Enhanced Solubility and Biological Activity of Dexibuprofen-Loaded Silica-Based Ternary Solid Dispersions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SDs
2.3. Preformulation Studies
2.4. Analysis of Drug Content
2.5. Solubility Studies
2.6. Practical Yield
2.7. Solid-State Characterization
2.7.1. Fourier-Transformed Infrared Spectroscopy (FTIR)
2.7.2. X-ray Diffraction (XRD)
2.7.3. Surface Morphology
2.7.4. Thermal Analysis
2.8. In Vitro Dissolution Studies
2.9. Properties of the Powder
Density and Flowability
2.10. Estimation of Gastroprotective Effect
2.10.1. Macroscopic Scoring
2.10.2. Microscopic Analysis
2.11. Anti-Inflammatory Effects
2.11.1. Serum TNF Alpha and IL-6
2.11.2. Histopathological Analysis of Paw
3. Results and discussion
3.1. Preformulation of SD
3.1.1. Evaluation and Optimization of BSDs
Drug Content, Solubility, and Percentage Yield of BSDs
3.1.2. Evaluation and Optimization of TSDs
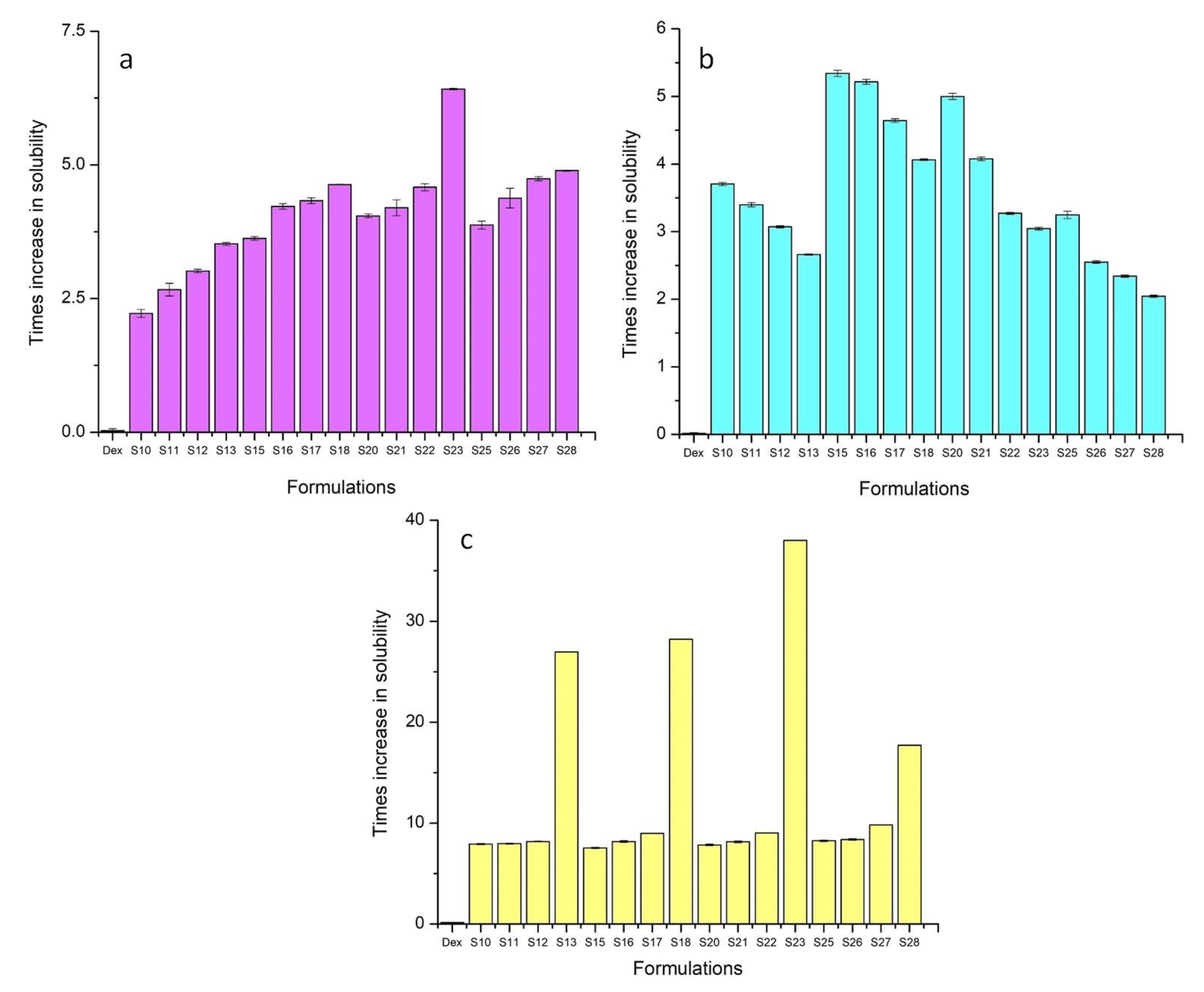
Drug content, Solubility, and Percentage Yield of TSDs
3.2. FTIR Analysis
3.3. Powder X-ray Diffraction Analysis
3.4. SEM Analysis
3.5. Thermal Analysis
3.5.1. Thermogravimetric Analysis (TGA)
3.5.2. Differential Scanning Calorimetry (DSC)
3.6. In Vitro Dissolution
3.7. Micromeritic Properties
3.8. Assessment of Gastro Protective Effect In Vivo
3.8.1. Macroscopic Scoring
3.8.2. Histopathological Analysis
3.9. Evaluation of Anti-Inflammatory Activity
3.9.1. Measurement of Serum IL-6 and TNF Alpha
3.9.2. Histopathology of Rat Paw
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FitzGerald, G.A.; Patrono, C. The Coxibs, Selective Inhibitors of Cyclooxygenase-2. N. Engl. J. Med. 2001, 345, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.Y.; Gwee, K.A.; Cheng, Y.K.; Yoon, K.H.; Hee, H.T.; Omar, A.R. Nonsteroidal anti-inflammatory drugs in chronic pain: Implications of new data for clinical practice. J. Pain Res. 2018, 11, 1937–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaehler, S.T.; Phleps, W.; Hesse, E. Dexibuprofen: Pharmacology, therapeutic uses and safety. Inflammopharmacology 2003, 11, 371–383. [Google Scholar] [CrossRef] [PubMed]
- El-Houssieny, B.M.; El-Dein, E.Z.; El-Messiry, H.M. Enhancement of solubility of dexibuprofen applying mixed hydrotropic solubilization technique. Drug Discov. Ther. 2014, 8, 178–184. [Google Scholar] [CrossRef]
- Hussain, T.; Waters, L.J.; Parkes, G.M.; Shahzad, Y. Microwave processed solid dispersions for enhanced dissolution of gemfibrozil using non-ordered mesoporous silica. Colloids Surfaces A: Physicochem. Eng. Asp. 2017, 520, 428–435. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, D. Investigation of molecular dissolution mechanism of ketoprofen binary and ternary solid dispersions by molecular dynamics simulations. Mol. Simul. 2017, 43, 1074–1080. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Salem, J.K.; Selmane, M.; Kodeh, F.S.; Ebtihan, H.A. Synthesis and structural characterization of ZnO and CuO nanoparticles supported mesoporous silica SBA-15. Chem. Phys. Lett. 2017, 667, 165–171. [Google Scholar] [CrossRef]
- Lodha, A.; Lodha, M.; Patel, A.; Chaudhuri, J.; Dalal, J.; Edwards, M.; Douroumis, D. Synthesis of mesoporous silica nanoparticles and drug loading of poorly water soluble drug cyclosporin A. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. S1), S92. [Google Scholar] [CrossRef]
- Ilevbare, G.A.; Liu, H.; Edgar, K.J.; Taylor, L.S. Maintaining Supersaturation in Aqueous Drug Solutions: Impact of Different Polymers on Induction Times. Cryst. Growth Des. 2013, 13, 740–751. [Google Scholar] [CrossRef]
- Collett, J.; Popli, H.; Kibbe, A. Poloxamer; Pharmaceutical Press: London, UK, 2000; pp. 385–388. [Google Scholar]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alkholifi, F.K.; Alharbi, K.S.; Mostafa, E.M.; Alanazi, A.S.; Gilani, S.J.; Musa, A.; et al. Formulation of Genistein-HP β Cyclodextrin-Poloxamer 188 Ternary Inclusion Complex: Solubility to Cytotoxicity Assessment. Pharmaceutics 2021, 13, 1997. [Google Scholar] [CrossRef] [PubMed]
- Chambin, O.; Jannin, V. Interest of multifunctional lipid excipients: Case of Gelucire® 44/14. Drug Dev. Ind. Pharm. 2005, 31, 527–534. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Balogh, A.; Vajna, B.; Farkas, A.; Patyi, G.; Kramarics, Á.; Marosi, G. Comparison of Electrospun and Extruded Soluplus®-Based Solid Dosage Forms of Improved Dissolution. J. Pharm. Sci. 2012, 101, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Lee, S.-E.; Jang, W.S.; Byeon, J.C.; Park, J.-S. Solid dispersion of dutasteride using the solvent evaporation method: Approaches to improve dissolution rate and oral bioavailability in rats. Mater. Sci. Eng. C 2018, 90, 387–396. [Google Scholar]
- Kenechukwu, F.C.; Ofokansi, K.C.; Ezugwu, R.O.; Attama, A.A. Improved dissolution and anti-inflammatory activity of ibuprofen-polyethylene glycol 8000 solid dispersion systems. Int. J. Pharm. Investig. 2016, 6, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolašinac, N.; Kachrimanis, K.; Homšek, I.; Grujić, B.; Đurić, Z.; Ibrić, S. Solubility enhancement of desloratadine by solid dispersion in poloxamers. Int. J. Pharm. 2012, 436, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Soundararajan, R.; Shanmugam, U.; Ramu, V. Development, characterization and solubility enhancement of comparative dissolution study of second generation of solid dispersions and microspheres for poorly water soluble drug. Asian J. Pharm. Sci. 2015, 10, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Burki, I.K.; Khan, M.K.; Khan, B.A.; Uzair, B.; Braga, V.A.; Jamil, Q.A. Formulation Development, Characterization, and Evaluation of a Novel Dexibuprofen-Capsaicin Skin Emulgel with Improved In Vivo Anti-inflammatory and Analgesic Effects. AAPS PharmSciTech 2020, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Nagira, S.; Yamamoto, H.; Kawashima, Y. Solid dispersion particles of amorphous indomethacin with fine porous silica particles by using spray-drying method. Int. J. Pharm. 2005, 293, 155–164. [Google Scholar] [CrossRef]
- Mouralova, K.; Kovar, J.; Klakurkova, L.; Bednar, J.; Benes, L.; Zahradnicek, R. Analysis of surface morphology and topography of pure aluminium machined using WEDM. Measurement 2018, 114, 169–176. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Minhas, M.U.; Batool, F.; Malik, N.S.; Rehman, M. Novel β-cyclodextrin nanosponges by chain growth condensation for solubility enhancement of dexibuprofen: Characterization and acute oral toxicity studies. J. Drug Deliv. Sci. Technol. 2020, 61, 102089. [Google Scholar] [CrossRef]
- Kojima, T.; Elliott, J.A. Incipient flow properties of two-component fine powder systems and their relationships with bulk density and particle contacts. Powder Technol. 2012, 228, 359–370. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Kumar, M.; Pathak, K.; Bhatt, S.; Saini, V. Surface Solid Dispersion and Solid Dispersion of Meloxicam: Comparison and Product Development. Adv. Pharm. Bull. 2017, 7, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saw, H.Y.; Davies, C.E.; Paterson, A.H.; Jones, J.R. Correlation between Powder Flow Properties Measured by Shear Testing and Hausner Ratio. Procedia Eng. 2015, 102, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, A.; Kumar, C.K.A.; Mishra, A. Synthesis, hydrolysis studies and phamacodynamic profiles of amide prodrugs of dexibuprofen with amino acids. J. Enzym. Inhib. Med. Chem. 2011, 26, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.U.; Ikraam, M.; Nadeem, M.; Khalid, S.H.; Asghar, S.; Khalid, I.; Irfan, M.; Islam, N.; Ajaz, N.; Khan, I.U. Fabrication, In Vitro and In Vivo Evaluation of Non-Ordered Mesoporous Silica-Based Ternary Solid Dispersions for Enhanced Solubility of Flurbiprofen. Pharmaceuticals 2022, 15, 856. [Google Scholar] [CrossRef]
- Akinnawo, O.O.; Anyasor, G.N.; Osilesi, O. Aqueous fraction of Alstonia boonei de Wild leaves suppressed inflammatory responses in carrageenan and formaldehyde induced arthritic rats. Biomed. Pharmacother. 2017, 86, 95–101. [Google Scholar] [CrossRef]
- Ma, H.; Wang, F.; Jiang, J.; Cheng, L.; Zhang, H.; Zhang, G. In vivo anti-inflammatory activity of Liquidambar formosana Hance infructescence extract. Trop. J. Pharm. Res. 2017, 16, 2403–2410. [Google Scholar] [CrossRef]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous silica nanoparticles in medicine—Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef]
- Vasanthavada, M.; Tong, W.-Q.; Joshi, Y.; Kislalioglu, M.S. Phase Behavior of Amorphous Molecular Dispersions I: Determination of the Degree and Mechanism of Solid Solubility. Pharm. Res. 2004, 21, 1598–1606. [Google Scholar] [CrossRef]
- Karekar, P.; Vyas, V.; Shah, M.; Sancheti, P.; Pore, Y. Physicochemical investigation of the solid dispersion systems of etoricoxib with poloxamer 188. Pharm. Dev. Technol. 2009, 14, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Savjani, J. Co-crystallization: An approach to improve the performance characteristics of active pharmaceutical ingredients. Asian J. Pharm. 2015, 9, 147–151. [Google Scholar] [CrossRef]
- Zi, P.; Zhang, C.; Ju, C.; Su, Z.; Bao, Y.; Gao, J.; Sun, J.; Lu, J.; Zhang, C. Solubility and bioavailability enhancement study of lopinavir solid dispersion matrixed with a polymeric surfactant—Soluplus. Eur. J. Pharm. Sci. 2019, 134, 233–245. [Google Scholar] [CrossRef]
- Bahl, D.; Hudak, J.; Bogner, R.H. Comparison of the Ability of Various Pharmaceutical Silicates to Amorphize and Enhance Dissolution of Indomethacin Upon Co-grinding. Pharm. Dev. Technol. 2008, 13, 255–269. [Google Scholar] [CrossRef]
- Soliman, M.; Khan, M. Preparation and in vitro characterization of a semi-solid dispersion of flurbiprofen with Gelucire 44/14 and Labrasol. Die Pharm. -Int. J. Pharm. Sci. 2005, 60, 288–293. [Google Scholar]
- Mehmood, Y.; Khan, I.U.; Shahzad, Y.; Yousaf, A.M.; Irfan, M.; Khalid, S.H.; Asghar, S.; Rasul, A.; Khan, N.R. Mesoporous Silica Nanoparticles-Based Bilayer Tablets: A New Strategy for Co-delivery of Velpatasvir and Sofosbuvir. Lat. Am. J. Pharm. 2022, 41, 283–293. [Google Scholar]
- Gupta, M.K.; Tseng, Y.-C.; Goldman, D.; Bogner, R.H. Hydrogen Bonding with Adsorbent During Storage Governs Drug Dissolution from Solid-Dispersion Granules. Pharm. Res. 2002, 19, 1663–1672. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Lee, B.-J.; Oh, D.H.; Kim, J.O.; Hong, M.J.; Jee, J.-P.; Kim, J.A.; Yoo, B.K.; Woo, J.S.; Yong, C.S.; et al. Enhanced oral bioavailability of dexibuprofen by a novel solid Self-emulsifying drug delivery system (SEDDS). Eur. J. Pharm. Biopharm. 2009, 72, 539–545. [Google Scholar] [CrossRef]
- Sireesha, M. Research on Formulation and Evaluation of Lipid Based Solid Dispersions of Lafutidine. J. Sci. Bioavailab 2017, 1, 2. [Google Scholar]
- Munir, R.; Hadi, A.; Khan, S.-U.; Asghar, S.; Irfan, M.; Khan, I.U.; Hameed, M.; Inam, S.; Islam, N.; Hassan, S.F.; et al. Solubility and Dissolution Enhancement of Dexibuprofen with Hydroxypropylbetacyclodextrin (HPβCD) and Poloxamers (188/407) Inclusion Complexes: Preparation and In Vitro Characterization. Polymers 2022, 14, 579. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, J.; Zhang, F.; Han, X.; Han, L.; Yang, M.; Zou, W. Preparation and evaluation of andrographolide solid dispersion vectored by silicon dioxide. Pharmacogn. Mag. 2016, 12 (Suppl. S2), S245. [Google Scholar] [PubMed]
- Giraldo, L.; López, B.; Pérez, L.; Urrego, S.; Sierra, L.; Mesa, M. Mesoporous silica applications. In Proceedings of the Macromolecular Symposia, Rio de Janeiro, Brazil; 2007; pp. 129–141. [Google Scholar]
- Munir, R.; Mashood, U.; Asgher, S.; Khan, I.U.; Irfan, M.; Inam, S.; Islam, N.; Ajaz, N.; Hassan, S.F.; Anwar, S. Solubility and dissolution enhancement of dexibuprofen by inclusion complexation with cyclodextrin. Lat. Am. J. Pharm. 2022, 41, 235–243. [Google Scholar]
- Tran, P.; Park, J.-S. Formulation of solid dispersion to improve dissolution and oral bioavailability of poorly soluble dexibuprofen. Pharm. Dev. Technol. 2021, 26, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Limnell, T.; Santos, H.A.; Mäkilä, E.; Heikkilä, T.; Salonen, J.; Murzin, D.Y.; Kumar, N.; Laaksonen, T.; Peltonen, L.; Hirvonen, J. Drug delivery formulations of ordered and nonordered mesoporous silica: Comparison of three drug loading methods. J. Pharm. Sci. 2011, 100, 3294–3306. [Google Scholar] [CrossRef]
- Fernández-Núñez, M.; Zorrilla, D.; Montes, A.; Mosquera, M.J. Ibuprofen Loading in Surfactant-Templated Silica: Role of the Solvent According to the Polarizable Continuum Model. J. Phys. Chem. A 2009, 113, 11367–11375. [Google Scholar] [CrossRef] [PubMed]
- Abuzara, S.M.; Hyun, S.-M.; Kim, J.-H.; Park, H.J.; Kim, M.-S.; Park, J.-S.; Hwang, S.-J. Enhancing the solubility and bioavailability of poorly water-soluble drugs using supercritical antisolvent (SAS) process. Int. J. Pharm. 2018, 538, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Miyoshi, K.; Ida, Y. Solubilization behavior of poorly soluble drugs with combined use of Gelucire 44/14 and cosolvent. J. Pharm. Sci. 2004, 93, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.; Papp, M.; Park, K.; Pinal, R. Hydrotropic Solubilization of Poorly Water-Soluble Drugs. J. Pharm. Sci. 2010, 99, 3953–3965. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Gupte, A. Mesoporous silica as a carrier for amorphous solid dispersion. arXiv preprint 2017, arXiv:1707.00036. [Google Scholar] [CrossRef] [Green Version]
- Pardhi, V.P.; Jain, K. Impact of binary/ternary solid dispersion utilizing poloxamer 188 and TPGS to improve pharmaceutical attributes of bedaquiline fumarate. J. Drug Deliv. Sci. Technol. 2021, 62, 102349. [Google Scholar] [CrossRef]
- Yıldırım, T.; Eylen, A.; Lule, S.; Erdener, S.E.; Vural, A.; Karatas, H.; Ozveren, M.F.; Dalkara, T.; Gursoy-Ozdemir, Y. Poloxamer-188 and citicoline provide neuronal membrane integrity and protect membrane stability in cortical spreading depression. Int. J. Neurosci. 2014, 125, 941–946. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, S.A.; Rizk, S.M.; Shahin, N.N. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem. Interactions 2015, 229, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Fulton, A.M. Selective cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res 2002, 62, 2343–2346. [Google Scholar]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Accounts Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, U.A.; LAlaofi, A.; Awan, Z.A.; Alqarni, H.M.; Alhakamy, N.A. Optimization of Thymoquinone-Loaded Coconut Oil Nanostructured Lipid Carriers for the Management of Ethanol-Induced Ulcer. AAPS PharmSciTech 2020, 21, 1–10. [Google Scholar]
- Chime, S.A.; Akpa, P.A.; Ugwuanyi, C.C.; Attama, A.A. Anti-Inflammatory and Gastroprotective Properties of Aspirin—Entrapped Solid Lipid Microparticles. Recent Patents Inflamm. Allergy Drug Discov. 2020, 14, 78–88. [Google Scholar] [CrossRef]
- Yao, H.; Yao, H.; Zhu, J.; Yu, J.; Zhang, L. Preparation and evaluation of a novel gastric floating alginate/poloxamer inner-porous beads using foam solution. Int. J. Pharm. 2012, 422, 211–219. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, X.; Fang, R.; Xiao, Z.; Jin, Y. 3D printed mold-based capsaicin candy for the treatment of oral ulcer. Int. J. Pharm. 2019, 568, 118517. [Google Scholar] [CrossRef]
- Nupane, S.; Srivastava, D.; Chaurasia, M.; Awasthi, H. Fabrication, In vitro and In vivo Characterization of Solid Dispersion- Microsphere Controlled Release System for Lornoxicam. Nanosci. Nanotechnology-Asia 2021, 11, 108–117. [Google Scholar] [CrossRef]
- Liang, H.; Li, Z.; Ren, Z.; Jia, Q.; Guo, L.; Li, S.; Zhang, H.; Hu, S.; Zhu, D.; Shen, D.; et al. Light-triggered NO-releasing nanoparticles for treating mice with liver fibrosis. Nano Res. 2020, 13, 2197–2202. [Google Scholar] [CrossRef]
- Ding, W.; Lin, H.; Hong, X.; Ji, D.; Wu, F. Poloxamer 188-mediated anti-inflammatory effect rescues cognitive deficits in paraquat and maneb-induced mouse model of Parkinson’s disease. Toxicology 2020, 436, 152437. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, H.; Bian, W.; Liu, Y.; Liu, X.; Ma, S.; Zheng, X.; Du, Z.; Zhang, K.; Ouyang, D. Molecular Interactions for the Curcumin-Polymer Complex with Enhanced Anti-Inflammatory Effects. Pharmaceutics 2019, 11, 442. [Google Scholar] [CrossRef] [PubMed]











| Sr. No. | Sample No. | Grade of Silica | Drug: Silica (w/w) |
|---|---|---|---|
| 1 | S1 | Syloid 244FP® | 1:1 |
| 2 | S2 | Syloid 244FP® | 1:2 |
| 3 | S3 | Syloid 244FP® | 1:4 |
| 4 | S4 | Syloid AL1FP® | 1:1 |
| 5 | S5 | Syloid AL1FP® | 1:2 |
| 6 | S6 | Syloid AL1FP® | 1:4 |
| 7 | S7 | Syloid XDP3150® | 1:1 |
| 8 | S8 | Syloid XDP3150® | 1:2 |
| 9 | S9 | Syloid XDP3150® | 1:4 |
| Sr. No. | Sample No. | Grade of Silica | Ratio of Drug to Silica (w/w) | Ternary Carrier | Percentage of Ternary Carrier (w/w) |
|---|---|---|---|---|---|
| 1 | S10, S11, S12, S13, and S14 | Syloid 244FP® | 1:1 | Gelucire 44/14® | 5%, 10%, 20%, 40%, 80% |
| 2 | S15, S16, S17, S18, and S19 | Syloid 244FP® | 1:1 | Gelucire 48/16® | 5%, 10%, 20%, 40%, 80% |
| 3 | S20, S21, S22, S23, and S24 | Syloid 244FP® | 1:1 | Poloxamer 188® | 5%, 10%, 20%, 40%, 80% |
| 4 | S25, S26, S27, S28, and S29 | Syloid 244FP® | 1:1 | Soluplus® | 5%, 10%, 20%, 40%, 80% |
| Silica | Particle Size (µm) | Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| Syloid 244FP® | 2.5–3.7 | 379 | 17 | 1.6 |
| Syloid ALIFP® | 6.5–8.1 | 605 | 26 | 0.3 |
| Syloid XDP3150® | 110 | 320 | 200 | 1.7 |
| Sr. No. | Formulation | Solubility (mg/mL) | % Drug | % Yield | ||
|---|---|---|---|---|---|---|
| Distilled H₂O | pH 1.2 | pH 6.8 | ||||
| 1 | Dex | 0.029 ± 0.04 | 0.016 ± 0.03 | 0.13 ± 0.01 | - | - |
| 2 | S1 | 0.18 ± 0.09 | 0.028 ± 0.014 | 3.12 ± 0.06 | 97 | 91 |
| 3 | S2 | 0.22 ± 0.15 | 0.036 ± 0.003 | 2.95 ± 0.02 | 93 | 93 |
| 4 | S3 | 0.24 ± 0.18 | 0.059 ± 0.008 | 2.91 ± 0.04 | 92 | 96 |
| 5 | S4 | 0.06 ± 0.002 | 0.002 ± 0.009 | 0.59 ± 0.006 | 66 | 83 |
| 6 | S5 | 0.09 ± 0.004 | 0.005 ± 0.005 | 0.63 ± 0.002 | 81 | 84 |
| 7 | S6 | 0.10 ± 0.001 | 0.006 ± 0.002 | 0.72 ± 0.001 | 86 | 87 |
| 8 | S7 | 0.05 ± 0.002 | 0.003 ± 0.01 | 0.66 ± 0.03 | 65 | 94 |
| 9 | S8 | 0.072 ± 0.003 | 0.007 ± 0.03 | 0.60 ± 0.01 | 59 | 95 |
| 10 | S9 | 0.073 ± 0.001 | 0.008 ± 0.01 | 0.58 ± 0.02 | 56 | 96 |
| Sr. No. | Formulation | Solubility Studies (mg/mL) | Drug Content | % Yield | ||
|---|---|---|---|---|---|---|
| Distilled H₂O | pH 1.2 | pH 6.8 | ||||
| 1 | S10 | 0.064 ± 0.07 | 0.062 ± 0.02 | 1.031 ± 0.07 | 70% | 94.28% |
| 2 | S11 | 0.076 ± 0.12 | 0.057 ± 0.03 | 1.036 ± 0.04 | 91% | 94.54% |
| 3 | S12 | 0.087 ± 0.03 | 0.052 ± 0.01 | 1.063 ± 0.04 | 94% | 95.83% |
| 4 | S13 | 0.101 ± 0.03 | 0.045 ± 0.01 | 3.51 ± 0.02 | 96% | 96.42% |
| 5 | S15 | 0.104 ± 0.03 | 0.090 ± 0.04 | 0.98 ± 0.07 | 60% | 89.52% |
| 6 | S16 | 0.121 ± 0.05 | 0.088 ± 0.03 | 1.061 ± 0.08 | 73% | 90.00% |
| 7 | S17 | 0.125 ± 0.05 | 0.079 ± 0.02 | 1.168 ± 0.02 | 82% | 96.66% |
| 8 | S18 | 0.133 ± 0.01 | 0.068 ± 0.01 | 3.67 ± 0.01 | 97% | 97.86% |
| 9 | S20 | 0.117 ± 0.04 | 0.084 ± 0.04 | 1.019 ± 0.07 | 58% | 91.43% |
| 10 | S21 | 0.121 ± 0.15 | 0.069 ± 0.02 | 1.058 ± 0.11 | 76% | 92.73% |
| 11 | S22 | 0.132 ± 0.07 | 0.055 ± 0.01 | 1.174 ± 0.02 | 80% | 94.16% |
| 12 | S23 | 0.185 ± 0.02 | 0.052 ± 0.02 | 4.943 ± 0.01 | 91% | 95.71% |
| 13 | S25 | 0.112 ± 0.07 | 0.055 ± 0.05 | 1.074 ± 0.07 | 65% | 87.62% |
| 14 | S26 | 0.126 ± 0.18 | 0.043 ± 0.01 | 1.090 ± 0.08 | 62% | 87.27% |
| 15 | S27 | 0.137 ± 0.04 | 0.039 ± 0.01 | 1.277 ± 0.01 | 56% | 84.16% |
| 16 | S28 | 0.141 ± 0.01 | 0.035 ± 0.02 | 2.303 ± 0.01 | 47% | 82.86% |
| Sr. No. | Samples | Bulk Density gm/mL | Tapped Density gm/mL | Carr’s Index% | Hausner’s Ratio | Angle of Repose (θ) | Remarks |
|---|---|---|---|---|---|---|---|
| 1 | Dex | 0.170 | 0.257 | 33.85 | 1.51 | 56 | Very poor |
| 2 | S1 | 0.234 | 0.270 | 13.33 | 1.15 | 31 | Good |
| 3 | S18 | 0.239 | 0.266 | 10.15 | 1.11 | 26 | Excellent |
| 4 | S23 | 0.234 | 0.259 | 9.65 | 1.10 | 23 | Excellent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asim, M.; Nazir, M.; Chauhdary, Z.; Irfan, M.; Khalid, S.H.; Asghar, S.; Usra; Felimban, R.I.; Majrashi, M.A.; Hazzazi, M.S.; et al. Enhanced Solubility and Biological Activity of Dexibuprofen-Loaded Silica-Based Ternary Solid Dispersions. Pharmaceutics 2023, 15, 399. https://doi.org/10.3390/pharmaceutics15020399
Asim M, Nazir M, Chauhdary Z, Irfan M, Khalid SH, Asghar S, Usra, Felimban RI, Majrashi MA, Hazzazi MS, et al. Enhanced Solubility and Biological Activity of Dexibuprofen-Loaded Silica-Based Ternary Solid Dispersions. Pharmaceutics. 2023; 15(2):399. https://doi.org/10.3390/pharmaceutics15020399
Chicago/Turabian StyleAsim, Muhammad, Marriam Nazir, Zunera Chauhdary, Muhammad Irfan, Syed Haroon Khalid, Sajid Asghar, Usra, Raed I. Felimban, Mohammed A Majrashi, Mohannad S. Hazzazi, and et al. 2023. "Enhanced Solubility and Biological Activity of Dexibuprofen-Loaded Silica-Based Ternary Solid Dispersions" Pharmaceutics 15, no. 2: 399. https://doi.org/10.3390/pharmaceutics15020399
APA StyleAsim, M., Nazir, M., Chauhdary, Z., Irfan, M., Khalid, S. H., Asghar, S., Usra, Felimban, R. I., Majrashi, M. A., Hazzazi, M. S., Alissa, M., Qahl, S. H., Hussain, G., Rasul, A., Chatha, S. A. S., & Khan, I. U. (2023). Enhanced Solubility and Biological Activity of Dexibuprofen-Loaded Silica-Based Ternary Solid Dispersions. Pharmaceutics, 15(2), 399. https://doi.org/10.3390/pharmaceutics15020399









