Application of Antiviral, Antioxidant and Antibacterial Glycyrrhiza glabra L., Trifolium pratense L. Extracts and Myristica fragrans Houtt. Essential Oil in Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Extracts and Essential Oil Preparation
2.2.1. Preparation of Glycyrrhiza glabra L. Extract
2.2.2. Preparation of Trifolium pratense L. Extract
2.2.3. Essential Oil Preparation
2.3. Chromatografical Analysis of Extracts
2.3.1. HPLC–PDA Conditions
2.3.2. GC-MS Conditions
2.4. Total Phenolic and Flavonoid Content and Antioxidant Activity
2.4.1. Determination of Total Phenolic Content
2.4.2. Determination of Total Flavonoid Content
2.4.3. ABTS Radical Scavenging Activity Assay
2.4.4. DPPH Radical Scavenging Activity Assay
2.4.5. Ferric Reducing Antioxidant Power (FRAP)
2.5. Antimicrobial Activity
2.5.1. Antibacterial Activity of Extracts
2.5.2. Antibacterial Activity of Essential Oil
2.6. Antiviral Activity
2.7. Emulsion Preparation
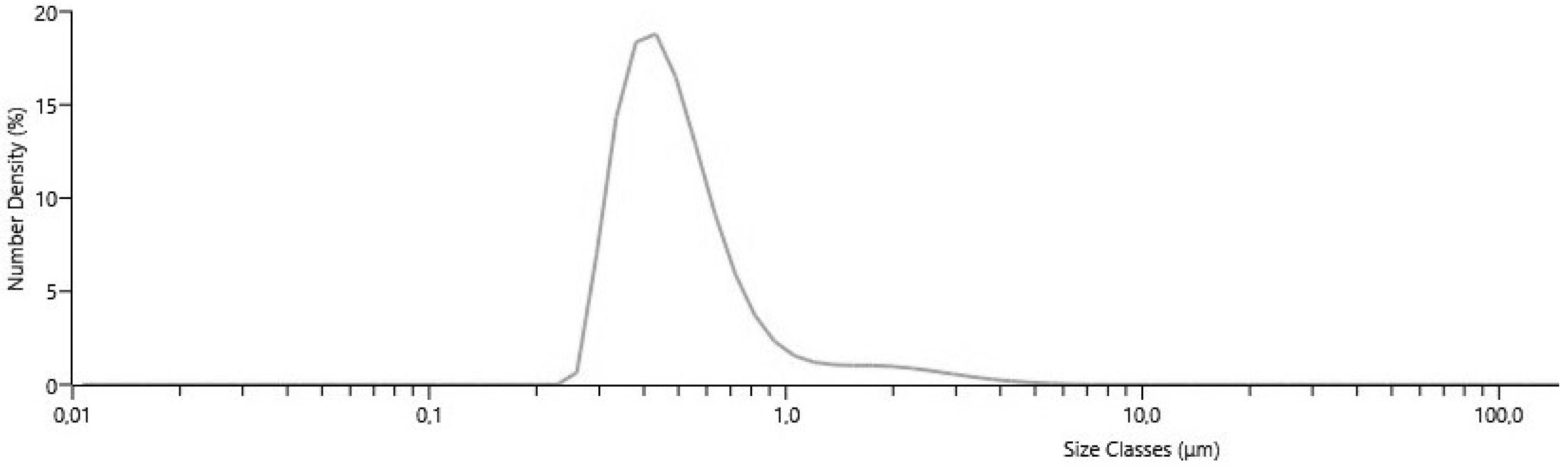
2.8. Particle Size and Distribution Measurements
2.9. Microcapsule Preparation Using the Extrusion Method
2.10. Physical Parameters of Microcapsules
2.10.1. Size of Dry and Wet Microcapsules
2.10.2. Firmness of Microcapsules
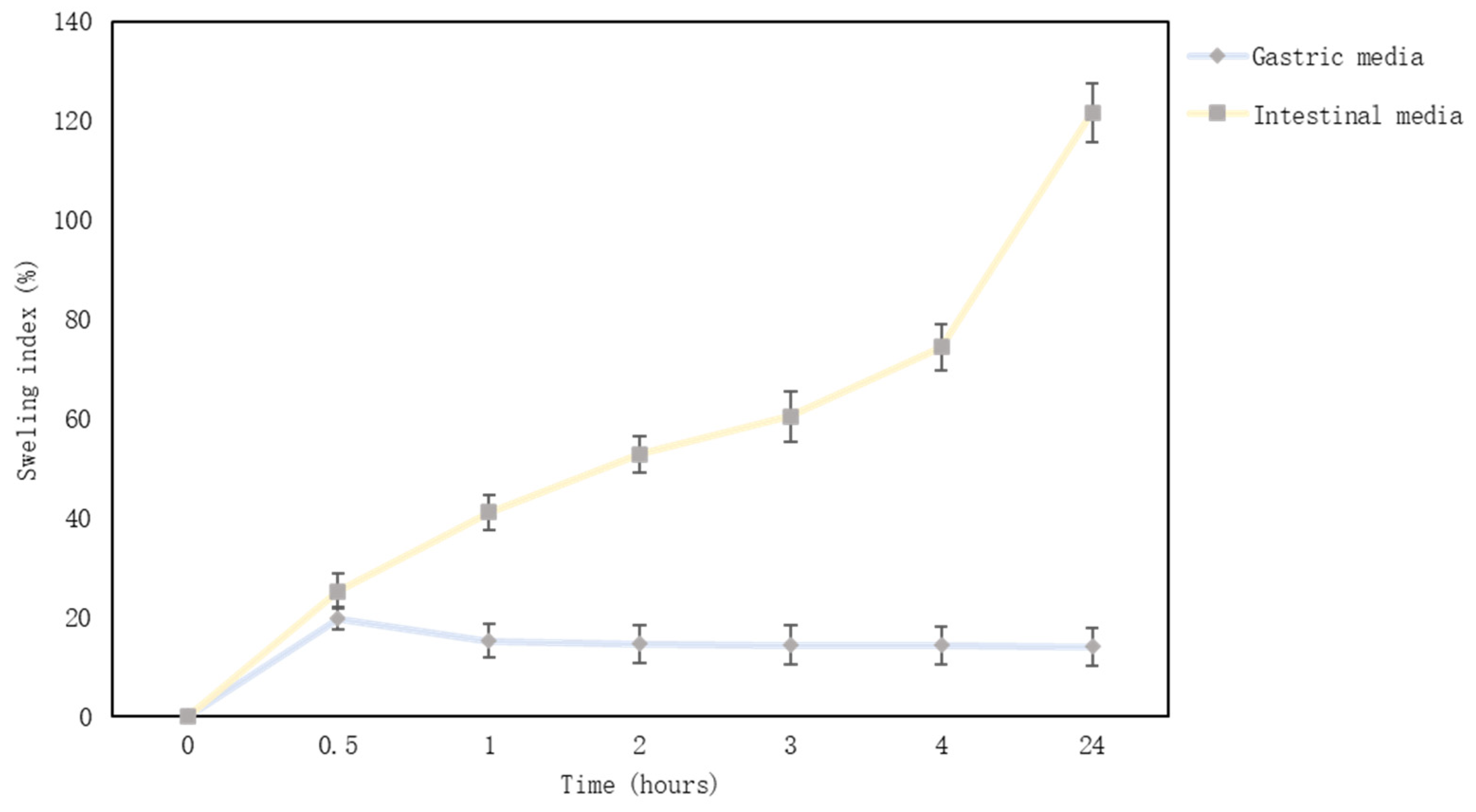
2.10.3. Swelling Characteristic of Microcapsules
2.11. Statistical Data Analysis
3. Results and Discussion
3.1. Quantification of Main Compounds in Plant Extracts and Essential Oil
3.2. Phenols and Antioxidant Activity of Extracts and Essential Oil
3.2.1. Total Phenolic and Flavonoid Content
3.2.2. Antioxidant Activity of Extracts and Essential Oil
3.3. Antimicrobial and Antiviral Activity
3.4. Antiviral Activity of Used Plant Extracts and Essential Oil
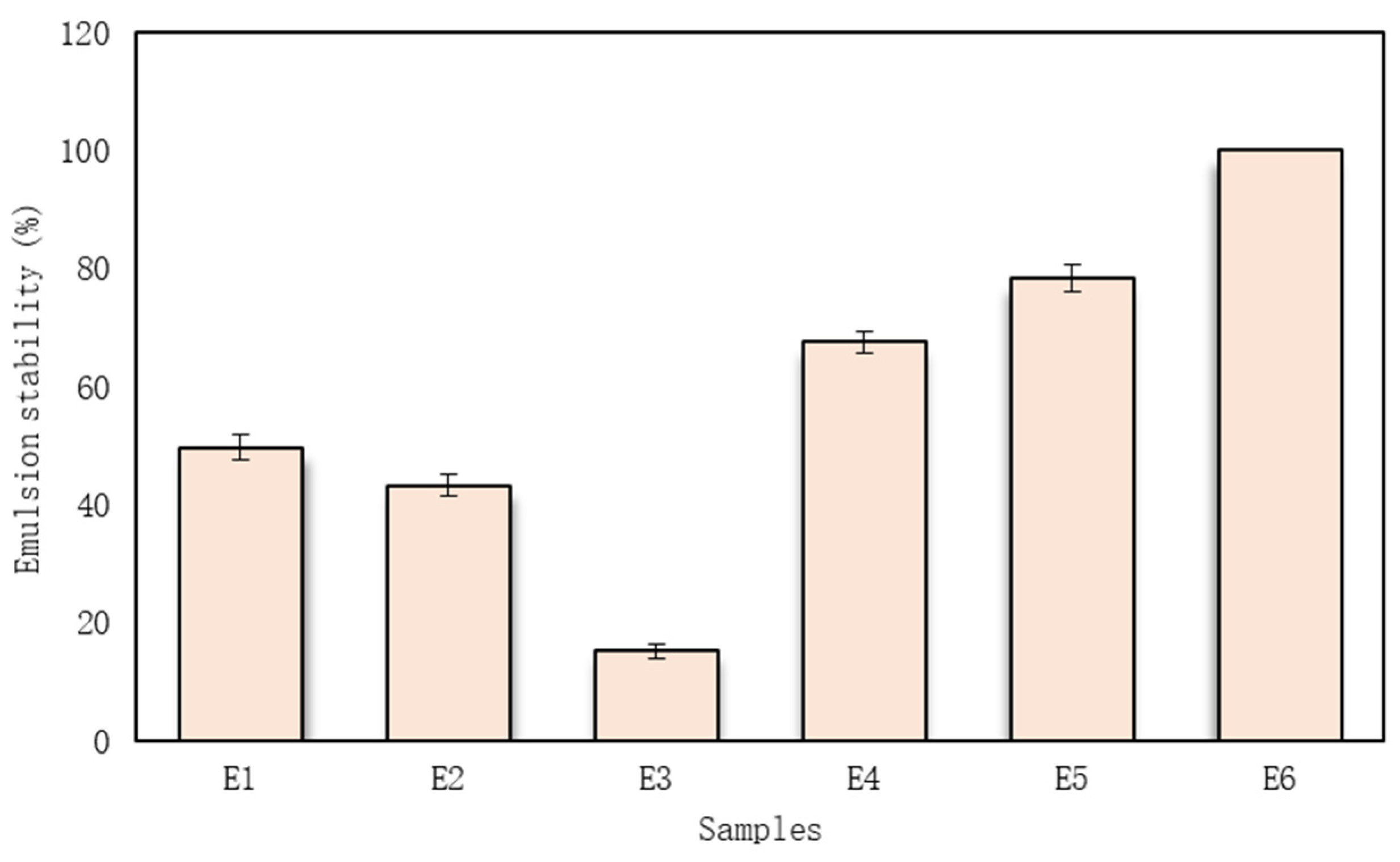
3.5. Emulsion Physical Stability
3.6. Microcapsules’ Physical Parameters
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K.; Bhargav, V.K. Influence of Different Solvents in Extraction of Phenolic Compounds from Vegetable Residues and Their Evaluation as Natural Sources of Antioxidants. J. Food Sci. Technol. 2014, 51, 2568–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starowicz, M.; Piskuła, M.; Achrem–Achremowicz, B.; Zieliński, H. Phenolic Compounds from Apples: Reviewing Their Occurrence, Absorption, Bioavailability, Processing, and Antioxidant Activity—A Review. Polish J. Food Nutr. Sci. 2020, 70, 321–336. [Google Scholar] [CrossRef]
- Vitale, S.G.; Caruso, S.; Rapisarda, A.M.C.; Cianci, S.; Cianci, A. Isoflavones, Calcium, Vitamin D and Inulin Improve Quality of Life, Sexual Function, Body Composition and Metabolic Parameters in Menopausal Women: Result from a Prospective, Randomized, Placebo-Controlled, Parallel-Group Study. Prz. Menopauzalny 2018, 17, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanadys, W.; Baranska, A.; Jedrych, M.; Religioni, U.; Janiszewska, M. Effects of Red Clover (Trifolium pratense) Isoflavones on the Lipid Profile of Perimenopausal and Postmenopausal Women—A Systematic Review and Meta-Analysis. Maturitas 2020, 132, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.A.; Abdel-Wadood, Y.A. The Potential of Glycyrrhizin and Licorice Extract in Combating COVID-19 and Associated Conditions. Phytomed. Plus 2021, 1, 100043. [Google Scholar] [CrossRef] [PubMed]
- Cianci, A.; Colacurci, N.; Paoletti, A.M.; Perino, A.; Cicinelli, E.; Maffei, S.; Di Martino, M.; Daguati, R.; Stomati, M.; Pilloni, M.; et al. Soy Isoflavones, Inulin, Calcium, and Vitamin D3 in Post-Menopausal Hot Flushes: An Observational Study. Clin. Exp. Obstet. Gynecol. 2015, 42, 743–745. [Google Scholar] [CrossRef]
- Malinowska, P. Effect of Flavonoids Content on Antioxidant Activity of Commercial Cosmetic Plant Extracts. Herba Pol. 2013, 59, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Ashokkumar, K.; Simal-Gandara, J.; Murugan, M.; Dhanya, M.K.; Pandian, A. Nutmeg (Myristica fragrans houtt.) Essential Oil: A Review on Its Composition, Biological, and Pharmacological Activities. Phyther. Res. 2022, 36, 2839–2851. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The Antiviral and Antimicrobial Activities of Licorice, a Widely-Used Chinese Herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, A.K.; Taha, R.M.; Mohajer, S.; Banisalam, B. Antioxidant Activity and Total Phenolic and Flavonoid Content of Various Solvent Extracts from In Vivo and In Vitro Grown Trifolium pratense L. (Red clover). Biomed Res. Int. 2015, 2015, 643285. [Google Scholar] [CrossRef] [Green Version]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Díez-Sales, O.; Ruiz-Saurí, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of Liquorice Extract by Liposomes and Hyalurosomes to Protect the Skin against Oxidative Stress Injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A Review on Phytochemicals, Pharmacological Activities, Drug Interactions, and Associated Toxicities of Licorice (Glycyrrhiza sp.). Food Front. 2021, 2, 449–485. [Google Scholar] [CrossRef]
- Vasile, C.; Sivertsvik, M.; Miteluţ, A.C.; Brebu, M.A.; Stoleru, E.; Rosnes, J.T.; Tănase, E.E.; Khan, W.; Pamfil, D.; Cornea, C.P.; et al. Comparative Analysis of the Composition and Active Property Evaluation of Certain Essential Oils to Assess Their Potential Applications in Active Food Packaging. Materials 2017, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kumar Singh, V.; Kumar Dwivedy, A.; Kumar Chaudhari, A.; Upadhyay, N.; Singh, A.; Krishna Saha, A.; Ray Chaudhury, S.; Prakash, B.; Dubey, N.K. Assessment of Chemically Characterised Myristica Fragrans Essential Oil against Fungi Contaminating Stored Scented Rice and Its Mode of Action as Novel Aflatoxin Inhibitor. Nat. Prod. Res. 2020, 34, 1611–1615. [Google Scholar] [CrossRef]
- Nikolic, V.; Nikolic, L.; Dinic, A.; Gajic, I.; Urosevic, M.; Stanojevic, L.; Stanojevic, J.; Danilovic, B. Chemical Composition, Antioxidant and Antimicrobial Activity of Nutmeg (Myristica fragrans houtt.) Seed Essential Oil. J. Essent. Oil-Bearing Plants 2021, 24, 218–227. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P. Applied Sciences Aromatherapy—A Review. Appl. Sci. 2022, 12, 4495. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Novac, C.Ș.; Filip, G.A.; Vlase, L.; Crișan, G. Red Clover and the Importance of Extraction Processes—Ways in Which Extraction Techniques and Parameters Affect Trifolium pratense L. Extracts’ Phytochemical Profile and Biological Activities. Processes 2022, 10, 2581. [Google Scholar] [CrossRef]
- Gupta, P.D.; Birdi, T.J. Development of Botanicals to Combat Antibiotic Resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A Review: Mechanism of Action of Antiviral Drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 205873842110026. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, A.; Kumoro, A.C.; Bahlawan, Z.A.S. Review Calcium Alginate Beads as Immobilizing Matrix of Functional Cells: Extrusion Dripping Method, Characteristics, and Application. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012017. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Nunthanid, J.; Luangtana-anan, M.; Weerapol, Y.; Puttipipatkhachorn, S. Alginate-Based Pellets Prepared by Extrusion/Spheronization: Effect of the Amount and Type of Sodium Alginate and Calcium Salts. Eur. J. Pharm. Biopharm. 2008, 69, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhang, X.; Chen, Z.; Shi, L.; Li, W. Preparation and Characterization of Hydroxyapatite-Sodium Alginate Scaffolds by Extrusion Freeforming. Ceram. Int. 2015, 41, 14029–14034. [Google Scholar] [CrossRef]
- Dolçà, C.; Ferrándiz, M.; Capablanca, L.; Franco, E.; Mira, E.; López, F.; García, D. Microencapsulation of Rosemary Essential Oil by Co-Extrusion/Gelling Using Alginate as a Wall Material. J. Encapsulation Adsorpt. Sci. 2015, 05, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Volić, M.; Pajić-Lijaković, I.; Djordjević, V.; Knežević-Jugović, Z.; Pećinar, I.; Stevanović-Dajić, Z.; Veljović, Đ.; Hadnadjev, M.; Bugarski, B. Alginate/Soy Protein System for Essential Oil Encapsulation with Intestinal Delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef]
- Kakita, H.; Kamishima, H. Some Properties of Alginate Gels Derived from Algal Sodium Alginate. J. Appl. Phycol. 2008, 20, 543–549. [Google Scholar] [CrossRef]
- Rhim, J.-W. Physical and Mechanical Properties of Water Resistant Sodium Alginate Films. LWT Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Goli, S.A.H.; Fathi, M. Encapsulation of Orange Essential Oil Using Cross-Linked Electrospun Gelatin Nanofibers. Food Bioprocess Technol. 2018, 11, 427–434. [Google Scholar] [CrossRef]
- Chan, E.S. Preparation of Ca-Alginate Beads Containing High Oil Content: Influence of Process Variables on Encapsulation Efficiency and Bead Properties. Carbohydr. Polym. 2011, 84, 1267–1275. [Google Scholar] [CrossRef]
- Yan, M.; Liu, B.; Jiao, X.; Qin, S. Preparation of Phycocyanin Microcapsules and Its Properties. Food Bioprod. Process. 2014, 92, 89–97. [Google Scholar] [CrossRef]
- Matulyte, I.; Kasparaviciene, G.; Bernatoniene, J. Development of New Formula Microcapsules from Nutmeg Essential Oil Using Sucrose Esters and Magnesium Aluminometasilicate. Pharmaceutics 2020, 12, 628. [Google Scholar] [CrossRef]
- Bah, M.G.; Bilal, H.M.; Wang, J. Fabrication and Application of Complex Microcapsules: A Review. Soft Matter 2020, 16, 570–590. [Google Scholar] [CrossRef]
- Pavlović, Ž.; Dedijer, S.; Stankovič Elesini, U.; Urbas, R. Structure of Microcapsules and Its Use in the Industry-Overview. GRID 2014: International Symposium on Graphic Engineering and Design. In Proceedings of 7th International Symposium GRID, Novi Sad, Serbia, 13–14 November 2014. [Google Scholar]
- Kazlauskaite, J.A.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. The Effect of Traditional and Cyclodextrin-Assisted Extraction Methods on Trifolium pratense L. (Red clover) Extracts Antioxidant Potential. Antioxidants 2022, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Matulyte, I.; Marksa, M.; Ivanauskas, L.; Kalveniene, Z.; Lazauskas, R.; Bernatoniene, J. GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica Fragrans Seeds Using Magnesium Aluminometasilicate as Excipient. Molecules 2019, 24, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruppach, H. Log 10 Reduction Factors in Viral Clearance Studies with Individual Process Steps. BioProcessing 2013, 12, 1538–8786. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Karkanis, A.; Martins, N.; Petropoulos, S.A.; Ferreira, I.C.F.R. Phytochemical Composition, Health Effects, and Crop Management of Liquorice (Glycyrrhiza glabra L.): A Medicinal Plant. Food Rev. Int. 2018, 34, 182–203. [Google Scholar] [CrossRef] [Green Version]
- Ong, E.S.; Len, S.M. Pressurized Hot Water Extraction of Berberine, Baicalein and Glycyrrhizin in Medicinal Plants. Anal. Chim. Acta 2003, 482, 81–89. [Google Scholar] [CrossRef]
- Charpe, T.W.; Rathod, V.K. Extraction of Glycyrrhizic Acid from Licorice Root Using Ultrasound: Process Intensification Studies. Chem. Eng. Process. Process Intensif. 2012, 54, 37–41. [Google Scholar] [CrossRef]
- Saviranta, N.M.; Anttonen, M.J.; von Wright, A.; Karjalainen, R.O. Red Clover (Trifolium pratense L.) Isoflavones: Determination of Concentrations by Plant Stage, Flower Colour, Plant Part and Cultivar. J. Sci. Food Agric. 2008, 88, 125–132. [Google Scholar] [CrossRef]
- Carneiro, S.; Costa Duarte, F.; Heimfarth, L.; Siqueira Quintans, J.; Quintans-Júnior, L.; Veiga Júnior, V.; Neves de Lima, Á. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekundayo, O.; Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A. Chemical Composition of Essential Oil of Myristica Fragrans Houtt (Nutmeg) from Nigeria. J. Essent. Oil-Bearing Plants 2003, 6, 21–26. [Google Scholar] [CrossRef]
- Valente, V.M.M.; Jham, G.N.; Dhingra, O.D.; Ghiviriga, I. Composition and Antifungal Activity of the Brazilian Myristica Fragrans Houtt Essential Oil. J. Food Saf. 2011, 31, 197–202. [Google Scholar] [CrossRef]
- Patthamakanokporn, O.; Puwastien, P.; Nitithamyong, A.; Sirichakwal, P.P. Changes of Antioxidant Activity and Total Phenolic Compounds during Storage of Selected Fruits. J. Food Compos. Anal. 2008, 21, 241–248. [Google Scholar] [CrossRef]
- Wakeel, A.; Jan, S.A.; Ullah, I.; Shinwari, Z.K.; Xu, M. Solvent Polarity Mediates Phytochemical Yield and Antioxidant Capacity of Isatis tinctoria. PeerJ 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Celaya, L.S.; Kolb, E.; Kolb, N. Solubility of Stevioside and Rebaudioside A in Water, Ethanol and Their Binary Mixtures. Int. J. Food Stud. 2016, 5, 158–166. [Google Scholar] [CrossRef]
- Kroyer, G.T. Red Clover Extract as Antioxidant Active and Functional Food Ingredient. Innov. Food Sci. Emerg. Technol. 2004, 5, 101–105. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Horvat, D.; Tucak, M.; Viljevac Vuletić, M.; Čupić, T.; Krizmanić, G.; Kovačević Babić, M. Phenolic Content and Antioxidant Activity of the Croatian Red Clover Germplasm Collection. Poljoprivreda 2020, 26, 3–10. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A Phytochemical and Pharmacological Review. Phyther. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Flythe, M.D.; Harrison, B.; Kagan, I.A.; Klotz, J.L.; Gellin, G.L.; Goff, B.; Aiken, G.E. Antimicrobial Activity of Red Clover (Trifolium pratense L.) Extract on Caprine Hyper Ammonia-Producing Bacteria. Agric Food Anal Bacteriol 2013, 3, 176–185. [Google Scholar]
- Khan, A.V.; Ahmed, Q.U.; Shukla, I.; Khan, A.A. Antibacterial Activity of Leaves Extracts of Trifolium alexandrinum Linn. against Pathogenic Bacteria Causing Tropical Diseases. Asian Pac. J. Trop. Biomed. 2012, 2, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zawiślak, A.; Francik, R.; Francik, S.; Knapczyk, A. Impact of Drying Conditions on Antioxidant Activity of Red Clover (Trifolium pratense), Sweet Violet (Viola Odorata) and Elderberry Flowers (Sambucus nigra). Materials 2022, 15, 3317. [Google Scholar] [CrossRef] [PubMed]
- Aisyah, Y.; Yunita, D.; Amanda, A. Antimicrobial Activity of Patchouli (Pogostemon cablin benth) Citronella (Cymbopogon nardus), and Nutmeg (Myristica fragrans) Essential Oil and Their Mixtures against Pathogenic and Food Spoilage Microbes. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 012020. [Google Scholar] [CrossRef]
- Hanif, M.A.; Bhatti, H.N.; Jamil, M.S.; Anjum, R.S.; Jamil, A.; Khan, M.M. Antibacterial and Antifungal Activities of Essential Oils Extracted from Medicinal Plants Using CO2 Supercritical Fluid Extraction Technology. Asian J. Chem. 2010, 22, 7787–7798. [Google Scholar]
- Vlaisavljevic, S.; Kaurinovic, B.; Popovic, M.; Djurendic-Brenesel, M.; Vasiljevic, B.; Cvetkovic, D.; Vasiljevic, S. Trifolium pratense L. as a Potential Natural Antioxidant. Molecules 2014, 19, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Ayfer ATEfi, D.; Turgay ERDO, Ö. Antimicrobial Activities of Various Medicinal and Commercial Plant Extracts. Turk. J. Biol. 2003, 27, 157–162. [Google Scholar]
- Xiao, M.; Tang, B.; Qin, J.; Wu, K.; Jiang, F. Properties of Film-Forming Emulsions and Films Based on Corn Starch/Sodium Alginate/Gum Arabic as Affected by Virgin Coconut Oil Content. Food Packag. Shelf Life 2022, 32, 100819. [Google Scholar] [CrossRef]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of Essential Oil in Emulsion Based Edible Films Prepared by Soy Protein Isolate-Gum Acacia Conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Wan Sulaiman, W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of Black Seed Oil in Alginate Beads as a Ph-Sensitive Carrier for Intestine-Targeted Drug Delivery: In Vitro, in Vivo and Ex Vivo Study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dima, C.; Pətraşcu, L.; Cantaragiu, A.; Alexe, P.; Dima, Ş. The Kinetics of the Swelling Process and the Release Mechanisms of Coriandrum sativum L. Essential Oil from Chitosan/Alginate/Inulin Microcapsules. Food Chem. 2016, 195, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Matyash, M.; Despang, F.; Ikonomidou, C.; Gelinsky, M. Swelling and Mechanical Properties of Alginate Hydrogels with Respect to Promotion of Neural Growth. Tissue Eng. Part C Methods 2014, 20, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Xie, X.; Zhang, X.; Zhang, J.; Wang, A. Preparation and Swelling Properties of PH-Sensitive Composite Hydrogel Beads Based on Chitosan-g-Poly (Acrylic Acid)/Vermiculite and Sodium Alginate for Diclofenac Controlled Release. Int. J. Biol. Macromol. 2010, 46, 356–362. [Google Scholar] [CrossRef] [PubMed]

| Samples | Total Phenols (mg GA/g dw) | Total Flavonoids (mg RU/g dw) |
|---|---|---|
| Glycyrrhiza glabra L. extract | 43.14 ± 0.06 | 16.89 ± 0.02 |
| Trifolium pratense L. extract | 74.00 ± 0.15 | 19.50 ± 0.04 |
| Myristica fragrans Houtt. Essential oil (1%) | 7.49 ± 0.04 | 6.84 ± 0.05 |
| Samples | DPPH (µg TE/g dw) | ABTS (µg TE/g dw) | FRAP (mg FS/g dw) |
|---|---|---|---|
| Glycyrrhiza glabra L. extract | 26.22 ± 0.27 | 524.67 ± 6.32 | 675.71 ± 4.61 |
| Trifolium pratense L. extract | 26.27 ± 0.31 | 638.55 ± 9.14 | 526.86 ± 3.21 |
| Myristica fragrans Houtt. Essential oil (1%) | 8.69 ± 0.01 | 92.14 ± 1.26 | 176.05 ± 0.12 |
| Reference Cultures of Microorganisms | Amount of Glycyrrhiza glabra L. Extract (mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 1.10 | |
| 1.0 | 0.75 | 0.5 | 0.25 | 0.1 | 0.075 | 0.05 | 0.025 | 0.01 | 0.0075 | |
| Staphylococcus aureus ATCC 25923 | S | S | S | S | S | S | S | S | S | N |
| Staphylococcus epidermidis ATCC 12228 | S | S | S | S | S | S | S | S | S | N |
| Enterococcus faecalis ATCC 29212 | S | S | S | S | S | S | S | S | S | N |
| Escherichia coli ATCC 25922 | N | N | N | N | N | N | N | N | N | N |
| Klebsiella pneumoniae ATCC 13883 | N | N | N | N | N | N | N | N | N | N |
| Pseudomonas aeruginosa ATCC 27853 | N | N | N | N | N | N | N | N | N | N |
| Bacillus cereus ATCC 11778 | S | S | S | S | S | N | N | N | N | N |
| Candida albicans ATCC 10231 | S | S | S | S | S | S | S | S | ± | N |
| Proteus vulgaris ATCC 8427 | N | N | N | N | N | N | N | N | N | N |
| Reference Cultures of Microorganisms | Amount of Clover Extract (mL) | Amount of Myristica fragrans Houtt. Essential Oil (µg) | ||||
|---|---|---|---|---|---|---|
| 2.1 | 2.2 | 2.3 | 2.4 | 2.5 | 4 | |
| 1.0 | 0.75 | 0.5 | 0.25 | 0.1 | 30.0 | |
| Staphylococcus aureus ATCC 25923 | S | ± | N | N | N | S |
| Staphylococcus epidermidis ATCC 12228 | S | S | N | N | N | N |
| Enterococcus faecalis ATCC 29212 | N | N | N | N | N | N |
| Escherichia coli ATCC 25922 | N | N | N | N | N | S |
| Klebsiella pneumoniae ATCC 13883 | N | N | N | N | N | S |
| Pseudomonas aeruginosa ATCC 27853 | N | N | N | N | N | N |
| Bacillus cereus ATCC 11778 | S | ± | N | N | N | N |
| Candida albicans ATCC 10231 | ± | N | N | N | N | S |
| Proteus vulgaris ATCC 8427 | N | N | N | N | N | N |
| Material, Dilution and Concentration | Virus Titre, TCID50 | Virus Reduction (TCID50) | ||||
|---|---|---|---|---|---|---|
| log10 | % | Reduction Factor | ||||
| Name | Dilution | Concentration | ||||
| Trifolium pratense L. extract | 1:7.5 | 4 mg/mL | NA | NA | NA | NA |
| 1:15 | 2 mg/mL * | 2.50 ± 0.26 | 2.72 | 99.82 | moderate | |
| 1:30 | 1 mg/mL * | 3.50 ± 0.00 | 1.74 | 98.23 | contributable | |
| 50% ethanol (control) | 1:15 | 3.33% | 5.22 ± 0.16 | - | - | - |
| 1:30 | 1.67% | 5.24 ± 0.16 | - | - | - | |
| Glycyrrhiza glabra L. extract | 1:15 | 13.3 mg/mL * | 3.50 ± 0.18 | 1.72 | 98.23 | contributable |
| 1:30 | 6.6 mg/mL * | 4.00 ± 0.19 | 1.24 | 94.38 | contributable | |
| Water (control) | 1:15 | 6.66% | 5.22 ± 0.16 | - | - | - |
| 1:30 | 3.33% | 5.24 ± 0.16 | - | - | - | |
| Myristica fragrans Houtt. essential oil ** | 1:15 | 13.3 µL/mL | 4.13 ± 0.18 | 1.10 | 92.50 | contributable |
| 1:30 | 6.6 µL/mL | 4.00 ± 0.18 | 1.24 | 94.38 | contributable | |
| Almond oil (control) | 1:15 | 13.3 µL/mL | 3.88 ± 0.18 | 1.34 | 95.80 | contributable |
| 1:30 | 6.6 µL/mL | 3.75 ± 0.16 | 1.49 | 96.84 | contributable | |
| IBV control | undiluted | 100% | 5.25 ± 0.16 | - | - | - |
| Plant Extract | Antiviral Effect Evaluated by CIA100 | |||
|---|---|---|---|---|
| Virus Pre-Treatment with Extract | Cell Pre-Treatment Prior to Infection | |||
| Prior to Infection | During Infection | After Infection | ||
| Trifolium pratense L. | + | + | - | - |
| Glycyrrhiza glabra L. | + | + | - | - |
| Ingredients | Samples and Ingredients Quantities | |||||
|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | |
| 4% sodium alginate solution (g) | 47.0 | 47.0 | 47.0 | 47.0 | 47.0 | 47.0 |
| Myristica fragrans Houtt. essential oil (mL) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Trifolium pratense L. extract (mL) | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 |
| Glycyrrhiza glabra L. extract (mL) | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 |
| Maltodextrin (g) | 12.0 | - | - | 8.6 | 10.3 | 6.2 |
| Inulin (g) | - | 12.0 | - | 3.4 | - | 4.3 |
| Gum Arabic (g) | - | - | 12.0 | - | 1.7 | 1.5 |
| Purified water (mL) | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazlauskaite, J.A.; Matulyte, I.; Marksa, M.; Lelesius, R.; Pavilonis, A.; Bernatoniene, J. Application of Antiviral, Antioxidant and Antibacterial Glycyrrhiza glabra L., Trifolium pratense L. Extracts and Myristica fragrans Houtt. Essential Oil in Microcapsules. Pharmaceutics 2023, 15, 464. https://doi.org/10.3390/pharmaceutics15020464
Kazlauskaite JA, Matulyte I, Marksa M, Lelesius R, Pavilonis A, Bernatoniene J. Application of Antiviral, Antioxidant and Antibacterial Glycyrrhiza glabra L., Trifolium pratense L. Extracts and Myristica fragrans Houtt. Essential Oil in Microcapsules. Pharmaceutics. 2023; 15(2):464. https://doi.org/10.3390/pharmaceutics15020464
Chicago/Turabian StyleKazlauskaite, Jurga Andreja, Inga Matulyte, Mindaugas Marksa, Raimundas Lelesius, Alvydas Pavilonis, and Jurga Bernatoniene. 2023. "Application of Antiviral, Antioxidant and Antibacterial Glycyrrhiza glabra L., Trifolium pratense L. Extracts and Myristica fragrans Houtt. Essential Oil in Microcapsules" Pharmaceutics 15, no. 2: 464. https://doi.org/10.3390/pharmaceutics15020464





