Properties and Bioapplications of Amphiphilic Janus Dendrimers: A Review
Abstract
:1. Introduction
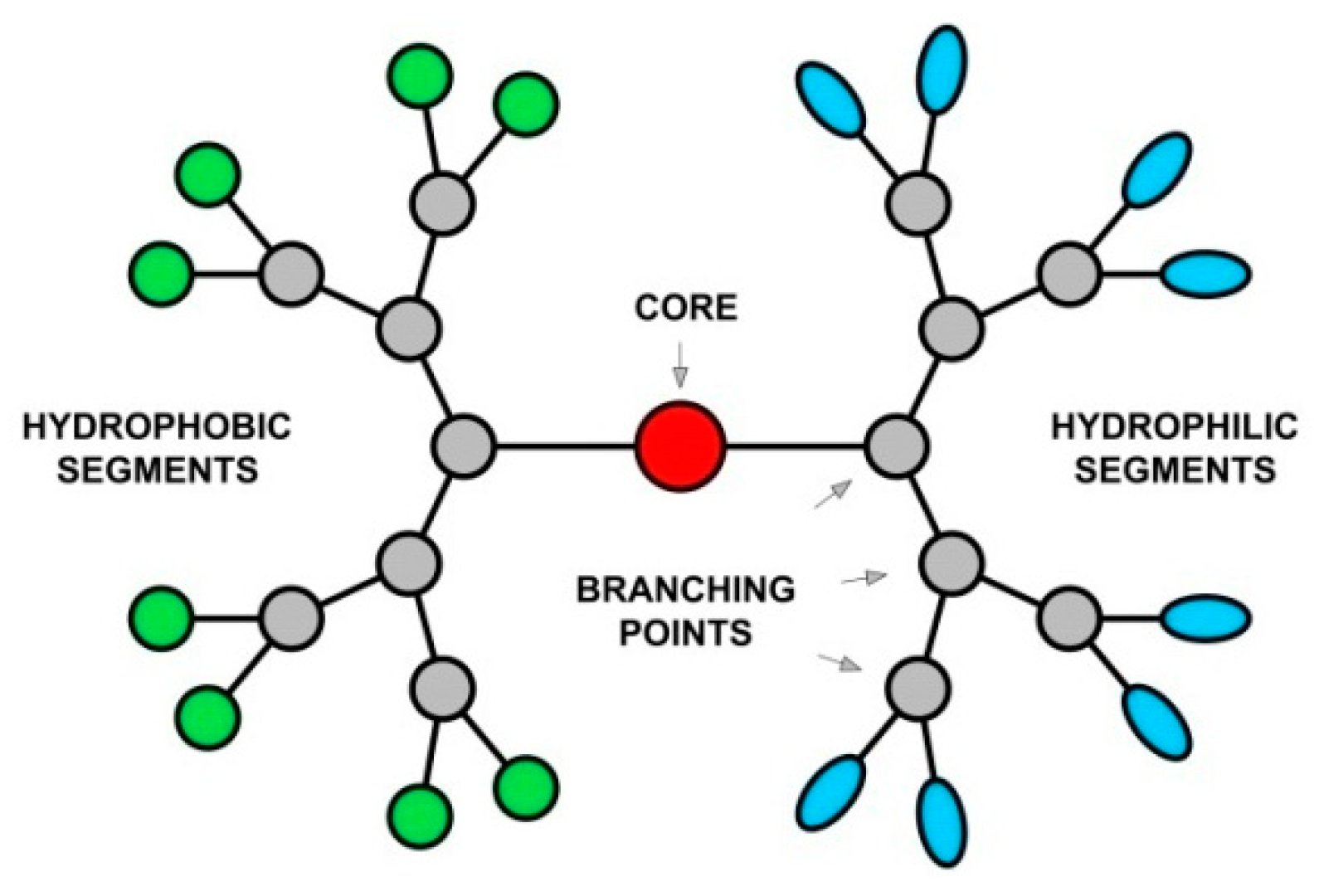
2. Definition and Nomenclature of Amphiphilic JDs
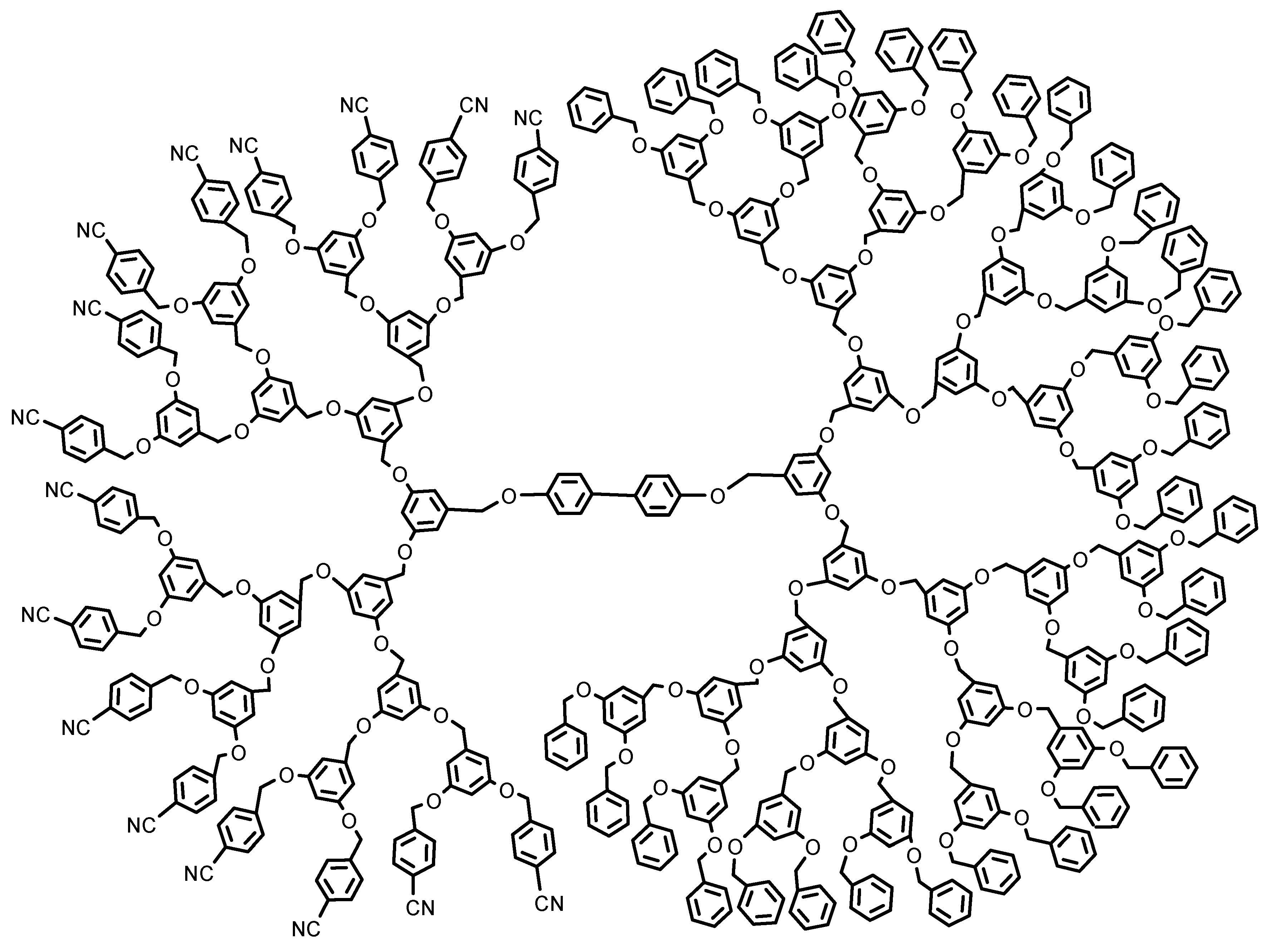
3. Classification of Amphiphilic JDs
4. Characteristics of Amphiphilic JDs and Dendrimersomes (DSs)
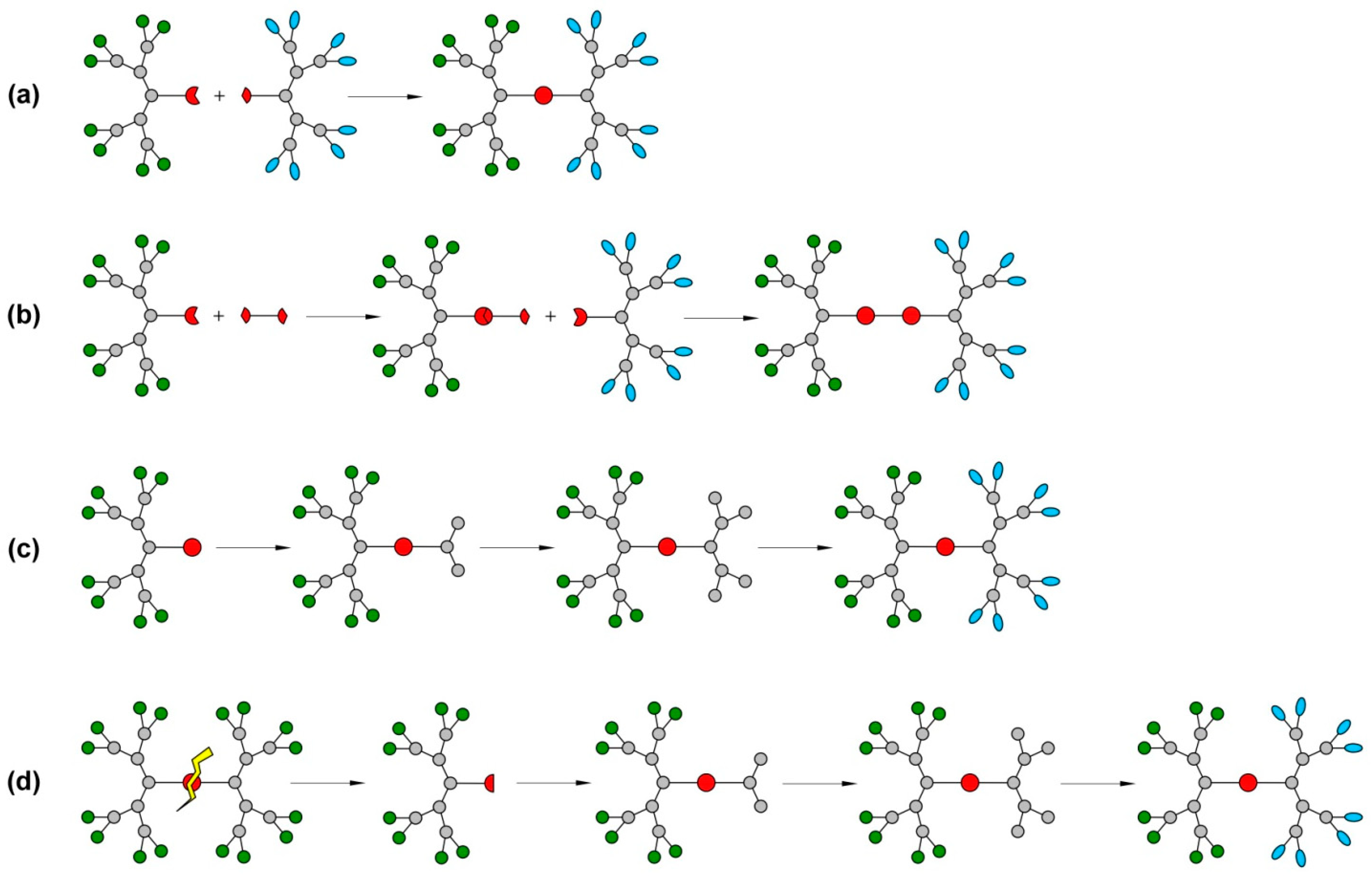
5. Synthesis of JDs
6. Characterization Methods
| Hydrophilic/Hydrophobic Dendrons | Methods | Tests | Results | Conclusions |
|---|---|---|---|---|
| TEG termini /Ferrocenyl (Fc) units [104] | 1H-NMR | Structure elucidation |
|
|
| MS | Molecular mass |
|
| |
| UV-Vis | Structure elucidation |
|
| |
| CV | Electrochemical properties Structure elucidation |
|
| |
| AFM | Self-assembly capability |
|
| |
| DLS | Self-assembly capability |
|
| |
| TEG termini /Ferrocenyl (Fc) units [93] | 1H-NMR | Structure elucidation |
|
|
| MS | Molecular mass |
|
| |
| UV-Vis | Structure elucidation |
|
| |
| (NH3+)8 [GMPA]/ [MPA](C17)4 [114] | 1H-NMR | Structure elucidation |
|
|
| MS | Molecular mass |
|
| |
| SEC | Structure elucidation |
|
| |
| TEM | Morphology of JD aggregates |
|
| |
| DLS | Morphology of JD aggregates |
|
| |
| bis-MPA (G1-G3)/F27-N3 [77] | 1H-NMR | Structure elucidation |
|
|
| 19F-NMR | Structure elucidation |
|
| |
| FTIR | Structure elucidation |
|
| |
| HR-MS (ESI) | Molecular mass |
|
| |
| SWAXS | Molecular organization of JDs in bulk |
|
| |
| POM | Molecular organization of JDs in bulk |
|
| |
| TGA | Molecular organization of JDs in bulk |
|
| |
| DSC | Molecular organization of JDs in bulk |
|
| |
| Cryo-TEM | Morphology of JD assemblies |
|
| |
| DLS | Morphology of JD assemblies |
|
| |
| 19F-NMR | Structure elucidation Morphology of JD assemblies |
|
|
7. Applications of Amphiphilic JDs
7.1. JDs as Stabilizing Agents
7.1.1. Drug Suspensions
7.1.2. Au and Ag Nanoparticles
7.2. JDs as Biological Membrane Mimics
7.2.1. Amphiphilic Janus Dendrimers (JDs)
7.2.2. Janus glycodendrimers (JGDs)
7.3. JDs as Nanocarriers
7.4. JDs as Protein Recruitment Enhancers
7.5. Vectors for Gene Delivery
7.5.1. DNA
7.5.2. Messenger RNA
7.5.3. Small Interference RNAs
7.6. JDs as MRI Traceable Probes
19F-MRI Traceable Probes
8. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Tomalia, D.A.; Fréchet, J.M.J. A Brief Historical Perspective. In Dendrimers and Other Dendritic Polymers; Fréchet, J.M.J., Tomalia, D.A., Eds.; John Wiley & Sons Ltd.: Baffins Lane, Chichester, West Sussex, UK, 2001; pp. xxvii–xxxix. [Google Scholar]
- Miguel, A.F. Dendritic design as an archetype for growth patterns in nature: Fractal and constructal views. Front. Phys. 2014, 2, 9. [Google Scholar] [CrossRef]
- Lanoue, V.; Cooper, H.M. Branching mechanisms shaping dendrite architecture. Dev. Biol. 2019, 451, 16–24. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. I. Gelation. J. Am. Chem. Soc. 1941, 63, 3083–3090. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. II. Trifunctional Branching Units. J. Am. Chem. Soc. 1941, 63, 3091–3096. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. III. Tetrafunctional Branching Units. J. Am. Chem. Soc. 1941, 63, 3096–3100. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vögtle, F. “Cascade”- and “Nonskid-Chain-like” Syntheses of Molecular Cavity Topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Newkome, G.R.; Yao, Z.-Q.; Baker, G.R.; Gupta, V.K. Micelles. Part 1. Cascade molecules: A new approach to micelles. A [27]-arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Seiler, M. Hyperbranched polymers: Phase behavior and new applications in the field of chemical engineering. Fluid Ph. Equilibria 2006, 241, 155–174. [Google Scholar] [CrossRef]
- Aulenta, F.; Hayes, W.; Rannard, S. Dendrimers: A new class of nanoscopic containers and delivery devices. Eur. Polym. J. 2003, 39, 1741–1771. [Google Scholar] [CrossRef]
- Hu, J.; Hu, K.; Cheng, Y. Tailoring the dendrimer core for efficient gene delivery. Acta Biomater. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Patel, P.; Patel, V.; Patel, P.M. Synthetic strategy of dendrimers: A review. J. Indian Chem. Soc. 2022, 99, 100514. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Patel, V.; Patel, P.; Patel, J.V.; Patel, P.M. Dendrimer as a versatile platform for biomedical application: A review. J. Indian Chem. Soc. 2022, 99, 100516. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Pedziwiatr-Werbicka, E.; Milowska, K.; Dzmitruk, V.; Ionov, M.; Shcharbin, D.; Bryszewska, M. Dendrimers and hyperbranched structures for biomedical applications. Eur. Polym. J. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Agatemor, C. Emerging Opportunities in the Biomedical Applications of Dendrimers. J. Inorg. Organomet. Polym. Mater. 2018, 28, 369–382. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, S.; Abourehab, M.A.S.; Sahebkar, A.; Kesharwani, P. Exploring dendrimer-based drug delivery systems and their potential applications in cancer immunotherapy. Eur. Polym. J. 2022, 177, 111471. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Mohgan, R.; Jong, J.S.J.; David, R.N.; Ngan, W.Y.; Chin, T.L.; Ting, S.; Kesharwani, P.; Gorain, B. Dendrimer-based delivery of macromolecules for the treatment of brain tumor. Biomater. Adv. 2022, 141, 213118. [Google Scholar] [CrossRef]
- Arora, V.; Abourehab, M.A.S.; Modi, G.; Kesharwani, P. Dendrimers as prospective nanocarrier for targeted delivery against lung cancer. Eur. Polym. J. 2022, 180, 111635. [Google Scholar] [CrossRef]
- Sathe, R.Y.; Bharatam, P.V. Drug-dendrimer complexes and conjugates: Detailed furtherance through theory and experiments. Adv. Colloid Interface Sci. 2022, 303, 102639. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Shaikh, A.; Kesharwani, P.; Gajbhiye, V. Dendrimer as a momentous tool in tissue engineering and regenerative medicine. J. Control. Release 2022, 346, 328–354. [Google Scholar] [CrossRef]
- Gorain, B.; Tekade, M.; Kesharwani, P.; Iyer, A.K.; Kalia, K.; Tekade, R.K. The use of nanoscaffolds and dendrimers in tissue engineering. Drug Discov. Today 2017, 22, 652–664. [Google Scholar] [CrossRef]
- Arkas, M.; Anastopoulos, I.; Giannakoudakis, D.A.; Pashalidis, I.; Katsika, T.; Nikoli, E.; Panagiotopoulos, R.; Fotopoulou, A.; Vardavoulias, M.; Douloudi, M. Catalytic Neutralization of water pollutants mediated by dendritic polymers. Nanomaterials 2022, 12, 445. [Google Scholar] [CrossRef]
- Devadas, B.; Periasamy, A.P.; Bouzek, K. A review on poly(amidoamine) dendrimer encapsulated nanoparticles synthesis and usage in energy conversion and storage applications. Coord. Chem. Rev. 2021, 444, 214062. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Laurent, R. Homogeneous catalysis with phosphorus dendrimer complexes. Coord. Chem. Rev. 2019, 389, 59–72. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Kuroda, H.; Yanagi, M.; Nanbu, H.; Juneja, L.R. Catalysis by Mesoporous Dendrimers. Top. Catal. 2009, 52, 634–642. [Google Scholar] [CrossRef]
- Fernandes, T.; Daniel-da-Silva, A.L.; Trindade, T. Metal-dendrimer hybrid nanomaterials for sensing applications. Coord. Chem. Rev. 2022, 460, 214483. [Google Scholar] [CrossRef]
- Thakare, S.; Shaikh, A.; Bodas, D.; Gajbhiye, V. Application of dendrimer-based nanosensors in immunodiagnosis. Colloids Surf. B Biointerfaces 2022, 209, 112174. [Google Scholar] [CrossRef]
- Loch, A.S.; Stoltzfus, D.M.; Burn, P.L.; Shaw, P.E. High-Sensitivity Poly(dendrimer)-Based Sensors for the Detection of Explosives and Taggant Vapors. Macromolecules 2020, 53, 1652–1664. [Google Scholar] [CrossRef]
- Sánchez, A.; Villalonga, A.; Martínez-García, G.; Parrado, C.; Villalonga, R. Dendrimers as Soft Nanomaterials for Electrochemical Immunosensors. Nanomaterials 2019, 9, 1745. [Google Scholar] [CrossRef]
- Xu, K.-F.; Jia, H.-R.; Liu, X.; Zhu, Y.-X.; Cong She, C.; Li, J.; Duan, Q.-Y.; Zhang, R.; Wu, F.-G. Fluorescent dendrimer-based probes for cell membrane imaging: Zebrafish epidermal labeling-based toxicity evaluation. Biosens. Bioelectron. 2022, 213, 114403. [Google Scholar] [CrossRef]
- McMahon, M.T.; Bulte, J.W.M. Two decades of dendrimers as versatile MRI agents: A tale with and without metals. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1496. [Google Scholar] [CrossRef]
- Qiao, Z.; Shi, X. Dendrimer-based molecular imaging contrast agents. Prog. Polym. Sci. 2015, 44, 1–27. [Google Scholar] [CrossRef]
- Roeven, E.; Scheres, L.; Smulders, M.M.J.; Zuilhof, H. Zwitterionic dendrimer—Polymer hybrid copolymers for self-assembling antifouling coatings. Eur. Polym. J. 2021, 156, 110578. [Google Scholar] [CrossRef]
- Riegert, D.; Bareille, L.; Laurent, R.; Majoral, J.-P.; Caminade, A.-M.; Chaumonnot, A. Silica Functionalized by Bifunctional Dendrimers: Hybrid Nanomaterials for Trapping CO2. Eur. J. Inorg. Chem. 2016, 2016, 3103–3110. [Google Scholar] [CrossRef]
- Ali, B.M.; Kumar, K.A.; Sultan Nasar, A. Fifth generation polyurethane dendrimers decorated with protected amine, free amine and blocked isocyanate end groups: Synthesis and electrolytic performance to increase the efficiency of Dye-Sensitized Solar Cell. ChemistrySelect 2019, 4, 12983–12991. [Google Scholar] [CrossRef]
- Ghann, W.; Kang, H.; Uddin, J.; Gonawala, S.J.; Mahatabuddin, S.; Ali, M.M. Dendrimer-based Nanoparticle for Dye Sensitized Solar Cells with Improved Efficiency. J. Nanomed. Nanotechnol. 2018, 9, 496. [Google Scholar] [CrossRef]
- Stoltzfus, D.M.; Ma, C.-Q.; Nagiri, R.C.R.; Clulow, A.J.; Bäuerle, P.; Burn, P.L.; Gentle, I.R.; Meredith, P. Thiophene dendrimer-based low donor content solar cells. Appl. Phys. Lett. 2016, 109, 103302. [Google Scholar] [CrossRef] [Green Version]
- Rajakumar, P.; Thirunarayanan, A.; Raja, S.; Ganesan, S.; Maruthamuthu, P. Photophysical properties and dye-sensitized solar cell studies on thiadiazole–triazole–chalcone dendrimers. Tetrahedron Lett. 2012, 53, 1139–1143. [Google Scholar] [CrossRef]
- Caminade, A.-M. Dendrimers, an Emerging Opportunity in Personalized Medicine? J. Pers. Med. 2022, 12, 1334. [Google Scholar] [CrossRef]
- Gudson, A.M.; Faraday, N.; Aita, J.S.; Kumar, S.; Mehta, I.; Choi, H.A.; Cleland, J.L.; Robinson, K.; McCullough, L.D.; Ng, D.K.; et al. Dendrimer nanotherapy for severe COVID-19 attenuates inflammation and neurological injury markers and improves outcomes in a phase2a clinical trial. Sci. Transl. Med. 2022, 14, eabo2652. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Kao, C.-L.; Selvaraj, A.; Peng, L. Solid-phase dendrimer synthesis: A promising approach to transform dendrimer construction. Mater. Today Chem. 2023, 27, 101285. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2020, 13, 65. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Bugno, J.; Hsu, H.-J.; Hong, S. Tweaking Dendrimers and Dendritic Nanoparticles for Controlled Nano-bio Interactions: Potential Nanocarriers for Improved Cancer Targeting. J. Drug Target. 2015, 23, 642–650. [Google Scholar] [CrossRef]
- Huang, D.; Wu, D. Biodegradable dendrimers for drug delivery. Mater. Sci. Eng. C 2018, 90, 713–727. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from “oxidative stress”. Drug Deliv. Trans. Res. 2020, 10, 259–270. [Google Scholar] [CrossRef]
- Leiro, V.; Spencer, A.P.; Magalhães, N.; Pêgo, A.P. Versatile fully biodegradable dendritic nanotherapeutics. Biomaterials 2022, 281, 121356. [Google Scholar] [CrossRef]
- Guan, X.-I.; Yang, X.-Q.; Lai, S.-J.; Ding, Y.-Y.; Zhang, J.-M.; Zhang, L.-Y.; Li, C.-H.; Tong, J.-H.; Lei, Z.-Q. Design and synthesis of biodegradable nonconjugated S—A—SAPAMAM dendrimers with unexpected Deep-red/NIR emission and cell membrane targeting ability for biological imaging. Mater. Des. 2022, 221, 110982. [Google Scholar] [CrossRef]
- Twibanire, J.K.; Grindley, T.B. Polyester Dendrimers: Smart Carriers for Drug Delivery. Polymers 2014, 6, 179–213. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface modification of PAMAM dendrimer improves its biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 815–817. [Google Scholar] [CrossRef]
- Nikolskaya, E.; Yabbarov, N.; Zhunina, O.; Tereshenko, O.; Mollaev, M.; Faustova, M.; Zamulaeva, I.; Severin, E. Influence of the doxorubicin conjugated PAMAM dendrimer surface charge on cytotoxic effects and intracellular trafficking routes in tumor cells. Mater. Today: Proc. 2017, 4, 6849–6855. [Google Scholar] [CrossRef]
- Cui, Y.; Liang, B.; Wang, L.; Zhu, L.; Kang, J.; Sun, H.; Chen, S. Enhanced biocompatibility of PAMAM dendrimers benefiting from tuning their surface charges. Mater. Sci. Eng. C 2018, 93, 332–340. [Google Scholar] [CrossRef]
- Kharwade, R.; More, S.; Warokar, A.; Agrawal, P.; Mahajan, N. Starburst pamam dendrimers: Synthetic approaches, surface modifications, and biomedical applications. Arab. J. Chem. 2020, 13, 6009–6039. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Wang, M.; Xu, H.; Wang, Z.; Li, Y.; Ding, B.; Gao, J. Effects of the surface charge of polyamidoamine dendrimers on cellular exocytosis and the exocytosis mechanism in multidrug-resistant breast cancer cells. J. Nanobiotechnol. 2021, 19, 135. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.-P. “Janus” dendrimers: Syntheses and properties. New J. Chem. 2012, 36, 217–226. [Google Scholar] [CrossRef]
- Casagrande, C.; Fabre, P.; Raphaël, E.; Veyssié, M. “Janus Beads”: Realization and behaviour at water/oil interfaces. Europhys. Lett. 1989, 9, 251–255. [Google Scholar] [CrossRef]
- de Gennes, P.-G. Soft matter. Angew. Chem. Int. Ed. 1992, 31, 842–845. [Google Scholar] [CrossRef]
- Sikwal, D.R.; Kalhapure, R.S.; Govender, T. An emerging class of amphiphilic dendrimers for pharmaceutical and biomedical applications: Janus amphiphilic dendrimers. Eur. J. Pharm. Sci. 2017, 97, 113–134. [Google Scholar] [CrossRef]
- Wooley, K.L.; Hawker, C.J.; Frechet, J.M. Polymers with controlled molecular architecture: Control of surface functionality in the synthesis of dendritic hyperbranched macromolecules using the convergent approach. J. Chem. Soc. Perkin Trans. 1991, 1, 1059–1076. [Google Scholar] [CrossRef]
- Wooley, K.L.; Hawker, C.J.; Frechet, J.M. Unsymmetrical three-dimensional macromolecules: Preparation and characterization of strongly dipolar dendritic macromolecules. J. Am. Chem. Soc. 1993, 115, 11496–11505. [Google Scholar] [CrossRef]
- Percec, V.; Wilson, D.A.; Leowanawat, P.; Wilson, C.J.; Hughes, A.D.; Kaucher, M.S.; Hammer, D.A.; Levine, D.H.; Kim, A.J.; Bates, F.S.; et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science 2010, 328, 1009–1014. [Google Scholar] [CrossRef]
- Peterca, M.; Percec, V.; Leowanawat, P.; Bertin, A. Predicting the Size and Properties of Dendrimersomes from the Lamellar Structure of Their Amphiphilic Janus Dendrimers. J. Am. Chem. Soc. 2011, 133, 20507–20520. [Google Scholar] [CrossRef]
- Percec, V.; Leowanawat, P.; Sun, H.-J.; Kulikov, O.; Nusbaum, C.D.; Tran, T.M.; Bertin, A.; Wilson, D.A.; Peterca, M.; Zhang, S.; et al. Modular synthesis of amphiphilic Janus glycodendrimers and their self-assembly into glycodendrimersomes and other complex architectures with bioactivity to biomedically relevant lectins. J. Am. Chem. Soc. 2013, 135, 9055–9077. [Google Scholar] [CrossRef]
- Zhang, S.; Moussodia, R.O.; Sun, H.J.; Leowanawat, P.; Muncan, A.; Nusbaum, C.D.; Chelling, K.M.; Heiney, P.A.; Klein, M.L.; André, S.; et al. Mimicking biological membranes with programmable glycan ligands self-assembled from amphiphilic Janus glycodendrimers. Angew. Chem. Int. Ed. 2014, 53, 10899–10903. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, Q.; Sherman, S.E.; Muncan, A.; Ramos Vicente, A.D.M.; Wang, Z.; Hammer, D.A.; Williams, D.; Chen, Y.; Pochan, D.J.; et al. Glycodendrimersomes from sequence-defined Janus glycodendrimers reveal high activity and sensor capacity for the agglutination by natural variants of human lectins. J. Am. Chem. Soc. 2015, 137, 13334–13344. [Google Scholar] [CrossRef]
- Xiao, Q.; Ludwig, A.-K.; Romanò, C.; Buzzacchera, I.; Sherman, S.E.; Vetro, M.; Vértesy, S.; Kaltner, H.; Reed, E.H.; Möller, M.; et al. Exploring functional pairing between surface glycoconjugates and human galectins using programmable glycodendrimersomes. Proc. Natl. Acad. Sci. USA 2018, 115, E2509–E2518. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Atochina-Vasserman, E.N.; Maurya, D.S.; Huang, N.; Xiao, Q.; Ona, N.; Liu, M.; Shahnawaz, H.; Ni, H.; Kim, K.; et al. One-Component Multifunctional Sequence-Defined Ionizable Amphiphilic Janus Dendrimer Delivery Systems for mRNA. J. Am. Chem. Soc. 2021, 143, 12315–12327. [Google Scholar] [CrossRef]
- Zhang, D.; Atochina-Vasserman, E.N.; Lu, J.; Maurya, D.S.; Xiao, Q.; Liu, M.; Adamson, J.; Ona, N.; Reagan, E.K.; Ni, H.; et al. The Unexpected Importance of the Primary Structure of the Hydrophobic Part of One-Component Ionizable Amphiphilic Janus Dendrimers in Targeted mRNA Delivery Activity. J. Am. Chem. Soc. 2022, 144, 4746–4753. [Google Scholar] [CrossRef]
- Filippi, M.; Martinelli, J.; Mulas, G.; Ferraretto, M.; Teirlinck, E.; Botta, M.; Tei, L.; Terreno, E. Dendrimersomes: A new vesicular nano-platform for MR-molecular imaging applications. Chem. Commun. 2014, 50, 3453–3456. [Google Scholar] [CrossRef]
- Filippi, M.; Patrucco, D.; Martinelli, J.; Botta, M.; Castro-Hartmann, P.; Tei, L.; Terreno, E. Novel stable dendrimersome formulation for safe bioimaging applications. Nanoscale 2015, 7, 12943–12954. [Google Scholar] [CrossRef]
- Plunkett, S.; El Khati, M.; Şencan, İ.; Porter, J.E.; Kumar, A.T.N.; Collins, J.E.; Sakadžić, S.; Vinogradov, S.A. In vivo Deep-Tissue Microscopy with UCNP/Janus-Dendrimers as Imaging Probes: Resolution at Depth and Feasibility of Ratiometric Sensing. Nanoscale 2020, 12, 2657–2672. [Google Scholar] [CrossRef]
- Rosati, M.; Acocella, A.; Pizzi, A.; Turtù, G.; Neri, G.; Demitri, N.; Nonappa; Raffaini, G.; Donnio, B.; Zerbetto, F.; et al. Janus-Type Dendrimers Based on Highly Branched Fluorinated Chains with Tunable Self-Assembly and 19F Nuclear Magnetic Resonance Properties. Macromolecules 2022, 55, 2486–2496. [Google Scholar] [CrossRef]
- Dengiz, C.; Breiten, B.; Gisselbrecht, J.P.; Boudon, C.; Trapp, N.; Schweizer, W.B.; Diederich, F. Synthesis and optoelectronic properties of Janus-dendrimer-type multivalent donor–acceptor systems. J. Org. Chem. 2015, 80, 882–896. [Google Scholar] [CrossRef]
- Gracia, I.; Feringán, B.; Serrano, J.L.; Termine, R.; Golemme, A.; Omenat, A.; Barberá, J. Functional Carbazole Liquid-Crystal Block Codendrimers withOptical and Electronic Properties. Chem. Eur. J. 2015, 21, 1359–1369. [Google Scholar] [CrossRef]
- Tang, R.; Zhou, S.; Cheng, Z.; Yu, G.; Peng, Q.; Zeng, H.; Guo, G.; Li, Q.; Li, Z. Janus second-order nonlinear optical dendrimers: Their controllable molecular topology and corresponding largely enhanced performance. Chem. Sci. 2017, 8, 340–347. [Google Scholar] [CrossRef]
- Iguarbe, V.; Concellón, A.; Termine, R.; Golemme, A.; Barberá, J.; Serrano, J.L. Making coaxial wires out of Janus dendrimers for efficient charge transport. ACS Macro Lett. 2018, 7, 1138–1143. [Google Scholar] [CrossRef]
- Iguarbe, V.; Barberá, J.; Serrano, J.L. Functional Janus dendrimers containing carbazole with liquid crystalline, optical and electrochemical properties. Liq. Cryst. 2020, 47, 301–308. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; Ma, B.; He, Y.-M.; Fan, Q.-H. Design and Synthesis of Janus-Type Chiral Dendritic Diphosphanes and Their Applications in Asymmetric Hydrogenation. Eur. J. Org. Chem. 2012, 2012, 6737–6744. [Google Scholar] [CrossRef]
- Choi, J.-W.; Cho, B.-K. Degree of chain branching-dependent assemblies and conducting behavior in ionic liquid crystalline Janus dendrimers. Soft Matter 2011, 7, 4045–4049. [Google Scholar] [CrossRef]
- Cho, B.-K. Spontaneous bulk organization of molecular assemblers based on aliphatic polyether and/or poly(benzyl ether) dendrons. Polym. J. 2012, 44, 475–489. [Google Scholar] [CrossRef]
- Percec, V.; Imam, M.R.; Peterca, M.; Leowanawat, P. Self-organizable vesicular columns assembled from polymers dendronized with semifluorinated Janus dendrimers act as reverse thermal actuators. J. Am. Chem. Soc. 2012, 134, 4408–4420. [Google Scholar] [CrossRef]
- Taabache, S.; Bertin, A. Vesicles from Amphiphilic Dumbbells and Janus Dendrimers: Bioinspired Self-Assembled Structures for Biomedical Applications. Polymers 2017, 9, 280. [Google Scholar] [CrossRef]
- Sherman, S.E.; Xiao, Q.; Percec, V. Mimicking complex biological membranes and their programmable glycan ligands with dendrimersomes and glycodendrimersomes. Chem. Rev. 2017, 117, 6538–6631. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, H.J.; Hughes, A.D.; Draghici, B.; Lejnieks, J.; Leowanawat, P.; Bertin, A.; Otero De Leon, L.; Kulikov, O.V.; Chen, Y.; et al. “Single–Single” amphiphilic Janus dendrimers self-assemble into uniform dendrimersomes with predictable size. ACS Nano 2014, 8, 1554–1565. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, S.; Wang, Z.; Sherman, S.E.; Moussodia, R.-O.; Peterca, M.; Muncan, A.; Williams, D.R.; Hammer, D.A.; Vértesy, S.; et al. Onion-like glycodendrimersomes from sequence-defined Janus glycodendrimers and influence of architecture on reactivity to a lectin. Proc. Natl. Acad. Sci. USA 2016, 113, 1162–1167. [Google Scholar] [CrossRef]
- Rodriguez-Emmenegger, C.; Xiao, Q.; Kostina, N.Y.; Sherman, S.E.; Rahimi, K.; Partridge, B.E.; Li, S.; Sahoo, D.; Reveron Perez, A.M.; Buzzacchera, I.; et al. Encoding biological recognition in a bicomponent cell-membrane mimic. Proc. Natl. Acad. Sci. USA 2019, 116, 5376–5382. [Google Scholar] [CrossRef]
- Xiao, Q.; Delbianco, M.; Sherman, S.E.; Perez, A.M.R.; Bharate, P.; Pardo-Vargas, A.; Rodriguez-Emmenegger, C.; Kostina, N.Y.; Rahimi, K.; Söder, D.; et al. Nanovesicles displaying functional linear and branched oligomannose self-assembled from sequence-defined Janus glycodendrimers. Proc. Natl. Acad. Sci. USA 2020, 117, 11931–11939. [Google Scholar] [CrossRef]
- Zhao, L.; Ling, Q.; Liu, X.; Hang, C.; Zhao, Q.; Liu, F.; Gu, H. Multifunctional triazolylferrocenyl Janus dendron: Nanoparticle stabilizer, smart drug carrier and supramolecular nanoreactor. Appl. Organometal. Chem. 2018, 32, e4000. [Google Scholar] [CrossRef]
- Najafi, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Janus-type dendrimers: Synthesis, properties, and applications. J. Mol. Liq. 2022, 347, 118396. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Jin, H.; Jiang, B.; Zhu, X.; Zhou, Y.; Lu, Z.; Yan, D. A supramolecular Janus hyperbranched polymer and its photoresponsive self-assembly of vesicles with narrow size distribution. J. Am. Chem. Soc. 2013, 135, 4765–4770. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Würthner, F. Morphology Control of Fluorescent Nanoaggregates by Co-Self-Assembly of Wedge- and Dumbbell-Shaped Amphiphilic Perylene Bisimides. J. Am. Chem. Soc. 2007, 129, 4886–4887. [Google Scholar] [CrossRef]
- Chen, C.; Posocco, P.; Liu, X.; Cheng, Q.; Laurini, E.; Zhou, J.; Liu, C.; Wang, Y.; Tang, J.; Col, V.; et al. Mastering dendrimer self-assembly for efficient siRNA delivery: From conceptual design to in vivo efficient gene silencing. Small 2016, 12, 3667–3676. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, T.; Ding, L.; Laurini, E.; Huang, Y.; Zhang, M.; Weng, Y.; Lin, S.; Chen, P.; Marson, D.; et al. A Dual Targeting Dendrimer-Mediated siRNA Delivery System for Effective Gene Silencing in Cancer Therapy. J. Am. Chem. Soc. 2018, 140, 16264–16274. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechét, J.M. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Walter, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593. [Google Scholar] [CrossRef]
- Grayson, S.M.; Frechét, J.M. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3867. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Pulgam, V.R.; Swanson, D.R.; Huang, B. Janus Dendrimers and Dendrons. U.S. Patent 7,977,452 B2, 12 July 2011. [Google Scholar]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications and the Future; Cambridge University Press: New York, NY, USA, 2012; pp. 64–75. [Google Scholar]
- Liu, Y.; Mu, S.; Liu, X.; Ling, Q.; Hang, C.; Ruiz, J.; Astruc, D.; Gu, H. Ferrocenyl Janus mixed-dendron stars and their stabilization of Au and Ag nanoparticles. Tetrahedron 2018, 74, 4777–4789. [Google Scholar] [CrossRef]
- Mu, S.; Ling, Q.; Liu, X.; Ruiz, J.; Astruc, D.; Gu, H. Supramolecular redox-responsive substrate carrier activity of a ferrocenyl Janus device. J. Inorg. Biochem. 2019, 193, 31–41. [Google Scholar] [CrossRef]
- Selin, M.; Nummelin, S.; Deleu, J.; Ropponen, J.; Viitala, T.; Lahtinen, M.; Koivisto, J.; Hirvonen, J.; Peltonen, L.; Kostiainen, M.A.; et al. High-Generation Amphiphilic Janus-Dendrimers as Stabilizing Agents for Drug Suspensions. Biomacromolecules 2018, 19, 3983–3993. [Google Scholar]
- Du, X.-J.; Wang, Z.-Y.; Wang, Y.-C. Redox-sensitive dendrimersomes assembled from amphiphilic Janus dendrimers for siRNA delivery. Biomater. Sci. 2018, 6, 2122–2129. [Google Scholar] [CrossRef]
- Yaddehige, M.L.; Chandrasiri, I.; Barker, A.; Kotha, A.K.; Williams, J.S.D.; Simms, B.; Kucheryavy, P.; Abebe, D.G.; Chougule, M.B.; Watkins, D.L. Structural and Surface Properties of Polyamidoamine (PAMAM)—Fatty Acid-based Nanoaggregates Derived from Self-assembling Janus Dendrimers. ChemNanoMat 2020, 6, 1833–1842. [Google Scholar] [CrossRef]
- Ohta, Y.; Abe, Y.; Hoka, K.; Baba, E.; Lee, Y.-P.; Dai, C.-A.; Yokozawa, T. Synthesis of amphiphilic, Janus diblock hyperbranched copolyamides and their self-assembly in water. Polym. Chem. 2019, 10, 4246–4251. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Wang, X.; Guo, D.; Duncan, T.M.; Luo, J. Zwitterionic Janus Dendrimer with distinct functional disparity for enhanced protein delivery. Biomaterials 2019, 215, 119233. [Google Scholar] [CrossRef]
- Najafi, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Synthesis of amphiphilic Janus dendrimer and its application in improvement of hydrophobic drugs solubility in aqueous media. Eur. Polym. J. 2020, 134, 109804. [Google Scholar] [CrossRef]
- Kostina, N.Y.; Söder, D.; Haraszti, T.; Xiao, Q.; Rahimi, K.; Partridge, B.E.; Klein, M.L.; Percec, V.; Rodriguez-Emmenegger, C. Enhanced concanavalin A binding to preorganized mannose nanoarrays in glycodendrimersomes revealed multivalent interactions. Angew. Chem. Int. Ed. 2021, 60, 8352–8360. [Google Scholar] [CrossRef]
- Laskar, P.; Somani, S.; Altwaijry, N.; Mullin, M.; Bowering, D.; Warzecha, M.; Keating, P.; Tate, R.J.; Leung, H.Y.; Dufès, C. Redox-sensitive, cholesterol-bearing PEGylated poly(propylene imine)-based dendrimersomes for drug and gene delivery to cancer cells. Nanoscale 2018, 10, 22830–22847. [Google Scholar] [CrossRef]
- San Anselmo, M.; Lancelot, A.; Egido, J.E.; Clavería-Gimeno, R.; Casanova, Á.; Serrano, J.L.; Hernández-Ainsa, S.; Abian, O.; Sierra, T. Janus Dendrimers to Assess the Anti-HCV Activity of Molecules in Cell-Assays. Pharmaceutics 2020, 12, 1062. [Google Scholar] [CrossRef]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Ciganda, R.; Gu, H.; Hernandez, R.; Escobar, A.; Martínez, A.; Yates, L.; Moya, S.; Ruiz, J.; Astruc, D. Electrostatic Assembly of Functional and Macromolecular Ferricinium Chloride-Stabilized Gold Nanoparticles. Inorg. Chem. 2017, 56, 2784–2791. [Google Scholar] [CrossRef]
- Rapakousiou, A.; Deraedt, C.; Gu, H.; Salmon, L.; Belin, C.; Ruiz, J.; Astruc, D. Mixed-Valent Click Intertwined Polymer Units Containing Biferrocenium Chloride Side Chains Form Nanosnakes that Encapsulate Gold Nanoparticles. J. Am. Chem. Soc. 2014, 136, 13995–13998. [Google Scholar] [CrossRef]
- Deraedt, C.; Rapakousiou, A.; Wang, Y.; Salmon, L.; Bousquet, M.; Astruc, D. Multifunctional Redox Polymers: Electrochrome, Polyelectrolyte, Sensor, Electrode Modifier, Nanoparticle Stabilizer, and Catalyst Template. Angew. Chem. Int. Ed. 2014, 53, 8445–8449. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Igartua, M.E.; Rapakousiou, A.; Salmon, L.; Moya, S.; Ruiz, J.; Astruc, D. Stabilization of AuNPs by monofunctional triazole linked to ferrocene, ferricenium, or coumarin and applications to synthesis, sensing, and catalysis. Inorg. Chem. 2014, 53, 11802–11808. [Google Scholar] [CrossRef]
- Wang, Y.; Salmon, L.; Ruiz, J.; Astruc, D. Metallodendrimers in three oxidation states with electronically interacting metals and stabilization of size-selected gold nanoparticles. Nat. Commun. 2014, 5, 3489. [Google Scholar] [CrossRef]
- Ciganda, R.; Irigoyen, J.; Gregurec, D.; Hernández, R.; Moya, S.; Wang, C.; Ruiz, J.; Astruc, D. Liquid–Liquid Interfacial Electron Transfer from Ferrocene to Gold(III): An Ultrasimple and Ultrafast Gold Nanoparticle Synthesis in Water under Ambient Conditions. Inorg. Chem. 2016, 55, 6361–6363. [Google Scholar] [CrossRef]
- Gu, H.; Ciganda, R.; Castel, P.; Vax, A.; Gregurec, D.; Irigoyen, J.; Moya, S.; Salmon, L.; Zhao, P.; Ruiz, J.; et al. Redox-Robust Pentamethylferrocene Polymers and Supramolecular Polymers, and Controlled Self-Assembly of Pentamethylferricenium Polymer-Embedded Ag, AgI, and Au Nanoparticles. Chem. Eur. J. 2015, 21, 18177–18186. [Google Scholar] [CrossRef]
- Rapakousiou, A.; Deraedt, C.; Irigoyen, J.; Wang, Y.; Pinaud, N.; Salmon, L.; Ruiz, J.; Moya, S.; Astruc, D. Synthesis and Redox Activity of “Clicked” Triazolylbiferrocenyl Polymers, Network Encapsulation of Gold and Silver Nanoparticles and Anion Sensing. Inorg. Chem. 2015, 54, 2284–2299. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Winnik, M.A.; Manners, I.J. Redox-Mediated Synthesis and Encapsulation of Inorganic Nanoparticles in Shell-Cross-Linked Cylindrical Polyferrocenylsilane Block Copolymer Micelles. J. Am. Chem. Soc. 2008, 130, 12921–12930. [Google Scholar] [CrossRef]
- Jia, L.; Tong, L.; Liang, Y.; Petretic, A.; Guerin, G.; Manners, I.; Winnik, M.A. Templated Fabrication of Fiber-Basket Polymersomes via Crystallization-Driven Block Copolymer Self-Assembly. J. Am. Chem. Soc. 2014, 136, 16676–16682. [Google Scholar] [CrossRef]
- Sui, X.; Feng, X.; Di Luca, A.; van Blitterswijk, C.A.; Moroni, L.; Hempenius, M.A.; Vancso, G.J. Poly(N-isopropylacrylamide)–poly(ferrocenylsilane) dual-responsive hydrogels: Synthesis, characterization and antimicrobial applications. Polym. Chem. 2013, 4, 337–342. [Google Scholar] [CrossRef]
- Sui, X.; Shui, L.; Cui, J.; Xie, Y.; Song, J.; van den Berg, A.; Hempenius, M.A.; Vancso, G.J. Redox-responsive organometallic microgel particles prepared from poly(ferrocenylsilane)s generated using microfluidics. Chem. Commun. 2014, 50, 3058–3060. [Google Scholar] [CrossRef]
- Enciso, A.E.; Doni, G.; Nifosì, R.; Palazzesi, F.; Gonzalez, R.; Ellsworth, A.A.; Coffer, J.L.; Walker, A.V.; Pavan, G.M.; Mohamed, A.A.; et al. Facile synthesis of stable, water soluble, dendron-coated gold nanoparticles. Nanoscale 2017, 9, 3128. [Google Scholar] [CrossRef]
- Shi, P.; Qu, Y.; Liu, C.; Khan, H.; Sun, P.; Zhang, W. Redox-Responsive Multicompartment Vesicles of Ferrocene-Containing Triblock Terpolymer Exhibiting On–Off Switchable Pores. ACS Macro Lett. 2016, 5, 88–93. [Google Scholar] [CrossRef]
- Morsbach, J.; Elbert, J.; Rüttiger, C.; Winzen, S.; Frey, H.; Gallei, M. Polyvinylferrocene-Based Amphiphilic Block Copolymers Featuring Functional Junction Points for Cross-Linked Micelles. Macromolecules 2016, 49, 3406–3414. [Google Scholar] [CrossRef]
- Torre, P.; Xiao, Q.; Buzzacchera, I.; Sherman, S.E.; Rahimi, K.; Kostina, N.Y.; Rodriguez-Emmenegger, C.; Möller, M.; Wilson, C.J.; Klein, M.L.; et al. Encapsulation of hydrophobic components in dendrimersomes and decoration of their surface with proteins and nucleic acids. Proc. Natl. Acad. Sci. USA 2019, 116, 15378–15385. [Google Scholar] [CrossRef]
- Yadavalli, S.S.; Xiao, Q.; Sherman, S.E.; Hasley, W.D.; Klein, M.L.; Goulian, M.; Percec, V. Bioactive cell-like hybrids from dendrimersomes with a human cell membrane and its components. Proc. Natl. Acad. Sci. USA 2019, 116, 744–752. [Google Scholar] [CrossRef]
- Murphy, P.V.; Romero, A.; Xiao, Q.; Ludwig, A.-K.; Jogula, S.; Shilova, N.V.; Singh, T.; Gabba, A.; Javed, B.; Zhang, D.; et al. Probing sulfatide-tissue lectin recognition with functionalized glycodendrimersomes. Iscience 2021, 24, 101919. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric lipid hybrid nanoparticles (plns) as emerging drug delivery platform—A comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloids Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Thoma, J.; Belegrinou, S.; Rossbach, P.; Grzelakowski, M.; Kita-Tokarczyk, K.; Meier, W. Membrane protein distribution in composite polymer-lipid thin films. Chem. Commun. 2012, 48, 8811–8813. [Google Scholar] [CrossRef]
- Xia, T.; Hao, W.; Shang, Y.; Xu, S.; Liu, H. Incorporation of Amphipathic Diblock Copolymer in Lipid Bilayer for Improving pH Responsiveness. Int. J. Polym. Sci. 2016, 2016, 5879428. [Google Scholar] [CrossRef]
- Buzzacchera, I.; Xiao, Q.; Han, H.; Rahimi, K.; Li, S.; Kostina, N.Y.; Toebes, B.J.; Wilner, S.E.; Möller, M.; Rodriguez-Emmenegger, C.; et al. Screening libraries of amphiphilic Janus dendrimers based on natural phenolic acids to discover monodisperse Unilamellar Dendrimersomes. Biomacromolecules 2019, 20, 712–727. [Google Scholar] [CrossRef]
- Joseph, A.; Wagner, A.M.; Garay-Sarmiento, M.; Aleksanyan, M.; Haraszti, T.; Söder, D.; Georgiev, V.N.; Dimova, R.; Percec, V.; Rodriguez-Emmenegge, C. Zwitterionic Dendrimersomes: A Closer Xenobiotic Mimic of Cell Membranes. Adv. Mater. 2022, 34, 2206288. [Google Scholar] [CrossRef]
- Kostina, N.Y.; Rahimi, K.; Xiao, Q.; Haraszti, T.; Dedisch, S.; Spatz, J.P.; Schwaneberg, U.; Klein, M.L.; Percec, V.; Möller, M.; et al. Membrane-mimetic Dendrimersomes engulf living bacteria via endocytosis. Nano Lett. 2019, 19, 5732–5738. [Google Scholar] [CrossRef]
- Kostina, N.Y.; Wagner, A.M.; Haraszti, T.; Rahimi, K.; Xiao, Q.; Klein, M.L.; Percec, V.; Rodriguez-Emmenegger, C. Unraveling topology-induced shape transformations in dendrimersomes. Soft Matter 2021, 17, 254–267. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, J.; Wei, Z.; Yu, D.; Zheng, S.; Wang, J.; Li, H.; Hua, Z.; Zhang, H.; Yang, G. Dynamic Glycopeptide Dendrimers: Synthesis and Their Controllable Self-Assembly into Varied Glyco-Nanostructures for the Biomimicry of Glycans. Biomacromolecules 2022, 23, 128–139. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Krishnan, N.; Perumal, D.; Atchimnaidu, S.; Harikrishnan, K.S.; Golla, M.; Kumar, N.M.; Kalathil, J.; Krishna, J.; Vijayan, D.K.; Varghese, R. Galactose-Grafted 2D Nanosheets from the Self-Assembly of Amphiphilic Janus Dendrimers for the Capture and Agglutination of Escherichia coli. Chem. Eur. J. 2020, 26, 1037–1041. [Google Scholar] [CrossRef]
- Lancelot, A.; Clavería-Gimeno, R.; Velázquez-Campoy, A.; Abian, O.; Serrano, J.L.; Sierra, T. Nanostructures based on ammonium-terminated amphiphilic Janus dendrimers as camptothecin carriers with antiviral activity. Eur. Polym. J. 2017, 90, 136–149. [Google Scholar] [CrossRef]
- Nazemi, A.; Gillies, E.R. Dendrimersomes with photodegradable membranes for triggered release of hydrophilic and hydrophobic cargo. Chem. Commun. 2014, 50, 11122–11125. [Google Scholar] [CrossRef]
- Zhang, X.; Rehm, S.; Safont-Sempere, M.M.; Wurthner, F. Vesicular Perylene Dye Nanocapsules as Supramolecular Fluorescent pH Sensor Systems. Nat. Chem. 2009, 1, 623–629. [Google Scholar] [CrossRef]
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983. [Google Scholar] [CrossRef]
- Fedeli, E.; Lancelot, A.; Serrano, J.L.; Calvo, P.; Sierra, T. Self-Assembling Amphiphilic Janus Dendrimers: Mesomorphic Properties and Aggregation in Water. New J. Chem. 2015, 39, 1960–1967. [Google Scholar] [CrossRef]
- Laskar, P.; Somani, S.; Mullin, M.; Tate, R.J.; Warzecha, M.; Bowering, D.; Keating, P.; Irving, C.; Leung, H.Y.; Dufès, C. Octadecyl chain-bearing PEGylated poly(propyleneimine)-based dendrimersomes: Physicochemical studies, redox-responsiveness, DNA condensation, cytotoxicity and gene delivery to cancer cells. Biomater. Sci. 2021, 9, 1431–1448. [Google Scholar] [CrossRef]
- Li, S.; Xia, B.; Javed, B.; Hasley, W.D.; Melendez-Davila, A.; Liu, M.; Kerzner, M.; Agarwal, S.; Xiao, Q.; Torre, P.; et al. Direct visualization of vesicle disassembly and reassembly using photocleavable dendrimers elucidates cargo release mechanisms. ACS Nano 2020, 14, 7398–7411. [Google Scholar] [CrossRef]
- García-Serradilla, M.; Risco, C.; Pacheco, B. Drug repurposing for new, efficient, broad spectrum antivirals. Virus Res. 2020, 264, 22–31. [Google Scholar] [CrossRef]
- Patil, A.; Mishra, V.; Thakur, S.; Riyaz, B.; Kaur, A.; Khursheed, R.; Patil, K.; Sathe, B. Nanotechnology Derived Nanotools in Biomedical Perspectives: An Update. Curr. Nanosci. 2019, 15, 137–146. [Google Scholar] [CrossRef]
- Su, C.; Liu, Y.; Li, R.; Wu, W.; Fawcett, J.P.; Gu, J. Absorption, distribution, metabolism and excretion of the biomaterials used in Nanocarrier drug delivery systems. Adv. Drug Deliv. Rev. 2019, 143, 97–114. [Google Scholar] [CrossRef]
- Lancelot, A.; González-Pastor, R.; Clavería-Gimeno, R.; Romero, P.; Abian, O.; Martín-Duque, P.; Serrano, J.L.; Sierra, T. Cationic poly(ester amide) dendrimers: Alluring materials for biomedical applications. J. Mater. Chem. B 2018, 6, 3956–3968. [Google Scholar] [CrossRef]
- Castillo-Rodríguez, I.O.; Hernández-Alducin, P.A.; Pedro-Hernández, L.D.; Barajas-Mendoza, I.; Ramírez-Ápan, T.; Martínez-García, M. Antileukemia and Anticolorectal Cancer Activity of Janus Dendrimer Conjugates with Naproxen and Ibuprofen. ChemistrySelect 2023, 8, e20220422. [Google Scholar] [CrossRef]
- Lv, J.; Fan, Q.; Wang, H.; Cheng, Y. Polymers for Cytosolic Protein Delivery. Biomaterials 2019, 218, 119358. [Google Scholar] [CrossRef]
- Lv, S.; Sylvestre, M.; Prossnitz, A.N.; Yang, L.F.; Pun, S.H. Design of Polymeric Carriers for Intracellular Peptide Delivery in Oncology Applications. Chem. Rev. 2021, 121, 11653–11698. [Google Scholar] [CrossRef]
- Cheng, Y. Design of Polymers for intracellular protein and peptide delivery. Chin. J. Chem. 2021, 39, 1443–1449. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Li, Y.; Lv, J.; Cheng, Y. Catechol-based polymers with high efficacy in cytosolic protein delivery. CCS Chem. 2022, 1–11. [Google Scholar] [CrossRef]
- Ren, L.; Lv, J.; Wang, H.; Cheng, Y. A Coordinative Dendrimer Achieves Excellent Efficiency in Cytosolic Protein and Peptide Delivery. Angew. Chem. Int. Ed. 2020, 59, 4711–4719. [Google Scholar] [CrossRef]
- Lv, J.; Liu, C.; Lv, K.; Wang, H.; Cheng, Y. Boronic acid-rich dendrimer for efficient intracellular peptide delivery. Sci. China Mater. 2020, 63, 620–628. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Gao, Y.; Cheng, Y. A manganese (II)-based coordinative dendrimer with robust efficiency in intracellular peptide delivery. Bioact. Mater. 2022, 9, 44–53. [Google Scholar] [CrossRef]
- Rong, G.; Wang, C.; Hu, J.; Li, Y.; Cheng, Y. Benzaldehyde-tethered fluorous tags for cytosolic delivery of bioactive peptides. J. Control. Release 2022, 351, 703–712. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kwon, S.H.; Lim, Y.-B. 3D2 Self-Assembling Janus Peptide Dendrimers with Tailorable Supermultivalency. Adv. Funct. Mater. 2019, 29, 1808020. [Google Scholar] [CrossRef]
- Falanga, A.; Del Genio, V.; Kaufman, E.A.; Zannella, C.; Franci, G.; Weck, M.; Galdiero, S. Engineering of Janus-Like Dendrimers with Peptides Derived from Glycoproteins of Herpes Simplex Virus Type 1: Toward a Versatile and Novel Antiviral Platform. Int. J. Mol. Sci. 2021, 22, 6488. [Google Scholar] [CrossRef]
- Xiao, Q.; Rivera-Martinez, N.; Raab, C.J.; Bermudez, J.G.; Good, M.C.; Klein, M.L.; Percec, V. Co-assembly of liposomes, Dendrimersomes, and Polymersomes with amphiphilic Janus dendrimers conjugated to Mono- and Tris-Nitrilotriacetic Acid (NTA, TrisNTA) enhances protein recruitment. Giant 2022, 9, 100089. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef]
- Apartsin, E.; Venyaminova, A.; Majoral, J.-P.; Caminade, A.-M. Dendriplex-Impregnated Hydrogels with Programmed Release Rate. Front. Chem. 2022, 9, 780608. [Google Scholar] [CrossRef]
- Gonçalves, G.A.R.; Paiva, R.M.A. Gene therapy: Advances, challenges and perspectives. Einstein 2017, 15, 369–375. [Google Scholar] [CrossRef]
- Laskar, P.; Somani, S.; Campbell, S.J.; Mullin, M.; Keating, P.; Tate, R.J.; Irving, C.; Leung, H.Y.; Dufès, C. Camptothecin-based dendrimersomes for gene delivery and redoxresponsive drug delivery to cancer cells. Nanoscale 2019, 11, 20058–20071. [Google Scholar] [CrossRef]
- Huang, X.; Kong, N.; Zhang, X.; Cao, Y.; Langer, R.; Tao, W. The landscape of mRNA nanomedicine. Nat. Med. 2022, 28, 2273–2287. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, D.; Liu, X.; Peng, L. Amphiphilic Dendrimer Vectors for RNA Delivery: State-of-the-Art and Future Perspective. Acc. Mater. Res. 2022, 3, 484. [Google Scholar] [CrossRef]
- Zhang, D.; Atochina-Vasserman, E.N.; Maurya, D.S.; Liu, M.; Xiao, Q.; Lu, J.; Lauri, G.; Ona, N.; Reagan, E.K.; Ni, H.; et al. Targeted Delivery of mRNA with One-Component Ionizable Amphiphilic Janus Dendrimers. J. Am. Chem. Soc. 2021, 143, 17975–17982. [Google Scholar] [CrossRef]
- Mahmoodi Chalbatani, G.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rad, M.R.; Marmari, V. Small interfering RNAs (siRNAs) in cancer therapy: A nano-based approach. Int. J. Nanomed. 2019, 2019, 3111–3128. [Google Scholar] [CrossRef]
- Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles Adv. Drug Deliv. Rev. 2020, 154–155, 64–78. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Vaiyapuri, R.; Zhang, L.; Chan, J.M. Lipid-coated polymeric nanoparticles for cancer drug delivery. Biomater. Sci. 2015, 3, 923–936. [Google Scholar] [CrossRef]
- Yang, X.-Z.; Dou, S.; Wang, Y.-C.; Long, H.-Y.; Xiong, M.-H.; Mao, C.-Q.; Yao, Y.-D.; Wang, J. Single-step assembly of cationic lipid-polymer hybrid nanoparticles for systemic delivery of siRNA. ACS Nano 2012, 6, 4955–4965. [Google Scholar] [CrossRef]
- Yu, T.; Liu, X.; Bolcato-Bellemin, A.; Wang, Y.; Liu, C.; Erbacher, P.; Qu, F.; Rocchi, P.; Behr, J.; Peng, L. An amphiphilic dendrimer for effective delivery of small interfering RNA and gene silencing in vitro and in vivo. Angew. Chem. Int. Ed. 2012, 51, 8478–8484. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Yu, T.; Chen, C.; Cheng, Q.; Sengupta, K.; Huang, Y.; Li, H.; Liu, C.; Wang, Y.; et al. Adaptive amphiphilic dendrimer-based nanoassemblies as robust and versatile siRNA delivery systems. Angew. Chem. Int. Ed. 2014, 53, 11822–11827. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Chen, C.; Tintaru, A.; Cao, Y.; Liu, J.; Ziarelli, F.; Tang, J.; Guo, H.; Rosas, R.; et al. A fluorinated bola-amphiphilic dendrimer for on-demand delivery of siRNA, via specific response to reactive oxygen species. Adv. Funct. Mater. 2016, 26, 8594–8603. [Google Scholar] [CrossRef]
- Nazemi, A.; Martínez, F.; Scholl, T.J.; Gillies, E.R. Biodegradable Dendritic Polymersomes as Modular, High-Relaxivity MRI Contrast Agents. RSC Adv. 2012, 2, 7971–7973. [Google Scholar] [CrossRef]
- Filippi, M.; Remotti, D.; Botta, M.; Terreno, E.; Tei, L. GdDOTAGA(C18)2: An efficient amphiphilic Gd(iii) chelate for the preparation of self-assembled high relaxivity MRI nanoprobes. Chem. Comm. 2015, 51, 17455–17458. [Google Scholar] [CrossRef]
- Filippi, M.; Catanzaro, V.; Patrucco, D.; Botta, M.; Tei, L.; Terreno, E. First in vivo MRI study on theranostic dendrimersomes. J. Control. Release 2017, 248, 45–52. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, X.; Chen, L.; Lin, H.; Gao, J. Multicolor 19F magnetic resonance imaging: A promising medical technique for in vivo visualization of multiple biological targets. Fundam. Res. 2022, in press. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Liu, X.; Jeong, E.K.; Yu, Y.B. Symmetry-guided design and fluorous synthesis of a stable and rapidly excreted imaging tracer for 19F MRI. Angew. Chem. Int. Ed. Engl. 2009, 48, 4755–4758. [Google Scholar] [CrossRef]
- Xiao, Q.; Rubien, J.D.; Wang, Z.; Reed, E.H.; Hammer, D.A.; Sahoo, D.; Heiney, P.A.; Yadavalli, S.S.; Goulian, M.; Wilner, S.E.; et al. Self-sorting and coassembly of fluorinated, hydrogenated, and hybrid Janus dendrimers into dendrimersomes. J. Am. Chem. Soc. 2016, 138, 12655–12663. [Google Scholar] [CrossRef] [Green Version]



| Encapsulated Drug | Hydrophilic/Hydrophobic Dendrons | Methods | Tests | Results | Conclusions |
|---|---|---|---|---|---|
| Indomethacin (IN) [106] | Propargyl-modified bis-MPA G3, G4/ Percec-type azide | DSC | Physical interactions Chemical compatibility |
|
|
| CAT | Drug wettability |
|
| ||
| MP-SPR | Solid−liquid interface interactions |
|
| ||
| DLS | Stabilization of IN suspensions |
|
| ||
| UV-Vis | Dissolution rate |
|
| ||
| Rhodamine B (RhB) [93] | TEG termini /Ferrocenyl (Fc) units | UV-Vis | Drug loading |
|
|
| Redox- responsive release |
|
| |||
| DLS | Redox- responsive release |
|
| ||
| Tiratricol (TRIAC) [114] Iopanoic acid (IA) [114] | (NH3+)8 [GMPA]/[MPA](C17)4 (NH3+)8 [GMPA]/[MPA](C17)2 | ITC | JD-drugs affinity |
|
|
| UV-Vis | Drug loading |
|
| ||
| TEM | Morphology of JD-drugs |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Căta, A.; Ienașcu, I.M.C.; Ştefănuț, M.N.; Roșu, D.; Pop, O.-R. Properties and Bioapplications of Amphiphilic Janus Dendrimers: A Review. Pharmaceutics 2023, 15, 589. https://doi.org/10.3390/pharmaceutics15020589
Căta A, Ienașcu IMC, Ştefănuț MN, Roșu D, Pop O-R. Properties and Bioapplications of Amphiphilic Janus Dendrimers: A Review. Pharmaceutics. 2023; 15(2):589. https://doi.org/10.3390/pharmaceutics15020589
Chicago/Turabian StyleCăta, Adina, Ioana Maria Carmen Ienașcu, Mariana Nela Ştefănuț, Dan Roșu, and Oana-Raluca Pop. 2023. "Properties and Bioapplications of Amphiphilic Janus Dendrimers: A Review" Pharmaceutics 15, no. 2: 589. https://doi.org/10.3390/pharmaceutics15020589
APA StyleCăta, A., Ienașcu, I. M. C., Ştefănuț, M. N., Roșu, D., & Pop, O.-R. (2023). Properties and Bioapplications of Amphiphilic Janus Dendrimers: A Review. Pharmaceutics, 15(2), 589. https://doi.org/10.3390/pharmaceutics15020589






