Recent Advances in Cancer Immunotherapy Delivery Modalities
Abstract
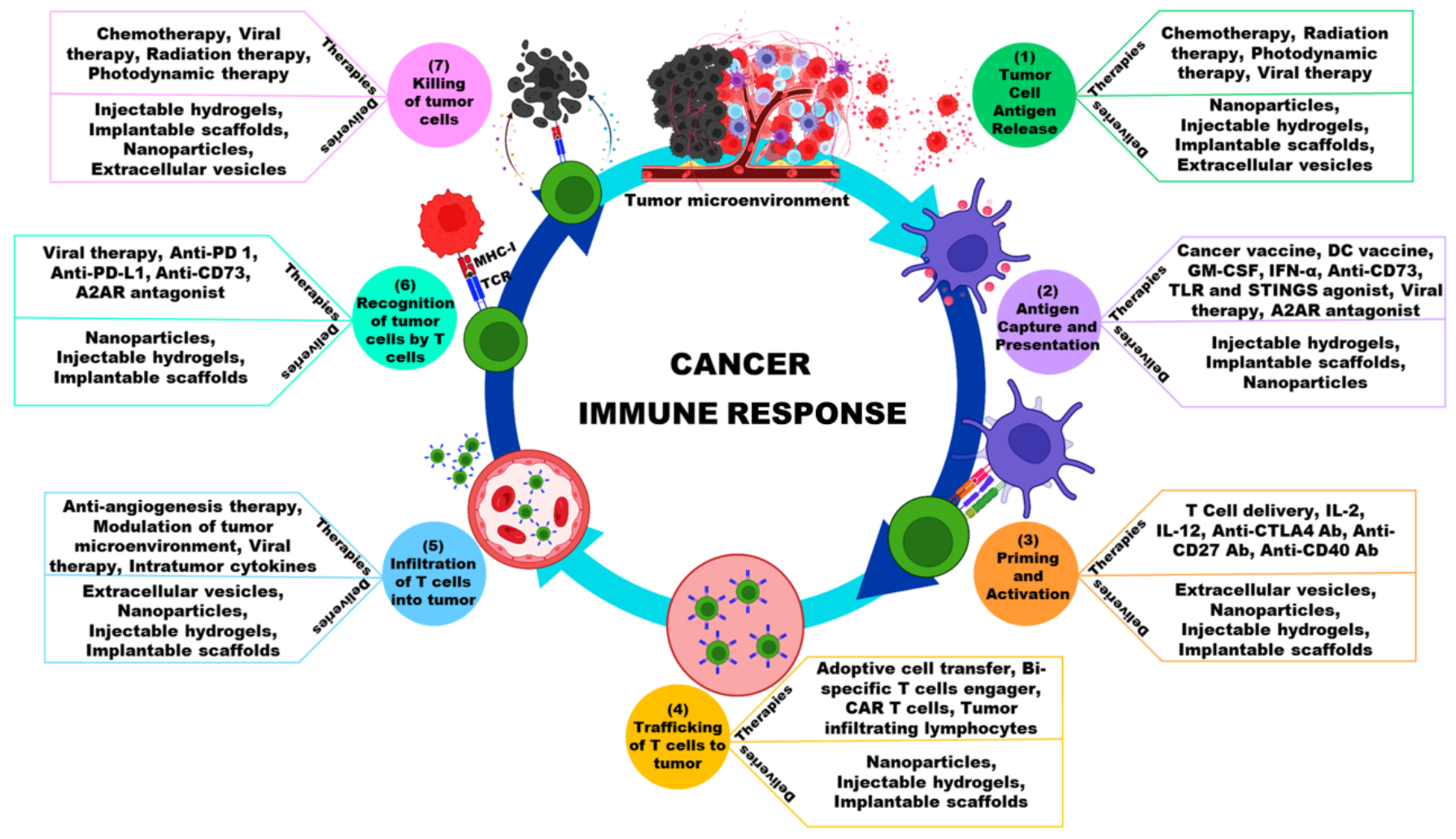
:1. Introduction
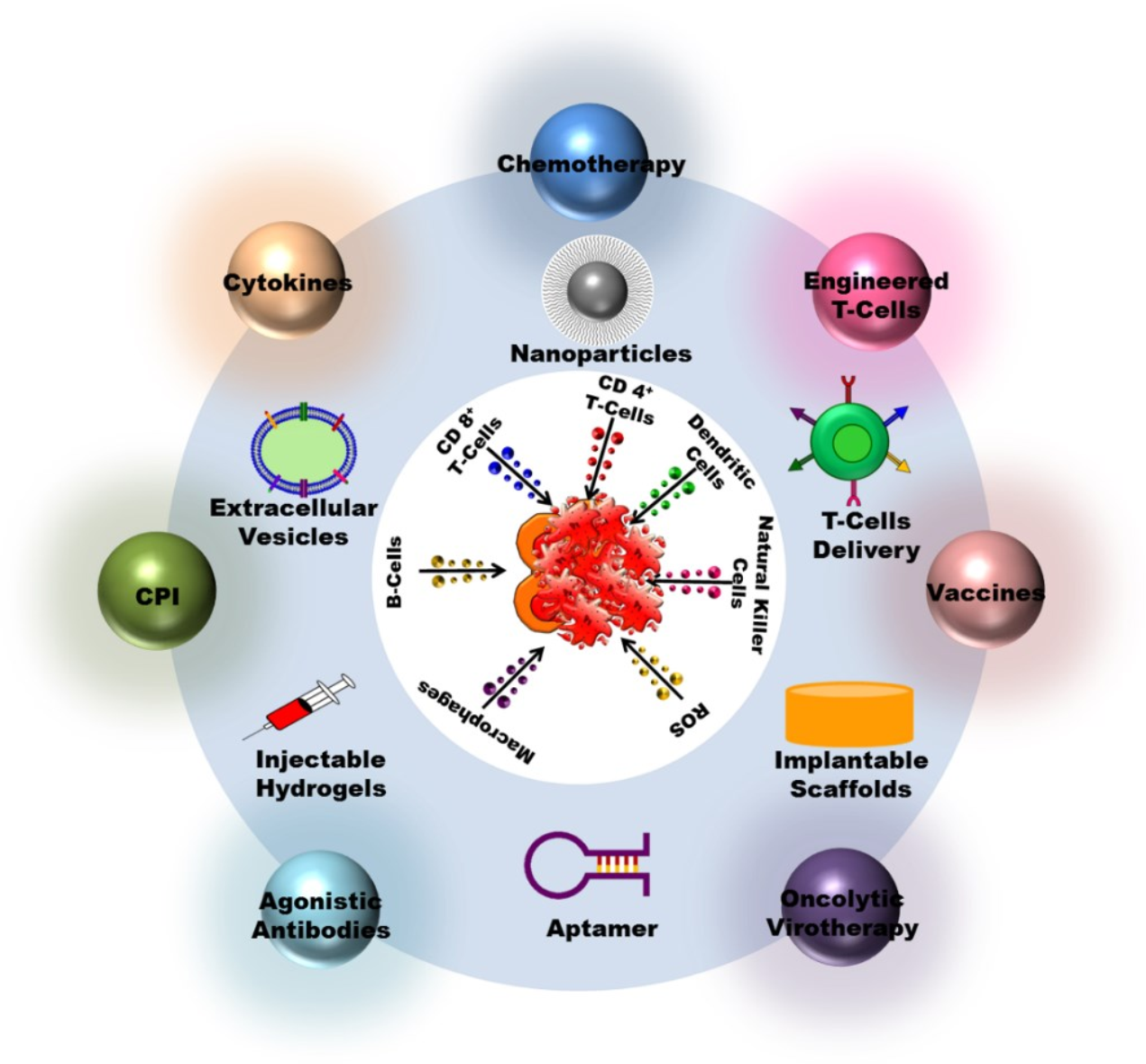
2. Cancer Immunotherapy Types
2.1. Checkpoint Inhibitors
2.2. Cytokines
2.3. Vaccinations
2.4. Antibodies That Are Agonistic
2.5. T Cells with Alternations
2.6. Virotherapy with Oncolytic Agents
3. Administration Mode
4. Cancer Immunotherapy Delivery Methods
4.1. Nanoparticles
4.2. Vesicles Extracellular
4.3. Biomaterials
4.4. T Cell Therapy Delivery Methods
5. Clinical Trails and Patents
6. Challenges and Future Progress
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yadav, D.; Kwak, M.; Chauhan, P.S.; Puranik, N.; Lee, P.C.W.; Jin, J.O. Cancer immunotherapy by immune checkpoint blockade and its advanced application using bio-nanomaterials. Semin. Cancer Biol. 2022, 86, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Benavente, S.; Sánchez-García, A.; Naches, S.; LLeonart, M.E.; Lorente, J. Therapy-Induced Modulation of the Tumor Microenvironment: New Opportunities for Cancer Therapies. Front. Oncol. 2020, 10, 582884. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Bondhopadhyay, B.; Sisodiya, S.; Chikara, A.; Khan, A.; Tanwar, P.; Afroze, D.; Singh, N.; Agrawal, U.; Mehrotra, R.; Hussain, S. Cancer immunotherapy: A promising dawn in cancer research. Am. J. Blood Res. 2020, 10, 375–385. [Google Scholar] [PubMed]
- Liu, M.; Guo, F. Recent updates on cancer immunotherapy. Precis. Clin. Med. 2018, 1, 65–74. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Naran, K.; Nundalall, T.; Chetty, S.; Barth, S. Principles of Immunotherapy: Implications for Treatment Strategies in Cancer and Infectious Diseases. Front. Microbiol. 2018, 9, 3158. [Google Scholar] [CrossRef]
- Wan, X.; Song, M.; Wang, A.; Zhao, Y.; Wei, Z.; Lu, Y. Microbiome Crosstalk in Immunotherapy and Antiangiogenesis Therapy. Front. Immunol. 2021, 12, 747914. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.; Karpinets, T.; Prieto, P.; Vicente, D.; Hoffman, K.; Wei, S.C. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Li, W.N.; Deng, Y.; Chu, Q.; Zhang, P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019, 447, 41–47. [Google Scholar] [CrossRef]
- Rivalland, G.; Scott, A.M.; John, T. Standard of care in immunotherapy trials: Challenges and considerations. Hum. Vaccin. Immunother. 2017, 13, 2164–2178. [Google Scholar] [CrossRef]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022, 20, 44. [Google Scholar] [CrossRef]
- Cunningham, N.; Lapointe, R.; Lerouge, S. Biomaterials for enhanced immunotherapy. APL Bioeng. 2022, 6, 041502. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar] [CrossRef]
- Munir, S.; Lundsager, M.T.; Jørgensen, M.A.; Hansen, M.; Petersen, T.H.; Bonefeld, C.M.; Friese, C.; Met, Ö.; Straten, P.T.; Andersen, M.H. Inflammation induced PD-L1-specific T cells. Cell Stress 2019, 3, 319–327. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Badrinath, N.; Jeong, S.N.; Woo, H.Y.; Heo, J. Overcoming Tumor Resistance to Oncolyticvaccinia Virus with Anti-PD-1-Based Combination Therapy by Inducing Antitumor Immunity in the Tumor Microenvironment. Vaccines 2020, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, S.; Zekiy, A.O.; Khanamir, R.A.; Zaman, B.A.; Ghayourvahdat, A.; Azimizonuzi, H.; Zamani, M. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022, 22, 2. [Google Scholar] [CrossRef]
- Bajwa, R.; Cheema, A.; Khan, T.; Amirpour, A.; Paul, A.; Chaughtai, S.; Patel, S.; Patel, T.; Bramson, J.; Gupta, V.; et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J. Clin. Med. Res. 2019, 11, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Franzin, R.; Netti, G.S.; Spadaccino, F.; Porta, C.; Gesualdo, L.; Stallone, G.; Castellano, G.; Ranieri, E. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Front. Immunol. 2020, 11, 574271. [Google Scholar] [CrossRef]
- Tang, X.Y.; Shi, A.P.; Xiong, Y.L.; Zheng, K.F.; Liu, Y.J.; Shi, X.G.; Jiang, T.; Zhao, J.B. Clinical Research on the Mechanisms Underlying Immune Checkpoints and Tumor Metastasis. Front. Oncol. 2021, 11, 693321. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Van Den Eeckhout, B.; Tavernier, J.; Gerlo, S. Interleukin-1 as Innate Mediator of T Cell Immunity. Front. Immunol. 2021, 11, 621931. [Google Scholar] [CrossRef]
- Mashima, H.; Zhang, R.; Kobayashi, T.; Hagiya, Y.; Tsukamoto, H.; Liu, T.; Iwama, T.; Yamamoto, M.; Lin, C.; Nakatsuka, R.; et al. Generation of GM-CSF-producing antigen-presenting cells that induce a cytotoxic T cell-mediated antitumor response. Oncoimmunology 2020, 9, 1814620. [Google Scholar] [CrossRef]
- Sanborn, R.E.; Schneiders, F.L.; Senan, S.; Gadgeel, S.M. Beyond Checkpoint Inhibitors: Enhancing Antitumor Immune Response in Lung Cancer. Am. Soc. Clin. Oncol. Educ. Book 2022, 41, 673–686. [Google Scholar] [CrossRef]
- Sadeghi Najafabadi, S.A.; Bolhassani, A.; Aghasadeghi, M.R. Tumor cell-based vaccine: An effective strategy for eradication of cancer cells. Immunotherapy 2022, 14, 639–654. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef]
- He, Q.; Gao, H.; Tan, D.; Zhang, H.; Wang, J.-z. mRNA cancer vaccines: Advances, trends and challenges. Acta Pharm. Sin. B 2022, 12, 2969–2989. [Google Scholar] [CrossRef]
- Fu, C.; Zhou, L.; Mi, Q.-S.; Jiang, A. DC-Based Vaccines for Cancer Immunotherapy. Vaccines 2020, 8, 706. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Zhou, W.-L.; Huang, Y.; Liang, X.; Jiang, L.; Yang, X.; Sun, J.; Li, Z.; Han, W.-D.; et al. Genetically engineered T cells for cancer immunotherapy. Signal Transduct. Target. Ther. 2019, 4, 35. [Google Scholar] [CrossRef]
- Hammerstrom, A.E.; Cauley, D.H.; Atkinson, B.J.; Sharma, P. Cancer immunotherapy: Sipuleucel-T and beyond. Pharmacotherapy 2011, 31, 813–828. [Google Scholar] [CrossRef]
- Saxena, M.; Balan, S.; Roudko, V.; Bhardwaj, N. Towards superior dendritic-cell vaccines for cancer therapy. Nat. Biomed. Eng. 2018, 2, 341–346. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Truong, C.S.; Yoo, S.Y. Oncolytic Vaccinia Virus in Lung Cancer Vaccines. Vaccines 2022, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Mardis, E.R. Neoantigens and genome instability: Impact on immunogenomic phenotypes and immunotherapy response. Genome Med. 2019, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Dahan, R. Next Generation CD40 Agonistic Antibodies for Cancer Immunotherapy. Front. Immunol. 2022, 13, 940674. [Google Scholar] [CrossRef]
- Vonderheide, R.H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu. Rev. Med. 2020, 71, 47–58. [Google Scholar] [CrossRef]
- van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar] [CrossRef]
- Chester, C.; Sanmamed, M.F.; Wang, J.; Melero, I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood 2018, 131, 49–57. [Google Scholar] [CrossRef]
- Segal, N.H.; Logan, T.F.; Hodi, F.S.; McDermott, D.; Melero, I.; Hamid, O.; Schmidt, H.; Robert, C.; Chiarion-Sileni, V.; Ascierto, P.A.; et al. Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody. Clin. Cancer Res. 2017, 23, 1929–1936. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Sznol, M.; Hu-Lieskovan, S.; Papadopoulos, K.P.; Patnaik, A.; Rasco, D.W.; Di Gravio, D.; Huang, B.; Gambhire, D.; Chen, Y.; et al. Phase Ib Study of Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Combination with Pembrolizumab (MK-3475) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 5349–5357. [Google Scholar] [CrossRef]
- Buchan, S.L.; Rogel, A.; Al-Shamkhani, A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 2018, 131, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, Y.J. Engineered T Cell Therapy for Cancer in the Clinic. Front. Immunol. 2019, 10, 2250. [Google Scholar] [CrossRef] [PubMed]
- Jogalekar, M.P.; Rajendran, R.L.; Khan, F.; Dmello, C.; Gangadaran, P.; Ahn, B.C. CAR T-Cell-Based gene therapy for cancers: New perspectives, challenges, and clinical developments. Front. Immunol. 2022, 13, 925985. [Google Scholar] [CrossRef] [PubMed]
- Scholler, J.; Brady, T.L.; Binder-Scholl, G.; Hwang, W.T.; Plesa, G.; Hege, K.M.; Vogel, A.N.; Kalos, M.; Riley, J.L.; Deeks, S.G.; et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012, 4, 132ra153. [Google Scholar] [CrossRef]
- Abou-El-Enein, M.; Elsallab, M.; Feldman, S.A.; Fesnak, A.D.; Heslop, H.E.; Marks, P.; Till, B.G.; Bauer, G.; Savoldo, B. Scalable Manufacturing of CAR T cells for Cancer Immunotherapy. Blood Cancer Discov. 2021, 2, 408–422. [Google Scholar] [CrossRef]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef]
- Al-Haideri, M.; Tondok, S.B.; Safa, S.H.; maleki, A.H.; Rostami, S.; Jalil, A.T.; Al-Gazally, M.E.; Alsaikhan, F.; Rizaev, J.A.; Mohammad, T.A.M.; et al. CAR-T cell combination therapy: The next revolution in cancer treatment. Cancer Cell Int. 2022, 22, 365. [Google Scholar] [CrossRef]
- Sengsayadeth, S.; Savani, B.N.; Oluwole, O.; Dholaria, B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. EJHaem 2022, 3, 6–10. [Google Scholar] [CrossRef]
- Ping, Y.; Liu, C.; Zhang, Y. T-cell receptor-engineered T cells for cancer treatment: Current status and future directions. Protein Cell 2018, 9, 254–266. [Google Scholar] [CrossRef]
- Sharpe, M.; Mount, N. Genetically modified T cells in cancer therapy: Opportunities and challenges. Dis. Model. Mech. 2015, 8, 337–350. [Google Scholar] [CrossRef]
- Santos Apolonio, J.; Lima de Souza Gonçalves, V.; Cordeiro Santos, M.L.; Silva Luz, M.; Silva Souza, J.V.; Rocha Pinheiro, S.L.; de Souza, W.R.; Sande Loureiro, M.; de Melo, F.F. Oncolytic virus therapy in cancer: A current review. World J. Virol. 2021, 10, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Y.; Badrinath, N.; Woo, H.Y.; Heo, J. Oncolytic Virus-Based Immunotherapies for Hepatocellular Carcinoma. Mediat. Inflamm. 2017, 2017, 5198798. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Doley, J.; Kumar, G.R.; Sahoo, A.P.; Tiwari, A.K. Oncolytic viruses & their specific targeting to tumour cells. Indian J. Med. Res. 2012, 136, 571–584. [Google Scholar] [PubMed]
- Badrinath, N.; Heo, J.; Yoo, S.Y. Viruses as nanomedicine for cancer. Int. J. Nanomed. 2016, 11, 4835–4847. [Google Scholar] [CrossRef]
- Jeong, S.N.; Yoo, S.Y. Novel Oncolytic Virus Armed with Cancer Suicide Gene and Normal Vasculogenic Gene for Improved Anti-Tumor Activity. Cancers 2020, 12, 1070. [Google Scholar] [CrossRef]
- Yang, L.; Gu, X.; Yu, J.; Ge, S.; Fan, X. Oncolytic Virotherapy: From Bench to Bedside. Front. Cell Dev. Biol. 2021, 9, 790150. [Google Scholar] [CrossRef]
- Ramesh, N.; Ge, Y.; Ennist, D.L.; Zhu, M.; Mina, M.; Ganesh, S.; Reddy, P.S.; Yu, D.C. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor--armed oncolytic adenovirus for the treatment of bladder cancer. Clin. Cancer Res. 2006, 12, 305–313. [Google Scholar] [CrossRef]
- Ruano, D.; López-Martín, J.A.; Moreno, L.; Lassaletta, Á.; Bautista, F.; Andión, M.; Hernández, C.; González-Murillo, Á.; Melen, G.; Alemany, R.; et al. First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol. Ther. 2020, 28, 1033–1042. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, J.Y.; Park, B.H.; Lee, D.E.; Kim, J.S.; Park, H.E.; Roh, M.S.; Je, J.E.; Yoon, J.H.; Thorne, S.H.; et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 2006, 14, 361–370. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Bang, S.Y.; Jeong, S.N.; Kang, D.H.; Heo, J. A cancer-favoring oncolytic vaccinia virus shows enhanced suppression of stem-cell like colon cancer. Oncotarget 2016, 7, 16479–16489. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Jeong, S.N.; Kang, D.H.; Heo, J. Evolutionary cancer-favoring engineered vaccinia virus for metastatic hepatocellular carcinoma. Oncotarget 2017, 8, 71489–71499. [Google Scholar] [CrossRef]
- Poh, A. First Oncolytic Viral Therapy for Melanoma. Cancer Discov. 2016, 6, 6. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Ailia, M.J.; Yoo, S.Y. In Vivo Oncolytic Virotherapy in Murine Models of Hepatocellular Carcinoma: A Systematic Review. Vaccines 2022, 10, 1541. [Google Scholar] [CrossRef]
- Shrestha, K.R.; Lee, D.H.; Chung, W.; Lee, S.W.; Lee, B.Y.; Yoo, S.Y. Biomimetic virus-based soft niche for ischemic diseases. Biomaterials 2022, 288, 121747. [Google Scholar] [CrossRef]
- Melero, I.; Castanon, E.; Alvarez, M.; Champiat, S.; Marabelle, A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat. Rev. Clin. Oncol. 2021, 18, 558–576. [Google Scholar] [CrossRef]
- Crittenden, M.R.; Thanarajasingam, U.; Vile, R.G.; Gough, M.J. Intratumoral immunotherapy: Using the tumour against itself. Immunology 2005, 114, 11–22. [Google Scholar] [CrossRef]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef]
- Porter, C.J.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef]
- Chaudhary, S.; Garg, T.; Murthy, R.; Rath, G.; Goyal, A.K. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J. Drug Target. 2014, 22, 871–882. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.; Ansari, D.; Sasor, A.; Andersson, R. SPARC: A potential prognostic and therapeutic target in pancreatic cancer. Pancreas 2015, 44, 1024. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Goldberg, S.L.; Feldman, E.J.; Rizzeri, D.A.; Hogge, D.E.; Larson, M.; Pigneux, A.; Recher, C.; Schiller, G.; Warzocha, K. Phase II, multicenter, randomized trial of CPX-351 (cytarabine: Daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer 2015, 121, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O. Eligard: Leuprolide acetate in a novel sustained-release delivery system. Urology 2003, 61, 25–31. [Google Scholar] [CrossRef]
- Podust, V.N.; Balan, S.; Sim, B.-C.; Coyle, M.P.; Ernst, U.; Peters, R.T.; Schellenberger, V. Extension of in vivo half-life of biologically active molecules by XTEN protein polymers. J. Control. Release 2016, 240, 52–66. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.H.; Park, C.G.; Lee, C.; Lim, B.Y.; Choy, Y.B. Implantable small device enabled with magnetic actuation for on-demand and pulsatile drug delivery. J. Control. Release 2018, 286, 224–230. [Google Scholar] [CrossRef]
- Roth, A.; Rohrbach, F.; Weth, R.; Frisch, B.; Schuber, F.; Wels, W.S. Induction of effective and antigen-specific antitumour immunity by a liposomal ErbB2/HER2 peptide-based vaccination construct. Br. J. Cancer 2005, 92, 1421–1429. [Google Scholar] [CrossRef]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Watarai, S.; Kono, K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials 2013, 34, 3042–3052. [Google Scholar] [CrossRef]
- Zheng, Y.; Stephan, M.T.; Gai, S.A.; Abraham, W.; Shearer, A.; Irvine, D.J. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J. Control. Release 2013, 172, 426–435. [Google Scholar] [CrossRef]
- Aoyama, K.; Kuroda, S.; Morihiro, T.; Kanaya, N.; Kubota, T.; Kakiuchi, Y.; Kikuchi, S.; Nishizaki, M.; Kagawa, S.; Tazawa, H.; et al. Liposome-encapsulated plasmid DNA of telomerase-specific oncolytic adenovirus with stealth effect on the immune system. Sci. Rep. 2017, 7, 14177. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Han, Y.; Zhang, J.; Liu, D.; Ma, G.; Li, C.; Liu, L.; Kong, D. Nanovaccine Incorporated with Hydroxychloroquine Enhances Antigen Cross-Presentation and Promotes Antitumor Immune Responses. ACS Appl. Mater. Interfaces 2018, 10, 30983–30993. [Google Scholar] [CrossRef]
- Reda, M.; Ngamcherdtrakul, W.; Nelson, M.A.; Siriwon, N.; Wang, R.; Zaidan, H.Y.; Bejan, D.S.; Reda, S.; Hoang, N.H.; Crumrine, N.A.; et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat. Commun. 2022, 13, 4261. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Song, B.; Ma, Z.; Zhang, Y.; Sun, H.; Wei, X.; Bai, Y.; Lu, X.; Zhang, P.; et al. Polymeric PD-L1 blockade nanoparticles for cancer photothermal-immunotherapy. Biomaterials 2022, 280, 121312. [Google Scholar] [CrossRef]
- Huang, K.-W.; Hsu, F.-F.; Qiu, J.T.; Qiu, J.T.; Chern, G.-J.; Lee, Y.-A.; Chang, C.-C.; Huang, Y.-T.; Sung, Y.-C.; Chiang, C.-C.; et al. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci. Adv. 2020, 6, eaax5032. [Google Scholar] [CrossRef]
- Roy, D.G.; Bell, J.C.; Bourgeois-Daigneault, M.C. Magnetic targeting of oncolytic VSV-based therapies improves infection of tumor cells in the presence of virus-specific neutralizing antibodies in vitro. Biochem. Biophys. Res. Commun. 2020, 526, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Tresilwised, N.; Pithayanukul, P.; Mykhaylyk, O.; Holm, P.S.; Holzmüller, R.; Anton, M.; Thalhammer, S.; Adigüzel, D.; Döblinger, M.; Plank, C. Boosting Oncolytic Adenovirus Potency with Magnetic Nanoparticles and Magnetic Force. Mol. Pharm. 2010, 7, 1069–1089. [Google Scholar] [CrossRef]
- Yin, Y.; Li, X.; Ma, H.; Zhang, J.; Yu, D.; Zhao, R.; Yu, S.; Nie, G.; Wang, H. In Situ Transforming RNA Nanovaccines from Polyethylenimine Functionalized Graphene Oxide Hydrogel for Durable Cancer Immunotherapy. Nano Lett. 2021, 21, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, W.; Wright, G.; Wang, A.Z.; Gu, Z. Inflammation-Triggered Cancer Immunotherapy by Programmed Delivery of CpG and Anti-PD1 Antibody. Adv. Mater. 2016, 28, 8912–8920. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, Q.; Zhao, X.; Zhao, R.; Wang, Y.; Wang, Y.; Liu, J.; Shang, Y.; Zhao, S.; Wu, T.; et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 2021, 20, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Kooijmans, S.A.; Aleza, C.G.; Roffler, S.R.; van Solinge, W.W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Zhang, H.; Xu, P.; Liu, J.; Ma, J.; Lv, M.; Li, D.; Katirai, F.; Shen, G.-X.; et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun. 2012, 3, 1282. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, F.; Liu, Y.; Wang, Z.; Yu, G.; Liang, B.; Niu, G.; Su, T.; Zhu, G.; Lu, G.; et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 2020, 6, eaaw6071. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, T.; Zhou, S.; Wang, W.; Lin, S.; Zhu, G. pH-Responsive STING-Activating DNA Nanovaccines for Cancer Immunotherapy. Adv. Ther. 2020, 3, 2000083. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Tian, B.; Liu, J.; Yang, L.; Zeng, L.; Chen, T.; Hong, A.; Wang, X. Nucleolin-targeted Extracellular Vesicles as a Versatile Platform for Biologics Delivery to Breast Cancer. Theranostics 2017, 7, 1360–1372. [Google Scholar] [CrossRef]
- Yuan, Z.; Kolluri, K.K.; Gowers, K.H.; Janes, S.M. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J. Extracell. Vesicles 2017, 6, 1265291. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Y.; Tang, K.; Zhang, H.; Yin, X.; Li, Y.; Xu, P.; Sun, Y.; Ma, R.; Ji, T.; et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016, 26, 713–727. [Google Scholar] [CrossRef]
- Morishita, M.; Takahashi, Y.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials 2016, 111, 55–65. [Google Scholar] [CrossRef]
- Liang, Q.; Bie, N.; Yong, T.; Tang, K.; Shi, X.; Wei, Z.; Jia, H.; Zhang, X.; Zhao, H.; Huang, W.; et al. The softness of tumour-cell-derived microparticles regulates their drug-delivery efficiency. Nat. Biomed. Eng. 2019, 3, 729–740. [Google Scholar] [CrossRef]
- Phuengkham, H.; Song, C.; Um, S.H.; Lim, Y.T. Implantable Synthetic Immune Niche for Spatiotemporal Modulation of Tumor-Derived Immunosuppression and Systemic Antitumor Immunity: Postoperative Immunotherapy. Adv. Mater. 2018, 30, e1706719. [Google Scholar] [CrossRef]
- Ali, O.A.; Huebsch, N.; Cao, L.; Dranoff, G.; Mooney, D.J. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 2009, 8, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.H.; Ren, L.; Kim, S.M.; Seo, S.H.; Jung, C.R.; Kim, D.S.; Noh, J.Y.; Lee, S.Y.; Lee, H.; Cho, M.Y.; et al. A three-dimensional hyaluronic acid-based niche enhances the therapeutic efficacy of human natural killer cell-based cancer immunotherapy. Biomaterials 2020, 247, 119960. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, M.; Zhang, J.; Wang, J.; Song, Y.; Shi, J.; Li, W.; Wu, G.; Ren, J.; Wang, Z.; et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology 2016, 5, e1074374. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Huang, P.; Niu, J.; Shi, G.; Zhang, C.; Kong, D.; Wang, W. Injectable polypeptide hydrogel for dual-delivery of antigen and TLR3 agonist to modulate dendritic cells in vivo and enhance potent cytotoxic T-lymphocyte response against melanoma. Biomaterials 2018, 159, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Zhang, X.; Yu, S.; Wen, D.; Hu, Q.; Ye, Y.; Bomba, H.; Hu, X.; Liu, Z.; et al. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. 2018, 10, eaan3682. [Google Scholar] [CrossRef]
- Xie, X.; Wang, H.; Williams, G.R.; Yang, Y.; Zheng, Y.; Wu, J.; Zhu, L.M. Erythrocyte Membrane Cloaked Curcumin-Loaded Nanoparticles for Enhanced Chemotherapy. Pharmaceutics 2019, 11, 429. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Zhang, Y.; Cao, F.; Dong, K.; Ren, J.; Qu, X. Erythrocyte Membrane Cloaked Metal-Organic Framework Nanoparticle as Biomimetic Nanoreactor for Starvation-Activated Colon Cancer Therapy. ACS Nano 2018, 12, 10201–10211. [Google Scholar] [CrossRef]
- Luk, B.T.; Fang, R.H.; Hu, C.-M.J.; Copp, J.A.; Thamphiwatana, S.; Dehaini, D.; Gao, W.; Zhang, K.; Li, S.; Zhang, L. Safe and Immunocompatible Nanocarriers Cloaked in RBC Membranes for Drug Delivery to Treat Solid Tumors. Theranostics 2016, 6, 1004–1011. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Chen, H.; Wei, J.; Qian, H.; Su, S.; Shao, J.; Wang, L.; Qian, X.; Liu, B. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: A new approach to enhance drug targeting in gastric cancer. Int. J. Nanomed. 2017, 12, 2129–2142. [Google Scholar] [CrossRef]
- Deng, G.; Sun, Z.; Li, S.; Peng, X.; Li, W.; Zhou, L.; Ma, Y.; Gong, P.; Cai, L. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano 2018, 12, 12096–12108. [Google Scholar] [CrossRef]
- Sridhar, P.; Petrocca, F. Regional Delivery of Chimeric Antigen Receptor (CAR) T-Cells for Cancer Therapy. Cancers 2017, 9, 92. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Waheed, S.; Li, Z.; Zhang, F.; Chiarini, A.; Armato, U.; Wu, J. Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J. Nanobiotechnology 2022, 20, 395. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Cao, J.; Gao, H. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J. Mater. Chem. B 2020, 8, 6765–6781. [Google Scholar] [CrossRef]
- Hosseini, M.; Haji-Fatahaliha, M.; Jadidi-Niaragh, F.; Majidi, J.; Yousefi, M. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1051–1061. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; He, C.; Riviere, J.E.; Monteiro-Riviere, N.A.; Lin, Z. Meta-Analysis of Nanoparticle Delivery to Tumors Using a Physiologically Based Pharmacokinetic Modeling and Simulation Approach. ACS Nano 2020, 14, 3075–3095. [Google Scholar] [CrossRef]
- Pudlarz, A.; Szemraj, J. Nanoparticles as Carriers of Proteins, Peptides and Other Therapeutic Molecules. Open Life Sci. 2018, 13, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced permeability and retention effect: A key facilitator for solid tumor targeting by nanoparticles. Photodiagnosis Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, B.; Cao, G.; Huang, W.; Jia, F.; Nie, G.; Wang, H. Direct Presentation of Tumor-Associated Antigens to Induce Adaptive Immunity by Personalized Dendritic Cell-Mimicking Nanovaccines. Adv. Mater. 2022, 34, 2205950. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Tang, W.; Wang, M.; Zhou, S.; Wang, H.; Wu, Z.; Hao, Z.; Li, Z.; Liu, L.; Xie, J. Protein Nanocage Mediated Fibroblast-Activation Protein Targeted Photoimmunotherapy To Enhance Cytotoxic T Cell Infiltration and Tumor Control. Nano Lett. 2017, 17, 862–869. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Yang, J.; Gao, H.-Y.; Shen, Y.; Jiang, L.; Zhou, C.; Li, Y.-F.; He, R.-R.; Liu, M. Folate-Conjugated Halloysite Nanotubes, an Efficient Drug Carrier, Deliver Doxorubicin for Targeted Therapy of Breast Cancer. ACS Appl. Nano Mater. 2018, 1, 595–608. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Lu, S.; Li, C.; Zhao, W.; Zhao, Y.; Yu, S.; Wang, T.; Sun, T. Nano micelles of cellulose-graft-poly (l-lactic acid) anchored with epithelial cell adhesion antibody for enhanced drug loading and anti-tumor effect. Mater. Today Commun. 2020, 22, 100764. [Google Scholar] [CrossRef]
- Chiang, C.-S.; Lin, Y.-J.; Lee, R.; Lai, Y.-H.; Cheng, H.-W.; Hsieh, C.-H.; Shyu, W.-C.; Chen, S.-Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018, 13, 746–754. [Google Scholar] [CrossRef]
- Badrinath, N.; Jeong, Y.I.; Woo, H.Y.; Bang, S.Y.; Kim, C.; Heo, J.; Kang, D.H.; Yoo, S.Y. Local delivery of a cancer-favoring oncolytic vaccinia virus via poly (lactic-co-glycolic acid) nanofiber for theranostic purposes. Int. J. Pharm. 2018, 552, 437–442. [Google Scholar] [CrossRef]
- Mohapatra, A.; Sathiyamoorthy, P.; Park, I.K. Metallic Nanoparticle-Mediated Immune Cell Regulation and Advanced Cancer Immunotherapy. Pharmaceutics 2021, 13, 1867. [Google Scholar] [CrossRef]
- Fang, X.; Lan, H.; Jin, K.; Gong, D.; Qian, J. Nanovaccines for Cancer Prevention and Immunotherapy: An Update Review. Cancers 2022, 14, 3842. [Google Scholar] [CrossRef]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef]
- Jin, L.; Yang, D.; Song, Y.; Li, D.; Xu, W.; Zhu, Y.; Xu, C.F.; Lu, Y.; Yang, X. In Situ Programming of Nanovaccines for Lymph Node-Targeted Delivery and Cancer Immunotherapy. ACS Nano 2022, 16, 15226–15236. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wu, X.; Chen, J.; Yang, M.; Ma, F.; Shi, L. In Situ Antigen-Capturing Nanochaperone Toward Personalized Nanovaccine for Cancer Immunotherapy. Small 2022, 18, 2203100. [Google Scholar] [CrossRef]
- Xu, J.; Lv, J.; Zhuang, Q.; Yang, Z.; Cao, Z.; Xu, L.; Pei, P.; Wang, C.; Wu, H.; Dong, Z.; et al. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; He, T.; Liu, P.; Yi, Z.; Zhu, S.; Liang, X.; Kang, E.; Gong, C.; Liu, X. Self-Adjuvanted Molecular Activator (SeaMac) Nanovaccines Promote Cancer Immunotherapy. Adv. Healthc. Mater. 2021, 10, 2002080. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Shen, P.; Wang, J.; Shen, Y.; Shen, Y.; Webster, T.J.; Deng, J. Applications of Inorganic Nanomaterials in Photothermal Therapy Based on Combinational Cancer Treatment. Int. J. Nanomed. 2020, 15, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Review: Organic nanoparticle based active targeting for photodynamic therapy treatment of breast cancer cells. Oncotarget 2020, 11, 2120–2136. [Google Scholar] [CrossRef]
- Kim, J.; Chun, S.H.; Amornkitbamrung, L.; Song, C.; Yuk, J.S.; Ahn, S.Y.; Kim, B.W.; Lim, Y.T.; Oh, B.K.; Um, S.H. Gold nanoparticle clusters for the investigation of therapeutic efficiency against prostate cancer under near-infrared irradiation. Nano Converg. 2020, 7, 5. [Google Scholar] [CrossRef]
- Lu, Z.; Bai, S.; Jiang, Y.; Wu, S.; Xu, D.; Zhang, J.; Peng, X.; Zhang, H.; Shi, Y.; Liu, G. Amplifying Dendritic Cell Activation by Bioinspired Nanometal Organic Frameworks for Synergistic Sonoimmunotherapy. Small 2022, 18, e2203952. [Google Scholar] [CrossRef]
- Alili, L.; Sack, M.; Karakoti, A.S.; Teuber, S.; Puschmann, K.; Hirst, S.M.; Reilly, C.M.; Zanger, K.; Stahl, W.; Das, S. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor–stroma interactions. Biomaterials 2011, 32, 2918–2929. [Google Scholar] [CrossRef]
- Mardhian, D.F.; Storm, G.; Bansal, R.; Prakash, J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. J. Control. Release 2018, 290, 1–10. [Google Scholar] [CrossRef]
- Hu, C.; Liu, X.; Ran, W.; Meng, J.; Zhai, Y.; Zhang, P.; Yin, Q.; Yu, H.; Zhang, Z.; Li, Y. Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials 2017, 144, 60–72. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, W.; Liang, C.; Shi, S.; Yu, X.; Chen, Q.; Sun, T.; Lu, Y.; Zhang, Y.; Guo, Q. Codelivery nanosystem targeting the deep microenvironment of pancreatic cancer. Nano Lett. 2019, 19, 3527–3534. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Sang, Y.; Deng, Q.; Cao, F.; Liu, Z.; You, Y.; Liu, H.; Ren, J.; Qu, X. Remodeling Macrophages by an Iron Nanotrap for Tumor Growth Suppression. ACS Nano 2021, 15, 19298–19309. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, J.; Zhu, X.; Liu, Y.; Wang, X.; Wang, H.; Yao, Y.; Gao, Y.; Chen, Z. Development of toll-like receptor agonist-loaded nanoparticles as precision immunotherapy for reprogramming tumor-associated macrophages. ACS Appl. Mater. Interfaces 2021, 13, 24442–24452. [Google Scholar] [CrossRef]
- Hu, X.-X.; He, P.-P.; Qi, G.-B.; Gao, Y.-J.; Lin, Y.-X.; Yang, C.; Yang, P.-P.; Hao, H.; Wang, L.; Wang, H. Transformable nanomaterials as an artificial extracellular matrix for inhibiting tumor invasion and metastasis. ACS Nano 2017, 11, 4086–4096. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, K.; Sun, J.; Zhang, T.; Zhang, Q.; Song, L.; Zhang, X.; Gu, N. Fabrication of hydrogel with cell adhesive micropatterns for mimicking the oriented tumor-associated extracellular matrix. ACS Appl. Mater. Interfaces 2014, 6, 10963–10968. [Google Scholar] [CrossRef]
- Grossman, M.; Ben-Chetrit, N.; Zhuravlev, A.; Afik, R.; Bassat, E.; Solomonov, I.; Yarden, Y.; Sagi, I. Tumor Cell Invasion Can Be Blocked by Modulators of Collagen Fibril Alignment That Control Assembly of the Extracellular MatrixDisruption of Collagen Fibril Alignment Attenuates Cancer. Cancer Res. 2016, 76, 4249–4258. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, Z.; Deng, J.; Lemons, P.K.; Arhontoulis, D.C.; Bowne, W.B.; Cheng, H. Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016, 16, 3268–3277. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Z.; Chen, B.; Dai, W.; Zhang, H.; He, B.; Wang, X.; Wang, Y.; Zhang, Q. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 2018, 25, 1495–1503. [Google Scholar] [CrossRef]
- Du, S.; Xiong, H.; Xu, C.; Lu, Y.; Yao, J. Attempts to strengthen and simplify the tumor vascular normalization strategy using tumor vessel normalization promoting nanomedicines. Biomater. Sci. 2019, 7, 1147–1160. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Du, B.; Li, X.; Liu, S.; Yang, X.-Y.; Ding, H.; Yang, W.; Pan, F.; Wu, X. Gold nanoparticle–mediated targeted delivery of recombinant human endostatin normalizes tumour vasculature and improves cancer therapy. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Gao, W.; Li, S.; Liu, Z.; Sun, Y.; Cao, W.; Tong, L.; Cui, G.; Tang, B. Targeting and destroying tumor vasculature with a near-infrared laser-activated “nanobomb” for efficient tumor ablation. Biomaterials 2017, 139, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Satterlee, A.B.; Rojas, J.D.; Dayton, P.A.; Huang, L. Enhancing nanoparticle accumulation and retention in desmoplastic tumors via vascular disruption for internal radiation therapy. Theranostics 2017, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Nedeva, C.; Mathivanan, S. Engineering Extracellular Vesicles for Cancer Therapy. Subcell. Biochem. 2021, 97, 375–392. [Google Scholar] [CrossRef]
- Elliott, R.O.; He, M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016, 5, e1071008. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Zhang, L.; Ban, L.; Chen, P.; Du, W.; Feng, X.; Liu, B.-F. Chemically Edited Exosomes with Dual Ligand Purified by Microfluidic Device for Active Targeted Drug Delivery to Tumor Cells. ACS Appl. Mater. Interfaces 2017, 9, 27441–27452. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, Y.; Li, B.; Xia, Y.; Wang, H.; Fu, C. Implantable and Injectable Biomaterial Scaffolds for Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2020, 8, 612950. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Park, J.; Shim, M.K.; Um, W.; Yoon, H.Y.; Ryu, J.H.; Lim, D.K.; Kim, K. Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics 2019, 9, 7906–7923. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xu, J.; Yang, Z.; Tong, R.; Dong, Z.; Wang, C.; Leong, K.W. Engineered biomaterials for cancer immunotherapy. MedComm 2020, 1, 35–46. [Google Scholar] [CrossRef]
- Sinha, A.; Choi, Y.; Nguyen, M.H.; Nguyen, T.L.; Choi, S.W.; Kim, J. A 3D Macroporous Alginate Graphene Scaffold with an Extremely Slow Release of a Loaded Cargo for In Situ Long-Term Activation of Dendritic Cells. Adv. Healthc. Mater. 2019, 8, e1800571. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Wang, B.; Fan, Q.; Yang, Q.; Xu, J.; Dai, H.; Xu, F.; Wang, C. Blood Clot Scaffold Loaded with Liposome Vaccine and siRNAs Targeting PD-L1 and TIM-3 for Effective DC Activation and Cancer Immunotherapy. ACS Nano 2023, 17, 760–774. [Google Scholar] [CrossRef]
- Ren, L.; Lim, Y.T. Degradation-Regulatable Architectured Implantable Macroporous Scaffold for the Spatiotemporal Modulation of Immunosuppressive Microenvironment and Enhanced Combination Cancer Immunotherapy. Adv. Funct. Mater. 2018, 28, 1804490. [Google Scholar] [CrossRef]
- Cai, M.-H.; Chen, X.-Y.; Fu, L.-Q.; Du, W.-L.; Yang, X.; Mou, X.-Z.; Hu, P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef]
- Liu, M.; Cao, Z.; Zhang, R.; Chen, Y.; Yang, X. Injectable Supramolecular Hydrogel for Locoregional Immune Checkpoint Blockade and Enhanced Cancer Chemo-Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 33874–33884. [Google Scholar] [CrossRef]
- Chen, M.; Tan, Y.; Dong, Z.; Lu, J.; Han, X.; Jin, Q.; Zhu, W.; Shen, J.; Cheng, L.; Liu, Z.; et al. Injectable Anti-inflammatory Nanofiber Hydrogel to Achieve Systemic Immunotherapy Post Local Administration. Nano Lett. 2020, 20, 6763–6773. [Google Scholar] [CrossRef]
- Song, H.; Yang, P.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. Injectable polypeptide hydrogel-based co-delivery of vaccine and immune checkpoint inhibitors improves tumor immunotherapy. Theranostics 2019, 9, 2299–2314. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, X.; Zhang, F.; Zhang, X.; Tang, F.; Han, Z.; Li, Y. TCR-T Immunotherapy: The Challenges and Solutions. Front. Oncol. 2021, 11, 794183. [Google Scholar] [CrossRef]
- Labanieh, L.; Majzner, R.G.; Mackall, C.L. Programming CAR-T cells to kill cancer. Nat. Biomed. Eng. 2018, 2, 377–391. [Google Scholar] [CrossRef]
- Grosskopf, A.K.; Labanieh, L.; Klysz, D.D.; Roth, G.A.; Xu, P.; Adebowale, O.; Gale, E.C.; Jons, C.K.; Klich, J.H.; Yan, J.; et al. Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci. Adv. 2022, 8, eabn8264. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Archibong, E.; Chen, Q.; Ruan, H.; Ahn, S.; Dukhovlinova, E.; Kang, Y.; Wen, D.; Dotti, G.; et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets. Nat. Biomed. Eng. 2021, 5, 1038–1047. [Google Scholar] [CrossRef]
- Stephan, S.B.; Taber, A.M.; Jileaeva, I.; Pegues, E.P.; Sentman, C.L.; Stephan, M.T. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 2015, 33, 97–101. [Google Scholar] [CrossRef]
- Liao, J.B.; Swensen, R.E.; Ovenell, K.J.; Hitchcock-Bernhardt, K.M.; Reichow, J.L.; Apodaca, M.C.; D’Amico, L.; Childs, J.S.; Higgins, D.M.; Buening, B.J. Phase II trial of albumin-bound paclitaxel and granulocyte macrophage colony-stimulating factor as an immune modulator in recurrent platinum resistant ovarian cancer. Gynecol. Oncol. 2017, 144, 480–485. [Google Scholar] [CrossRef]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef]
- Calmeiro, J.; Carrascal, M.; Gomes, C.; Falcão, A.; Cruz, M.T.; Neves, B.M. Biomaterial-based platforms for in situ dendritic cell programming and their use in antitumor immunotherapy. J. ImmunoTherapy Cancer 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Sonneveld, P.; Hajek, R.; Nagler, A.; Spencer, A.; Bladé, J.; Robak, T.; Zhuang, S.H.; Harousseau, J.L.; Orlowski, R.Z.; Investigators, D.M.S. Combined pegylated liposomal doxorubicin and bortezomib is highly effective in patients with recurrent or refractory multiple myeloma who received prior thalidomide/lenalidomide therapy. Cancer 2008, 112, 1529–1537. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.-P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act. In. Sarc): A multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Gargett, T.; Abbas, M.N.; Rolan, P.; Price, J.D.; Gosling, K.M.; Ferrante, A.; Ruszkiewicz, A.; Atmosukarto, I.I.; Altin, J.; Parish, C.R. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol. Immunother. 2018, 67, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.; Maksymiuk, A.; Goss, G.; Soulieres, D.; Marshall, E.; Cormier, Y.; Ellis, P.M.; Price, A.; Sawhney, R.; Beier, F. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): Phase IIB randomized, multicenter, open-label trial. J. Cancer Res. Clin. Oncol. 2011, 137, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Somerville, R.P.; Lu, T.; Yang, J.C.; Sherry, R.M.; Feldman, S.A.; McIntyre, L.; Bot, A.; Rossi, J.; Lam, N. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol. Ther. 2017, 25, 2245–2253. [Google Scholar] [CrossRef]
- Morgan, R.A.; Johnson, L.A.; Davis, J.L.; Zheng, Z.; Woolard, K.D.; Reap, E.A.; Feldman, S.A.; Chinnasamy, N.; Kuan, C.-T.; Song, H. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum. Gene Ther. 2012, 23, 1043–1053. [Google Scholar] [CrossRef]
- Gauthier, J.; Bezerra, E.D.; Hirayama, A.V.; Fiorenza, S.; Sheih, A.; Chou, C.K.; Kimble, E.L.; Pender, B.S.; Hawkins, R.M.; Vakil, A. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood 2021, 137, 323–335. [Google Scholar] [CrossRef]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty years of cancer nanomedicine: Success, frustration, and hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef]
- Nayak, P.P.; Narayanan, A.; Badekila, A.K.; Kini, S. Nanomedicine in cancer clinics: Are we there yet? Curr. Pathobiol. Rep. 2021, 9, 43–55. [Google Scholar] [CrossRef]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 1–30. [Google Scholar] [CrossRef]
- Das, K.P. Nanoparticles and Convergence of Artificial Intelligence for Targeted Drug Delivery for Cancer Therapy: Current Progress and Challenges. Front. Med.Technol. 2023, 4, 1067144. [Google Scholar] [CrossRef]
| Product Name | Therapy | Type | Cancers Approved | Approved Year |
|---|---|---|---|---|
| Roferon-A | Recombinant IFNα2a | Cytokine | Hairy cell leukemia, follicular lymphoma, melanoma, Kaposi sarcoma | 1986 |
| Intron-A | Recombinant IFNα2b | Cytokine | Hairy cell leukemia, follicular lymphoma, melanoma, Kaposi sarcoma | 1986 |
| Aldesleukin | Recombinant IL-2 | Cytokine | Melanoma and kidney cancer | 1992 |
| Sipuleucel-T | Autologous PBMCs activated with Recombinant human PAP–GM-CSF | Cell-Based Cancer Vaccine | Prostate cancer | 2010 |
| Ipilimumab | CTL A4 mAb | ICI | Melanoma | 2011 |
| Nivolumab | Anti PD-L1 (PD-L1 mAb) | ICI | Melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer | 2014 |
| Pembrolizumab | Anti PD-L1 (PD-L1 mAb) | ICI | Melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. | 2014 |
| T-Vec (Talimogene laherparepvec) | GE Oncolytic HSV1 with GM-CSF | Oncolytic Virus | Melanoma | 2015 |
| Atezolizumab | Anti PD-L1 (PD-L1 mAb) | ICI | Urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer, small cell lung cancer, hepatocellular carcinoma, and alveolar soft part sarcoma. | 2016 |
| Tisagenlecleucel | CD19-specific CAR-T cells | Adoptive cell therapy | B cell acute lymphocytic leukemia and non- Hodgkin lymphoma | 2017 |
| Axicabtagene ciloleucel | CD19-specific CAR-T cells | Adoptive cell therapy | Large B cell lymphoma | 2017 |
| Brexucabtagene autoleucel | CD19-specific CAR-T cells | Adoptive cell therapy | Mantle cell lymphoma (MCL) and acute lymphoblastic leukemia (ALL) | 2020 |
| Lisocabtagene maraleucel | CD19-specific CAR-T cells | Adoptive cell therapy | B cell non-Hodgkin lymphoma | 2021 |
| Idecabtagene vicleucel | B cell Maturation antigen (BCMA) | Adoptive cell therapy | Multiple myeloma | 2021 |
| Ciltacabtagene autoleucel | BCMA | Adoptive cell therapy | Multiple myeloma | 2022 |
| Opdualag | PD1 blocking and Anti-LAG-3 | ICI | Melanoma | 2022 |
| Route of Administration | Advantages | References |
|---|---|---|
| Oral Administration |
| [78,79,80] |
| Intravenous Administration |
| [81,82,83] |
| Subcutaneous Administration |
| [84,85,86] |
| Delivery Technology | Types/Source | Cargo | Cancer Type | Reference |
|---|---|---|---|---|
| Nanoparticles | Liposomes | ErbB2/HER2 peptide | Renal carcinoma | [87] |
| OVA | Thymoma | [88] | ||
| ACT-cell-specific antibodies and Interleukin-2 (IL-2) | Melanoma | [89] | ||
| Plasmid encoding telomerase-specific oncolytic adenovirus | Colorectal cancer | [90] | ||
| Polymer | OVA and Hydroxychloroquine | Thymoma | [91] | |
| PLK1 inhibitor and PD-L1 antibody, | NSCLC | [92] | ||
| IR780 and PD-L1 antagonist | Colorectal cancer | [93] | ||
| Dendrimer | PD-L1 siRNA and IL-2 encoding plasmid DNA | HCC | [94] | |
| Inorganic nanocarriers | Vesicular stomatitis virus, | Colorectal cancer | [95] | |
| Adenovirus | Pancreatic cancer, Colorectal cancer | [96] | ||
| mRNA-encoding OVA and R848 | Melanoma | [97] | ||
| RNA/DNA Technology | Anti-PD-1 antibody and CpG oligodeoxynucleotides, | Melanoma | [98] | |
| OVA | Melanoma | [99] | ||
| Exosomes | Let-7a miRNA | Breast cancer | [100] | |
| EGFR nanobodies | Epidermal | [101] | ||
| Cisplatin | Ovarian cancer and Hepatocarcinoma | [102] | ||
| Nanovaccine | Peptide neoantigen (Adpgk) and R848 and CpG | Colorectal cancer | [103] | |
| cyclic dimeric guanosine monophosphate (CDG) | melanoma | [104] | ||
| Extracellular Vesicles | Dendritic cells | VEGF siRNA | Breast cancer | [105] |
| Bone Marrow-Derived MSC | TRAIL | lung Cancer | [106] | |
| A549 Lung Carcinoma ells (Human) | Doxorubicin | Lung carcinoma | [107] | |
| B16-F10 melanoma cells (Mouse) | CpG DNA | Melanoma | [108] | |
| H22 Hepatocarcinoma cells (Mouse) | Doxorubicin, 5-FU | Hepatocarcinoma | [109] | |
| Implantable Scaffolds | Collagen and HA cross-linking scaffold | GEM, poly(I:C) | Breast cancer | [110] |
| PLG scaffold | GM-CSF, CpG-ODNs | Melanoma | [111] | |
| Hyaluronic acid scaffold | CAR-NK cells | Breast cancer | [112] | |
| Injectable Scaffolds | Alginate Hydrogel | Celecoxib, PD-1 antibody | Melanoma, Breast cancer | [113] |
| PEGylated poly(L-valine) hydrogel | TCL, poly(I:C) | Melanoma | [114] | |
| ROS-degradable hydrogel | GEM, PD-L1 antibody | Melanoma, Breast cancer | [115] | |
| Cell-Based Delivery | Erythrocyte | Curcumin | Liver cancer | [116] |
| Glucose oxidase, Tirapazamine | Colon cancer | [117] | ||
| DOX | Lymphoma | [118] | ||
| Cytotoxic T cells | Taxol | Gastric cancer | [119] | |
| NK cell | TCPP | Breast cancer | [120] | |
| Car-T Cells | Glioblastoma, hepatic colorectal metastases, peritoneal carcinomatosis, pleural mesothelioma, mesothelioma | [121] |
| Delivery Modalities | Immunotherapy Classes | Advantages | Disadvantages |
|---|---|---|---|
| Nanoparticles |
|
|
|
| Extracellular Vesicles |
|
|
|
| Implantable Scaffolds |
|
|
|
| Injectable Scaffolds |
|
|
|
| Cell-Based Delivery |
|
|
|
| Clinical Trial Identifier | Phase | Treatment | Therapy | Delivery Modalities | References |
|---|---|---|---|---|---|
| NCT00466960 | II | Sargramostim and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer That Did Not Respond to Previous Chemotherapy | Combined Therapy (Chemotherapy and Cytokine) | Nanoparticle | [187] |
| NCT02410733 | I | Evaluation of the Safety and Tolerability of i.v. Administration of a Cancer Vaccine in Patients with Advanced Melanoma (Lipo-MERIT) | Vaccine | Liposome | [188] |
| NCT01753089 | I | Dendritic Cell Activating Scaffold in Melanoma | Cell Therapy | Scaffold | [189] |
| NCT00103506 | III | Study of DOXIL/CAELYX (Pegylated Liposomal Doxorubicin) and VELCADE (Bortezomib) or VELCADE Monotherapy for the Treatment of Relapsed Multiple Myeloma | Chemotherapy | Liposome | [190] |
| NCT02379845 | II/III | NBTXR3 Crystalline Nanoparticles and Radiation Therapy in Treating Randomized Patients in Two Arms with Soft Tissue Sarcoma of the Extremity and Trunk Wall | Radiotherapy | Nanoparticle | [191] |
| NCT01052142 | I | Safety Study of a Liposomal Vaccine to Treat Malignant Melanoma | Vaccine | Liposome | [192] |
| NCT00157209 | IIb | Phase 2b Randomized Controlled Study of Tecemotide (L-BLP25) for Immunotherapy of NSCLC (Non-Small Cell Lung Cancer) | Vaccine | Liposome | [193] |
| NCT00924326 | I/II | CAR T Cell Receptor Immunotherapy for Patients With B-cell Lymphoma | CAR-T | [194] | |
| NCT01454596 | I/II | CAR T Cell Receptor Immunotherapy Targeting EGFRvIII for Patients with Malignant Gliomas Expressing EGFRvIII | CAR-T | [195] | |
| NCT01865617 | I/II | Laboratory Treated T Cells in Treating Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia, Non-Hodgkin Lymphoma, or Acute Lymphoblastic Leukemia | CAR-T | [196] |
| Patent Number | Inventors | Title |
|---|---|---|
| US20090010948A1 | Fang Ping Huang, Yu Xiao Chen, Kwan Man | Anti-tumor vaccines delivered by dendritic cells devoid of interleukin-10 |
| US20040156846A1 | Wolfgang Daum, Gerald DeNardo, Diane Ellis-Busby, Alan Foreman, Douglas Gwost, Erik Handy, Robert Ivkov | Therapy via targeted delivery of nanoscale particles using L6 antibodies |
| WO2017151727A1 | Zhen GU, Chao Wang, Yanqi YE | Enhanced cancer immunotherapy by microneedle patch-assisted delivery |
| US20160361268A1 | Chih-Peng Liu, Ya-Chin Lo, Ming-Cheng Wei, Maggie LU, Shuen-Hsiang CHOU, Shih-Ta Chen, Hsiang-Wen TSENG | Intralymphatic delivery of hyaluronan nanoparticle for cancer metastasis |
| WO2011097384A2 | Dapeng Zhou, Li Chun, Patrick Hwu | Tumor targeted delivery of immunomodulators by nanoplymers |
| US8785371B2 | Rameshwar Patil, Eggehard Holler, Keith L. Black, Julia Y. Ljubimova | Drug delivery of temozolomide for systemic based treatment of cancer |
| US20160346204A1 | Wenbin Lin, Chunbai He, Demin Liu | Nanoscale carriers for the delivery or co-delivery of chemotherapeutics, nucleic acids and photosensitizers |
| US9610250B2 | Tarek M. Fahmy, Eric STERN, Richard A. Flavell, Jason Park, Alyssa Siefert, Stephen H. Wrzesinski | Nanolipogel vehicles for controlled delivery of different pharmaceutical agents |
| US20080044484A1 | Boris Minev | Use of polymeric nanoparticles for vaccine delivery |
| US20040038406A1 | Gretchen Unger, Beverly Lundell | Nanoparticle delivery systems and methods of use thereof |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthukutty, P.; Woo, H.Y.; Ragothaman, M.; Yoo, S.Y. Recent Advances in Cancer Immunotherapy Delivery Modalities. Pharmaceutics 2023, 15, 504. https://doi.org/10.3390/pharmaceutics15020504
Muthukutty P, Woo HY, Ragothaman M, Yoo SY. Recent Advances in Cancer Immunotherapy Delivery Modalities. Pharmaceutics. 2023; 15(2):504. https://doi.org/10.3390/pharmaceutics15020504
Chicago/Turabian StyleMuthukutty, Palaniyandi, Hyun Young Woo, Murali Ragothaman, and So Young Yoo. 2023. "Recent Advances in Cancer Immunotherapy Delivery Modalities" Pharmaceutics 15, no. 2: 504. https://doi.org/10.3390/pharmaceutics15020504
APA StyleMuthukutty, P., Woo, H. Y., Ragothaman, M., & Yoo, S. Y. (2023). Recent Advances in Cancer Immunotherapy Delivery Modalities. Pharmaceutics, 15(2), 504. https://doi.org/10.3390/pharmaceutics15020504







