.; Reusch, K.; Paudel, A.; Khinast, J. Towards predicting the product quality in hot-melt extrusion: Small scale extrusion. Int. J. Pharm. X 2020, 2, 100062. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J.; Kotta, S. 3D Printing in medicine: Technology overview and drug delivery applications. Ann. 3D Print. Med. 2021, 4, 100037. [Google Scholar] [CrossRef]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Electrospinning based on benign solvents: Current definitions, implications and strategies. Green Chem. 2022, 24, 2347–2375. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.S.; Kumar, S.; (Tony) Zhou, Q. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies. Acta Pharm. Sin. B 2021, 11, 2505–2536. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Musazzi, U.M.; Franzé, S.; Selmin, F.; Minghetti, P. Orodispersible dosage forms: Biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 2018, 23, 251–259. [Google Scholar] [CrossRef]
- Zayed, G.M.; El Rasoul, S.A.; Ibrahim, M.A.; Saddik, M.S.; Alshora, D.H. In vitro and in vivo characterization of domperidone-loaded fast dissolving buccal films. Saudi Pharm. J. 2020, 28, 266–273. [Google Scholar] [CrossRef]
- Kiefer, O.; Fischer, B.; Breitkreutz, J. Fundamental Investigations into Metoprolol Tartrate Deposition on Orodispersible Films by Inkjet Printing for Individualised Drug Dosing. Pharmaceutics 2021, 13, 247. [Google Scholar] [CrossRef]
- Gupta, M.S.; Kumar, T.P.; Davidson, R.; Kuppu, G.R.; Pathak, K.; Gowda, D.V. Printing Methods in the Production of Orodispersible Films. AAPS PharmSciTech 2021, 22, 129. [Google Scholar] [CrossRef]
- Bala, R.; Khanna, S.; Pawar, P.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67. [Google Scholar] [CrossRef]
- Arun, A.; Amrish, C.; Vijay, S.; Kamla, P. Fast Dissolving Oral Films: An Innovative Drug Delivery System and Dosage Form. Int. J. ChemTech Res. 2010, 2, 576–583. [Google Scholar]
- Mahboob, M.B.H.; Riaz, T.; Jamshaid, M.; Bashir, I.; Zulfiqar, S. Oral Films: A Comprehensive Review. Int. Curr. Pharm. J. 2016, 5, 111–117. [Google Scholar] [CrossRef]
- Vlachojannis, C.; Al-Ahmad, A.; Hellwig, E.; Chrubasik, S. Listerine® Products: An Update on the Efficacy and Safety. Phyther. Res. 2016, 30, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, K.; Winnicka, K. How to assess orodispersible film quality? A review of applied methods and their modifications. Acta Pharm. 2019, 69, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.S. OTC product: Theraflu Thin Strips. J. Am. Pharm. Assoc. 2006, 46, e7. [Google Scholar] [CrossRef]
- Hampton, L.M.; Nguyen, D.B.; Edwards, J.R.; Budnitz, D.S. Cough and cold medication adverse events after market withdrawal and labeling revision. Pediatrics 2013, 132, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Orally Dissolving Film Strips could Pose Hazard to Kids. Available online: https://www.inquirer.com/philly/blogs/healthcare/Orally-dissolving-film-strips-could-pose-hazard-to-kids.html (accessed on 17 October 2022).
- Buck, M.L. Alternative Forms of Oral Drug Delivery for Pediatrics. Pediatr. Pharm. 2013, 19. Available online: https://www.medscape.com/viewarticle/807030 (accessed on 3 January 2023).
- FDA. Suboxone (Sublingual Film)—Center for Drug Evaluation and Research. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022410Orig1s000Approv.pdf (accessed on 3 January 2023).
- BioDelivery Sciences Receives FDA Approval for BUNAVAILTM (Buprenorphine and Naloxone) Buccal Film for the Maintenance Treatment of Opioid Dependence. Available online: https://bdsi.gcs-web.com/news-releases/news-release-details/biodelivery-sciences-receives-fda-approval-bunavailtm (accessed on 3 January 2023).
- BioDelivery Sciences Provides an Update of Anticipated 2014 Milestones. Available online: https://www.prnewswire.com/news-releases/biodelivery-sciences-provides-an-update-of-anticipated-2014-milestones-239591691.html (accessed on 3 January 2023).
- Enabling Healthier Lives. The Global Leaden in Consumer Health and Hygiene. Reckitt Benckiser Group Plc. Annual Report and Financial Statements. 2013. Available online: https://www.annualreports.com/HostedData/AnnualReportArchive/r/LSE_RB_2013.pdf (accessed on 3 January 2023).
- Gupta, M.S.; Kumar, T.P.; Gowda, D.V. Orodispersible Thin Film: A new patient-centered innovation. J. Drug Deliv. Sci. Technol. 2020, 59, 101843. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, C.; Song, I.-O.; Lee, B.-J.; Kang, C.-Y.; Park, J.-B. Investigation of Patient-Centric 3D-Printed Orodispersible Films Containing Amorphous Aripiprazole. Pharmaceuticals 2022, 15, 895. [Google Scholar] [CrossRef]
- Pechová, V.; Gajdziok, J.; Muselík, J.; Vetchý, D. Development of Orodispersible Films Containing Benzydamine Hydrochloride Using a Modified Solvent Casting Method. AAPS PharmSciTech. 2018, 19, 2509–2518. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Khalid, G.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized orodispersible films by hot melt ram extrusion 3d printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef]
- Qin, Z.; Jia, X.; Liu, Q.; Kong, B.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Łyszczarz, E.; Brniak, W.; Szafraniec-Szczęsny, J.; Majka, T.M.; Majda, D.; Zych, M.; Pielichowski, K.; Jachowicz, R. The impact of the preparation method on the properties of orodispersible films with aripiprazole: Electrospinning vs. casting and 3D printing methods. Pharmaceutics 2021, 13, 1122. [Google Scholar] [CrossRef] [PubMed]
- Citra Rani, K.; Parfati, N.; Luh Dewi Aryani, N.; Nuniek Winantari, A.; Wahyu Fitriani, E.; Trias Pradana, A.; Nawatila, R.; Rizky Putranti, A.; Irine, F.; Angelica, F.; et al. pharmaceutics Development, Evaluation, and Molecular Docking of Oral Dissolving Film of Atenolol. Pharmaceutics 2021, 13, 1727. [Google Scholar] [CrossRef]
- Talekar, D.S.; Haware, R.V.; Dave, R.H. Evaluation of self-nanoemulsifying drug delivery systems using multivariate methods to optimize permeability of captopril oral films. Eur. J. Pharm. Sci. 2019, 130, 215–224. [Google Scholar] [CrossRef]
- Domokos, A.; Balogh, A.; Dénes, D.; Nyerges, G.; Ződi, L.; Farkas, B.; Marosi, G.; Nagy, Z.K. Continuous manufacturing of orally dissolving webs containing a poorly soluble drug via electrospinning. Eur. J. Pharm. Sci. 2019, 130, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, Y.; Maqbool, M.; Hussain, T.; Yousaf, A.M.; Khan, I.U.; Mahmood, T.; Jamshaid, M. Natural and semisynthetic polymers blended orodispersible films of citalopram. Nat. Prod. Res. 2020, 34, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Kokab, A.; Afzal, H.; Shoaib, Q.U.A.; Hameed, M.; Manzoor, M.; Batool, N.; Ijaz, Q.A. Formulation and characterization of orodispersible film containing diltiazem hydrochloride with taste masked effects. Pak. J. Pharm. Sci. 2022, 35, 1007–1014. [Google Scholar] [PubMed]
- Chachlioutaki, K.; Tzimtzimis, E.K.; Tzetzis, D.; Chang, M.-W.; Ahmad, Z.; Karavasili, C.; Fatouros, D.G. Electrospun Orodispersible Films of Isoniazid for Pediatric Tuberculosis Treatment. Pharmaceutics 2020, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.G.; Rathod, V.; Basim, P.; Gajera, B.; Dave, R.H. Understanding the Impact of Multi-factorial Composition on Efficient Loading of the Stable Ketoprofen Nanoparticles on Orodispersible Films Using Box-Behnken Design. J. Pharm. Siences 2022, 111, P1451–P1462. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Aodsab, N.; Promyos, P.; Panraksa, P.; Udomsom, S.; Jantrawut, P. Fabrication of Hydroxypropyl Methylcellulose Orodispersible Film Loaded Mirtazapine Using a Syringe Extrusion 3D Printer. Sci. Pharm. 2022, 90, 68. [Google Scholar] [CrossRef]
- Tian, Y.; Visser, J.C.; Klever, J.S.; Woerdenbag, H.J.; Frijlink, H.W.; Hinrichs, W.L.J. Orodispersible films based on blends of trehalose and pullulan for protein delivery. Eur. J. Pharm. Biopharm. 2018, 133, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, E.A.; Scarpa, M.; Abdelhakim, H.E.; Bukhary, H.A.; Craig, D.Q.M.; Barker, S.A.; Orlu, M. A potential alternative orodispersible formulation to prednisolone sodium phosphate orally disintegrating tablets. Pharmaceutics 2021, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, L.; Luo, C.; Wang, Y.; Wang, H.; Chen, F.; Xiang, X. Development, In Vitro and In Vivo Evaluation of Racecadotril Orodispersible Films for Pediatric Use. AAPS PharmSciTech 2021, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.A.; Li, G.; Song, L.; Gao, B.; Huang, L.; Luan, D.; Iqbal, H.; Cao, Q.; Menaa, F.; Lee, B.-J.; et al. Rizatriptan-Loaded Oral Fast Dissolving Films: Design and Characterizations. Pharmaceutics 2022, 14, 2687. [Google Scholar] [CrossRef]
- No Sinha, S.; Thapa, S.; Singh, S.; Dutt, R.; Verma, R.; Pandey, P.; Mittal, V.; Rahman, M.H.; Kaushik, D. Development of Biocompatible Nanoparticles of Tizanidine Hydrochloride in Orodispersible Films: In vitro Characterization, Ex vivo Permeation, and Cytotoxic Study on Carcinoma Cells. Curr Drug Deliv. 2022, 19, 1061–1072. [Google Scholar] [CrossRef]
- Niese, S.; Quodbach, J. European Journal of Pharmaceutics and Biopharmaceutics Formulation development of a continuously manufactured orodispersible fi lm containing warfarin sodium for individualized dosing. Eur. J. Pharm. Biopharm. 2019, 136, 93–101. [Google Scholar] [CrossRef]
- Manda, P.; Popescu, C.; Juluri, A.; Janga, K.; Kakulamarri, P.R.; Narishetty, S.; Murthy, S.N.; Repka, M.A. Micronized Zaleplon Delivery via Orodispersible Film and Orodispersible Tablets. AAPS PharmSciTech 2018, 19, 1358–1366. [Google Scholar] [CrossRef]
- Ehtezazi, T.; Algellay, M.; Hardy, A. Next Steps in 3D Printing of Fast Dissolving Oral Films for Commercial Production. Recent Pat. Drug Deliv. Formul. 2020, 14, 5–20. [Google Scholar] [CrossRef]
- Gupta, M.S.; Kumar, T.P.; Gowda, D.V.; Rosenholm, J.M. Orodispersible films: Conception to quality by design. Adv. Drug Deliv. Rev. 2021, 178, 113983. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simões, S. Oral films: Current status and future perspectives. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef]
- Andrew, H.; Teresa, H.; Sophie, J.A.P. Oral Presentation—Abstract O216. Predicted savings to the UK National Health Service from switching to generic antiretrovirals, 2014–2018. J. Int. AIDS Soc. 2014, 17, 19497. [Google Scholar]
- Products|KYUKYU PHARMACEUTICAL CO., LTD. Available online: https://www.qqp.co.jp/en/product/ (accessed on 3 January 2023).
- Pharmaceutical Drugs ODM&OEM—C.L.Pharm. Available online: http://clpharm.com/product-information/pharmaceutical-drugs-odmoem/?jet-smart-filters=epro-posts/default&jet_paged=1 (accessed on 3 January 2023).
- Chloraseptic (Benzocaine and Menthol Strips) Information—Drugs.com. Available online: https://www.drugs.com/cdi/chloraseptic-benzocaine-and-menthol-strips.html (accessed on 3 January 2023).
- Fluor-I-Strips, A.T. Indications, Side Effects, Warnings—Drugs.com. Available online: https://www.drugs.com/cdi/fluor-i-strips-a-t.html (accessed on 3 January 2023).
- KYNMOBI®(Apomorphine HCI) Sublingual Film|for Patients. Available online: https://www.kynmobi.com/ (accessed on 3 January 2023).
- Ondansetron ODF—APR.ch. Available online: https://www.apr.ch/apr-pharma-products/medical-prescription/ondansetron-odf/ (accessed on 3 January 2023).
- Ora-Film: Indications, Side Effects, Warnings—Drugs.com. Available online: https://www.drugs.com/cdi/ora-film.html (accessed on 3 January 2023).
- IntelGenx—RIZAPORT®. Available online: https://www.intelgenx.com/index.php?option=com_content&view=category&layout=blog&id=33&Itemid=158 (accessed on 3 January 2023).
- Our Products—Norgine. Available online: https://norgine.com/products/ (accessed on 3 January 2023).
- Patient Information for SUBOXONE®(Buprenorphine and Naloxone) Sublingual Film (CIII). Available online: https://www.suboxone.com/ (accessed on 3 January 2023).
- Sympazan: Uses, Dosage, Side Effects & Warnings—Drugs.com. Available online: https://www.drugs.com/sympazan.html (accessed on 3 January 2023).
- SYMPAZAN (Clobazam) Oral Film|Official Caregivers Site. Available online: https://www.sympazan.com/ (accessed on 3 January 2023).
- Zentrip (Film, Soluble) Sato Pharmaceutical Co., Ltd. Available online: https://www.drugs.com/otc/116987/zentrip.html (accessed on 3 January 2023).
- ZUPLENZ—Ondansetron Film. Fortovia Therapeutics, Inc. Available online: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0b1dab95-b64b-4133-bcad-a65b332ab320 (accessed on 3 January 2023).
- About Us—C.L.Pharm. Available online: http://clpharm.com/company/#pr_history (accessed on 3 January 2023).
- Benadryl Allergy Quick Dissolve (strip) McNeil Consumer Healthcare Division of McNeil-PPC, Inc. Available online: https://www.drugs.com/otc/106077/benadryl-allergy-quick-dissolve.html (accessed on 3 January 2023).
- Produkte—Hexal.de. Available online: https://www.hexal.de/hcp/produkte (accessed on 3 January 2023).
- Bernard, D.B. OTC Product: Gas-X Thin Strips. J. Am. Pharm. Assoc. 2007, 47, 432. [Google Scholar] [CrossRef] [PubMed]
- Hiforce 100 ODS—Healing Pharma India Pvt Ltd.—Pharmaceutical Third Party Manufacturer. Available online: https://www.healingpharma.in/product-tag/hiforce-100-ods-for-export-only/ (accessed on 3 January 2023).
- Ivyfilm—Online Vitamins & Natural Medication. Available online: https://www.feelhealthy.co.za/buy-online-south-africa/ivyfilm-2/ (accessed on 3 January 2023).
- Jack & Jill Thin Strips Cough Side Effects: Common, Severe, Long Term—Drugs.com. Available online: https://www.drugs.com/sfx/jack-jill-thin-strips-cough-side-effects.html (accessed on 3 January 2023).
- NiQuitin Strips Mint 2.5 mg Oral Film 60 Oral Films. Available online: https://www.doorsteppharmacy.com/product/niquitin-strips-mint-2-5mg-oral-film-60-oral-films/ (accessed on 3 January 2023).
- Orajel Kids Sore Throat Relief Strips, 28 mg, Scooby-Doo, Cool Cherry. Available online: https://www.festfoods.com/shop/orajel_kids_sore_throat_relief_strips_28_mg_scooby_doo_cool_cherry/p/2598279 (accessed on 3 January 2023).
- Pedia-Lax Quick Dissolve Strips Side Effects: Common, Severe, Long Term—Drugs.com. Available online: https://www.drugs.com/sfx/pedia-lax-quick-dissolve-strips-side-effects.html (accessed on 3 January 2023).
- SUDAFED PE Quick-Dissolve Strips Cherry Menthol. Available online: https://www.medshopexpress.com/sudafed-pe-quick-dissolve-strips-cherry-menthol-10-ea# (accessed on 3 January 2023).
- Zolmitriptan Oral Dispersible Film. Available online: https://adisinsight.springer.com/drugs/800032266 (accessed on 3 January 2023).
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef] [PubMed]
- About Us—Aavishkar. Available online: https://aavishkar.com/about-us/ (accessed on 3 January 2023).
- Klingmann, V.; Pohly, C.E.; Meissner, T.; Mayatepek, E.; Möltner, A.; Flunkert, K.; Breitkreutz, J.; Bosse, H.M. Acceptability of an orodispersible film compared to syrup in neonates and infants: A randomized controlled trial. Eur. J. Pharm. Biopharm. 2020, 151, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Orlu, M.; Woerdenbag, H.J.; Scarpa, M.; Kiefer, O.; Kottke, D.; Sjöholm, E.; Öblom, H.; Sandler, N.; Hinrichs, W.L.J.; et al. Oromucosal films: From patient centricity to production by printing techniques. Expert Opin. Drug Deliv. 2019, 16, 981–993. [Google Scholar] [CrossRef]
- Products—Aavishkar. Available online: http://aavishkar.com/products/ (accessed on 3 January 2023).
- Thinsol—Oral Strips. Available online: https://thinsol.com/#services (accessed on 3 January 2023).
- POCKETPAKS® Cool Mint Fresh Breath Strips. LISTERINE®. Available online: https://www.listerine.com/on-the-go-oral-health/listerine-pocketpaks-cool-mint (accessed on 3 January 2023).
- Anti-Snoring Oral Strips|Snoreeze. Available online: https://snoreeze.com/product/snoreeze-oral-strips/ (accessed on 3 January 2023).
- Tusheel 30 Orodispersible Films—Farmacia Internacional. Available online: https://www.farmacia-internacional.net/en/home/15518-tusheel-30-orodispersible-films.html (accessed on 3 January 2023).
- Vitafol Strips.—Healthy Pregnancy. Available online: https://vitafol.com/more-strip/&cd=13&hl=pl&ct=clnk&gl=pl (accessed on 3 January 2023).


| Disease or Therapeutic Indications or Drug Action | API | Manufacturing Method | Film-Forming Polymers | References |
|---|---|---|---|---|
| Painkiller | Acetaminophen | Hot-melt ram-extrusion 3D printing | Maltodextrins | [119] |
| Painkiller | Acetaminophen | FDM 3D printing | PVA | [81] |
| Antipyretic | Acetylsalicylic acid | Electrospinning | Pullulan, chitosan | [120] |
| Oropharyngeal candidiasis | Amphotericin B | Solvent casting | Dextrose-derived polymers; HMPC/HPC | [121] |
| Schizophrenia | Aripiprazole | Solvent casting | PVA | [45] |
| Schizophrenia | Aripiprazole | 3D printing | PEO | [61] |
| Schizophrenia | Aripiprazole | 3D printing and solvent casting | PVA | [46] |
| Schizophrenia | Aripiprazole | 3D printing and solvent casting | PVA, HPC | [117] |
| Schizophrenia | Aripiprazole | 3D printing and solvent casting and electrospinning | PVA | [122] |
| Hypertension | Atenolol | Solvent casting | HMPC/CMC-Na/Na-alginate | [123] |
| Antiseptic | Benzydamine hydrochloride | Solvent casting | Maltodextrin | [118] |
| Antiseptic | Benzydamine hydrochloride | 3D printing | Maltodextrin | [71] |
| Hypertension | Captopril | Solvent casting | HPMC | [124] |
| Hypertension | Carvedilol | Electrospinning | PVPK30, hydroxypropyl-β-cyclodextrin | [125] |
| Infections of the respiratory tract | Cefixime trihydrate | Solvent casting | HPMC | [10] |
| Depression | Citalopram | Solvent casting | HPMC, Okra biopolymer | [126] |
| Anti-inflammatory | Diclofenac sodium | Solvent casting | HPMC E3 | [72] |
| Anti-inflammatory | Diclofenac sodium | Solvent casting | HPMC | [50] |
| Angina and hypertension | Diltiazem hydrochloride | Solvent casting | HPMC, CMC | [127] |
| Alzheimer’s disease | Donepezil hydrochloride | Solvent casting | HPMC, hydroxypropyl-β-cyclodextrin | [75] |
| Alzheimer’s disease | Donepezil hydrochloride | Solvent casting | HPMC | [36,74] |
| Allergy | Ebastine | Solvent casting | HPMC | [44] |
| Migraine | Eletriptan hydrobromide | Solvent casting | PVA | [11] |
| Hypertension | Enalapril | Solvent casting | HPMC, HPC, HEC | [64] |
| Hypertension | Enalapril and hydrochlorothiazide | Solvent casting | HPC/PVA | [53] |
| Migraine | Frovatriptan succinate monohydrate | Solvent casting | HPMC E15, chitosan | [83] |
| Painkiller, anti-inflammatory | Ibuprofen | Solvent casting | HPC | [67] |
| Painkiller, anti-inflammatory | Ibuprofen | Solvent casting | PVP | [70] |
| Tuberculosis | Isoniazid | Electrospinning | Pullulan/HPMC | [128] |
| Painkiller | Ketoprofen | Solvent casting | HPMC | [129] |
| Hepatitis C virus infection | Ledipasvir and sofosbuvir | Solvent casting | MC (methyl cellulose) | [9] |
| Allergy | Loratidine | Solvent casting | PVA/PVP; HMPC | [66] |
| Heart diseases | Metoprolol | Inkjet printing | HPMC | [101] |
| Depression | Mirtazapine | 3D printing | HPMC | [130] |
| Painkiller, anti-inflammatory | Naproxen, anthraquinone | Solvent casting | Pharmacoat 606 (HPMC) | [34] |
| Schizophrenia | Olanzapine | Hot-melt ram-extrusion 3D printing and solvent casting | Maltodextrin | [85] |
| Schizophrenia | Olanzapine | 3D printing based on hot-melt pneumatic extrusion (HMPE) | Polyethylene oxide, poloxamer 407, poloxamer188, PVP VA64 | [47] |
| Therapeutic proteins | Ovalbumin, lysozyme, β-galactosidase | Solvent casting | Pullulan, trehalose | [131] |
| Adrenal insufficiency | Prednisolone | Solvent casting | PVA | [37] |
| Adrenal insufficiency | Prednisolone sodium phosphate | Electrospinning | PVA | [132] |
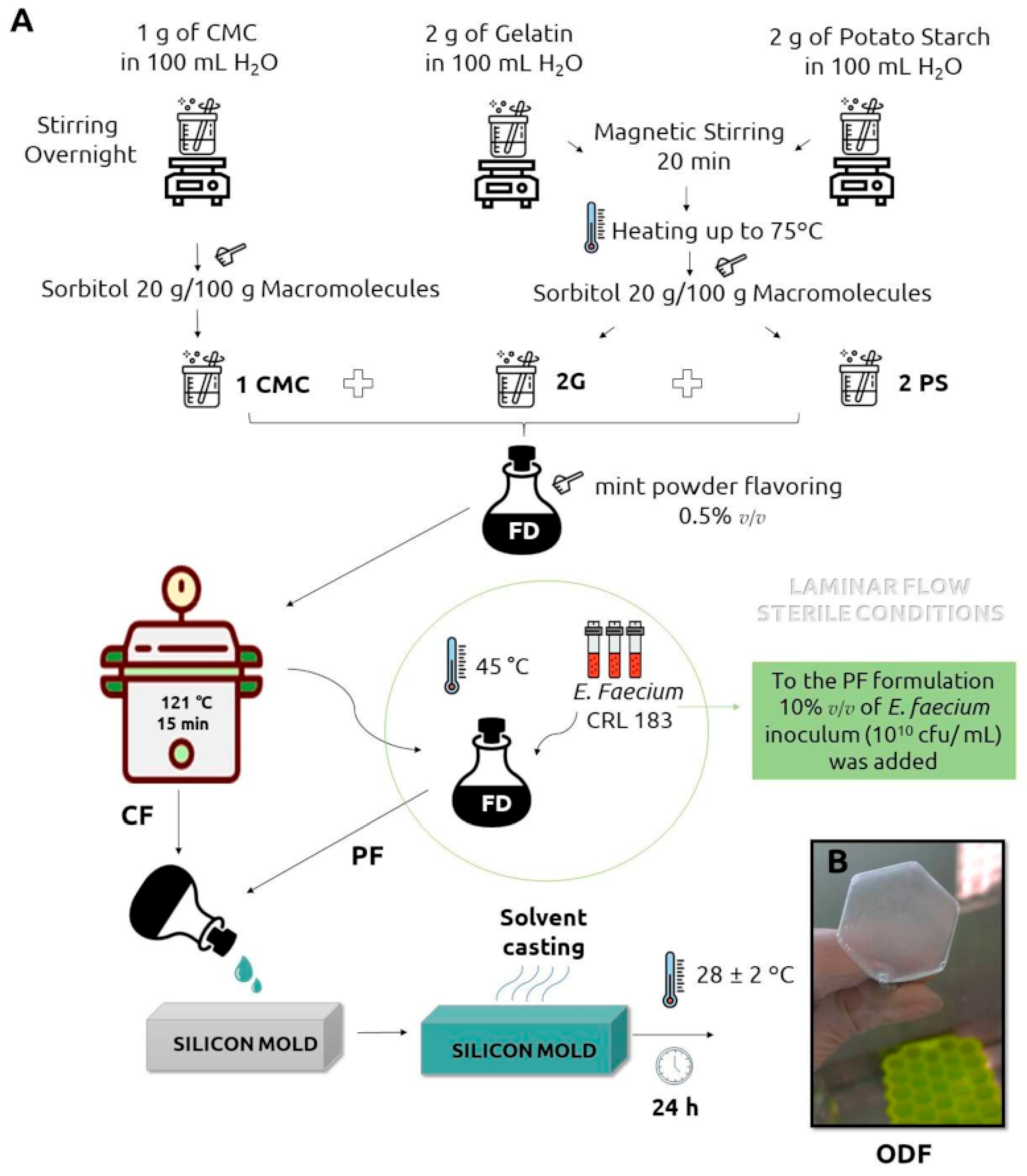
| Candida spp. infections | Probiotic bacteria Enterococcus faecium CRL183 | Solvent casting | CMC | [20] |
| Diarrhea | Racecadotril | Solvent casting | PVA | [133] |
| Anti-migraine | Rizatriptan | Solvent casting | Maltodextrin, pullulan | [134] |
| Parkinson’s disease | Ropinirole HCl | Solvent casting | HPMC 603 | [14] |
| Allergy | Rupatadine fumarate | Solvent casting | HPMC | [23] |
| Allergy | Rupatadine fumarate | Electrospinning | HPMC | [77] |
| Migraine and associated nausea and vomiting | Sumatriptan-prochlorperazine | Solvent casting | PA | [51] |
| Muscle realaxant | Tizanidine hydrochloride | Solvent casting | Chitosan-alginate | [135] |
| Erectile dysfunction | Vardenafil | Solvent casting | PVP/MC/sodium alginate (SA)/polyvinylpyrrolidone K 30 (PVP) | [73] |
| Anticoagulant | Warfarin | Solvent casting (continuously working pilot-scale coating bench) | HPMC, HPC, PVA | [136] |
| Anticoagulant | Warfarin | 3D printing | PVA, HPC | [15] |
| Anticoagulant | Warfarin | Semi-solid extrusion 3D printing and 2D inkjet printing | HPMC | [16] |
| Insomnia | Zaleplon | Solvent casting | Lycoat® RS 720 | [137] |
| Drug Name | The Active Substance | Dose | Region | Producer | Drug Action/Application | References |
|---|---|---|---|---|---|---|
| Amlodipine OD Film “QQ” | Amlodipine Besilate |
| Japan | Kyukyu Pharmaceutical Co., Ltd. | Hypertension, angina pectoris | [142] |
| Cendom ODF | Tadalafil |
| South Korea | C.L.Pharm Co., Ltd. | Treatment for erectile dysfunction | [143] |
| Chloraseptic strips | Benzocaine, menthol |
| USA | Prestige Brands | Sore throat relief | [144] |
| Cool Strip ODF | Cetylpyridinium Chloride |
| South Korea | C.L.Pharm Co., Ltd. | Pharyngitis, tonsillitis, and stomatitis treatment | [143] |
| Donepezil Hydrochloride OD Film “EE” | Donepezil Hydrochloride |
| Japan | Kyukyu Pharmaceutical Co., Ltd. | Inhibiting the progression of dementia symptoms associated with dementia of the Alzheimer’s type and dementia with Lewy bodies | [142] |
| Fluor-I-Strips A.T. | Fluorescein Sodium |
| USA | Wyeth Ayerst Laboratories | Specially prepared sterile ophthalmic strip for diagnostic use | [145] |
| Hemoramin ODF | Folic acid, Ferric Hydroxide Polymaltose |
| South Korea | C.L.Pharm Co., Ltd. | Prevention and treatment for iron-deficiency anemia | [143] |
| Ignis ODF | Sildenafil |
| South Korea | C.L.Pharm Co., Ltd. | Treatment for erectile dysfunction | [143] |
| Kynmobi | Apomorphine HCl | Sublingual film 10 mg, 15 mg, 20 mg, 25 mg, 30 mg | Sunovion Pharmaceuticals Inc. | Acute, intermittent treatment of “off” episodes in patients with Parkinson’s disease | [146] | |
| Loratadine OD Film 10 mg “MOCHIDA” | Loratadine |
| Japan | Kyukyu Pharmaceutical Co., Ltd. | Allergic rhinitis, urticaria, itch associated with skin diseases (eczema/dermatitis, pruritus cutaneous) | [142] |
| Neutoin ODF | Donepezil HCl |
| South Korea | C.L.Pharm Co., Ltd. | Treatment of dementia | [143] |
| New Pezil ODF | Donepezil |
| Korea | C.L.Pharm Co., Ltd. | Treatment for Alzheimer—dementia | [143] |
| Olopatadine Hydrochloride OD Film “MARUHO” | Olopatadine Hydrochloride |
| Japan | Kyukyu Pharmaceutical Co., Ltd. | Allergic rhinitis, urticaria, itch associated with skin diseases | [142] |
| Ondansetron Rapidfilm ODF | Ondansetron |
| Germany | Applied Pharma Research/Labtec | Prevention and treatment of chemo- and radiotherapy induced nausea and vomiting | [147] |
| Ora-film | Benzocaine Strips | USA | Treats mouth sores. | [148] | ||
| Rizaport | Rizatriptan Film |
| Spain | Intelgenx | Selective 5-HT1B/1D receptor agonist indicated for the treatment of migraines | [149] |
| Sentrip ODF—tadalafil | Tadalafil |
| Korea | C.L.Pharm Co., Ltd. | Treatment for erectile dysfunction | [143] |
| Setofilm ODF | Ondansetron |
| Europe, Australia, and New Zealand | Norgine | Prophylaxis and treatment of acute and delayed nausea and vomiting | [150] |
| Suboxone | Buprenorphine and Naloxone sublingual film | Buprenorphine/Naloxone:
| USA, UE | Indivior UK Limited | Treatment of opioid dependence | [151] |
| Sympazan | Clobazam oral film |
| Aquestive Therapeutics | Treats the symptoms of seizures | [152,153] | |
| Vinix ODF | Sildenafil |
| Korea | C.L.Pharm Co., Ltd. | Treatment for erectile dysfunction | [143] |
| Voglibose OD Film “QQ” | Voglibose |
| Japan | Kyukyu Pharmaceutical Co., Ltd. | Improvement of postprandial hyperglycemia in diabetes mellitus | [142] |
| Zentrip | Meclizine hydrochloride |
| Sato Pharmaceutical Co., Ltd. | Prevention and treatment of the nausea, vomiting, or dizziness associated with motion sickness | [154] | |
| Zolpidem Tartrate OD Film “MOCHIDA” | Zolpidem Tartrate |
| Japan | Kyukyu Pharmaceutical Co., Ltd. | Insomnia | [142] |
| Zuplenz | Ondansetron film |
| Aquestive Therapeutics | Prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy, including cisplatin ≥ 50 mg/m2 | [155] | |
| Zyris ODF | Tadalafil |
| South Korea | C.L.Pharm Co., Ltd. | Treatment for erectile dysfunction | [143] |
| Drug Name | The Active Substance | Dose | Region | Producer | Drug Action/Application | References |
|---|---|---|---|---|---|---|
| Antimal ODF | Primaquine |
| Korea | C.L.Pharm Co., Ltd. | Antiprotozoal | [156] |
| Benadryl Allergy Quick dissolve strips | Diphenhydramine HCl |
| USA, EU | McNeil Consumer Healthcare Division of McNeil-PPC, Inc | Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies | [157] |
| Donepezil HCl Hexal SF | Donepezil |
| EU | Hexal AG | Treatment of mild to moderately severe Alzheimer’s dementia | [158] |
| Gas-X thin strips | Simethicone |
| Novartis Consumer Health | Flatulence | [159] | |
| Hiforce 100 ODS | Sildenafil |
| Indie | Healing Pharma | Treatment for erectile dysfunction | [160] |
| IvyFilm | Dried extract from Ivy leaves (Hedera helix L., folium); |
| Africa | LAMAR INTERNATIONAL | Loosens mucus in the airway | [161] |
| Jack & Jill Thin Strips Cough | Dextromethorphan |
| USA, Canada | The Buckley’s Company | Antitussives | [162] |
| Niquitin strips | Nicotine |
| EU | Omega Pharma Ltd. | Relieve and/or prevent craving and nicotine withdrawal sympoms associated with tobacco dependence | [163] |
| Olanzapin Hexal SF | Olanzapine |
| EU | Hexal AG | Schizophrenia | [158] |
| Orajel Kids Sore Throat Relief Strips | Pectin |
| Canada, USA | Church & Dwight, Inc. | Soothes pain & irritation of throat | [164] |
| Pedia Lax Quick dissolve strips | Standardized sennosides |
| USA | Fleet Company | Constipation | [165] |
| Ramea ODF | Ramosetron HCl |
| Korea | C.L.Pharm Co., Ltd. | Antiemetic | [156] |
| Risperidon Hexal SF | Risperidone |
| EU | Hexal AG | Schizophrenia | [158] |
| Sudafed PE Quick dissolve strips | Phenylephrine HCl | USA | Pfizer | Decongestant | [166] | |
| Theraflu Thin Strips multi symptom | Diphenhydramine hydrochloride |
| USA | Novartis Consumer Health | Treat symptoms of allergies and the common cold. | [28] |
| Triaminic Thin Strip | Dextromethorphan HBr/Phenylephrine HCl |
| USA | Novartis Consumer Health | Cough suppressant, nasal decongestant | [27] |
| Zolmitriptan Renantos Schmelzfilm | Zolmitriptan |
| EU | Renantos | Antimigraine | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferlak, J.; Guzenda, W.; Osmałek, T. Orodispersible Films—Current State of the Art, Limitations, Advances and Future Perspectives. Pharmaceutics 2023, 15, 361. https://doi.org/10.3390/pharmaceutics15020361
Ferlak J, Guzenda W, Osmałek T. Orodispersible Films—Current State of the Art, Limitations, Advances and Future Perspectives. Pharmaceutics. 2023; 15(2):361. https://doi.org/10.3390/pharmaceutics15020361
Chicago/Turabian StyleFerlak, Jan, Weronika Guzenda, and Tomasz Osmałek. 2023. "Orodispersible Films—Current State of the Art, Limitations, Advances and Future Perspectives" Pharmaceutics 15, no. 2: 361. https://doi.org/10.3390/pharmaceutics15020361
APA StyleFerlak, J., Guzenda, W., & Osmałek, T. (2023). Orodispersible Films—Current State of the Art, Limitations, Advances and Future Perspectives. Pharmaceutics, 15(2), 361. https://doi.org/10.3390/pharmaceutics15020361







