Mesoporous Silica-Based Nanoplatforms Are Theranostic Agents for the Treatment of Inflammatory Disorders
Abstract
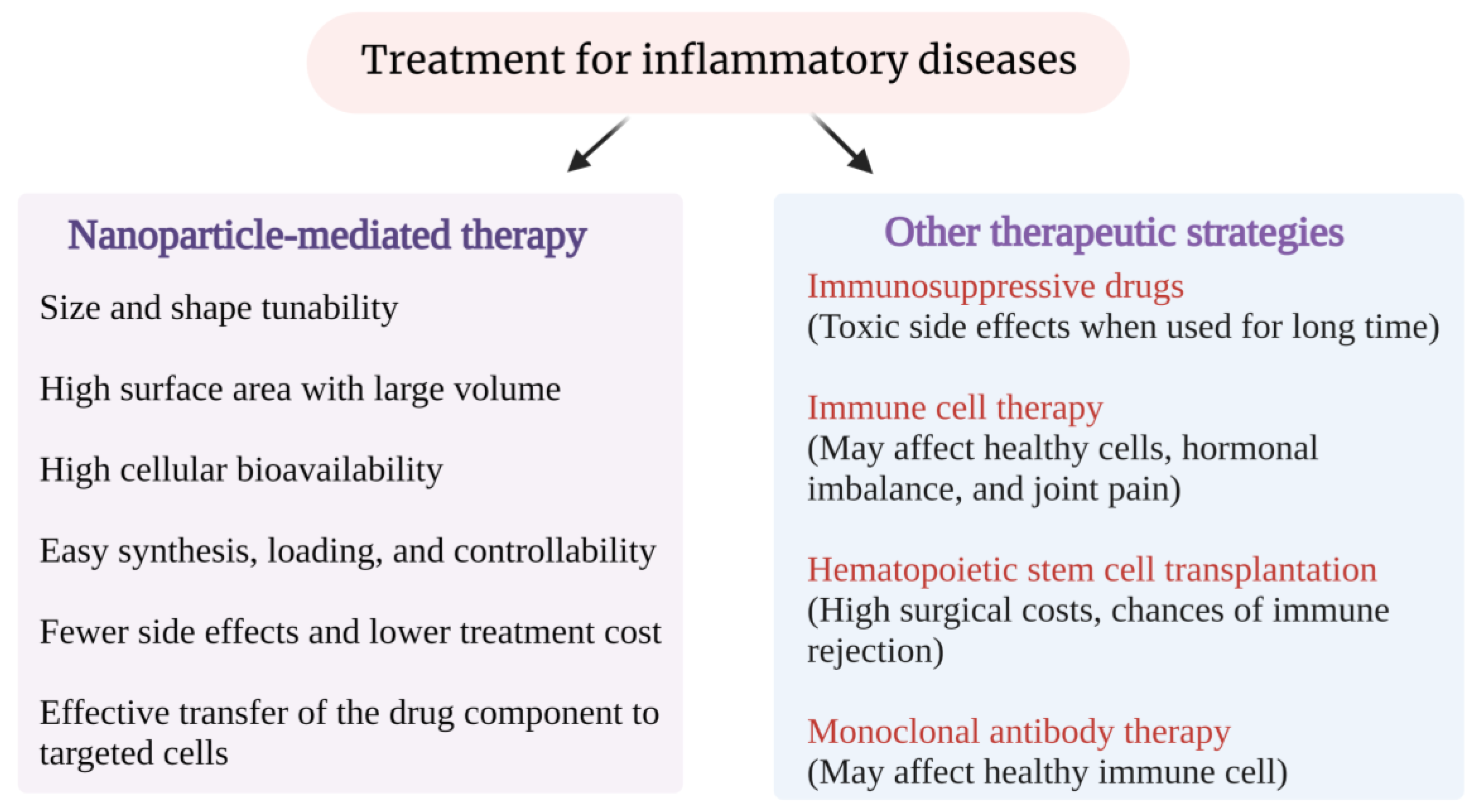
1. Introduction
1.1. Nanomaterial-Based Drug Delivery Systems
1.2. Nanoparticles and Their Role in Medicine
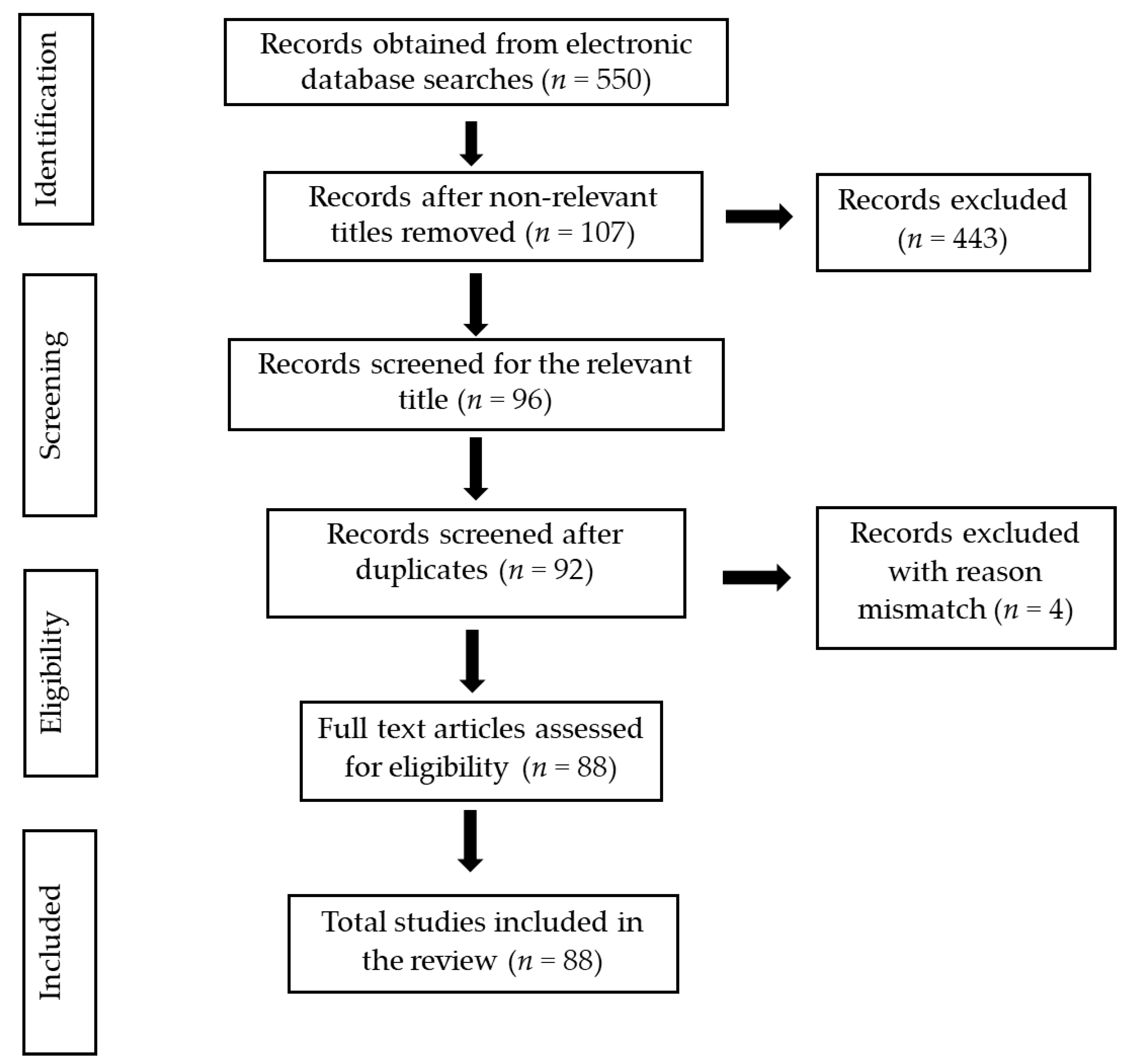
2. Methods
3. Mesoporous Silica Nanoparticles (MSN)
3.1. Types of MSNs
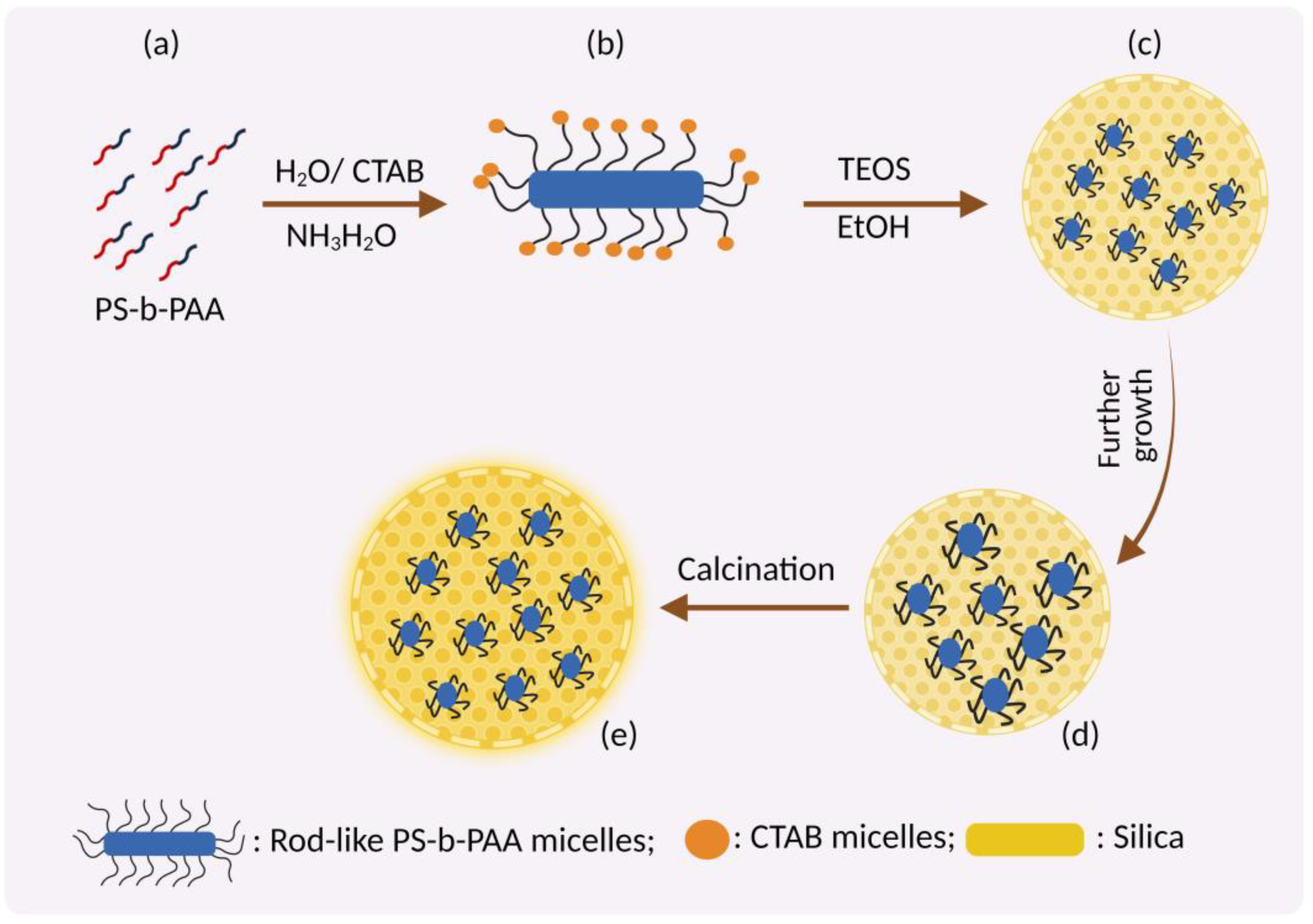
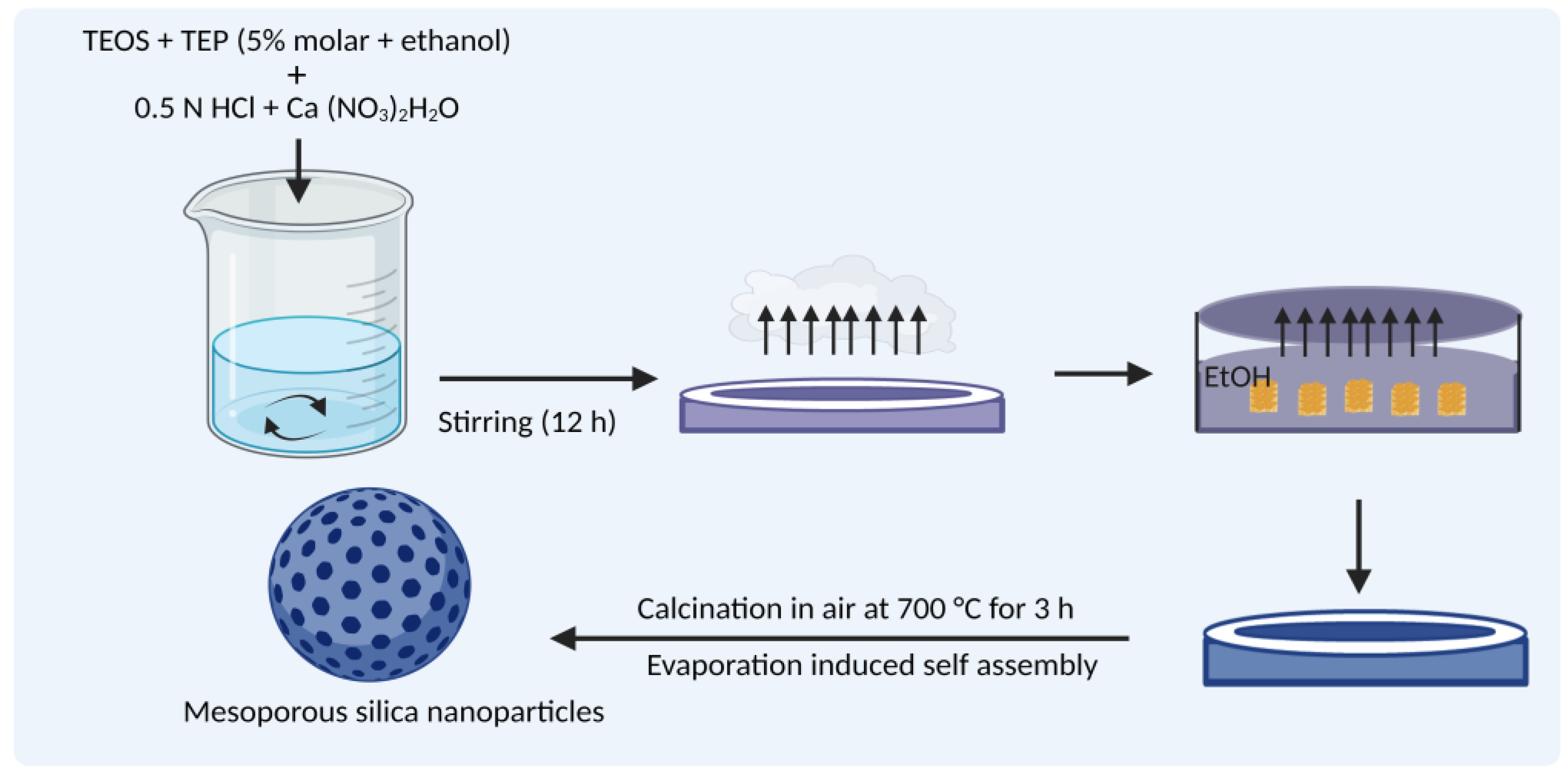
3.2. Fabrication of MSNs
- Cationic surfactants: Surfactants such as cetyl trimethyl ammonium chloride (CTAC), cetyl pyridinium chloride (CPyC), and cetyl tri methyl ammonium bromide (CTAB) with positively charged hydrophilic trimethyl ammonium/pyridinium groups as polar head and a sixteen-carbon hydrocarbon chain nonpolar tail act as a cationic surfactant.
- Anionic surfactant: Low-cost, eco-friendly anionic surfactants such as sodium salts of alkyl carboxylic acids, phosphoric acid, and sulfonic acid with a negatively charged polar head and hydrocarbon nonpolar tail are widely used as an anionic surfactant.
- Non-ionic surfactants: Non-ionic triblock copolymers such as alkyl poly (ethylene oxide) (PEO) oligomeric surfactants, poly (alkylene oxide) block copolymers, Triton X-100, polysorbate, and pluronic F 127 with a non-dissociable hydrophilic head which cannot ionize in aqueous solution act as a non-ionic surfactant.
- Amphoteric/zwitterionic surfactants: Includes surfactants with a net neutral charge such as sodium dodecyl benzene sulfonate (SDBS), sodium dodecyl sulphate (SDS), phospholipids, betaines/sulfobetaine, amino acids, propyl ortho-silicate (TPOS), trimethoxy silane (TMS) and sodium metasilicate (Na2SiO3).
3.3. Mechanism of Formation of MSN
3.3.1. Swelling Shrinking Mechanism
3.3.2. Sol-Gel Method
3.3.3. Evaporation-Induced Self-Assembly (EISA)
3.3.4. Removal of Surfactant after Synthesis
- For biomedical applications, the cytotoxic nature of surfactants affects living cells due to its interaction with the phospholipid layer of cells leading to lysis [55].
- Surfactants reduce the pore size and volume, affecting the drug-loading efficiency and release kinetics [56].
- The presence of surfactants affects surface functionalization.
3.3.5. Factors That Influence MSN Particle Synthesis
- (a)
- Rate of silica interaction and condensation
- (b)
- Assembly kinetics, nucleation, and growth rates
- (c)
- Charge of silica: The rate of silane hydrolysis and condensation of the siloxane bond depends strongly on the charge states.
- (d)
- pH of reaction mixture: The silica charge depends on the reaction mixture’s pH. Hydrolysis of the Si–OR bond in silanes occurs faster in an acidic/basic environment compared to a neutral solution. At a pH below the isoelectric point (IEP of silica—2.0), the silica is positively charged, and its charge density increases with a decrease in pH. In reaction mixtures with a pH above the IEP, the silica becomes negatively charged and the charge density increases with the pH. At pH 2–4, the negatively charged silicates interact with positively charged surfactants via electrostatic and hydrogen bond interactions. At pH 4–7, the negative charge density of silicate increases; hence interaction with the surfactant occurs only through the electrostatic force [62].
- (e)
- Co-surfactants used: Alcohols such as ethanol and butanol influence the pore size, shape, and flexibility when their concentration increases. An increase in co-surfactant concentration disrupts the spherical shape of MSNs, forming amorphous particles with disordered pore sizes.
- (f)
- Solvent used: Alcohols such as ethanol, propanol, butanol, and pentanol enhance the pore formation in mesopores. The channel rotations of mesoporous materials are also modified by alcohol. Removal of surfactants after the synthesis of MSNs is promoted by alcohol with a high boiling point which prevents aggregation of MSNs. Long-chain alcohols help MSNs transit from one phase to another.
- (g)
- Silica sources: Sodium silicates, colloidal solutions, and organosilanes form mesoporous silicate structures more rapidly than other precursors [63].
- (h)
- Temperature: The critical temperature for determining the final properties of MSNs is between 10 and 130 °C, within which 25 °C is the optimum temperature [64].
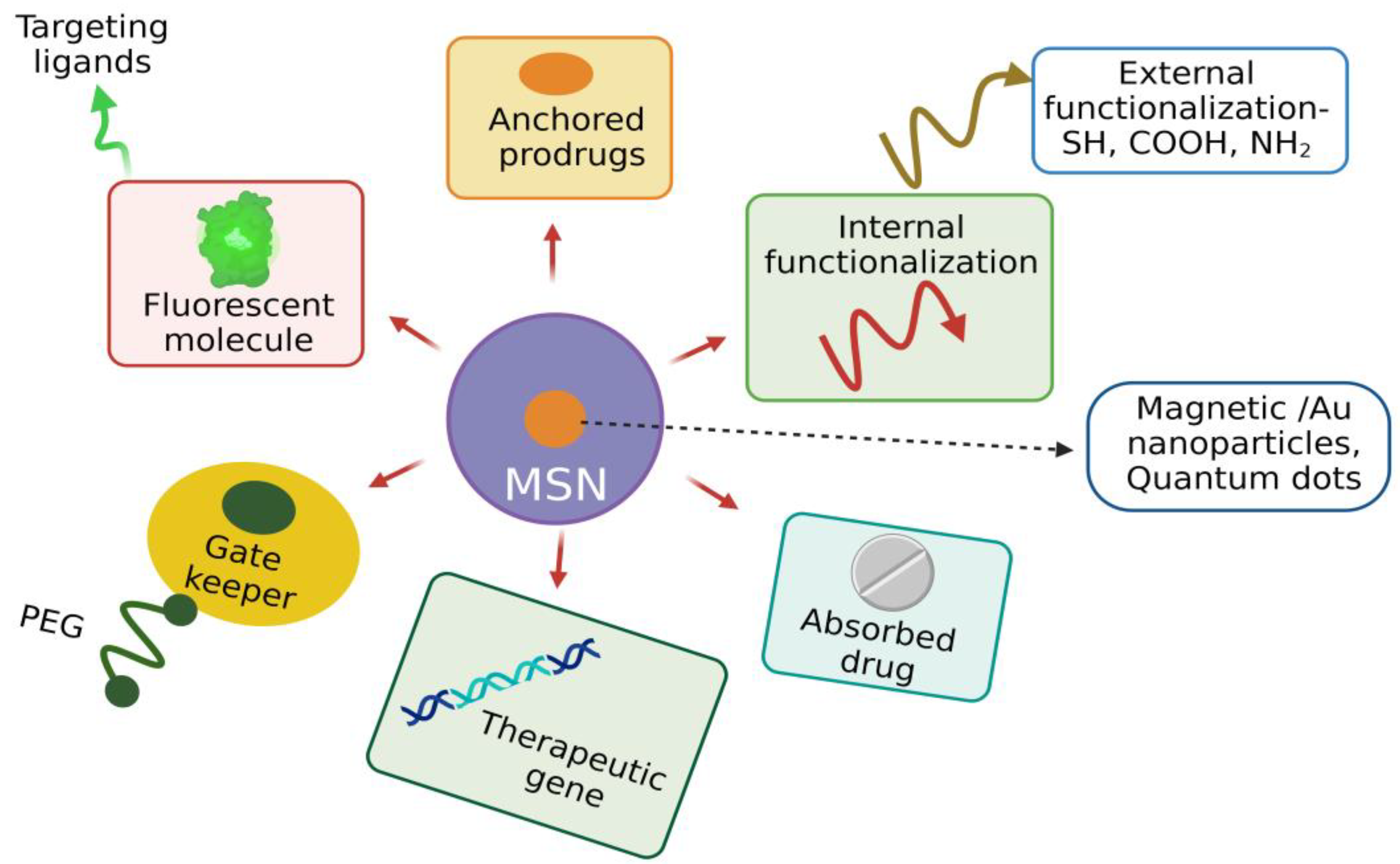
3.3.6. Functionalization of MSNs
3.4. Unique Structural Features of MSNs Suitable for Biomedical Applications
3.4.1. Ordered and Tunable Pore Structure
3.4.2. Biocompatibility
3.4.3. Biodistribution
3.4.4. Biodegradability and Clearance
4. MSNs: Theranostic Tool for Inflammatory Diseases
4.1. Anti-Inflammatory Properties of MSNs
4.2. MSNs in Airway Inflammation
4.3. MSNs in Neuroinflammation
4.4. MSNs in Inflammatory Bowel Disease
4.5. MSNs in Arthritis Inflammation
4.6. MSNs as a Theranostic Tool for other Diseases
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef] [PubMed]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. Methods Mol. Biol. 2018, 1803, 57–79. [Google Scholar] [PubMed]
- Lindahl, H.; Bryceson, Y.T. Neuroinflammation associated with inborn errors of immunity. Front. Immunol. 2022, 12, 827815. [Google Scholar] [CrossRef]
- Zhu, F.D.; Hu, Y.J.; Yu, L.; Zhou, X.G.; Wu, J.M.; Tang, Y.; Qin, D.L.; Fan, Q.Z.; Wu, A.G. Nanoparticles: A Hope for the Treatment of Inflammation in CNS. Front. Pharmacol. 2021, 12, 683935. [Google Scholar] [CrossRef]
- Cerqueira, S.R.; Ayad, N.G.; Lee, J.K. Neuroinflammation Treatment via Targeted Delivery of Nanoparticles. Front. Cell. Neurosci. 2020, 14, 576037. [Google Scholar] [CrossRef]
- Saha, R.N.; VasanthaKumar, S.; Bende, G.; Snehalatha, M. Nanoparticulate drug delivery systems for cancer chemotherapy. Mol. Membr. Biol. 2010, 27, 215–231. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Hong, S.H.; Choi, Y. Mesoporous silica-based nanoplatforms for the delivery of photodynamic therapy agents. J. Pharm. Investig. 2018, 48, 3–17. [Google Scholar] [CrossRef]
- Lu, A.H.; Schüth, F. Nanocasting: A versatile strategy for creating nanostructured porous materials. Adv. Mater. 2006, 18, 1793–1805. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef]
- Siefker, J.; Karande, P.; Coppens, M.O. Packaging biological cargoes in mesoporous materials: Opportunities for drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity, and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Guliants, V.V.; Carreon, M.A.; Lin, Y.S. Ordered mesoporous and macroporous inorganic films and membranes. J. Membr. Sci. 2004, 235, 53–72. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Mesoporous materials as elements of modern drug delivery systems for anti-inflammatory agents: A review of recent achievements. Pharmaceutics 2022, 14, 1542. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Mitophagy Induced by Metal Nanoparticles for Cancer Treatment. Pharmaceutics 2022, 14, 2275. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles - properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.D.; Doane, T.; Burda, C.; Basilion, J.P. Nanoparticles for imaging and treating brain cancer. Nanomedicine 2013, 8, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Foy, S.P.; Manthe, R.L.; Foy, S.T.; Dimitrijevic, S.; Krishnamurthy, N.; Labhasetwar, V. Optical imaging and magnetic field targeting of magnetic nanoparticles in tumors. ACS Nano 2010, 4, 5217–5224. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G. Nanomedicines for cancer therapy: AN update of FDA approved ad those under various stages of development. SOJ Pharm. Pharm. Sci. 2014, 1, 13. [Google Scholar]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Chen, S.; Zhang, H.; Zhou, J.; Fan, H.M.; Liang, X.J. Magnetic nanomaterials for advanced regenerative medicine: The promise and challenges. Adv. Mater. 2019, 31, 1804922. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Herbaj, S.; Redmond, J.; McCarthy, H.O.; Dunne, N.J. Calcium phosphate nanoparticles-based systems for RNAi delivery: Applications in bone tissue regeneration. Nanomaterials 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Sim, T.M.; Tarini, D.; Dheen, S.T.; Bay, B.H.; Srinivasan, D.K. Nanoparticle-based technology approaches to the management of neurological disorders. Int. J. Mol. Sci. 2020, 21, 6070. [Google Scholar] [CrossRef] [PubMed]
- Siegemund, T.; Paulke, B.R.; Schmiedel, H.; Bordag, N.; Hoffmann, A.; Harkany, T.; Tanila, H.; Kacza, J.; Härtig, W. Thioflavins released from nanoparticles target fibrillar amyloid beta in the hippocampus of APP/PS1 transgenic mice. Int. J. Dev. Neurosci. 2006, 24, 195–201. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Schlachetzki, F.; Zhang, Y.; Boado, R.J.; Pardridge, W.M. Gene therapy of the brain: The trans-vascular approach. Neurology 2004, 62, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Deeba, F.; Umer, F.; Nainan, D. Applications of nanoparticles in treatment of respiratory disorders. Life Sci. 2022, 3, 55–62. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Reiner, Ž.; Carbone, F.; Montecucco, F.; Sahebkar, A. The therapeutic potential of nanoparticles to reduce inflammation in Atherosclerosis. Biomolecules 2019, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef]
- Kumar, D.; Sailaja Chirravuri, S.V.; Shastri, N.R. Impact of surface area of silica particles on dissolution rate and oral bioavailability of poorly water-soluble drugs: A case study with aceclofenac. Int. J. Pharm. 2014, 461, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Sábio, R.M.; Meneguin, A.B.; Ribeiro, T.C.; Silva, R.R.; Chorilli, M. New insights towards mesoporous silica nanoparticles as a technological platform for chemotherapeutic drugs delivery. Int. J. Pharm. 2019, 564, 379–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luz, Z.; Goldfarb, D. EPR studies of the formation mechanism of the mesoporous materials MCM-41 and MCM-50. J. Phys. Chem. B. 1997, 101, 7087–7094. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B. 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Ordered mesoporous materials for drug delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Schumacher, K.; Ravikovitch, P.I.; Du Chesne, A.; Neimark, A.V.; Unger, K.K. Characterisation of MCM-48 materials. Langmuir 2000, 16, 4648–4654. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Ukmar, T.; Planinšek, O. Ordered mesoporous silicates as matrices for controlled release of drugs. Acta Pharm. 2010, 60, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Tadjarodi, A.; Jalalat, V.; Zare-Dorabei, R. Synthesis and characterization of functionalized SBA-15 mesoporous silica by N, N0 - bis(salicylidene)ethylenediamine schiff-base. J. Nanostruct. 2013, 3, 477–482. [Google Scholar]
- Jammaer, J.; Aerts, A.; D’Haen, J.; Seo, J.W.; Martens, J.A. Convenient synthesis of ordered mesoporous silica at room temperature and quasi-neutral pH. J. Mater. Chem. 2009, 19, 8290–8293. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Kim, S.-G.; Iskandar, F.; Okuyama, K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Heikkilä, T.; Salonen, J.; Tuura, J.; Hamdy, M.S.; Mul, G.; Kumar, N.; Salmi, T.; Murzin, D.Y.; Laitinen, L.; Kaukonen, A.M.; et al. Mesoporous silica material TUD-1 as a drug delivery system. Int. J. Pharm. 2007, 331, 133–138. [Google Scholar] [CrossRef]
- Grun, M.; Unger, K.K.; Matsumoto, A.; Tsutsumi, K. Novel Pathways for the Preparation of Mesoporous MCM-41 Materials: Control of Porosity and Morphology. Microporous Mesoporous Mater. 1999, 27, 207–216. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Zhi, Z.; Jiang, T.; Wang, S. Facile synthesis of 3D cubic mesoporous silica microspheres with a controllable pore size and their application for improved delivery of a water-insoluble drug. J. Colloid Interface Sci. 2011, 363, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, L.; Bonnyay, D.; Mahnke, K.; Rivera, M.; Nussenzweig, M.C.; Steinman, R.M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002, 196, 1627–1638. [Google Scholar] [CrossRef]
- Mirzaei, M.; Zarch, M.B.; Darroudi, M.; Sayyadi, K.; Keshavarz, S.T.; Sayyadi, J.; Fallah, A.; Maleki, H. Silica mesoporous structures: Effective nanocarriers in drug delivery and nanocatalysts. Appl. Sci. 2020, 10, 7533. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica nanoparticles—a versatile tool for the treatment of bacterial infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- Blin, J.L.; Impéror-Clerc, M. Mechanism of self-assembly in the synthesis of silica mesoporous materials: In situ studies by X-ray and neutron scattering. Chem. Soc. Rev. 2013, 42, 4071–4082. [Google Scholar] [CrossRef]
- Yi, Z.; Dumée, L.F.; Garvey, C.J.; Feng, C.; She, F.; Rookes, J.E.; Mudie, S.; Cahill, D.M.; Kong, L. A new insight into growth mechanism and kinetics of mesoporous silica nanoparticles by in situ small angle X-ray scattering. Langmuir 2015, 31, 8478–8487. [Google Scholar] [CrossRef] [PubMed]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of “sol–gel” chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Fontecave, T.; Boissiere, C.; Baccile, N.; Plou, F.J.; Sanchez, C. Using evaporation-induced self-assembly for the direct drug templating of therapeutic vectors with high loading fractions, tunable drug release, and controlled degradation. Chem. Mater. 2013, 25, 4671–4678. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, B.; Shang, Y.; Huang, X.; Dong, H.; Liu, H.; Chen, W.; Gui, R.; Li, J. Development of a nano-drug delivery system based on mesoporous silica and its anti-lymphoma activity. Appl. Nanosci. 2020, 10, 3431–3442. [Google Scholar] [CrossRef]
- Hoffmann, F.; Fröba, M. Vitalising porous inorganic silica networks with organic functions—PMOs and related hybrid materials. Chem. Soc. Rev. 2011, 40, 608–620. [Google Scholar] [CrossRef]
- Fuertes, A.B. Synthesis of ordered nanoporous carbons of tunable mesopore size by templating SBA-15 silica materials. Microporous Mesoporous Mater. 2004, 67, 273–281. [Google Scholar] [CrossRef]
- Yang, L.M.; Wang, Y.J.; Luo, G.S.; Dai, Y.Y. Simultaneous removal of copolymer template from SBA-15 in the crystallization process. Microporous Mesoporous Mater. 2005, 81, 107–114. [Google Scholar] [CrossRef]
- Büchel, G.; Denoyel, R.; Llewellyn, P.L.; Rouquerol, J. In situ surfactant removal from MCM-type mesostructures by ozone treatment. J. Mater. Chem. 2001, 11, 589–593. [Google Scholar] [CrossRef]
- Manet, S.; Schmitt, J.; Impéror-Clerc, M.; Zholobenko, V.; Durand, D.; Oliveira, C.L.; Pedersen, J.S.; Gervais, C.; Baccile, N.; Babonneau, F.; et al. Kinetics of the formation of 2D-hexagonal silica nanostructured materials by nonionic block copolymer templating in solution. J. Phys. Chem. B 2011, 115, 11330–11344. [Google Scholar] [CrossRef]
- Tella, J.O.; Adekoya, J.A.; Ajanaku, K.O. Mesoporous silica nanocarriers as drug delivery systems for anti-tubercular agents: A review. R. Soc. Open Sci. 2022, 9, 220013. [Google Scholar] [CrossRef]
- Yu, J.; Shi, J.L.; Chen, H.R.; Yan, J.N.; Yan, D.S. Effect of inorganic salt addition during synthesis on pore structure and hydrothermal stability of mesoporous silica. Microporous Mesoporous Mater. 2001, 46, 153–162. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Kleitz, F.; Marlow, F.; Stucky, G.D.; Schüth, F. Mesoporous silica fibers: Synthesis, internal structure, and growth kinetics. Chem. Mater. 2001, 13, 3587–3595. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Selvaraj, S. Mesoporous silica nanoparticles: Importance of surface modifications and its role in drug delivery. RSC Adv. 2014, 4, 14328–14334. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic– inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, Y.; Wu, D.; Sun, Y. A study of carboxylic-modified mesoporous silica in controlled delivery for drug famotidine. J. Solid State Chem. 2006, 179, 1513–1520. [Google Scholar] [CrossRef]
- Hartono, S.B.; Gu, W.; Kleitz, F.; Liu, J.; He, L.; Middelberg, A.P.; Yu, C.; Lu, G.Q.; Qiao, S.Z. Poly-L-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano 2012, 6, 2104–2117. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Chen, J.; Li, J.; Xu, Y.; Wu, D.; Sun, Y. Drug delivery from hydrophobic-modified mesoporous silicas: Control via modification level and site-selective modification. J. Solid State Chem. 2010, 183, 76–83. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanoparticles: Structural design and applications. J. Mater. Chem. 2010, 20, 7924–7937. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, L.; Xing, F.; Lin, H. Controlled synthesis of monodispersed mesoporous silica nanoparticles: Particle size tuning and formation mechanism investigation. Microporous Mesoporous Mater. 2016, 225, 238–244. [Google Scholar] [CrossRef]
- Radu, D.R.; Lai, C.Y.; Wiench, J.W.; Pruski, M.; Lin, V.S.Y. Gatekeeping layer effect: A poly (lactic acid)-coated mesoporous silica nanosphere-based fluorescence probe for detection of amino-containing neurotransmitters. J. Am. Chem. Soc. 2004, 126, 1640–1641. [Google Scholar] [CrossRef]
- Ye, F.; Laurent, S.; Fornara, A.; Astolfi, L.; Qin, J.; Roch, A.; Martini, A.; Toprak, M.S.; Muller, R.N.; Muhammed, M. Uniform mesoporous silica coated iron oxide nanoparticles as a highly efficient, nontoxic MRI T (2) contrast agent with tunable proton relaxivities. Contrast Media Mol. Imaging 2012, 7, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.; González, B.; Lozano, D.; Doadrio, A.L.; Colilla, M.; Izquierdo-Barba, I. Nanoantibiotics Based in Mesoporous Silica Nanoparticles: New Formulations for Bacterial Infection Treatment. Pharmaceutics 2021, 13, 2033. [Google Scholar] [CrossRef] [PubMed]
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; MacCuaig, W.M.; Jain, A.; Walters, K.B.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 1, 570. [Google Scholar] [CrossRef] [PubMed]
- Ahir, M.; Upadhyay, P.; Ghosh, A.; Sarker, S.; Bhattacharya, S.; Gupta, P.; Ghosh, S.; Chattopadhyay, S.; Adhikary, A. Delivery of dual miRNA through CD44-targeted mesoporous silica nanoparticles for enhanced and effective triple-negative breast cancer therapy. Biomater. Sci. 2020, 8, 2939–2954. [Google Scholar] [CrossRef]
- Paris, J.L.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Co-Delivery of Drugs and Nucleic Acids in Oncology: A Review. Pharmaceutics 2020, 12, 526. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Lim, W.Q.; Luo, Z.; Phua, S.Z.F.; Huo, R.; Li, L.; Li, K.; Dai, L.; Liu, J.; et al. A Transferrin-Conjugated Hollow Nanoplatform for Redox-Controlled and Targeted Chemotherapy of Tumor with Reduced Inflammatory Reactions. Theranostics 2018, 8, 518–532. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and organosilica nanoparticles: Physical chemistry, biosafety, delivery strategies, and biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Teng, X.; Huang, X.; Liu, H.; Chen, D.; Ren, J.; He, J.; Tang, F. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials 2011, 32, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Hamidi, M. Nanoarchitectured mesoporous silica-based drug-delivery systems: Toward perfect nanomedicine. In Nanoarchitectonics for Smart Delivery and Drug Targeting, 1st ed.; Holban, A.M., Grumezescu, A., Eds.; Elsevier: Cambridge, MA, USA, 2016; pp. 345–377. [Google Scholar]
- He, Q.; Zhang, Z.; Gao, F.; Li, Y.; Shi, J. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: Effects of particle size and PEGylation. Small 2011, 7, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Liu, T.; Li, L.; Liu, H.; Chen, D.; Tang, F. The absorption, distribution, excretion, and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013, 34, 2565–2575. [Google Scholar] [CrossRef]
- He, Q.; Shi, J.; Zhu, M.; Chen, Y.; Chen, F. The three-stage in vitro degradation behavior of mesoporous silica in simulated body fluid. Microporous Mesoporous Mater. 2010, 131, 314–320. [Google Scholar] [CrossRef]
- Burns, A.A.; Vider, J.; Ow, H.; Herz, E.; Penate-Medina, O.; Baumgart, M.; Larson, S.M.; Wiesner, U.; Bradbury, M. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 2009, 9, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Roy, I.; Ohulchanskky, T.Y.; Vathy, L.A.; Bergey, E.J.; Sajjad, M.; Prasad, P.N. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano 2010, 4, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Cho, W.S.; Choi, M.; Kim, S.J.; Han, B.S.; Kim, S.H.; Kim, H.O.; Sheen, Y.Y.; Jeong, J. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol. Lett. 2009, 189, 177–183. [Google Scholar] [CrossRef]
- Chen, G.; Teng, Z.; Su, X.; Liu, Y.; Lu, G. Unique biological degradation behavior of stöber mesoporous silica nanoparticles from their interiors to their exteriors. J. Biomed. Nanotechnol. 2015, 11, 722–729. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Gao, Y.; Shi, J.; Li, Y. Intracellular localization, and cytotoxicity of spherical mesoporous silica nano-and microparticles. Small 2009, 5, 2722–2729. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Fu, C.; Tan, L.; Meng, X.; Liu, H. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomedicine 2015, 11, 1915–1924. [Google Scholar] [CrossRef]
- Rancan, F.; Gao, Q.; Graf, C.; Troppens, S.; Hadam, S.; Hackbarth, S.; Kembuan, C.; Blume-Peytavi, U.; Rühl, E.; Lademann, J.; et al. Skin penetration and cellular uptake of amorphous silica nanoparticles with variable size, surface functionalization, and colloidal stability. ACS Nano 2012, 6, 6829–6842. [Google Scholar] [CrossRef]
- Chen, F.; Wang, G.; Griffin, J.I.; Brenneman, B.; Banda, N.K.; Holers, V.M.; Backos, D.S.; Wu, L.P.; Moghimi, S.M.; Simberg, D. Complement Proteins Bind to Nanoparticle Protein Corona and Undergo Dynamic Exchange. Nat. Nanotechnol. 2016, 12, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, S.J.; Park, J.Y.; Kim, S.H.; Kwon, S.; Jung, Y.; Khang, D. Unfolded Protein Corona Surrounding Nanotubes Influence the Innate and Adaptive Immune System. Adv. Sci. 2021, 8, 2004979. [Google Scholar] [CrossRef]
- Biswas, S.; Lopes de Faria, J. Hypertension induces oxidative stress but not macrophage infiltration in the kidney in the early stage of experimental diabetes mellitus. Am. J. Nephrol. 2006, 26, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease—a double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, W.; Li, K.; Zhang, K.; Lin, C.; Han, R.; Lu, C.; Wang, Y.; Chen, H.; Sun, F.; et al. Metformin attenuates gefitinib-induced exacerbation of pulmonary fibrosis by inhibition of TGF-β signaling pathway. Oncotarget 2015, 6, 43605–43619. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.; Jerard, C.; Michael, B.P.; Suresh, S.; Ramachandran, R. Mesoporous silica loaded caffeine inhibits inflammatory markers in lipopolysaccharide-activated rat macrophage cells. J. Appl. Pharm. Sci. 2018, 8, 124–131. [Google Scholar]
- Bao, L.; Dou, G.; Tian, R.; Lv, Y.; Ding, F.; Liu, S.; Zhao, R.; Zhao, L.; Zhou, J.; Weng, L.; et al. Engineered neutrophil apoptotic bodies ameliorate myocardial infarction by promoting macrophage efferocytosis and inflammation resolution. Bioact. Mater. 2021, 9, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Zhang, Y.; Lin, C.; Han, R.; Wang, Y.; Wu, D.; Zheng, J.; Lu, C.; Tang, L.; He, Y. pH-responsive theranostic nanoplatform of ferrite and ceria co-engineered nanoparticles for anti-inflammatory. Front. Bioeng. Biotechnol. 2022, 10, 983677. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Wiener-Kronish, J.P. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am. Rev. Respir. Dis. 1990, 142, 1250–1257. [Google Scholar] [CrossRef]
- Ward, P.A. Acute lung injury: How the lung inflammatory response works. The Eur. Respir. J. Suppl. 2003, 44, 22s–23s. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, A.; Sancho, M.; Bisbal, V.; Amorós, P.; Marcos, M.D.; Orzáez, M.; Sancenón, F.; Martínez-Máñez, R. Targeted-lung delivery of dexamethasone using gated mesoporous silica nanoparticles. A new therapeutic approach for acute lung injury treatment. J. Control. Release 2021, 337, 14–26. [Google Scholar] [CrossRef]
- Wigenstam, E.; Rocksén, D.; Ekstrand-Hammarström, B.; Bucht, A. Treatment with dexamethasone or liposome-encapsuled vitamin E provides beneficial effects after chemical-induced lung injury. Inhal. Toxicol. 2009, 21, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Gulin-Sarfraz, T.; Jonasson, S.; Wigenstam, E.; von Haartman, E.; Bucht, A.; Rosenholm, J.M. Feasibility study of mesoporous silica particles for pulmonary drug delivery: Therapeutic treatment with dexamethasone in a mouse model of airway inflammation. Pharmaceutics 2019, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta, S.; Hussein, M.; Nasser, H.; Ali, A. Multidisciplinary role of mesoporous silica nanoparticles in brain regeneration and cancers: From crossing the blood-brain barrier to treatment. Part. Part. Syst. Charact. 2019, 36, 1900195. [Google Scholar] [CrossRef]
- Baghirov, H.; Karaman, D.; Viitala, T.; Duchanoy, A.; Lou, Y.R.; Mamaeva, V.; Pryazhnikov, E.; Khiroug, L.; de Lange Davies, C.; Sahlgren, C.; et al. Feasibility study of the permeability and uptake of mesoporous silica nanoparticles across the blood-brain barrier. PLoS ONE 2016, 11, e0160705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dunphy, D.R.; Jiang, X.; Meng, H.; Sun, B.; Tarn, D.; Xue, M.; Wang, X.; Lin, S.; Ji, Z.; et al. Processing pathway dependence of amorphous silica nanoparticle toxicity: Colloidal vs pyrolytic. J. Am. Chem. Soc. 2012, 134, 15790–15804. [Google Scholar] [CrossRef]
- Wang, J.; Doré, S. Inflammation after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2007, 27, 894–908. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.G.; Jeong, H.G.; Kang, D.W.; Nam, M.J.; Kim, C.K.; Kim, D.Y.; Choi, I.Y.; Ki, S.K.; Kim, S.I.; Han, J.H.; et al. Customized lipid-coated magnetic mesoporous silica nanoparticle doped with ceria nanoparticles for theragnosis of intracerebral hemorrhage. Nano Res. 2018, 11, 3582–3592. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xiao, B.; Tang, G.; Yu, J.; Wang, W.; Xu, G.; Ye, X. pH-responsive delivery of H2 through ammonia borane-loaded mesoporous silica nanoparticles improves recovery after spinal cord injury by moderating oxidative stress and regulating microglial polarization. Regen. Biomater. 2021, 8, rbab058. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Patel, S.P.; VanRooyen, J.L.; Sullivan, P.G.; Rabchevsky, A.G. Cellular and subcellular oxidative stress parameters following severe spinal cord injury. Redox Biol. 2016, 8, 59–67. [Google Scholar] [CrossRef]
- Yang, T.; Jin, Z.; Zhihao, W.; Zhao, P.; Zhao, B.; Fan, M.; Lihua, C.; Wang, T.; Su, B.; He, Q. Intratumoral high-payload delivery and acidresponsive release of H2 for efficient cancer therapy using the ammonia borane-loaded mesoporous silica nanomedicine. Appl. Mater. Today. 2018, 11, 136–143. [Google Scholar] [CrossRef]
- Ma, D.; Shen, H.; Chen, F.; Liu, W.; Zhao, Y.; Xiao, Z.; Wu, X.; Chen, B.; Lu, J.; Shao, D.; et al. Inflammatory microenvironment-responsive nanomaterials promote spinal cord injury repair by targeting IRF5. Adv. Healthc. Mater. 2022, 11, e2201319. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Teruel, A.H.; Pérez-Esteve, É.; González-Álvarez, I.; González-Álvarez, M.; Costero, A.M.; Ferri, D.; Parra, M.; Gaviña, P.; Merino, V.; Martínez-Mañez, R.; et al. Smart gated magnetic silica mesoporous particles for targeted colon drug delivery: New approaches for inflammatory bowel diseases treatment. J. Control. Release 2018, 281, 58–69. [Google Scholar] [CrossRef]
- Teruel, A.H.; Pérez-Esteve, É.; González-Álvarez, I.; González-Álvarez, M.; Costero, A.M.; Ferri, D.; Gaviña, P.; Merino, V.; Martínez-Máñez, R.; Sancenón, F. Double drug delivery using capped mesoporous silica microparticles for the effective treatment of inflammatory bowel disease. Mol. Pharm. 2019, 16, 2418–2429. [Google Scholar] [CrossRef] [PubMed]
- Dudzińska, E.; Gryzinska, m.; Ognik, k.; Gil-Kulik, P.; Kocki, J. Oxidative stress and effect of treatment on the oxidation product decomposition processes in IBD. Oxidative Med. Cell. Longev. 2018, 2018, 7918261. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Dawulieti, J.; Shi, F.; Yang, C.; Qin, Q.; Shi, T.; Wang, L.; Hu, H.; Sun, M.; Ren, L.; et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease. Sci. Adv. 2022, 8, eabj2372. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, Y.; Min, K.T.; Hong, S. Dexamethasone-loaded radially mesoporous silica nanoparticles for sustained anti-inflammatory effects in rheumatoid arthritis. Pharmaceutics 2022, 14, 985. [Google Scholar] [CrossRef]
- Wan, L.; Wang, Y.; Tan, X.; Sun, Y.; Luo, J.; Zhang, H. Biodegradable lubricating mesoporous silica nanoparticles for osteoarthritis therapy. Friction 2022, 10, 68–79. [Google Scholar] [CrossRef]
- Kumar, A.; Ahuja, M. Carboxymethyl gum kondagogu: Synthesis, characterization and evaluation as mucoadhesive polymer. Carbohydr. Polym. 2012, 90, 637–643. [Google Scholar] [CrossRef]
- Bera, H.; Boddupalli, S.; Nandikonda, S.; Kumar, S.; Nayak, A.K. Alginate gel-coated oil entrapped alginate-tamarind gum-magnesium stearate buoyant beads of risperidone. Int. J. Biol. Macromol. 2015, 78, 102–111. [Google Scholar] [CrossRef]
- Bera, H.; Nadimpalli, J.; Kumar, S.; Vengala, P. Kondogogu gum-Zn+2-pectinate emulgel matrices reinforced with mesoporous silica for intragastric furbiprofen delivery. Int. J. Biol. Macromol. 2017, 104, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.; Sealy, B.; Combes, V.; Morsch, M.; Garcia-Bennett, A.E. Enhanced antioxidant effects of the anti-inflammatory compound probucol when released from mesoporous silica particles. Pharmaceutics 2022, 14, 502. [Google Scholar] [CrossRef] [PubMed]
- Parra-Robert, M.; Zeng, M.; Shu, Y.; Fernández-Varo, G.; Perramón, M.; Desai, D.; Chen, J.; Guo, D.; Zhang, X.; Morales-Ruiz, M.; et al. Mesoporous silica coated CeO2 nanozymes with combined lipid-lowering and antioxidant activity induce long-term improvement of the metabolic profile in obese Zucker rats. Nanoscale 2021, 13, 8452–8466. [Google Scholar] [CrossRef]
- Lyu, N.; Zhao, Y.; Xiang, J.; Fan, X.; Huang, C.; Sun, X.; Xu, J.; Xu, Z.P.; Sun, J. Inhibiting corneal neovascularization by sustainably releasing anti-VEGF and anti-inflammation drugs from silica-thermogel nanohybrids. Mater. Biol. Appl. 2021, 128, 112274. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, K.R.; Gao, H.L.; Zhou, Y.; Yan, B.B.; Yang, C.; Zhang, Z.Y.; Dong, L.; Chen, S.M.; Xu, R.; et al. Activating proper inflammation for wound-healing acceleration via mesoporous silica nanoparticle tissue adhesive. Nano Res. 2020, 13, 373–379. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, H.; Yang, S.; Xi, Z.; Tang, T.; Yin, R.; Zhang, W. Electrospun PLGA membrane incorporated with andrographolide-loaded mesoporous silica nanoparticles for sustained antibacterial wound dressing. Nanomedicine 2018, 13, 2881–2899. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Huang, J.; Xu, Z.; Li, X.; Yang, J.; Huang, H.; Tang, S.; Chai, Y.; Lin, J.; et al. Mesoporous silica-coated silver nanoparticles as ciprofloxacin/siRNA carriers for accelerated infected wound healing. J. Nanobiotechnol. 2022, 20, 386. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle-based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Zhou, J.; Kong, L.; Dai, Y.; Zhang, X.; Song, W.; Zhu, C. An injectable photo-cross-linking silk hydrogel system augments diabetic wound healing in orthopaedic surgery through spatiotemporal immunomodulation. J. Nanobiotechnol. 2022, 20, 232. [Google Scholar] [CrossRef]
- Zou, Z.; Wen, S.; Li, Y.; An, J.; Wu, Q.; Tong, L.K.; Mei, X.; Tian, H.; Wu, C. Novel lactoferrin-functionalized manganese-doped silica hollow mesoporous nanoparticles loaded with resveratrol for the treatment of ischemic stroke. Mater. Today Adv. 2022, 15, 100262. [Google Scholar] [CrossRef]
- Song, K.; Tang, Z.; Song, Z.; Meng, S.; Yang, X.; Guo, H.; Zhu, Y.; Wang, X. Hyaluronic acid-functionalized mesoporous silica nanoparticles loading simvastatin for targeted therapy of atherosclerosis. Pharmaceutics 2022, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, Z.; Pu, H.; Li, W.; Liu, J.; Zhao, Z.; Lu, X.; Lin, K.; Li, B. Degradable co-delivery nanoplatforms for inflammation-targeted therapy against atherosclerosis. Appl. Mater. Today. 2021, 25, 101214. [Google Scholar] [CrossRef]
- Peng, X.; Liang, Y.; Yin, Y.; Liao, H.; Li, L. Development of a hollow mesoporous silica nanoparticles vaccine to protect against house dust mite induced allergic inflammation. Int. J. Pharm. 2018, 549, 115–123. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.; Yang, Q.; Ding, Q.; Wang, R.; Li, Z.; Fang, Y.; Liao, J.; Qi, W.; Chen, K.; et al. A novel dendritic mesoporous silica based sustained hydrogen sulfide donor for the alleviation of adjuvant-induced inflammation in rats. Drug deliv. 2021, 28, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.; Trewyn, B.G.; Lin, V.S. Effect of surface functionalization of MCM-41- type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Niu, X.; Ma, K.; Huang, P.; Grothe, J.; Kaskel, S.; Zhu, Y. Graphene quantum dots-capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapy. Small 2017, 13, 1602225. [Google Scholar] [CrossRef]
- Huang, D.M.; Hung, Y.; Ko, B.S.; Hsu, S.C.; Chen, W.H.; Chien, C.L.; Tsai, C.P.; Kuo, C.T.; Kang, J.C.; Yang, C.S.; et al. Highly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells: Implication for stem cell tracking. FASEB J. 2005, 19, 2014–2016. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Wu, S.H.; Yao, M.; Lu, C.W.; Lin, Y.S.; Hung, Y.; Mou, C.Y.; Chen, Y.C.; Huang, D.M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials 2007, 28, 2959–2966. [Google Scholar] [CrossRef]
- Huang, D.M.; Chung, T.H.; Hung, Y.; Lu, F.; Wu, S.H.; Mou, C.Y.; Yao, M.; Chen, Y.C. Internalization of mesoporous silica nanoparticles induces transient but not sufficient osteogenic signals in human mesenchymal stem cells. Toxicol. Appl. Pharmacol. 2008, 231, 208–215. [Google Scholar] [CrossRef]
- Choi, J.K.; Park, J.Y.; Lee, S.; Choi, Y.A.; Kwon, S.; Shin, M.J.; Yun, H.S.; Jang, Y.H.; Kang, J.; Kim, N.; et al. Greater plasma protein adsorption on mesoporous silica nanoparticles aggravates atopic dermatitis. Int. J. Nanomed. 2022, 17, 4599–4617. [Google Scholar] [CrossRef]
- Scarino, A.; Noel, A.; Renzi, P.M.; Cloutier, Y.; Vincent, R.; Truchon, G.; Tardif, R.; Charbonneau, M. Impact of emerging pollutants on pulmonary inflammation in asthmatic rats: Ethanol vapors and agglomerated TiO2 nanoparticles. Inhal. Toxicol. 2012, 24, 528–538. [Google Scholar] [CrossRef]
- Han, H.; Park, Y.H.; Park, H.J.; Lee, K.; Um, K.; Park, J.W.; Lee, J.H. Toxic and adjuvant effects of silica nanoparticles on ovalbumin-induced allergic airway inflammation in mice. Respir. Res. 2016, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.C.; Quan, L.; Zhou, C.; Zhan, Q.Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine 2018, 13, 1495–1512. [Google Scholar] [PubMed]
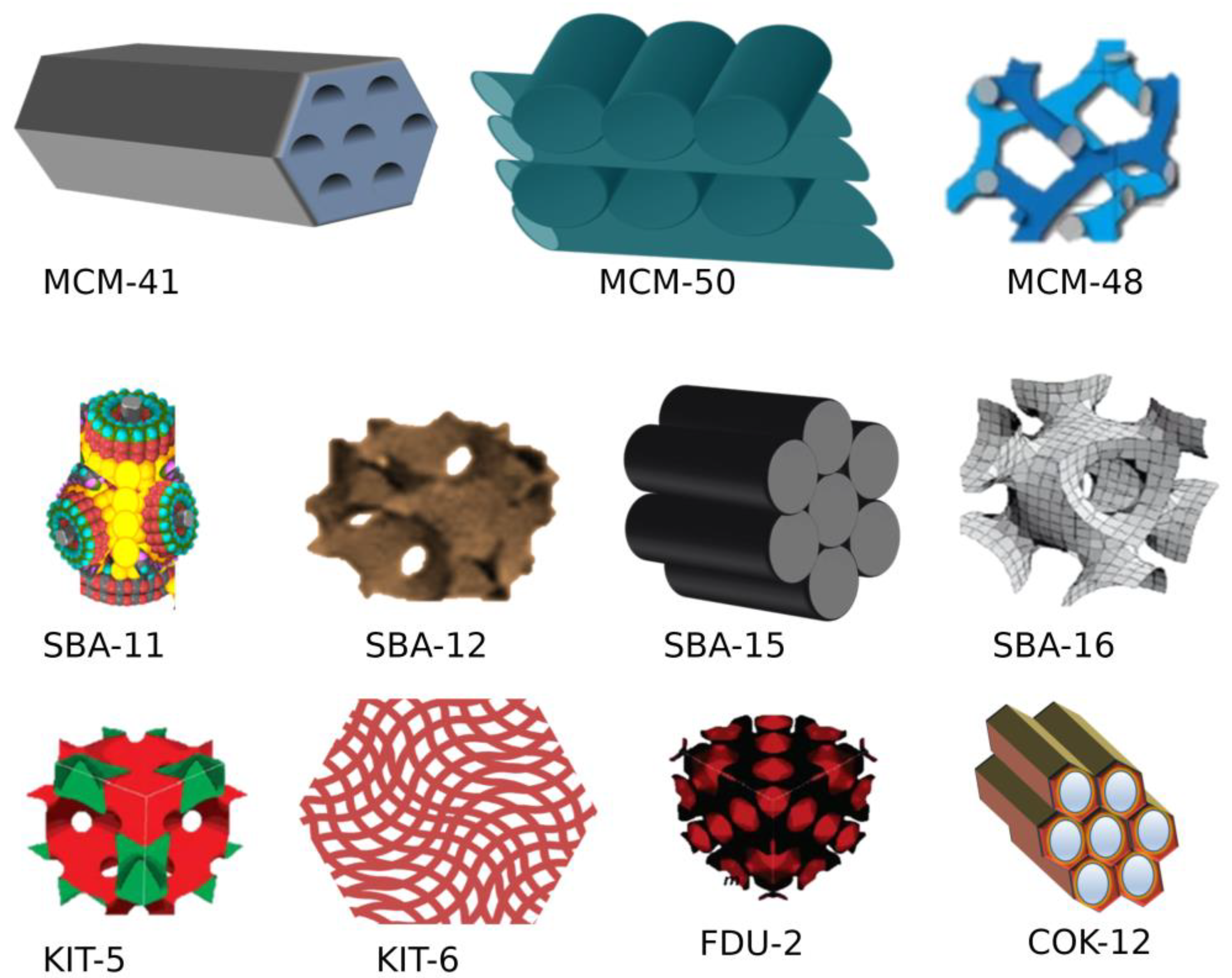

| S. No. | MSN Family | Type | Pore Symmetry | Pore Size (nm) | Pore Volume (cm3/g) | Application | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | M41S | MCM-41 | 2D hexagonal P6mm unidirectional | 1.5 to 8 | >10 | Drug delivery, adsorbent, catalysis, biosensor | [38] |
| MCM-48 | 3-D cubic Ia3d | 2 to 5 | >10 | [39] | |||
| MCM-50 | 3D lamellar P2 | 2 to 5 | >10 | Excellent adsorbent and catalysis | [40] | ||
| 2 | SBA | SBA-11 | 3-D cubic Pm3m | 2.1 to 3.6 | 0.68 | [41] | |
| SBA-12 | 3D hexagonal P63/mmc | 3.1 | 0.83 | [42] | |||
| SBA-15 | 2D hexagonal p6mm | 6 | 1.17 | Drug delivery, adsorbent, catalysis, biosensor | [42] | ||
| SBA-16 | Cubic Im3m | 5 to 15 | 0.91 | [43] | |||
| 3 | KIT | KIT-5 | Cubic Im3m | 9.3 | 0.45 | - | [42] |
| 4 | COK | COK-12 | Hexagonal P6m | 5.8 | 0.45 | - | [44] |
| 5 | Modern MSN | Hollow MSN | - | - | - | - | [45] |
| Nanocomposite and Drug | Model | Results Obtained | Ref. |
|---|---|---|---|
| Anti-oxidant property | |||
| Anionic mesoporous silica-6 (AMS-6) with Probucol (PB) | Zebrafish and human brain microvascular endothelial cells |
| [132] |
| Mesoporous silica-coated cerium oxide (CeO2) nano enzyme | Obese Zucker rats and human hepatic cell line (HepG2 cells). |
| [133] |
| Corneal neovascularization | |||
| Mesoporous silica nanoparticles loaded with anti-VEGF bevacizumab (BEV) incorporated into cyclosporin A (CSA) thermo gel. (BEV@MSN-CsA@Thermogel) | Male New Zealand white rabbits, primary human tenon’s fibroblasts, human corneal epithelial cells, human corneal endothelial cells, and human umbilical vein endothelial cells. |
| [134] |
| Wound healing | |||
| Three-dimensional (3D) dendritic mesoporous silica nanoparticles | Sprague Dawley Rats |
| [135] |
| Poly (lactic-co-glycolic acid) nanofibrous wound dressing incorporated with andrographolide-loaded mesoporous silica nanoparticles (PLGA/Andro-MSNs). | Staphylococcus aureus-infected wound mice and human keratinocyte cell line (HaCat) |
| [136] |
| A core-shell structured silver core embedded with mesoporous silica-based nanoplatform co-load with ciprofloxacin and TNF-α and Si TNF-α (AMPC@siTNF-α) | BALB/c mice and RAW 264.7 macrophage cells from immortalized mouse myoblast cell line |
| [137] |
| MSN decorated with Ceria (MSN-Ceria) | Male Sprague Dawley rats and human skin keratinocytes (HaCaT cells) |
| [138] |
| Photocurable methacryloxylated silk fibroin hydrogel (Sil-MA) system, co-encapsulated with metformin-loaded mesoporous silica microspheres (MET@ MSNs) and silver nanoparticles (Ag NPs). | Streptozotocin (STZ)-induced diabetic C57BL/6 mice, EA. vy926, L929, RAW264.7 cells |
| [139] |
| Ischemic stroke | |||
| Lactoferrin functionalized hollow mesoporous manganese doped silica nanoparticles (LHMMSN) loaded with resveratrol (RES) | Sprague Dawley rats and rat nerve cells (PC12), mouse brain microvascular endothelial cells (bEnd.3 cells) and mouse microglial cells (BV2) |
| [140] |
| Atherosclerosis | |||
| MSNs were loaded with the lipid-lowering drug simvastatin (SIM) and gated with hyaluronic acid (HA) coating (SIM@HA-MSN). | Male C57BL/6 mice, mouse macrophages (Raw264.7 cells), and human umbilical vein endothelial cells (HUVECs) |
| [141] |
| IL-1 receptor antagonist (IL-1Ra) loaded copper doped MSNs (IL-1Ra@Cu-MSNs) | Healthy C57BL/6 male mice, Apolipoprotein E knockout (ApoE−/−) mice and Raw264.7 macrophage cells |
| [142] |
| Induced inflammation | |||
| House dust mite (HDM) allergen Dermatophagoides farina and Dermatophagoides (Der f2) loaded with hollow MSN (HMSN) | Pathogen-free female BALB/c mice |
| [143] |
| Dendritic MSNs with sustained H2S delivery system consisted of S-propargyl-cysteine (SPRC@DMSN) | Adjuvant-induced arthritis (AIA) rat model and bone-marrow-derived monocytes isolated from healthy mice |
| [144] |
| Cancer | |||
| Hollow mesoporous silica nanoparticles (HMSNs) with transferrin (Tf) targeting moieties | MDA-MB-231 cell lines, RAW264.7 macrophage cells |
| [79] |
| Mesoporous silica nanoparticles loaded with anti-tumor component harmine (HM@MSN) | Lymphoma model of Balb/c mice, human lymphoma cell line Daudi, Raw264.7 cells |
| [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivamaruthi, B.S.; Thangaleela, S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. Mesoporous Silica-Based Nanoplatforms Are Theranostic Agents for the Treatment of Inflammatory Disorders. Pharmaceutics 2023, 15, 439. https://doi.org/10.3390/pharmaceutics15020439
Sivamaruthi BS, Thangaleela S, Kesika P, Suganthy N, Chaiyasut C. Mesoporous Silica-Based Nanoplatforms Are Theranostic Agents for the Treatment of Inflammatory Disorders. Pharmaceutics. 2023; 15(2):439. https://doi.org/10.3390/pharmaceutics15020439
Chicago/Turabian StyleSivamaruthi, Bhagavathi Sundaram, Subramanian Thangaleela, Periyanaina Kesika, Natarajan Suganthy, and Chaiyavat Chaiyasut. 2023. "Mesoporous Silica-Based Nanoplatforms Are Theranostic Agents for the Treatment of Inflammatory Disorders" Pharmaceutics 15, no. 2: 439. https://doi.org/10.3390/pharmaceutics15020439
APA StyleSivamaruthi, B. S., Thangaleela, S., Kesika, P., Suganthy, N., & Chaiyasut, C. (2023). Mesoporous Silica-Based Nanoplatforms Are Theranostic Agents for the Treatment of Inflammatory Disorders. Pharmaceutics, 15(2), 439. https://doi.org/10.3390/pharmaceutics15020439








