A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future
Abstract
:1. Introduction
2. The Advantages of 3D Printing Technology in Pharmaceuticals
2.1. Personalized Medicine for Special Populations
2.2. Precise Control of Drug Release
2.3. Rapid Integration of Production
3. Principle of BJ-3DP Technology and Applications in the Pharmaceutical Industry
3.1. The Principle of BJ-3DP Technology
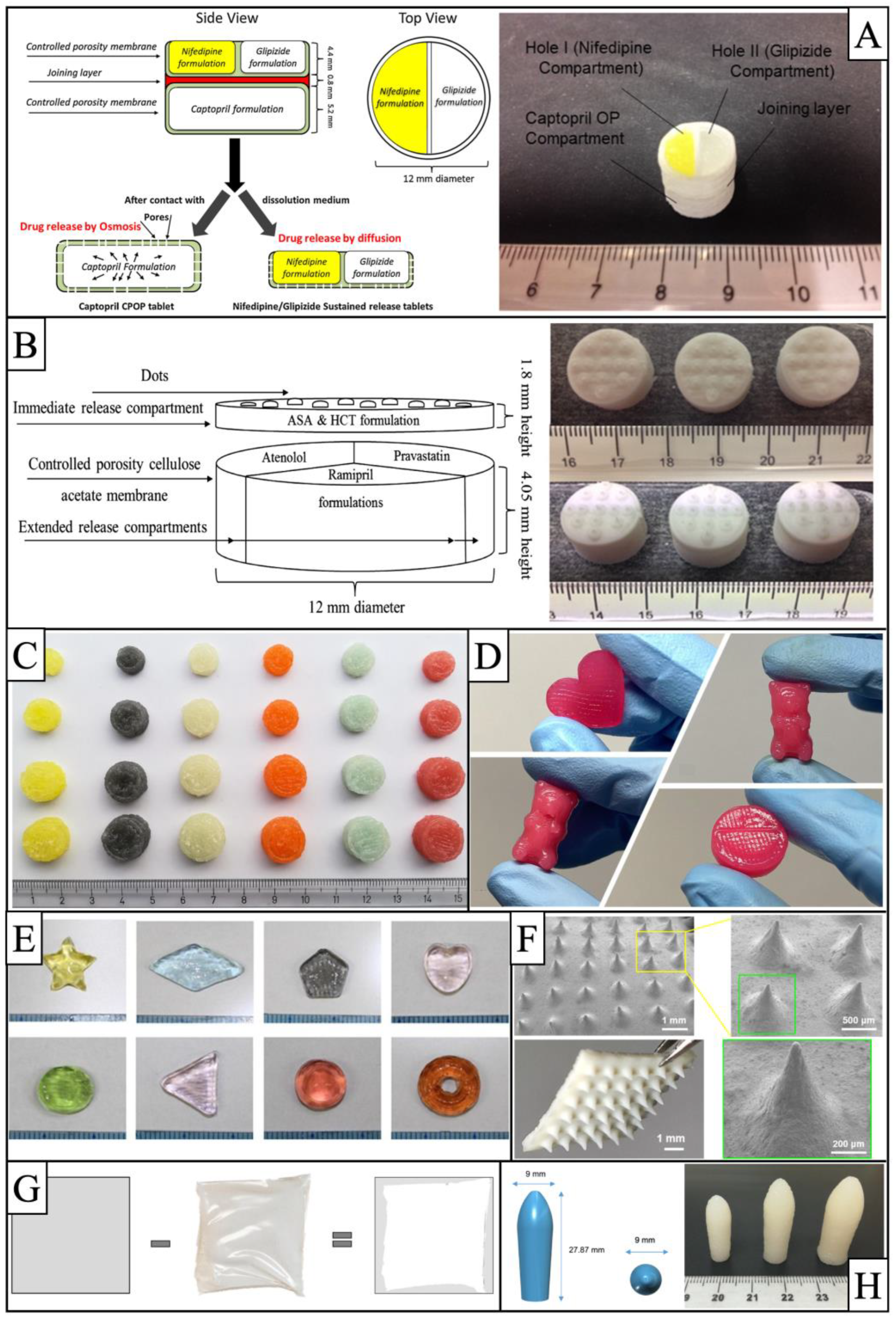
3.2. BJ-3DP Technology in Pharmaceuticals
| Dosage | Powder Bed | Ink | API and the Parts Where It Exist | Release Behavior | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Excipients | Binder | Solvent | Binder | Excipients | ||||||
| Rapid release preparations | Instant-dissolving Tablets | MCC, mannitol, colloidal silicon dioxide | PVP K30 | Isopropanol aqueous solution | PVP K30 | Glycerin | Levetiracetam (65%) | Powder | Dispersion (<30 s) and drug release (2.5 min > 90%) | [52] |
| Fast disintegration tablet, low-dose preparations | MCC | PVP | Water-ethanol | PVP | Polysorbate, sodium lauryl sulfate | Quinapril Hydrochloride (2%) | Powder | Drug release (30 min > 80%) | [32] | |
| Fast disintegration Tablet (hydrophobic API) | MCC, spray dried PVP (with clotrimazole) | PVP | Water-ethanol | PVP | Polysorbate, sodium lauryl sulfate | Clotrimazole (11%) | Powder | Drug release (30 min > 80%) | ||
| Fast disintegration | Lactose monohydrate | Kollidon VA64 | Water | Kollidon VA64 | Red liquid food dye | Indomethacin (10%) | Powder | Disintegration time (<10 s) | [53] | |
| Dispersive tablets | Lactose monohydrate, spray-dried lactose monohydrate, MCC, mannitol, silica | PVP K25 | Water-ethanol | Polyethylene glycol 1500 | - | Ketoprofen (20%) | Powder | Disintegration time (<25 s) | [54] | |
| Slow-release and controlled-release preparations | Tablets with near zero order release | Kollidon SR, HPMC | - | Drug containing area: water | PVP K17 | Tween 20 | Pseudoephedrine hydrochloride (50%) | Ink | Near constant release rate, 100% release at 8,12,16 h for different formulations | [55] |
| Drug-free area: 75% ethanol and 25% water | Triethyl citrate | |||||||||
| Zero-level release preparations | HPMC E50, colloidal silicon dioxide | PVP K30 | Aqueous 90% ethanol | Ethyl cellulose | - | Acetaminophen (80%) | Powder | 98% of drugs released linearly in 12 h | [56] | |
| Zero-level release preparations | Lactose, HPMC E50 | PVP K30 | Aqueous 75% ethanol | PVP K30 | Glycerol | Diclofenac sodium | Ink | 98% of drugs released linearly in 12 h | [57] | |
| Compound preparations | Compound dispersible tablets with multichamber structure | MCC, mannitol, colloidal silicon dioxide | PVP K30 | Isopropanol aqueous solution | PVP K30 | Glycerin | Levetiracetam (65%) in powder; pyridoxine hydrochloride (4.5%) in ink | Two drug release (both 5 min = 100%) | [58] | |
| Multi-drug combinations with compartments for compounding | calcium sulfate hemihydrate | - | Aqueous 5% ethanol | - | Tween 80 | - | 90% lisinopril released in 24 h, above 60% spironolactone released in 24 h | [59] | ||
| Poly (ethylene glycol) diacrylate, PEG200, ethanol | Lisinopril (40 mg/mL) in ink | |||||||||
| Spironolactone (20 mg/mL) in ink | ||||||||||
| Scaffold | Bone scaffold | β-tricalcium phosphate (β-TCP), Fe2O3, SiO2 | - | - | - | - | [60] | |||
| Bone scaffold | β-TCP, SiO2, ZnO | - | - | - | - | [61] | ||||
| Bone scaffold | β-TCP, MgO, ZnO | - | - | - | - | [62] | ||||
| Bone scaffold | Hydroxyapatite microsphere | - | Water | PVP, polyvinyl alcohol, polyacryl amide | - | - | [63] | |||
| Biodegradable composite scaffold | Calcium sulfate hemihydrate | 2-pyrrolidone | - | - | - | [64] | ||||
4. Principle of FDM Technology and Applications in the Pharmaceuticals
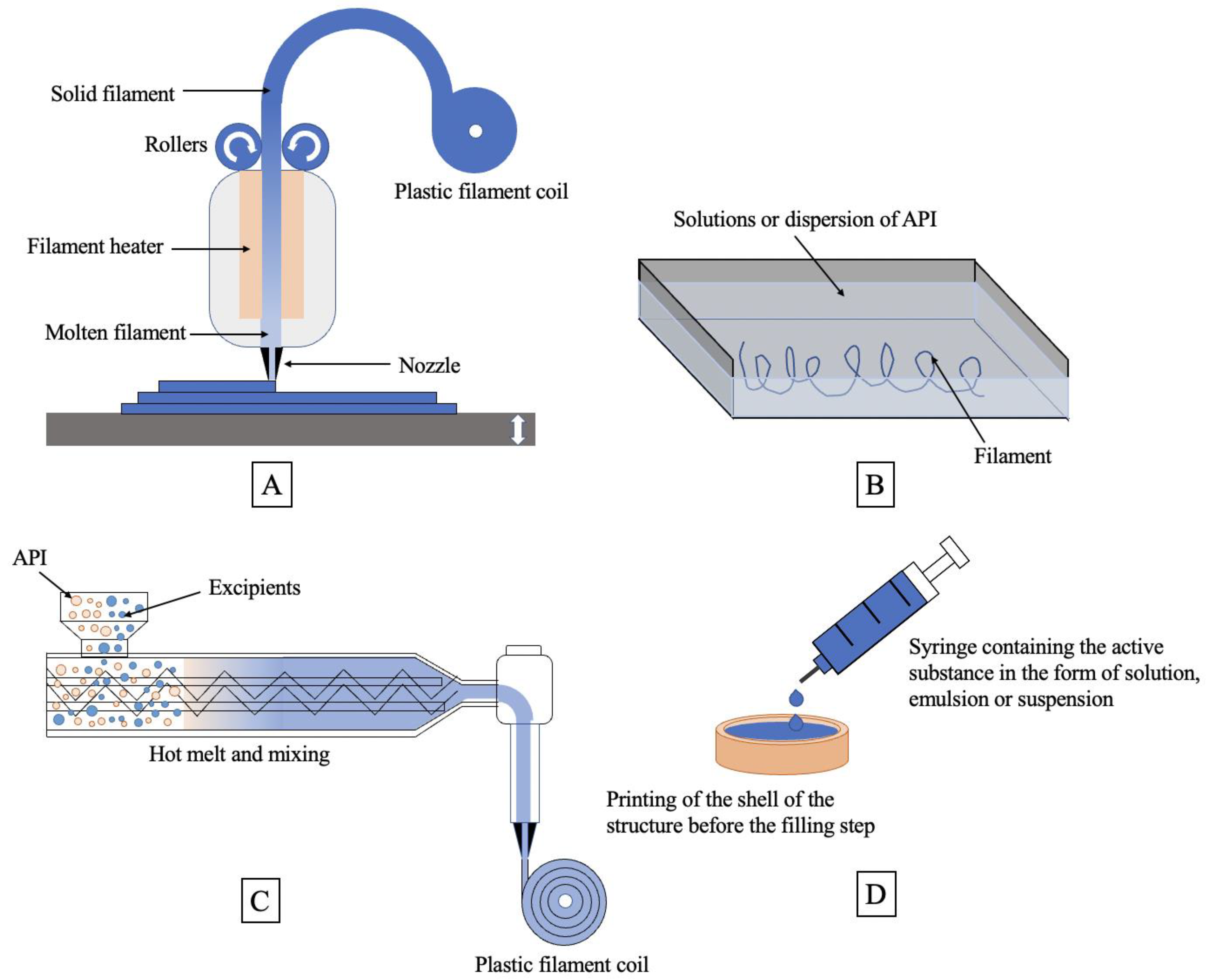
4.1. The Principle of FDM Technology
4.2. FDM Technology in Pharmaceuticals
5. Principle of SSE Technology and Applications in the Pharmaceutical Industry
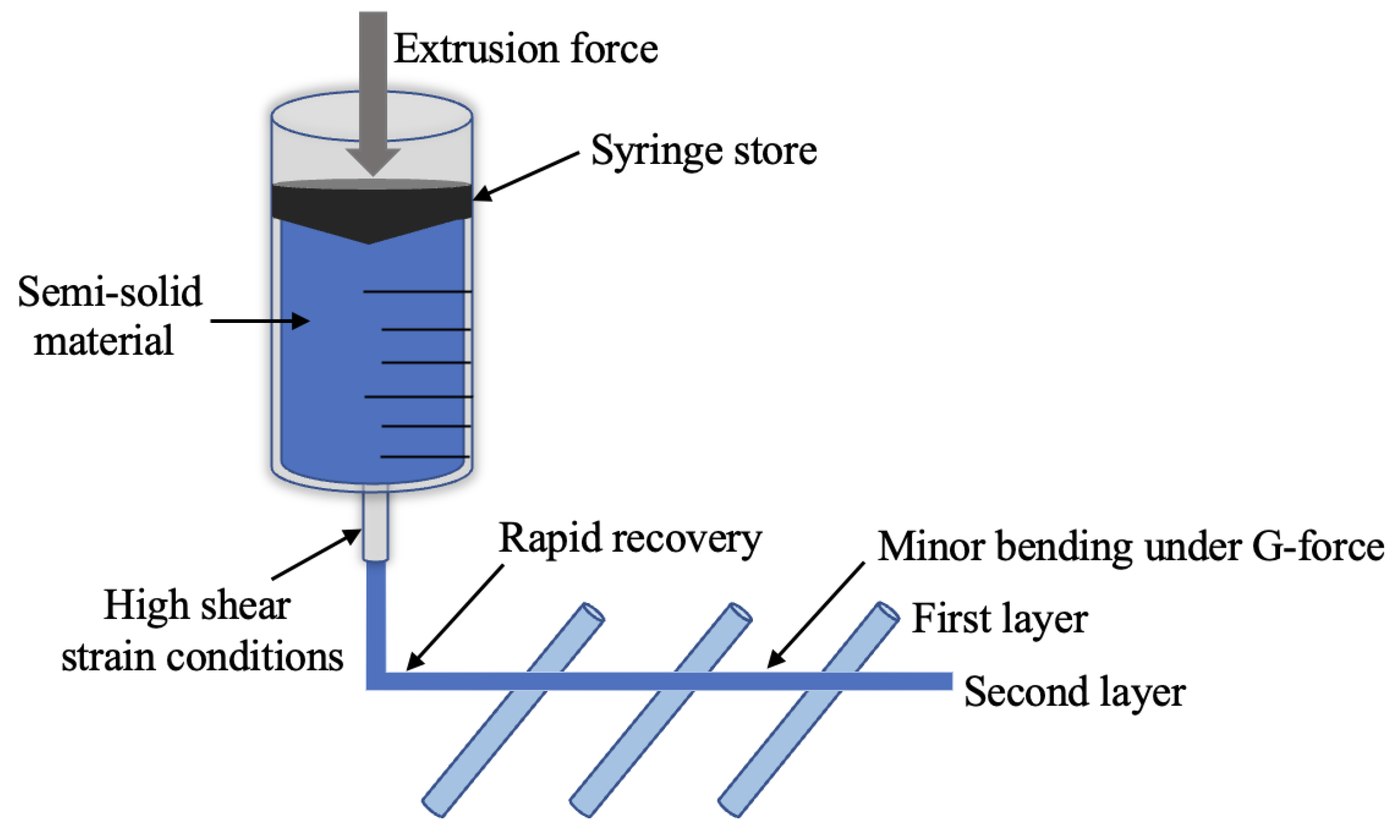
5.1. The Principle of SSE Technology
5.2. SSE Technology in Pharmaceuticals
6. Principle of MED Technology and Applications in the Pharmaceutical Industry
6.1. The Principle of MED Technology
6.2. MED Technology in Pharmaceuticals
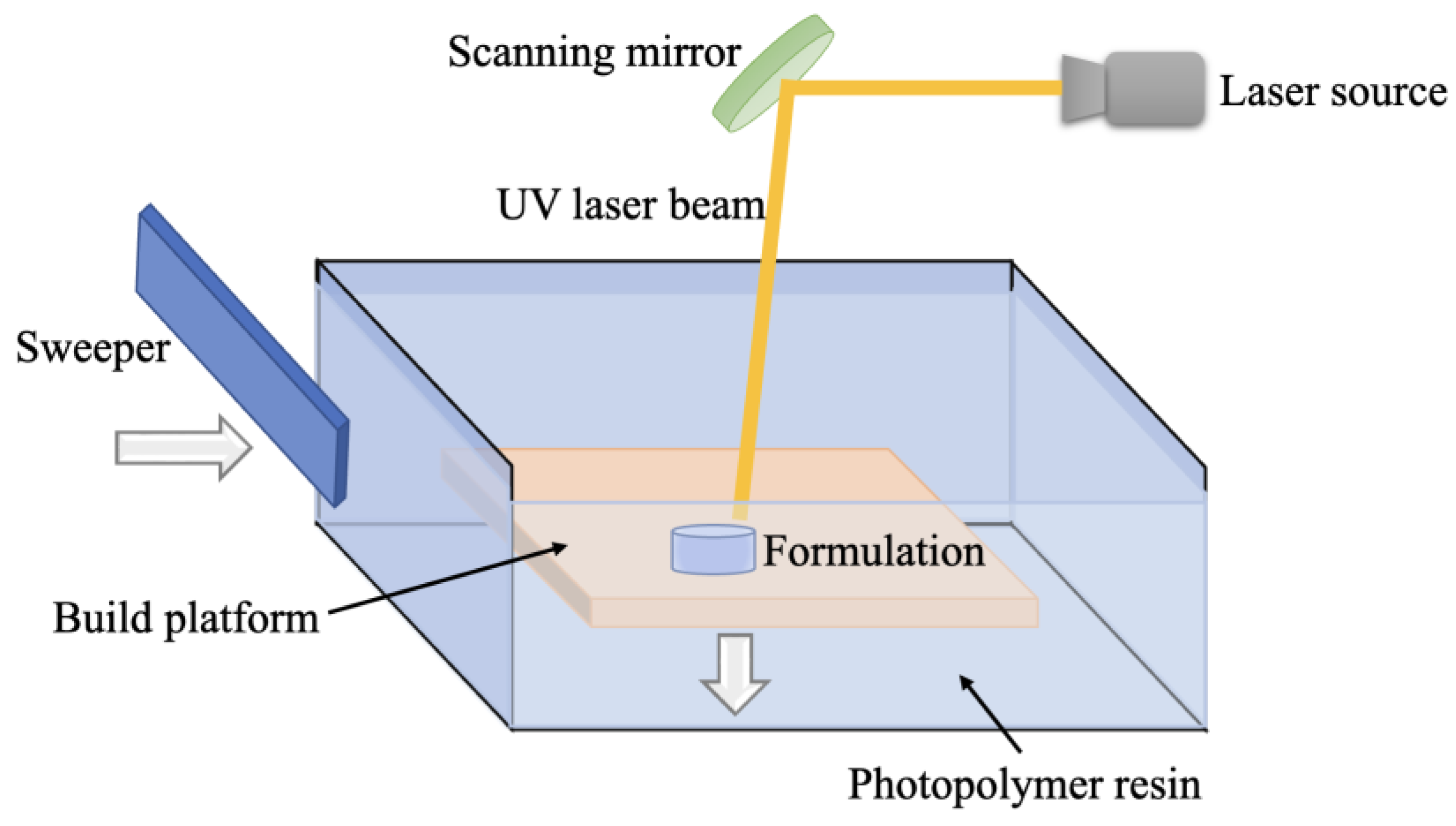
7. Principle of SLA Technology and Applications in the Pharmaceutical Industry
7.1. The Principle of SLA Technology
7.2. SLA Technology in Pharmaceuticals
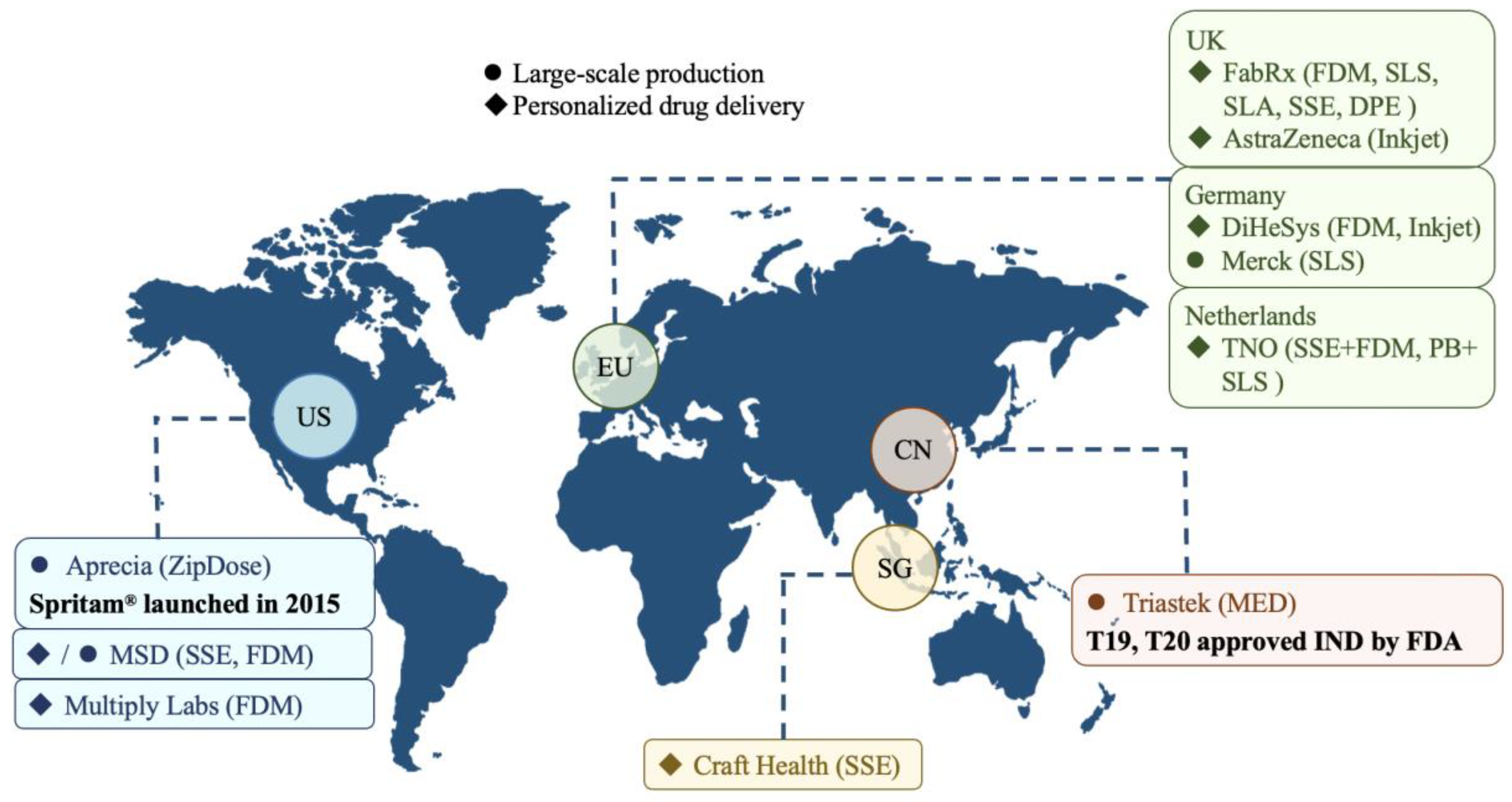
8. Progress in Commercialization of the 3D Printed Drug Industry
8.1. Large-Scale Production
8.2. Personalized Drug Delivery
9. Policies and Regulations in the Field of 3D Printed Drugs
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| BJ-3DP | Binder jet 3D printing |
| CDE | Center for drug evaluation |
| DPE | Direct powder extrusion |
| ETT | Emerging technology team |
| FDM | Fused deposition modeling |
| GMP | Good manufacturing practice |
| HME | Hot melt extrusion |
| HPMC | Hydroxypropyl methylcellulose |
| MCC | Microcrystalline cellulose |
| MED | Melt extrusion deposition |
| PB | Powder binding |
| PI | Photoinitiator |
| PVP | Polyvinylpyrrolidone |
| SA | Surface area |
| SLA | Stereo lithography appearance |
| SLS | Selective laser sintering |
| SSE | Semisolid extrusion |
| UK | United Kingdom |
| US | United States |
| UV | Ultraviolet |
References
- Bethany, C.G.; Jayda, L.E.; Sarah, Y.L.; Chengpeng, C.; Dana, M.S. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Belhabib, S.; Guessasma, S. Compression Performance of Hollow Structures: From Topology Optimisation to Design 3D Printing. Int. J. Mech. Sci. 2017, 133, 728–739. [Google Scholar] [CrossRef]
- Ishita, M.; Gurvinder, K.; Amir, S.; Aneesah, M.; Cato, T.L. Progress in 3D Bioprinting Technology for Tissue/Organ Regenerative Engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Jiménez, M.; Romero, L.; Domínguez, I.A.; del Mar Espinosa, M.; Domínguez, M. Additive Manufacturing Technologies: An Overview about 3D Printing Methods and Future Prospects. Complexity 2019, 2019, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Trenfield, S.J.; Madla, C.M.; Basit, A.W.; Gaisford, S. The Shape of Things to Come: Emerging Applications of 3D Printing in Healthcare. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer International Publishing: Cham, Switzerland, 2018; Volume 31, pp. 1–19. ISBN 978-3-319-90754-3. [Google Scholar]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Patel, V.; Shah, J. 3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems. AAPS PharmSciTech 2020, 21, 220. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Madla, C.; Hatton, G.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the Future: Recent Advances of 3D Printing in Drug Delivery and Healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [Green Version]
- Cader, H.K.; Rance, G.A.; Alexander, M.R.; Gonçalves, A.D.; Roberts, C.J.; Tuck, C.J.; Wildman, R.D. Water-Based 3D Inkjet Printing of an Oral Pharmaceutical Dosage Form. Int. J. Pharm. 2019, 564, 359–368. [Google Scholar] [CrossRef]
- Mancilla-De-la-Cruz, J.; Rodriguez-Salvador, M.; An, J.; Chua, C.K. Three-Dimensional Printing Technologies for Drug Delivery Applications: Processes, Materials, and Effects. Int. J. Bioprinting 2022, 8, 622. [Google Scholar] [CrossRef]
- Giangreco, N.P.; Elias, J.E.; Tatonetti, N.P. No Population Left behind: Improving Paediatric Drug Safety Using Informatics and Systems Biology. Br. J. Clin. Pharmacol. 2022, 88, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Itoh, H.; Matsuo, H.; Nakajima, K. Differences in Pharmaceutical Intervention Triggers for the Optimization of Medication by Patient Age: A University Hospital Study. Biol. Pharm. Bull. 2021, 44, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, O. Metabolism and Pharmacokinetics in Children and the Elderly. Expert Opin. Drug Metab. Toxicol. 2007, 3, 147–148. [Google Scholar] [CrossRef]
- Pratico, A.D.; Longo, L.; Mansueto, S.; Gozzo, L.; Barberi, I.; Tiralongo, V.; Salvo, V.; Falsaperla, R.; Vitaliti, G.; La Rosa, M.; et al. Off-Label Use of Drugs and Adverse Drug Reactions in Pediatric Units: A Prospective, Multicenter Study. Curr. Drug Saf. 2018, 13, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, G.; Patil, S.; Girdhar, V.; Chellappan, K.; Gupta, G.; Dua, K. 3D-Printing: An Emerging and a Revolutionary Technology in Pharmaceuticals. Panminerva Med. 2018, 60, 622. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Fullbrook, D.H.G.; Vilain, L.; Derrar, Y.; Nandi, U.; Grau, C.; Morales, A.; Hooper, G.; Hiezl, Z.; Douroumis, D. Personalised Tasted Masked Chewable 3D Printed Fruit-Chews for Paediatric Patients. Pharmaceutics 2021, 13, 1301. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Nandi, U.; Scoutaris, N.; Sanfo, K.; Alexander, B.; Gong, Y.; Hui, H.-W.; Kumar, S.; Douroumis, D. Personalised Paediatric Chewable Ibuprofen Tablets Fabricated Using 3D Micro-Extrusion Printing Technology. Int. J. Pharm. 2022, 626, 122135. [Google Scholar] [CrossRef]
- Preis, M.; Öblom, H. 3D-Printed Drugs for Children-Are We Ready Yet? AAPS PharmSciTech 2017, 18, 303–308. [Google Scholar] [CrossRef]
- Lafeber, I.; Ruijgrok, E.J.; Guchelaar, H.-J.; Schimmel, K.J.M. 3D Printing of Pediatric Medication: The End of Bad Tasting Oral Liquids?-A Scoping Review. Pharmaceutics 2022, 14, 416. [Google Scholar] [CrossRef]
- van Kampen, E.E.M.; Willemsteijn, L.; Ruijgrok, E.J. 3D Printing of Drugs: Expanding the Options for Child-Tailored Pharmacotherapy. Arch. Dis. Child 2021. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Duran Piñeiro, G.; Giraldez Montero, J.M.; Lamas Diaz, M.J.; Gonzalez Barcia, M.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L.; et al. Automated Therapy Preparation of Isoleucine Formulations Using 3D Printing for the Treatment of MSUD: First Single-Centre, Prospective, Crossover Study in Patients. Int. J. Pharm. 2019, 15, 118497. [Google Scholar] [CrossRef]
- Roulon, S.; Soulairol, I.; Lavastre, V.; Payre, N.; Cazes, M.; Delbreilh, L.; Alié, J. Production of Reproducible Filament Batches for the Fabrication of 3D Printed Oral Forms. Pharmaceutics 2021, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, M.; Chen, W.; Liu, H.; Tan, D.; Shen, S.; Lei, Y.; Xue, L. 3D Printing of Bioinspired Compartmentalized Capsular Structure for Controlled Drug Release. J. Zhejiang Univ. Sci. B 2021, 22, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Deng, F.; Wang, B.; Wu, Y.; Luo, Q.; Zuo, X.; Liu, X.; Cao, L.; Li, M.; Lu, H.; et al. Melt Extrusion Deposition (MEDTM) 3D Printing Technology—A Paradigm Shift in Design and Development of Modified Release Drug Products. Int. J. Pharm. 2021, 602, 120639. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-Solid Extrusion 3D Printing in Drug Delivery and Biomedicine: Personalised Solutions for Healthcare Challenges. J. Control. Release Off. J. Control. Release Soc. 2021, 332, 367–389. [Google Scholar] [CrossRef]
- Smith, D.M.; Kapoor, Y.; Klinzing, G.R.; Procopio, A.T. Pharmaceutical 3D Printing: Design and Qualification of a Single Step Print and Fill Capsule. Int. J. Pharm. 2018, 544, 21–30. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Madla, C.M.; Basit, A.W.; Gaisford, S. Binder Jet Printing in Pharmaceutical Manufacturing. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer International Publishing: Cham, Switzerland, 2018; Volume 31, pp. 41–54. ISBN 978-3-319-90754-3. [Google Scholar]
- Chen, G.; Xu, Y.; Chi Lip Kwok, P.; Kang, L. Pharmaceutical Applications of 3D Printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Ameeduzzafar; Alruwaili, N.K.; Rizwanullah, M.; Abbas Bukhari, S.N.; Amir, M.; Ahmed, M.M.; Fazil, M. 3D Printing Technology in Design of Pharmaceutical Products. Curr. Pharm. Des. 2018, 24, 5009–5018. [Google Scholar] [CrossRef]
- Kozakiewicz-Latała, M.; Nartowski, K.P.; Dominik, A.; Malec, K.; Gołkowska, A.M.; Złocińska, A.; Rusińska, M.; Szymczyk-Ziółkowska, P.; Ziółkowski, G.; Górniak, A.; et al. Binder Jetting 3D Printing of Challenging Medicines: From Low Dose Tablets to Hydrophobic Molecules. Eur. J. Pharm. Biopharm. 2022, 170, 144–159. [Google Scholar] [CrossRef]
- Prasad, L.K.; Smyth, H. 3D Printing Technologies for Drug Delivery: A Review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, V.; Bonn, D.; Martin, J.Y.; Vovelle, L. Controlling Droplet Deposition with Polymer Additives. Nature 2000, 405, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Fromm, J.E. Numerical Calculation of the Fluid Dynamics of Drop-on-Demand Jets. IBM J. Res. Dev. 1984, 28, 322–333. [Google Scholar] [CrossRef]
- Jang, D.; Kim, D.; Moon, J. Influence of Fluid Physical Properties on Ink-Jet Printability. Langmuir 2009, 25, 2629–2635. [Google Scholar] [CrossRef]
- Range, K.; Feuillebois, F. Influence of Surface Roughness on Liquid Drop Impact. J. Colloid Interface Sci. 1998, 203, 16–30. [Google Scholar] [CrossRef]
- Levin, B.Z.; Hobbs, P.V. Splashing of Water Drops on Solid and Wetted Surfaces: Hydrodynamics and Charge Separation. Philos. Trans. R. Soc. Lond. 1971, 269, 555–585. [Google Scholar] [CrossRef]
- Yarin, A.L. Drop Impact Dynamics: Splashing, Spreading, Receding, Bouncing…. Annu. Rev. Fluid Mech. 2006, 38, 159–192. [Google Scholar] [CrossRef]
- Stow, C.D.; Hadield, M.G. An Experimental Investigation of Fluid Flow Resulting from the Impact of a Water Drop with an Unyielding Dry Surface. Proc. R. Soc. Lond. A Math. Phys. Sci. 1981, 373, 419–441. [Google Scholar] [CrossRef]
- Stow, C.D.; Stainer, R.D. The Physical Products of a Splashing Water Drop. J. Meteorol. Soc. Jpn. 1977, 55, 518–532. [Google Scholar] [CrossRef] [Green Version]
- Scoutaris, N.; Alexander, M.R.; Gellert, P.R.; Roberts, C.J. Inkjet Printing as a Novel Medicine Formulation Technique. J. Control. Release 2011, 156, 179–185. [Google Scholar] [CrossRef]
- Hirshfield, L.; Giridhar, A.; Taylor, L.S.; Harris, M.T.; Reklaitis, G.V. Dropwise Additive Manufacturing of Pharmaceutical Products for Solvent-Based Dosage Forms. J. Pharm. Sci. 2014, 103, 496–506. [Google Scholar] [CrossRef]
- Meléndez, P.A.; Kane, K.M.; Ashvar, C.S.; Albrecht, M.; Smith, P.A. Thermal Inkjet Application in the Preparation of Oral Dosage Forms: Dispensing of Prednisolone Solutions and Polymorphic Characterization by Solid-State Spectroscopic Techniques. J. Pharm. Sci. 2008, 97, 2619–2636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Müllertz, A.; Rantanen, J. Structured Approach for Designing Drug-Loaded Solid Products by Binder Jetting 3D Printing. Eur. J. Pharm. Sci. 2022, 178, 106280. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.M.; Borland, S.W.; Giordano, R.A.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Solid Free-Form Fabrication of Drug Delivery Devices. J. Control. Release 1996, 40, 77–87. [Google Scholar] [CrossRef]
- Wang, Y.; Müllertz, A.; Rantanen, J. Additive Manufacturing of Solid Products for Oral Drug Delivery Using Binder Jetting Three-Dimensional Printing. AAPS PharmSciTech 2022, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, X.; Chen, R.; Li, J.; Gao, J.; Zhang, H.; Liu, N.; Gao, X.; Zheng, A. Innovative Color Jet 3D Printing of Levetiracetam Personalized Paediatric Preparations. Asian J. Pharm. Sci. 2021, 16, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Mehta, T.; Sansare, S.; Sharifi, L.; Ma, A.W.K.; Chaudhuri, B. Pharmaceutical Applications of Powder-Based Binder Jet 3D Printing Process—A Review. Adv. Drug Deliv. Rev. 2021, 177, 113943. [Google Scholar] [CrossRef]
- Brunello, G.; Sivolella, S.; Meneghello, R.; Ferroni, L.; Gardin, C.; Piattelli, A.; Zavan, B.; Bressan, E. Powder-Based 3D Printing for Bone Tissue Engineering. Biotechnol. Adv. 2016, 34, 740–753. [Google Scholar] [CrossRef]
- Lin, K.-F.; He, S.; Song, Y.; Wang, C.-M.; Gao, Y.; Li, J.-Q.; Tang, P.; Wang, Z.; Bi, L.; Pei, G.-X. Low-Temperature Additive Manufacturing of Biomimic Three-Dimensional Hydroxyapatite/Collagen Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 6905–6916. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Hong, X.; Han, X.; Liu, B.; Li, X.; Zhang, H.; Gao, J.; Liu, N.; Gao, X.; et al. Taste Masking Study Based on an Electronic Tongue: The Formulation Design of 3D Printed Levetiracetam Instant-Dissolving Tablets. Pharm. Res. 2021, 38, 831–842. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Jin, J.; Yan, J.; Dong, X.; Chaudhuri, B.; Nagapudi, K.; Ma, A.W.K. Development of a Pilot-Scale HuskyJet Binder Jet 3D Printer for Additive Manufacturing of Pharmaceutical Tablets. Int. J. Pharm. 2021, 605, 120791. [Google Scholar] [CrossRef] [PubMed]
- Kreft, K.; Lavrič, Z.; Stanić, T.; Perhavec, P.; Dreu, R. Influence of the Binder Jetting Process Parameters and Binder Liquid Composition on the Relevant Attributes of 3D-Printed Tablets. Pharmaceutics 2022, 14, 1568. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Tejwani Motwani, M.R.; Roach, W.J.; Kay, J.L.; Yoo, J.; Surprenant, H.L.; Monkhouse, D.C.; Pryor, T.J. Development of near Zero-Order Release Dosage Forms Using Three-Dimensional Printing (3-DP) Technology. Drug Dev. Ind. Pharm. 2006, 32, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Yang, X.L.; Huang, W.D.; Liu, J.; Wang, Y.G.; Xu, H. Tablets With Material Gradients Fabricated by Three-Dimensional Printing. J. Pharm. Sci. 2007, 96, 2446–2456. [Google Scholar] [CrossRef]
- Yu, D.; Liu, J.; Yang, Y.; Yang, X. Studies on Preparation of Controlled-Release Delivery Systems with Drug Gradients Using Three Dimensional Printing Technique. Zhongguo Yaoxue Zazhi (1989) 2006, 41, 1080–1083. [Google Scholar]
- Hong, X.; Han, X.; Li, X.; Li, J.; Wang, Z.; Zheng, A. Binder Jet 3D Printing of Compound LEV-PN Dispersible Tablets: An Innovative Approach for Fabricating Drug Systems with Multicompartmental Structures. Pharmaceutics 2021, 13, 1780. [Google Scholar] [CrossRef]
- Acosta-Vélez, G.; Linsley, C.; Zhu, T.; Wu, W.; Wu, B. Photocurable Bioinks for the 3D Pharming of Combination Therapies. Polymers 2018, 10, 1372. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Banerjee, D.; Robertson, S.; Vahabzadeh, S. Enhanced In Vivo Bone and Blood Vessel Formation by Iron Oxide and Silica Doped 3D Printed Tricalcium Phosphate Scaffolds. Ann. Biomed. Eng. 2018, 46, 1241–1253. [Google Scholar] [CrossRef]
- Nandi, S.K.; Fielding, G.; Banerjee, D.; Bandyopadhyay, A.; Bose, S. 3D Printed β-TCP Bone Tissue Engineering Scaffolds: Effects of Chemistry on in Vivo Biological Properties in a Rabbit Tibia Model. J. Mater. Res. 2018, 33, 1939–1947. [Google Scholar] [CrossRef]
- Ke, D.; Bose, S. Effects of Pore Distribution and Chemistry on Physical, Mechanical, and Biological Properties of Tricalcium Phosphate Scaffolds by Binder-Jet 3D Printing. Addit. Manuf. 2018, 22, 111–117. [Google Scholar] [CrossRef]
- Chai, W.; Wei, Q.; Yang, M.; Ji, K.; Guo, Y.; Wei, S.; Wang, Y. The Printability of Three Water Based Polymeric Binders and Their Effects on the Properties of 3D Printed Hydroxyapatite Bone Scaffold. Ceram. Int. 2020, 46, 6663–6671. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kim, J.; Han, G.; Kim, D.; Cheon, K.-H.; Lee, H.; Kim, H.-E.; Kim, Y.-J.; Jang, T.-S.; Jung, H.-D. 3D-Printed Biodegradable Composite Scaffolds with Significantly Enhanced Mechanical Properties via the Combination of Binder Jetting and Capillary Rise Infiltration Process. Addit. Manuf. 2021, 41, 101988. [Google Scholar] [CrossRef]
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. Fused Deposition Modeling (FDM), the New Asset for the Production of Tailored Medicines. J. Control. Release 2021, 330, 821–841. [Google Scholar] [CrossRef]
- Awad, A.; Gaisford, S.; Basit, A.W. Fused Deposition Modelling: Advances in Engineering and Medicine. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer International Publishing: Cham, Switzerland, 2018; Volume 31, pp. 107–132. ISBN 978-3-319-90754-3. [Google Scholar]
- Goole, J.; Amighi, K. 3D Printing in Pharmaceutics: A New Tool for Designing Customized Drug Delivery Systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Quodbach, J.; Bogdahn, M.; Breitkreutz, J.; Chamberlain, R.; Eggenreich, K.; Elia, A.G.; Gottschalk, N.; Gunkel-Grabole, G.; Hoffmann, L.; Kapote, D.; et al. Quality of FDM 3D Printed Medicines for Pediatrics: Considerations for Formulation Development, Filament Extrusion, Printing Process and Printer Design. Ther. Innov. Regul. Sci. 2022, 56, 910–928. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. A Graphical Review on the Escalation of Fused Deposition Modeling (FDM) 3D Printing in the Pharmaceutical Field. J. Pharm. Sci. 2020, 109, 2943–2957. [Google Scholar] [CrossRef]
- Korte, C.; Quodbach, J. Formulation Development and Process Analysis of Drug-Loaded Filaments Manufactured via Hot-Melt Extrusion for 3D-Printing of Medicines. Pharm. Dev. Technol. 2018, 23, 1117–1127. [Google Scholar] [CrossRef]
- Nober, C.; Manini, G.; Carlier, E.; Raquez, J.-M.; Benali, S.; Dubois, P.; Amighi, K.; Goole, J. Feasibility Study into the Potential Use of Fused-Deposition Modeling to Manufacture 3D-Printed Enteric Capsules in Compounding Pharmacies. Int. J. Pharm. 2019, 569, 118581. [Google Scholar] [CrossRef]
- Parulski, C.; Jennotte, O.; Lechanteur, A.; Evrard, B. Challenges of Fused Deposition Modeling 3D Printing in Pharmaceutical Applications: Where Are We Now? Adv. Drug Deliv. Rev. 2021, 175, 113810. [Google Scholar] [CrossRef]
- Wang, P.; Zou, B.; Xiao, H.; Ding, S.; Huang, C. Effects of Printing Parameters of Fused Deposition Modeling on Mechanical Properties, Surface Quality, and Microstructure of PEEK. J. Mater. Process. Technol. 2019, 271, 62–74. [Google Scholar] [CrossRef]
- Mania, S.; Ryl, J.; Jinn, J.-R.; Wang, Y.-J.; Michałowska, A.; Tylingo, R. The Production Possibility of the Antimicrobial Filaments by Co-Extrusion of the PLA Pellet with Chitosan Powder for FDM 3D Printing Technology. Polymers 2019, 11, 1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basa, B.; Jakab, G.; Kállai-Szabó, N.; Borbás, B.; Fülöp, V.; Balogh, E.; Antal, I. Evaluation of Biodegradable PVA-Based 3D Printed Carriers during Dissolution. Materials 2021, 14, 1350. [Google Scholar] [CrossRef] [PubMed]
- Alhijjaj, M.; Belton, P.; Qi, S. An Investigation into the Use of Polymer Blends to Improve the Printability of and Regulate Drug Release from Pharmaceutical Solid Dispersions Prepared via Fused Deposition Modeling (FDM) 3D Printing. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2016, 108, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.; Belton, P.; Nollenberger, K.; Clayden, N.; Reading, M.; Craig, D.Q.M. Characterisation and Prediction of Phase Separation in Hot-Melt Extruded Solid Dispersions: A Thermal, Microscopic and NMR Relaxometry Study. Pharm. Res. 2010, 27, 1869–1883. [Google Scholar] [CrossRef]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-Printed Oral Pharmaceutical Dosage Forms: Feedstock Filament Properties and Characterization for Fused Deposition Modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, M.E.; Swain, Z.R.; Banbury, C.R.; Phan, D.D.; Edwards, D.A. The Performance of the Hot End in a Plasticating 3D Printer. J. Rheol. 2017, 61, 229–236. [Google Scholar] [CrossRef]
- Ilyés, K.; Kovács, N.K.; Balogh, A.; Borbás, E.; Farkas, B.; Casian, T.; Marosi, G.; Tomuță, l.; Nagy, Z.K. The Applicability of Pharmaceutical Polymeric Blends for the Fused Deposition Modelling (FDM) 3D Technique: Material Considerations-Printability-Process Modulation, with Consecutive Effects on in Vitro Release, Stability and Degradation. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2019, 129, 110–123. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Bowland, C.C.; Naskar, A.K. A General Method to Improve 3D-Printability and Inter-Layer Adhesion in Lignin-Based Composites. Appl. Mater. Today 2018, 12, 138–152. [Google Scholar] [CrossRef]
- Syrlybayev, D.; Zharylkassyn, Z.; Seisekulova, A.; Akhmetov, M.; Perveen, A.; Talamona, D. Optimisation of Strength Properties of FDM Printed Parts-A Critical Review. Polymers 2021, 13, 1587. [Google Scholar] [CrossRef]
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3D/4D Printing of Polymers: Fused Deposition Modelling (FDM), Selective Laser Sintering (SLS), and Stereolithography (SLA). Polymers 2021, 13, 3101. [Google Scholar] [CrossRef]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient Acceptability of 3D Printed Medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D Scanning and 3D Printing as Innovative Technologies for Fabricating Personalized Topical Drug Delivery Systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-Specific 3D Scanned and 3D Printed Antimicrobial Polycaprolactone Wound Dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Firth, J.; Basit, A.W.; Gaisford, S. The Role of Semi-Solid Extrusion Printing in Clinical Practice. In 3D Printing of Pharmaceuticals; Springer International Publishing: Cham, Switzerland, 2018; Volume 31, pp. 133–151. ISBN 978-3-319-90754-3. [Google Scholar]
- Hospodiuk, M.; Moncal, K.K.; Dey, M.; Ozbolat, I.T. Extrusion-Based Biofabrication in Tissue Engineering and Regenerative Medicine. In 3D Printing and Biofabrication; Ovsianikov, A., Yoo, J., Mironov, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 255–281. ISBN 978-3-319-45443-6. [Google Scholar]
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of Release Modifiers to Modulate Drug Release from Fused Deposition Modelling (FDM) 3D Printed Tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef]
- Duty, C.; Ajinjeru, C.; Kishore, V.; Compton, B.; Hmeidat, N.; Chen, X.; Liu, P.; Hassen, A.A.; Lindahl, J.; Kunc, V. What Makes a Material Printable? A Viscoelastic Model for Extrusion-Based 3D Printing of Polymers. J. Manuf. Process. 2018, 35, 526–537. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, J.; Feng, C.; Shi, H.; Zhao, G.; Bian, Y. Rheological Behavior, 3D Printability and the Formation of Scaffolds with Cellulose Nanocrystals/Gelatin Hydrogels. J. Mater. Sci. 2020, 55, 15709–15725. [Google Scholar] [CrossRef]
- Zidan, A.; Alayoubi, A.; Coburn, J.; Asfari, S.; Ghammraoui, B.; Cruz, C.N.; Ashraf, M. Extrudability Analysis of Drug Loaded Pastes for 3D Printing of Modified Release Tablets. Int. J. Pharm. 2019, 554, 292–310. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D Printing of Five-in-One Dose Combination Polypill with Defined Immediate and Sustained Release Profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D Printing of Tablets Containing Multiple Drugs with Defined Release Profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Rodríguez-González, D.; Alejandro Fernández, M.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D Printed Gummies: Personalized Drug Dosage in a Safe and Appealing Way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D Printing of Gummy Drug Formulations Composed of Gelatin and an HPMC-Based Hydrogel for Pediatric Use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Kang, D.; Liu, B.; Zhang, H.; Wang, Z.; Gao, X.; Zheng, A. Feasibility of Developing Hospital Preparation by Semisolid Extrusion 3D Printing: Personalized Amlodipine Besylate Chewable Tablets. Pharm. Dev. Technol. 2022, 27, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid Extrusion 3D Printing of Propranolol Hydrochloride Gummy Chewable Tablets: An Innovative Approach to Prepare Personalized Medicine for Pediatrics. AAPS PharmSciTech 2022, 23, 166. [Google Scholar] [CrossRef]
- El Aita, I.; Breitkreutz, J.; Quodbach, J. On-Demand Manufacturing of Immediate Release Levetiracetam Tablets Using Pressure-Assisted Microsyringe Printing. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2019, 134, 29–36. [Google Scholar] [CrossRef]
- El Aita, L.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-Printing with Precise Layer-Wise Dose Adjustments for Paediatric Use via Pressure-Assisted Microsyringe Printing. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2020, 157, 59–65. [Google Scholar] [CrossRef]
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Jiang, X.; Shi, X. 3D Printing of Extended-Release Tablets of Theophylline Using Hydroxypropyl Methylcellulose (HPMC) Hydrogels. Int. J. Pharm. 2020, 591, 119983. [Google Scholar] [CrossRef]
- Sjöholm, E.; Sandler, N. Additive Manufacturing of Personalized Orodispersible Warfarin Films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Huang, H.; Li, J.; Liu, H.; Guo, Z.; Xue, L.; Liu, S.; Lei, Y. Assisted 3D Printing of Microneedle Patches for Minimally Invasive Glucose Control in Diabetes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111299. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology. Pharmaceutics 2020, 12, 56. [Google Scholar] [CrossRef] [Green Version]
- Seoane-Viaño, I.; Ong, J.J.; Luzardo-Álvarez, A.; González-Barcia, A.; Basit, A.; Otero-Espinar, F.J.; Goyanes, A. 3D Printed Tacrolimus Suppositories for the Treatment of Ulcerative Colitis. Asian J. Pharm. Sci. 2021, 16, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-Printed Microneedles in Biomedical Applications. iScience 2020, 24, 102012. [Google Scholar] [CrossRef]
- Martinez, P.R.; Basit, A.W.; Gaisford, S. The History, Developments and Opportunities of Stereolithography. 3D Print. Pharm. 2018, 31, 55–79. [Google Scholar]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical Characterization of 3D-Printed Polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Q.; Wang, J. A Review of Stereolithography: Processes and Systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Heller, C.; Schwentenwein, M.; Russmueller, G.; Varga, F.; Stampfl, J.; Liska, R. Vinyl Esters: Low Cytotoxicity Monomers for the Fabrication of Biocompatible 3D Scaffolds by Lithography Based Additive Manufacturing. J. Polym. Sci. A Polym. Chem. 2009, 47, 6941–6954. [Google Scholar] [CrossRef]
- Jiang, F.; Drummer, D. Curing Kinetic Analysis of Acrylate Photopolymer for Additive Manufacturing by Photo-DSC. Polymers 2020, 12, 1080. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, I.H.; Cho, D.-W. Laser Scanning Path Generation Considering Photopolymer Solidification in Micro-Stereolithography. Microsyst. Technol. 2005, 11, 158–167. [Google Scholar] [CrossRef]
- Lin, J.-T.; Lalevee, J.; Cheng, D.-C. A Critical Review for Synergic Kinetics and Strategies for Enhanced Photopolymerizations for 3D-Printing and Additive Manufacturing. Polymers 2021, 13, 2325. [Google Scholar] [CrossRef]
- Miedzińska, D.; Gieleta, R.; Popławski, A. Experimental Study on Influence of Curing Time on Strength Behavior of SLA-Printed Samples Loaded with Different Strain Rates. Materials 2020, 13, 5825. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D Printing with Polymers: Challenges among Expanding Options and Opportunities. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D Printing of Oral Modified-Release Dosage Forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of Geometry on the Drug Release Profiles of Stereolithographic (SLA) 3D-Printed Tablets. AAPS PharmSciTech 2018, 19, 3355–3361. [Google Scholar] [CrossRef]
- Yadav, V.; Sharma, P.K.; Murty, U.S.; Mohan, N.H.; Thomas, R.; Dwivedy, S.K.; Banerjee, S. 3D Printed Hollow Microneedles Array Using Stereolithography for Efficient Transdermal Delivery of Rifampicin. Int. J. Pharm. 2021, 605, 120815. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Scoutaris, N.; Economidou, S.N.; Giraud, C.; Chowdhry, B.Z.; Donnelly, R.F.; Douroumis, D. 3D Printed Microneedles for Anticancer Therapy of Skin Tumours. Mater. Sci. Eng. C 2020, 107, 110248. [Google Scholar] [CrossRef]
- Economidou, S.; Pissinato Pere, C.; Okereke, M.; Douroumis, D. Optimisation of Design and Manufacturing Parameters of 3D Printed Solid Microneedles for Improved Strength, Sharpness, and Drug Delivery. Micromachines 2021, 12, 117. [Google Scholar] [CrossRef]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.C.; Lamprou, D.A.; Douroumis, D. 3D Printed Microneedle Patches Using Stereolithography (SLA) for Intradermal Insulin Delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Economidou, S.N.; Uddin, M.J.; Marques, M.J.; Douroumis, D.; Sow, W.T.; Li, H.; Reid, A.; Windmill, J.F.C.; Podoleanu, A. A Novel 3D Printed Hollow Microneedle Microelectromechanical System for Controlled, Personalized Transdermal Drug Delivery. Addit. Manuf. 2021, 38, 101815. [Google Scholar] [CrossRef]
- Yeung, C.; Chen, S.; King, B.; Lin, H.; King, K.; Akhtar, F.; Diaz, G.; Wang, B.; Zhu, J.; Sun, W.; et al. A 3D-Printed Microfluidic-Enabled Hollow Microneedle Architecture for Transdermal Drug Delivery. Biomicrofluidics 2019, 13, 064125. [Google Scholar] [CrossRef] [Green Version]






| Types of 3D Printing Technology | Technical Characteristics | Advantages | Disadvantages | |||
|---|---|---|---|---|---|---|
| Preprocessing | Print Processing | Postprocessing | ||||
| BJ-3DP | Prefabricated powder bed or ink containing drug | Room temperature/heating | Removal and recovery of powders, drying of preparations |
|
| |
| Material Extrusion | FDM | Prefabricated filamentous containing drugs | Heating | Removal of support material/none |
|
|
| SSE | Prefabricated semi-solid materials containing drugs | Room temperature/heating | Drying/none |
|
| |
| MED | None | 25–250 °C | None |
|
| |
| SLA | Prefabricated polymer monomers containing drugs | Photopolymerization | Separation from unreacted polymer monomers and re-curing |
|
| |
| Microneedle Types | Resin Materials | API | Microneedle Shapes | Research Findings | Ref. |
|---|---|---|---|---|---|
| Coated microneedle | Biocompatible Class I resin: Dental SG | Insulin | Pyramid and flat spear shaped | Microneedles prepared by SLA technology penetrate better than metal microneedles; they help to lower glucose levels quickly and maintain them for longer than direct insulin injections. | [122] |
| Hollow microneedle | Class IIa biocompatible resin which is a mixture of methacrylic acid esters and photo initiator comprised of (in % w/w) >70% methacrylic oligomer, <20% glycol methacrylate, <5% pentamethyl-piperidyl sebacate, and <5% phosphine oxide | - | Syringe-shaped | The microfluidic microneedle device prepared by SLA technology allows for the homogeneous mixing of multiple fluids at different flow rates for transdermal delivery, making it particularly suitable for preclinical studies of multiple drug treatments. | [124] |
| Hollow microneedle | Biocompatible class I resin: methacrylic oligomers and phosphine oxides as photo initiators | Insulin | Cone-shaped with top and side openings | Combining 3D printing, microneedles, and microelectromechanical systems, a novel device for multifunctional and drug-controlled transdermal drug delivery, has been successfully prepared and its feasibility has been demonstrated by drug delivery. | [123] |
| Coated microneedle | Class I biocompatible resin | Cisplatin | Cross-shaped | Demonstrates the potential of 3D-printed microneedles for transdermal delivery of the anticancer drug cisplatin in nude mice, where cisplatin is sufficiently permeable to achieve high anticancer activity and tumor regression. | [120] |
| Hollow microneedle | Biocompatible Class I resin | Rifampicin | With subapical holes present in a quarter of the needle tip | Microneedles with subacute holes at the tip quarter were designed for transdermal drug delivery of the antibiotic rifampicin, with effective penetration and ideal bioavailability in SD rats. | [119] |
| Coated microneedle | Biocompatible Class I acrylic resin: Dental SG | Insulin | Cone, pyramid, spear-shaped | The effects of geometry and manufacturing parameters on the quality and performance of microneedles are investigated to optimize the ability of 3D-printed microneedles for drug delivery. | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. https://doi.org/10.3390/pharmaceutics15020416
Wang S, Chen X, Han X, Hong X, Li X, Zhang H, Li M, Wang Z, Zheng A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics. 2023; 15(2):416. https://doi.org/10.3390/pharmaceutics15020416
Chicago/Turabian StyleWang, Shanshan, Xuejun Chen, Xiaolu Han, Xiaoxuan Hong, Xiang Li, Hui Zhang, Meng Li, Zengming Wang, and Aiping Zheng. 2023. "A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future" Pharmaceutics 15, no. 2: 416. https://doi.org/10.3390/pharmaceutics15020416






